Summary
Background
A previous randomized controlled trial showed contralateral seventh cervical nerve (CC7) cross transfer to be safe and effective in restoring the arm function of spastic arm paralysis patients in a specified population. Guidance on indications, safety and expected long-term improvements of the surgery are needed for clinical practice.
Methods
This is a retrospective, multicenter, propensity score-matched cohort study. All patients registered between 2013 and 2019 with unilateral spastic arm paralysis over 1 year who were registered at one of five centers in China and South Korea were included. Patients received CC7 cross transfer or rehabilitation treatment in each center. Primary outcome was the change in the upper-extremity Fugl–Meyer (UEFM) score from baseline to 2-year follow-up; larger increase indicated better functional improvements.
Findings
The analysis included 425 eligible patients. After propensity score matching, 336 patients who were 1:1 matched into surgery and rehabilitation groups. Compared to previous trial, patient population was expanded on age (< 12 and > 45 years old), duration of disease (< 5 years) and severity of paralysis (severe disabled patients with UEFM < 20 points). In matched patients, the overall increases of UEFM score from preoperative evaluation to 2-year follow-up were 15.14 in the surgery group and 2.35 in the rehabilitation group (difference, 12.79; 95% CI: 12.02–13.56, p < 0.001). This increase was 16.58 at 3-year and 18.42 at 5-year follow-up compared with the surgery group baseline. Subgroup analysis revealed substantial increase on UEFM score in each subgroup of age, duration of disease, severity of paralysis and cause of injury. No severe complication or disabling sequela were reported in the surgery group.
Interpretation
This study showed that CC7 cross transfer can provide effective, safe and stable functional improvements in long-term follow-up, and provided evidences for expanding the indications of the surgery to a wider population of patients with hemiplegia.
Keywords: Contralateral seventh cervical nerve cross transfer, Hemiplegia, Spastic arm paralysis, Real-world observation, Multicenter study
Research in context.
Evidence before this study
We searched PubMed and Wanfang database for studies published before the submission and reconfirmed the results in this revision with the term of contralateral C7 neve (CC7) cross transfer and hemiplegia or spastic arm paralysis. Apart from the first case report, first cohort study and the one randomized controlled clinical trial (RCT) from our team, four other studies reported 13 patients’ outcomes with follow-up of less than six months. In the RCT study published in 2018 from our group, CC7 cross transfer surgery has been proved to be effective in restoring spastic arm's motor function in 18 hemiplegic patients aged between 12 to 45 years old with a duration for more than 5 years. Whether this surgery could apply to a more inclusive population in a large-cohort analysis requires more evidences from clinical practice.
Added value of this study
Through a multicenter (Chinese and Korean) cohort study with five-years follow-up, it showed that among hemiplegia patients, no matter for what kind of central cerebral injury (Stroke, Traumatic brain injury or cerebral palsy), CC7 cross transfer can all provide effective, safe, and stable motor functional improvements in patients from childhood to old aged. Meaningful improvements were obtained for several years (1 to 5 years) after the original injury. This study provided valuable guidance for patient selection and prognostic prediction in daily practice.
Implications of all the available evidence
By connecting the paralyzed arm to the contralesional hemisphere, CC7 nerve cross transfer represents a brand-new approach and direction in restoring the motor function after central cerebral injury and provides opportunities for insights into the essence of adult brain plasticity and neural regeneration.
Alt-text: Unlabelled box
Introduction
Cerebral injuries such as stroke, cerebral palsy or traumatic brain injuries, lead to long-term disability in the chronic stage.1, 2, 3 While two-thirds of patients succeed in walking independently after an injury, less than half regain basic upper limb functions 6 months or longer after the injury, typically suffering unilateral spastic limb paralysis.4, 5, 6 Strategies to regain function of the paralyzed hand predominantly focus on repairing the injured hemisphere and reducing spasticity of the upper limb, but substantial improvement is rare.7, 8, 9
In 1872, Brown-Sequard announced his conjecture “one side of the brain is sufficient for the functional integrity of both sides of the body” in The New England Journal of Medicine (Boston Medical and Surgical Journal at that time).10 This conjecture is supported by the evidence that infants and children who receive hemispherectomy for intractable epilepsy can gradually rebuild the motion and sensation of the contralateral limb,11,12 and the fact that involvement of the contralesional hemisphere during recovery of hand dexterity movement after stroke.13,14 These findings indicate that to exploit the potential of the contralesional hemisphere is a potential direction to treat unilateral spastic arm paralysis.
In 2018, we reported the results of treating spastic arm paralysis after chronic brain injury using a new surgical procedure, contralateral seventh cervical nerve (CC7) cross transfer surgery. In this surgery, the seventh cervical nerve of the intact side is mobilized to the paralyzed side and coapted with the ipsilesional seventh cervical nerve; therefore, the paralyzed hand is connected to the contralesional hemisphere. Through a randomized, controlled clinical trial (abbreviated to CONCENT), we showed that CC7 is safe and effective for the treatment of spastic arm paralysis,15 consist with previous reports.16,17 In the CONCENT study, patients were aged 12–45 years and had a disease duration of at least 5 years. Although sex and functional state at baseline were not restricted, patients included were male and mostly had a baseline score in the upper extremity of the Fugl–Meyer (UEFM) scale between 20–40 points. Whether this surgery could be effective in a more inclusive population needs to be explored.
In clinical practice, hemiplegia could be caused by different etiologies, and the extent of functional loss varies according to the lesion area. Moreover, the expected functional improvements need to be approximately anticipated. In this study, we provided this information based on follow-up data from five centers and subgroup analysis, which could be used as a clinical guidance for the usage of CC7 transfer for unilateral spastic arm paralysis.
Methods
Study design and oversight
This was a retrospective cohort study involving patients with unilateral spastic arm paralysis from four centers in China and one center in South Korea who underwent CC7 cross transfer surgery or rehabilitation alone between August 1, 2013 and January 1, 2019. The locations of four centers in China are from Shanghai, Yinchuan, Nantong and Jiaxing, and the center in Korea is from Seoul. All centers included rehabilitation and surgical departments that cooperated for the treatment of spastic arm paralysis. The institutional review board of the Hua Shan Hospital of Fudan University approved this study. Hospitals received approval to enroll patients with individual patient's consent or waiver authorization and exemption from subsequent review by their own institutional review boards. The study was approved by the institutional review board of Huashan Hospital and registered on www.chictr.org.cn (number: ChiCTR2000033670). This study has been conducted according to the STROBE guidelines.
Treatments and follow-up
The patients in this study were identified from the electronic medical records of clinics or inpatient departments. Patients were eligible for inclusion if they had a documented history of chronic cerebral injury and unilateral spastic paralysis, manifesting mainly as spasticity and unilateral upper limb disability for at least 1 year. Assessments of UEFM scores before surgery and at least once after the surgery were also required. Exclusion criteria were history of surgeries including orthomorphic surgery, selective posterior rhizotomy, or functional reconstructive surgery of the upper limb; severe comorbidities or surgical contradictions.
Standard operation protocols were used throughout patient screening and treatment, and single-blinded functional evaluation before and after surgery was performed in each center. Treatments included CC7 cross transfer surgery or regular rehabilitation treatment. In the surgery group, standard surgical procedures were performed by experienced surgeons, as described in the article introducing surgical technique.18 In most patients, the length of the contralateral C7 nerve was long enough for direct coaptation with the ipsilateral C7 nerve after cross transfer. In patient who's contralateral C7 nerve was not long enough, the sural nerve was used as graft to bridge the gap between the donor and recipient nerves. In the rehabilitation group, patients received regular rehabilitation for at least 6 months within the first year after registry. The only between-group difference in rehabilitation was the use of a special immobilizing cast for four weeks after the surgery. The detailed procedure and rehabilitation process were described in the supporting information of supplement.
The data used for analysis were collected from medical records including outpatient registries, inpatient medical histories, nurse records, medical orders, and follow-up materials. Other information, including baseline characteristics, details of surgery, adverse events and medication use, and duration and type of rehabilitation, were also collected.
Outcomes
The primary outcome was the change in UEFM score between baseline and the 2-year follow-up. The UEFM scale was designed to assess recovery after stroke19,20 and the patients included in this study manifested mainly hemiplegia or hemiparesis. The UEFM scale measures 33 items, each scored from 0 to 2, with 0 indicating “cannot perform,” 1 indicating “perform partially,” and 2 indicating “performs fully” (total score ranges from 0 to 66, with higher scores indicating better function).
Secondary outcomes included changes from baseline to the 2-year follow-up for Modified Ashworth Scale scores (MAS) and active range of motion. The MAS scores measures spasticity at the elbow, forearm, wrist, thumb and digits two through five on a scale from zero to five, with higher values indicating more spasticity. The active range of motion for the elbow and wrist and for forearm rotation was measured as the range through which a joint could be actively moved and was compared between the range at baseline and at the 2-year follow-up.
Two participant-reported questionnaires were used in this study. The first was the participant-reported quality of life questionnaire, which consisted of six items including total satisfaction with the treatment, change of self-caring ability, reduction in family burden of caring, increase in efficiency and ability of daily activities, relief of pain or discomfort, and relief of anxiety or depression. Each question was analyzed independently and scores were rated from one to five, with higher scores indicating better satisfaction. The second questionnaire was designed for the rehabilitation group to explore the reason why they choose conventional rehabilitation over the CC7 cross transfer surgery (Figure S2 in the supplement).
Safety outcomes include all adverse events related to surgery or rehabilitation, and changes in sensory and muscle strength of the arm and hand related to transection of bilaterally C7 nerve root over a period of 6 months. Initial adverse events refer to any incidences related to the surgery within 1 month, and persistent adverse events referred to any discomfort emerging after the surgery and that existed for more than 6 months after the surgery.
Propensity score matching
To reduce selection bias for the type of treatment and confounding factors in comparisons of the functional outcomes of the two groups, propensity score matching was used to create well-balanced groups.21 The following characteristics were included as covariates: age, sex, education level, body-mass index, smoking history, comorbidity including diabetes mellitus and hypertension, duration of disease, functional assessments at baseline, cause of injury, paralyzed side, and which center the patient was treated. The propensity score was calculated in 425 patients with a multivariate logistic regression model, and patients were 1:1 matched by caliper restriction to the nearest neighbor without replacement. To ensure close matches, we used calipers of width equal to 0.2 of the pooled standard deviation of the logit of the propensity score, which will eliminate approximately 99% of the bias due to the measured confounders.22 Standardized mean difference (SMD) was used to examine the balance of covariate distribution between treatment groups in the propensity score matched sample.
Statistical analysis
Descriptive statistics were used to report the characteristics of the patients at baseline. For continuous variables, Student's t-tests or Wilcoxon rank-sum tests were used for between-group comparisons. For discrete variables, chi-square or Fisher's exact tests were used for between-group comparisons. After propensity matching, intergroup comparisons of the continuous outcomes of changes from baseline to the 2-year follow-up were performed by means of analysis of covariance to adjust baseline measures.21 Two-tailed P values of 0.05 were considered to indicate statistical significance.
Post-hoc subgroup analyses were related to the main characters of the study population in previous study, “CONCENT”. Subgroups were determined by whether they were in accord with CONCENT population (CONCENT-eligible) or different from it (CONCENT-ineligible). Four main factors were involved, including age, sex, duration of paralysis, and UEFM score at baseline. The subgroups of population comprised CONCENT-eligible (patients who meet all the criteria in CONCENT) and CONCENT-ineligible (patients who did not). Then, each factor was evaluated separately. For age, the CONCENT-eligible subgroup was 12 to 45 years old and the CONCENT-ineligible subgroups were below 12 years and above 45 years. The severity of paralysis was determined by the UEFM score at baseline and was CONCENT-eligible (20-40 points), and two CONCENT-ineligible subgroups (<20 points, and > 40 points). Similar subgroup settings were used on sex (male and female) and duration (below 5 years, and 5 years or longer).
Further analyses were performed on regular factors, including education level, cause of injury, and side of paralysis. For cause of injury, patients were divided into five subgroups, hemorrhagic stroke, ischemic stroke, cerebral palsy, traumatic brain injury and encephalitis. To evaluate the influence of adulthood on the outcomes, subgroups of age were also divided into groups including age below 18 years, 18–45 years, and age over 45 years. The main software used in this study is R (version: 3.6.2. http://www.r-project.org/). To do the propensity-score matching, we used the Matchlt package (version 4.2.0).
Role of the funding source
The sponsor of the study had no role in the data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study population
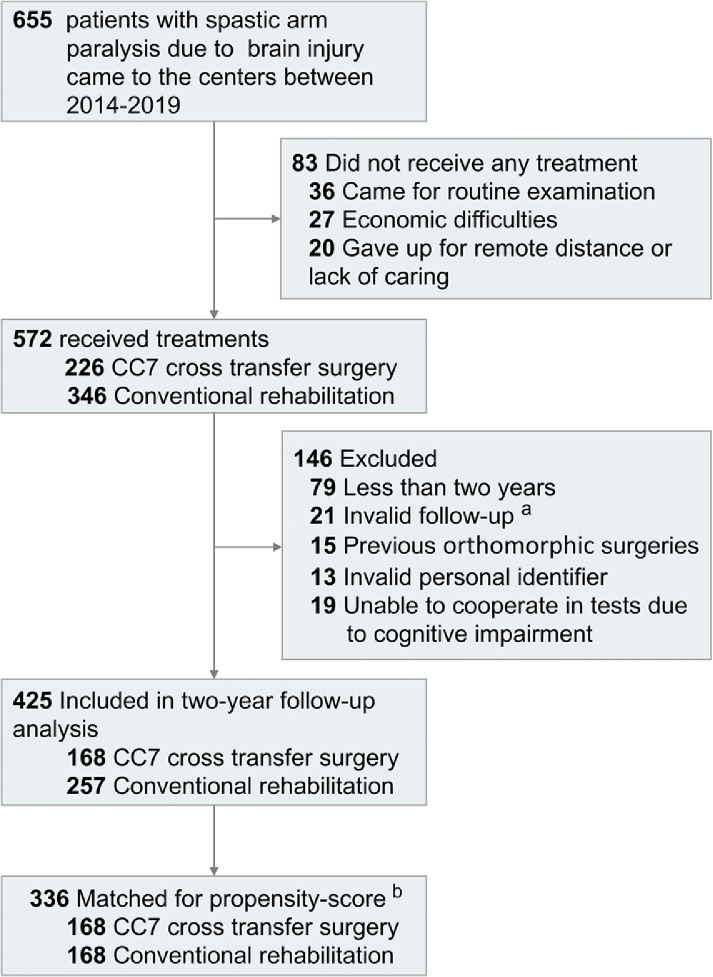
We identified 655 eligible patients with spastic arm paralysis resulting from chronic cerebral injury of different etiologies. Of these, 226 underwent CC7 cross transfer surgery, and 346 received conventional rehabilitation treatment. Among them, 425 completed 2-year follow-ups and were included in analyses and propensity-score matching (see patient flow diagram, Figure 1).
Figure 1.
Patient flow diagram. a Patients for whom valid data were missing or for whom there was incomplete UEFM scale follow-up. b Patients for whom data was available at the 2-year follow-up were matched for propensity-score and subsequently analyzed.
Among the unmatched groups, patients in the surgery group were quantitatively younger comparing to the rehabilitation group (median [range]: 36.5 [5–69] vs 43.0 [4–76]), a longer duration of paralysis and similar baseline UEFM score. After propensity score matching, 336 patients were 1:1 matched, comprising well-balanced CC7 cross transfer (n = 168) and rehabilitation alone groups (n = 168), as shown in the flow chart (Figure 1). The median age of the matched patients was 38 (range: 4–69) years old, and the median duration of paralysis was 4.0 (range: 1–38) years. Male patients comprise 83.9% of the whole population. There were no significant differences between the matched groups in the matching variables of basic characteristics and disease related characteristics. Baseline characteristics and coexisting conditions before and after propensity-score matching are shown in Table 1.
Table 1.
Patients’ baseline characteristics before and after propensity-score matching.
| Propensity Score Matching, No (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Before |
After |
|||||||
| Characteristics | Surgery | Rehabilitation | P value | SMD | Surgery | Rehabilitation | P value | SMD |
| Demographic Characteristics | ||||||||
| No. of patients | 168 | 257 | 168 | 168 | ||||
| Age | ||||||||
| Mean (SD) | 35.8 (14.8) | 39.6(14.5) | 0.010 | 0.255 | 35.8 (14.8) | 38.1 (14.6) | 0.16 | 0.155 |
| Median(range) | 36.5 (5.0,69.0) | 43.0(4.0,76.0) | 36.5 (5.0,69.0) | 39.5 (4.0,65.0) | ||||
| Sex | ||||||||
| Female | 26 (15.5) | 43 (16.7) | 0.84 | 0.034 | 26 (15.5) | 28 (16.7) | 0.88 | 0.032 |
| Male | 142 (84.5) | 214 (83.3) | 142 (84.5) | 140 (83.3) | ||||
| Education | ||||||||
| Junior high or below | 44 (26.2) | 101 (39.3) | 0.0073 | 0.282 | 44 (26.2) | 47 (28.0) | 0.81 | 0.040 |
| High school and above | 124 (73.8) | 156 (60.7) | 124 (73.8) | 121 (72.0) | ||||
| BMI—-mean (SD) | 23.4 (2.5) | 23.7 (3.5) | 0.25 | 0.118 | 23.4 (2.5) | 23.7 (3.4) | 0.33 | 0.105 |
| Comorbidities | ||||||||
| Diabetes Mellitus | 35 (20.8) | 60 (23.3) | 0.63 | 0.061 | 35 (20.8) | 34 (20.2) | 1.0 | 0.015 |
| Hypertension | 69 (41.1) | 114 (44.4) | 0.57 | 0.066 | 69 (41.1) | 77 (45.8) | 0.44 | 0.096 |
| Smoking habit | 37 (22.0) | 66 (25.7) | 0.46 | 0.086 | 37 (22.0) | 37 (22.0) | 1.00 | <0.001 |
| Disease Characteristics | ||||||||
| Paralyzed side | ||||||||
| Left | 94 (56.0) | 132 (51.4) | 0.41 | 0.092 | 94 (56.0) | 89 (53.0) | 0.66 | 0.060 |
| Right | 74 (44.0) | 125 (48.6) | 74 (44.0) | 79 (47.0) | ||||
| Cause of injury | ||||||||
| Stroke | <.0001 | 0.490 | 0.057 | 0.335 | ||||
| Hemorrhagic | 63 (37.5) | 136 (52.9) | 63(37.5) | 82(48.8) | ||||
| Ischemic | 39 (23.2) | 72 (28.0) | 39(23.2) | 40(23.8) | ||||
| Cerebral palsy | 27 (16.1) | 24 (9.3) | 27(16.1) | 23(13.7) | ||||
| Traumatic brain injury | 32 (19.0) | 24 (9.3) | 32(19.0) | 22(13.1) | ||||
| Encephalitis | 7 (4.2) | 1 (0.4) | 7(4.2) | 1(0.6) | ||||
| Duration of disease | ||||||||
| Mean (SD) | 7.1 (7.1) | 6.4 (6.6) | 0.31 | 0.099 | 7.1 (7.1) | 6.9 (7.4) | 0.80 | 0.028 |
| Median(range) | 5.0 (1.0,38.0) | 4.0 (1.0,33.0) | 5.0 (1.0,38.0) | 4.0(1.0,33.0) | ||||
| Baseline UEFM a score | ||||||||
| Mean (SD) | 24.8 (12.6) | 24.5 (12.2) | 0.81 | 0.024 | 24.8(12.6) | 24.5 (12.8) | 0.81 | 0.026 |
| Median(range) | 24.0 (4.0,59.0) | 23.0 (3.0,60.0) | 24.0(4.0,59.0) | 23.0 (3.0,60.0) | ||||
| Baseline MAS b score— Mean(SD) | ||||||||
| Elbow | 2.12 (0.79) | 2.13 (0.77) | 0.90 | 0.012 | 2.12 (0.79) | 2.17 (0.80) | 0.58 | 0.060 |
| Forearm rotation | 2.30 (0.76) | 2.39 (0.80) | 0.28 | 0.109 | 2.30 (0.76) | 2.38 (0.81) | 0.37 | 0.098 |
| Wrist | 2.42 (0.84) | 2.40 (0.91) | 0.89 | 0.014 | 2.42 (0.84) | 2.46 (0.93) | 0.62 | 0.054 |
| Thumb | 2.49 (0.88) | 2.33 (0.94) | 0.066 | 0.184 | 2.49 (0.88) | 2.45 (0.93) | 0.63 | 0.053 |
| Fingers 2–5 | 2.19 (0.92) | 2.33 (0.98) | 0.14 | 0.148 | 2.19 (0.92) | 2.25 (1.02) | 0.57 | 0.061 |
| Baseline Range of motion—degree c | ||||||||
| Elbow | 77.95 (30.79) | 70.80 (30.76) | 0.021 | 0.232 | 77.95(30.79) | 72.77 (33.06) | 0.14 | 0.16 |
| Forearm rotation | 39.26 (27.25) | 31.74 (21.81) | 0.0021 | 0.305 | 39.26(27.25) | 34.23 (22.12) | 0.064 | 0.20 |
| Wrist | 54.76 (23.96) | 47.45 (30.12) | 0.0091 | 0.269 | 54.76(23.96) | 50.82 (31.33) | 0.20 | 0.14 |
| Center | ||||||||
| Huashan | 132 (78.6) | 194 (75.5) | 0.54 | 0.073 | 132(78.6) | 129(76.8) | 0.79 | 0.043 |
| Other | 36 (21.4) | 63 (24.5) | 36(21.4) | 39(23.2) | ||||
All the characteristics listed here were involved as covariates for matching. BMI: body mass index. SMD: standardized mean difference.
UEFM: The Upper-Extremity Fugl-Meyer scale, a measure of motor impairment; scores range from 0 to 66, with higher scores indicating better function.
MAS: The Modified Ashworth Scale is a measure of spasticity (muscle tone) in the paralyzed arm; Scores range from 0 to 5 at each of five joints, with higher scores indicating more severe spasticity.
Range of motion measures the range through which a joint can be actively moved.
Outcomes
The mean change in UEFM scores between baseline and the 2-year follow-up was significantly higher in the surgery group compared with that in the rehabilitation group (mean change: 15.14 and 2.35, respectively; mean difference: 12.79; 95% CI: 12.02–13.56) (Table 2).
Table 2.
Changes of primary and secondary outcomes in matched cohorts.
| Mean (SD) |
Effect size (Cohen's d) | Mean (95% CI) | P value | ||
|---|---|---|---|---|---|
| Surgery | Rehabilitation | Adjusted Mean difference | |||
| Number of patients | 168 | 168 | |||
| Primary outcome | |||||
| Change in UEFM score from baseline to 2-year follow-up | |||||
| Total | 15.14 (4.78) | 2.35 (1.79) | 3.55(3.20∼3.89) | 12.79 (12.02, 13.56) | < 0.0001 |
| CONCENT-eligible a | 18.00 (4.86) | 2.08 (1.26) | 4.23(2.90∼5.53) | 16.35 (13.79, 18.91) | < 0.0001 |
| CONCENT-ineligible b | 14.82 (4.67) | 2.37 (1.82) | 3.52(3.17∼3.88) | 12.44 (11.65, 13.24) | < 0.0001 |
| Secondary outcomes | |||||
| Changes in MAS score from baseline to 2-year follow-up c | |||||
| Elbow | |||||
| Total | -0.88 (0.58) | -0.13 (0.55) | -0.88(-1.11∼-0.66) | -0.76 (- 0.87, -0.64) | < 0.0001 |
| CONCENT-eligible | -0.94 (0.66) | -0.08 (0.28) | -1.89(-2.76∼-1.01) | -0.92 (-1.33, -0.51) | 0.00016 |
| CONCENT-ineligible | -0.87 (0.57) | -0.14 (0.57) | -0.82(-1.06∼-0.59) | -0.74 (-0.87, -0.61) | < 0.0001 |
| Forearm rotation | |||||
| Total | -0.97 (0.73) | -0.20 (0.53) | -0.91(-1.14∼-0.69) | -0.78 (-0.91, -0.64) | < 0.0001 |
| CONCENT-eligible | -1.12 (0.78) | -0.23 (0.44) | -1.21(-1.99∼-0.41) | -0.90 (-1.37, -0.43) | 0.00078 |
| CONCENT-ineligible | -0.95 (0.72) | -0.20 (0.54) | -0.88(-1.12∼-0.65) | -0.76 (-0.90, -0.62) | < 0.0001 |
| Wrist | |||||
| Total | -1.10 (0.72) | -0.18 (0.63) | -1.03(-1.25∼-0.80) | -0.93 (-1.07, -0.79) | < 0.0001 |
| CONCENT-eligible | -1.53 (0.80) | -0.15 (0.38) | -1.47(-2.28∼-0.64) | -1.11 (-1.55, -0.67) | < 0.0001 |
| CONCENT-ineligible | -1.05 (0.70) | -0.19 (0.65) | -1(-1.24∼-0.76) | -0.89 (- 1.03, -0.74) | <0.0001 |
| Thumb | |||||
| Total | -1.37 (0.62) | -0.23 (0.51) | -1.17(-1.40∼-0.94) | -1.14 (-1.25, -1.02) | <0.0001 |
| CONCENT-eligible | -1.59 (0.62) | -0.62 (0.77) | -0.12(-0.84∼0.61) | -0.68 (-1.23, -0.14) | 0.021 |
| CONCENT-ineligible | -1.34 (0.62) | -0.19 (0.47) | -1.28(-1.52∼-1.03) | -1.16 (-1.27, -1.04) | < 0.0001 |
| Fingers 2–5 | |||||
| Total | -1.04 (0.75) | -0.17 (0.59) | -0.96(-1.19∼-0.74) | -0.88 (-1.02, -0.75) | < 0.0001 |
| CONCENT-eligible | -1.12 (0.49) | -0.31 (0.85) | -1.37(-2.17∼-0.56) | -0.84 (-1.20, -0.48) | < 0.0001 |
| CONCENT-ineligible | -1.03 (0.77) | -0.16 (0.56) | -0.94(-1.17∼-0.70) | -0.88 (-1.03, -0.74) | <0.0001 |
| Changes in ROM from baseline to 2-year follow-up d | |||||
| Elbow | |||||
| Total | 30.54 (14.88) | -4.03 (6.14) | 1.05(0.82∼1.28) | 35.11 (32.77, 37.45) | < 0.0001 |
| CONCENT-eligible | 35.00 (15.31) | -3.08 (6.30) | 1.73(0.87∼2.57) | 39.16 (30.80, 47.52) | < 0.0001 |
| CONCENT-ineligible | 30.03 (14.79) | -4.11 (6.14) | 1.01(0.77∼1.24) | 34.66 (32.23, 37.08) | < 0.0001 |
| Forearm rotation | |||||
| Total | 38.21 (15.94) | -2.26 (3.59) | 1.70(1.45∼1.95) | 41.14 (38.76, 43.53) | < 0.0001 |
| CONCENT-eligible | 39.12 (15.64) | -1.54 (3.15) | 2.21(1.27∼3.12) | 40.49 (32.01, 48.97) | < 0.0001 |
| CONCENT-ineligible | 38.11 (16.02) | -2.32 (3.63) | 1.67(1.41∼1.93) | 41.17 (38.68, 43.67) | <0.0001 |
| Wrist | |||||
| Total | 38.54 (16.13) | -2.08 (3.97) | 1.45(1.21∼1.69) | 41.05 (38.61, 43.49) | < 0.0001 |
| CONCENT-eligible | 45.00 (15.61) | -2.31 (4.39) | 1.60(0.75∼2.42) | 44.88 (36.45, 53.31) | < 0.0001 |
| CONCENT-ineligible | 37.81 (16.08) | -2.06 (3.95) | 1.44(1.19∼1.69) | 40.40 (37.85, 42.95) | < 0.0001 |
CONCENT-eligible denotes the patients who were eligible according the previous RCT criteria in this study.
CONCENT-ineligible refers to the patients who were not eligible according the previous RCT criteria in this study.
MAS refers to the Modified Ashworth Scale, a measure of spasticity (muscle tone) in the paralyzed arm; Scores range from 0 to 5 at each of five joints, with higher scores indicating more severe spasticity. Negative numbers indicate decrease and positive numbers indicate increase in spasticity from baseline to 2-year follow-up.
ROM refers to the Range of motion of the paralyzed arm
In secondary outcomes, changes in Modified Ashworth Scale scores between baseline and the 2-year follow-up showed significant improvement in the surgery group at all joints (difference: elbow, −0.76; forearm rotation, −0.78; wrist, −0.93; thumb, −1.14; fingers 2–5, −0.88). Differences between the surgery and rehabilitation groups in changes to the active range of motion between baseline and the 2-year follow-up were 35.11 (95% CI: 32.77–37.45) at the elbow, 41.14 (95% CI: 38.76–43.53) for forearm rotation, and 41.05 (95% CI: 38.61–43.39) at the wrist (Table 2).
Post-hoc subgroup analysis
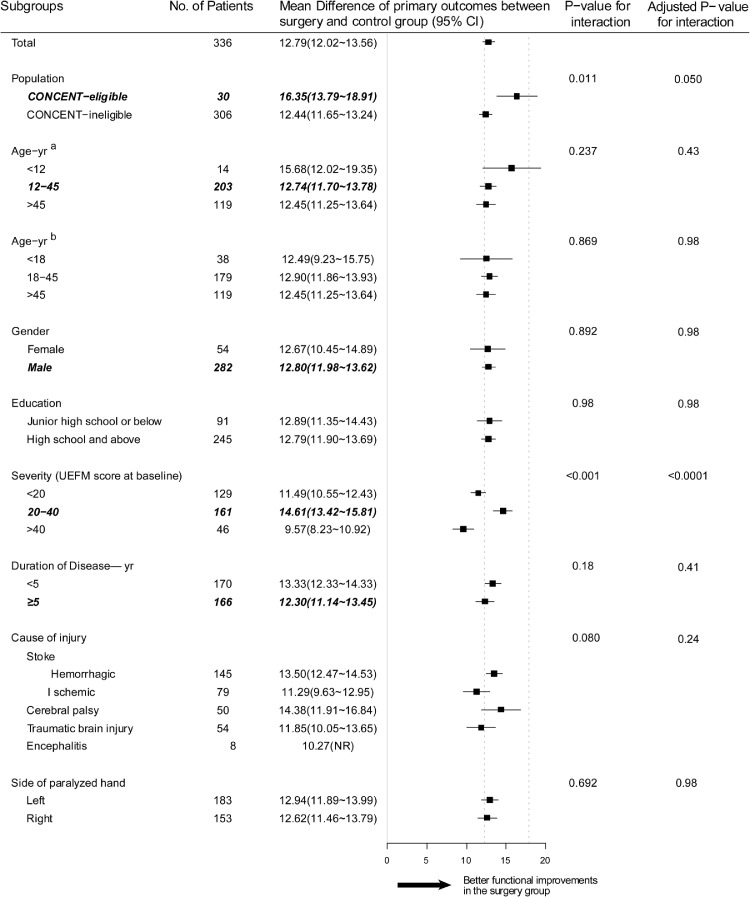
In the subgroup analysis, a more pronounced difference in UEFM score changes was seen in the CONCENT-eligible patients comparing to CONCENT-ineligible patients (16.35 vs 12.44) between the surgery and rehabilitation group. With regard to the four factors related to the criteria set in CONCENT, no statistically significant differences were seen in subgroup analyses on the factors of age, sex and duration of disease (Figure 2). In subgroups of severity at baseline, the CONCENT-eligible subgroup (20–40 points) acquired statistically larger improvement comparing to CONCENT-ineligible subgroups (below 20 points; over 40 points). However, the mean difference did not exceed the minimal clinical important difference (MCID) for the upper extremity of 5.25 points in chronic stroke.23
Figure 2.
Forest plot presenting differences in changes of UEFM score for each subgroup between matched cohorts of surgery and rehabilitation groups.
Subgroup analyses show that the patients who acquired a larger increase were in the subgroup of patients with a UEFM score of 20–40 points at baseline compared with subgroups with UEFM scores below 20 or over 40, while subgroups for different sex, education, and paralyzed side had similar functional improvements. Italic bold text represents subgroups concordant with the CONCENT criteria. Areas between dotted lines indicate the confidence intervals of the differences in the UEFM score changes between the surgery and rehabilitation groups in the CONCENT study. a: Age subgroup divided according to CONCENT-eligible or CONCENT-ineligible. b. Age subgroup divided according to adulthood.
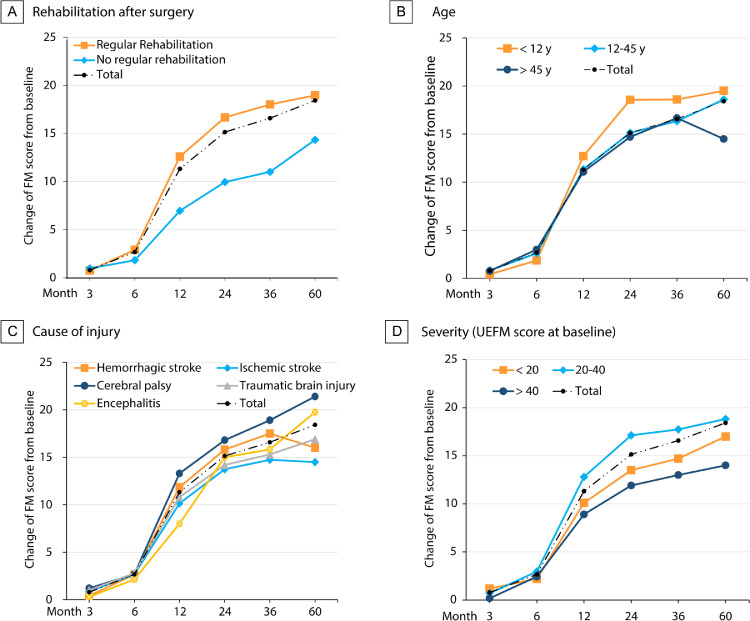
In the surgery group, 39 patients needed nerve graft because of inadequate length of the contralateral C7 nerve. The average length of grafted nerve was 1.81 (range, 1–3) centimeters. No significant differences in the change of UEFM scores were seen between the patients who needed nerve graft and those who received direct coaptation. With regard to post-operative rehabilitation, patients who received rehabilitation for 6 months or longer after surgery acquired larger increase in UEFM score from baseline to 2-year follow-up (mean 16.66, 95%CI: 15.95–17.38), compared with patients who did not (mean 9.95, 95%CI: 9.03-10.86. Figure S1).
Longitudinal data
The changes of UEFM score in patients of the surgery group and each subgroup were shown in Figure 3. At each follow-up time point, the number of patients participated were 168 (baseline), 131 (month 3), 126 (month 6), 165(year 1), 168(year 2), 64(year 3, 62 for the range of motion test), and 26 (year 5). In the surgery group, the UEFM score increased significantly within 2 years and became stable between 2 and 5 years after surgery. The change in UEFM score from baseline to the 3-year follow-up was 16.58 (95% CI: 15.33–17.82), and to the 5-year follow-up was 18.42 (95% CI: 16.49–20.35). The largest increase was seen from 6 to 12 months (8.96, 95% CI: 8.07–9.85), followed by from 12 to 24 months after surgery (3.81, 95% CI: 3.27–4.34). In the rehabilitation subgroup, patients who received rehabilitation after surgery acquired the largest UEFM score increase from month 6 to month 12 (9.89, 95% CI: 8.85–10.92), compared with patients who did not receive rehabilitation (5.63, 95% CI: 4.61–6.65, Table S3).
Figure 3.
Longitudinal data of change of UEFM score in the surgery group.
The overall trend is shown as a dotted-dashed line in each panel. Subgroups are shown as different lines including subgroups of (A) rehabilitation after surgery, (B) age according to CONCENT criteria, (C) cause of injury and (D) severity of disease. Rehabilitation after surgery was judged according to whether the patients received regular rehabilitation for at least 6 months after surgery. Severity of disease was determined by the UEFM score at baseline. The numbers of patients in each subgroup who participated in the follow-up are listed in Table S3 in the supplement.
The changes of MAS score which reflects the spasticity of each joint were shown in Figure S4 and table S3. The spasticity was significantly lower at month 3 comparing to baseline, and regained at month 6. Since the follow-up at year 1, the spasticity of the five joints were gradually decreased at each time-point.
Safety
No severe complication or disabling sequela adverse events were reported in the surgery and control group. Among the adverse events, pain on the shoulder, back or limb was most common discomfort (in 98 patients, 58%) within 1 month after the surgery, while most of them disappeared within 6 months (the pain sustained for more than six months in 1 patient and disappeared at 1-year follow-up). 194 events of numbness and increase of tactile threshold on the intact hand were reported within 1 month, and all absent at month 6. 244 events of changes in muscle strength on the intact side was report, while 190 were reported on the affected side within 1 month, decrease in muscle strength on the intact side still presented in 8 patients at month 6, but was absent at the 1-year follow-up (see Table S5; Table S4 and Figure S3 in supplement).
Sensitivity analysis
In the Chinese population, the difference in mean UEFM score changes from baseline to the 2-year follow-up between surgery and rehabilitation groups was 13.19 (95% CI: 12.48–13.89). We also applied a propensity score weighting model and acquired a difference of 12.59 (95% CI: 11.89–13.29) in all 425 eligible patients. In addition to the propensity-score weighting model, we also applied a multivariate model to verify the results, and acquired mean UEFM score differences of 12.61 (95% CI: 11.86–13.35) from baseline to the 2-year follow-up between surgery and rehabilitation groups (Table S6).
Discussion
In this study, patients were mostly CONCENT-ineligible and represented a large percentage of the hemiplegia population. Overall, patients acquired substantial functional improvements and decreases in spasticity of the paralyzed arm over a 2-year follow-up period following CC7 cross transfer compared with patients who received rehabilitation only. These improvements on the functional of the paralyzed arm were stable in patients who participated in 5-year follow-up. While temporal discomfort and weakness of muscles in the intact arm were seen, which was mainly caused by neurotomy of the C7 nerve root, no severe complication or disabling sequela were seen. These results gave confidence to the safety and effectiveness of applying CC7 cross transfer in real-world practice.
Subgroup analysis (Figure 2) provided evidence for applying the CC7 cross transfer surgery to patients with older age, shorter duration of hemiplegia and severely disabled patients. In terms of age, functional improvements in patients aged over 45 years old were close to those of patients aged below 45, and no severe complications occurred. While the incidence of stroke doubles for each decade after age 55,24, 25 these results provide confidence for applying this surgery in older patients. For severity of paralysis, patients with a UEFM score below 20 points or even below 10 points who were considered to have a poor potential for recovery26, 27, 28 showed substantial improvements in function and decrease in spasticity. Patients with a better baseline functional state (UEFM over 40 points) had a modest functional improvement compared with other subgroups, which may be attributed to the “ceiling effect”, while their self-reported outcomes indicated that they acquired improvements in a practical way (Table S2). In terms of duration, patients who were paralyzed for less than 5 years acquired quantitatively but not statistically increase on UEFM score compared with patients who were paralyzed for more than 5 years. This favors the concept of early intervention that CC7 cross transfer surgery could be performed as soon as functional recovery reaches a plateau after the onset of disease. Considering there may be a country-wise difference, we performed sensitivity analysis by leaving the Korean population out. The results showed that leaving the Korean population out or not, the primary outcome is consistent (Table S6).
Longitudinal follow-up provided useful information as to whether the functional improvements were caused by neurotomy of C7 on the paralyzed side or by the reinnervation of the transferred C7 from the intact side. Neurotomy of C7 mostly led to decreased spasticity, which occurred shortly after surgery. Although stable decreases in spasticity are commonly seen after dorsal rhizotomy of the brachial plexus, concerns of spasticity recurrence remain.29,30 Moreover, neurotomy of a single nerve is unlikely to compare to the effect of dorsal rhizotomy in which three or more roots are transected to decrease spasticity of the whole arm.31 Generally, regeneration of the donor C7 nerve would take 1 to 1.5 years to reach the forearm, but the recipient C7 nerve can give out long branches and innervate muscles at the level of the shoulder and elbow.32,33 Therefore, the increased UEFM scores were more likely to be caused by regeneration of the transferred C7 nerve root from the intact side, since larger increase were seen at 1-year follow-up and 2-year follow-up compared with 6-month follow-up.
The subgroup analysis provided useful information for the guidance of patient inclusion, anticipatory management of functional outcomes for different patients, and confidence for long-term stability of functional improvements. The inclusion criteria are expanded on aspects of age, duration of paralysis and severity of paralysis. The importance of rehabilitation is also emphasized after the surgery, which could largely affect the final improvements. These findings are useful since it represents outcomes from real-world practice in different centers, which provided essential information for clinical practice in daily work.
The major limitation of the present study was the retrospective design, which is associated with selection bias and hidden confounders, even after close matching of main characteristics; therefore, the results should be interpreted with caution. Randomized controlled clinical trials recruiting patients receiving CC7 nerve cross transfer matched with patients receiving sham C7 transection surgery alone are required, but are difficult to perform because of ethical concerns. Patients of young age, with severe comorbidities, or low baseline function are unsuitable to be included in clinical trials, but they also require improvement to hand function through surgery. Accordingly, in the absence of randomized clinical trials, our propensity-score matched analysis of a robust series with durable follow-up provides evidence of the safety, effectiveness, and stability of CC7 cross transfer for hemiplegia after cerebral injury.
In conclusion, CC7 nerve cross transfer surgery can provide effective, safe and stable functional improvements for unilateral spastic arm paralysis patients aged 4–69 years old, male or female and with chronic cerebral injuries sustained at least 1 year previously from stroke, traumatic brain injury, or cerebral palsy.
Declaration of interests
We declare that all authors have no other funding, financial relationships, or conflicts of interest to disclose.
Acknowledgments
Contributors
WD Xu, JG Xu, YD Gu participated in the concept and design of the study. JT Feng, Tie Li, SS Kim, JH Shin, QZ Chen, YP Gong, YC Sun, ZX Zhao, Ning Zhu, JH Cao, Wen Fang, Bin Chen, Song Zhen, Zhu Xu, Xin Jin, YD Shen, YQ Qiu, Su Jiang, HW Yin, Ying Ying, Jie Li, Jie Jia, Ying Liu participated in the data collection and preparation, and were responsible for the raw data associated with this study. WD Xu, MZ Lv, NQ Zhao, JT Feng, Tie Li, YD Shen, Chuantao Zuo, Jie Jia, JG Xu, YD Gu participated in the data analysis and interpretation. WD Xu, JT Feng, Tie Li, MZ Lv, NQ Zhao drafted the article. All of the authors critically revised the article and reviewed the submitted version of the manuscript. WD Xu approved the final version of the manuscript and decided the submission on behalf of all the authors.
Data sharing statement
The data used in this study would be provided upon reasonable request to the corresponding author after publication.
Funding
This study was funded by the National Natural Science Foundation of China (82021002, 81830063 and 82072539), Shanghai Clinical Center for limb function reconstruction project (2017ZZ01006), National Key R&D Program of China (2017YFC0840100 and 2017YFC0840106), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101258.
Appendix. Supplementary materials
Reference
- 1.Willeit P., Toell T., Boehme C., et al. STROKE-CARD care to prevent cardiovascular events and improve quality of life after acute ischaemic stroke or TIA: a randomised clinical trial. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauser K., Burke J.F., Reeves M.J., Barsan W.G., Levine D.A. A systematic review and critical appraisal of quality measures for the emergency care of acute ischemic stroke. Ann Emerg Med. 2014;64(3):235–244. doi: 10.1016/j.annemergmed.2014.01.034. e235. [DOI] [PubMed] [Google Scholar]
- 3.Zhou M., Wang H., Zeng X., et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobkin B.H. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352(16):1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsu A.P., Abrams G.M., Byl NN. Poststroke upper limb recovery. Semin Neurol. 2014;34(5):485–495. doi: 10.1055/s-0034-1396002. [DOI] [PubMed] [Google Scholar]
- 6.Langhorne P., Coupar F., Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 7.Raghavan P. Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am. 2015;26(4):599–610. doi: 10.1016/j.pmr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winstein C.J., Wolf S.L., Dromerick A.W., et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA. 2016;315(6):571–581. doi: 10.1001/jama.2016.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollock A., St George B., Fenton M., Firkins L. Top 10 research priorities relating to life after stroke–consensus from stroke survivors, caregivers, and health professionals. Int J Stroke. 2014;9(3):313–320. doi: 10.1111/j.1747-4949.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 10.Ropper A.H. Two centuries of neurology and psychiatry in the Journal. N Engl J Med. 2012;367(1):58–65. doi: 10.1056/NEJMra1104781. [DOI] [PubMed] [Google Scholar]
- 11.Küpper H., Kudernatsch M., Pieper T., et al. Predicting hand function after hemidisconnection. Brain. 2016;139(Pt 9):2456–2468. doi: 10.1093/brain/aww170. a journal of neurology. [DOI] [PubMed] [Google Scholar]
- 12.Holloway V., Gadian D.G., Vargha-Khadem F., Porter D.A., Boyd S.G., Connelly A. The reorganization of sensorimotor function in children after hemispherectomy. A functional MRI and somatosensory evoked potential study. Brain. 2000;123(Pt 12):2432–2444. doi: 10.1093/brain/123.12.2432. a journal of neurology. [DOI] [PubMed] [Google Scholar]
- 13.McPherson J.G., Chen A., Ellis M.D., Yao J., Heckman C.J., Dewald J.P.A. Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J Physiol. 2018;596(7):1211–1225. doi: 10.1113/JP274968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva C.C., Silva A., Sousa A., et al. Co-activation of upper limb muscles during reaching in post-stroke subjects: an analysis of the contralesional and ipsilesional limbs. J Electromyogr Kinesiol. 2014;24(5):731–738. doi: 10.1016/j.jelekin.2014.04.011. official journal of the International Society of Electrophysiological Kinesiology. [DOI] [PubMed] [Google Scholar]
- 15.Zheng M.X., Hua X.Y., Feng J.T., et al. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med. 2018;378(1):22–34. doi: 10.1056/NEJMoa1615208. [DOI] [PubMed] [Google Scholar]
- 16.Xu W.D., Hua X.Y., Zheng M.X., Xu J.G., Gu YD. Contralateral C7 nerve root transfer in treatment of cerebral palsy in a child: case report. Microsurgery. 2011;31(5):404–408. doi: 10.1002/micr.20877. [DOI] [PubMed] [Google Scholar]
- 17.Hua X.Y., Qiu Y.Q., Li T., et al. Contralateral peripheral neurotization for hemiplegic upper extremity after central neurologic injury. Neurosurgery. 2015;76(2):187–195. doi: 10.1227/NEU.0000000000000590. discussion 195. [DOI] [PubMed] [Google Scholar]
- 18.Xu W.D. Surgical technique of Xu’s CC7 procedure “contralateral C7 to C7 cross nerve transfer through a trans longus colli, prespinal route for treating spastic arm”. Oper Neurosurg. 2020;20(1):61–68. doi: 10.1093/ons/opaa325. (Hagerstown) [DOI] [PubMed] [Google Scholar]
- 19.Gladstone D.J., Danells C.J., Black S.E. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabilitation Neural Repair. 2002;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 20.Fugl-Meyer A.R., Jaasko L., Leyman I., Olsson S., Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 21.Austin P.C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 22.Haukoos J.S., Lewis R.J. The propensity score. JAMA. 2015;314(15):1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page S.J., Fulk G.D., Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 24.Boehme A.K., Esenwa C., Elkind M.S.V. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thrift A.G., Thayabaranathan T., Howard G., et al. Global stroke statistics. Int J Stroke. 2017;12(1):13–32. doi: 10.1177/1747493016676285. [DOI] [PubMed] [Google Scholar]
- 26.Pollock A., Farmer S.E., Brady M.C., et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD010820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo A.C., Guarino P.D., Richards L.G., et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward H.O., McIldowie B., Kibble S., Squire A., Carson-Stevens A. Rehabilitation after stroke. Financial implications for survivors of stroke. BMJ. 2013;347:f4999. doi: 10.1136/bmj.f4999. (Clinical research ed) [DOI] [PubMed] [Google Scholar]
- 29.Bertelli J.A., Ghizoni M.F., Michels A. Brachial plexus dorsal rhizotomy in the treatment of upper-limb spasticity. J Neurosurg. 2000;93(1):26–32. doi: 10.3171/jns.2000.93.1.0026. [DOI] [PubMed] [Google Scholar]
- 30.Guenot M., Lee J.W., Nasirinezhad F., Sagen J. Deafferentation pain resulting from cervical posterior rhizotomy is alleviated by chromaffin cell transplants into the rat spinal subarachnoid space. Neurosurgery. 2007;60(5):919–925. doi: 10.1227/01.NEU.0000255435.29118.3D. discussion 919-925. [DOI] [PubMed] [Google Scholar]
- 31.Lin H., Hou C., Chen A., Xu Z. Long-term outcome of division of the C8 nerve root for spasticity of the hand in cerebral palsy. J Hand Surg. 2010;35(7):558–562. doi: 10.1177/1753193410368200. European volume. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y., Xu J., Chen L., Wang H., Hu S. Long term outcome of contralateral C7 transfer: a report of 32 cases. Chin Med J. 2002;115(6):866–868. (Engl) [PubMed] [Google Scholar]
- 33.Yang G., Chang K.W., Chung KC. A systematic review of outcomes of contralateral C7 transfer for the treatment of traumatic brachial plexus injury: part 2. donor-site morbidity. Plast Reconstr Surg. 2015;136(4):480e–489e. doi: 10.1097/PRS.0000000000001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.