Abstract
Objective
The aim of this study was to explore the clinical efficacy of conventional heparin anticoagulation in combination with apixaban in the treatment of patients with cerebral venous thrombosis (CVT) and its influence on serum D-dimer (D-D) and fibrinogen (FIB).
Methods
One hundred and fifty-seven consecutive CVT patients admitted to our hospital from January 1, 2006, to December 31, 2013, were allocated into two groups according to the different treatment methods, of which 95 cases received standard anticoagulation therapy (standard group (SG)) and the remaining 62 cases were given apixaban therapy (research group (RG)). The curative effects and the changes of coagulation function during the treatment, as well as the incidence of adverse reactions, were analyzed in the two groups. The changes of D-D and FIB levels before treatment and at days 1, 4, and 7 posttreatment were detected.
Results
In treatment efficacy, RG was superior to SG. No evident difference was observed in the incidence of adverse events or coagulation function between the two groups. At day 1 posttreatment, D-D level was increased largely in both SG and RG, but the increase was much more significant in RG. However, D-D level was decreased gradually with time in both groups, and the reduction was more notable in RG. The FIB level in SG declined gradually with time after treatment and was higher than that in RG at the same time point. In RG, FIB was decreased gradually at day 1 and day 4 posttreatment, and its level at day 7 posttreatment showed no difference compared with that at day 4 posttreatment. Spearman's analysis identified that the higher the D-D level or the lower the FIB level at day 1 posttreatment was, the better the treatment efficacy was. After seven-day treatment, the lower the level of D-D and FIB was, the better the therapeutic effect was. Logistic analysis indicated that age, time of diagnosis, deep vein thrombosis (DVT), Glasgow Coma Scale (GCS) score, infection, Apixaban, D-D, and FIB all independently affect the treatment effect of patients.
Conclusions
The combined use of Apixaban with heparin is high-performing and safe in the treatment of CVT. The changes of D-D and FIB levels during the treatment are strongly linked to the therapeutic effect, which can be used as plausible evaluation indexes for the efficacy of CVT.
1. Introduction
Cerebral venous thrombosis (CVT), also known as cerebral venous sinus thrombosis (CVST), has an annual incidence of approximately 1.32-1.57/100,000 people [1, 2]. It is a cerebrovascular disease with focal cerebral edema, venous cerebral infarction, epilepsy, and intracranial hypertension as the most prominent clinical characteristics, which often involves young adults and women of childbearing age and children [3, 4].
Although the mortality rate of CVT has been profoundly reduced with the improvement of treatment and diagnostic techniques, the mortality rate of severe CVT is still as high as 34.2% [5].
If CVT can be identified correctly at an early stage, patients could receive appropriate treatment in time, such as anticoagulation, intracranial pressure reduction, or neurosurgery. Thus, most patients diagnosed and treated at an early stage could have a favorable prognosis [6, 7]. After diagnosis by computed tomography (CT) or magnetic resonance (MR) venography, the standard treatment is to use heparin for anticoagulation first and then warfarin for anticoagulation [8]. It was reported that the treatment with anticoagulation for CVT was first demonstrated to be beneficial in a prospective study in 1991 [2]. The adoption of direct oral anticoagulants (DOAC), including dabigatran (a direct thrombin inhibitor) and the direct factor Xa (FXa) inhibitors such as apixaban, provides a viable alternative to vitamin K antagonists (VKA) or heparins for treating venous thrombosis and preventing stroke in atrial fibrillation patients in both the acute and longer-term phases. DOAC has been proved that it is more effective than VKA through more predictable pharmacokinetics, and it displayed similar efficacy to VKA in the treatment of acute venous thromboembolism (VTE), similarly, if not better, bleeding and mortality rates [1–6]. Subsequently, increased prescribing of these medications was shown in the last decade.
Apixaban belonged to an oral FXa inhibitor, which exerted an inhibitory effect on both free and clot-bound factor Xa, and also, it got approval in the clinical use of several thromboembolic disorders including reduction of stroke risk in nonvalvular atrial fibrillation, thromboprophylaxis following hip or knee replacement surgery, the treatment of deep vein thrombosis (DVT) or pulmonary embolism, and prevention of recurrent DVT and pulmonary embolism [4–6]. Apixaban was reported to be as effective as warfarin in the treatment of DVT and pulmonary embolism [9, 10]. More importantly, apixaban has many advantages including predictable pharmacokinetics and pharmacodynamics, low number of drug and food interactions, and relatively wide therapeutic window. However, only case reports and small sample studies have so far described the application of apixaban in CVT patients. Rao et al. [11] tracked a CVT patient who received apixaban in their study. During the follow-up, the patient had good tolerance to apixaban without any bleeding complications, and CT scan showed thrombolysis and recanalization, suggesting that apixaban may be a safe and viable method to treat CVT. Covut et al. [12] reported the therapeutic effect of apixaban on 5 CVT patients. Only 2 patients developed lower gastrointestinal bleeding 15 days after discharge. MR/CT venography showed that no patients had complete recanalization, 2 (40%) patients had partial recanalization, and 3 (60%) patients had no recanalization. During the treatment, no patients experienced clinically significant hemorrhage, nor did they experience thromboembolic events.
In this context, the therapeutic effect of apixaban in 62 CVT patients was analyzed, and the changes of D-dimer (D-D) and fibrinogen (FIB) levels in patients' peripheral blood were detected, so as to evaluate the therapeutic effect of Apixaban in this disease.
2. Materials and Methods
2.1. Study Participants
This study is a prospective cohort analysis, with 157 CVT patients admitted to our hospital between January 1, 2006, and December 31, 2013, and enrolled. The following are the inclusion criteria: all patients were diagnosed as CVT by X-ray and CT angiography and received oral anticoagulation therapy for at least 3 to 6 months. The following are the exclusion criteria: patients excluded were those (1) aged <18 years; (2) with severe liver and kidney dysfunction, mental abnormality, consciousness disorder, or inability to swallow oral drugs; (3) with CVT and central nervous system infection or severe head trauma; (4) with planned CVT surgical procedures; (5) with tumors; or (6) incomplete clinical data. This study conforms to the Declaration of Helsinki and has been ratified by the hospital's Medical Ethics Committee. All patients have signed the informed consent form.
2.2. Treatment Methods
Patients were divided into two groups based on different treatment methods. Of them, 95 patients were given standard anticoagulation therapy (standard group (SG)), and the other 62 patients were treated with apixaban therapy (research group (RG)). Standard treatment began with subcutaneous injection of low-molecular-weight enoxaparin (1 mg/kg) twice daily for 5-15 days. Warfarin was not used until the acute phase. In principle, warfarin and heparin were used repeatedly for 3-5 days, and heparin was withdrawn when the International Normalized Ratio (INR) of warfarin reached 2.0-3.0. The INR was detected regularly to adjust the dosage of warfarin, and the treatment duration was ≤24 weeks. After discontinuation of intravenous heparin therapy, apixaban was administered orally twice a day at a dose of 5 mg and measured 5 weeks later.
2.3. Follow-Up
After discharge, the patients were followed up by interview, which lasted for 6 months and was conducted once a month.
2.4. Outcome Measures
The following are the outcome measures:
Clinical efficacy: the efficacy was evaluated referring to the score standards of Clinical neurological impairment in Stroke Patients (1995).
Basically cured: the patients' functional impairment score reduced by 91-100%, and the degree of disability was grade 0.
Significantly improved: the patient's functional impairment score decreased by 46%-90%, and the degree of disability was 1-3.
Improved: the patient's functional impairment score decreased by 18%-45%.
No change: the patient's functional impairment score decreased by about 17%.
Deteriorated: the patient's functional impairment score decreased or increased by more than 18%.
Death: total effective rate = number of cases with (basically cured + significantly improved + improved)/total number of cases∗100%.
Statistics on adverse events: severe bleeding is defined as significant bleeding with a hemoglobin drop of more than 2 g/dL and/or requiring blood product infusion of more than 2 U, when the patient should stop the study medication.
Coagulation function analysis: the coagulation function, including activated partial thromboplastin time (APTT), partial prothrombin time (PT), and platelet (PLT), was compared between the two series before and after the last treatment.
Determination of D-D and FIB levels: fasting venous blood was collected before treatment and 1, 4, and 7 days after treatment for the determination of D-D and FIB via the SysmexCA7000 automatic blood coagulation analyzer (Hisenmeikang, Japan), specifically by immunoturbidimetry.
2.5. Statistical Analysis
SPSS19.0 was used for statistical analysis of the data. The counting data were represented by n (%), and the differences between groups were compared by a χ2 test. The measurement data were recorded as the mean ± SD. One-way ANOVA was used for comparison among groups, LSD test for back testing, repeated measures ANOVA for comparison at different time points, and LSD test for post hoc testing. Correlation analysis was done by Spearman's analysis, and the risk factors affecting efficacy were determined by logistic analysis. P < 0.05 was considered statistically significant.
3. Results
3.1. General Information
A total of 157 CVT patients were included in this study, including 95 cases in SG and 62 cases in RG. Basic data analysis revealed no statistical difference between RG and SG in terms of sex ratio, age, CVT diagnosis method, time of admission, time of diagnosis, hospitalization time, symptoms, DVT, GCS score, and infection (Table 1).
Table 1.
Comparison of clinical data between the two groups (n (%)).
| Standard group (n = 95) | Research group (n = 62) | χ 2/t | P | |
|---|---|---|---|---|
| Gender | 0.045 | 0.833 | ||
| Male | 43 (45.26) | 27 (43.55) | ||
| Female | 52 (54.74) | 35 (56.45) | ||
| Age (years old) | 2.860 | 0.582 | ||
| <30 | 11 (11.58) | 8 (12.90) | ||
| 30-39 | 25 (26.31) | 10 (16.13) | ||
| 40-49 | 30 (31.58) | 22 (35.48) | ||
| 50-59 | 14 (14.74) | 13 (20.97) | ||
| ≥60 | 15 (15.79) | 9 (14.52) | ||
| Diagnostic method | 3.502 | 0.321 | ||
| CT combined with ductography | 58 (61.05) | 32 (51.61) | ||
| MRI combined with ductography | 24 (25.26) | 24 (38.71) | ||
| CT combined with CT venography | 5 (5.26) | 3 (4.84) | ||
| MRI combined with MR venography | 8 (8.42) | 3 (4.84) | ||
| Admission time (d) | 4 ± 3 | 5 ± 4 | 1.786 | 0.076 |
| Time of diagnosis (d) | 7 ± 4 | 8 ± 5 | 1.386 | 0.168 |
| Hospitalization time (d) | 16 ± 8 | 17 ± 10 | 0.693 | 0.490 |
| Symptoms | 1.483 | 0.686 | ||
| Aphasia | 21 (22.11) | 10 (16.13) | ||
| Dyskinesia | 28 (29.47) | 23 (37.10) | ||
| Mental disorder | 16 (16.84) | 9 (14.52) | ||
| Intracranial hypertension | 30 (31.53) | 20 (32.26) | ||
| Headache | 88 | 39 | ||
| Deep vein thrombosis | 0.209 | 0.648 | ||
| Yes | 10 (10.53) | 8 (12.90) | ||
| No | 85 (89.47) | 54 (87.10) | ||
| GCS score | 1.312 | 0.519 | ||
| 13-15 | 64 | 46 | ||
| 9-12 | 18 | 11 | ||
| ≤8 | 13 | 5 | ||
| Infection | 2.026 | 0.155 | ||
| Yes | 17 (17.89) | 6 (9.68) | ||
| No | 78 (82.11) | 56 (90.32) | ||
| C-reactive protein (mg/L) | 10.25 ± 1.25 | 10.34 ± 1.36 | 0.215 | 0.830 |
| Homocysteine (μmol/L) | 16.45 ± 1.55 | 16.50 ± 1.50 | 0.098 | 0.923 |
| Drinking | 0.993 | 0.319 | ||
| Yes | 49 (51.58) | 37 (59.68) | ||
| No | 46 (48.42) | 25 (40.32) | ||
| Smoking | 0.338 | 0.561 | ||
| Yes | 43 (45.26) | 31 (50.00) | ||
| No | 52 (54.74) | 31 (50.00) |
GCS: Glasgow Coma Scale; CT: computed tomography; MRI: magnetic resonance.
3.2. Therapeutic Effect Analysis
The total effective rate of patients in RG was higher than that in SG. RG had higher proportion of patients who were basically cured and lower proportion of patients who showed no change after treatment than SG (Table 2).
Table 2.
Treatment effect analysis of patients in the two groups (n (%)).
| Standard group (n = 95) | Research group (n = 62) | χ 2 | P | |
|---|---|---|---|---|
| Basically cured | 9 (9.47) | 26 (41.94) | 22.821 | <0.001 |
| Significantly improved | 22 (23.16) | 22 (35.48) | 2.826 | 0.093 |
| Improved | 50 (52.63) | 12 (19.35) | 17.385 | <0.001 |
| No change | 10 (10.53) | 1 (1.61) | 4.575 | 0.032 |
| Deteriorated or dead | 4 (4.21) | 1 (1.61) | 0.821 | 0.365 |
| Total effective rate | 81 (85.26) | 60 (96.77) | 5.431 | 0.020 |
3.3. Adverse Event Analysis
Statistical analysis showed no difference in the incidence of single adverse event or total adverse event between the two series (Table 3).
Table 3.
Adverse event analysis in the two groups.
| Standard group (n = 95) | Research group (n = 62) | χ 2 | P | |
|---|---|---|---|---|
| Recurrence | 0 (0.00) | 0 (0.00) | — | — |
| Intracranial hemorrhage | 3 (3.16) | 2 (3.23) | 0.441 | 0.659 |
| Gastrointestinal bleeding | 2 (2.11) | 1 (1.61) | 0.376 | 0.707 |
| Venous thrombosis | 3 (3.16) | 1 (1.61) | 0.082 | 0.934 |
| Thrombocytopenia | 2 (2.11) | 1 (1.61) | 0.376 | 0.707 |
| Abdominal discomfort | 3 (3.16) | 2 (3.23) | 0.441 | 0.659 |
| Elevated liver enzymes | 1 (1.05) | 0 (0.00) | Fisher | >0.999 |
| Depression | 7 (7.37) | 5 (4.84) | 0.113 | 0.737 |
| Total incidence | 21 (22.11) | 12 (19.35) | 0.171 | 0.679 |
3.4. Comparison of Coagulation Function between the Two Series
APTT, PT, and PLT identified no evident differences between the two series before and after treatment (P > 0.05) (Figure 1).
Figure 1.
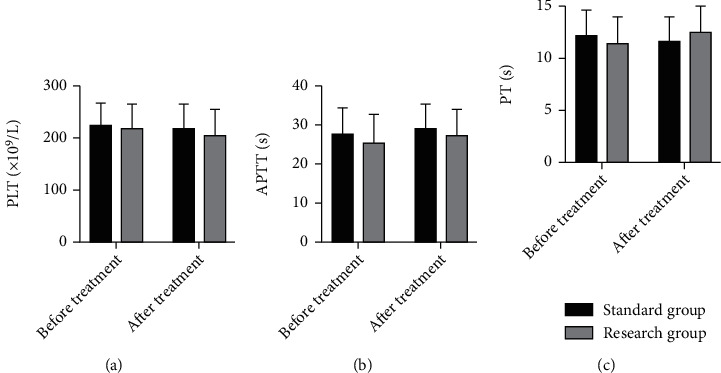
Comparison of coagulation function between the two groups. (a) Comparison of PLT levels between the two groups before and after treatment. (b) Comparison of APTT levels between the two groups before and after treatment. (c) Comparison of PT levels between the two groups before and after treatment. APTT: activated partial thromboplastin time; PT: partial prothrombin time; PLT: platelet.
3.5. Changes of D-D and FIB Levels in the Two Series during Treatment
The pretreatment D-D and FIB levels showed no marked differences between the two series. On posttreatment day 1, D-D increased observably in both SG and RG, but the increase was more significant in RG. However, D-D decreased gradually with time in both series, and the reduction was more notable in RG. The FIB level in SG declined gradually with time after treatment and was higher than that in RG at the same time point. In RG, FIB decreased gradually on posttreatment day 1 and day 4, and its level at 7 days after treatment showed no difference compared with that at 4 days after treatment (Figure 2).
Figure 2.
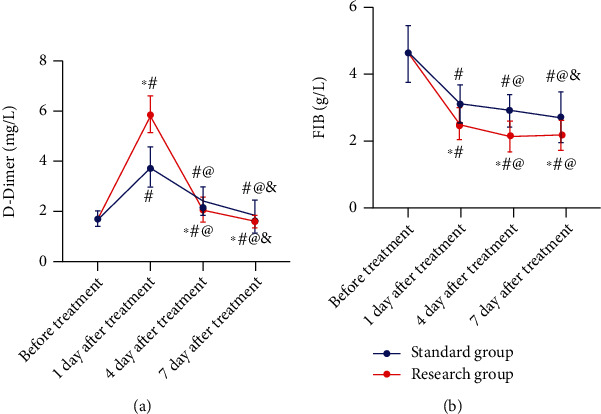
Changes of D-dimer and FIB levels in the two groups during treatment. (a) Changes in D-dimer levels. (b) Changes in FIB levels. ∗P < 0.05vs. standard group at the same time point; #P < 0.05vs. before treatment; @P < 0.05vs. one day after treatment; &P < 0.05vs. four days after treatment. FIB: fibrinogen.
3.6. Correlation of D-D and FIB Levels with Therapeutic Effect
We analyzed the correlation of D-D and FIB with therapeutic effect one day and seven days after treatment. It was identified that the higher the D-D level or the lower the FIB level one day after treatment, the better the treatment effect. Seven days after treatment, the lower the levels of D-D and FIB, the better the therapeutic effect (Figure 3).
Figure 3.
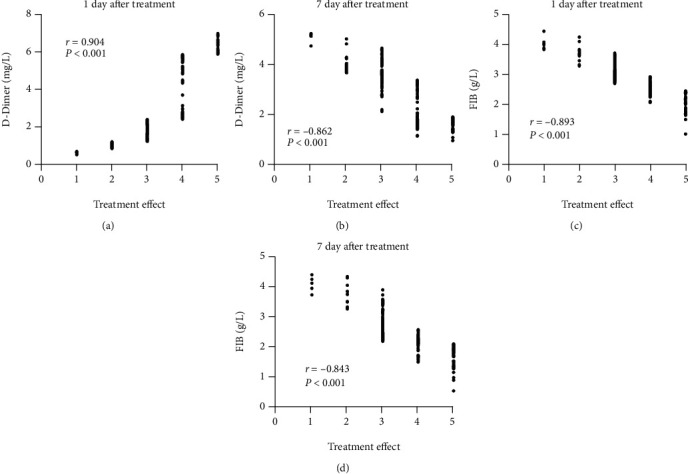
Correlation of D-dimer and FIB levels with therapeutic effect. (a) Relationship between D-dimer and therapeutic effect one day after treatment. (b) Relationship between D-dimer and therapeutic effect 7 days after treatment. (c) Relationship between FIB and therapeutic effect one day after treatment. (d) Relationship between FIB and therapeutic effect 7 days after treatment. FIB: fibrinogen.
3.7. Analysis of Risk Factors Affecting Curative Effect
In order to analyze the factors influencing the curative effect of patients, we established a logistic model. The results identified that age, time of diagnosis, DVT, GCS score, infection, apixaban, D-D, and FIB can independently influence the curative effect of patients (Table 4).
Table 4.
Logistic analysis of curative effect.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | ||
| Gender | Male vs. female | 0.561 | 1.358 (0.484-3.683) | ||
| Age | <40 vs. ≥40 years | 0.031 | 2.167 (1.072-4.381) | 0.048 | 1.654 (1.014-2.716) |
| Hospitalization time (d) | Unclassified | 0.124 | 2.023 (0.825-4.960) | ||
| Time of diagnosis (d) | Unclassified | 0.001 | 3.266 (1.644-6.475) | 0.004 | 3.017 (1.426-6.316) |
| Hospitalization time (d) | Unclassified | 0.039 | 1.036 (1.576-5.887) | 0.382 | 1.688 (0.521-5.552) |
| Deep vein thrombosis | Yes vs. no | 0.013 | 1.842 (1.152-2.977) | 0.035 | 2.763 (1.093-5.388) |
| GCS score | >8 vs. ≤8 | 0.025 | 1.536 (0.284-1.987) | 0.038 | 1.155 (0.653-2.118) |
| Infection | Yes vs. no | 0.001 | 9.379 (6.482-13.166) | 0.018 | 5.477 (1.247-23.318) |
| Drinking | Yes vs. no | 0.034 | 2.158 (1.063-4.372) | 0.739 | 1.227 (0.393-3.897) |
| Smoking | Yes vs. no | 0.031 | 2.601 (1.068-6.269) | 0.381 | 1.688 (0.531-5.552) |
| Complications | Yes vs. no | 0.046 | 1.287 (0.739-2.286) | 0.085 | 0.977 (0.188-1.964) |
| Treatment methods | Standard vs. apixaban | 0.004 | 3.017 (1.426-6.318) | 0.023 | 2.979 (1.185-7.648) |
| D-dimer | Unclassified | 0.001 | 1.006 (1.664-5.411) | 0.006 | 1.984 (1.232-3.264) |
| FIB | Unclassified | 0.025 | 1.545 (0.284-1.979) | 0.036 | 0.878 (0.394-1.194) |
GCS: Glasgow Coma Scale; FIB: fibrinogen.
4. Discussion
CVT is a rare cause of stroke, and the prognosis of patients is good. However, due to persistent neuropsychiatric symptoms and cognitive problems, approximately 1/4 of patients still cannot resume normal life and work [13, 14]. The current guidelines for the treatment of CVT are consistent, and anticoagulation is still the mainstay treatment method, with heparin or low-molecular-weight-heparin (LMWH) as the main drug [15, 16]. The optimal duration for anticoagulation treatment is usually 3 to 12 months. However, the condition of some severe CVT patients deteriorates after anticoagulation therapy [17], so the treatment strategy of CVT should be improved.
As an Xa inhibitor, apixaban has been approved in many countries for several indications [3–6]. The results of the key phase III clinical trials supporting its approval demonstrated that apixaban is an important alternative to existing anticoagulant therapies, such as VKAs or aspirin or LMWH, with an improved benefit-risk profile. Apixaban is an oral anticoagulant that can inhibit the production of thrombin by blocking the coagulation pathway both in vivo and in vitro. This paper analyzed the efficacy of apixaban in CVT patients on the basis of LMWH. The results exhibited that the patients supplemented with apixaban had better therapeutic effect. According to the score standards of clinical neurological impairment in stroke patients (1995), the basic recovery rate of patients treated with apixaban is 41.94%, which is notably higher than that of patients treated with LMWH (9.47%). Meanwhile, apixaban supplementation does not increase additional adverse reactions and has no significant effect on the coagulation function of the patients, suggesting that apixaban enjoys promising efficacy and safety in CVT treatment.
It is often applied to prevent thrombosis in cancer patients due to its merits in administration route, convenient use, and cost [18, 19]. Apixaban has been shown to reduce massive bleeding and VTE recurrence in the treatment of cancer-related venous thromboembolism (VTE) [20], as well as to prevent venous thrombosis and pulmonary embolism in the limbs of cancer patients [20, 21]. In the retrospective study of acute VTE, apixaban also showed no less efficacy and safety than rivaroxaban [22, 23]. However, in the current research on apixaban in treating CTV, the number of reported cases is small, although all of them show the positive effect of apixaban in treating CVT [11, 12]. Our research included 62 patients who received apixaban, which, to a certain extent, supplemented the evidence that apixaban actively treated CVT. We hope that more relevant studies will be conducted to provide a large number of case data.
The coagulation and fibrinolysis state were altered in CVT patients, and the changes play an important role in thrombolytic therapy [24, 25]. We analyzed the changes of D-D and FIB levels in CVT patients and found that the changes were closely linked to the treatment effect. The higher the D-D level in CVT patients one day after treatment, the better the curative effect; 7 days after treatment, the lower the D-D level of CVT patients, the better the curative effect, while the FIB level of CVT patients kept decreasing after treatment. D-D is a specific degradation product of cross-linked fibrin, and its increased level reflects secondary hyperfibrinolytic activity [26]. Higher D-D levels are found to be associated with thrombotic enlargement and acute attacks of symptoms. Although low D-D levels could not rule out CVT in patients with subacute or chronic diseases [27], D-D can be a reliable index for prognosis assessment and treatment guidance in patients with cerebral infarction [28, 29]. The final process of thrombus formation is the formation of soluble fibrin from FIB through the action of thrombin, and the content of FIB in the patient's body decreases. Generally speaking, FIB begins to decrease within 24 hours after thrombolytic therapy [30–32]. While there are currently few or even no reports on the correlation of D-D and FIB with treatment effect of CVT, more researches will be needed to provide more relevant evidence.
During CVT treatment, warfarin needs routine monitoring and dose adjustment, heparin requires intravenous administration, and there is a risk of heparin-induced thrombocytopenia. Apixaban has a high binding rate to plasma protein, and its metabolism depends on the liver and kidney, so it cannot be cleared through hemodialysis. Once uncontrollable bleeding occurs, it will be very dangerous. Hence, it is necessary to constantly detect the changes of physiological state of patients during medication. Though a randomized trial is undertaken, one thing is for sure: given the rarity of CVT, it will only succeed through collaboration of a large number of hospitals. Follow-up should be extended to observe the efficacy of them in CVT patients. More researches should be done to explore the underlying mechanism of apixaban/heparin or their combined use in vivo.
To sum up, apixaban combined with heparin is effective and safe in treating CVT, and the changes of D-D and FIB levels during treatment are closely related to the therapeutic effect, all indicating that their combined use can be valid evaluation indexes for the efficacy of CVT.
Acknowledgments
The work was supported by the Medical Science Research Project Plan of Hebei Province (20200262).
Data Availability
All the raw data could be accessed by contacting the corresponding author if necessary.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Devasagayam S., Wyatt B., Leyden J., Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke . 2016;47(9):2180–2182. doi: 10.1161/STROKEAHA.116.013617. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho J. M., Zuurbier S. M., Aramideh M., Stam J. The incidence of cerebral venous thrombosis. Stroke . 2012;43(12):3375–3377. doi: 10.1161/STROKEAHA.112.671453. [DOI] [PubMed] [Google Scholar]
- 3.Stam J. Thrombosis of the cerebral veins and sinuses. The New England Journal of Medicine . 2005;352(17):1791–1798. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 4.Scheffer I. E., Berkovic S., Capovilla G., et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia . 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y., Tian X., Wang X. Diagnosis and treatment of cerebral venous thrombosis: a review. Frontiers in Aging Neuroscience . 2018;10:p. 2. doi: 10.3389/fnagi.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee D. J., Ahmadpour A., Binyamin T., Dahlin B. C., Shahlaie K., Waldau B. Management and outcome of spontaneous cerebral venous sinus thrombosis in a 5-year consecutive single-institution cohort. J Neurointerv Surg . 2017;9(1):34–38. doi: 10.1136/neurintsurg-2015-012237. [DOI] [PubMed] [Google Scholar]
- 7.Sassi S. B., Touati N., Baccouche H., Drissi C., Romdhane N. B., Hentati F. Cerebral venous thrombosis: a Tunisian Monocenter study on 160 patients. Clinical and Applied Thrombosis/Hemostasis . 2017;23(8):1005–1009. doi: 10.1177/1076029616665168. [DOI] [PubMed] [Google Scholar]
- 8.Saposnik G., Barinagarrementeria F., Brown R. D., Jr., et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke . 2011;42(4):1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 9.Woller S. C., Stevens S. M., Johnson S. A., et al. Apixaban for routine Management of Upper Extremity Deep Venous Thrombosis (ARM-DVT): methods of a prospective single-arm management study. Res Pract Thromb Haemost . 2019;3(3):340–348. doi: 10.1002/rth2.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandernach M. W., Beyth R. J., Rajasekhar A. Apixaban for the prophylaxis and treatment of deep vein thrombosis and pulmonary embolism: an evidence-based review. Therapeutics and Clinical Risk Management . 2015;11:1273–1282. doi: 10.2147/TCRM.S68010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao S. K., Ibrahim M., Hanni C. M., et al. Apixaban for the treatment of cerebral venous thrombosis: a case series. Journal of the Neurological Sciences . 2017;381:318–320. doi: 10.1016/j.jns.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Covut F., Kewan T., Perez O., Flores M., Haddad A., Daw H. Apixaban and rivaroxaban in patients with cerebral venous thrombosis. Thrombosis Research . 2019;173:77–78. doi: 10.1016/j.thromres.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Koopman K., Uyttenboogaart M., Vroomen P. C., van der Meer J., De Keyser J., Luijckx G. J. Long-term sequelae after cerebral venous thrombosis in functionally independent patients. Journal of Stroke and Cerebrovascular Diseases . 2009;18(3):198–202. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Hiltunen S., Putaala J., Haapaniemi E., Tatlisumak T. Long-term outcome after cerebral venous thrombosis: analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. Journal of Neurology . 2016;263(3):477–484. doi: 10.1007/s00415-015-7996-9. [DOI] [PubMed] [Google Scholar]
- 15.Field T. S., Hill M. D. Cerebral venous thrombosis. Stroke . 2019;50(6):1598–1604. doi: 10.1161/STROKEAHA.119.025334. [DOI] [PubMed] [Google Scholar]
- 16.Einhaupl K., Stam J., Bousser M. G., et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. European Journal of Neurology . 2010;17(10):1229–1235. doi: 10.1111/j.1468-1331.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho J. M., Seelig R., Bousser M. G., Canhao P., Ferro J. M., Stam J. Treatment variations in cerebral venous thrombosis: an international survey. Cerebrovascular Diseases . 2011;32(3):298–300. doi: 10.1159/000330646. [DOI] [PubMed] [Google Scholar]
- 18.Carrier M., Abou-Nassar K., Mallick R., et al. Apixaban to prevent venous thromboembolism in patients with cancer. The New England Journal of Medicine . 2019;380(8):711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 19.Agnelli G., Becattini C., Meyer G., et al. Apixaban for the treatment of venous thromboembolism associated with cancer. The New England Journal of Medicine . 2020;382(17):1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 20.McBane R. D., 2nd, Wysokinski W. E., le-Rademacher J. G., et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. Journal of Thrombosis and Haemostasis . 2020;18(2):411–421. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 21.Wysokinski W. E., Houghton D. E., Casanegra A. I., et al. Comparison of apixaban to rivaroxaban and enoxaparin in acute cancer-associated venous thromboembolism. American Journal of Hematology . 2019;94(11):1185–1192. doi: 10.1002/ajh.25604. [DOI] [PubMed] [Google Scholar]
- 22.Dawwas G. K., Brown J., Dietrich E., Park H. Effectiveness and safety of apixaban versus rivaroxaban for prevention of recurrent venous thromboembolism and adverse bleeding events in patients with venous thromboembolism: a retrospective population-based cohort analysis. Lancet Haematol . 2019;6(1):e20–e28. doi: 10.1016/S2352-3026(18)30191-1. [DOI] [PubMed] [Google Scholar]
- 23.Bott-Kitslaar D. M., McBane R. D., Casanegra A. I., et al. Apixaban and rivaroxaban in patients with acute venous thromboembolism. Mayo Clinic Proceedings . 2019;94(7):1242–1252. doi: 10.1016/j.mayocp.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Arauz A., Arguelles N., Jara A., Guerrero J., Barboza M. A. Thrombin-activatable fibrinolysis inhibitor polymorphisms and cerebral venous thrombosis in Mexican mestizo patients. Clinical and Applied Thrombosis/Hemostasis . 2018;24(8):1291–1296. doi: 10.1177/1076029618766267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopytek M., Zabczyk M., Natorska J., Malinowski K. P., Undas A. Effects of direct oral anticoagulants on thromboelastographic parameters and fibrin clot properties in patients with venous thromboembolism. Journal of Physiology and Pharmacology . 2020;71(1) doi: 10.26402/jpp.2020.1.03. [DOI] [PubMed] [Google Scholar]
- 26.Kearon C., de Wit K., Parpia S., et al. Diagnosis of pulmonary embolism withd-Dimer adjusted to clinical probability. The New England Journal of Medicine . 2019;381(22):2125–2134. doi: 10.1056/NEJMoa1909159. [DOI] [PubMed] [Google Scholar]
- 27.Hiltunen S., Putaala J., Haapaniemi E., Salonen O., Tatlisumak T. D-dimer and clinicoradiologic features in cerebral venous thrombosis. Journal of the Neurological Sciences . 2013;327(1-2):12–14. doi: 10.1016/j.jns.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Wang R., Wei Y., Teng J. Levels of plasma N-terminal pro-brain natriuretic peptide and D-dimer on the prognosis of patients with acute cerebral infarction. Pak J Med Sci . 2018;34(4):855–858. doi: 10.12669/pjms.344.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Li S., Chen W., et al. The potential value of D-dimer to fibrinogen ratio in diagnosis of acute ischemic stroke. Journal of Stroke and Cerebrovascular Diseases . 2020;29(8, article 104918) doi: 10.1016/j.jstrokecerebrovasdis.2020.104918. [DOI] [PubMed] [Google Scholar]
- 30.Bai Y., Shi M., Yang X., et al. The value of FDP/FIB and D-dimer/FIB ratios in predicting high-risk APL- related thrombosis. Leukemia Research . 2019;79:34–37. doi: 10.1016/j.leukres.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Induruwa I., Moroi M., Bonna A., et al. Platelet collagen receptor glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. Journal of Thrombosis and Haemostasis . 2018;16(2):389–404. doi: 10.1111/jth.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Moerloose P., Boehlen F., Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Seminars in Thrombosis and Hemostasis . 2010;36(1):007–017. doi: 10.1055/s-0030-1248720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the raw data could be accessed by contacting the corresponding author if necessary.


