Summary
Background
Air pollution is a risk factor for poor cognitive function, while a plant-based dietary pattern is associated with better cognitive function. We aimed to explore their interaction with cognitive function among older adults.
Methods
We used a prospective cohort of old individuals, including 6525 participants of the Chinese Longitudinal Healthy Longevity Survey (CLHLS), aged 65-110 years and with normal cognition at baseline. Air pollution measurement was derived using satellite-derived annual average fine particulate matter (PM2.5) concentrations based on residential locations. Plant-based diet index (PDI) was calculated using survey responses to assess the dietary pattern. Repeated measures of the Mini-Mental State Examination (MMSE) were utilized to assess cognitive function. We applied the Cox proportional hazard regression to explore the associations and further stratified the analysis by PDI.
Findings
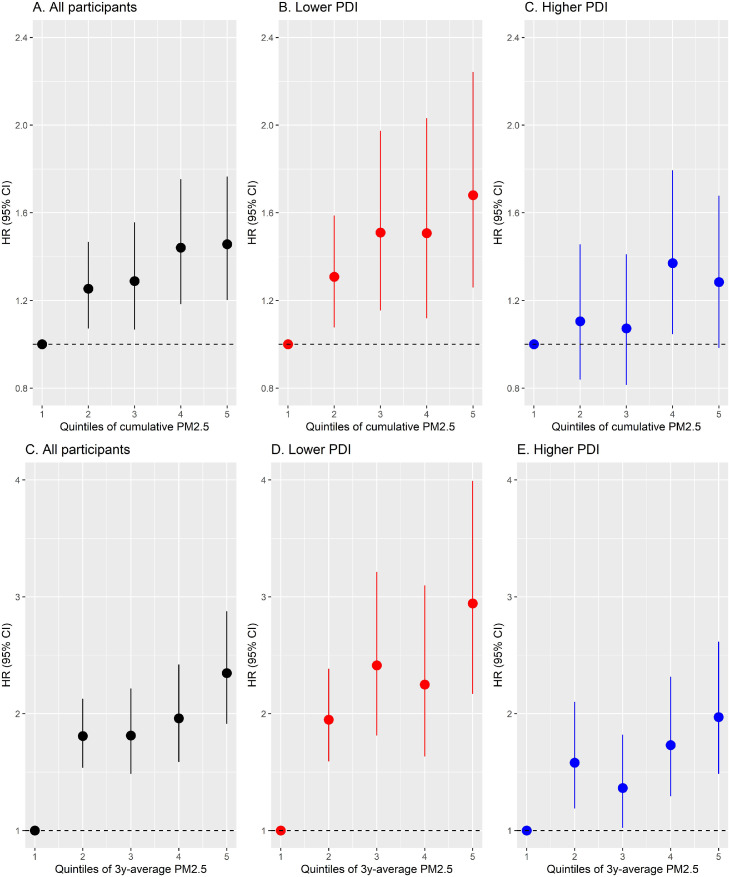
During a median of 5·6-year follow-up, 1537 (23·6%) out of 6525 participants with normal cognition at baseline developed poor cognitive function (MMSE <18). Living in areas with the highest quintile of cumulative PM2.5 was associated with a 46% increase in the risk of developing poor cognitive function (hazard ratio (HR): 1·46, 95% confidence interval (CI): 1·20, 1·77), compared to those living in areas with the lowest quintile. We observed a significant interaction between cumulative PM2.5 and PDI (p-interaction: 0·04), with the corresponding associations of cumulative PM2.5 being more pronounced among participants with lower PDI (HR: 1·68, 95% CI: 1·26, 2·24) than those with higher PDI (HR: 1·28, 95% CI: 0·98, 1·68).
Interpretation
Plant-based dietary pattern may attenuate detrimental impacts of PM2.5 on cognitive function among older adults. Adherence to the plant-based dietary pattern could be used to prevent adverse neurological effects caused by air pollution, especially in developing regions.
Keywords: Cognitive function, Air pollution, Plant-based dietary pattern, Healthy longevity
Research in context.
Evidence before this study
We searched PubMed, Web of Science, and Google Scholar for the studies on air pollution, plant-based dietary patterns, and cognitive function, published in English up to September 1st, 2021. We used a combination of search terms, including “air pollution”, “fine particulate matter”, “PM2.5”, “plant-based dietary pattern”, “cognition”, and “cognitive function”. We found consistent evidence on higher levels of air pollution and worse cognitive function from numerous observational studies in different places such as North America, Europe, and Asia. Adherence to the plant-based dietary pattern was associated with better cognitive function, observed in most observational studies. There was only one published abstract of a doctoral dissertation on Mediterranean-like diet, air pollution, and cognitive function. This cross-sectional study of women aged 54-55 years old from Germany reported that those with a lower Mediterranean diet score were more vulnerable to cognitive decline induced by air pollution, although the interaction between air pollution and the Mediterranean diet was not significant.
Added value of this study
To the best of our knowledge, this is the first epidemiological study exploring the interaction between air pollution and plant-based dietary pattern on cognitive function among older adults with normal cognition. Our study found that the effects of long-term exposure to PM2.5 on the risks of developing poor cognitive function were stronger among older adults with lower adherence to the plant-based dietary pattern, compared to those with higher adherence.
Implications of all the available evidence
Our findings suggest higher adherence to the plant-based dietary pattern may be beneficial to poor cognitive function induced by long-term PM2.5 exposure. Promoting the plant-based dietary pattern may be a strategy to reduce the effects of PM2.5 on neurological health.
Alt-text: Unlabelled box
Background
Incidences of dementia are growing worldwide because of increased life expectancy, with 50 million dementia cases and counting. The case number is projected to increase to 152 million by 2050. A recent 2020 Lancet Commission on dementia prevention, intervention, and care identified air pollution as one of 12 modifiable risk factors that could prevent or delay dementia.1 Estimates on the contribution of air pollution on dementia are rapidly emerging, although it is not yet formally recognized as a risk factor outcome pair in the Global Burden of Disease Study, or the new WHO Air Quality Guideline 2021. However, estimates suggest about 2·1 million incident dementia cases could be attributable to ambient exposure to fine particulate matter (PM2.5) pollution in 2015.2 Air pollutants could directly elicit inflammatory changes and oxidative stress in the brain and increase the risk of cardiometabolic diseases, ultimately increasing the risks of dementia and cognitive decline.3
Plant foods, such as vegetables and fruits, often rich in nutrients including polyphenols, flavonoids, antioxidant vitamins, could reduce inflammation and oxidative stress in the central nervous system.4, 5, 6, 7 Evolving evidence showed that the plant-based dietary pattern might be associated with better neurological health.8,9 The Singapore Chinese Health Study of 16,948 men and women reported that plant-based dietary patterns in middle life were associated with lower risks of cognitive impairment in late life.10 Another cohort of 12,062 participants from Taiwan found that vegetarians had a 38% lower risk of dementia compared with non-vegetarians.11 Nutritional solution or intervention for air pollution induced cardiopulmonary was introduced in several studies. These findings suggested plant-based food with higher antioxidants could reduce oxidative stress and inflammation in cardiovascular disease and other chronic inflammatory diseases induced by air pollution.12, 13, 14 Currently, no studies have looked at the plant-based dietary pattern and the association between air pollution and cognitive decline.
Given the positive association between plant-based dietary patterns and cognitive function and the opposite association of air pollution and cognitive function, it is plausible to hypothesize that it may modify the association between air pollution and cognitive function. Our study aimed to test this hypothesis and add additional dimensions to food, air pollution, and dementia.
Methods
Study population
Initiated in 1998, the CLHLS aimed to study determinants of healthy longevity. The CLHLS has a nationally representative sample, with participants recruited from 22 provinces in China. The CLHLS applied a multistage, stratified cluster sampling in 631 randomly selected cities and counties where the Han Chinese are the largest majority. These sample sites represent about 85% of the Chinese population. A more detailed description of the sampling design can be found elsewhere.15
Our study used the 2008 wave of the CLHLS. We excluded participants who were younger than 65 years old since age group of 65 and over had a vast majority of dementia cases, had missing values in the dietary pattern (less than 2%) and covariates, and with poor cognitive function at baseline (Mini-Mental State Examination (MMSE) <18). In total, 6525 participants were included in the analysis. More details on participant inclusion and exclusion can be found in Figure S1.
PM2.5 exposure assessment
Based on participants’ residential addresses, we calculated the ground-level concentrations of PM2.5 from the Atmospheric Composition Analysis Group. It combined the remote sensing from National Aeronautics and Space Administration's Moderate Resolution Imaging Spectroradiometer, Multiangle Imaging SpectroRadiometer, and Sea-viewing Wide Field-of-view Sensor satellite instruments, vertical profiles derived from the GEOS-Chem chemical transport model, and calibration to ground-based observations of PM2.5 using geographically weighted regression.16 We calculated annual PM2.5 from 1998 to 2014, at 1 km2 spatial resolution, which was the longest and the highest resolution exposure dataset available in China.17 Additionally, our estimations were highly consistent with cross-validated concentrations from monitors (R2=0·81) and another exposure dataset (R2=0·81) in China.16,17
We calculated two exposure measures to reflect long-term exposure for each participant. We averaged the estimated PM2.5 concentrations from recruitment to a diagnosis of poor cognitive function (MMSE <18), death, loss to follow-up, or the end of follow-up (September 2014) to indicate cumulative exposure. We also averaged the estimated exposures for three years prior to a diagnosis of poor cognitive function, death, loss to follow-up, or the end of follow-up (September 2014) to indicate 3-year average exposure, which suggested recent accumulated exposure levels.
Cognitive function assessment
We used the Mini-Mental State Examination (MMSE) to measure cognitive function, adapted to the Chinese language. The MMSE in the CLHLS has been validated for reliability in prior findings.18,19 The reliability of the MMSE scale is high (Cronbach's a=0·96).20 MMSE assesses cognitive function in five dimensions, including orientation, registration, attention and calculation, recall, and language.21 We scored each question as zero (wrong or unable to answer) or one (correct),22 and the score ranged from 0 to 30. Higher scores indicated better cognitive function.23 We defined MMSE scores to ≥18 as normal cognitive function and <18 as poor cognitive function. This cut-off point was typically used in other prior research studies.19,23,24 We also used the MMSE score as a continuous variable in a secondary analysis.
Dietary assessment
We evaluated the plant-based dietary pattern by constructing the plant-based diet index (PDI), an adapted approach used by Satija et al.25 We included 16 food groups for the assessment, using dietary data collected by a simplified food frequency questionnaire. The included food groups covered the most common food consumed in the daily diet in China. The plant food included whole/refined grain, vegetable oil, fresh fruit, fresh vegetable, legume, garlic, nut, tea, salt-preserved vegetable, and sugar. The animal food included animal fat, dairy product, egg, fish, and meat.
We scored the PDI according to intake frequency. Although servings or quintiles of intake are commonly used, using a non-quantitative food frequency questionnaire to assess dietary patterns has been demonstrated to be reliable and valid in some studies.26, 27, 28 In addition, previous studies also showed that frequency of intake is more important than portion size to distinguish between high and low consumption of fruits and vegetables.29 The CLHLS recorded the intake frequency as “almost everyday” or “≥1 time/week” or “≥1 time/month” or “occasionally” or “rarely or never” for most food groups, including legume, garlic, nut, tea, salt-preserved vegetables, sugar, eggs, fish, meat, and dairy products. The CLHLS recorded the intake frequency of fruits and fresh vegetables as “almost everyday” or “quite often” or “occasionally” or “rarely or never”. We scored 5 for the most frequent consumption, and 1 for the least frequent consumption of plant food (positive scores). We scored 1 for the most frequent consumption, and 5 for the least frequent consumption of animal food (reverse scores). We scored 5 for the consumption of whole grain, vegetable oil, and refined grain, and scored 1 for the consumption of animal fat. More details on constructing and scoring PDI can be found in Table S1. PDI score ranges from 16 to 80 theoretically. Higher PDI scores indicated more frequent consumption of plant food. We first ranked the PDI scores of all participants and then divided them into two half according the median level. Those with lower half scores were defined as lower PDI. Those with higher half scores were defined as higher PDI.
Covariates
We assessed demographic characteristics, socioeconomic status, health behaviour, and health status. The covariates included age (years), sex (male or female), urban/rural residence, education (with or without formal education), main occupation before age 60 (professional work like professional and technical personnel, government, and management or non-professional work like agriculture, fishing, service, industry, and housework), financial status (financial independence or dependence), regular exercise (yes or no), and geographic regions (Central China, Eastern China, Northeastern China, Northern China, Northwestern China, Southern China, Southwestern China). We calculated the social and leisure activity index by taking into consideration seven activities: gardening, personal outdoor activities (like square dancing and Tai Chi), raising poultry or pets, reading, playing cards or mah-jong, listening to the radio or watching TV, and participating in organized social activities.30 Each activity was scored as zero (no) or one (yes), and the index ranged from zero to seven. We defined never smokers as those who neither smoked in the past nor at the time of the interview, former smokers as those who smoked in the past but not at the time of the interview, and current smokers as those who smoked at the time of interview. We defined never, former, and current drinkers using a similar evaluation.
Statistical analysis
We summarized the baseline characteristics using descriptive statistics. We used the Cox proportional hazard regression to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between long-term PM2.5 exposure and risks of developing poor cognitive function (MMSE <18) during the follow-up. We stratified the analysis by PDI scores and individual food groups. We plotted the stratified analysis to see whether the associations differed by PDI. We also plotted three knots cubic splines to explore non-linearity between PM2.5 exposure and risks of developing poor cognitive function. Wald test was used to assess whether the observed relationships were linear or nonlinear. We further stratified the associations of PM2.5 exposure, PDI, and cognitive function by sex. The regression models were multivariable-adjusted for age, sex, urban/rural residence, education, main occupation before age 60, financial status, social and leisure activity, smoking and drinking status, regular exercise, and geographic regions. We conducted extra sensitivity analysis to explore the potential bias. Given the relatively old age among our participants, there may be bias caused by competing risk from death. Therefore, we applied the competing risk model as a sensitivity analysis. In addition, we conducted another sensitivity analysis to have the regression models additionally adjusted for intake of vitamin A/C/E supplements, body mass index (BMI), and health status of five cardiometabolic diseases (hypertension, diabetes, heart disease, cerebrovascular disease, and dyslipidemia). Cardiometabolic diseases were the risk factors of poor cognitive function.31 We also updated the regressional models by adjusting for baseline MMSE score. Furthermore, we applied MMSE scores as a continuous variable in the generalized estimating equation model to look at changes in the score as an outcome variable. Since education could significantly influence MMSE score, we applied education-specific cut-off points to categorize MMSE score.10 Specifically, we used the MMSE score of 18, 20, and 24 as the cut-off points for the participants without formal education, primary school education (1-6 years), and secondary school or higher education (>6 years), respectively. Lastly, we examined the associations between PM2.5 exposure, PDI, and changes in cognitive function by using ordinary logistic regression. All statistical analysis was conducted by using R software (version: 3·6·2, R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined by p<0·05 in two-sided testing.
Ethical approval
The study protocol was approved by the Institutional Review Board, Duke University (Pro00062871), and the Biomedical Ethics Committee, Peking University (IRB00001052-13074). Paper-based informed consent was signed and collected from all participants.
Role of the funding sources
The funders of the study had no role in study design; collection, analysis, and interpretation of data; or manuscript drafting. The corresponding author had full access to the data and had full responsibility for the final submission of the manuscript.
Results
Table 1 presented the baseline characteristics of 6525 CLHLS participants with normal cognitive function at baseline. Their mean age was 81 (standard deviation (SD): 10·8) years old, 50·8% were females, 17·2% were urban residents, and 48·0% had formal education. About 21·8% and 20·7% were current smokers and drinkers. Mean BMI was 20·9 (SD: 3·5) kg/m2. Cumulative PM2.5 during the follow-up period ranged from 9 to 106 μg/m3, and the mean value was 49·3 μg/m3. The mean PDI was 49·6. PDI score was slightly higher among the participants living in the areas with a higher level of PM2.5. There were no distinguished changes in participants’ characteristics during the follow-up surveys (see Table S2).
Table 1.
Baseline characteristics of participants by quintiles of cumulative PM2.5.
| Characteristics | Total | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-value |
|---|---|---|---|---|---|---|---|
| N | 6525 | 1305 | 1305 | 1305 | 1305 | 1305 | |
| Cumulative PM2.5 (μg/m3)* | 49·3 (13·8) | 31·0 (6·4) | 41·2 (1·9) | 48·8 (2·4) | 56·8 (2·9) | 69·0 (7·0) | <0·001 |
| PDI score* | 49·6 (6·1) | 47·3 (6·9) | 47·8 (6·0) | 50·4 (5·5) | 51·5 (5·3) | 51·3 (5·1) | <0·001 |
| PDI score (range) | 25-70 | 25-67 | 26-64 | 32-70 | 28-68 | 35-66 | |
| Age, years* | 80·8 (10·8) | 81.4 (10·1) | 81·9 (10·0) | 79·6 (9·6) | 80·1 (9·8) | 80·8 (10·7) | <0·001 |
| Sex, females | 3317 (50·8) | 656 (50·2) | 681 (52·2) | 640 (49·0) | 655 (50·2) | 685 (52·5) | 0·338 |
| Urban residence | 1125 (17·2) | 117 (9·0) | 124 (9·5) | 302 (23·1) | 271 (20·8) | 311 (23·8) | <0.001 |
| With formal education | 3132 (48·0) | 573 (43·9) | 679 (52·0) | 689 (52·8) | 629 (48·2) | 562 (43·1) | <0·001 |
| With professional work | 578 (8·9) | 107 (8·2) | 91 (7·0) | 112 (8·6) | 122 (9·3) | 146 (11·2) | 0·003 |
| Financial independence | 2296 (35·2) | 376 (28·8) | 360 (27·6) | 503 (38·5) | 535 (41·0) | 522 (40·0) | <0·001 |
| Social and leisure activity index* | 2.7 (1·4) | 2.8 (1·5) | 2.7 (1·4) | 2.8 (1·4) | 2.7 (1·5) | 2.6 (1·5) | 0·027 |
| With regular exercise | 2318 (35·5) | 464 (35·6) | 477 (36·6) | 497 (38·1) | 400 (30·7) | 480 (36·8) | 0·001 |
| Smoking status | <0·001 | ||||||
| Never smoker | 4070 (62·4) | 877 (67·2) | 882 (67·6) | 721 (55·2) | 807 (61·8) | 783 (60·0) | |
| Former smoker | 1031 (15·8) | 203 (15·6) | 165 (12·6) | 245 (18·8) | 190 (14·6) | 228 (17·5) | |
| Current smoker | 1424 (21·8) | 225 (17·2) | 258 (19·8) | 339 (26·0) | 308 (23·6) | 294 (22·5) | |
| Alcohol consumption | 0·001 | ||||||
| Never drinker | 4293 (65·8) | 872 (66·8) | 880 (67·4) | 824 (63·2) | 835 (64·0) | 882 (67·6) | |
| Former drinker | 878 (13·5) | 207 (15·9) | 159 (12·2) | 188 (14·4) | 167 (12·8) | 157 (12·0) | |
| Current drinker | 1354 (20·7) | 226 (17·3) | 266 (20·4) | 293 (22·4) | 303 (23·2) | 266 (20·4) | |
| Geographic region | <0·001 | ||||||
| Central China | 1007 (15·4) | 27 (2·1) | 136 (10·4) | 163 (12·5) | 332 (25·4) | 349 (26·7) | |
| East China | 2442 (37·4) | 323 (24·8) | 128 (9·8) | 428 (32·8) | 827 (63·4) | 736 (56·4) | |
| Northeast China | 459 (7·0) | 112 (8.6) | 64 (4·9) | 239 (18·3) | 44 (3·4) | 0 (0) | |
| North China | 290 (4·4) | 21 (1·6) | 26 (2·0) | 30 (2·3) | 6 (0·5) | 207 (15·9) | |
| Northwest China | 74 (1·1) | 31 (2·4) | 8 (0·6) | 2 (0·2) | 26 (2·0) | 7 (0·5) | |
| South China | 1467 (22.5) | 662 (50·7) | 688 (52·7) | 117 (9·0) | 0 (0) | 0 (0) | |
| Southwest China | 786 (12·0) | 129 (9·9) | 255 (19·5) | 326 (25·0) | 70 (5·4) | 6 (0·5) | |
| Body mass index (kg/m2)* | 20·9 (3·5) | 19.8 (3·1) | 20·2 (3·2) | 21.5 (3·5) | 21·3 (3·7) | 21·9 (3·7) | <0·001 |
| Daily intake of vitamin A/C/E supplements | 389 (6.0) | 50 (3·8) | 50 (3·8) | 77 (5·9) | 96 (7.4) | 116 (8·9) | <0·001 |
| Hypertension⁎⁎ | 1445 (22·1) | 246 (18·9) | 234 (17.9) | 257 (19·7) | 314 (24·1) | 394 (30·2) | <0·001 |
| Diabetes⁎⁎ | 189 (2·9) | 24 (1·8) | 28 (2·1) | 37 (2·8) | 42 (3·2) | 58 (4·4) | <0·001 |
| Heart diseases⁎⁎ | 623 (9·5) | 82 (6·3) | 64 (4·9) | 143 (11·0) | 152 (11·6) | 182 (13·9) | <0·001 |
| Cerebrovascular disease⁎⁎ | 344 (5·3) | 53 (4·1) | 37 (2·8) | 77 (5·9) | 71 (5·4) | 106 (8·1) | <0·001 |
| Dyslipidemia⁎⁎ | 114 (1·7) | 16 (1·2) | 14 (1·1) | 19 (1·5) | 24 (1·8) | 41 (3·1) | <0·001 |
Number (%) were reported.
Abbreviations: PDI: plant-based diet index; PM2.5: fine particulate matter.
mean (standard deviation) was reported.
Cases of hypertension, diabetes, heart diseases, cerebrovascular disease, and dyslipidemia were self-reported.
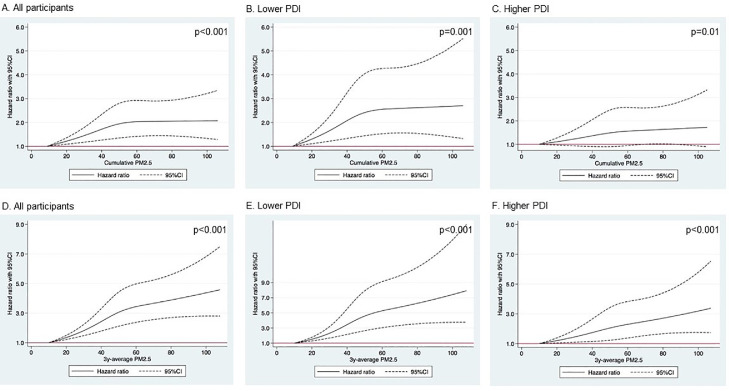
Table 2, Figure 1, and Figure 2 showed the risks of developing poor cognitive function under long-term PM2.5 exposure. During a median of 5·6-year follow-up, 1537 (23·6%) participants with normal cognition at baseline developed poor cognitive function. Living in the areas with the highest quintile of cumulative PM2.5 (range: 62-106 μg/m3) was associated with a 46% increase in the risk of developing poor cognitive function (HRQ5vsQ1 for cumulative PM2.5: 1·46, 95% CI: 1·20, 1·77), compared to those living in the areas with the lowest quintile (range: 9-38 μg/m3). Per 10 μg/m3 increase in cumulative PM2.5 was related to a 10% increase in the risk of developing poor cognitive function (HR for cumulative PM2.5: 1·10, 95% CI: 1·04, 1·15). Their associations were non-linear (Figure 2, p<0.001). We also observed a significant interaction between cumulative PM2.5 and PDI (p-value of interaction: 0·04), with the corresponding associations of cumulative PM2.5 being much more pronounced among participants with lower PDI (HRQ5vsQ1=1·68, 95% CI: 1·26, 2·24), compared to those with higher PDI (HRQ5vsQ1=1·28, 95% CI: 0·98, 1·68). Similar associations and the significant modification by the plant-based dietary pattern were also observed for 3-year average PM2.5. The results of the main analysis were consistent with the results of sensitivity analysis by adjusting for extra covariates (see Table S3), adjusting for baseline MMSE score (see Table S4), using competing risk models (see Table S5), using the MMSE score as a continuous variable (see Table S6), using education-specific cut-off points for MMSE score (see Table S7). The inverse associations between PM2.5 exposure and changes in MMSE scores during the follow-up survey were also consistent with main results (see Table S8). We further stratified the associations of PM2.5, PDI, and cognitive function by sex (see Figure S2). Among the participants with lower PDI, the effect estimates of PM2.5 on the risks of developing poor cognitive function were slightly higher among males than females.
Table 2.
The association between long-term PM2.5 exposure and risks of developing poor cognitive function, stratified by plant-based dietary index.
| N of MMSE<18 | N of risk | Cumulative PM2.5 |
3-year average PM2.5 |
|||
|---|---|---|---|---|---|---|
| Range (μg/m3) | HR (95% CI) | Rang (μg/m3) | HR (95% CI) | |||
| All participants | ||||||
| PM2.5 by quintiles | ||||||
| Quintile 1 | 248 | 1305 | 9-38 | Ref | 10-38 | Ref |
| Quintile 2 | 376 | 1305 | 38-44 | 1·25 (1·07, 1·47) | 38-44 | 1·81 (1·54, 2·13) |
| Quintile 3 | 276 | 1305 | 44-52 | 1·29 (1·07, 1·56) | 44-52 | 1·81 (1·48, 2·22) |
| Quintile 4 | 259 | 1305 | 52-62 | 1·44 (1·18, 1·75) | 52-61 | 1·96 (1·59, 2·42) |
| Quintile 5 | 378 | 1305 | 62-106 | 1·46 (1·20, 1·77) | 61-109 | 2·35 (1·91, 2·88) |
| Per 10 μg/m3 increase in PM2.5 | 1537 | 6525 | ·· | 1·10 (1·04, 1·15) | ·· | 1·19 (1·14, 1·25) |
| Stratified by PDI | ||||||
| Lower PDI | ||||||
| PM2.5 by quintiles | ||||||
| Quintile 1 | 200 | 803 | 9-38 | Ref | 10-38 | Ref |
| Quintile 2 | 236 | 796 | 38-44 | 1·31 (1·08, 1·59) | 38-44 | 1·95 (1·59, 2·38) |
| Quintile 3 | 140 | 583 | 44-52 | 1·51 (1·15, 1·97) | 44-52 | 2·41 (1·81, 3·21) |
| Quintile 4 | 113 | 524 | 52-62 | 1·51 (1·12, 2·03) | 52-61 | 2·25 (1·63, 3·10) |
| Quintile 5 | 172 | 557 | 62-106 | 1·68 (1·26, 2·24) | 61-108 | 2·94 (2·17, 3·99) |
| Per 10 μg/m3 increase in PM2.5 | 861 | 3263 | ·· | 1·13 (1·05, 1·21) | ·· | 1·26 (1·17, 1·35) |
| Higher PDI | ||||||
| By quintiles | ||||||
| Quintile 1 | 105 | 502 | 10-38 | Ref | 10-38 | Ref |
| Quintile 2 | 112 | 509 | 38-44 | 1·11 (0·84, 1·46) | 38-44 | 1·58 (1·19, 2·10) |
| Quintile 3 | 127 | 722 | 44-52 | 1·07 (0·81, 1·41) | 44-52 | 1·36 (1·02, 1·82) |
| Quintile 4 | 155 | 781 | 52-62 | 1·37 (1·05, 1·79) | 52-61 | 1·73 (1·29, 2.31) |
| Quintile 5 | 177 | 748 | 62-105 | 1·28 (0·98, 1·68) | 61-107 | 1·97 (1·48, 2·62) |
| Per 10 μg/m3 increase in PM2.5 | 676 | 3262 | ·· | 1·07 (1·01, 1·14) | ·· | 1·15 (1·07, 1·22) |
The regression models were multivariable-adjusted for age (years), sex (male or female), urban/rural residence, education (with or without formal education), occupation before age 60 (professional or non-professional work), financial status (financial independence or dependence), social and leisure activity, smoking and drinking status (never, former or current smokers/drinkers), regular exercise (yes or no), and geographic regions (Central China, Eastern China, Northeastern China, Northern China, Northwestern China, Southern China, Southwestern China).
Abbreviations: MMSE: Mini-Mental State Examination; PDI: plant-based diet index; PM2.5: fine particulate matter
Figure 1.
Hazard ratios (95% CI) of developing poor cognitive function by quintiles of long-term PM2.5 exposure, stratified by plant-based diet index.
Figure 2.
Cubic splines for long-term PM2.5 exposure and risks of developing poor cognitive function, stratified by plant-based diet index.
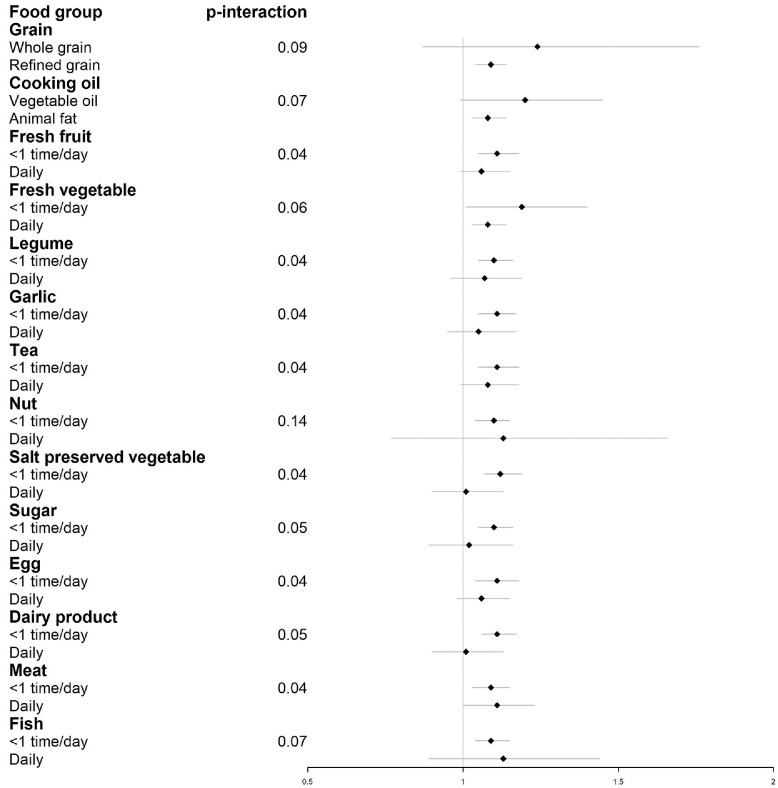
Figure 3 reported the risks of developing poor cognitive function by per 10-μg/m3 increase in cumulative PM2.5 exposure, stratified by individual food group. The risk estimates of cumulative PM2.5 exposure on developing poor cognitive function were lower among the participants with daily intake of fresh fruit, fresh vegetable, legume, garlic, tea, salted preserved vegetable, sugar, egg, dairy product, and less frequent (<1 time/per day) intake of meat and fish. Similar results were also found for 3-year average PM2.5 exposure (see Figure S3).
Figure 3.
Hazard ratios (95% CI) of developing poor cognitive function by per 10-μg/m3 increase in cumulative PM2.5 exposure, stratified by individual food group.
Discussion
In this prospective cohort study of 6525 Chinese older adults with normal cognitive function at baseline, we found living in the areas with the highest quintiles of cumulative PM2.5 was associated with a 46% increase in the risk of developing poor cognitive function. The associations were significantly modified by the plant-based dietary pattern. The association of cumulative PM2.5 with cognitive function was attenuated with a 28% increased risk and marginally significant among those with higher PDI, in contrast with a 68% increase in the risk of developing a poor cognitive function outcome among those with lower PDI. Our findings indicated that higher adherence to the plant-based dietary pattern might be beneficial to poor cognitive function induced by long-term PM2.5 exposure.
There is scarce evidence on whether the association between long-term PM2.5 exposure and cognitive function differed by the plant-based dietary pattern. To the best of our knowledge, there was only one published abstract from a doctoral dissertation on a relevant topic from Germany. The author reported that women with a low Mediterranean diet score were more vulnerable to cognitive decline induced by air pollution, although the interaction between air pollution and the Mediterranean diet was not significant.32 Emerging evidence showed that plant-based dietary pattern was beneficial to other health outcomes associated with PM2.5 exposure. A prospective cohort of 548,845 participants with a follow-up of 17 years in the United States found that those with a higher Mediterranean diet score had significantly lower cardiovascular mortality rates associated with PM2.5 and nitrogen dioxide (NO2) exposures.14 The Northeast Cohort Study of China reported that pregnant women who had a higher intake of animal food had higher risks of gestational diabetes mellitus associated with NO2 and carbon monoxide (CO) exposures.33 A study of 501 children from Portugal showed that the association between PM2.5 and asthma was stronger among those having a pro-inflammatory diet, in contrast with those having an anti-inflammatory diet with more fresh vegetables and fruits, suggesting that inflammatory characteristics of diet may modify the association.34 Potential benefits of the plant-based dietary pattern on various health outcomes induced by air pollution were also reported in other contexts.35,36
Rich antioxidants and anti-inflammatory nutrients from the plant-based dietary pattern may help explain how it could modify the association between long-term PM2.5 exposure and cognitive function. First, the nutrients like unsaturated fatty acids from vegetable oil were related to lower risks of PM2.5 associated cardiometabolic diseases,14,37 through suppressing arrhythmias, modulating autonomic function, and its anti-thrombotic, anti-inflammatory, and vasodilatory effects.13 While cardiometabolic diseases were risk factors of poor cognitive function.38 Additionally, vegetables and fruits have rich nutrients, including polyphenols, antioxidant vitamins, and dietary fiber, which could reduce inflammation and oxidative stress induced by ambient pollutant exposures in the central nervous system,4, 5, 6, 7,39,40 ultimately influencing the pathogenesis of neurodegenerative disorder.41,42 Although many studies showed antioxidant and anti-inflammatory effects of specific foods or nutrients like n-3 fatty acids, antioxidant vitamins, their effects on cognitive function among the non-demented population were controversial in practice.7,43, 44, 45 There is still a lack of mechanistic evidence in the perspective of the plant-based dietary pattern.
The plant-based dietary pattern maybe not only beneficial to air pollution associated health outcomes, but also decrease air pollution produced by agriculture, and relevant disease burden. The global simulation showed that agriculture, largely due to animal farming, is the largest contributing sector to PM2.5 in eastern United States, Russia, East Asia, and Europe.46,47 For instance, food-related PM2.5 pollution caused 15,900 annual deaths in the United States. About 80% of these deaths could be attributable to animal-based food.48 A 50% decrease in agricultural ammonia emissions would reduce annual PM2.5 concentration levels by 11%, 8%, and 5% in Europe, North America, and East Asia respectively.47 Promoting the plant-based dietary pattern at the population level may be a cost-effective strategy for decreasing the relevant burden of diseases induced by air pollution.
In our study, inverse associations between PM2.5 exposure and risks of developing poor cognitive function were stronger among males than females. Sex differences may be due to sex-linked biological differences or gender differences in activity patterns, coexposures, or accuracy of measurement.49 However, the current evidence on sex differences in the air pollution effects is not consistent. For example, a Korean study reported women had a higher risk of cognitive decline associated with PM2.5 exposure than men.50 However, no sex difference was reported in another cohort of older adults in the United States.51
The strengths of our study included using a national-representative sample of older adults with relatively large sample size, reliable PM2.5 exposure, and considering a wide range of confounding variables for adjustment. Our study setting covers diverse geographic regions (22 provinces) in China with average annual PM2.5 levels ranging from 9 to 106 μg/m3. PM2.5 concentrations are generally higher in the northern than southern areas, which could be partly explained by a higher density of coal consumption and heavy industries and favorable atmospheric conditions for accumulation, formation, and processing of aerosols in the northern areas.52 The wide distribution of both PDI and PM2.5 allows us to have heterogeneity which aids the robustness of our findings. Our study also provided evidence from East Asia to a western-centric nutrition research paradigm. Lastly, to our knowledge, our study was the first one of its kind and the novel findings may provide new perspectives against poor cognitive function induced by PM2.5 exposure.
Our study also had several limitations. First, residual confounding may not be ruled out. With the mean age of 81 years old at the time of the interview, our participants were mainly born between the 1900s and 1940s, when China experienced societal instability, disrupted economic activities, and war. It requires careful consideration when generalizing our findings to the general population of older adults, and to other populations. Some demographic characteristics cannot be fully controlled for in the study. The status of cardiometabolic diseases was self-reported and unverified by the clinical diagnosis in the CLHLS. The prevalence was underreported, but it shall not bias our results since they were not the key covariates. Second, we used intake frequency to score the diets, rather than using the servings or quintiles of intake per day. We do not have information on portion sizes, which did not allow us to adjust for total energy intake. But the frequency of intake may be more important than portion size to distinguish between high and low consumption of fruits and vegetables.29 A further complication is that our food questionnaire may not be standardized enough to be comparable with other nutrition studies and face challenges translating into nutrition recommendations. Diet questionnaires are notoriously difficult to be made generalizable across cultures and populations. But several studies have demonstrated the reliability and validity of using non-quantitative food frequency questionnaires to assess dietary patterns.26, 27, 28 Thirdly, we used MMSE rather in lieu of clinical diagnoses, but the adapted Chinese version used in our study was demonstrated reliable and valid in prior research.18,19 Additionally, there may be survival bias and competing risks from death given the relatively advanced age of participants (mean age: 81 years old). But our sensitivity analysis by using competing risk models showed consistent results, which supported the robustness of the main analysis.
Conclusions
We found that the effects of long-term PM2.5 exposure on developing poor cognitive function were lower among the participants with higher PDI. Our findings suggested that higher adherence to the plant-based dietary pattern may benefit poor cognitive function induced by long-term PM2.5 exposure. Promoting the plant-based dietary pattern may be a strategy to reduce the effects of PM2.5 on neurological health.
Declaration of interests
The authors declared no competing interests.
Acknowledgments
Contributors
JSJ and CY conceived and designed the study design. A Zhu conducted statistical analysis and drafted the manuscript. A Zhu, HC, JS, XW, ZL, A Zhao, XS, LY, YZ, CY, and JSJ helped interpret data. All authors contributed to the interpretation of findings, provided revisions to the manuscript, and approved the final manuscript.
Acknowledgments
Not applicable.
Funding
Collections of the Chinese Longitudinal Healthy Longevity Surveys (CLHLS) datasets analyzed in this paper were jointly supported by the National Key R&D Program of China (2018YFC2000400), National Natural Sciences Foundation of China (72061137004), the United States National Institute of Aging/ National Institute of Health (P01AG031719) and Duke/Duke-NUS/ RECA(Pilot)/2019/0051. This study was also supported by Zhejiang University Education Foundation Global Partnership Fund (to C Yuan) and Research Fund, Vanke School of Public Health, Tsinghua University (to JS Ji).
Data sharing statements
CLHLS data is available through the request portal at the Center for Healthy Aging and Development Studies, Peking University.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2021.100372.
Contributor Information
Changzheng Yuan, Email: chy478@zju.edu.cn.
John S. Ji, Email: johnji@tsinghua.edu.cn.
Appendix. Supplementary materials
References
- 1.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England). 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ru M, Brauer M, Lamarque JF, Shindell D. Exploration of the Global Burden of Dementia Attributable to PM2.5: What Do We Know Based on Current Evidence? GeoHealth. 2021;5(5) doi: 10.1029/2020GH000356. e2020GH000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schikowski T, Altuğ H. The role of air pollution in cognitive impairment and decline. Neurochemistry international. 2020;136 doi: 10.1016/j.neuint.2020.104708. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Tsao RJCOiFS. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. 2016;8:33–42. [Google Scholar]
- 5.Ricker MA, Haas WC. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2017;32(3):318–325. doi: 10.1177/0884533617700353. [DOI] [PubMed] [Google Scholar]
- 6.Shishtar E, Rogers GT, Blumberg JB, Au R, Jacques PF. Long-term dietary flavonoid intake and change in cognitive function in the Framingham Offspring cohort. Public Health Nutr. 2020;23(9):1576–1588. doi: 10.1017/S136898001900394X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh SW, Kim HS, Han JH, Bae JB, Oh DJ, Han JW, et al. Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis. Nutrients. 2020;12(4) doi: 10.3390/nu12041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medawar E, Huhn S, Villringer A, Veronica Witte A. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry. 2019;9(1):226. doi: 10.1038/s41398-019-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajaram S, Jones J, Lee GJ. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Advances in nutrition (Bethesda, Md) 2019;10(Suppl_4):S422. doi: 10.1093/advances/nmz081. -s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Song X, Chen GC, Neelakantan N, van Dam RM, Feng L, et al. Dietary pattern in midlife and cognitive impairment in late life: a prospective study in Chinese adults. Am J Clin Nutr. 2019;110(4):912–920. doi: 10.1093/ajcn/nqz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin M, Chiu T, Chang C, Lin M. The impact of a plant-based dietary pattern on dementia risk: a prospective cohort study. Innovation in Aging. 2019;3(Suppl 1):S734. [Google Scholar]
- 12.Péter S, Holguin F, Wood LG, Clougherty JE, Raederstorff D, Antal M, et al. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients. 2015;7(12):10398–10416. doi: 10.3390/nu7125539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochimica et biophysica acta. 2016;1860(12):2891–2898. doi: 10.1016/j.bbagen.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR, et al. Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation. 2019;139(15):1766–1775. doi: 10.1161/CIRCULATIONAHA.118.035742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Y, Poston DL, Vlosky DA, Gu D. Springer Science & Business Media; 2008. Healthy longevity in China: Demographic, socioeconomic, and psychological dimensions. [Google Scholar]
- 16.Ji JS, Zhu A, Lv Y, Shi X. Interaction between residential greenness and air pollution mortality: analysis of the Chinese Longitudinal Healthy Longevity Survey. The Lancet Planetary health. 2020;4(3):e107. doi: 10.1016/S2542-5196(20)30027-9. -e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T, Zhang Y, Wang J, Xu D, Yin Z, Chen H, et al. All-cause mortality risk associated with long-term exposure to ambient PM2· 5 in China: a cohort study. The Lancet Public Health. 2018;3(10):e470. doi: 10.1016/S2468-2667(18)30144-0. -e7. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet (London, England) 2017;389(10079):1619–1629. doi: 10.1016/S0140-6736(17)30548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Li T, Lv Y, Kraus VB, Zhang Y, Mao C, et al. Fine Particulate Matter and Poor Cognitive Function among Chinese Older Adults: Evidence from a Community-Based, 12-Year Prospective Cohort Study. Environ Health Perspect. 2020;128(6):67013. doi: 10.1289/EHP5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun R, Gu D. Air pollution, economic development of communities, and health status among the elderly in urban China. Am J Epidemiol. 2008;168(11):1311–1318. doi: 10.1093/aje/kwn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Xu H, Pan W, Wu B. Association between tooth loss and cognitive decline: A 13-year longitudinal study of Chinese older adults. PloS one. 2017;12(2) doi: 10.1371/journal.pone.0171404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 24.Matusik P, Tomaszewski K, Chmielowska K, Nowak J, Nowak W, Parnicka A, et al. Severe frailty and cognitive impairment are related to higher mortality in 12-month follow-up of nursing home residents. Arch Gerontol Geriatr. 2012;55(1):22–24. doi: 10.1016/j.archger.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, et al. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS medicine. 2016;13(6) doi: 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammadifard N, Sajjadi F, Maghroun M, Alikhasi H, Nilforoushzadeh F, Sarrafzadegan N. Validation of a simplified food frequency questionnaire for the assessment of dietary habits in Iranian adults: Isfahan Healthy Heart Program, Iran. ARYA atherosclerosis. 2015;11(2):139–146. [PMC free article] [PubMed] [Google Scholar]
- 27.Saeedi P, Skeaff SA, Wong JE, Skidmore PM. Reproducibility and Relative Validity of a Short Food Frequency Questionnaire in 9-10 Year-Old Children. Nutrients. 2016;8(5) doi: 10.3390/nu8050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong JE, Parnell WR, Black KE, Skidmore PM. Reliability and relative validity of a food frequency questionnaire to assess food group intakes in New Zealand adolescents. Nutr J. 2012;11:65. doi: 10.1186/1475-2891-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashfield-Watt PA, Welch AA, Day NE, Bingham SA. Is 'five-a-day' an effective way of increasing fruit and vegetable intakes? Public Health Nutr. 2004;7(2):257–261. doi: 10.1079/PHN2003524. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Y, Gu D, Purser J, Hoenig H, Christakis N. Associations of environmental factors with elderly health and mortality in China. American Journal of Public Health. 2010;100(2):298–305. doi: 10.2105/AJPH.2008.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Wischniowski K. The protective role of a Mediterranean-like diet on the cognitive function of elderly women exposed to long-term air pollution 2016.
- 33.Hehua Z, Yang X, Qing C, Shanyan G, Yuhong Z. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ Int. 2021;147 doi: 10.1016/j.envint.2020.106347. [DOI] [PubMed] [Google Scholar]
- 34.Mendes MM, Darling AL, Hart KH, Morse S, Murphy RJ, Lanham-New SA. Impact of high latitude, urban living and ethnicity on 25-hydroxyvitamin D status: A need for multidisciplinary action? Journal of Steroid Biochemistry and Molecular Biology. 2019;188:95–102. doi: 10.1016/j.jsbmb.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Romieu I, Téllez-Rojo MM, Lazo M, Manzano-Patiño A, Cortez-Lugo M, Julien P, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005;172(12):1534–1540. doi: 10.1164/rccm.200503-372OC. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Cai L, Gui Z, Wang S, Zeng X, Lai L, et al. Air pollution-associated blood pressure may be modified by diet among children in Guangzhou. China. J Hypertens. 2020;38(11):2215–2222. doi: 10.1097/HJH.0000000000002521. [DOI] [PubMed] [Google Scholar]
- 37.AlEssa HB, Malik VS, Yuan C, Willett WC, Huang T, Hu FB, et al. Dietary patterns and cardiometabolic and endocrine plasma biomarkers in US women. Am J Clin Nutr. 2017;105(2):432–441. doi: 10.3945/ajcn.116.143016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyall DM, Celis-Morales CA, Anderson J, Gill JM, Mackay DF, McIntosh AM, et al. Associations between single and multiple cardiometabolic diseases and cognitive abilities in 474 129 UK Biobank participants. European heart journal. 2017;38(8):577–583. doi: 10.1093/eurheartj/ehw528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et biophysica acta. 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative medicine and cellular longevity. 2017;2017 doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain, behavior, and immunity. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes SC, Holroyd-Leduc JM, Poulin MJ, Hogan DB. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Canadian geriatrics journal: CGJ. 2015;18(4):231–245. doi: 10.5770/cgj.18.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Cunha NM, Georgousopoulou EN, Dadigamuwage L, Kellett J, Panagiotakos DB, Thomas J, et al. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: a 10-year systematic review of randomised controlled trials. The British journal of nutrition. 2018;119(3):280–298. doi: 10.1017/S0007114517003452. [DOI] [PubMed] [Google Scholar]
- 45.Rutjes AW, Denton DA, Di Nisio M, Chong LY, Abraham RP, Al-Assaf AS, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12(12) doi: 10.1002/14651858.CD011906.pub2. Cd011906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer SE, Tsigaridis K, Miller RJGRL. Significant atmospheric aerosol pollution caused by world food cultivation. 2016;43(10):5394–5400. [Google Scholar]
- 47.Lavaine E, Majerus P, Treich NJRoA. Food, Studies E. Health, air pollution, and animal agriculture. 2020;101(4):517–528. [Google Scholar]
- 48.Domingo NGG, Balasubramanian S, Thakrar SK, Clark MA, Adams PJ, Marshall JD, et al. Air quality-related health damages of food. Proc Natl Acad Sci U S A. 2021;118(20) doi: 10.1073/pnas.2013637118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia T, Fang F, Montgomery S, Fang B, Wang C, Cao YJAQ, Atmosphere, et al. Sex differences in associations of fine particulate matter with non-accidental deaths: an ecological time-series study. 2021;14(6):863–872. [Google Scholar]
- 50.Kim H, Noh J, Noh Y, Oh SS, Koh SB, Kim C. Gender Difference in the Effects of Outdoor Air Pollution on Cognitive Function Among Elderly in Korea. Frontiers in public health. 2019;7:375. doi: 10.3389/fpubh.2019.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulick ER, Elkind MSV, Boehme AK, Joyce NR, Schupf N, Kaufman JD, et al. Long-term exposure to ambient air pollution, APOE-ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int. 2020;136 doi: 10.1016/j.envint.2019.105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang YL, Cao F. Fine particulate matter (PM 2.5) in China at a city level. Sci Rep. 2015;5:14884. doi: 10.1038/srep14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.