Abstract
HER2-positive breast cancer represents 15%-20% of breast malignancies and is characterized by an aggressive behavior and high recurrence rates. Anti-HER2-directed agents represent the mainstay of treatment of patients with HER2-positive metastatic breast cancer (MBC). In this review we propose a treatment algorithm for patients with HER2-positive MBC based on the currently available literature on the topic. The combination of trastuzumab, pertuzumab and a taxane (THP) remains the preferred first-line therapy in most scenarios. Results of trials recently presented at the European Society for Medical Oncology (ESMO) Congress 2021 might have direct clinical impact in the second- and later-line settings. The randomized DESTINY-BREAST03 study compared trastuzumab deruxtecan (T-DXd) with trastuzumab emtansine (T-DM1) in patients previously treated with trastuzumab and a taxane. T-DXd significantly improved progression-free survival and showed a trend towards improved overall survival, establishing this agent as preferred second-line therapy. Treatment with T-DM1, or the combination of tucatinib, trastuzumab and capecitabine, are considered reasonable options after second-line therapy. For subsequent lines, trastuzumab duocarmazine, neratinib plus capecitabine or the continuation of trastuzumab with different chemotherapy partners are valid options. For patients experiencing disease relapse up to 6 months after completion of adjuvant therapy, as well as for those relapsing within 12 months from the completion of pertuzumab-based adjuvant treatment, we recommend T-DXd as preferred first-line option. For those relapsing between 6 and 12 months after non-pertuzumab-based adjuvant treatment, we recommend first-line THP. Finally, for patients with active brain metastasis, tucatinib-based combination represents a suitable second-line option.
Key words: breast neoplasms, trastuzumab deruxtecan, trastuzumab duocarmazine, ado-trastuzumab emtansine, HER-2 protein
Highlights
-
•
Treatment landscape for patients with HER2-positive metastatic breast cancer is rapidly evolving.
-
•
Trastuzumab deruxtecan improved outcomes in comparison with T-DM1 and should now be the preferred second-line therapy.
-
•
T-DM1 and tucatinib-based therapy are valid third-line options.
-
•
Toxicity profile should be taken into account when considering different later-line treatment options.
Background
Approximately 15%-20% of breast cancers (BC) harbor human epidermal growth factor receptor 2 (HER2) overexpression and/or amplification, defining a subset of BC historically associated with aggressive behavior, high recurrence rates, and worse prognosis.1 The development of HER2-targeted therapies altered the natural course of HER2-positive early and metastatic breast cancer (MBC) by significantly decreasing relapse rates for patients treated with curative intent and prolonging survival of patients with metastatic disease.2,3 Since the introduction of trastuzumab, several HER2-targeted agents have been developed and demonstrated clinical activity.4 These new alternatives increase the complexity of the decision-making process, especially regarding the different treatment sequence possibilities for each clinical setting. In this editorial, we summarize the currently available evidence for the systemic treatment of HER2/neu-overexpressing MBC, considering the time elapsed since the completion of adjuvant treatments and the presence of central nervous system (CNS) metastases, with a particular focus on recently presented data.
Disease relapse 12 months after completing adjuvant treatment or de novo metastatic disease
For patients with disease relapse 1 year after completing adjuvant treatment or ‘de novo’ HER2-positive MBC, the combination of trastuzumab, pertuzumab, and a taxane (THP) is usually the preferred treatment regimen based on data from the CLEOPATRA study.5 In this phase III trial, 808 patients with HER2-positive MBC were treated with trastuzumab and docetaxel and were randomly assigned to receive pertuzumab or placebo. After ∼8 years of follow-up, the overall survival (OS) was improved by 16.3 months in the pertuzumab arm [median OS 57.1 versus 40.8 months, hazard ratio (HR) 0.69, 95% confidence (CI) 0.58-0.8].5 This OS benefit was also observed in the subgroup of 376 patients that received (neo)adjuvant treatment (HR 0.70, 95% CI 0.53-0.93). However, only 11% of the patients included in this trial were previously exposed to trastuzumab in the early setting. If well tolerated, we recommend at least six cycles of docetaxel (or equivalent period of taxane-based chemotherapy) followed by trastuzumab and pertuzumab maintenance, combined with endocrine therapy for patients with hormone receptor-positive disease.
In the second-line setting, ado-trastuzumab emtansine (T-DM1) has been considered the standard therapeutic approach after demonstrating improved progression-free survival (PFS) and OS in comparison with lapatinib/capecitabine in the EMILIA trial.6 Recently presented at the European Society for Medical Oncology (ESMO) Congress 2021, DESTINYBreast-03 is a phase III trial in which 524 patients with HER2-positive MBC previously treated with a taxane and trastuzumab (60% with prior exposure to pertuzumab) were randomly assigned to receive T-DM1 or fam-trastuzumab deruxtecan-nxki (T-DXd), an antibody-drug conjugate (ADC) composed of an anti-HER2 antibody and a cytotoxic topoisomerase I inhibitor. With a median follow-up of 16.2 months for T-DXd and 15.3 months for T-DM1, treatment with T-DXd resulted in a significant improvement in PFS (HR 0.28, 95% CI 0.22-0.37, P = 7.8 × 10-22), with a 12-month PFS rate of 75.8% with T-DXd versus 34.1% with T-DM1, providing compelling evidence of the activity of a new agent in this setting. The PFS superiority of T-DXd was sustained across subgroups. A strong trend for OS benefit was also observed favoring the T-DXd arm (HR 0.56, 95% CI 0.36-0.86, P = 0.0071). The rate of all-grade treatment-related adverse events (AEs) was numerically higher in the T-DXd arm (98.1% versus 86.6%), as did the rate of grade ≥3 AEs (45.1% versus 39.8%) and the proportion of patients with drug-related AE associated with discontinuation (12.8% versus 5.0%). Although drug-related interstitial lung disease (ILD)/pneumonitis was reported in 10.5% of patients receiving T-DXd, with it being the most common cause of treatment discontinuation in this arm, the number of high-grade ILD was low, with two cases (0.8%) of grade 3 and no grades 4/5 ILD in the T-DXd arm.7 Based on these results, T-DXd will replace T-DM1 as the standard second-line therapy.
Although several studies have evaluated different therapeutic strategies after progression to two previous lines of anti-HER2 therapy, there are currently no data to support one preferred specific approach after progression to T-DXd. Understanding the mechanisms of resistance to this agent, as well the patterns of cross-resistance among different ADCs (and other anti-HER2 agents), will have important implications for optimizing treatment sequencing. Until such data are available, different factors such as drug availability, treatment costs, toxicity profile, presence of comorbidities, prior exposure to different anti-HER2 agents, site of metastasis, and personal preferences will influence decision making.8
In the phase III HER2CLIMB trial, tucatinib, an oral tyrosine kinase inhibitor (TKI), was compared with placebo, both in combination with trastuzumab and capecitabine, in 612 patients previously treated with trastuzumab, pertuzumab, and T-DM1.9 After a follow-up of 29.6 months, the tucatinib-containing arm was associated with a 5.5-month improvement in OS (HR 0.73, P = 0.004) and with increased PFS (HR 0.57, P < 0.00001).10 Also during the ESMO Congress 2021, results from the phase III SYD985.002/TULIP were presented, evaluating another ADC, [vic]trastuzumab duocarmazine, in comparison with physician's treatment choice (PTC), in patients who had received at least two prior lines of treatment, or previous treatment with T-DM1. In this trial, PFS was 7.0 months for [vic]trastuzumab duocarmazine and 4.9 months for PTC (HR 0.64, 95% CI 0.49-0.84, P = 0.002). At the time of this analysis, OS difference favoring [vic]trastuzumab duocarmazine was not statistically significant (HR 0.83, 95% CI 0.62-1.09, P = 0.153). Although rates of treatment-related AE of any grade were similar between treatment arms (96.5% versus 96.4%), rates of grade ≥ 3 AEs were numerically higher in the [vic]trastuzumab duocarmazine arm (52.8% versus 48.2%). Importantly, 78.1% of patients treated with [vic]trastuzumab duocarmazine experienced eye toxicity, a significant proportion of which (21.2%) was grade ≥3. Eye toxicity was also the cause of dose modifications in 22.9% of patients and led to treatment discontinuation in 20.8% of the patients in this arm. These AEs occurred despite several risk mitigation strategies implemented in the trial, such as the exclusion of patients with prior keratitis, use of prophylactic lubricating eye drops, regular eye exams by an ophthalmologist, and treatment discontinuation in case of grade ≥3 keratitis. Thus, eye toxicity is a major factor to be considered when considering [vic]trastuzumab duocarmazine since this class of AEs could have significant impact on the quality of life. ILD/pneumonitis was also observed with this agent in 7.6% of patients, with 2.4% grade ≥3 events and two fatal cases (0.7%).11
Continued HER2 blockade is considered standard clinical practice during the disease course and several options are now available for the treatment of patients with trastuzumab-, pertuzumab-, and ADC-pretreated HER2-positive MBC. In the phase III NALA trial, neratinib, an irreversible pan-HER TKI, in combination with capecitabine was compared with lapatinib plus capecitabine in patients with HER2-positive MBC with ≥ 2 previous HER2-directed regimens (41.7% and 54.3% with prior exposure to pertuzumab and T-DM1, respectively).12 Neratinib was associated with an improvement in PFS (HR 0.76, 95% CI 0.63-0.93), but no significant OS benefit (HR 0.88, 95% CI 0.72-1.07).12 In the phase III SOPHIA trial, patients experiencing progression after two or more prior anti-HER2 therapies (91.2% and >99% previously treated with T-DM1 and pertuzumab, respectively) received PTC in combination with margetuximab, an Fc-engineered anti-HER2 monoclonal antibody aiming at increasing activation of innate and adaptive anti-ERBB2 immune responses, or trastuzumab.13 Margetuximab led to an improvement in PFS (HR 0.76, 95% CI 0.59-0.98, P = 0.03) without significant impact in OS (HR 0.89, 95% CI 0.69-1.13, P = 0.33) in the second planned interim analysis.13 Although there is no direct evidence supporting any ideal treatment sequence, neratinib, margetuximab, lapatinib, or trastuzumab in combination with different chemotherapy agents can be considered as later-line options, while T-DXd, T-DM1, or tucatinib-based combination may offer interesting alternatives, if not previously used in earlier lines.14
Disease relapse up to 12 months after completing adjuvant treatment
For patients presenting disease relapse during or within 6 months after completing adjuvant therapy, the preferred treatment option is T-DXd based on the data from the DESTINYBreast-03 trial.7 For patients with MBC diagnosed between 6 and 12 months after completing adjuvant therapy, if the regimen used in the adjuvant setting included pertuzumab, T-DXd would be the preferred treatment, also based on the results of the DESTINYBreast-03 trial.7 Although both populations (so called ‘rapid progressors’) were represented in DESTINYBreast-03, absence of head-to-head comparison between T-DXd and THP precludes an absolute exclusion of the role of THP in this setting. Interestingly, a retrospective real-world cohort of early-relapsing patients demonstrated PFS and OS superiority of THP over T-DM1 in patients with time-to-relapse ≤6 months or between 6 and 12 months.15 In view of the lack of consensus or compelling data regarding the standard treatment of relapses with a treatment-free interval between 6 and 12 months after exposure to pertuzumab-free adjuvant regimens (trastuzumab alone or T-DM1), our preferred approach is to use THP in this first-line setting.
Relapse with central nervous system metastases
Approximately one-third of patients with HER2-positive MBC will ultimately develop CNS metastases. Despite this high incidence of CNS metastases, the survival after CNS involvement is higher in patients with HER2-positive MBC compared to other subtypes, primarily due to better systemic and cranial disease control provided by anti-HER2 agents. The initial treatment of symptomatic brain metastases (BM) is traditionally local with neurosurgery and/or radiotherapy, depending on the number of metastases, performance status, and systemic disease control.16 These techniques provide good local control but do not effectively prevent future CNS events. An exploratory analysis of the CLEOPATRA trial showed longer median time to development of CNS metastases as first site of disease progression in pertuzumab arm (15.0 versus 11.9 months, HR 0.58, 95% CI 0.39-0.85),17 meaning that despite the low blood–brain barrier (BBB) permeability, these monoclonal antibodies still play a role in first-line MBC with BM. Moreover, trials evaluating ADC, such as EMILIA (T-DM1) and DESTINY-Breast03 (T-DXd), included patients with CNS metastases (19.8% and 23.8%, respectively).6,7 Presented at San Antonio Breast Cancer Symposium 2021, the updated subgroup analysis of 82 patients with stable BM at baseline in DESTINY-Breast03 demonstrated an improvement in median PFS from 3 months with T-DM1 to 15 months with T-DXd (HR 0.25, 95% CI 0.31-0.45). This finding positions this drug as preferred second-line therapy after THP also in case of CNS involvement.18 Small molecules such as anti-HER2TKI consistently demonstrated the ability to penetrate the BBB, showing activity in the CNS with intracranial overall response rates from 47% to 66%.19, 20, 21 However, only HER2CLIMB (tucatinib) included patients with active CNS metastases, demonstrating survival benefit for the addition of tucatinib to trastuzumab/capecitabine in patients with BM (HR 0.58, 95% CI 0.40-0.85), even in the subpopulation of patients with active BM (HR 0.49, 95% CI 0.30-0.80). These data potentially place this agent after THP in case of active CNS metastases not requiring immediate local treatment, considering that head-to-head comparisons between tucatinib and ADC are not available.
Conclusion
Treatment of patients with HER2-positive MBC is rapidly evolving as new agents demonstrate increased efficacy in different clinical settings. While THP remains the standard first-line, trastuzumab deruxtecan has now established its role as the preferred therapy in the second-line setting. Tucatinib in combination with trastuzumab/capecitabine remains a valid option particularly in patients with BM. A plethora of subsequent treatment options are then available for later lines. Further studies are required to better understand mechanisms of resistance to these agents, thus allowing treatment sequencing optimization.
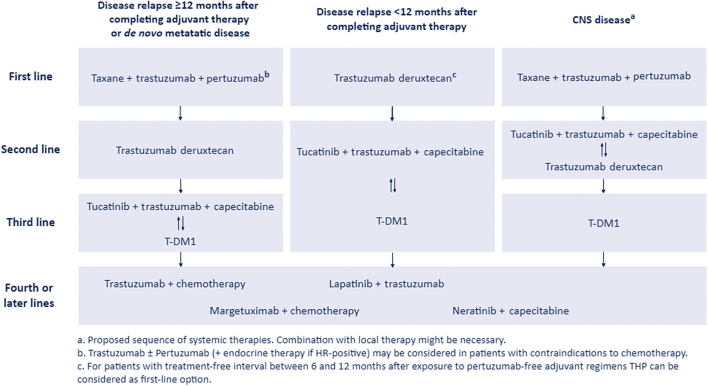
Figure 1.
Proposed treatment algorithm.
Acknowledgments
Funding
None declared.
Disclosure
GNM Travel/accommodations/expenses: Roche and Bayer; DMB Honoraria and advisory board fees from Janssen, Pfizer, Merck Sharp & Dohme, Angelini, AstraZeneca, and Novartis, and meeting/travel grants from LEO Farmacêuticos, Merck Sharp & Dohme, Ipsen, Janssen, Roche, Laboratórios Vitória, and Novartis; EdA Honoraria and/or advisory board from Roche/GNE, Novartis, Seattle Genetics, Zodiac, Libbs, and Pierre Fabre. Travel grants from Roche/GNE and GSK/Novartis. Research grant to my institution from Roche/GNE, Astra-Zeneca, GSK/Novartis, and Servier.
References
- 1.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 2.Dawood S., Broglio K., Buzdar A.U., Hortobagyi G.N., Giordano S.H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley R., Braybrooke J., Gray R., et al. Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22(8):1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain S.M., Miles D., Kim S.B., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 6.Diéras V., Miles D., Verma S., et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes J., Kim S., Chung W. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32(suppl 5):S1283–S1346. [Google Scholar]
- 8.Martínez-Sáez O., Prat A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract. 2021;17(10):594–604. doi: 10.1200/OP.21.00172. [DOI] [PubMed] [Google Scholar]
- 9.Murthy R.K., Loi S., Okines A., et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 10.Curigliano G., Mueller V., Borges V.F., et al. Updated results of tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB) JCO. 2021;39(suppl 15):1043. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Saura C., O'Shaughnessy J., Aftimos P. LBA15 - Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician's choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32(suppl 5):S1283–S1346. [Google Scholar]
- 12.Saura C., Oliveira M., Feng Y.H., et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo H.S., Im S.A., Cardoso F., et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennari A., André F., Barrios C.H., et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer†. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Schettini F., Conte B., Buono G., et al. T-DM1 versus pertuzumab, trastuzumab and a taxane as first-line therapy of early-relapsed HER2-positive metastatic breast cancer: an Italian multicenter observational study. ESMO Open. 2021;6(2):100099. doi: 10.1016/j.esmoop.2021.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Rhun E., Guckenberger M., Smits M., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32(11):1332–1347. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Swain S.M., Baselga J., Miles D., et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. doi: 10.1093/annonc/mdu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvitz S., Kim S.B., Chung W. GS3-01. Trastuzumab deruxtecan vs trastuzumab emtansine in patients with HER2+ metastatic breast cancer: Results of the randomized phase 3 study DESTINY-Breast03. San Antonio Breast Cancer Symposium. 2021 [Google Scholar]
- 19.Lin N.U., Borges V., Anders C., et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachelot T., Romieu G., Campone M., et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 21.Freedman R.A., Gelman R.S., Anders C.K., et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]