Abstract
Recently, multifunctional drug delivery systems (DDSs) have been designed to provide a comprehensive approach with multiple functionalities, including diagnostic imaging, targeted drug delivery, and controlled drug release. Chitosan-based drug nanoparticles (CSNPs) systems are employed as diagnostic imaging and delivering the drug to particular targeted sites in a regulated manner. Drug release is an important factor in ensuring high reproducibility, stability, quality control of CSNPs, and scientific-based for developing CSNPs. Several factors influence drug release from CSNPs, including composition, composition ratio, ingredient interactions, and preparation methods. Early, CSNPs were used for improving drug solubility, stability, pharmacokinetics, and pharmacotherapeutics properties. Chitosan has been developed toward a multifunctional drug delivery system by exploring positively charged properties and modifiable functional groups. Various modifications to the polymer backbone, charge, or functional groups will undoubtedly affect the drug release from CSNPs. The drug release from CSNPs has a significant influence on its therapeutic actions. Our review's objective was to summarize and discuss the relationship between the modification in CSNPs as multifunctional delivery systems and drug release properties and kinetics of the drug release model. Kinetic models help describe the release rate, leading to increased efficiency, accuracy, the safety of the dose, optimizing the drug delivery device's design, evaluating the drug release rate, and improvement of patient compatibility. In conclusion, almost all CSNPs showed bi-phasic release, initial burst release drug in a particular time followed controlled manner release in achieving the expected release, stimuli external can be applied. CSNPs are a promising technique for multifunctional drug delivery systems.
Keywords: Multifunction delivery system, Bi-phasic release, Burst release, Controlled release
Multifunction delivery system; Bi-phasic release; Burst release; Controlled release.
1. Introduction
Nanomedicine is rapidly evolving toward multifunctional DDSs, and it can be used as a diagnostic tool, targeted delivery, and a controlled delivery system [1]. Nanomedicine has been engineered to be incredibly small, allowing them to travel more easily inside the human body while possessing structurally unique, chemical, electronic, magnetic, electrical, and biological properties [1, 2, 3]. Advantages of nanomedicine are in vivo long-circulating in vivo, improved drug bioavailability, breaking through biological barriers [4], enhancing deep-tissue penetration, and cancer cell uptake. Furthermore, active targeting and the effects of passive targeting enable polymeric nanoparticles (PNPs) to specifically target cancer cells by detecting the expression of surface receptors on tumours [4, 5, 6, 7].
Throughout the treatment cycle, the primary aim of drug therapy for any disease is to obtain and sustain the drug's optimal therapeutic concentration at the site of action. PNPs can control the release of drugs over a long period, thus increasing the therapeutic index of pharmacology activity of agents [8]. PNPs' enhanced bioavailability and less adverse effects result in favorable anticancer outcomes as compared to free medicines [8,9].
Characterization of NPs is critical to ensure the desired behaviour in vitro and in vivo [10]. The in vitro release rate and mechanism profile are strongly influenced by the structure, composition, composition ratio, and interaction between drug and polymer [11]. This information accommodates a scientific and predictive approach to the design and development of DDSs. In vitro release studies are generally indirect measures of drug availability in the early stages of product development, quality control to support batch release clinically and biologically effective, assessment of formulation factors, and manufacturing methods that affect bioavailability [12].
Chitosan (CS) is a natural, hydrophilic, positively charged polymer with easy fabrication, good biocompatibility, and similarity of flexibility to natural tissue [2,6,11,13]. Drug release from CSNPs is governed by polymer swelling, adsorbed drugs, drug diffusion, polymer erosion or degradation, and a combination of erosion and degradation [14,15].
CSNPs have been extensively used for bioactive compounds because of their high physicochemical stability, ability to enhance the bioavailability, non-toxicity, and potential targeted [16]. CSNPs are used as a diagnostic tool, targeted drug delivery, and a controlled delivery system [17,18]. CS modifications may be carried out using functional groups by various physical and chemical processes like grafting, crosslinking, complexation, and mixing with polymers [19]. Modification or adding other substances to the formulation, such as crosslinker, copolymer, conjugates, or drug, is needed to reach multifunction DDSs. The encapsulated drug in CSNPs is typically formed through hydrogen bonds, Van der Waals interactions including hydrophobic and electrostatic interactions, or monomer polymerization. This modification of CS will have consequences in changing CSNPs properties, specialty drug release. CSNPs have shown great potential with extensive use in active substance administration, diagnostics, and other sectoral uses [20], including personal medicine [21]. These advances raise questions about the safety of NPs. Nanotoxicological studies are needed to produce safe NPs and a comprehensive toxicity study of existing NPs [22]. The availability of safe and non-toxic polymers or materials attracts them, due to its biodegradable and biocompatible qualities. Cs was determined to be generally harmless, like mesoporous carbon nanoparticles, organosilica and hydroxyapatite (HAP) nanoparticles [23].
2. Polymeric nanoparticles
NPs are made of solid polymer carriers in submicron size (10–1000 nm) [10]. PNPs can overcome the biological barriers, protecting and delivering a drug to target cells [24,25]. The PNPs can be formulated as dendrimers, micelles, nanogels, and nanocapsules. PNPs are widely used for controlled release in the pharmaceutical industry, using different formulations and manufacturing methods [14,15]. PNPs offer a remarkable guarantee that they exhibit outstanding physicochemical properties - size, surface load, hydrophilicity, and hydrophobia - and are thus regarded as a future carrier of drugs and medicinal products [10]. The advantage characteristics can reduce a load of medicine and time for patient convenience [26].
2.1. Drug release from polymeric nanoparticles
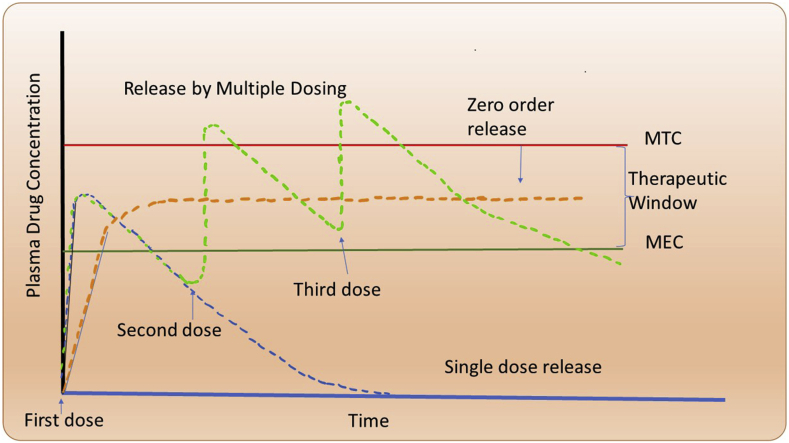
The therapeutic window is well-defined as the region between the minimum toxic concentration (MTC) and minimum effective concentration (MEC) [27]. A single big dosage of a medicine has hazardous adverse effects and quickly drops below the MEC (Figure 1). If there is more excellent stability in the plasma's drug levels, multiple administration of a particular formulation can help; however, noncompliance can be a problem if patients are dosed intermittently. Long-term release of zero-order drugs is being pursued in practice. However, excessive release can reduce the therapeutic efficacy, resulting in delayed drug delivery [28]. Hence, pulsatile or stimulus-responsive drug administration is investigated [27,29].
Figure 1.
Multiple dosing results in plasma drug concentration profiles (green line) and zero-order release (orange line). Two minimum toxic concentration (MTC) levels form the range, and the MEC displays the therapeutic windows.
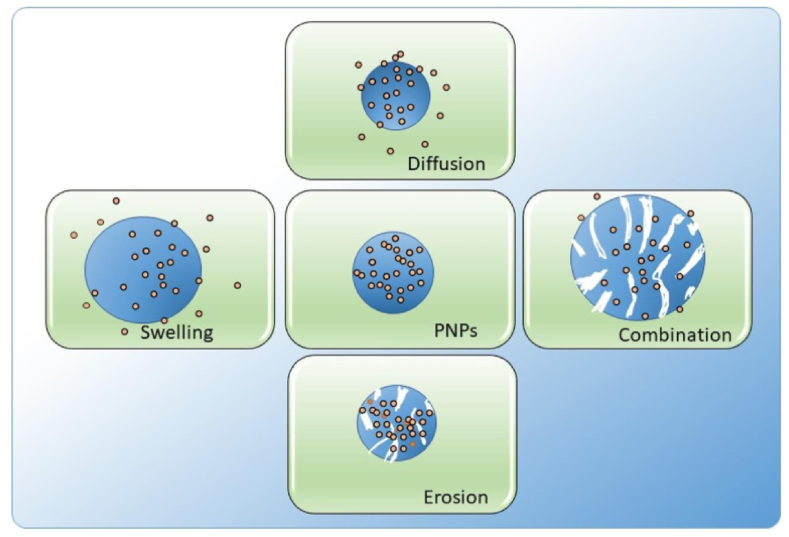
Several factors influence drug release, including drug composition (drug, polymer, and adjuvants) and preparation method. The mechanism by which a drug pulls away from its carrier is summarized below (Figure 2). All factors will give different properties to the final NPs product, namely swelling ability, matrix density, and degradation properties. The drug release from PNPs is affected by swelling of polymers, diffusion of adsorbed materials, diffusion of the drug through a polymer matrix, polymer erosion or degradation, and a combination of erosion and degradation [30, 31, 32].
Figure 2.
Mechanism of drug release from PNPs.
An essential step in bringing benefits to PNPs is the characterization of their complicated release phase since it is distant from conventional DDSs [33].
Additionally, while generating NPs, it is necessary to identify and comprehend the unique processes and complications involved in the release process and their differentiation and comprehension of their respective characteristics [34]. Controlled release of a drug from a carrier may be achieved by:
2.1.1. Diffusion-controlled release
The most applicable mechanism for drug release is the diffusion control mechanism. The diffusion mechanism occurs when the drug or active substance passes through the polymer NPs matrix, which acts as a controlled release device. The rate of drug release decreases when the active agent has a longer mileage [27,34,35]. The substance moves through the interior of the polymer matrix towards the release medium. The diffusion barrier is formed by polymer chains, which restrict the drug from moving. Swelling or erosion may also be associated with diffusion. Mathematically, diffusion is described by Fick's Law of Diffusion [15].
Some assumptions must be made to obtain Fick's law parameters, such as maintaining a pseudo-steady state during the release process, the diameter of the drug particles less than the average distance of the diffusion of the drug through the polymer matrix, and the media around the NPs in sink conditions [36,37]. The drug molecules will be distributed throughout the polymer matrix because no membrane acts as a diffusion barrier in a matrix-type system. This system will show a high initial release rate, followed by a decreased release rate related to the diffusion distance of the drug molecule with the solution medium [4,27,38]. The relationship between the release rate increases and the diffusion coefficient is Directly proportional, as the release rate increases as the diffusion coefficient increases [34].
An osmotic-controlled release is found in the PNPs with a semipermeable membrane, which relies on water and polymer chains relaxing. Removing the drugs from the drug-loaded core will allow water to flow into the carrier (with a high drug concentration). Maintenance of constant concentration gradient, the zero-order release profile is reached [27,38]. Hydrophilic polymers can form a gel when a water-bearing hydrophilic system is added to the water system. The polymer will absorb water, will expand, and then diffuse [39].
2.1.2. The swelling-controlled release
The polymer chains break down when the polymer interacts with the surrounding media, releasing the medication from the polymer matrix. Before the polymer degrades, it swells [40]. Diffusion is affected by hydrophilicity, degree of swelling, and density of polymer chains [15,41]. Swelling of the polymer matrix occurs by a non-Fickian diffusion process, where the active substance is delivered concurrently via erosion and diffusion. The relaxation constant affects the matrix swelling device with slab, spherical and cylindrical geometries. The more significant the value of the relaxation constant, the slower the drug is released from the matrix. Still, the diffusion constant does not affect them. The Weibull model seems most applicable to describe the release process. The Weibull model appears to be flexible enough to account for the influence of system characteristics on the release process and is more suitable for determining the drug release profile of swellable PNPs in vivo [42].
2.1.3. Erosion and degradation-controlled release
Polymer erosion involves swelling, diffusion, and dissolution. Erosion is divided into (a) Homogeneous erosion occurs when polymers erode uniformly throughout the matrix, (b) Heterogeneous erosion occurs when polymers erode from the surface to the inner core. Polymer breakdown can be triggered by the surrounding media or by the presence of enzymes and the pH of the media, the content of the polymer, and water absorption. The drug release is regulated by the type of polymer, internal bonding, adjuvant, and the shape and size of the NPs [15]. Because of the distance of water diffusion and the domain size of crystallization, polymer breakdown is considerably accelerated in small-scale NPs [27,43]. The polymer may show no normal surface erosion but shows signs of mass degradation [27,44]. Biodegradable polymeric systems are preferred because the breakdown results of compounds can be safely removed from the body without harming the body in the long run [45]. The pharmaceutical drug-polymer conjugates are released by hydrolytic or enzymatic cleavage at target tissues. The cleavage rate governs the kinetics of drug release [27].
2.1.4. Stimuli-controlled release
Drug release can use internal or external stimuli. Internal stimuli are directed directly at diseased tissue and have been shown to increase the selectivity of drug action. This requires the incorporation of appropriate substances into PNPs that are activated by certain endogenous stimuli. PNPs offer tumour-targeting selectivity and efficiency, as is the tumour microenvironment (e.g. pH and redox) [27]. External stimuli are applied through external factors such as temperature, electromagnetic and magnetic fields, or ultrasonic waves. This strategy is advantageous in terms of delivering drugs exactly to the destination and minimizing side effects [46,47].
2.2. In vitro release methods for nanoparticles
The in vitro release of the NPs must take pH and agitation into account. The administration of NPs depends on the target location [48, 49, 50]. During in vitro release studies, agitation is required to keep the dosage forms from aggregating. The release media is influenced by the solubility, stability of the drug, the test's sensitivity, and the test procedure. The in vitro method is also used in sampling and buffer replacement techniques [12,45,51]. To ensure the quality of the CSNPs, in vitro release analysis is very important, but currently, no compendial standards or regulations apply. Direct comparisons between different systems are difficult due to the variety of testing methods [12,45,51]. Drug release from NPs can be assessed using:
-
a.
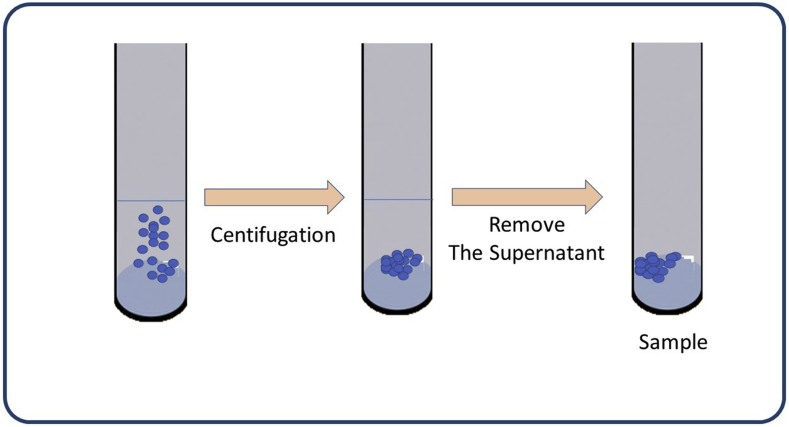
Sample and Separate (SS).
SS is the simplest, practical, popular method for determining the drug release of NPs. The NPs containing the drug is suspended in a container with a fixed amount of release medium, then analyzed accordingly (Figure 3). Various factors such as vessel size, agitating, sample separation methods, and sampling volume may be adjusted. The amount of media needed for the release study determines the type of container used [12,52].
-
b.
Continuous Flow (CF)
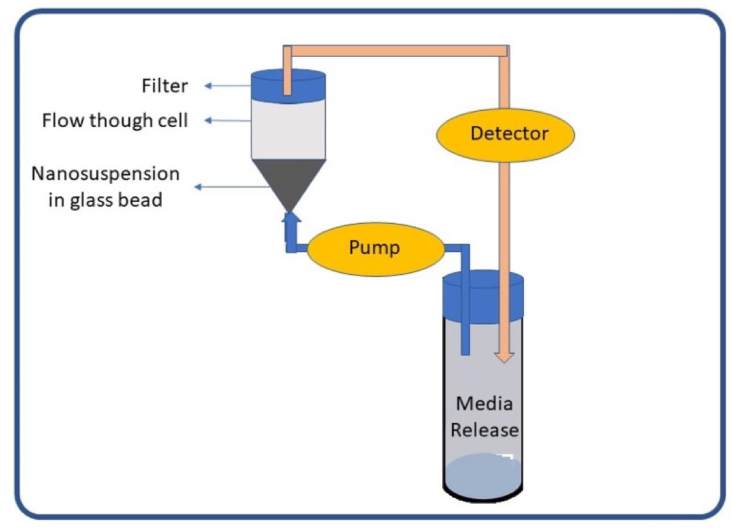
Figure 3.
A fluid cell containing the sample, a pump, and a water bath is a continuous-flow device in closed or open-end configurations (Figure 4). The media constantly circulates through the column containing the NPs in a closed system, then the amount of drug re-leased is analyzed accordingly [52].
Figure 4.
Continuous flow methods [12].
The literature on the technique of using the CF method in nanoparticulate dosage forms is still rare. The flow rate is determined by the type of pump and filter used. The CF approach replaces the media using closed-loop and open systems. Low flow rates indicate slow or incomplete release from NPs [12].
-
c.
Dialysis Method (DM)
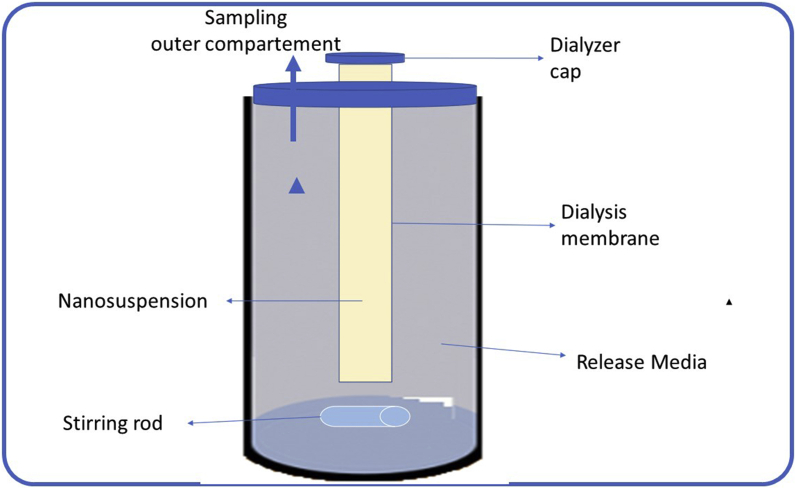
The DM is the most widely used method for determining drug release from NPs dosage forms [29]. The dosage forms are physically separated, allowing for convenient sampling at regular intervals. In the literature, differences in DM are found in arrangement, container size, and molecular weight (MW) cut-off [12].
The NPs in an inverted dialysis setup are swirled to reduce the unstirred water layer in the outer compartment. The interior compartment is sampled for drug release testing (Figure 5). Adjoining dialysis arrangement, where the dialysis membrane separates the donor and recipient cells, sampling is carried out from the recipient cell and the vertical Franz diffusion cell, containing the same medium stirred with a magnetic stirrer [12].
Figure 5.
Dialysis method [12].
2.3. Modeling drug release
Drug release from NPs plays an essential role in determining its pharmacological effects [42]. The drug release kinetics of NPs is an important feature of the formulation and as a quality control one. In vitro release kinetics is also a prerequisite to look for in vitro-in vivo (IVIVC) correlations, which will be an illustration of the performance of in vivo formulations [53]. Drug release data under simulated physiological conditions is essential in preclinical development, and will serve as the basis for evaluation of drug formulations and regulatory approvals. Prediction of in vivo drug release through in vitro methods for nanoformulations is becoming widely developed [54]. Mathematical models have many advantages, including predicting drug release mechanisms, assisting in formulation development, and building controlled drug release systems [12]. The dosage forms of NPs are complicated, and the assessment of drug release is complex. Therefore, the use of in vitro drug release data to predict and characterize drug substances' in vivo performance can be considered the rational development of controlled release formulations [42,55,56].
The models are The Korsmeyer-Peppas model, Higuchi Model, First-order Release Kinetics Model, Zero-order Release Kinetics Model, Weibull Release Model, Two-film Theory Mathematical Model [57].
3. Chitosan-based nanoparticles
3.1. Chitosan
CS is a widely available, sustainable biomaterial for health care product development and tissue engineering [58,59]. CS has been proven biodegradable, bio-compatible, biorenewable, non-toxic, non-allergenic, bioadhesive, does not contain anti-genic properties and is environmentally friendly [41,58,60]. CS has antibacterial, anti-tumor properties and was classified as GRAS (generally recognized as safe) by the FDA in 2001 [58].
The biodegradation profile will affect the drug release so that it becomes the basis for the selection of natural polymers. Drug release from natural polymers takes place relatively quickly from NPs because they decompose within a few hours. In contrast, synthetic polymers provide prolonged drug release because they can withstand degradation in the body for long periods of time, days or even weeks [61]. CS consists of covalently linked monosaccharide units, in the manufacture of NPs providing stability or adequacy of functional groups and ease of functionalization [62,63].
CS is a naturally positively charged polysaccharide (polycation polymer) [64, 65, 66], has a good affinity for negatively charged bioactive molecules, such as antigens, antibodies, enzymes, cytokines, and polyanionic polymers [58]. CS can interact with the blood coagulation process (e.g., platelets, red blood cells, coagulation factors), accelerating hemostasis, increasing monocyte/macrophage migration, and stimulating collagen synthesis. CS with a regulated degree of deacetylation strongly affects cell-polymer interaction on cell death by breaking the cell membrane. CS prevents tumour cell growth by proliferating cytolytic T lymphocytes because of its anti-tumour effect and bone regeneration approach [62]. The molecular structure of CS is similar to that of collagen and can be applied to mimic the extracellular matrix [67].
CS has three different functional groups: primary alcohol, secondary alcohol, and –NH2 groups, which inhibit the growth of various bacteria and fungi. Functional groups are helpful in biosensors, separation membranes, tissue engineering, and wastewater treatment. Through ion exchange and complex reactions, they can adsorb various metal ions through their amino group chelation sites [68]. Changes in pH, concentration, MW, degree of crosslinking, CS polymerization, and polydispersity index (PDI) will determine the overall characteristics of CS polymers [14,69]. CS can be fabricated into various nano-morphologies (nanofilms, nanofibers, NPs, nanocapsules, nanomembranes, nanosponges, nanoscaffolds, and hydrogels) [60,70]. CS is extensively researched in the preparation of NPs drug delivery and release control. CS can interact with negatively charged polymers, macromolecules, and certain in-organic polyanions. Synthetic polymers normally advantage from high but adaptable mechanical properties, while they often suffer from poor biocompatibility and/or biodegradability [19].
CS is generally amorphous which is almost insoluble in water due to strong intermolecular hydrogen bonds between polymer chains. However, CS can dissolve in aqueous solution when the pH is below 6, due to protonation of the amino groups. Increasing the solubility in alkaline solutions can be done by several chemical modifications, including the attachment of the carboxymethyl group to the CS structure and retaining its cationic structure [19]. CS is a natural sugar, and is highly bioactive, showing it as a cell-compatible drug carrier [71].
3.2. Chitosan nanoparticles
CSNPs are used for developing the stimuli-responsive NPs to carry chemo drugs to deliver specifically at cancer locations without causing any toxicity to normal cells [72]. Because of excellent biocompatibility, durability, low toxicity, simple preparation methods, and versatility of administration routes, CSNPs give several benefits [73]. Due to the activation of the RES, CSNPs might be extremely unstable in the systemic circulation [74].
Drug encapsulation is a critical area of biomedicine because it provides numerous benefits such as increased drug stability, distribution, activity, and bioactivity expansion by preventing pharmaceuticals from premature degradation, all of which are linked with low side effects [75]. CSNPs inhibit bacterial growth and antibacterial function and inhibit bacterial uptake by the stomach and intestines [76].
Many research shows that CSNPs and their derivates have broad-spectrum efficacy in biomedical purposes, enhancing solubility, stability, and bioavailability for the hydro-phobic drug. The other using for a diagnostic tool, targeted drug delivery, and a controlled delivery system, for example:
-
1)
Improve the pharmacokinetics and pharmacodynamics profile.
PEGylated CSNPs are contributing long-circulating and targeted accumulation of rosuvastatin [18]. Compared to CSNPs, PEGylation decreased macrophage identification of the NPs, allowing them to circulate continuously in the blood for a more extended period [42]. PEGylated is an ideal graft forming polymer due to its solubility in water and organic solvents, low toxicity, high biocompatibility, and biodegradability [79]. The novel amphiphilic CSNPs may serve as effective carriers for hydrophobic medicines used in tissue engineering [58].
-
2)
Diagnosis of cancer cells/photoimaging.
CSNPs are used as a photodiagnostic agent for cancer cells, as a transporter of 5-aminoluvanonic acid inside the cell and over the lipophilic obstacle, to inhibit bacterial absorption of 5-ALA, and as a source of PpIX [78].
-
3)
Overcome biological barrier.
CSNPs form ionic connections with endothelial cells, allowing medicines to penetrate the BBB through adsorptive transcytosis [77].
-
4)
Across intestinal epithelial cell layer
Amphiphilic polymers such as n-octyl-n-arginine-CS, arginine-modified CS (CS–N-Arg), or amphiphilic CS derivatives with arginine may facilitate drug transport through the intestinal epithelial cell layer [80].
-
5)
Dual-delivery system
A dual-delivery method for biodegradable bone grafts constructed by inserting CS microspheres in calcium sulfate is capable of effectively loading and releasing antibiotics and growth agents concurrently [58].
-
6)
Targeting
In vitro cytotoxicity and in vivo anticancer effect of Doxorubicin Transferrin palmitoyl Glycol CS (DOX-TF-PGCS) demonstrated that DOX-HGC could avoid the toxicity of the free drug after systemic administration [18].
-
7)
Control release
CSNPs produced in this study could be used for sustain release with amiodarone and may serve as a paradigm for regulated administration of a variety of antiarrhythmic medications [81].
Numerous methods have been proposed to enhance CS characteristics and expand its application range, including crosslinking, graft copolymerization, complexation, chemical modification, and blending [19].
3.2.1. Preparing methods
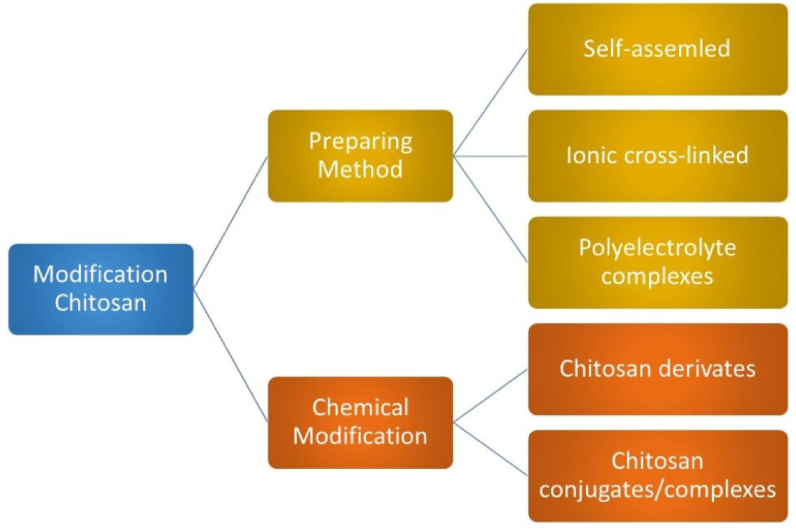
CSNPs can divide into three groups based on their preparation methods (Figure 6) [82]: (a) Self-assembled, hydrophobic chains are spontaneously collected to form reservoirs for drugs that dissolve in aqueous phases, while hydrophilic chains act as shells around the nucleus affected by the aqueous phase [83]; (b) Ionic crosslinked, electrostatic interaction is used to create ionic crosslinked NPs by using the cationic property of CS (the backbone's amino groups) interact with a polyanionic crosslinker such as tripolyphosphate (TPP), CaCl2, Na2SO4 [84]. The physical characteristics of the NPs (surface size and charge) are easily adapted to the processing parameters of the ionic crosslinking, which impacts the encapsulation efficiency and drug release [85]. (c) Polyelectrolyte complexes, If CS is mixed with negatively charged polyelectrolyte in a solution and the polymer chain interact to form a CSNPs polyelectrolyte complex. The manufacturing procedure is simple and does not use toxic reagents. When CS is mixed with a negatively charged polyelectrolyte in solution, the polymer chains interact with each other to form a strong but reversible electrostatic network without the use of crosslinking [86].
Figure 6.
Preparation methods and chemical modifications of CS.
3.2.2. Chemical modifications
The effectiveness of native CS transfection is low due to in vivo instabilities and insufficient cellular release. Developing effective delivery systems needed chemical modifications [86]. First, NPs based on CS derivatives can be chemically modified because of hydroxyl, acetamido, and amine functional groups [[87], [88]]. Second, CS polymer conjugates/complexes, conjugation, or the addition of functional polymers in CS can mask CS weakness and achieve more efficient transfection [86]. Modified CS is used to modulate the carrier's functionality to adjust delivery properties to those required for the desired application [15].
CS contains three types of reactive functional groups, an amino group on the C-2 position for each deacetylated unit and primary and secondary hydroxyl groups at the C-6 and C-3 positions, respectively, for each repeating unit. CS has been modified mainly to improve solubility and therefore widen its applications. The most investigated and promising CS derivatives are quaternized alkyl CS (e.g., trimethyl CS), N-alkyl and N-benzyl CS, N-acyl CS (acetyl, propionyl, butyryl, hexanoyl, octanoyl, lauroyl, palmitoyl, benzoyl), N-carboxyalkyl, and aryl CS (e.g., N-carboxymethyl and N-carboxybenzyl CS), O-carboxyalkyl (e.g., O-carboxymethyl CS) and N-carboxyacyl- CS (derived from anhydrides such as maleic, succinic, glutaric, phthalic anhydride), phosphorylated CS and thiolated CS (CS-thioglycolic acid, CS-2- iminothiolane) [[63], [77]].
CS derivatives are semi-synthetic aminopolysaccharides with unique properties, various marvellous functionalities, and a wide range of applications in research and industrial areas [89].
3.2.3. Key factors in CSNPs development
-
1)
Crystallinity
Since CS is a heterogeneous polymer consisting of D-glucosamine and N-acetyl-D-glucosamine units, its properties depend on its structure and composition [90]. Partial alignment of the polymer molecular chains, which affects the physical and chemical properties of the polymer. Polymers that have high crystallinity are high melting, low flexibility, and low solvent penetration. Polymer crystallinity refers to the proportion of the crystalline region in the polymer sample to the amorphous region [[14], [91]]. Water molecules can pass across amorphous areas, which are permeable. The monomers' composition regulates the crystallinity and affects flexibility, swelling, solubility, and degradation rates. When low MW polymers are used, A high crystalline degree leads to slower drug release conditions. In high MW, the effect on drug release is reduced [[92], [93]].
The quantification of crystallinity is the crystallinity index (CI) calculated from the connection between the distinctive X-ray diffraction peaks. Quantitative CI is important because these properties affect swelling, porous, hydration, and absorption. According to the source and chain arrangement, CS exists in three polymorphic phases with varying degrees of crystallinity (α, β, and ϒ). CS is a semi-crystalline polymer that is commercially available, while CI is a function of DD [60].
The solubility of CS in aqueous solution and its capacity to form complexes depend mainly on the degree of deacetylation and crystallinity [68]. The less deacetylated the sample, the higher the crystallinity. CS chain packaging and crystallinity are important parameters in the utilization in various fields [90].
The method used to synthesize CS from chitin determines its crystallinity; solid CS synthesized from chitin has a more amorphous structure than CS synthesized using the suspension method [91]. Reducing the MW and decreasing crystallinity of CS by random deacetylation generally increases its solubility in dilute acids and allows processing of its solution into various bead shapes [42].
-
2)
Glass Transition (Tg)
Polymers, copolymers, biopolymers, and polymer-based composites all have a glass transition temperature. Tg drops in some circumstances when the interaction between the polymer and the nanofiller develops free surfaces and expands in size due to the attractive interactions formed by the wetted interface [94]. Their glass transition temperature influences the polymer physicochemical properties of the polymer [14,95].
Tg was determining if the amorphous region is in “glasslike” or “rubberlike” condition. The polymer is in a “glasslike” condition if the state is below Tg and is affected by low flexibility and low diffusion rate. The polymer is in a “rubberlike” condition if the state is above Tg [14,95]. This condition will allow for faster water and drug molecule mass transfer throughout the matrix. Developing effective PNPs needs a balance between crystalline and amorphous states [14,96,97]. If the transition between 140 and 150 degrees Celsius is a β relaxation transition, the degree of deacetylation, which is proportional to the quantity of side group (acetamino or amino group), will effect this transition. If the transition between 140 and 150 °C is part of the α relaxation, the degree of deacetylation has no effect on Tg, since the relaxation simply refers to the motion of segments in the main chain [98].
The glass transition temperatures of the nanocomposite matrices are utilized to investigate the effect of nanofillers on their thermal characteristics. Thermal property variations as a function of loading are also utilized to infer changes in the molecular packing of polymer chains due to the polymer-nanofiller interface [99]. Incorporating NPs disrupts the CS chains, resulting in a decrease in the system's free volume. As a result, the polymer's properties, such as free volume and chain conformation, deviated from their bulk behaviour [100].
-
3)
Molecular Weight
CS classification can be divided into oligochitosan (16 kDa), low MW-CS (LMW) (>16 kDa–190 kDa), medium MW-CS (MMW) (>190 kDa–300 kDa), and high MW-CS (HMW).) (>300 kDa). This division is not yet universal [63]. MW affects CS bioactivity. The lower the MW indicates the more significant bioactivity. The degree of deacetylation and MW are the main parameters affecting wound healing efficiency and antimicrobial properties [19].
The lower the MW the higher the solubility, CS with MW below 9 kDa shows much better solubility in water. CS above 30 kDa still require acid to dissolve in water. Acetic acid most popular, many acid can also dissolve CS in water, except for phosphoric acid [101].
MW of degraded polymer affects the release and Cmax of the drug in plasma. Low MW CS polymer is more soluble, less viscous, less crystalline, degrades faster. Low MW polymers have high elasticity, and the matrix is more plastic, causing the pore size to increase.
Low MW polymers produce smaller NPs, resulting in altered drug release kinetics, longer exposure to the bloodstream, reduced accumulation in the liver and spleen, and more efficient drug delivery [19]. Cellular toxicity is dependent on the concentration and MW of CSNPs [77], meaning lower toxicity is associated with less MW and vice versa.
The higher weight CS molecules are broken down and completely to a lower level. Carbon (44.11%), Nitrogen (7.97%), and Hydrogen (6.84%) are the main constituents of CS [26].
-
4)
Degree of deacetylation (DD)
DD of CS is determined by the ratio between the N-acetyl-D-glucosamine and D-glucosamine units [60]. The CS structure is exclusive because the primary amine is at the C-2 position of the glucosamine residue, increasing its functionalization [26]. DD indicates the concentration of the amino group in the molecule and the protonation level of the -NH2 functional group [101]. The degree of deacetylation determines the properties of CS, namely hydrophobicity, solubility, and toxicity. The manufacturing conditions used to make CS have an effect on its DD and MW. CS in the market has a DD of between 70% and 85% [77], soluble in acidic medium but insoluble at neutral medium.
The degree of deacetylation to form different assemblies and vesicles [102]. The lower DD promotes the absorption function of the molar compounds [26]. DD CS regulates its physicomechanical features. Although medium/high MW CS with acetylation degree less than 40% will tend to agglomerate, CS with acetylation ranging from 40% to 60% are soluble even at physiological pH [26].
A higher DD indicates a stronger biologic effect. to realize the biological effect of CS and water solubility [101].
The polycationic amino groups give CS mucoadhesive properties, prolonging the time spent at the target region and thereby boosting membrane absorption [77].
The critical step in production is that the acetamide group is converted to an amino group during deacetylation of the C-2 position of chitin (-NH2). DD is an important parameter affecting the structural and functional properties of polymers and the specific materials that follow them [103].
-
5)
Polarity
CS, a naturally occurring linear copolymer of β-(1→4)-2-acetamido-D-glucose and β- (1→4)-2-amino-D-glucose processed by partial deacetylation of chitin [104,105]. CS is a compound that combines electrostatic stabilization due to its positive charge and steric stabilization [100]. Due to the protonation of amino groups, its hydrophilic character enables high solubility in acidic conditions. The existence of a surface charge and the potential of altering the molecule using electrostatically charged chemicals stimulate the usage of CS [106].
To develop effective DDS polymers, factors affecting solubility such as chemical properties, structure, and degree of crystallinity must be considered [41].
Polymolecularity generally increases with MW and branched structure, leading to an increase in hydrophilicity. Hydrophobic drug release is controlled by surface erosion of polymers; The ratio of hydrophobic to hydrophilic qualities in the polymer backbone determines its breakdown rate. Copolymers with hydrophobic and hydrophilic moieties often provide more predictable physical properties such as drug release rates [35,107].
The solubility and polycationic characteristics of CS are essential for the production of further derivatives. They are typically strong bases, with a pKa of 6.3 for the main amino group. As a result, these biopolymers are soluble in diluted acidic solutions with a pH of 6. H+ ions in medium protonate the -NH2 group, forming (NH3)+ at low pH values. As a consequence of this transformation, the CS structure was converted into a polycationic polymer. When the pH reaches 6.0, deprotonation occurs, rendering CS insoluble [35].
Significant research has been carried out to improve polymer membranes' hydrophilic and anti-fouling properties, such as modification with other polymers through mixing the polymer with a third compound [108].
-
6)
Size
Determination of particle size and morphology are two important parameters in nanotechnology [109]. Sreekumar et al. evaluated the effect of several factors, namely the chitosans degree of acetylation and the degree of polymerisation, degree of space occupancy, polymer concentration, and degree of crosslinking with TPP (NH2/PO4 molar ratio) on the ability of chitosans to form substances by ionic crosslinking and the effect of such parameters on the average hydrodynamic diameter of the particles formed [103].
As the amount of TPP increased, so did the number of TPP molecules available to attach to the free amino groups in chitosan. The anion may have been introduced to the nanoparticle during creation to promote cross-linking between chitosan chains, which would account for the decrease in CSNPs size as TPP increased. Internal cross-linking improves the strength of the chitosan chains inside the particle, further condensing and shrinking it. Cross-linking decreases the amount of available primary amino groups on CS, hence preventing nanoparticles from self-aggregating. This is consistent with the fact that the nanoparticles are smaller and have a lower PDI value. This interaction has been defined in terms of polymeric micelles, which helps to understand the chitosan polymer and cross-linker dynamics in our system [110].
The pH of chitosan used also favored the formation of smaller-sized nanoparticles. The pH of the CS utilized encouraged the creation of nanoparticles with a smaller diameter. Due to a lesser degree of amine protonation, chitosan chains are more constricted at pH 5 than in more acidic settings. Whenever cross-linked with TPP, a highly compressed CS chain produces particles that are much denser. However, adding TPP reduces the pH of the CNP solution, resulting in increased protonation of amine groups. Protonation may cause the ionic bonds between the chitosan and the TPP in CSNPs to break down, resulting in agglomeration of the nanoparticles. Centrifugation resulted in a reduction in the size of CSNPs [110].
NPs size influences particle biodistribution, excretion and medication delivery. Small molecule (5nm) NP is readily removed by the kidneys, resulting in slower blood circulation. The liver and spleen ultimately remove larger particles. Degradation rate impacts particle dispersion [111].
3.3. Surface properties of CSNPs
Several strategies have been established for surface engineering (modification, functionalization, and grafting) of various NPs [112]. The potential of CS as a cationic polymer provides fascinating properties regarding interactions with oppositely charged materials, especially mucosal surfaces (via sugar groups such as sialic acid) and nucleic acids [86]. Increasing the content of amine groups with the possibility of chemical modification will increase the selectivity and adsorption capacity [71].
Strategies for developing for surface engineering CSNPs.
-
1.
CSNPs have polycanionic NPsthat interact negatively with the cell membrane and enhancing drug absorption. They are not ideal for systemic delivery, however, since they bind to proteins and cells in the blood and are quickly opsonized and removed by the renal system, resulting in embolism. CSNPs samples were formed through the cross-linking of chitosan chains via amino group ionic interactions. The fraction of free primary amino groups remaining in the CSNPs [110]. The charge on the PNP surface is important, which can affect its penetration. Positively charged NPs and negatively charged cell membranes interact electrostatically, will direct the internalization of endocytotic NPs. Positively charged particles also showed higher macrophage uptake and clearance compared to neutral surface charge NPs, and showed higher toxicity than anionic and neutral NPs. Charged particles can also have a negative effect on the integrity of the BBB. The NP charge also affects protein corona formation, which also affects receptor binding. The zeta potential involves determining the surface charge [113].
The surface of CS-derived NPs usually has a positive charge [26]. The polycationic CS molecule has a strong interaction with the surface of microbial cells, causing a gradual shrinking of the cell membrane and ultimately cell death. Similarly, the bactericidal activity of CS is ascribed to electrostatic interactions between the (NH3)+ CS groups and the phosphoryl groups of the cell membrane component phospholipid. Polycationicity of CS interacts with anionic surface antigen components (lipopolysaccharides and proteins) of microorganisms, contributing to intracellular response: changes in the permeability barrier. It also inhibits bacterial development by blocking nutrient access into the cell and binding to DNA, preventing protein and RNA production [60].
Using a charged polysaccharide derivative enabled the scientists to modify the surface charge of the nanocapsules and improve their stability. Acute oral toxicity studies demonstrated the capsules were non-toxic nanotransporters, opening the door for biological applications of CSNPs [106].
-
2.
Surface roughness: NPs with a rougher surface have a greater concentration and interact with more proteins following intravenous injection (IV) [78].
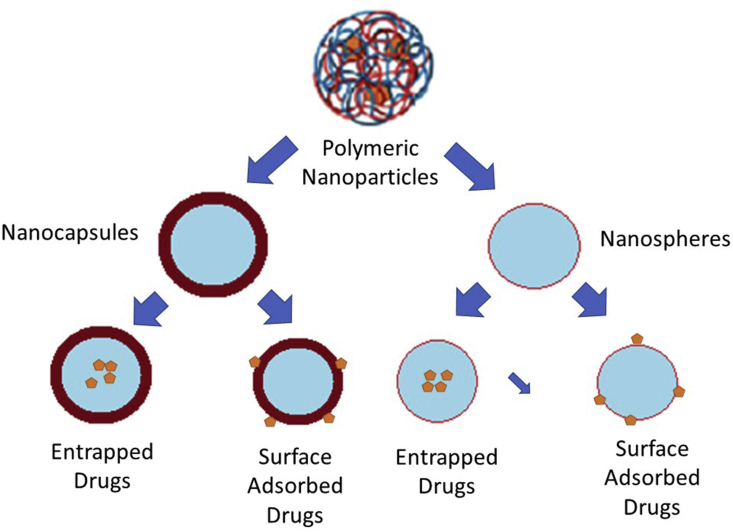
CSNPs can be found either in nanocapsules or nanosphere (Figure 7). Drug release usually occurs when the polymer degrades and diffuses [114].
Figure 7.
Type of biodegradable NPs.
Polymers can be used to engineer the pore surface chemistry as a place for the adsorption of NPs. Porosity can be varied pore size, ratio, and shape. The polymer material is crosslinked, which will promote better stability in various conditions such as extreme pH or temperature [108]. PNPs may have a drug encapsulated within or surface-adsorbed on their polymeric core [109]. The hydrophilic, hydrophobic arrangement of the polymer interface will affect the interaction with the surrounding fluid, especially in wettability. Organic and inorganic nanomaterials will give a high difference for pore-forming [115]. NPs properties affect the rate of releases such as porous, porosity, surface area, and biodegradation rate. The drug is entrapped through the surface of the matrix or is scattered within it. Because of its large surface area, a porous stationary phase would be an excellent stationary phase for analyte molecule adsorption. The research indicates that tiny molecules may have a larger initial burst release rate owing to their trapping capacity or surface absorption [33].
-
3.
Small substances and proteins such as transferrin albumin antibodies are used as active targeting moieties to specifically target tumor tissue [78].
-
4.
A variety of natural and synthetic polymers, including as PEG, PEI, polyglycerols, hydrophilic polyacrylates, CS, and dextran, are stealth-inducing molecules (prolonging the drug plasma half-life and improving the accumulation of these NPs in the target tissue) [78].
Due to the high instability of CSNPs in the systemic circulation as a result of RES activation, the NP surface is often modified with poly-ethylene glycol (PEG), a hydrophilic polyether molecule that has been shown in vitro and in vivo to be non-toxic to brain cells. By inhibiting NCs from interacting with cellular or serum proteins, PEG may help minimize cytotoxicity and macrophage uptake. Finally, since the CS structure has a low solubility and permeability at physiological pH, functionalizing it helps reduce systemic adverse effects while increasing drug loading and cellular uptake and managing drug release [77].
The NPs surface chemistry may be further complicated by controlled ligand exchange. One example of ligand exchange is to change the NPs surface from hydrophobic to hydrophilic. NPs synthesized in nonaqueous media covered with long-chain ligands (such as trioctylphosphine oxide, oleic acid, and oleylamine) are hydrophobic.
-
5.
Label/drug binding scaffolds provides drug binding sites, light sensitizers, and imaging molecules [78].
3.4. Biocompatibility
CSNPs have large surface area, zeta potential and provides superior activity [116]. The precise and unique characteristic feature of CS such as biodegradability, biocompatibility, very low-toxicity and non-immunogenicity make this a good vehicle for drug administration [117]. Chitosan is highly biocompatible; it does not lead to allergic reactions or rejection and is degraded to nontoxic amino sugars in tissues Chitosan nanoparticle is a drug carrier with the some advantage of slow and controlled drug release, which improves drug solubility and stability, efficacy and reduces toxicity. Nanoparticles are generally made up of biocompatible polymers, to reduce their rapid clearance from circulation by the reticuloendothelial system [118].
4. Drug release from CSNPs
The drug release from CSNPs has a significant influence on its therapeutic actions.
Different shapes and sizes of CSNPs impact releases of the drug due to their physicochemical properties. The ability to absorb water or the rate and rate of degradation, chemical composition, MW, solubility, and crystallinity of the materials that make up the NPs, will affect the release of the NPs. Even drug-drug or drug-polymer interactions appear to significantly affect drug release from the delivery system [30]. CSNPs are used to regulate release, increase the bioavailability of degraded substances and enhance absorption of hydrophilic drugs at target sites [119]. The process of making CSNPs can be done by crosslinking with anions, precipitation, complex coacervation, modified emulsification, ionotropic glassing, precipitation-chemical glutaraldehyde crosslinking, and thermal crosslinking. The choice of manufacturing method depends on the requirements of the particle size, thermal and chemical stability of the drug molecule, reproduction of the kinetic release profile, stability, and residual toxicity of the final product [120].
The release of the drug from the polymer can be regulated by one of the following mechanisms: (a) The surface of the polymer matrix is eroded, (b) the breaking of polymer bonds at the surface or in the bulk of the matrix, or (c) Diffusion of the loaded drug. In many cases, a mixture of the three procedures can be used to release the drug [51].
The release of the drug from CSNPs is also influenced by pH due to the solubility of CS. CS derivatives can design the release of the drug in accordance with the expected pharmacokinetic profile of the drug [15]. To see the effect of CSNPs modification, see Table 1.
Table 1.
Drug release from CSNPs.
| Preparing Method | CS Composition | Agents of Drugs | Result of Research | Drug Release Assay | Effect | Drug Release Models | Reference |
|---|---|---|---|---|---|---|---|
| Self-aggregated. | Carboxymethyl CS (CMC)-based nanocarriers | Curcumin | d = 41.27 nm and 87.35 nm. Monodispersed | SS methods | The highest release rates of curcumin in simulated intestinal systems (pH = 6.86) | [67] | |
| Ionic gelation method. | CS-Alginate NPs | Doxorubicin (DOX) | d = 100 nm in size | Dialysis methods | The release of DOX in CS-Alginate NPs was retarded significantly due to the encapsulation. | DOX is quickly released in the initial 10 h then slower or/and controlled for the next 72 h | [121] |
| Polyelectrolyte complex (PEC) | Salecan-CS. | Vitamin C (VC) | It was observed an interconnected, highly porous architecture. | SS methods | The swelling is great influence on the controlled release of VC. | The release mechanism agreed well with the Ritger-Peppas model | [122] |
| Emulsification and Ionotropic gelation technique | Encapsulated CS NPs | Capsaicin (CAP) | (d) = 180 nm and Efficient Entrapment 70% | Dialysis methods. | CSNPs controlled and sustained release of CAP. | Release kinetics followed the Weibull model with Fickian diffusion | [123] |
| Ionic crosslinked | Freeze-dried CS scaffolds | Diclofenac sodium | Porous CS scaffolds loaded with model drug diclofenac sodium | SF methods | A swelling-dependent release is affected by significant changes in pore size and porosity. Crosslinking affects increasing compactness and decreasing overall porosity. | Release kinetics followed Higuchi model with Fickian diffusion | [124] |
| The ionic gelation method | CS and alginate NPs | Lovastatin (LS) | d = 68 nm–171.8 nm | SS methods | The NPs' drug release rate is pH and lovastatin content-dependent. | Hixson-Crowell model. LS is quickly released from the NPs in the initial 10 h | [125] |
| Ionic gelation method | CS porous NPs | Oil Red O and Rhodamine B. | Denser hydrogels and Highly interconnected macropores, around 50–200 nm and relatively uniformly around 50–100 nm, | SF methods | A fast release for 1 h, then reaching the equilibrium concentration | [126] | |
| Ionic interaction with dextran sulfate (DS) as a crosslinker | CS NPs | Double-stranded siRNA | Irregular morphology, d = 353–1083 nm. | SS methods | DS and CS concentration influenced the size of NPs. | Quickly released from the NPs in the initial 6 h | [68] |
| A more negatively charged DS was added | |||||||
| Ionic gelation method | CSNPs. | Hydrocortisone (HC) | Nonspherical. d = 243 ± 12 nm to 337 ± 13 nm | SF method, A Franz diffusion cell | The swelling ratios increase with the pH of incubating media was increased. Particle size and EE (Entrapment Efficiency) of HC loaded as the CS concentration was increased. | quickly released from the NPs in the initial 6 h | [76] |
| Ionic gelation method | CSNPs | Ganciclovir (GCV) | d = 121.20 ± 2.7 and EE = 85.15 ± 1.1% | The assay was carried out in diffusion cell apparatus in phosphate buffer pH 6.8. | The incorporation of GCV into CSNPs results in enhanced permeability, which may, in turn, increase the overall oral absorption of the drug. | Higuchi model quickly released from the NPs in the initial 10 h | [127] |
| Polymer conjugation | Glucose- CS NPs (GCNPs), | Doxorubicin (DOX) | Spherical d = 187.9 nm and a -15.43 mV | Dialysis method | After incubation for 30 h, the accumulative release rates of DOX/GCNPs and DOX/CSNPs were 19% and 21%, respectively. | first, the release profile was mainly a diffusion-controlled process of DOX molecules from the hydrophobic micro-domains; second, the sustained and constant release, maintain the drug concentration at a therapeutic level. | [128] |
| Polymer Conjugates | Functionalized nanohybrid hydrogel using L-Histidine (HIS) conjugated CS | Naringenin, Quercetin and Curcumin. | The nanohybrid hydrogels exhibited highly porous three-dimensional crosslinked structures as a result of the electrostatic interaction between the conjugated polymeric backbone and the ZnO NPs | Drug release was conducted for the optimal drug-loaded condition using 100 mL of buffer medium (PBS buffers with 2% Tween 80 at physiological pH conditions of 5.0, 6.8, and 7.4). The drug release was quantified periodically by analyzing the buffer release medium using UV–Vis spectroscopy up to 12 h. | Improved stability due to HIS conjugation and the pH gives a steady swelling rate. quickly released from the NPs in the initial follow by controlled release | The Korsmeyer – Peppas model, a non-Fickian diffusion-based mechanism, polymer erosion. Drug release kinetics from CHGZ hydrogel predicted a non-Fickian diffusion-based mechanism along with a molecular diffusion mechanism. | [39] |
| Polymer conjugates. | L-leucine conjugated CS NPs. | Diltiazem hydrochloride (DH) | a bi-modal distribution, d = 32 nm, and 388.74 nm. | SS method | The higher dispersibility was attributed to the L-leucine conjugate and hydrophobic crosslinks' amphiphilic environment, and the release profile reflects the more significant swelling. | The Korsmeyer-Peppas model, Fickian diffusion. A big initial burst followed by roughly 1–2 weeks of sustained release. | [136] |
| Conjugates Reaction. | CS–steroid conjugates NPs | Diosgenin monoesters | Dialysis Methods | The steroid releases pH-dependent and the hydrolysis of the ester linkage between the steroid and CS. | Zero-order kinetics during the first 12 h. | [137] |
In the ionic gelation method, crosslinking is formed by hydrogen bonding between the polar groups on the polymer chains. In contrast, crosslinking is formed by covalent bonds between different functional groups on the polymer chains facilitated by special crosslinking agents [129]. They were covalently crosslinking agents formaldehyde, glutaraldehyde or genipin, or other compounds with several reactive chemical functions, undesirable in drug delivery applications [130]. Drug release from CSNPs is more pH-dependent. The drug's pKa and pH values and the release media were expected to influence release behaviour [49]. At higher pH, crosslinking will be reduced, the extent to which the counterion converts positively charged amino groups (NH3+) of CS into the unionized state [76,131]. NPs were kept in a shrinkage state and drug released due to the attractive electrostatic interaction between anions and CS.
Furthermore, at an acidic pH, the electrostatic attraction between protonated amine groups and H2O molecules was more significant, dissolves CS polymers, resulting in NPs matrix erosion and rapid drug release [123]. NPs swelled significantly and even dissociated rapidly, resulting in the quick model drug release [132]. The cumulative release of drugs from CSNPs could indicate that drug release was faster at lower pH than at neutral or higher pH [133].
George et al. discovered a much faster rate of release in polymer conjugates at the of 5.0 than at 6.8 and 7.4. Protonation under acidic conditions will increase swelling in an acidic medium could account for these release characteristics [39]. The swelling of CSNPs, at a higher pH value (7.4) may increase, which would allow release media to penetrate deeper into the polymer matrix, converting the glassy polymer to a rubbery state [134]. Drug molecules were able to diffuse out of the CSNPs more quickly and release quickly [126].
Adding polymer conjugates such as L-Histidine (HIS), Alginate, and Emulsification and Ionotropic Gelation Technique have opposite charges could more stabilize charge and control release against different pH [39]. Due to the –NH2 groups (-NH3 +) protonation, CS is highly positively charged at low pH, The interaction of H+ ions (at acidic pH) with cations on the CS surface, which limits the polymer's hydrolysis, may explain why drug release from NPs occurs quicker in a neutral environment than in an acidic one [135].
The mechanism of drug release from CSNPs: (a) Release of adsorbed or trapped drug in the surface layer of particles; (b) Diffusion through the swollen rubber matrix, and (c) Release caused by polymer erosion, breakdown, hydrolysis, or degradation of the NPs backbone, resulting in long-term drug release [36]. Drug release from CSNPs shows a characteristic biphasic pattern characterized by a rapid initial release followed by a slow-er, controlled-release [39,68,76,125,136,137]. In the case of the hydrogel, the biphasic form of discharge is more prominent [130]. The drug that is absorbed and trapped in the surface layer of CSNPs dissolves immediately upon contact with the media. This phenomenon causes an explosive effect. The structure of the CSNPs carrier will influence the kinetics of drug release. If the NPs are in the form of nanocapsules, they will show a low initial burst release. The small size of the CSNPs will also indicate an initial blast release. The NPs diameter decreases, the specific surface area increases, and the path length to the drug surface decreases. CSNPs can be modified to reduce initial blast release, Zheng et al. modified conventional PLGA microspheres with alginate and CS [138]. Interfacial deposition of hydrophobic polymers was used to create nanocapsules. Drug release is also regulated utilizing a mixture of nanospheres and nanocapsules [27].
The bi-phasic release type helps determine the release pattern, especially achieving therapeutic levels rapidly and maintaining controlled release [136]. The pattern of bi-phasic drug release also occurs in CSNPs, which are made by crosslinking (ionically and covalently). On conjugated CSNPs demonstrated a faster release, roughly twice that of unconjugated CSNPs [125].
The kinetic analysis of drug release from CSNP can be carried out by mathematical models such as the zero-order model, first-order model, the Higuchi model, the Hixson-Crowell cube root law, the Baker-Lonsdale time kinetics equation, and the Peppas exponential model [139]. The diffusion-controlled release kinetics were investigated using the zero-order, first-order, and Higuchi models. The Hixson-Crowell model was used to study dissolution-type release kinetics due to changes in surface area or particle diameter [140]. As shown in Table 1, the model release from CSNPs has various models: the zero-order [137], Hixson-Crowell [125], Higuchi [127], Weibull [123], and the Korsmeyer-Peppas [136].
Table 1 shows that CSNPs can transport drugs with efficient loading and controlled drug release. Two processes can make the drug loading in CSNPs: coalescence simultaneously as particle preparation and incubation (after particle formation) [140]. In this case, drug loading is done by adding the drug during the NPs formulation process.
The amount of surface charge decreased significantly after drug filling, but all remained positively charged (empty, conjugated, and drug-laden). The surface charge reduction is because of the accumulation of some drugs on the surface [140]. The adsorbed drug will determine the surface smoothness of the CSNPs, in which drug molecules occupy the fully porous hydrogel. HIS-CHGZ hydrogels include histidine and have a smoother drug-loaded surface than non-conjugated hydrogels. CUR, which is very hydrophobic, has a rather rough surface, followed by NRG and QE [39].
The greater the drug loading, the more the drug dissolved in the hydrated polymeric matrix, resulting in a higher diffusional driving force and quicker drug release from the conjugate NPs [141]. This phenomenon is similar to the release of another hydrophilic drug from CSNPs.: propranolol hydrochloride, sodium diclofenac, Diltiazem HCl, and vitamin C [122]. Apart from showing higher drug release rates, conjugated NPs also continued to release drug for a period of twice as long as other CSNPs because of the higher percentage of a drug charge and the hydrophobic environment predominating to the surface [136]. The timing of drug release can be engineered via encapsulation and air-drying. Shrinkage and thickness of the structure caused by the drying process can inhibit diffusion [126]. In general, drug release follows more than one type of mechanism, as shown in Table 1.
Drug release from CSNPs was determined by the particle morphology, size, extent of crosslinking, the active substance's physicochemical characteristics, pH, polarity of the dissolution matrix, and enzymes' presence. Chemical and enzyme catalysis are the primary mechanisms by which CS is degraded, with the latter being the predominant process in vivo [140]. The degradation of CS depends on the MW, degree of deacetylation [142], crosslinking agents [143] used for NPs preparation, concentrations, enzymes [136], and the media's pH [144]. Incubation of NPs with lysozyme resulted in a decrease in the size of the NP. Increased lysozyme concentration accelerates the reduction in NPs size [136]. The degradation rate of glutaraldehyde crosslinked CS NPs was faster than the TPP crosslinked CSNPs, both high and low MW. This degradation promotes higher drug release [61,136].
The drug release from the CSNPs can be set to the oral delivery system. As showed by Liu et al., curcumin is released at the fastest concentration in the simulated intestinal system (pH = 6.86), meeting the standard of oral drug administration [67]. These findings imply that NPs are more suited to the basic environment of the colon, colon, and rectal mucosa than they are to the acid environment [125].
Drug release from CSNPs systems is dependent on the degree of crosslinking, the morphology, the size, the density of the particulate system, the physicochemical characteristics of the drug, and the presence of an adjuvant in this research. Additionally, in vitro release is dependent on the pH, polarity, and enzyme content of the dissolving medium.
pH-responsive delivery devices may take advantage of the body's pH gradients between normal and malignant tissue, or between extracellular space and particular cell compartments. Previously, we synthesized pH-responsive CS-tripolyphosphate NPs loaded with doxorubicin (DOX- CS-NPs). Additionally, we examined its antiproliferative efficacy in vitro against a variety of tumor cells. The improved anti-tumor efficacy of these NPs in vitro is examined in this study, using conditions that simulate both physiological (pH 7.4) and tumor extracellular environments (pH 6.6). CS-NPs were synthesized through ionotropic gelation using the pH-sensitive adjuvant 77KS, which is derived from the amino acid lysine [145].
The multifunctional components used to change the surface of NPs for oral administration have the potential to generate “smart” NPs with pH-triggered and tailored release mechanisms [146].
There are many reports about the dangers of nanoparticles, especially those that dominate metal and metal oxide NPs. After acute systemic exposure, the mediating NP toxicity in target organs are reactive oxygen species (ROS) in liver, spleen and kidney, DNA damage resulting in cell cycle arrest and apoptosis, modification of protein structure and function and impaired inflammation of membrane integrity [22,147,148], inhibiting antioxidant effects, and mitochondrial dysfunction [22]. Metal and metal oxide NPs such as silver, zinc, copper oxide, uraninite, and cobalt oxide have also been found to cause DNA damage [149]. Gold NPs may induce kidney injury, which can be amplified synergistically as a consequence of interactions with chemicals or medications [150]. In general, most NPs tend to accumulate in mononuclear phagocytic system organs such as liver and spleen, resulting in toxic effects on exposure to nanoparticles including monocytes, platelets, leukocytes, dendritic cells, and macrophages [151,152]. For CSNPs there have been no reports showing symptoms such as metal and metal oxide NPs. Due to the nature of biocompatibility and non-toxic properties.
5. Future perspectives
In the future decades, the true influence of new polymeric NPs technologies on clinical trials will be obvious [14]. Although specifically receptive to disease pathology, the next generation of nano-enabled drug delivery products will increase medication performance, patient compliance, and therapeutic success. The use of stimuli-responsive materials allows for more precision control of drug release kinetics and sites. When designing stimuli-responsive systems and manufacturing challenges, other factors must be considered, such as that few of these materials have been tested in vivo. However, emerging technologies have a long way to go before being transformed into clinically practical products.
Our knowledge of drug release mechanisms has improved because of mechanistic studies and mathematical modelling. Precision mathematical modelling can optimize the design of CSNPs with predetermined release properties. In vitro-in vivo correlation (IVIVC) concepts for CSNPs DDSs will be aided by the novelty method of in vitro release study, reducing regulatory burden and speeding clinical translation.
Despite recent research and development of nanomedicines, the success rate of clinical translation remains poor, with patients in clinical settings benefiting from nanomedicines only through a reduction in side effects [18]. As an adjuvant in a number of high-quality human vaccines, CS also has the potential to be used in oral or mucoadhesive medication formulations [26]. Due to their poor reproducibility and batch-to-batch variations will be a challenge for the next step in the drug delivery system.
6. Conclusion
CSNPs can transfer active ingredients in a controlled manner, improving therapeutic efficacy while reducing systemic side effects and increasing patient compliance. Small sizes and a large surface area are advantages of CSNPs, ready to modify for targeted delivery.
The properties of CS, such as intermolecular and intramolecular hydrogen bonding and cationic charge in acidic media, make it an excellent DDSs candidate. The foundation for further optimization will be laid by a better understanding of nanocarrier mechanisms, opening more exciting possibilities for improving macromolecule administration.
The dialysis method is the most commonly used method for determining drug re-lease from CSNPs dosage forms. The release of drugs from CSNPs followed a typical bi-phasic pattern, with a rapid initial release followed by a slower and continuous release (controlled release) and more pH-dependent. The zero-order model, Hixson-Crowell model, Higuchi model, Weibull model, and Korsmeyer-Peppas model predict drug release from CSNPs. It depicts three-drug release mechanisms from CSNPs. This discovery strongly supports the modification of CS polymer, the selection of materials, formulations, the manufacture of better CS-based preparations, and to obtain the expected release.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Minister of Research and Higher Education for funding this project through grant number 1207/UN6.3.1/PT.00/2021 and Universitas Padjadjaran Academic Leadership Grants 2021 grant number 2021 1959/UN6.3.1/PT.00/2021.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:1–34. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Sayed A., Kamel M. Advances in nanomedical applications: diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2020;27:19200–19213. doi: 10.1007/s11356-019-06459-2. [DOI] [PubMed] [Google Scholar]
- 3.Zahin N., Anwar R., Tewari D., Kabir M.T., Sajid A., Mathew B., Uddin M.S., Aleya L., Abdel-Daim M.M. Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ. Sci. Pollut. Res. 2020;27:19151–19168. doi: 10.1007/s11356-019-05211-0. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi S.A.A., Saleh A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceut. J. 2018;26:64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Din F. ud, Aman W., Ullah I., Qureshi O.S., Shafique S., Zeb A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017;12:7291–7309. doi: 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catarata R., Azim N., Bhattacharya S., Zhai L. Controlled drug release from polyelectrolyte-drug conjugate nanoparticles. J. Mater. Chem. B. 2020;8:2887–2894. doi: 10.1039/d0tb00012d. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell M.J., Billingsley M.M., Haley R.M., Langer R., Wechsler M.E., Peppas N.A. Engineering precision nanoparticles. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidar M.K., Demirbolat G.M., Timur S.S., Gürsoy R.N., Nemutlu E., Ulubayram K., Öner L., Eroğlu H. Atorvastatin-loaded nanosprayed chitosan nanoparticles for peripheral nerve injury. Bioinsp. Biomim. Nanobiomater. 2020;9:74–84. [Google Scholar]
- 9.Edis Z., Wang J., Waqas M.K., Ijaz M., Ijaz M. Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int. J. Nanomed. 2021;16:1313–1330. doi: 10.2147/IJN.S289443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A.K., Thareja S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cell Nanomed. Biotechnol. 2019;47:524–539. doi: 10.1080/21691401.2018.1561457. [DOI] [PubMed] [Google Scholar]
- 11.Sowjanya M., Debnath S., Lavanya P., Thejovathi R., Babu M.N. Polymers used in the designing of controlled drug delivery system. Res. J. Pharm. Technol. 2017;10:903. [Google Scholar]
- 12.D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharm. 2014;2014:1–12. [Google Scholar]
- 13.Ashrafi H., Azadi A. Chitosan-based hydrogel nanoparticle amazing behaviors during transmission electron microscopy. Int. J. Biol. Macromol. 2016;84:31–34. doi: 10.1016/j.ijbiomac.2015.11.089. [DOI] [PubMed] [Google Scholar]
- 14.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 2016;116:2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammed M.A., Syeda J.T.M., Wasan K.M., Wasan E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9 doi: 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorasitthiyanukarn F.N., Muangnoi C., Rojsitthisak P., Rojsitthisak P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021;256:117426. doi: 10.1016/j.carbpol.2020.117426. [DOI] [PubMed] [Google Scholar]
- 17.Karthika V., AlSalhi M.S., Devanesan S., Gopinath K., Arumugam A., Govindarajan M. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci. Rep. 2020;10:1–17. doi: 10.1038/s41598-020-76015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu J.H., Yoon H.Y., Sun I.C., Kwon I.C., Kim K. Tumor-targeting glycol chitosan nanoparticles for cancer heterogeneity. Adv. Mater. 2020;32:1–40. doi: 10.1002/adma.202002197. [DOI] [PubMed] [Google Scholar]
- 19.Seidi F., Khodadadi Yazdi M., Jouyandeh M., Dominic M., Naeim H., Nezhad M.N., Bagheri B., Habibzadeh S., Zarrintaj P., Saeb M.R., et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021;183:1818–1850. doi: 10.1016/j.ijbiomac.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Zielinska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulikova T., Abduraimova A., Molkenova A., Em S., Duisenbayeva B., Han D.W., Atabaev T.S. Mesoporous silica decorated with gold nanoparticles as a promising nanoprobe for effective CT X-ray attenuation and potential drug delivery. Nano-Struct. Nano-Obj. 2021;26:100712. [Google Scholar]
- 22.Najahi-Missaoui W., Arnold R.D., Cummings B.S. Safe nanoparticles: are we there yet? Int. J. Mol. Sci. 2021;22:1–22. doi: 10.3390/ijms22010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X., Wu S., Du X. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon N. Y. 2016;101:135–142. [Google Scholar]
- 24.Christopher M., S A.M.L. Principles of nanoparticle design for overcoming biological. Physiol. Behav. 2016;176:100–106. [Google Scholar]
- 25.Gagliardi M., Bardi G., Gamucci O., Mazzolai B. Targeted drug delivery across biological barriers using polymer nanoparticles. Ther. Deliv. Meth. Concise Overv. Emerg. Areas. 2013:96–109. [Google Scholar]
- 26.Rashki S., Asgarpour K., Tarrahimofrad H., Hashemipour M., Ebrahimi M.S., Fathizadeh H., Khorshidi A., Khan H., Marzhoseyni Z., Salavati-Niasari M., et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021;251:117108. doi: 10.1016/j.carbpol.2020.117108. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.H., Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2016;125:75–84. doi: 10.1016/j.ces.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laracuente M.L., Yu M.H., McHugh K.J. Zero-order drug delivery: state of the art and future prospects. J. Contr. Release. 2020;327:834–856. doi: 10.1016/j.jconrel.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Awad N.S., Paul V., Alsawaftah N.M., Haar G., Allen T.M., Pitt W.G., Husseini G.A. Ultrasound-responsive nanocarriers in cancer treatment. Review. 2020 doi: 10.1021/acsptsci.0c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iacob A.T., Lupascu F.G., Apotrosoaei M., Vasincu I.M., Tauser R.G., Lupascu D., Giusca S.E., Caruntu I., Profire L. Recent biomedical approaches for chitosan based materials as drug delivery nanocarriers. Pharmaceutics. 2021;13:1–36. doi: 10.3390/pharmaceutics13040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safdara R., Omara A.A., Arunagirib A., Regupathic I., Thanabalan M. Potential of Chitosan and its derivatives for controlled drug release applications – a review. J. Drug Deliv. Sci. Technol. 2018 [Google Scholar]
- 32.W.C. Y., Hani A., R D.C. The role of chitosan on oral delivery of peptide- loaded nanoparticle formulation. J. Drug Target. 2017 doi: 10.1080/1061186X.2017.1400552. 0, 000. [DOI] [PubMed] [Google Scholar]
- 33.Pourtalebi Jahromi L., Ghazali M., Ashrafi H., Azadi A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahya I., Ahmed L., Omara A., Eltayeb M., Atif R., Eldeen T.S. Mathematical modeling of diffusion controlled drug release profiles from nanoparticles. Int. J. Res. Sci. Innov. 2019;VI:287–291. [Google Scholar]
- 35.Varma M.V.S., Kaushal A.M., Garg A., Garg S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Healthc. Technol. Rev. 2004;2:43–57. [Google Scholar]
- 36.Lisa L., Peppas N.A., Yin F., Boey C., Venkatraman S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011;418:28–41. doi: 10.1016/j.ijpharm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Han X., Zhang X. Elsevier; 2017. Modelling Degradation of Biodegradable Polymers. [Google Scholar]
- 38.Ho G., Beom S., Lee J., Weon C. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017 0, 0. [Google Scholar]
- 39.George D., Maheswari P.U., Begum K.M.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: kinetics and in-vitro biological studies. Carbohydr. Polym. 2020:236. doi: 10.1016/j.carbpol.2020.116101. [DOI] [PubMed] [Google Scholar]
- 40.Arnold A.E., Czupiel P., Shoichet M. Engineered polymeric nanoparticles to guide the cellular internalization and trafficking of small interfering ribonucleic acids. J. Contr. Release. 2017;259:3–15. doi: 10.1016/j.jconrel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Bahram M., Mohseni N. Emerging Concepts in Analysis and Applications of Hydrogels. 2016. An introduction to hydrogels and some recent applications; pp. 9–39. [Google Scholar]
- 42.Azadi S., Ashrafi H., Azadi A. Mathematical modeling of drug release from swellable polymeric nanoparticles. J. Appl. Pharmaceut. Sci. 2017;7:125–133. [Google Scholar]
- 43.Plazek D.J. Effect of crosslink density on the creep behavior of natural rubber vulcanizates. J. Polym. Sci. Polym. Phys. 1966;4:745–763. [Google Scholar]
- 44.Burkersrodaa F. von, Schedlb L., Opfericha G.A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 45.Song R., Murphy M., Li C., Ting K., Soo C., Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018;12:3117–3145. doi: 10.2147/DDDT.S165440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu B., Zhou G., Xiao F., He Q., Zhang J. Stimuli-responsive poly(ionic liquid) nanoparticles for controlled drug delivery. J. Mater. Chem. B. 2020;8:7994–8001. doi: 10.1039/d0tb01352h. [DOI] [PubMed] [Google Scholar]
- 47.Abdo G.G., Zagho M.M., Khalil A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emergent. Mater. 2020;3:407–425. [Google Scholar]
- 48.Yetisgin A.A., Cetinel S., Zuvin M., Kosar A., Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25:1–31. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mudie D.M., Amidon G.L., Amidon G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010;7:1388–1405. doi: 10.1021/mp100149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajesh S., J.W.L Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2000;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weng J., Tong H.H.Y., Chow S.F. In vitro release study of the polymeric drug nanoparticles: development and validation of a novel method. Pharmaceutics. 2020;12:1–18. doi: 10.3390/pharmaceutics12080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amatya S., Park E.J., Park J.H., Kim J.S., Seol E., Lee H., Choi H., Shin Y.H., Na D.H. Drug release testing methods of polymeric particulate drug formulations. J. Pharm. Investig. 2013;43:259–266. [Google Scholar]
- 53.Modi S., Anderson B.D. Vol. 10. 2013. Determination of Drug Release Kinetics from Nanoparticles: Overcoming Pitfalls of the Dynamic Dialysis Method. [DOI] [PubMed] [Google Scholar]
- 54.D’Addio S.M., Bukari A.A., Dawoud M., Bunjes H., Rinaldi C., Prud’Homme R.K. Determining drug release rates of hydrophobic compounds from nanocarriers. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2016;374 doi: 10.1098/rsta.2015.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zambito Y., Pedreschi E., Di Colo G. Is dialysis a reliable method for studying drug release from nanoparticulate systems? - a case study. Int. J. Pharm. 2012;434:28–34. doi: 10.1016/j.ijpharm.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed L., Yahya I., Eltayeb M. Computational modeling of drug release profiles from swellable polymeric nanoparticles. Proc. Int. Conf. Comput. Control. Electr. Electron. Eng. 2019 ICCCEEE 2019 2019. [Google Scholar]
- 57.Wang J., Langemann H. Unsteady two-film model for mass transfer accopanied by chemical reaction. Chem. Eng. Sci. 1994;49:3457–3463. [Google Scholar]
- 58.Tao F., Ma S., Tao H., Jin L., Luo Y., Zheng J., Xiang W., Deng H. Chitosan-based drug delivery systems: from synthesis strategy to osteomyelitis treatment – a review. Carbohydr. Polym. 2021;251:117063. doi: 10.1016/j.carbpol.2020.117063. [DOI] [PubMed] [Google Scholar]
- 59.Choudhary P., Ramalingam B., Das S.K. Fabrication of chitosan-reinforced multifunctional graphene nanocomposite as antibacterial scaffolds for hemorrhage control and wound-healing application. ACS Biomater. Sci. Eng. 2020;6:5911–5929. doi: 10.1021/acsbiomaterials.0c00923. [DOI] [PubMed] [Google Scholar]
- 60.Shoueir K.R., El-Desouky N., Rashad M.M., Ahmed M.K., Janowska I., El-Kemary M. Chitosan based-nanoparticles and nanocapsules: overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021;167:1176–1197. doi: 10.1016/j.ijbiomac.2020.11.072. [DOI] [PubMed] [Google Scholar]
- 61.Prabaharan M., Mano J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. J. Deliv. Target. Ther. Agents. 2005;12:41–57. doi: 10.1080/10717540590889781. [DOI] [PubMed] [Google Scholar]
- 62.Idrees H., Zaidi S.Z.J., Sabir A., Khan R.U., Zhang X., Hassan S.U. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials. 2020;10:1–22. doi: 10.3390/nano10101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conti S. 2019. Biomedical Studies on Chitosan Materials and Polyoxometalate Nanocomposites as Versatile Anticancer and Antimicrobial Drug Prototypes; Zurich. [Google Scholar]
- 64.Ficai A., Grumezescu A.M. 2017. Nanostructures in Therapeutic Medicine Series: Nanostructures for Cancer Therapy. [Google Scholar]
- 65.Kizaloglu A., Kilicay E., Karahaliloglu Z., Hazer B., Denkbas E.B. The preparation of chitosan membrane improved with nanoparticles based on unsaturated fatty acid for using in cancer-related infections. J. Bioact. Compat Polym. 2020;35:328–350. [Google Scholar]
- 66.Yhee J.Y., Koo H., Lee D.E., Choi K., Kwon I.C., Kim K. Multifunctional chitosan nanoparticles for tumor imaging and therapy. Adv. Polym. Sci. 2011;243:139–162. [Google Scholar]
- 67.Liu Q., Li Y., Yang X., Xing S., Qiao C., Wang S., Xu C., Li T. O-Carboxymethyl chitosan-based pH-responsive amphiphilic chitosan derivatives: characterization, aggregation behavior, and application. Carbohydr. Polym. 2020;237:116112. doi: 10.1016/j.carbpol.2020.116112. [DOI] [PubMed] [Google Scholar]
- 68.Katas H., Abdul M., Raja G., Lam K.L. 2013. Development of Chitosan Nanoparticles as a Stable Drug Delivery System for Protein/siRNA. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lucio D., Martínez-Ohárriz M.C. Chitosan: strategies to increase and modulate drug release rate. Biol. Act. Appl. Mar. Polysacc. 2017 [Google Scholar]
- 70.Nazeerb N., Ahmeda M. Polymer Science and Nanotechnology. Vol. 2. Elsevier; 2020. Polymers in medicine; pp. 319–333. [Google Scholar]
- 71.M Ahmed E., Saber D., Abd Elaziz K., Alghtani A.H., Felemban B.F., Ali H.T., Megahed M. Chitosan-based nanocomposites: preparation and characterization for food packing industry. Mater. Res. Express. 2021:8. [Google Scholar]
- 72.Su S., Kang P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials. 2020;10 doi: 10.3390/nano10040656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdul Khalil H.P.S., Saurabh C.K., Adnan A.S., Nurul Fazita M.R., Syakir M.I., Davoudpour Y., Rafatullah M., Abdullah C.K., Haafiz M.K.M., Dungani R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: properties and their applications. Carbohydr. Polym. 2016;150:216–226. doi: 10.1016/j.carbpol.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 74.Ghassemi B., Nayeri F.D., Hosseini R. Vol. 9. 2021. The effects of chitosan nanoparticles on genes expression of artemisinin synthase in suspension culture of Artemisia annua L : a comparative study; pp. 190–203. [Google Scholar]
- 75.Saravanakumar K., Mariadoss A.V.A., Sathiyaseelan A., Wang M.H. Synthesis and characterization of nano-chitosan capped gold nanoparticles with multifunctional bioactive properties. Int. J. Biol. Macromol. 2020;165:747–757. doi: 10.1016/j.ijbiomac.2020.09.177. [DOI] [PubMed] [Google Scholar]
- 76.Katas H., Hussain Z., Ling T.C. Chitosan nanoparticles as a percutaneous drug delivery system for hydrocortisone. J. Nanomater. 2012;2012 [Google Scholar]
- 77.Caprifico A.E., Foot P.J.S., Polycarpou E., Calabrese G. Overcoming the blood-brain barrier: functionalised chitosan nanocarriers. Pharmaceutics. 2020;12:1–20. doi: 10.3390/pharmaceutics12111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghorbani F., Kokhaei P., Ghorbani M., Eslami M. Application of different nanoparticles in the diagnosis of colorectal cancer. Gene Rep. 2020;21:100896. [Google Scholar]
- 79.Du J., Hsieh Y. Lo PEGylation of chitosan for improved solubility and fiber formation via electrospinning. Cellulose. 2007;14:543–552. [Google Scholar]
- 80.Chen Z., Han S., Yang X., Xu L., Qi H., Hao G., Cao J., Liang Y., Ma Q., Zhang G., et al. Overcoming multiple absorption barrier for insulin oral delivery using multifunctional nanoparticles based on chitosan derivatives and hyaluronic acid. Int. J. Nanomed. 2020;15:4877–4898. doi: 10.2147/IJN.S251627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buyuk N.I., Arayici P.P., Derman S., Mustafaeva Z., Yucel S. Synthesis of chitosan nanoparticles for controlled release of amiodarone. Indian J. Pharmaceut. Sci. 2020;82:131–138. [Google Scholar]
- 82.Zhang X., Niu S., Williams G.R., Wu J., Chen X., Zheng H., Zhu L.M. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr. Polym. 2019;221:84–93. doi: 10.1016/j.carbpol.2019.05.081. [DOI] [PubMed] [Google Scholar]
- 83.Quiñones J.P., Peniche H., Peniche C. Vol. 10. 2018. Chitosan based self-assembled nanoparticles in drug delivery; pp. 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moody C.T., Palvai S., Brudno Y. Click cross-linking improves retention and targeting of refillable alginate depots. Acta Biomater. 2020;112:112–121. doi: 10.1016/j.actbio.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loutfy S.A., Elberry M.H., Farroh K.Y., Mohamed H.T., Mohamed A.A., Mohamed E.B., Faraag A.H.I., Mousa S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomed. 2020;15:2699–2715. doi: 10.2147/IJN.S241702. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Zhang E., Xing R., Liu S., Qin Y., Li K., Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019;222:115004. doi: 10.1016/j.carbpol.2019.115004. [DOI] [PubMed] [Google Scholar]
- 87.Thomas D., Thomas S. Biopolymer Nanocomposites: Processing. Properties, and Applications; 2013. Chemical modification of chitosan and its biomedical application; pp. 33–51. [Google Scholar]
- 88.Kahya N. Water soluble chitosan derivatives and their biological activities : a review. iMedPub J. 2019;5:1–11. [Google Scholar]
- 89.Mohammadi Z., Eini M., Rastegari A., Tehrani M.R. Chitosan as a machine for biomolecule delivery: a review. Carbohydr. Polym. 2021;256:117414. doi: 10.1016/j.carbpol.2020.117414. [DOI] [PubMed] [Google Scholar]
- 90.Jaworska M., Sakurai K., Gaudon P., Guibal E. Influence of chitosan characteristics on polymer properties. I: crystallographic properties. Polym. Int. 2003;52:198–205. [Google Scholar]
- 91.Eldsäter C., Erlandsson B., Renstad R., Albertsson A.C., Karlsson S. The biodegradation of amorphous and crystalline regions in film-blown poly(ε-caprolactone) Polymer. 2000;41:1297–1304. [Google Scholar]
- 92.Bikiaris D.N., Papageorgiou G.Z., Papadimitriou S.A., Karavas E., Avgoustakis K. Novel biodegradable polyester poly(propylene succinate): synthesis and application in the preparation of solid dispersions and nanoparticles of a water-soluble drug. AAPS Pharm. Sci. Tech. 2009;10:138–146. doi: 10.1208/s12249-008-9184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pantani R., Sorrentino A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stabil. 2013;98:1089–1096. [Google Scholar]
- 94.Catalan K.N., Corrales T.P., Forero J.C., Romero C.P., Acevedo C.A. Glass transition in crosslinked nanocomposite scaffolds of Gelatin/chitosan/hydroxyapatite. Polymers. 2019;11:1–7. doi: 10.3390/polym11040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balasubramanian S., Devi A., Singh K.K., Bosco S.J.D., Mohite A.M. Application of glass transition in food processing. Crit. Rev. Food Sci. Nutr. 2016;56:919–936. doi: 10.1080/10408398.2012.734343. [DOI] [PubMed] [Google Scholar]
- 96.Joy N., Samavedi S. Identifying specific combinations of matrix properties that promote controlled and sustained release of a hydrophobic drug from electrospun meshes. ACS Omega. 2020;5:15865–15876. doi: 10.1021/acsomega.0c00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sánchez A., Mejía S.P., Orozco J. Recent advances in polymeric nanoparticle-encapsulated drugs against intracellular infections. Molecules. 2020;25:1–45. doi: 10.3390/molecules25163760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong Y., Ruan Y., Wang H., Zhao Y., Bi D. Studies on glass transition temperature of chitosan with four techniques. J. Appl. Polym. Sci. 2004;93:1553–1558. [Google Scholar]
- 99.Sharma S.K., Sudarshan K., Sahu M., Pujari P.K. Investigation of free volume characteristics of the interfacial layer in poly(methyl methacrylate)-alumina nanocomposite and its role in thermal behaviour. RSC Adv. 2016;6:67997–68004. [Google Scholar]
- 100.Lamarra J., Damonte L., Rivero S., Pinotti A. Structural insight into chitosan supports functionalized with nanoparticles. Adv. Mater. Sci. Eng. 2018;2018 [Google Scholar]
- 101.Kou S., Gabriel), Peters L.M., Mucalo M.R. Vol. 169. Elsevier B.V; 2021. Chitosan: A Review of Sources and Preparation Methods. [DOI] [PubMed] [Google Scholar]
- 102.Jana P., Shyam M., Singh S., Jayaprakash V., Dev A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021;142:110155. [Google Scholar]
- 103.Sreekumar S., Goycoolea F.M., Moerschbacher B.M., Rivera-Rodriguez G.R. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-23064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sagita E., Syahdi R.R., Arrahman A. Synthesis of polymer-drug conjugates using natural polymer : what , why and how. Pharm. Sci. Res. 2018;5:97–115. [Google Scholar]
- 105.Hassani S., Laouini A., Fessi H., Charcosset C. Preparation of chitosan-TPP nanoparticles using microengineered membranes - effect of parameters and encapsulation of tacrine. Colloids Surf. A Physicochem. Eng. Asp. 2015;482:34–43. [Google Scholar]
- 106.Szafraniec-Szczęsny J., Janik-Hazuka M., Odrobińska J., Zapotoczny S. Polymer capsules with hydrophobic liquid cores as functional nanocarriers. Polymers. 2020;12:1–25. doi: 10.3390/polym12091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petersen R.S., Nielsen L.H., Rindzevicius T., Boisen A., Keller S.S. Controlled drug release from biodegradable polymer matrix loaded in microcontainers using hot punching. Pharmaceutics. 2020;12:1–13. doi: 10.3390/pharmaceutics12111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fathanah U., Machdar I., Riza M., Arahman N., Lubis M.R., Yusuf M. The improvement of hydrophilic property of polyethersulfone membrane with chitosan as additive. J. Rekayasa Kimia Lingkungan. 2020;15:53–61. [Google Scholar]
- 109.Khanmohammadi M., Elmizadeh H., Ghasemi K. Investigation of size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran. J. Pharm. Res. (IJPR) 2015;14:665–675. [PMC free article] [PubMed] [Google Scholar]
- 110.Masarudin M.J., Cutts S.M., Evison B.J., Phillips D.R., Pigram P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015;8:67–80. doi: 10.2147/NSA.S91785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang W., Mehta A., Tong Z., Esser L., Voelcker N.H. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv. Sci. 2021;8:1–32. doi: 10.1002/advs.202003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu G., Liu Y., Sweeney S., Chen S. Vol. 1. Elsevier; 2018. Functionalization and Grafting of Nanoparticle Surfaces. [Google Scholar]
- 113.Peltonen L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv. Drug Deliv. Rev. 2018;131:101–115. doi: 10.1016/j.addr.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerf. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Fan J., Zhang Z., Wang Y., Lin S., Yang S. Photo-responsive degradable hollow mesoporous organosilica nanoplatforms for drug delivery. J. Nanobiotechnol. 2020;18:1–14. doi: 10.1186/s12951-020-00642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Alfy E.A., El-Bisi M.K., Taha G.M., Ibrahim H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020;161:1247–1260. doi: 10.1016/j.ijbiomac.2020.06.118. [DOI] [PubMed] [Google Scholar]
- 117.Uǧuz H., Goyal A., Meenpal T., Selesnick I.W., Baraniuk R.G., Kingsbury N.G., Haiter Lenin A., Mary Vasanthi S., Jayasree T., Adam M., et al. Ce pte d M us pt. J. Phys. Energy. 2020;2:1–31. [Google Scholar]
- 118.Yemisci M., Caban S., Fernandez-megia E., Capan Y., Couvreur P., Dalkara T. Chitosan Nanoparticles for Targeted Brain Delivery. Vol. 1727. Springer Science+Business Media, LLC 2018; 2017. Preparation and characterization of biocompatible chitosan nanoparticles for targeted brain delivery of peptides; pp. 443–454. [DOI] [PubMed] [Google Scholar]
- 119.Bhatt P., Trehan S., Inamdar N., Mourya V.K., Misra A. INC; 2021. Polymers in Drug Delivery: an Update. [Google Scholar]
- 120.Mitra A., Dey B. Chitosan microspheres in novel drug delivery systems. Indian J. Pharmaceut. Sci. 2011;73:355–366. doi: 10.4103/0250-474X.95607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katuwavila N.P., Perera A.D.L.C., Samarakoon S.R., Soysa P., Karunaratne V., Amaratunga G.A.J., Karunaratne D.N. Chitosan-alginate nanoparticle system efficiently delivers doxorubicin to MCF-7 cells. J. Nanomater. 2016;2016 [Google Scholar]
- 122.Hu X., Wang Y., Zhang L., Xu M. Formation of self-assembled polyelectrolyte complex hydrogel derived from salecan and chitosan for sustained release of Vitamin C. Carbohydr. Polym. 2020;234:115920. doi: 10.1016/j.carbpol.2020.115920. [DOI] [PubMed] [Google Scholar]
- 123.Kulpreechanan N., Sorasitthiyanukarn F.N. Evaluation of in vitro release kinetics of capsaicin-loaded chitosan nanoparticles using DDsolver. Int. J. Res. Pharm. Sci. 2020;11:4555–4559. [Google Scholar]
- 124.Dathathri E., Thakur G., Koteshwara K.B., Anil Kumar N.V., Rodrigues F.C. Investigating the effect of freezing temperature and cross-linking on modulating drug release from chitosan scaffolds. Chem. Pap. 2020;74:1759–1768. [Google Scholar]
- 125.Thai H., Thuy Nguyen C., Thi Thach L., Thi Tran M., Duc Mai H., Thi Thu Nguyen T., Duc Le G., Van Can M., Dai Tran L., Long Bach G., et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020;10:1–15. doi: 10.1038/s41598-020-57666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Y., Chen G., Murray P., Zhang H. Porous chitosan by crosslinking with tricarboxylic acid and tuneable release. SN Appl. Sci. 2020;2:1–10. [Google Scholar]
- 127.Patel R., Gajra B., Rh P., Patel G. Ganciclovir loaded chitosan nanoparticles : preparation and nanomedicine & nanotechnology. J. Nanomed. Nanotechnol. 2016;7:1–8. [Google Scholar]
- 128.Li J., Ma F.K., Dang Q.F., Liang X.G., Chen X.G. Glucose-conjugated chitosan nanoparticles for targeted drug delivery and their specific interaction with tumor cells. Front. Mater. Sci. 2014;8:363–372. [Google Scholar]
- 129.Rizwan M., Yahya R., Hassan A., Yar M., Azzahari A.D., Selvanathan V., Sonsudin F., Abouloula C.N. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers. 2017;9 doi: 10.3390/polym9040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peers S., Montembault A., Ladavière C. Chitosan hydrogels for sustained drug delivery. J. Contr. Release. 2020;326:150–163. doi: 10.1016/j.jconrel.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 131.Vakili M., Rafatullah M., Salamatinia B., Abdullah A.Z., Ibrahim M.H., Tan K.B., Gholami Z., Amouzgar P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohydr. Polym. 2014;113:115–130. doi: 10.1016/j.carbpol.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 132.Shu X.Z., Zhu K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002;54:235–243. doi: 10.1016/s0939-6411(02)00052-8. [DOI] [PubMed] [Google Scholar]
- 133.Sabra R., Billa N. Gastrointestinal delivery of APIs from chitosan nanoparticles. Chitin Chitosan - Physicochem. Prop. Ind. Appl. 2020 [Google Scholar]
- 134.Agnihotri S.A., Mallikarjuna N.N., Aminabhavi T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Contr. Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 135.Tingting C., Shunying L., Wenting Z., Zhi L., Qingbing Z. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J. Microencapsul. 2019 doi: 10.1080/02652048.2019.1604846. 0, 000. [DOI] [PubMed] [Google Scholar]
- 136.Muhsin M.D.A., George G., Beagley K., Ferro V., Wang H., Islam N. Effects of chemical conjugation of l -leucine to chitosan on dispersibility and controlled release of drug from a nanoparticulate dry powder inhaler formulation. Mol. Pharm. 2016;13:1455–1466. doi: 10.1021/acs.molpharmaceut.5b00859. [DOI] [PubMed] [Google Scholar]
- 137.Pérez Quiñones J., Szopko R., Schmidt C., Peniche Covas C. Novel drug delivery systems: chitosan conjugates covalently attached to steroids with potential anticancer and agrochemical activity. Carbohydr. Polym. 2011;84:858–864. [Google Scholar]
- 138.Vaghani S.S., Patel M.M., Satish C.S. Synthesis and characterization of pH-sensitive hydrogel composed of carboxymethyl chitosan for colon targeted delivery of ornidazole. Carbohydr. Res. 2012;347:76–82. doi: 10.1016/j.carres.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 139.Samy M., Abd El-Alim S.H., Rabia A.E.G., Amin A., Ayoub M.M.H. Formulation, characterization and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020;156:783–791. doi: 10.1016/j.ijbiomac.2020.04.112. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y., Sun M., Wang T., Chen X., Wang H. Chitosan-based self-assembled nanomaterials: their application in drug delivery. View. 2021;2:20200069. [Google Scholar]
- 141.Rawal S.U., Patel M.M. Elsevier; 2018. Lipid Nanoparticulate Systems: Modern Versatile Drug Carriers. [Google Scholar]
- 142.Kunjachan S., Jose S. Understanding the mechanism of ionic gelation for synthesis of chitosan nanoparticles using qualitative techniques. Asian J. Pharm. 2010 [Google Scholar]
- 143.Islam N., Dmour I., Taha M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Al-Nerawi N.K., Alsharif S.S.M., Dave R.H. Preparation of chitosan-TPP nanoparticles : the influence of chitosan polymeric properties and formulation variables. Int. J. Appl. Pharm. 2018;10:60–65. [Google Scholar]
- 145.Nogueira-Librelotto D.R., Scheeren L.E., Macedo L.B., Vinardell M.P., Rolim C.M.B. pH-Sensitive chitosan-tripolyphosphate nanoparticles increase doxorubicin-induced growth inhibition of cervical HeLa tumor cells by apoptosis and cell cycle modulation. Colloids Surf. B Biointerf. 2020;190:110897. doi: 10.1016/j.colsurfb.2020.110897. [DOI] [PubMed] [Google Scholar]
- 146.Han Y., Gao Z., Chen L., Kang L., Huang W., Jin M., Wang Q., Bae Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B. 2019;9:902–922. doi: 10.1016/j.apsb.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weiss M., Fan J., Claudel M., Sonntag T., Didier P., Ronzani C., Lebeau L., Pons F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 2021;19:1–18. doi: 10.1186/s12951-020-00747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raj S., Kumar D. Biochemical Toxicology - Heavy Metals and Nanomaterials. IntechOpen; 2020. Biochemical toxicology: heavy metals and nanomaterials; pp. 1–15. [Google Scholar]
- 149.Seabra A.B., Durán N. Nanotoxicology of metal oxide nanoparticles. Metals. 2015;5:934–975. [Google Scholar]
- 150.Isoda K., Tanaka A., Fuzimori C., Echigoya M., Taira Y., Taira I., Shimizu Y., Akimoto Y., Kawakami H., Ishida I. Toxicity of gold nanoparticles in mice due to nanoparticle/drug interaction induces acute kidney damage. Nanoscale Res. Lett. 2020;15 doi: 10.1186/s11671-020-03371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Damasco J.A., Ravi S., Perez J.D., Hagaman D.E., Melancon M.P. Understanding nanoparticle toxicity to direct a safe-by-design approach in cancer nanomedicine. Nanomaterials. 2020;10:1–41. doi: 10.3390/nano10112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hassan N., Oyarzun-Ampuero F., Lara P., Guerrero S., Cabuil V., Abou-Hassan A., Kogan M. Flow chemistry to control the synthesis of nano and microparticles for biomedical applications. Curr. Top. Med. Chem. 2014;14:676–689. doi: 10.2174/1568026614666140118213915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.