Abstract
The Portulaca oleracea L. commonly known as purslane is distributed all over the world and easily grows in diverse soil and climatic conditions. It has been traditionally used as a nutritious and ethnomedicinal food across the globe. Various studies have shown that the plant is a rich source of various important phytochemicals such as flavonoids, alkaloids, terpenoids, proteins, carbohydrates, and vitamins such as A, C, E, and B, carotenoids and minerals such as phosphorus, calcium, magnesium and zinc. It is particularly very important because of the presence of a very high concentration of omega-3- fatty acids especially α-linolenic acid, gamma-linolenic acid and linoleic acid, which are not generally synthesized in terrestrial plants. Various parts of purslane are known for ethnomedicinal and pharmacological uses because of its anti-inflammatory, antidiabetic, skeletal muscle relaxant, antitumor, hepatoprotective, anticancer, antioxidant, anti-insomnia, analgesic, gastroprotective, neuroprotective, wound healing and antiseptic activities. Due to multiple benefits of purslane, it has become an important wonder crop and various scientists across the globe have shown much interest in it as a healthy food for the future. In this review, we provide an update on the phytochemical and nutritional composition of purslane, its usage as nutritional and an ethnomedicinal plant across the world. We further provide a detailed account on ethnopharmacological studies that have proved the ethnomedicinal properties of purslane.
Keywords: Portulaca oleracea, Traditional food plants, Purslane, Omega-3-fatty acids, Ethnobotanical knowledge
Highlights
-
•
Purslane is one of the richest terrestrial sources of omega-3-fatty acids essential for healthy human growth and development.
-
•
It is an important traditional food and ethnomedicinal plant with potential to mitigate hunger and improve public wellbeing.
-
•
Purslane easily grows in wide climate and edaphic zones making it a wonderful home garden nutritious food at doorsteps.
-
•
Its rich phytochemical composition makes it an important pharmacological and nutraceutical food for future readiness.
Portulaca oleracea, Traditional food plants, Purslane, Omega-3-fatty acids, Ethnobotanical knowledge.
1. Introduction
The Portulaca oleracea L. commonly known as purslane is an important member of the family Portulacaceae Juss [1]. It has a worldwide distribution and grows mainly in the tropics and subtropics and it has its centres of origin in South America and Africa [1, 2]. The term “Portulaca” is derived from two Latin words ('Porto' means ‘to carry’ and lac means ‘milk’) which means the presence of milky juice in the plant [3, 4]. Several studies show that purslane is very important because of its nutritional [5, 6, 7], medicinal [3, 8, 9, 10, 11], phytoremediation properties [12, 13] and aesthetic value [12]. Purslane has been used in various parts of the world as folk medicine and traditional food since ancient times [3, 14]. Several ethnobotanical studies suggest that it is used by indigenous communities as an important medicine against several ailments such as diabetes, urinary infections, kidney and cardiovascular diseases, diarrhoea, headache and ulcers to name a few and against snake and insect bites [15, 16, 17, 18, 19, 20]. Its use as an ethno medicinal plant is reported from almost all the continents suggesting its huge importance in the healthcare of the indigenous communities. Recent advancements in the quantitative tools for the analysis of phytochemicals has led to the identification of several hundred metabolites from various parts of the purslane [5, 7, 10, 18, 21, 22]. Using ethnobotanical leads, scientists have tested the efficacy of purslane as a medicinal plant using in vitro as well as in vivo studies and have found impressive results on its pharmacological potential [23, 24, 25, 26, 27, 28, 29, 30]. The confirmation of the medicinal uses against the diseases using modern scientific studies provide evidence in support of the ethno medicinal properties of the purslane. Phytochemical studies have shown that purslane is one of the richest terrestrial sources of ω-3 and ω-6 fatty acids, ascorbic acid, tocopherols, glutathione and β-carotene [31, 32, 33, 34] suggesting its nutraceutical potential. It is found that it contains nearly 300–400 mg of alpha-linolenic acid, 12.2 mg of α-tocopherol, 26.6 mg of ascorbic acid, 1.9 mg of β-carotene, and 14.8 mg of glutathione per 100 g of fresh weight in its leaves [33, 35]. Purslane is also an important source of specialised metabolites such as alkaloids, catecholamines, phenolic acids, anthocyanins, flavonoids, lignans, terpenoids and betalains [20, 36, 37, 38, 39, 40, 41]. Some of these metabolites have been proved to possess health promoting benefits to humans. In this review, we provide comprehensive details of the importance of purslane with an emphasis on its ethnomedicinal use, phytochemical, nutritional richness, and pharmacological potential. This article discusses the ethnomedicinal importance, phytochemical composition and pharmacological potential of purslane. Research articles from various databases such as scopus, pubmed, sciencedirect and google scholar have been used to consolidate the ethnobotanical importance of purslane, its phytochemical constitution, nutritional composition and its pharmacological potential. Figure 1 shows purslane in its natural habitat growing profusely.
Figure 1.
Purslane plant in its natural habitat.
2. Ethnomedicinal importance of purslane
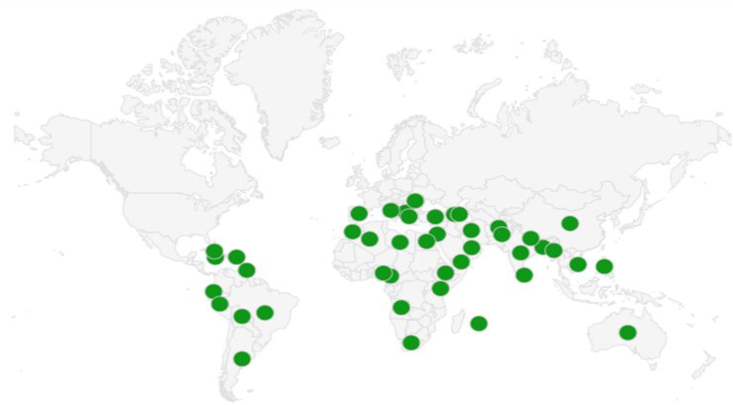
People from many countries have been traditionally using purslane for medicinal and nutritional purposes. Various parts of the plant are used against a number of diseases such as diarrhoea and throat infections, diabetes, obesity, toothache, asthma, ulcers, snake bites, jaundice and dysentery [15, 16, 17, 19, 20, 42, 43]. Its use for various ailments is mentioned in the ancient texts such as Materia Medica by Dioscorides, Canon of Medicine by Avicenna and Charaka Samhita, a Sanskrit text on Indian traditional medicine [44, 45, 46]. It is also used in the Chinese and Persian Traditional Systems of Medicines [44]. The Chinese Traditional Medicine has strong prescriptions of purslane for various ailments [44, 47]. It is known by various names such as Khorfeh in Persian, Loni or Lonna in Ayurvedic system of medicine, Kulfaa and Khurfaa, Tukhme khurfa in the Unani system of medicine, Paruppu Keerai and Pulli Keerai in Siddha system of medicine and Ma Chi Xian or Chang Shou Cai in Chinese traditional medicine [44, 48, 49, 50]. Its use as traditional medicine is well documented recently by many researchers from various countries. Various ethnomedicinal uses of purslane across globe along with the part(s) used are shown in Table 1. Figure 2 depicts the ethnobotanical usage reports of purslane across 44 countries.
Table 1.
Ethno-medicinal uses of purslane in treating various diseases across the globe.
| Country | Ethnomedicinal use | Reference(s) |
|---|---|---|
| Asia | ||
| Afghanistan | Seeds are used against diarrhea and throat infections. | [19] |
| Armenia | Leaves and stem are used against liver, gastric, kidney, and bladder diseases and as a hypoglycemic agent. | [51] |
| Azerbaijan | Infusion of leaves is used against diabetes. | [52] |
| Bangladesh | Dried seeds are used for toothache and asthma. | [17] |
| China | Used against dysentery, swellings, abnormal uterine bleeding (AUB), bleeding of hemorrhoids, erysipelas, and eczema. It is also used against snake bites and insect bites. | [15, 16, 20] |
| India | Used as an Ayurveda medicine against diseases of lungs, liver, kidney, bladder and bowel burning sensation, coughing and neurasthenia. | [53, 54, 55, 56] |
| Iran | Roots, leaves and seeds of purslane are used for the treatment of diabetes mellitus. | [57, 58] |
| Jordan | Seeds are used as blood purifiers and as aphrodisiacs. | [59] |
| Myanmar | Leaves used for kidney disease treatment and as a laxative. | [60] |
| Nepal | Leaves are used for scurvy, kidney and cardiovascular disorders. Juice is drunk for blood purification. | [42, 43] |
| Pakistan | Aerial parts are used in the treatment of urinary and digestive problems. Seeds are demulcent, diuretic and vermifuge. | [61, 62] |
| Philippines | Used for treatment of kidney infections. | [63] |
| Sri Lanka | Leaves are used against ulcers, wounds, burns and skin diseases. | [15, 64] |
| UAE | Aerial parts are used as a medicine to reduce fever. | [44] |
| Vietnam | The whole plant is used as an antibacterial, anathematic and anti-inflammatory agent. | [65, 66, 67] |
| Yemen | Leaves are used for gastric pain. | [68] |
| Europe | ||
| Albania | It is used to treat musculoskeletal disorders. | [69, 70] |
| Italy | Used to treat headache, stomach, intestine and kidney pains. | [71] |
| Greece | Used to cure inflammation. | [72, 73] |
| Romania | External bath for weakness and sickness. | [74] |
| Spain | Consumption of aerial parts for blood pressure regulation. | [75] |
| Turkey | Leaves are used to cure diarrhoea, diabetes, headache, ulcers, urinary disorders, wounds and constipation. | [76, 77, 78] |
| Australia | ||
| Australia | Aerial parts are eaten to cure scurvy, irritations, inflammations and as a diuretic and antibiotic agent. | [15] |
| Africa | ||
| Algeria | It is used against dyspepsia and also as a diuretic. | [79] |
| Angola | Whole plant is used for burns. | [80] |
| Cameroon | Shoot with leaves is used against headaches and poisoning. | [81] |
| Egypt | Used as a vegetable as well as medicinal plants and also spice. | [56] |
| Kenya | Whole plant is crushed and boiled with other herbs and used against cancer. | [82] |
| Ethiopia | Cooked leaves are eaten for gastritis, peptic ulcers and constipation. Application of crushed leaves on skin is used to cure fungal infections. | [83] |
| Libya | Used against headache, migraine and as a revulsant and vermicide. | [84] |
| Morocco | Cooked leaves are consumed to cure hypercholesterolemia. | [54] |
| Nigeria | Used as a diuretic and for the treatment of burns. | [15] |
| South Africa | Crushed and taken orally with warm water against tuberculosis. Application of crushed leaves is used against lymphatic filariasis. P. quadrifida is also used against lice and sores. Other uses: infections, pain relief | [85, 86, 87, 88] |
| Mauritius | Decoction of plants is used as an astringent and diuretic. Also used against inflammation and worms. Root and leaf decoction used as anthelmintics. | [89] |
| South America | ||
| Argentina | Used as an antipyretic. | [90] |
| Bolivia | Raw leaves are eaten against nephrolithiasis. | [91] |
| Brazil | Used against hemorrhoids. | [92] |
| Ecuador | Aqueous infusion of fresh leaves used against internal inflammation, infections, gastritis and kidney diseases. | [93] |
| Peru | Bark, leaf and sap used against diarrhea, fever and liver problems. | [94] |
| North America | ||
| Dominica, West Indies | Used for treatment of intestinal worms. | [95] |
| Republic of Trinidad and Tobago | Used against urinary problems, "cooling" and for lowering high cholesterol. | [96] |
| Jamaica | Used against urinary diseases. | [96] |
| Cuba | Used against kidney infections, inflammation and digestive disorders. | [97] |
Figure 2.
World map showing ethnomedicinal reports of purslane in 44 countries against various diseases.
3. Phytochemical richness of purslane
Considering the ethnobotanical relevance of purslane, dissection of its phytochemical composition is important. Various studies have attempted phytochemical analysis of P. oleracea [18, 21, 50, 98]. Qualitative and quantitative tools have been employed to study the phytoconstituents present in various parts of P. oleracea [21, 99, 100, 101, 102]. Studies show that it contains many important metabolites that provide health benefits [3, 27, 99]. The chemical composition of purslane varies during its growth stages [5]. It has been revealed that purslane contains abundant quantities of proteins, starch and essential amino acids [5, 50]. Considerably higher level of fat is found in leaf and stem and fiber content was found in leaves but not in stem [103]. Apart from being a source of primary metabolites, it contains varying quantities of specialised metabolites (earlier known as secondary metabolites) such as alkaloids, saponins, tannins, flavonoids, cardiac glycosides, terpenoids, phenolic acids and organic acids [18, 102]. The presence of diverse bioactive compounds in varying concentrations should be essentially responsible for its multiple biological activities [22, 44, 89]. The different parts of the purslane have been subjected to the quantification of various bioactive compounds. It has been found that water extract of purslane flowers have greater phenolic content than stem and leaves and the leaves show higher concentrations of total flavonoids and ascorbic acid [22]. The leaves also contain higher β-carotene content than stem [104]. Purslane is known for very high amounts of omega-3-fatty acids [105]. Wild genotypes of P. oleracea contain nearly 188.48 ± 6.35 mg/100 g of omega-3-fatty acid [18]. Therefore purslane is known as one of the richest terrestrial sources of omega-3- fatty acids [7]. A total of 85 metabolites belonging to different classes such as alkaloids, fatty acids, phenolic acids and amino acids were identified in three species namely P. oleracea, P. rausii and P. granulatostellulata [106]. This study reported methoxylated flavone glycosides, O and C-flavonoids, and four cyclodopa alkaloids namely oleracein A, C, K and N from purslane [106]. In addition to the identification of previously known oleraceins (oleraceins A, B, C, N, J, and U), Fernández-Poyatos et al. [107] reported two new cyclo-dopa amides namely oleraceins X and Y from purslane. Lei et al. [108] isolated four new cerebrosides and five known compounds viz. portulacerebroside A, B, C and D from P. oleracea. Nemzer et al. [18] identified widely known flavonoids, such as quercetin, kaempferol, isorhamnetin and naringenin in purslane. Several other phytoconstituents including 48 fatty acyl/lipids, 11 flavonoids and its derivatives, seven carbohydrates, two glycosylated hydroxy-cinnamic acid derivatives and miscellaneous terpenoids, steroids, lignan and purine nucleosides were identified [100]. The two dimensional structures of the main important metabolites found in purslane are presented in Table 2. The following subheadings explain various classes of specialized metabolites with individual metabolites that have been reported from purslane.
Table 2.
Important phytochemicals reported from purslane with their two dimensional structures.
| Phytochemical | Structure | Reference |
|---|---|---|
| Alkaloids | ||
| Oleracein A | 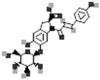 |
[109] |
| Oleracein B | 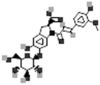 |
[109] |
| Oleracein C | 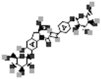 |
[109] |
| Oleracein D | 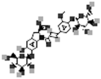 |
[109] |
| Oleracein E | 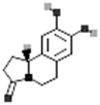 |
[109] |
| Oleracein K | 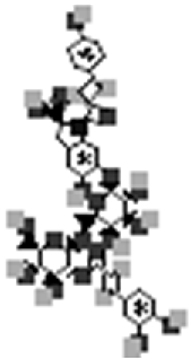 |
[110] |
| Oleracein L |  |
[28] |
| Scopoletin | 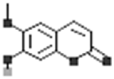 |
[50] |
| Aurantiamide | 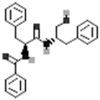 |
[50] |
| Aurantiamide acetate | 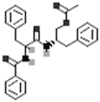 |
[50] |
| N-cis-Feruloyloctopamine | 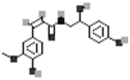 |
[50] |
| N-trans-Feruloyloctopamine | 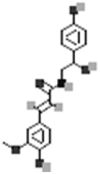 |
[50] |
| N-cis-Feruloyltyramine | 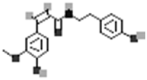 |
[50] |
| (3R)-3,5-Bis(3-methoxy-4-hydroxyphenyl)-2,3-dihydro-2(1H)-pyridinone | 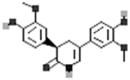 |
[50] |
| N-trans-Feruloyltyramine | 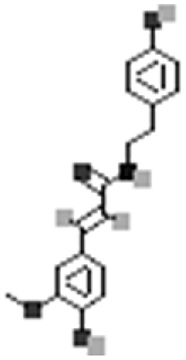 |
[50] |
| Indole-3-aldehyde | 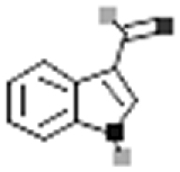 |
[50] |
| Catecholamines | ||
| Dopamine | 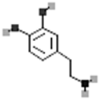 |
[3] |
| Noradrenaline | 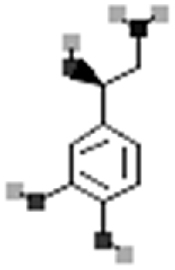 |
[111] |
| Flavonoid | ||
| Kaempferol | 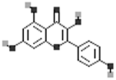 |
[50] |
| Apigenin | 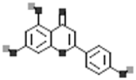 |
[50] |
| Luteolin | 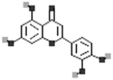 |
[50] |
| Myricetin | 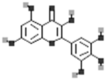 |
[50] |
| Quercetin | 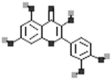 |
[50] |
| Genistein | 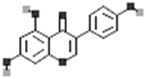 |
[50] |
| Genistin | 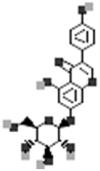 |
[50] |
| 2,2′-Dihydroxy-4′,6′-dimethoxychalcone | 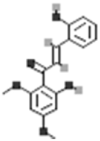 |
[50] |
| Isorhamnetin | 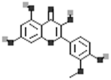 |
[18] |
| Naringenin | 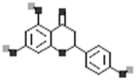 |
[18] |
| Terpenoids | ||
| Portuloside A | 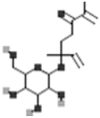 |
[50] |
| Portulene | 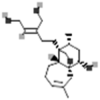 |
[38] |
| Lupeol | 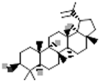 |
[50] |
| Friedelane | 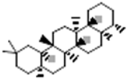 |
[50] |
| Taraxerol | 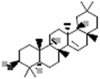 |
[112] |
| Lupeol | 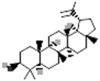 |
[3] |
| Phenolic acids | ||
| Caffeic acid | 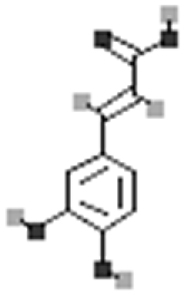 |
[113] |
| p-coumaric acid | 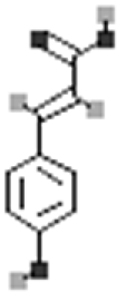 |
[114] |
| Ferulic acid | 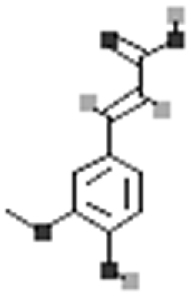 |
[113] |
| Gallic acid | 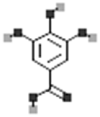 |
[113] |
| Gentisic acid | 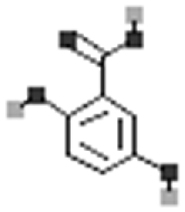 |
[115] |
| Benzoic acid | 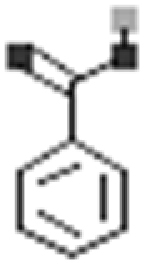 |
[115] |
| Anisic acid | 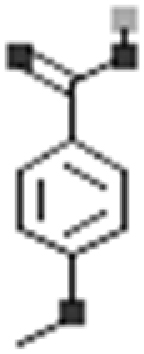 |
[115] |
| Vanillic acid | 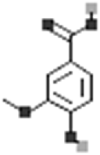 |
[113] |
| Anthocyanins | ||
| Delphinidin-3-glucoside | 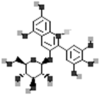 |
[115] |
| Cyanidin-3-glucoside | 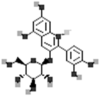 |
[115] |
| Pelargonidin-3-glucoside | 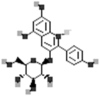 |
[115] |
| Lignans | ||
| (+)-Syringaresinol | 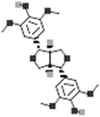 |
[116] |
| (+)-Lirioresinol A | 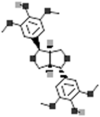 |
[116] |
| Fatty acids | ||
| Α-linolenic acid | [7] | |
| Linoleic acid | 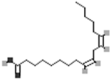 |
[117] |
4. Diverse metabolites belonging to different classes of specialised metabolites reported in purslane and their individual bioactivities
4.1. Alkaloids
Alkaloids are one of the most important groups of naturally occurring organic compounds with numerous pharmaceutical and medicinal uses [118]. Several alkaloids such as oleraceins, oleracins, trollisine, scopoletin and oleraisoindole have been reported in P. oleracea [39, 40, 48, 109, 119, 120]. Two new alkaloids namely (3R)-3,5-bis(3-methoxy4-hydroxyphenyl)-2,3-dihydro-2(1H)-pyridinone and 1,5-dimethyl-6-phenyl-1,2-dihydro-1,2,4-triazin-3(2H)-one were characterized from purslane by Tian et al. [121] and reported their moderate to high cytotoxic activity against different human cancer cell lines [122]. Three phenolic alkaloids namely oleracein A, oleracein B and oleracein E isolated from P. oleracea showed antioxidant activities [123]. Oleracein E and oleracein L from P. oleracea show hypoglycemic and antidiabetic activities [28]. Sun et al. [124] have demonstrated neuroprotective potential of oleracein E using in vitro and in vivo models. Xiu et al. [49] isolated soyalkaloid A for the first time from P. oleracea and reported its antioxidant activity using 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assay. In addition to several known compounds, Xiu et al. [48] reported a new alkaloid named, oleraurea from P. oleracea and interestingly it possesses anticholinesterase activity suggesting its important role in Alzheimer's disease as cholinesterase inhibitor [48]. Jin et al. [125] isolated three new isoquinoline alkaloids namely 1-(5′-hydroxymethyl furan-2-yl)-6,7-dihydroxy-3,4-dihydroisoquinoline, 1-(furan-2-yl)-6,7-dihydroxy-3,4-dihydroisoquinoline and 2-(furan-2-ylmethyl)-6,7- dihydroxy-3,4-dihydroisoquinoline-2-ium from purslane [125]. Ten new oleraceins (H, I, K, L, N, O, P, Q, R, S) along with four indoline amide glucosides have been characterized from P. oleracea by Jiao et al. [110]. The authors further found that oleraceins K and L showed higher antioxidant activities than vitamin C [110]. Zhao et al. [120] isolated a new lactam alkaloid namely oleraciamide D from P. oleracea in addition to five known alkaloids namely indole-3-aldehyde, portulacatone, N-trans-feruloyloctopamine, N-trans-feruloyl-3′-O-methyldopamine and N-trans-feruloyltyramine. The oleraciamide D posseses cytotoxic activity in human neuroblastoma SH-SY5Y cells [120].
4.2. Catecholamines (CAs)
Catecholamines are a group of amines that are produced in humans as neurotransmitters [126]. They are also synthesized in plants under stressful conditions and play diverse roles including growth and development [127]. Recent studies show that purslane is a rich source of a number of CAs with potential benefits. Dopamine is one of the most important catecholaminergic neurotransmitters synthesized in purslane [3]. High concentration of noradrenaline (norepinephrine) is found in purslane leaves [111]. Endress et al. [128] have reported the existence of adrenaline (epinephrine), another important catecholamine from P. grandiflora callus. The presence of noradrenaline and dopamine in purslane is further confirmed by several other studies [16, 129, 130]. Hu et al. [131] identified 22 compounds from a water extract of purslane and nine of them were found to be catecholamine derivatives. Martins et al. [132] have demonstrated neuroprotective effect of purslane in wistar rats. Earlier studies have shown multiple benefits of dopamine against neurodegenerative diseases such as Parkinson's disease, Schizophrenia and Huntington's disease [133]. Interestingly, dopamine and noradrenaline were detected in the purslane extracts administered to the rats that showed neuroprotective effects [132] suggesting its role as an important neuroprotectant. The existence of catecholamines in purslane provides evidence in favour of its neuroprotective activities and suggests its potential applications against neurodegenerative diseases. However, further clinical studies must be done to clearly understand the neuroprotective effects and its application for various neurodegenerative diseases in humans.
4.3. Phenolic acids
Phenolic acids are important specialized metabolites found in plants that are derivatives of benzoic and cinnamic acids [134, 135]. They are carboxylic acid groups possessing phenolic compounds [136]. Number of phenolic acids such as caffeic acid, p-coumaric acid, ferulic acid, gallic acid, gentisic acid, benzoic acid and anisic acid have been reported from purslane [114, 115]. The occurrence of caffeic, gallic, vanillic, ferulic and syringic acids was detected by Santiago-Saenz et al. [113].
4.4. Flavonoids
Plant flavonoids are a large group of naturally occurring phenyl chromones found in various parts of a plant such as root, stem, flower and fruit [137]. Flavonoids are known for various biological roles in humans because of their anti-oxidative, anti-inflammatory, antitumor, antiviral and antibacterial activities [138]. The flavonoids also play a protective role against coronary diseases and help in vascular activity [139]. Several flavonoids such as apigenin, kaempferol, luteolin, quercetin, isorhamnetin, kaempferol-3-O-glucoside and rutin have been isolated from purslane [114]. Xu et al. [140] have identified five flavonoids namely kaempferol, apigenin, myricetin, quercetin and luteolin using capillary electrophoresis with electrochemical detection. Use of quercetin isolated from purslane significantly improved learning and memory in mice suggesting its neuroprotective effects [141]. Santiago-Saenz et al. [113] have quantified myricetin in puralane. Three flavonoids oleracone C, D and E were identified for the first time by [142]. Nayaka et al. [143] isolated apigenin, a flavonoid from purslane and proved its antibacterial properties against certain bacterial pathogens such as Pseudomonas aeruginosa, Salmonella typhimurium, Proteus mirabilis, Klebsiella pneumoniae and Enterobacter aerogenes.
4.5. Anthocyanins
Anthocyanins are polyphenolic compounds that are found in plants as red, purple or blue coloured pigments [144, 145, 146, 147]. Anthocyanins are also very important for human health and have been found to play several beneficial roles against a number of diseases [146, 147]. Anthocyanins that have been reported from purslane are Delphinidin-3,5-glucoside, Cyanidin-3,5-glucoside, Pelargonidin-3,5-glucoside, Delphinidin-3-glucoside, Cyanidin-3-glucoside and Pelargonidin-3-glucoside [115].
4.6. Homoisoflavonoids (HIFs)
Homoisoflavonoids are another important group of specialised plants found in plants [148]. They are different from flavonoids as they possess one extra carbon which is absent in flavonoids and more than 300 HIFs have been identified in plants till date [149]. They are known for antimicrobial, antimutagenic, anti-inflammatory, antidiabetic and antioxidant activities [150, 151, 152]. The purslane is known to synthesize several important HIFs. Two new HIFs identified as 3-(2-hydroxybenzyl)-6,8-dimethoxy-4H-chromen-4-one (oleracone J) and 3-(2-hydroxybenzyl)-6,8-dimethoxychroman-4-one (oleracone K) were recently isolated by Duan et al. [153]. Nemzer et al. [18] identified four HIFs viz. portulacanones A–D from aerial parts of purslane. Yang et al. [142] isolated a unique HIF namely oleracone C. Another important HIF known as (E)-5- hydroxy-7- methoxy-3-(20- hydroxybenzyl)-4- chromanone (HM-Chromanone) isolated from P. oleracea showed protective effect against apoptosis of β cells of pancreas induced by glucose suggesting its antidiabetic properties [154]. It has also been found that HIFs show inhibitory activity against 5-lipoxygenase enzyme, which is responsible for inflammation in patients with asthma, allergic rhinitis, and osteoarthritis [152]. These studies suggest that P. oleracea is rich in HIFs and can play important roles against multiple diseases.
4.7. Lignans
Lignans are polyphenols formed by the dimerization of monolignols and they play defensive roles in plants against microorganisms [155]. Ma et al. [116] identified four lignans namely oleralignan, (+)-syringaresinol, (+)-lirioresinol A and monomethyl 3,3′,4,4′-tetrahydroxy-δ-truxinate and it was found that oleralignan was altogether new lignan reported from it. Further, evaluation of DPPH scavenging assay of the four lignans suggested good antioxidant activity [116].
4.8. Terpenoids
Terpenoids also known as isoprenoids are isoprene derived organic compounds synthesised in many organisms including plants [156]. Elkhayat et al. [38] reported portulene as a new diterpene from purslane. Apart from that, a number of other terpenoids identified in purslane are portuloside A, portuloside B, portulene, lupeol, friedelane, taraxerol, portaraxeroic acid A and portaeaxeroic acid B [3, 112].
4.9. Fatty acids (FAs)
A number of fatty acids have been isolated and identified from various parts of purslane plants. Studies have shown that purslane contains higher total fatty acid content than commonly used vegetables such as spinach, red leaf lettuce, mustard and buttercrunch lettuce [35]. Purslane is a nutritious vegetable crop rich in the polyunsaturated fatty acids (PUFA) and it is especially known for omega-3 (α-linolenic acid) and omega-6 (linoleic acid) fatty acids which are essential for human health [3, 117]. It has been proved that purslane contains higher levels of α-linolenic acid than spinach [7]. It is recommended that healthy diets should be enriched with foods having higher Omega 3/Omega 6 ratio. Interestingly, the leaves of purslane have a very high Omega 3/Omega 6 ratio [117, 157]. Simopoulos and Salem [34] have further reported the presence of eicosapentaenoic acid (EPA), another important omega-3 FA in purslane. Omara-Alwala et al. [105] reported that Purslane also contains docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA) which are also omega 3 fatty acids. The presence of essential fatty acids in purslane is highly beneficial for vegan and vegetarians whose diets generally lack essential omega-3-fatty acids. Few studies have also suggested enrichment of vegan and vegetarian diets with purslane for omega-3-FA requirements [7, 158].
4.10. Betalains
Betalains such as betacyanins and betaxanthins are water soluble nitrogenous plant pigments that impart red-violet and the yellow-orange coloration to fruits and vegetables [159, 160, 161]. Trezzini and Zrÿd [162] reported the presence of two new betalains namely portulacaxanthin II and portulacaxanthin III from the petals of P. grandiflora. Other betalains reported from P. grandiflora are portulacaxanthin, dopaxanthin, vulgaxanthin I, miraxanthin V and betanin [162, 163].
4.11. Other phytoconstituents
Purslane also contains many other phytoconstituents. Studies have shown the presence of organic acids such as oxalic acid, butanedioic acid, phenylpropionic acid, p-hydroxybenzoic acid, lauric acid, vanillic acid, myristic acid, pentadecanoic acid, palmitoleic acid, palmitic acid, heptadecanoic acid, oleic acid, stearic acid, arachidic acid, behenic acid, 3-Quinolinecarboxylic acid, Indole-carboxylic acid, catechol, lonchocarpic acid, fumaric, aconitic, citric, malic and oxalic acids and phenolic compounds such as 3- caffeoylquinic acid and 5-caffeoylquinic acid from different parts of P. oleracea plant [3, 29, 50, 157, 164]. Oxalic acid and citric acid are the most abundant organic acids in purslane [157, 165]. Allantoin, an end product of purine catabolism, has been reported from purslane [166]. A study reported the presence of N,N′-dicyclohexylurea, β-sitosterol and β-sitosteryl glucoside in purslane [167].
5. Purslane is nutritionally rich traditional food plant with huge nutraceutical potential
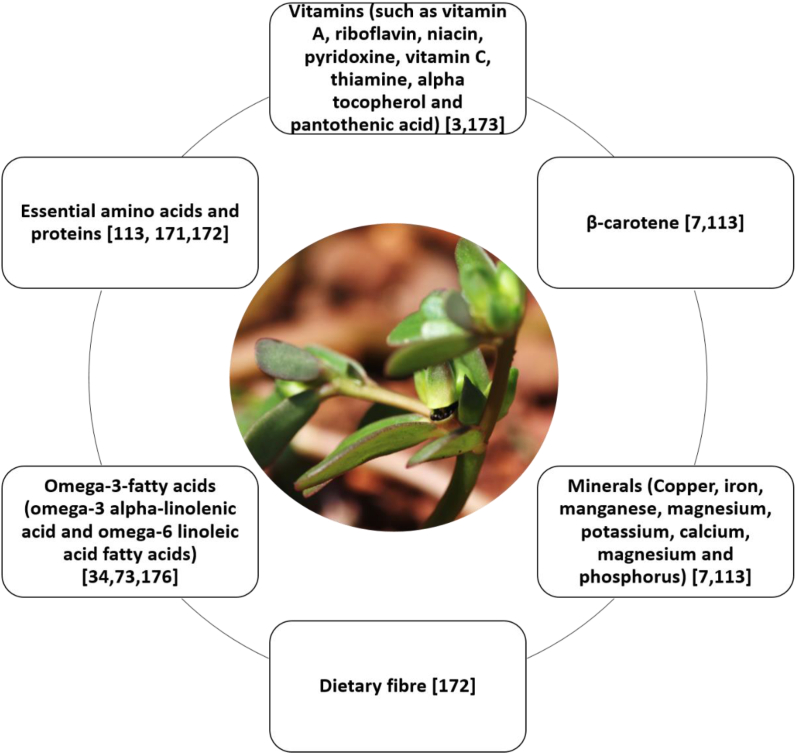
Purslane is a nutritionally rich plant with nutraceutical potential because of the presence of health promoting phytoconstituents [168]. It has been used by people across the globe as a traditional food plant [169, 170]. The purslane leaves contain 23–24 % of proteins [171, 172]. It is observed that protein, ash and fiber content in purslane is higher than that of wheat flour [172]. Presence of considerable amounts of essential dietary minerals such as copper, iron, manganese, magnesium, potassium, calcium, magnesium and phosphorus is reported in purslane [171]. It is a rich source of potassium (494 mg/100 g), magnesium (68 mg/100 g) and calcium (65 mg/100 g) [7]. Results obtained by Santiago-Saenz et al. [113] also suggest that purslane contains significant amounts of protein, fibre and inorganic nutrients such as Fe, Cu, Mn, Zn, B, P, Ca, Mg and K [113]. Mohamed and Hussein [5] found the presence of significant amounts of total solids in roots. The total solids and protein levels vary in different growth stages. Significant variation in total phosphorus, calcium, potassium, iron, manganese, and copper found in relation with growth stages. Purslane is also a rich source of vitamins such as vitamin A, riboflavin, niacin, pyridoxine, vitamin C, thiamin, α-tocopherol and pantothenic acid [3, 173]. It has been found that purslane contains higher amounts of β-carotene, vitamin C and α-tocopherol than spinach [7, 113]. Occurrence of antioxidant molecules suggests that intake of purslane can help overcome oxidative stress [174]. Because of the presence of a number of important nutritional components, common purslane is an important herb and possesses high nutritional potential [175]. Presence of omega-3 fatty acids in purslane makes it one of the few terrestrial sources of omega-3-fatty acids [34, 176]. It is known as a superfood as omega- 3- fatty acids play an important role in prevention of coronary artery diseases, diabetes, arthritis, cancer, and other inflammatory and autoimmune disorders [73]. The presence of vitamin A in purslane makes it a good candidate for people with vision impairments and vitamin A deficiency [18]. Islanders on Atolls in the South Pacific consume purslane because of the presence of macro and micronutrients [177]. The main nutraceutical constituents of purslane are presented in Figure 3 and their individual concentrations are shown in Table 3.
Figure 3.
Main nutraceutical constituents found in purslane.
Table 3.
Main nutraceutical constituents and their concentration found in purslane.
| Nutritional constituent | Concentration | Reference(s) |
|---|---|---|
| Crude protein (% DW) | 23.47 | [172] |
| Carbohydrate (% DW) | 40.67 | [172] |
| Crude lipid (% DW) | 5.26 | [172] |
| Crude fibre (% DW) | 8.00 | [172] |
| Ash (% DW) | 22.66 | [172] |
| Zinc (mg/100 g) | 5.83 ± 0.08 | [172] |
| Calcium (mg/100 g) | 131.44 ± 3.21 | [172] |
| Iron (mg/100 g) | 72.14 ± 505 | [172] |
| Magnesium (mg/100 g) | 66.47 ± 1.43 | [172] |
| Sodium (mg/100 g) | 571.41 ± 16.63 | [172] |
| Potassium (mg/100 g) | 2842.38 ± 91.68 | [172] |
| Manganese (mg/100 g) | 9.75 ± 1.02 | [172] |
| Phosphorus (mg/100 g) | 79.7 | [178] |
| Carotenes (mg/100 g) | 89.2 | [173] |
| Lipids (mg/100 g) | 3.81 | [173] |
| B1 – thiamine (mg/100 g) | 0.047 | [7, 168] |
| B2 – riboflavin (mg/100 g) | 0.112 | [7, 168] |
| B3 – niacin (mg/100 g) | 0.480 | [7, 168] |
| B5 - pantothenic acid (mg/100 g) | 0.036 | [7, 168] |
| B6 – pyridoxine (mg/100 g) | 0.073 | [7, 168] |
| B9 – folates (mg/100 g) | 0.012 | [7, 168] |
| Ascorbic acid (mg/g) | 2.27 (stem) to 3.99 (leaves) | [7] |
| α-tocopherol (mg/100 g) | 26.6 mg | [7] |
| Omega-3-fatty acid (mg/100 g) | 188.48 ± 6.35 | [18] |
| Linoleic acid (LA, mg/100 g) | 34.0 ± 5.2 | [117] |
| α-linolenic acid (LNA, mg/100 g) | 132.8 ± 22.0 | [117] |
| LNA/LA ratio | 5.2 ± 0.03 | [117] |
| α-carotene (mg/100 g) | 0.009 | [168] |
| β-carotene (mg/g) | 0.29 (stem) to 0.58 (leaves) | [7] |
| Lutein (mg/100 g) | 5.4 | [168] |
| Zeaxanthin (mg/100 g) | 0.19 | [168] |
6. Ethnopharmacological potential of purslane
Purslane is a medicinally important herb [103] as it possesses an array of medicinally important phytochemicals [7, 21, 114]. The use of purslane as a medicine and food in China have a history of thousands of years [47]. There are also clues regarding use of purslane for respiratory diseases in ancient Iranian medical books [179]. Ethnobotanical studies suggest that it has been an important part of traditional medicine among various cultures in different parts of this world [71, 78, 93, 96]. The qualitative and quantitative analysis of phytochemical composition also supports its pharmacological value. Various metabolic studies have deciphered its phytochemical composition [99, 100, 102, 106]. Because of its importance in various cultures as an important ethnomedicinal plant, scientists have taken strides to prove its pharmacological potential against various diseases such as diabetes, cancers, neural diseases, asthma, obesity and bacterial and viral diseases using various models including in vivo studies and cell lines [24, 73, 100, 180]. Main ethnopharmacological properties of purslane are depicted in Figure 4. Following subheadings provide details about ethnopharmacological roles and properties of purslane.
Figure 4.
Ethnopharmacological properties of the main phytochemicals found in purslane.
6.1. Antioxidant potential
The phytochemical composition of purslane points towards its antioxidant potential and various studies have proved this using various assays [178]. Several parts of purslane including its leaves, stem and flowers have been used to test its antioxidant potential. Lim and Quah [181] showed that methanolic extract of six cultivars of purslane showed strong antioxidant activity. However, it was found that flowers show highest antioxidant activity and it is linked with higher total phenolic content, ascorbic acid, β-carotene and omega- 3 fatty acids [22]. Antioxidants are very important for human health as they reduce risk of cell damage by free radicals [7]. Several studies have investigated and proved the antioxidant potential of purslane [27, 30, 142, 176]. Several compounds have been isolated from purslane and their antioxidant activities have been proved. For example, phenolic alkaloids such as oleracein A, oleracein B and oleracein E showed antioxidant activities [123]. Comparative analysis of raw and steamed purslane extracts showed reduction in the antioxidant activities following its steaming [107]. The extracts of the aerial parts of the P. quadrifida also showed DPPH radical scavenging activity [182]. Dkhil et al. [183] showed antioxidant potential of P. oleracea using adult Wister albino male rats. The study further found inhibition in lipid peroxidation, nitric oxide in liver, kidney and testis of rats. Taken together all these results suggest that purslane has health promoting effects and gives protection against the free radicals.
6.2. Muscle relaxant potential
Muscle relaxant properties of purslane have been investigated by a number of researchers and found promising results [184, 185, 186, 187, 188, 189, 190, 191]. The muscle relaxant properties and neuromuscular activities of purslane extract may be due to the higher concentration of K+ ions [192].
6.3. Anticancer potential
Cancer is one of the leading causes of deaths globally. Many researchers in the world are working on the prospecting of anticancer diseases against a variety of cancer types. Many drugs currently used against cancer such as taxol, epipodophyllotoxin, Vincristine, Vinblastine, paclitaxel, docetaxel, camptothecin and irinotecan have plant based origins [193, 194]. Therefore, it is not surprising if purslane also contains many such important anticancer molecules. Purslane has shown promising results as an anticancer plant against several cancer types. It was found that extract of purslane has an inhibitory effect on nodule formation in colon cancer stem cells [195]. It was also effective against ulcerative colitis in rats and mice [26, 196]. Use of a unique polysaccharide component (POP) from purslane showed antitumor effects in in vivo models [197]. Water soluble purslane extract showed inhibitory role against cervical cancer cell growth in vitro and in vivo models [198]. It has also been proved effective against gastric cancer [199]. Seed oil of purslane showed significant cytotoxicity against human liver cancer (HepG2) and human lung cancer (A-549) cell lines and inhibited cell growth [24]. Portulacerebroside A (PCA) isolated from purslane showed effectiveness against acute myeloid leukemia [200]. Rahimi [201] showed NF-κB inhibitor activity of the purslane extract. The extract also showed cytotoxicity and apoptogenic activity on human glioblastoma cancer cell line (U-87). The anticancer activities of purslane proves its potential in the development of future drugs against various types of cancer.
6.4. Antimicrobial potential
Purslane is also known to possess antibacterial and antifungal properties [38]. A lectin from the roots of P. elatior showed bacteriostatic activity against Enterococcus faecalis, Pseudomonas aeruginosa and Staphylococcus aureus and antifungal properties against Candida albicans, C. parapsilosis, C. krusei, and C. tropicalis [202]. Cerebrosides such as portulacerebroside (A-D) showed significant antibacterial effects against the enteropathogenic bacteria suggesting its role against bacillary dysentery [108]. Soliman et al. [203] showed antibacterial activities of purslane extract against S. aureus, P. aeruginosa, E. coli, including Acinetobacter baumannii and Klebsiella pneumoniae, a multidrug resistant bacteria. Same study showed antifungal activity of purslane extract against C. albicans. El-Desouky et al. [204] found antifungal activities against three species of Aspergillus namely A. flavus, A. ochraceus and A. parasiticus. Recently Tleubayeva et al. [205] showed that carbon dioxide extract of purslane possess antibacterial activities against E. coli, S. aureus, B. subtilis, and antifungal activity against C. albicans.
6.5. Anti-inflammatory and immunomodulatory potential
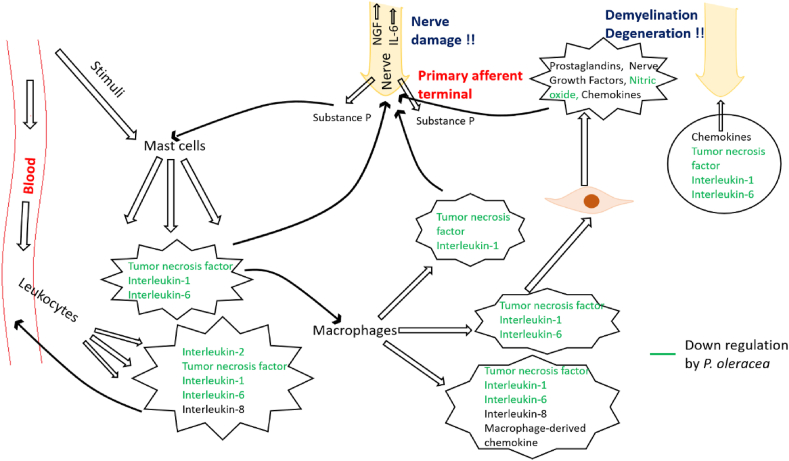
Several studies have confirmed the anti-inflammatory activity of purslane [39, 41, 206, 207, 208, 209, 210]. It is reported that purslane also suppresses lung inflammation [27]. Di Cagno et al. [211] found effectiveness of fermented purslane juice against intestinal inflammation and epithelial injury in caco-2 cell lines. Samarghandian et al. [212] observed the effectiveness of aqueous extract of purslane against inflammation in streptozotocin induced diabetic mice through prevention of oxidative stress. purslane extract also exerts immunomodulatory effects in rats [213]. The purslane showed promising results against oral lichen planus (OLP), a chronic inflammatory and immune-mediated disease [214]. Subsequent to receiving a stimuli, degranulation of mast cells occur and pro-inflammatory cytokines tumor necrosis factor (TNF), interleukin-1 (IL-1) and interleukin-6 (IL-6) are released from mast cells, which in turn lead to stimulation of macrophages that produce inflammatory cytokines and chemokines. They stimulate tissue cells to produce cytokines, chemokines and non-cytokine inflammatory mediators such as prostaglandins (PGs), Nerve growth factor (NGF), and nitric oxide (NO). Then the inflammatory cytokines and non-cytokine inflammatory mediators activate nociceptor terminals of the nerve cells to release neurotransmitters such as Substance P (SP) leading to neurogenic inflammations such as demyelination and degeneration. Polysaccharides from P. oleracea down regulate the inflammatory cytokines (TNF, IL-1 and IL-6) and NO. Furthermore, it upregulates production of anti-inflammatory cytokines (Figure 5). Polysaccharides complexes isolated from aerial parts of purslane shown to be stimulate CD4+/CD25+ and CD8+/CD25+ (human T-cells), CD14+ and CD64+ cells (activated phagocytes) and also enhanced IL-6 production from human white blood cells and Peyer's patch cells [215]. Few other studies have also shown immunomodulatory activities of purslane using various models and cell lines [216, 217]. The immunomodulatory activities of various bioactive components of purslane suggest its high value for the discovery of novel drugs that are immunomodulatory and anti-inflammatory.
Figure 5.
Regulation of inflammatory cytokines by polysaccharides present in purslane.
6.6. Antiviral potential
Only a few studies have reported the antiviral potential of purslane. Water extract of purslane showed antiviral activity against influenza A virus (IAV) infection [218]. Dong et al. [219] showed that pectic polysaccharide isolated from purslane showed activities against herpes simplex virus type 2 (HSV-2).
6.7. Anti-obesity and antidiabetic potential
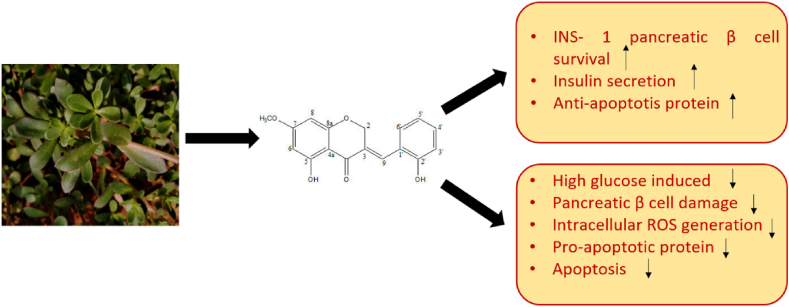
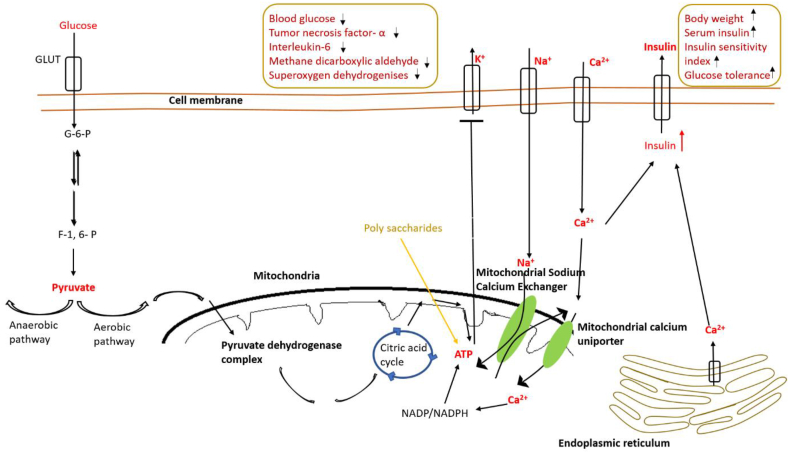
Li et al. [220] confirmed the anti-obesity potential of purslane by checking its anti-adipogenic activity in 3T3-L1 cells. Sicari et al. [114] also observed antioxidant and hypoglycaemic potential or purslane. Its anti-diabetic potential is evaluated by several researchers independently [25, 221, 222, 223]. Polysaccharides from purslane were found to induce the secretion of insulin in insulin-secreting β-cell line cells (INS-1 cells) suggesting its important potential roles in diabetic patients [222]. Similar results were obtained by Park and Han [224]. It has been found that purslane significantly improved liver injury of liver in streptozotocin-induced diabetic mice [224, 225]. Park et al. [226] have shown the molecular mechanisms that govern HM-chromanone induced antidiabetic potential of purslane. Their study further found that HM-chromanone promotes glucose uptake and glycogen biosynthesis through the activation of PI3K/AKT, CaMKKβ-AMPK and GSK3 α/β pathways respectively. Figure 6 represents HM-chromanone mediated antidiabetic properties of purslane. Shift from glucose tolerance state to impaired glucose tolerance leads to diabetes. Closure of ATP- sensitive K+ channels and activation of Na+ and Ca2+ channels increase Ca2+ influx into the cell thus secretion of insulin also increases leading to reduced blood glucose levels. Polysaccharides from purslane enhance ATP production which improves depolarized cell membrane potential via closure of K+ channels. Subsequent increase in Ca2+ entry improves insulin secretion. Polysaccharides from purslane also result in body weight, serum insulin, insulin sensitivity index and glucose tolerance and decrease the risk of diabetes by decreasing the level of TNF- α (Tumor necrosis factor-α), Interleukin-6 (IL-6), Methane dicarboxylic aldehyde (MDA) and Superoxygen dehydrogenases (SOD). The detailed mechanism of action is represented in Figure 7.
Figure 6.
HM-chromanone mediated antidiabetic activities of purslane.
Figure 7.
Regulation of insulin secretion from INS-1 pancreatic β cells and antidiabetic activities of purslane polysaccharides.
6.8. Neuroprotective potential
Neuroprotective effect of seed and aqueous extract of purslane in rats and mice was observed by several researchers [100, 227, 228]. Betacyanins from purslane were found to be effective against the D-galactose induced neurotoxicity in mice [29]. Truong et al. [180] have found that purslane extract acts as a neuroprotectant against Parkinson's disease because of the presence of levodopa and dopamine. Polysaccharides extracted from Purslane played protective roles against lead induced memory impairment in rats [229]. Several alkaloids such as oleracea [48] and oleraisoindole [119] showed anticholinesterase activity. The hydroalcoholic extract of purslane showed potential of sleep improvements and may be an important plant for the insomnia patients in future [230]. Catecholic isoquinolines have been known to show β 2-Adrenergic receptor agonist activity [125].
6.9. Gastro and hepatoprotective potential
Farkhondeh and Samarghandian [231] have reviewed gastroprotective and hepatoprotective effects of purslane [231]. Detailed review of hepatoprotective role is given by Farkhondeh et al. [232]. Anusha et al. [53] found that aqueous extract of purslane in combination with lycopene showed hepatoprotective results against carbon tetrachloride induced hepatotoxicity in rats. The seeds of purslane are very effective against nonalcoholic fatty liver disease which is the most common form of chronic liver disease [233, 234]. The gastro and hepatoprotective properties of purslane also suggest its use in the treatment of lung and liver related diseases.
6.10. Antiasthmatic potential
Purslane is known to possess bronchodilatory and anti-asthmatic effects [179, 235]. Iyekowa et al. [236] found improvement of bronchial asthma in histamine dihydrochloride induced asthmatic guinea pigs. The phytochemical analysis of the extract administered showed the occurrence of tannins, steroids, flavonoids, saponins, and alkaloids suggesting their antiasthmatic potential.
6.11. Wound healing potential
Topical application of crude extract of aerial parts of the purslane in excised wounds of house mouse (JVI-1) showed healing activity suggesting its role in healing [237].
6.12. Other pharmacological properties
Purslane also possesses other pharmacological properties such as effectiveness against abnormal uterine bleeding [238, 239]. It has also shown pancreatic protective roles [240]. Polysaccharides from purslane show anti fatigue results [241]. Ethanolic extract of purslane showed anti-nociceptive activity in rats [207, 242]. It is well known for antiseptic, antispasmodic, diuretic, vermifuge, antiscorbutic, analgesic, anti-inflammatory activities and, anti-ascorbic, antipyretic, and antitussive effects [109, 181, 243, 244].
7. Conclusions and future directions
Purslane is a very important nutritional vegetable with huge nutraceutical and pharmacological potential. Due to the presence of very important traits it possesses, it is considered as an important crop for the future. Although purslane is a wonder crop, it remains one of the underutilized crops across the world, but there is evidence for its usage in traditional foods and ethnomedicinal systems in various countries. Being the eighth most distributed plant in the world, its consumption as an important food and medicine can improve the health of the people besides providing nutrition. The existence of an array of important bioactive compounds and nutritional components makes it a nutraceutical plant. There are global efforts to discover new water efficient crops due to the scarcity of the water globally [245]. In order to address the issue of water scarcity, the Global Framework on Water Scarcity in Agriculture of FAO has suggested saline agriculture as one of the important strategies under water scarce situations in the future [245]. Purslane is highly tolerant to salinity conditions and can be promoted as a biosaline crop for future food and nutritional security. The purslane cultivation must be attempted using saline water. Purslane is rich in omega -3-fatty acids that can be further exploited to reduce pressure on the sea ecology due to overfishing for omega-3-resources. Additionally it is an important source of omega-3-fatty acids for vegetarians and vegans as their diets lack omega-3-fatty acids which is very crucial for the health of humans. Increasing pollution in the seas and risks posed by the seafoods on human health, purslane can act as an alternative source of omega-3 fatty acids [246]. Therefore, omega-3-fatty acid rich diets supplied by purslane can improve the health of the people [247]. In nutshell, purslane is an important future crop for achieving sustainable development goals 1, 2 and 3 on no poverty, zero hunger and good health and wellbeing of the people respectively. The pharmacological studies show multiple medicinal uses of purslane. The results also suggest that novel drugs can be obtained from purslane in future against many diseases. The phytochemical richness of purslane can therefore be exploited for prospecting future plant derived drugs.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge Central University of Kerala, India for the support during this study.
References
- 1.Ocampo G., Columbus J.T. Molecular phylogenetics, historical biogeography, and chromosome number evolution of Portulaca (Portulacaceae) Mol. Phylogenet. Evol. 2012;63:97–112. doi: 10.1016/j.ympev.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Nyffeler R., Eggli U. Disintegrating portulacaceae: a new familial classification of the suborder portulacineae (Caryophyllales) based on molecular and morphological data. Taxon. 2010;59:227–240. [Google Scholar]
- 3.Chugh V., Mishra V., Sharma K. Purslane (Portulaca oleracea L.): an underutilized wonder plant with potential pharmacological value. Pharm. J. 2019;8:236–246. [Google Scholar]
- 4.Uddin M.K., Quan L., Hasan M.M., Motmainna, Madom M.S. Purslane: a perspective plant source of nutrition and antioxident. Plant Arch. 2020;20:1624–1630. [Google Scholar]
- 5.Mohamed A.I., Hussein A.S. Chemical composition of purslane (Portulaca oleracea) Plant Foods Hum. Nutr. 1994;45:1–9. doi: 10.1007/BF01091224. [DOI] [PubMed] [Google Scholar]
- 6.Petropoulos S.A., Fernandes Â., Dias M.I., Vasilakoglou I.B., Petrotos K., Barros L., Ferreira I.C.F.R. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants. 2019;8 doi: 10.3390/antiox8080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uddin M.K., Juraimi A.S., Hossain M.S., Nahar M.A.U., Ali M.E., Rahman M.M. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014;2014 doi: 10.1155/2014/951019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abd El-Azime A.S.H., Hussein E.M., Ashry O.M. Synergestic effect of aqueous purslane (Portulaca oleracea L.) extract and fish oil on radiation-induced damage in rats. Int. J. Radiat. Biol. 2014;90:1184–1190. doi: 10.3109/09553002.2014.926040. [DOI] [PubMed] [Google Scholar]
- 9.Ahangarpour A., Oroojan A.A., Khorsandi L., Lamoochi Z. Effect of hydro alcoholic extract of Purslane (Portulaca oleracea L.) on kidney of aging model induced by D-galactose in female mice, Iran. J. Pharmacol. Ther. 2015;14:10–15. [Google Scholar]
- 10.Chowdhary C.V., Meruva A., Naresh K., Elumalai R.K.A. A review on phytochemical and pharmacological profile of Portulaca oleracea Linn. (Purslane) Int. J. Res. Ayurveda Pharm. 2013;4:34–37. [Google Scholar]
- 11.Miraj S. Healing properties of Purslane: a systematic review study. Der Pharm. Lett. 2016;8:437–441. [Google Scholar]
- 12.Ashrafi A., Zahedi M., Soleimani M. Effect of Co-planted purslane (Portulaca oleracea L.) on Cd accumulation by sunflower in different levels of Cd contamination and salinity: a pot study. Int. J. Phytoremediation. 2015;17:853–860. doi: 10.1080/15226514.2014.981239. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari K.K., Dwivedi S., Mishra S., Srivastava S., Tripathi R.D., Singh N.K., Chakraborty S. Phytoremediation efficiency of Portulaca tuberosa rox and Portulaca oleracea L. naturally growing in an industrial effluent irrigated area in Vadodra, Gujrat, India. Environ. Monit. Assess. 2008;147:15–22. doi: 10.1007/s10661-007-0093-5. [DOI] [PubMed] [Google Scholar]
- 14.Xiang L., Guo D.-X., Ju R., Ma B., Lei F., Du L.-J. Cyclic dipeptides from Portulaca oleracea. Chin. Tradit. Herb. Drugs. 2007;38:1622–1625. [Google Scholar]
- 15.Belcheff E. Polished Publishing Group; 2012. A Medical Intuitive Reveals the Wonders of Purslane.https://books.google.co.in/books?id=tBK2PIufeUsC [Google Scholar]
- 16.Chen C.-J., Wang W.-Y., Wang X.-L., Dong L.-W., Yue Y.-T., Xin H.-L., Ling C.-Q., Li M. Anti-hypoxic activity of the ethanol extract from Portulaca oleracea in mice. J. Ethnopharmacol. 2009;124:246–250. doi: 10.1016/j.jep.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Faruque M.O., Feng G., Khan M.N.A., Barlow J.W., Ankhi U.R., Hu S., Kamaruzzaman M., Uddin S.B., Hu X. Qualitative and quantitative ethnobotanical study of the Pangkhua community in Bilaichari Upazilla, Rangamati district, Bangladesh. J. Ethnobiol. Ethnomed. 2019;15:8. doi: 10.1186/s13002-019-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemzer B., Al-Taher F., Abshiru N. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem. 2020;320 doi: 10.1016/j.foodchem.2020.126621. [DOI] [PubMed] [Google Scholar]
- 19.Younos C., Fleurentin J., Notter D., Mazars G., Mortier F., Pelt J.-M. Repertory of drugs and medicinal plants used in traditional medicine of Afghanistan. J. Ethnopharmacol. 1987;20:245–290. doi: 10.1016/0378-8741(87)90052-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H., Wang Y., Liu Y., Xia Y., Tang T. Analysis of flavonoids in Portulaca oleracea L. by UV-vis spectrophotometry with comparative study on different extraction technologies. Food Anal. Methods. 2010;3:90–97. [Google Scholar]
- 21.Negi S. Quantitative phytochemical analysis of Portulaca oleracea Linn. growing in unpolluted and polluted area. Pharma Innov. J. 2018;7:619–621. [Google Scholar]
- 22.Siriamornpun S., Suttajit M. Microchemical components and antioxidant activity of different morphological parts of Thai wild purslane (Portulaca oleracea) Weed Sci. 2010;58:182–188. [Google Scholar]
- 23.Al-Sheddi E.S., Farshori N.N., Al-Oqail M.M., Al-Massarani S.M., Al Salem A.M., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Portulaca oleracea Linn seed extract ameliorates hydrogen peroxide-induced cell death in human liver cells by inhibiting reactive oxygen species generation and oxidative stress. Trop. J. Pharmaceut. Res. 2016;15:1643–1649. [Google Scholar]
- 24.Al-Sheddi E.S., Farshori N.N., Al-Oqail M.M., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Portulaca oleracea seed oil exerts cytotoxic effects on human liver cancer (HepG2) and human lung cancer (A-549) cell lines. Asian Pac. J. Cancer Prev. APJCP. 2015;16:3383–3387. doi: 10.7314/apjcp.2015.16.8.3383. [DOI] [PubMed] [Google Scholar]
- 25.Bai Y., Zang X., Ma J., Xu G. Anti-diabetic effect of Portulaca oleracea L. Polysaccharideandits mechanism in diabetic rats. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Dong L. Protective effect of purslane in a rat model of ulcerative colitis. Zhongguo Zhongyao Zazhi. 2011;36:2727–2730. [PubMed] [Google Scholar]
- 27.Rahimi V.B., Ajam F., Rakhshandeh H., Askari V.R. A pharmacological review on Portulaca oleracea L.: focusing on anti-inflammatory, anti- oxidant, immuno-modulatory and antitumor activities. J. Pharmacopuncture. 2019;22:7–15. doi: 10.3831/KPI.2019.22.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roozi H., Bojar M.N.A., Eidi V., Ali K.N.R. Effects of oleracein e and oleracein L from Portulaca oleracea on Cell Survival, antioxidant and antidiabetic efficacy on β-TC-6 pancreatic cell line. Indian J. Pharmaceut. Sci. 2019;81:681–689. [Google Scholar]
- 29.Wang C.-Q., Yang G.-Q. Betacyanins from Portulaca oleracea L. ameliorate cognition deficits and attenuate oxidative damage induced by D-galactose in the brains of senescent mice. Phytomedicine. 2010;17:527–532. doi: 10.1016/j.phymed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Yahyazadeh Mashhadi S., Askari V., Ghorani V., Jelodar G., Boskabady M. The effect of Portulaca oleracea and α-linolenic acid on oxidant/antioxidant biomarkers of human peripheral blood mononuclear cells. Indian J. Pharmacol. 2018;50:177–184. doi: 10.4103/ijp.IJP_737_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melilli M.G., Pagliaro A., Scandurra S., Gentile C., Di Stefano V. Omega-3 rich foods: durum wheat spaghetti fortified with Portulaca oleracea. Food Biosci. 2020;37 doi: 10.3390/foods9060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simopoulos A.P. Karger; Basel: 2011. Healthy Agriculture, Healthy Nutrition, Healthy People. [Google Scholar]
- 33.Simopoulos A.P., Norman H.A., Gillaspy J.E., Duke J.A. Common purslane: a source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr. 1992;11:374–382. doi: 10.1080/07315724.1992.10718240. [DOI] [PubMed] [Google Scholar]
- 34.Simopoulos A.P., Salem N. Purslane: a terrestrial source of omega-3 fatty acids. N. Engl. J. Med. 1986;315:833. doi: 10.1056/nejm198609253151313. [DOI] [PubMed] [Google Scholar]
- 35.Simopoulos A.P., Tan D.-X., Manchester L.C., Reiter R.J. Purslane: a plant source of omega-3 fatty acids and melatonin. J. Pineal Res. 2005;39:331–332. doi: 10.1111/j.1600-079X.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 36.Bhuiyan Md.N.H., Adachi T. Efficient regeneration from hypocotyl cultures of betalain forming plant, Portulaca sp. cv. “Jewel”: stimulatory effect of thidiazuron. Plant Biotechnol. 2002;19:57–61. [Google Scholar]
- 37.Bhuiyan N.H., Murakami K., Adachi T. Variation in betalain content and factors affecting the biosynthesis in Portulaca sp. “Jewel” cell cultures. Plant Biotechnol. 2002;19:369–376. [Google Scholar]
- 38.Elkhayat E.S., Ibrahim S.R.M., Aziz M.A. Portulene, a new diterpene from Portulaca oleracea L. J. Asian Nat. Prod. Res. 2008;10:1039–1043. doi: 10.1080/10286020802320590. [DOI] [PubMed] [Google Scholar]
- 39.Gu Y., Leng A., Zhang W., Ying X., Stien D. A novel alkaloid from Portulaca oleracea L. and its anti-inflammatory activity. Nat. Prod. Res. 2020:1–6. doi: 10.1080/14786419.2020.1795855. [DOI] [PubMed] [Google Scholar]
- 40.Jiang M., Zhang W., Yang X., Xiu F., Xu H., Ying X., Stien D. An isoindole alkaloid from Portulaca oleracea L. Nat. Prod. Res. 2018;32:2431–2436. doi: 10.1080/14786419.2017.1419226. [DOI] [PubMed] [Google Scholar]
- 41.Li C.-Y., Meng Y.-H., Ying Z.-M., Xu N., Hao D., Gao M.-Z., Zhang W.-J., Xu L., Gao Y.-C., Ying X.-X. Three novel alkaloids from Portulaca oleracea L. And their anti-inflammatory effects. J. Agric. Food Chem. 2016;64:5837–5844. doi: 10.1021/acs.jafc.6b02673. [DOI] [PubMed] [Google Scholar]
- 42.Joshi A.R., Joshi K. Indigenous knowledge and uses of medicinal plants by local communities of the Kali Gandaki Watershed Area, Nepal. J. Ethnopharmacol. 2000;73:175–183. doi: 10.1016/s0378-8741(00)00301-9. [DOI] [PubMed] [Google Scholar]
- 43.Singh A., Singh M.P., Tewari D. Wild plants used as vegetable in Rupandehi district of Nepal and their ethnomedicinal importance. J. Nat. Hist. Mus. 2015;26:111. [Google Scholar]
- 44.Iranshahy M., Javadi B., Iranshahi M., Jahanbakhsh S.P., Mahyari S., Hassani F.V., Karimi G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017;205:158–172. doi: 10.1016/j.jep.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Khare C.P. Routledge; 2015. Ayurvedic Pharmacopoeial Plant Drugs: Expanded Therapeutics.https://www.routledge.com/Ayurvedic-Pharmacopoeial-Plant-Drugs-Expanded-Therapeutics/Khare/p/book/9781466589995 [Google Scholar]
- 46.Osbaldeston T.A. IBIDIS Press; Johannesburg, South Africa: 2000. Dioscorides de materia medica (Written in Greek in the first century of the common era and translated by TA osbaldeston and RPA Wood in a new indexed version in modern English)https://cupdf.com/document/dioscorides-materia-medica-565b30844b5f8.html [Google Scholar]
- 47.Chen D., Yao J., Liu T., Zhang H., Li R., Zhang Z., Gu X. Research and application of Portulaca oleracea in pharmaceutical area. Chin. Herb. Med. 2019;11:150–159. [Google Scholar]
- 48.Xiu F., Li X., Zhang W., He F., Ying X., Stien D. A new alkaloid from Portulaca oleracea L. and its antiacetylcholinesterase activity. Nat. Prod. Res. 2019;33:2583–2590. doi: 10.1080/14786419.2018.1460833. [DOI] [PubMed] [Google Scholar]
- 49.Xiu F., Ying Z., Ying X., Yang G. Pharmacokinetic studies of soyalkaloid A from Portulaca oleracea L. using ultra high-performance liquid chromatography electrospray ionization quadrupole–time of flight mass spectrometry and its antioxidant activity. Biomed. Chromatogr. 2019;33 doi: 10.1002/bmc.4399. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y.-X., Xin H.-L., Rahman K., Wang S.-J., Peng C., Zhang H., Portulaca oleracea L. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/925631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nanagulyan S., Zakaryan N., Kartashyan N., Piwowarczyk R., Łuczaj Ł. Wild plants and fungi sold in the markets of Yerevan (Armenia) J. Ethnobiol. Ethnomed. 2020;16:26. doi: 10.1186/s13002-020-00375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jafarirad S., Rasoulpour I. Pharmaceutical ethnobotany in the Mahabad (West Azerbaijan) biosphere reserve: ethno-pharmaceutical formulations, nutraceutical uses and quantitative aspects. Braz. J. Pharm. Sci. 2019;55 [Google Scholar]
- 53.Anusha M., Venkateswarlu M., Prabhakaran V., Shareen Taj S., Pushpa Kumari B., Ranganayakulu D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011;43:563–567. doi: 10.4103/0253-7613.84973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaachouay N., Benkhnigue O., Fadli M., El Ibaoui H., Zidane L. Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02191. e02191–e02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prabhu S., Vijayakumar S., Morvin Yabesh J.E., Prakashbabu R., Murugan R. An ethnobotanical study of medicinal plants used in pachamalai hills of Tamil Nadu, India. J. Herb. Med. 2021;25 [Google Scholar]
- 56.Sultana A., Raheman K. Portulaca oleracea Linn: a global panacea with ethnomedicinal and pharmacological potential. Int. J. Pharm. Pharmaceut. Sci. 2013;5:33–39. [Google Scholar]
- 57.Ghahramani R., Eidi M., Ahmadian H., Nomani M.H., Abbasi R., Alipour M., Anissian A. Anti-diabetic effect of Portulaca oleracea (purslane) seeds in alloxan-induced diabetic rats. Int. J. Med. Lab. 2016;3:282–289. [Google Scholar]
- 58.Mosaddegh M., Naghibi F., Moazzeni H., Pirani A., Esmaeili S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012;141:80–95. doi: 10.1016/j.jep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Lev E., Amar Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J. Ethnopharmacol. 2002;82:131–145. doi: 10.1016/s0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 60.DeFilipps R.A., Krupnick G.A. The medicinal plants of Myanmar. PhytoKeys. 2018;102:1–341. doi: 10.3897/phytokeys.102.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aziz M.A., Adnan M., Khan A.H., Rehman A.U., Jan R., Khan J. Ethno-medicinal survey of important plants practiced by indigenous community at Ladha subdivision, South Waziristan agency, Pakistan. J. Ethnobiol. Ethnomed. 2016;12 doi: 10.1186/s13002-016-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ullah M.F. Antioxidative and xanthine oxidase inhibitory activities and phytochemical screening of the hydro-alcoholic extract of mace, aril of Myristica fragrans: implication as an adjuvant therapy in gout. Int. J. Food Prop. 2017;20:694–703. [Google Scholar]
- 63.Olowa L., Demayo C.G. Ethnobotanical uses of medicinal plants among the Muslim Maranaos in Iligan city, Mindanao, Philippines. Adv. Environ. Biol. 2015;9:204+. [Google Scholar]
- 64.Ranil R.H.G., Pushpakumara G., Fonseka R.M., Fonseka H., Bandaranayake P.C.G., Weerakkody W.A.P., Ariyaratne W.M.T.P., De Silva A.N., Gunawardena N.P.T. In: Agric. Res. Sustain. Food Syst. Sri Lanka Vol. 2 Purs. Adv. De Silva R.P., Pushpakumara G., Prasada P., Weerahewa J., editors. Springer Singapore; Singapore: 2020. Utilizing neglected crop genetic resources for food and nutritional security: special reference to indigenous vegetables of Sri Lanka; pp. 39–66. [Google Scholar]
- 65.Britta M. Ogle, Ho Thi Tuyet, Hoang Nghia Duyet, Nguyen Nhut Xuan Dung, food, feed or medicine: the multiple functions of edible wild plants in Vietnam. Econ. Bot. 2003;57:103–117. [Google Scholar]
- 66.W.H.O.R.O. for the W. Pacific . WHO Regional Office for the Western Pacific; Manila: 1990. Medicinal Plants in Viet Nam.https://apps.who.int/iris/handle/10665/207579 [Google Scholar]
- 67.Sam H.V., Baas P., Kessler P.J.A. Traditional medicinal plants in ben En National Park, Vietnam, Blumea - Biodivers. Evol. Biogeogr. Plants. 2008;53:569–601. [Google Scholar]
- 68.Hussein S., Dhabe A. Ethnobotanical study of folk medicinal plants used by villagers in Hajjah district - Republic of Yemen. J. Med. Plants Stud. 2018;6:24–30. [Google Scholar]
- 69.González-Tejero M.R., Casares-Porcel M., Sánchez-Rojas C.P., Ramiro-Gutiérrez J.M., Molero-Mesa J., Pieroni A., Giusti M.E., Censorii E., de Pasquale C., Della A., Paraskeva-Hadijchambi D., Hadjichambis A., Houmani Z., El-Demerdash M., El-Zayat M., Hmamouchi M., ElJohrig S. Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008;116:341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 70.Pieroni A., Dibra B., Grishaj G., Grishaj I., Maçai S.G. Traditional phytotherapy of the Albanians of Lepushe, Northern Albanian Alps. Fitoterapia. 2005;76:379–399. doi: 10.1016/j.fitote.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Bosi G., Guarrera P., Rinaldi R., Mazzanti M. Plants Cult. Seeds Cult. Herit. Eur.; 2009. Ethnobotany of purslane (Portulaca oleracea L.) in Italy and morphobiometric analyses of seeds from archaeological sites in the Emilia Romagna Region (Northern Italy) pp. 129–139. [Google Scholar]
- 72.Brussell D.E. Medicinal plants of Mt. Pelion, Greece. Econ. Bot. 2004;58:S174–S202. [Google Scholar]
- 73.Simopoulos A.P. The traditional diet of Greece and cancer. Eur. J. Cancer Prev. 2004;13:219–230. doi: 10.1097/01.cej.0000130011.99148.07. [DOI] [PubMed] [Google Scholar]
- 74.Petran M., Dragos D., Gilca M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s–1970s) J. Ethnobiol. Ethnomed. 2020;16:15. doi: 10.1186/s13002-020-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carrió E., Vallès J. Ethnobotany of medicinal plants used in Eastern Mallorca (Balearic Islands, Mediterranean sea) J. Ethnopharmacol. 2012;141:1021–1040. doi: 10.1016/j.jep.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 76.Cakilcioglu U., Turkoglu I. An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey) J. Ethnopharmacol. 2010;132:165–175. doi: 10.1016/j.jep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 77.Sargin S.A. Plants used against obesity in Turkish folk medicine: a review. J. Ethnopharmacol. 2021;270 doi: 10.1016/j.jep.2021.113841. [DOI] [PubMed] [Google Scholar]
- 78.Yeşil Y., İnal İ. Ethnomedicinal plants of Hasankeyf (Batman-Turkey) Front. Pharmacol. 2021;11:2511. doi: 10.3389/fphar.2020.624710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baziz K., tinhinen Maougal R., Amroune A. An ethnobotanical survey of spontaneous plants used in traditional medicine in the region of Aures, Algeria. Eur. J. Ecol. 2021;6:49–69. [Google Scholar]
- 80.Urso V., Signorini M., Tonini M., Bruschi P. Wild medicinal and food plants used by communities living in Mopane woodlands of southern Angola: results of an ethnobotanical field investigation. J. Ethnopharmacol. 2015;177:126–139. doi: 10.1016/j.jep.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 81.Jiofack T., Fokunang C., Guedje N.M. Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon. Afr. J. Pharm. Pharmacol. 2009;3:144–150. [Google Scholar]
- 82.Kipkore W., Wanjohi B., Rono H., Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J. Ethnobiol. Ethnomed. 2014;10:24. doi: 10.1186/1746-4269-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belayneh A., Asfaw Z., Demissew S., Bussa N.F. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J. Ethnobiol. Ethnomed. 2012;8:42. doi: 10.1186/1746-4269-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El-Mokasabi F.M., Al-Sanousi M.F., El-Mabrouk R.M. Taxonomy and ethnobotany of medicinal plants in Eastern region of Libya. J. Environ. Sci. Toxicol. Food Technol. 2008;12:14–23. [Google Scholar]
- 85.Adebayo S.A., Amoo S.O. South African botanical resources: a gold mine of natural pro-inflammatory enzyme inhibitors? South Afr. J. Bot. 2019;123:214–227. [Google Scholar]
- 86.Komoreng L., Thekisoe O., Lehasa S., Tiwani T., Mzizi N., Mokoena N., Khambule N., Ndebele S., Mdletshe N. An ethnobotanical survey of traditional medicinal plants used against lymphatic filariasis in South Africa. South Afr. J. Bot. 2017;111:12–16. [Google Scholar]
- 87.Mhlongo L.S., Van Wyk B.-E. Zulu medicinal ethnobotany: new records from the Amandawe area of KwaZulu-Natal, South Africa. Ethnobotany. 2019;122:266–290. [Google Scholar]
- 88.Semenya S.S., Maroyi A. Ethnobotanical survey of plants used by Bapedi traditional healers to treat tuberculosis and its opportunistic infections in the Limpopo Province, South Africa. Ethnobotany. 2019;122:401–421. [Google Scholar]
- 89.Suroowan S., Pynee K.B., Mahomoodally M.F. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. Ethnobotany. 2019;122:189–213. [Google Scholar]
- 90.Scarpa G. Medicinal plants used by the criollos of Northwestern Argentine Chaco. J. Ethnopharmacol. 2004;91:115–135. doi: 10.1016/j.jep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez E.C., Sandi Y.E., Kokoska L. Ethnobotanical inventory of medicinal plants used in the Bustillo province of the Potosi department, Bolivia. Fitoterapia. 2003;74:407–416. doi: 10.1016/s0367-326x(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 92.Netala S., Asha Priya M., Pravallika R., Naga Tejasri S., Shabreen Sumaiya M., Nandini Kumari S. Comparative pharmacognostic studies on three species of Portulaca. Int. J. Pharmacogn. Phytochem. Res. 2014;6:704–714. [Google Scholar]
- 93.Tene V., Malagón O., Finzi P.V., Vidari G., Armijos C., Zaragoza T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007;111:63–81. doi: 10.1016/j.jep.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 94.Jovel E.M., Cabanillas J., Towers G.H.N. An ethnobotanical study of the traditional medicine of the Mestizo people of Suni Miraño, Loreto, Peru. J. Ethnopharmacol. 1996;53:149–156. doi: 10.1016/0378-8741(96)01437-7. [DOI] [PubMed] [Google Scholar]
- 95.Quinlan M.B., Quinlan R.J., Nolan J.M. Ethnophysiology and herbal treatments of intestinal worms in Dominica, West Indies. J. Ethnopharmacol. 2002;80:75–83. doi: 10.1016/s0378-8741(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 96.Lans C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomed. 2006;2:45. doi: 10.1186/1746-4269-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Escalona Arranz J. An ethnobotanical survey of medicinal plants used by inhabitants of Holguín, Eastern Region, Cuba. Boletin Latinoam. Caribe Plantas Med. Aromat. 2018;17:160–196. [Google Scholar]
- 98.Bhat R.S., Al-Daihan S. Phytochemical constituents and antibacterial activity of some green leafy vegetables. Asian Pac. J. Trop. Biomed. 2014;4:189–193. doi: 10.1016/S2221-1691(14)60230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abourashed E.A., El-Alfy A.T. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.) Phytochem. Rev. 2016;15:1035–1056. doi: 10.1007/s11101-016-9469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farag O.M., Abd-Elsalam R.M., Ogaly H.A., Ali S.E., El Badawy S.A., Alsherbiny M.A., Li C.G., Ahmed K.A. Metabolomic profiling and neuroprotective effects of purslane seeds extract against acrylamide toxicity in rat’s brain. Neurochem. Res. 2021;46:819–842. doi: 10.1007/s11064-020-03209-6. [DOI] [PubMed] [Google Scholar]
- 101.Venkateshwari V., Vijayakumar A., Vijayakumar A.K., Reddy L.P.A., Srinivasan M., Rajasekharan R. Leaf lipidome and transcriptome profiling of Portulaca oleracea: characterization of lysophosphatidylcholine acyltransferase. Planta. 2018;248:347–367. doi: 10.1007/s00425-018-2908-8. [DOI] [PubMed] [Google Scholar]
- 102.Zaman S., Bilal M., Du H., Che S. Morphophysiological and comparative metabolic profiling of purslane genotypes (Portulaca oleracea L.) under salt stress. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/4827045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ezeabara C. Comparative determination of phytochemical, proximate and mineral compositions in various parts of Portulaca oleracea L. J. Plant Sci. 2014;2:293–298. [Google Scholar]
- 104.Liu L., Howe P., Zhou Y.-F., Xu Z.-Q., Hocart C., Zhang R. Fatty acids and β-carotene in Australian purslane (Portulaca oleracea) varieties. J. Chromatogr., A. 2000;893:207–213. doi: 10.1016/s0021-9673(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 105.Omara-Alwala T.R., Mebrahtu T., Prior D.E., Ezekwe M.O. Omega-three fatty acids in purslane (Portulaca oleracea) tissues. J. Am. Oil Chem. Soc. 1991;68:198–199. [Google Scholar]
- 106.Farag M.A., Shakour Z.T.A. Metabolomics driven analysis of 11 Portulaca leaf taxa as analysed via UPLC-ESI-MS/MS and chemometrics. Phytochemistry. 2019;161:117–129. doi: 10.1016/j.phytochem.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Fernández-Poyatos M.P., Llorent-Martínez E.J., Ruiz-Medina A. Phytochemical composition and antioxidant activity of Portulaca oleracea: influence of the steaming cooking process. Foods. 2021;10 doi: 10.3390/foods10010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lei X., Li J., Liu B., Zhang N., Liu H. Separation and identification of four new compounds with antibacterial activity from Portulaca oleracea L. Molecules. 2015;20:16375–16387. doi: 10.3390/molecules200916375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiang L., Xing D., Wang W., Wang R., Ding Y., Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66:2595–2601. doi: 10.1016/j.phytochem.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 110.Jiao Z.-Z., Yue S., Sun H.-X., Jin T.-Y., Wang H.-N., Zhu R.-X., Xiang L. Indoline amide glucosides from Portulaca oleracea: isolation, structure, and DPPH radical scavenging activity. J. Nat. Prod. 2015;78:2588–2597. doi: 10.1021/acs.jnatprod.5b00524. [DOI] [PubMed] [Google Scholar]
- 111.Feng P.C., Haynes L.J., Magnus K.E. High concentration of (−)-Noradrenaline in Portulaca oleracea L. Nature. 1961;191 doi: 10.1038/1911108a0. 1108–1108. [DOI] [PubMed] [Google Scholar]
- 112.Mir S.R., Ali M. Taraxerane-type triterpenoids from Portulaca oleracea Linn. seeds. Indian J. Chem. B Org. 2016;55B:119–122. [Google Scholar]
- 113.Santiago-Saenz Y.O., Hernández-Fuentes A.D., Monroy-Torres R., Cariño-Cortés R., Jiménez-Alvarado R. Physicochemical, nutritional and antioxidant characterization of three vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as potential sources of phytochemicals and bioactive compounds. J. Food Meas. Charact. 2018;12:2855–2864. [Google Scholar]
- 114.Sicari V., Loizzo M.R., Tundis R., Mincione A., Pellicanò T.M. Portulaca oleracea L. (Purslane) extracts display antioxidant and hypoglycaemic effects. J. Appl. Bot. Food Qual. 2018;91:39–46. [Google Scholar]
- 115.Silva R., Carvalho I.S. In vitro antioxidant activity, phenolic compounds and protective effect against DNA damage provided by leaves, stems and flowers of Portulaca oleracea (Purslane) Nat. Prod. Commun. 2014;9:45–50. [PubMed] [Google Scholar]
- 116.Ma Y., Bao Y., Zhang W., Ying X., Stien D. Four lignans from Portulaca oleracea L. and its antioxidant activities. Nat. Prod. Res. 2020;34:2276–2282. doi: 10.1080/14786419.2018.1534852. [DOI] [PubMed] [Google Scholar]
- 117.Palaniswamy U.R., McAvoy R.J., Bible B.B. Stage of harvest and polyunsaturated essential fatty acid concentrations in purslane (Portulaca oleraceae) leaves. J. Agric. Food Chem. 2001;49:3490–3493. doi: 10.1021/jf0102113. [DOI] [PubMed] [Google Scholar]
- 118.Dey P., Kundu A., Kumar A., Gupta M., Lee B.M., Bhakta T., Dash S., Kim H.S. In: Recent advances in natural products analysis. Sanches Silva Ana, Fazel Nabavi Seyed, Saeedi Mina, Mohammad Nabavi Seyed., editors. Elsevier; 2020. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids) pp. 505–567. [Google Scholar]
- 119.Ma Y., Li X., Zhang W., Ying X., Stien D. A trace alkaloid, oleraisoindole A from Portulaca oleracea L. and its anticholinesterase effect. Nat. Prod. Res. 2021;35:350–353. doi: 10.1080/14786419.2019.1627356. [DOI] [PubMed] [Google Scholar]
- 120.Zhao C., Ying Z., Tao X., Jiang M., Ying X., Yang G. A new lactam alkaloid from Portulaca oleracea L. and its cytotoxity. Nat. Prod. Res. 2018;32:1548–1553. doi: 10.1080/14786419.2017.1385022. [DOI] [PubMed] [Google Scholar]
- 121.Tian J.-L., Liang X., Gao P.-Y., Li D.-Q., Sun Q., Li L.-Z., Song S.-J. Two new alkaloids from Portulaca oleracea and their cytotoxic activities. J. Asian Nat. Prod. Res. 2014;16:259–264. doi: 10.1080/10286020.2013.866948. [DOI] [PubMed] [Google Scholar]
- 122.Tian J.-L., Liang X., Gao P.-Y., Li L.-Z., Song S.-J. Chemical constituents of Portulaca oleracea. Chem. Nat. Compd. 2015;51:760–761. [Google Scholar]
- 123.Yang Z., Liu C., Xiang L., Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytother Res. 2009;23:1032–1035. doi: 10.1002/ptr.2742. [DOI] [PubMed] [Google Scholar]
- 124.Sun H., He X., Liu C., Li L., Zhou R., Jin T., Yue S., Feng D., Gong J., Sun J., Ji J., Xiang L. Effect of Oleracein E, a Neuroprotective tetrahydroisoquinoline, on rotenone-induced Parkinson’s disease cell and animal models. ACS Chem. Neurosci. 2017;8:155–164. doi: 10.1021/acschemneuro.6b00291. [DOI] [PubMed] [Google Scholar]
- 125.Jin T.-Y., Li S.-Q., Jin C.-R., Shan H., Wang R.-M., Zhou M.-X., Li A.-L., Li L.-Y., Hu S.-Y., Shen T., Xiang L. Catecholic isoquinolines from Portulaca oleracea and their anti-inflammatory and β 2 -Adrenergic receptor agonist activity. J. Nat. Prod. 2018;81:768–777. doi: 10.1021/acs.jnatprod.7b00762. [DOI] [PubMed] [Google Scholar]
- 126.Kuklin A.I., Conger B.V. Catecholamines in plants. J. Plant Growth Regul. 1995;14:91. [Google Scholar]
- 127.Kulma A., Szopa J. Catecholamines are active compounds in plants. Plant Sci. 2007;172:433–440. [Google Scholar]
- 128.Endress R., Jäger A., Kreis W. Catecholamine biosynthesis dependent on the dark in betacyanin-forming Portulaca callus. J. Plant Physiol. 1984;115:291–295. doi: 10.1016/S0176-1617(84)80101-7. [DOI] [PubMed] [Google Scholar]
- 129.Weng Q., Yuan K., Zhang H., Xiong J., Wang C., Xu G. Determination of dopamine and norepinephrine in Portulaca oleracea L. by micellar electrokinetic capillary chromatography with amperometric detection. Chin. J. Chromatogr. Se Pu. 2005;23:18–21. [PubMed] [Google Scholar]
- 130.Yue M.-E., Jiang T.-F., Shi Y.-P. Simultaneous determination of noradrenaline and dopamine in Portulaca oleracea L. by capillary zone electrophoresis. J. Separ. Sci. 2005;28:360–364. doi: 10.1002/jssc.200400045. [DOI] [PubMed] [Google Scholar]
- 131.Hu S., Chai W.C., Xu L., Li S., Jin C., Zhu R., Yang L., Zhang R., Tang K., Li P., Yang E., Chang W., Shen T., Semple S., Venter H., Xiang L. Catecholic alkaloid sulfonates and aromatic nitro compounds from Portulaca oleracea and screening of their anti-inflammatory and anti-microbial activities. Phytochemistry. 2021;181 doi: 10.1016/j.phytochem.2020.112587. [DOI] [PubMed] [Google Scholar]
- 132.Martins W.B., Rodrigues S.A., Silva H.K., Dantas C.G., De Lucca Júnior W., Filho L.X., Cardoso J.C., Gomes M.Z. Neuroprotective effect of Portulaca oleracea extracts against 6-hydroxydopamine-induced lesion of dopaminergic neurons. An. Acad. Bras. Cienc. 2016;88:1439–1450. doi: 10.1590/0001-3765201620150574. [DOI] [PubMed] [Google Scholar]
- 133.Klein M.O., Battagello D.S., Cardoso A.R., Hauser D.N., Bittencourt J.C., Correa R.G. Dopamine: functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019;39:31–59. doi: 10.1007/s10571-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chandrasekara A. In: Encycl. Food Chem. Melton L., Shahidi F., Varelis P., editors. Academic Press; Oxford: 2019. Phenolic acids; pp. 535–545. [Google Scholar]
- 135.Vincente A.R., Manganaris G.A., Ortiz C.M., Sozzi G.O., Crisosto C.H. In: Postharvest Handl. third ed. Florkowski W.J., Shewfelt R.L., Brueckner B., Prussia S.E., editors. Academic Press; San Diego: 2014. Chapter 5 - nutritional quality of fruits and vegetables; pp. 69–122. [Google Scholar]
- 136.Kumar N., Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019;24 doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Middleton M.D.,.E., Jr. Biological properties of plant flavonoids: an overview. Int. J. Pharmacogn. 1996;34:344–348. [Google Scholar]
- 138.Cushnie T.P.T., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 139.Cazarolli L.H., Zanatta L., Alberton E.H., Figueiredo M., Folador P., Damazio R.G., Pizzolatti M.G., Silva F. Flavonoids: prospective drug candidates. Mini Rev. Med. Chem. 2008;8:1429–1440. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 140.Xu X., Yu L., Chen G. Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2006;41:493–499. doi: 10.1016/j.jpba.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 141.Lu J., Zheng Y., Luo L., Wu D., Sun D., Feng Y. Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav. Brain Res. 2006;171:251–260. doi: 10.1016/j.bbr.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 142.Yang X., Zhang W., Ying X., Stien D. New flavonoids from Portulaca oleracea L. and their activities. Fitoterapia. 2018;127:257–262. doi: 10.1016/j.fitote.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 143.Nayaka H.B., Londonkar R.L., Umesh M.K., Tukappa A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014;2014:175851. doi: 10.1155/2014/175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61 doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lila M.A. Anthocyanins and human health: an in vitro investigative approach. J. Biomed. Biotechnol. 2004;2004:673916. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mukherjee P.K. In: Qual. Control Eval. Herb. Drugs. Mukherjee P.K., editor. Elsevier; 2019. Chapter 20 - phyto-pharmaceuticals, nutraceuticals and their evaluation; pp. 707–722. [Google Scholar]
- 147.Valavanidis A., Vlachogianni T. In: Stud. Nat. Prod. Chem. Atta-ur-Rahman, editor. Elsevier; 2013. Chapter 8 - plant polyphenols: recent advances in epidemiological research and other studies on cancer prevention; pp. 269–295. [Google Scholar]
- 148.Castelli M.V., López S.N. In: Stud. Nat. Prod. Chem. Atta-ur-Rahman, editor. Elsevier; 2017. Chapter 9 - homoisoflavonoids: occurrence, biosynthesis, and biological activity; pp. 315–354. [Google Scholar]
- 149.Lin L.-G., Liu Q.-Y., Ye Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014;80:1053–1066. doi: 10.1055/s-0034-1383026. [DOI] [PubMed] [Google Scholar]
- 150.Abegaz B.M., Mutanyatta-Comar J., Nindi M. Naturally occurring homoisoflavonoids: phytochemistry, biological activities and synthesis. Nat. Prod. Commun. 2007;2 1934578X0700200418. [Google Scholar]
- 151.Melilli M.G., Pagliaro A., Bognanni R., Scandurra S., Di Stefano V. Antioxidant activity and fatty acids quantification in Sicilian purslane germplasm. Nat. Prod. Res. 2020;34:26–33. doi: 10.1080/14786419.2018.1560291. [DOI] [PubMed] [Google Scholar]
- 152.Siddaiah V., Rao C.V., Venkateswarlu S., Krishnaraju A.V., Subbaraju G.V. Synthesis, stereochemical assignments, and biological activities of homoisoflavonoids. Bioorg. Med. Chem. 2006;14:2545–2551. doi: 10.1016/j.bmc.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 153.Duan L., Tao H.W., Hao X.J., Gu Q.Q., Zhu W.M. Cytotoxic and antioxidative phenolic compounds from the traditional Chinese medicinal plant, myristica fragrans. Planta Med. 2009;75:1241–1245. doi: 10.1055/s-0029-1185506. [DOI] [PubMed] [Google Scholar]
- 154.Park J., Park J.-E., Seo Y.-W. J.-S. Han., 5,7-Dimethoxy-3-(2′-hydroxybenzyl)-4-chromanone inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2019;863 doi: 10.1016/j.ejphar.2019.172683. [DOI] [PubMed] [Google Scholar]
- 155.Heldt H.-W., Piechulla B. In: Plant Biochem. fourth ed. Heldt H.-W., Piechulla B., editors. Academic Press; San Diego: 2011. 18 - phenylpropanoids comprise a multitude of plant secondary metabolites and cell wall components; pp. 431–449. [Google Scholar]
- 156.Tholl D. In: Biotechnol. Isoprenoids. Schrader J., Bohlmann J., editors. Springer International Publishing; Cham: 2015. Biosynthesis and biological functions of terpenoids in plants; pp. 63–106. [Google Scholar]
- 157.Oliveira I., Valentão P., Lopes R., Andrade P.B., Bento A., Pereira J.A. Phytochemical characterization and radical scavenging activity of Portulaca oleraceae L. leaves and stems. Microchem. J. 2009;92:129–134. [Google Scholar]
- 158.Davis B.C., Kris-Etherton P.M. Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am. J. Clin. Nutr. 2003;78:640S–646S. doi: 10.1093/ajcn/78.3.640S. [DOI] [PubMed] [Google Scholar]
- 159.Azeredo H.M.C. Betalains: properties, sources, applications, and stability – a review. Int. J. Food Sci. Technol. 2009;44:2365–2376. [Google Scholar]
- 160.Rodriguez-Amaya D.B. In: Encycl. Food Chem. Melton L., Shahidi F., Varelis P., editors. Academic Press; Oxford: 2019. Betalains; pp. 35–39. [Google Scholar]
- 161.Villaño D., García-Viguera C., Mena P. In: Encycl. Food Health. Caballero B., Finglas P.M., Toldrá F., editors. Academic Press; Oxford: 2016. Colors: health effects; pp. 265–272. [Google Scholar]
- 162.Trezzini G.F., Zrÿd J.-P. Two betalains from Portulaca grandiflora. Phytochemistry. 1991;30:1897–1899. [Google Scholar]
- 163.Gandía-Herrero F., Escribano J., García-Carmona F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta. 2005;222:586–593. doi: 10.1007/s00425-005-0004-3. [DOI] [PubMed] [Google Scholar]
- 164.Wang H., Zhang L., Wang Y. Isolating and identifying organic acids from Portulaca oleracea and determining their anti-cyanobacterial activity. Pol. J. Environ. Stud. 2017;26:441–445. [Google Scholar]
- 165.Petropoulos S.Α., Karkanis A., Fernandes Â., Barros L., Ferreira I.C.F.R., Ntatsi G., Petrotos K., Lykas C., Khah E. Chemical composition and yield of six genotypes of common purslane (Portulaca oleracea L.): an alternative source of omega-3 fatty acids. Plant Foods Hum. Nutr. 2015;70:420–426. doi: 10.1007/s11130-015-0511-8. [DOI] [PubMed] [Google Scholar]
- 166.Selamoglu Z. Allantoin as metabolic compound. J. Tradit. Med. Clin. Naturop. 2018;7:e143. [Google Scholar]
- 167.Rashed A., Afifi F., Shehadeh M., Taha M. Investigation of the active constituents of Portulaca oleraceae L. (Portulacaceae) growing in Jordan. Pak. J. Pharm. Sci. 2004;17:37–45. [PubMed] [Google Scholar]
- 168.Petropoulos S., Karkanis A., Martins N., Ferreira I.C.F.R. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol. 2016;55:1–10. [Google Scholar]
- 169.Welcome A.K., Van Wyk B.-E. An inventory and analysis of the food plants of southern Africa. Ethnobotany. 2019;122:136–179. [Google Scholar]
- 170.Manzanero-Medina G.I., Vásquez-Dávila M.A., Lustre-Sánchez H., Pérez-Herrera A. Ethnobotany of food plants (quelites) sold in two traditional markets of Oaxaca, Mexico. South Afr. J. Bot. 2020;130:215–223. [Google Scholar]
- 171.Aberoumand A. Identification of fatty acids in edible wild plants by gas chromatography. Food Anal. Methods. 2009;2:208–211. [Google Scholar]
- 172.Almasoud A.G., Salem E. Nutritional quality of purslane and its crackers, Middle East. J. Appl. Sci. 2014;4:448–454. [Google Scholar]
- 173.Guil-Guerrero J.L., Rodríguez-García I. Lipids classes, fatty acids and carotenes of the leaves of six edible wild plants. Eur. Food Res. Technol. 1999;209:313–316. [Google Scholar]
- 174.Arruda S.F., Siqueira E.M.A., Souza E.M.T. Malanga (Xanthosoma sagittifolium) and purslane (Portulaca oleracea) leaves reduce oxidative stress in vitamin A-deficient rats. Ann. Nutr. Metab. 2004;48:288–295. doi: 10.1159/000081075. [DOI] [PubMed] [Google Scholar]
- 175.Simopoulos A.P., Norman H.A., Gillaspy J.E. Purslane in human nutrition and its potential for world agriculture. World Rev. Nutr. Diet. 1995;77:47–74. doi: 10.1159/000424465. [DOI] [PubMed] [Google Scholar]
- 176.Alam M.A., Juraimi A.S., Rafii M.Y., Abdul Hamid A., Aslani F., Hasan M.M., Mohd Zainudin M.A., Uddin M.K. Evaluation of antioxidant compounds, antioxidant activities, and mineral composition of 13 collected purslane (Portulaca oleracea L.) accessions. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/296063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lyons G., Dean G., Tongaiaba R., Halavatau S., Nakabuta K., Lonalona M., Susumu G. Macro-and micronutrients from traditional food plants could improve nutrition and reduce non-communicable diseases of islanders on atolls in the South Pacific. Plants. 2020;9:1–15. doi: 10.3390/plants9080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alam M.A., Nadirah T.A., Mohsin G.M., Saleh M., Moneruzzaman K.M., Aslani F., Juraimi A.S., Alam M.Z. Antioxidant compounds, antioxidant activities, and mineral contents among underutilized vegetables. Int. J. Veg. Sci. 2021;27:157–166. [Google Scholar]
- 179.Malek F., Boskabady M.H., Boroushaki M.T., Tohidi M. Bronchodilatory effect of Portulaca oleracea in airways of asthmatic patients. J. Ethnopharmacol. 2004;93:57–62. doi: 10.1016/j.jep.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 180.Truong H.K.T., Huynh M.A., Vuu M.D., Dang T.P.T. Evaluating the potential of Portulaca oleracea L. For Parkinson’s disease treatment using a Drosophila model with dUCH -Knockdown. Park. Dis. 2019;2019 doi: 10.1155/2019/1818259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lim Y.Y., Quah E.P.L. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007;103:734–740. [Google Scholar]
- 182.Desta Z.Y., Cherie D.A. Determination of antioxidant and antimicrobial activities of the extracts of aerial parts of Portulaca quadrifida. Chem. Cent. J. 2018;12 doi: 10.1186/s13065-018-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dkhil M.A., Moniem A.E.A., Al-Quraishy S., Saleh R.A. Antioxidant effect of purslane (Portulaca oleracea) and its mechanism of action. J. Med. Plants Res. 2011;5:1589–1593. [Google Scholar]
- 184.Okwuasaba F., Ejike C., Parry O. Effects of extracts of Portulaca oleracea on skeletal muscle in vitro. J. Ethnopharmacol. 1987;21:55–63. doi: 10.1016/0378-8741(87)90094-8. [DOI] [PubMed] [Google Scholar]
- 185.Okwuasaba F., Ejike C., Parry O. Comparison of the skeletal muscle relaxant properties of Portulaca oleracea extracts with dantrolene sodium and methoxyverapamil. J. Ethnopharmacol. 1987;20:85–106. doi: 10.1016/0378-8741(87)90082-1. [DOI] [PubMed] [Google Scholar]
- 186.Okwuasaba F., Parry O., Ejike C. Investigation into the mechanism of action of extracts of Portulaca oleracea. J. Ethnopharmacol. 1987;21:91–97. doi: 10.1016/0378-8741(87)90098-5. [DOI] [PubMed] [Google Scholar]
- 187.Okwuasaba F., Ejike C., Parry O. Skeletal muscle relaxant properties of the aqueous extract of Portulaca oleracea. J. Ethnopharmacol. 1986;17:139–160. doi: 10.1016/0378-8741(86)90054-1. [DOI] [PubMed] [Google Scholar]
- 188.Parry O., Marks J.A., Okwuasaba F.K. The skeletal muscle relaxant action of Portulaca oleracea: role of potassium ions. J. Ethnopharmacol. 1993;40:187–194. doi: 10.1016/0378-8741(93)90067-f. [DOI] [PubMed] [Google Scholar]
- 189.Parry O., Okwuasaba F., Ejike C. Effect of an aqueous extract of Portulaca oleracea leaves on smooth muscle and rat blood pressure. J. Ethnopharmacol. 1988;22:33–44. doi: 10.1016/0378-8741(88)90228-0. [DOI] [PubMed] [Google Scholar]
- 190.Parry O., Okwuasaba F.K., Ejike C. Skeletal muscle relaxant action of an aqueous extract of Portulaca oleracea in the rat. J. Ethnopharmacol. 1987;19:247–253. doi: 10.1016/0378-8741(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 191.Parry O., Okwuasaba F., Ejike C. Preliminary clinical investigation into the muscle relaxant actions of an aqueous extract of Portulaca oleracea applied topically. J. Ethnopharmacol. 1987;21:99–106. doi: 10.1016/0378-8741(87)90099-7. [DOI] [PubMed] [Google Scholar]
- 192.Habtemariam S., Harvey A.L., Waterman P.G. The muscle relaxant properties of Portulaca oleracea are associated with high concentrations of potassium ions. J. Ethnopharmacol. 1993;40:195–200. doi: 10.1016/0378-8741(93)90068-g. [DOI] [PubMed] [Google Scholar]
- 193.Greenwell M., Rahman P.K.S.M. Medicinal plants: their use in anticancer treatment. Int. J. Pharma Sci. Res. 2015;6:4103–4112. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Habtemariam S., Lentini G. Plant-derived anticancer agents: lessons from the pharmacology of geniposide and its aglycone, genipin. Biomedicines. 2018;6 doi: 10.3390/biomedicines6020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jin H., Chen L., Wang S., Chao D. Portulaca oleracea extract can inhibit nodule formation of colon cancer stem cells by regulating gene expression of the notch signal transduction pathway. Tumor Biol. 2017;39 doi: 10.1177/1010428317708699. [DOI] [PubMed] [Google Scholar]
- 196.Kong R., Luo H., Wang N., Li J., Xu S., Chen K., Feng J., Wu L., Li S., Liu T., Lu X., Xia Y., Shi Y., Zhou Y., He W., Dai Q., Zheng Y., Lu J. Portulaca extract attenuates development of dextran sulfate sodium induced colitis in mice through activation of PPAR γ. PPAR Res. 2018;2018 doi: 10.1155/2018/6079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shen H., Tang G., Zeng G., Yang Y., Cai X., Li D., Liu H., Zhou N. Purification and characterization of an antitumor polysaccharide from Portulaca oleracea L. Carbohydr. Polym. 2013;93:395–400. doi: 10.1016/j.carbpol.2012.11.107. [DOI] [PubMed] [Google Scholar]
- 198.Zhao R., Gao X., Cai Y., Shao X., Jia G., Huang Y., Qin X., Wang J., Zheng X. Antitumor activity of Portulaca oleracea L. polysaccharides against cervical carcinoma in vitro and in vivo. Carbohydr. Polym. 2013;96:376–383. doi: 10.1016/j.carbpol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 199.Li Y., Hu Y., Shi S., Jiang L. Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. Int. J. Biol. Macromol. 2014;68:113–116. doi: 10.1016/j.ijbiomac.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 200.Ye Q., Zhang N., Chen K., Zhu J., Jiang H. Effects of portulacerebroside a on apoptosis of human leukemia HL60 cells and p38/JNK signaling pathway. Int. J. Clin. Exp. Pathol. 2015;8:13968–13977. [PMC free article] [PubMed] [Google Scholar]
- 201.Baradaran Rahimi V., Mousavi S.H., Haghighi S., Soheili-Far S., Askari V. Cytotoxicity and apoptogenic properties of the standardized extract of Portulaca oleracea on glioblastoma multiforme cancer cell line (U-87): a mechanistic study. EXCLI J. 2019;18:165–186. doi: 10.17179/excli2019-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.da Silva J.D.F., da Silva S.P., da Silva P.M., Vieira A.M., de Araújo L.C.C., de Albuquerque Lima T., de Oliveira A.P.S., do Nascimento Carvalho L.V., da Rocha Pitta M.G., de Melo Rêgo M.J.B., Pinheiro I.O., Zingali R.B., do Socorro de Mendonça Cavalcanti M., Napoleão T.H., Paiva P.M.G. Portulaca elatior root contains a trehalose-binding lectin with antibacterial and antifungal activities. Int. J. Biol. Macromol. 2019;126:291–297. doi: 10.1016/j.ijbiomac.2018.12.188. [DOI] [PubMed] [Google Scholar]
- 203.Soliman S.S.M., Semreen M.H., El-Keblawy A.A., Abdullah A., Uppuluri P., Ibrahim A.S. Assessment of herbal drugs for promising anti-Candida activity. BMC Compl. Alternative Med. 2017;17 doi: 10.1186/s12906-017-1760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.El-Desouky T.A. Evaluation of effectiveness aqueous extract for some leaves of wild edible plants in Egypt as anti-fungal and anti-toxigenic. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tleubayeva M.I., Datkhayev U.M., Alimzhanova M., Ishmuratova M.Y., Korotetskaya N.V., Abdullabekova R.M., Flisyuk E.V., Gemejiyeva N.G. Component composition and antimicrobial activity of CO2 extract of Portulaca oleracea, growing in the territory of Kazakhstan. Sci. World J. 2021;2021 doi: 10.1155/2021/5434525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Allahmoradi E., Taghiloo S., Omrani-Nava V., Shobeiri S.S., Tehrani M., Ebrahimzadeh M.A., Asgarian-Omran H. Anti-inflammatory effects of the Portulaca oleracea hydroalcholic extract on human peripheral blood mononuclear cells. Med. J. Islam. Repub. Iran. 2018;32 doi: 10.14196/mjiri.32.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.He Y., Long H., Zou C., Yang W., Jiang L., Xiao Z., Li Q., Long S. Anti-nociceptive effect of Portulaca oleracea L. ethanol extracts attenuated zymosan-induced mouse joint inflammation via inhibition of Nrf2 expression. Innate Immun. 2021;27:230–239. doi: 10.1177/1753425921994190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Meng X.-L., Xue L., Zhang Z.-W., Gui X.-J., Chen Y.-H., Tang J.-F., Li W.-X., Cao Y.-J., Zhao Y., Li X.-L. Effect of Portulaca oleracea polysaccharide on immunological function in mice with cyclophosphamide-induced immunosuppression. Chin. J. New Drugs. 2017;26:1296–1300. [Google Scholar]
- 209.Meng Y., Ying Z., Xiang Z., Hao D., Zhang W., Zheng Y., Gao Y., Ying X. The anti-inflammation and pharmacokinetics of a novel alkaloid from Portulaca oleracea L. J. Pharm. Pharmacol. 2016;68:397–405. doi: 10.1111/jphp.12526. [DOI] [PubMed] [Google Scholar]
- 210.Miao L., Tao H., Peng Y., Wang S., Zhong Z., El-Seedi H., Dragan S., Zengin G., Cheang W.S., Wang Y., Xiao J. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019;290:239–245. doi: 10.1016/j.foodchem.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 211.Di Cagno R., Filannino P., Vincentini O., Cantatore V., Cavoski I., Gobbetti M. Fermented Portulaca oleracea L. Juice: a novel functional beverage with potential ameliorating effects on the intestinal inflammation and epithelial injury. Nutrients. 2019;11 doi: 10.3390/nu11020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Samarghandian S., Borji A., Farkhondeh T. Attenuation of oxidative stress and inflammation by Portulaca oleracea in streptozotocin-induced diabetic rats. J. Evid.-Based Complement. Altern. Med. 2017;22:562–566. doi: 10.1177/2156587217692491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kaveh M., Eidi A., Nemati A., Boskabady M.H. The extract of Portulaca oleracea and its constituent, alpha linolenic acid affects serum oxidant levels and inflammatory cells in sensitized rats, Iran. J. Allergy Asthma Immunol. 2017;16:256–270. [PubMed] [Google Scholar]
- 214.Agha-Hosseini F., Borhan-Mojabi K., Monsef-Esfahani H.-R., Mirzaii-Dizgah I., Etemad-Moghadam S., Karagah A. Efficacy of purslane in the treatment of oral lichen planus. Phytother Res. 2010;24:240–244. doi: 10.1002/ptr.2919. [DOI] [PubMed] [Google Scholar]
- 215.Georgiev Y.N., Ognyanov M.H., Kiyohara H., Batsalova T.G., Dzhambazov B.M., Ciz M., Denev P.N., Yamada H., Paulsen B.S., Vasicek O., Lojek A., Barsett H., Antonova D., Kratchanova M.G. Acidic polysaccharide complexes from purslane, silver linden and lavender stimulate Peyer’s patch immune cells through innate and adaptive mechanisms. Int. J. Biol. Macromol. 2017;105:730–740. doi: 10.1016/j.ijbiomac.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 216.Park Y.M., Lee H.Y., Kang Y.G., Park S.H., Lee B.G., Park Y.J., Oh H.G., Moon D.I., Kim Y.P., Park D.S., Lee H.M., jin Kim O., Yang H.-J., Kim M.J., Lee Y.-R. Immune-enhancing effects of Portulaca oleracea L.– based complex extract in cyclophosphamide-induced splenocytes and immunosuppressed rats. Food Agric. Immunol. 2019;30:13–24. [Google Scholar]
- 217.Catap E.S., Kho M.J.L., Jimenez M.R.R. In vivo nonspecific immunomodulatory and antispasmodic effects of common purslane (Portulaca oleracea Linn.) leaf extracts in ICR mice. J. Ethnopharmacol. 2018;215:191–198. doi: 10.1016/j.jep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 218.Li Y.-H., Lai C.-Y., Su M.-C., Cheng J.-C., Chang Y.-S. Antiviral activity of Portulaca oleracea L. against influenza A viruses. J. Ethnopharmacol. 2019;241:112013. doi: 10.1016/j.jep.2019.112013. [DOI] [PubMed] [Google Scholar]
- 219.Dong C.-X., Hayashi K., Lee J.-B., Hayashi T. Characterization of structures and antiviral effects of polysaccharides from Portulaca oleracea L. Chem. Pharm. Bull. (Tokyo). 2010;58:507–510. doi: 10.1248/cpb.58.507. [DOI] [PubMed] [Google Scholar]
- 220.Lee J.I., Oh J.H., Kong C.-S., Seo Y. Evaluation of anti-adipogenic active homoisoflavonoids from Portulaca oleracea. Z. Naturforsch. C Biosci. 2019;74:265–273. doi: 10.1515/znc-2018-0114. [DOI] [PubMed] [Google Scholar]
- 221.Gong F., Li F., Zhang L., Li J., Zhang Z., Wang G. Hypoglycemic effects of crude polysaccharide from purslane. Int. J. Mol. Sci. 2009;10:880–888. doi: 10.3390/ijms10030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hu Q., Niu Q., Song H., Wei S., Wang S., Yao L., Li Y.-P. Polysaccharides from Portulaca oleracea L. regulated insulin secretion in INS-1 cells through voltage-gated Na+ channel. Biomed. Pharma. 2019;109:876–885. doi: 10.1016/j.biopha.2018.10.113. [DOI] [PubMed] [Google Scholar]
- 223.Lee J.H., Park J.E., Han J.S. Portulaca oleracea L. extract reduces hyperglycemia via PI3k/Akt and AMPK pathways in the skeletal muscles of C57BL/Ksj-db/db mice. J. Ethnopharmacol. 2020;260 doi: 10.1016/j.jep.2020.112973. [DOI] [PubMed] [Google Scholar]
- 224.Park J.E., Han J.S. Portulaca oleracea L. Extract promotes insulin secretion via a K+ATP channel dependent pathway in INS-1 pancreatic β-cells. Nutr. Res. Pract. 2018;12:183–190. doi: 10.4162/nrp.2018.12.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zheng G., Mo F., Ling C., Peng H., Gu W., Li M., Chen Z. Portulaca oleracea L. Alleviates liver injury in streptozotocin-induced diabetic mice. Drug Des. Dev. Ther. 2018;12:47–55. doi: 10.2147/DDDT.S121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Park J.E., Seo Y., Han J.S. HM-chromanone, a component of Portulaca oleracea L., stimulates glucose uptake and glycogen synthesis in skeletal muscle cell. Phytomedicine. 2021;83 doi: 10.1016/j.phymed.2021.153473. [DOI] [PubMed] [Google Scholar]
- 227.Abdel Moneim A.E. The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol. Disord. - Drug Targets. 2013;12:830–841. doi: 10.2174/18715273113129990081. [DOI] [PubMed] [Google Scholar]
- 228.Hongxing Z., Nancai Y., Guofu H., Jianbo S., Yanxia W., Hanju H., Qian L., Wei M., Yandong Y., Hao H. Neuroprotective effects of purslane herb aquenous extracts against d-galactose induced neurotoxicity. Chem. Biol. Interact. 2007;170:145–152. doi: 10.1016/j.cbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 229.Tao H., Ye D.-L., Wu Y.-L., Han M.-M., Xue J.-S., Liu Z.-H., Chen X.-T., Wang H.-L. The protective effect of polysaccharide extracted from Portulaca oleracea L. against Pb-induced learning and memory impairments in rats. Int. J. Biol. Macromol. 2018;119:617–623. doi: 10.1016/j.ijbiomac.2018.07.138. [DOI] [PubMed] [Google Scholar]
- 230.Hamedi S., Forouzanfar F., Rakhshandeh H., Arian A. Hypnotic effect of Portulaca oleracea on pentobarbital-induced sleep in mice. Curr. Drug Discov. Technol. 2019;16:198–203. doi: 10.2174/1570163815666180308142543. [DOI] [PubMed] [Google Scholar]
- 231.Farkhondeh T., Samarghandian S. The therapeutic effects of Portulaca oleracea L. in hepatogastric disorders. Gastroenterol. Hepatol. 2019;42:127–132. doi: 10.1016/j.gastrohep.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 232.Farkhondeh T., Samarghandian S., Azimi-Nezhad M., Hozeifi S. The hepato-protective effects of Portulaca oleracea L. Extract: Review. Curr. Drug Discov. Technol. 2019;16:122–126. doi: 10.2174/1570163815666180330142724. [DOI] [PubMed] [Google Scholar]
- 233.Gheflati A., Adelnia E., Nadjarzadeh A. The clinical effects of purslane (Portulaca oleracea) seeds on metabolic profiles in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. Phytother Res. 2019;33:1501–1509. doi: 10.1002/ptr.6342. [DOI] [PubMed] [Google Scholar]
- 234.Mohamed D.A., Essa H.A., Mohamed R.S. Purslane and garden cress seeds as source of unconventional edible oils for prevention of hyperlipidemia. Pakistan J. Biol. Sci. 2019;22:537–544. doi: 10.3923/pjbs.2019.537.544. [DOI] [PubMed] [Google Scholar]
- 235.Khazdair M.R., Anaeigoudari A., Kianmehr M. Anti-asthmatic effects of Portulaca oleracea and its constituents, a review. J. Pharmacopuncture. 2019;22:122–130. doi: 10.3831/KPI.2019.22.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Iyekowa O., Uzama-Avenbuan O., Edema M., Enadeghe O., Stanley I. Antiasthmatic activity of Portulaca oleracea Linn. Sky J. Biochem. Res. 2012;1:1–6. [Google Scholar]
- 237.Rashed A.N., Afifi F.U., Disi A.M. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003;88:131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 238.Khanam B., Begum W., Tipo F.A., Naim M. Effect of Tukhme Khurfa (Purslane seeds) in abnormal uterine bleeding: a prospective study. Adv. Integr. Med. 2021;8(3):193–198. [Google Scholar]
- 239.Shobeiri S.F., Sharei S., Heidari A., Kianbakht S. Portulaca oleracea L. in the treatment of patients with abnormal uterine bleeding: a pilot clinical trial. Phytother Res. 2009;23:1411–1414. doi: 10.1002/ptr.2790. [DOI] [PubMed] [Google Scholar]
- 240.Ahangarpour A., Oroojan A.A., Khorsandi L., Lamoochi Z. Effect of hydroalcoholic extract of purslane (Portulaca oleracea L.) on diabetic variables in D-galactose induced aging mouse model. Acta Endocrinol. (Copenh.). 2018;14:24–29. doi: 10.4183/aeb.2018.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xu Z., Shan Y. Anti-fatigue effects of polysaccharides extracted from Portulaca oleracea L. in mice. Indian J. Biochem. Biophys. 2014;51:321–325. [PubMed] [Google Scholar]
- 242.Radhakrishnan R., Zakaria M.N.M., Islam M.W., Chen H.B., Kamil M., Chan K., Al-Attas A. Neuropharmacological actions of Portulaca oleraceae L v. sativa (Hawk) J. Ethnopharmacol. 2001;76:171–176. doi: 10.1016/s0378-8741(01)00230-6. [DOI] [PubMed] [Google Scholar]
- 243.Chan K., Islam M.W., Kamil M., Radhakrishnan R., Zakaria M.N.M., Habibullah M., Attas A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. J. Ethnopharmacol. 2000;73:445–451. doi: 10.1016/s0378-8741(00)00318-4. [DOI] [PubMed] [Google Scholar]
- 244.Islam M.W., Zakaria M.N.M., Radhakrishnan R., Habibullah M., Chan K. Evaluation of analgesic activity of the aerial parts of Portulaca oleracea v. sativa and its comparison with two related species. J. Pharm. Pharmacol. 1998;50:226. [Google Scholar]
- 245.FAO . 2021. WASAG - the Global Framework on Water Scarcity in Agriculture in a Changing Climate.http://www.fao.org/land-water/overview/wasag/es/ [Google Scholar]
- 246.Domingo J.L. Omega-3 fatty acids and the benefits of fish consumption: is all that glitters gold? Environ. Int. 2007;33:993–998. doi: 10.1016/j.envint.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 247.Muthoni J., Nyamongo D.O. Traditional food crops and their role in food and nutritional security in Kenya. J. Agric. Food Inf. 2010;11:36–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.