Abstract
Infectious bronchitis virus (IBV) is a causative agent that causes severe economic losses in the poultry industry worldwide. Papain-like protease (PLpro) is a nonstructural protein encoded by IBV. It has deubiquitinating enzyme activity, which can remove the ubiqutin modification from the protein in nuclear factor kappa-B (NF-κB) and interferon regulatory factor 7 (IRF7) signaling pathway, so as to negatively regulate the host's innate immune response to promote viral replication. In this study, PLpro was selected as the target to screen antiviral agents against IBV. Through protein prokaryotic expression technology, we successfully expressed the active IBV PLpro. Among the 16 natural products, myricetin showed the strongest inhibitory effect on IBV PLpro. Next, we tested the antiviral activity of myricetin against IBV and verified whether it can exert antiviral activity by inhibiting the deubiquitinating activity of PLpro. The results showed that myricetin can significantly inhibit IBV replication in primary chicken embryo kidney (CEK) cells and it can significantly upregulate the transcription levels in the NF-κB and IRF7 signaling pathways. Moreover, we verified that myricetin can increase the ubiquitin modification level on tumor necrosis factor receptor-associated factor 3 and 6 (TRAF3 and TRAF6) reduced by IBV PLpro. In conclusion, these results indicated that myricetin exerts antiviral activity against IBV by inhibiting the deubiquitinating activity of PLpro, which can provide new perspective for the prevention and treatment of IBV.
Key words: infectious bronchitis virus, papain-like protease, deubiquitinating activity, myricetin
INTRODUCTION
Avian infectious bronchitis is an acute contact respiratory infectious disease in poultry caused by infectious bronchitis virus (IBV). IBV is the prototype of gammacoronaviruses which has been reported in the United States as early as the 1930s (Cavanagh, 2007; Li et al., 2011; Lin and Chen, 2017). Nowadays, IBV is widespread all over the world and has become one of the important diseases causing severe economic losses in the poultry industry (Armesto et al., 2009; Promkuntod et al., 2014; Doyle et al., 2018). Although the poultry industry extensively vaccinated against IBV, it's still difficult to control the disease due to the continuous emergence of new serotypes and variants (De Wit et al., 2011; Chen et al., 2014; Chhabra et al., 2016; Jordan, 2017; Lin and Chen, 2017; Zhang et al., 2018).
IBV replication is a highly coordinated process that involves complex mechanisms to protect the viral genome and proteins from the host's antiviral defense mechanisms (Liao et al., 2011). After IBV entered host cells, the two large polyprotein precursors (polyprotein 1a and 1ab) will be translated. Polyprotein 1a and 1ab will be further processed into 15 nonstructural proteins (nsp2 to nsp16) by 2 virally encoded cysteine proteases (papain-like protease and 3CL protease) (Armesto et al., 2009). These nonstructural proteins are mainly involved in the process of viral transcription and replication (Phillips et al., 2012; Kong et al., 2015).
Papain-like protease (PLpro) is encoded by the open reading frame 1a, comprising the catalytic domain of nonstructural protein 3 (nsp3) (Yu et al., 2017). IBV PLpro can not only process the polyproteins to play a key role in the process of viral replication, but also act as a deubiquitinating enzyme to block the host innate immune response pathways (Yu et al., 2017; Hartenian et al., 2020; Ojha et al., 2021). In vitro characterization of PLpro enzyme activity showed that it can recognize and hydrolyze the ubiquitin and ubiquitin-like protein interferon-stimulated gene 15 (ISG15), which both carry the LXGG recognition motif at the C-terminal (Lim and Liu, 1998; Lim et al., 2000). Ubiquitin and ubiquitin-like proteins are critical molecules for the post-translational modification and they can regulate key biological processes such as enzymatic activity and cell signaling (Nanduri et al., 2013). PLpro, as a deubiquitinating enzyme, can remove ubiquitin and ubiquitin-like modifications from proteins in innate immune pathways to escape the host immune response. Actually, not only for IBV or coronaviruses, deubiquitinating enzymes have widely been reported as promising drug targets for other viral infections (Nanduri et al., 2013; Báez-Santos et al., 2015; Setz et al., 2017; Atkins et al., 2020).
Due to the dual activities in viral replication and blocking host immune response, PLpro is considered as a potential target for developing antiviral agents against IBV (Kong et al., 2015; Ojha et al., 2021). In fact, IBV used to be a virus that was difficult to culture in vitro. However, in recent years, a large number of studies have confirmed that IBV can be cultured in primary chicken embryo kidney (CEK) cells (Li et al., 2011; Zhang et al., 2018; Chen et al., 2019), which has strongly promoted the development of IBV in vitro research and made it possible to screen antiviral agents in vitro models.
MATERIALS AND METHODS
Virus, Cells, and Reagents
The IBV strain M41 (China Institute of Veterinary Drug Control) was adapted and propagated in primary CEK cells. The 50% tissue culture infective dose (TCID50) was determined as 10–7.24 /100 μL.
The study was approved by the Ethics Committee of Sichuan Agricultural University according to The Regulation of Experimental Animal Management. The primary CEK cells were isolated from chicken embryo, and cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The HEK 293T cells (ATCC CRL-11268) were obtained from Fuheng Biology Co., Ltd (Shanghai, China), and cultured in DMEM with 10% FBS.
The plasmids pET-32a(+) and pcDNA3.1(+) were obtained from Sangon Biotech Co., Ltd (Shanghai, China). The pcDNA3.1-HA-Ub construction plasmid was purchased from Transvector Co., Ltd (Chengdu, China). The anti-Flag tag monoclonal antibody, the anti-Myc tag monoclonal antibody and the anti-β-actin monoclonal antibody were purchased from Proteintech Co., Ltd (Wuhan, China). The anti-HA monoclonal antibody was purchased from Boster Biological Technology Co. Ltd (Wuhan, China). Myricetin was purchased from Meilunbio Co., Ltd (Dalian, China). NP-40 lysis buffer was obtained from Solarbio Co., Ltd (Beijing, China). SureBeads protein G Magnetic Beads were purchased from Bio-Rad (Hercules, CA). Ubiquitin-7-amido-4-methylcoumarin (UB-AMC) was purchased from Biovision (Milpitas, CA).
Construction of Recombinant Plasmids
The gene fragment of IBV PLpro was amplified from IBV-infected CEK cells by PCR with the addition of 6 × His and Myc tag in primers respectively, and subsequently it was connected to the pET-32a(+) or pcDNA3.1(+) expression vectors to build recombinant plasmids. The recombinant plasmids were transformed into the E. coli DH5α cells, and transformants were selected on Luria-Bertani plates containing 100 mg/mL ampicillin. PCR screening was performed to confirm whether these recombinant plasmids were constructed successfully.
Expression of IBV PLpro
The pET-32a-IBV-PLpro recombinant plasmid was transformed into the E. coli BL21(DE3) cells for protein expression. 10 mL overnight cultures of E. coli BL21 were seeded into 1,000 mL fresh Luria Bertani medium containing 50 mg/mL ampicillin. When the OD600 value of E. coil BL21 cells reached to 0.6, 0.2 mM isopropyl β-D-1-thiogalactopyranoside was added to induce the protein expression for 6 h at 28°C. The E. coil BL21 cells were harvested by centrifugation at 6,000 rpm for 10 min at 4°C. Purification of proteins was performed according to the instructions of His-tag protein purification kit (Beyotime Biotechnology, Shanghai, China).
IBV PLpro Inhibition Assay
Referring to the method of Cho et al. (2013), UB-AMC was selected as the substrate to detect the deubiquitinating activity, so as to screen the natural products with inhibitory effect on PLpro deubiquitinating activity. Reactions were performed in a total volume 50 μL, which contained 40 μL purified IBV PLpro, 10 μM UB-AMC substrate in DMSO, 1 mM DTT and 100 μM natural products. The fluorescence intensity was detected by full-wavelength multifunctional enzyme plate analyzer (Ex/EM = 350/440), and continuous monitoring was conducted for 30 min. The regression equation was fitted according to the fluorescence intensity curve under the kinetic mode, and the slope of the regression equation was the enzymatic reaction speeding. The changes of enzymatic reaction speeding can reflect the influence on PLpro deubiquitinating activity after incubated with different natural products. Then, the concentration of natural products in the reaction system was reduced to 50 μM, 20 μM, and 10 μM, and so on for further screening. The natural product with the minimum half inhibition concentration (IC50) value on PLpro was selected for following studies.
Cytotoxicity of Myricetin on CEK Cells
To determine the safe concentration ranges of myricetin on CEK cells, we used cell counting kit-8 (CCK-8) to evaluate the cytotoxicity of myricetin on CEK cells according to manufacturer's instruction. Briefly, CEK cells were added at a density of 1 × 104 cells per well to a 96-well plate and incubated for 24 h at 37°C. Then, the culture media was removed and replaced with DMEM containing different concentrations of myricetin (1 μM–1,000 μM). After cultured in a 5% CO2 incubator at 37°C for 48 h, 10 μL CCK-8 solution was added to each well and incubated at 37°C in a CO2 cell incubator for 30 min, then the absorbance rates were measured at 450 nm by microplate reader.
The Half Inhibition Concentration for Myricetin against IBV Infection
CEK cells were seeded in a 96-well plate and incubated at 37°C with 5% CO2 for 24 h. Then, the cells were washed twice using serum-free DMEM and infected with 0.1 multiplicity of infection (MOI) IBV. After 2 h postinfection, the media was removed and the myricetin in different concentration (1.56–100 μM) was added to treat for 48 h. After incubation for 48 h, 10 μL CCK-8 solution was added to each well and incubated at 37°C in a CO2 cell incubator for 30 min, then the absorbance rates were measured at 450 nm by microplate reader. The half inbihition concentration (IC50) was calculated using the Reed-Muench formula as follows: IC50 = C*2−S (S = N-1+(H-R)/(H-L)).
The Antiviral Effect Assay
Following the same cell culture, virus infection and drug treatment methods as above, to evaluate the antiviral effect of myricetin against IBV directly, the total RNA of cells was collected at 4 h, 8 h, 12 h, 18 h, 24 h, 36 h, and 48 h postinfection respectively and the transcription levels of IBV N gene were tested by qRT-PCR.
RNA Extraction, Reverse Transcription, and qRT-PCR
The total RNA of the samples was prepared with the Trizol reagent (Biomed, Beijing, China) based on the manufacturer's descriptions. Reverse transcription of cDNA was performed by using reverse transcription kits according to the manufacturer's protocol (Thermo Fisher Scientific, MA) . qRT-PCR was used to evaluate the transcription levels of target genes. The reaction system of qRT-PCR included 5 μL SYBR Green Mix, 3 μL RNA-free Water, 1 μL cDNA templates and 1 μL a pair of specific primers (0.5 μL forward primer and 0.5 μL reverse primer). All the sequences of forward and reverse primers were shown in Table 1. The qRT-PCR procedure was set as follows: 95°C for 5 min, followed 40 cycles of 95°C for 10 s and 60°C for 30 s, with final extension of 72°C for 60 s. According to the fluorescence threshold results (Ct values), the relative levels of mRNA expression were calculated using the 2−△△Ct method (Zheng et al., 2020), which normalized with β-actin gene as internal standard.
Table 1.
The primers for qRT-PCR.
| Gene | Sequences (5’-3’) | Accession No. |
|---|---|---|
| IBV N | Forward: CAAAGGTGGAAGAAAACCAGT | NC_001451.1 |
| Reverse: ATTTAGTATCAGCACCCTTAGCAG | ||
| TRAF6 | Forward: CAATAGAAAGCACGTATGACC | XM_015287208.2 |
| Reverse: AGCATTACAGTAACTTGGCAT | ||
| TAB1 | Forward: TCAGCTCCAAACCGTTC | NM_001006240.2 |
| Reverse: ATCATAGCCATTGAAGACACC | ||
| IKKβ | Forward: ACAGCCAAGAAATAGTACGG | NM_001031397.1 |
| Reverse: AGCATAAATGACTCGGACCT | ||
| IL-1β | Forward: CCCGCCTTCCGCTACACC | NM_204524.1 |
| Reverse: TGACTCCAGCACGAAGCAC | ||
| IL-6 | Forward: TAGAGAAAATCACCATGCACCT | NM_204628.1 |
| Reverse: TTCAAATAGCGAACGGCCCTC | ||
| TNF-α | Forward: GCCTATGCCAACAAGTACACC | NM_204267.1 |
| Reverse: ACGCTCCTGACTCATAGCAGA | ||
| MDA5 | Forward: ATGTATAAGGCCATTCGAC Reverse: TTCTTATATGTCTTACGCTGA |
AB371640.1 |
| MAVS | Forward: AACAACTCATCTCACGCCGAA Reverse: TGAAATCAGAGCGATGCCAA |
MF289560.1 |
| TRAF3 | Forward: TAGATATAATTCAATGGCACT | XM_015287827.2 |
| Reverse: ACAATTATTGAAATGACACGAA | ||
| IRF7 | Forward: CGTATCTTCCGCATCCCT Reverse: GCCTTGAAGATCTCCACGTC |
KP096419.1 |
| IFN-α | Forward: CCTCGCAACCTTCACC Reverse: AACCAGGCACGAGCTT |
KF923375.1 |
| IFN-β | Forward: TCCAGCTCCTTCAGAATACGG Reverse: ATGGCTGCTTGCTTCTTGTCC |
GU119897.1 |
| β-actin | Forward: CAACACAGTGCTGTCTGGTGGTA Reverse: ATCGTACTCCTGCTTGCTGATCC |
NM_205518.1 |
Transfection and Co-Immunoprecipitation
When the 293T cells reached to 70% to 90% abundance, transfection was performed according to the Lipofectamine 3,000 instructions. The plasmid and Lipofectamine 3,000 were diluted into DMEM respectively. The diluted plasmid DNA was added into the diluted Lipofectamine 3,000 (1:1 ratio), and after incubating for 15 min, the mixture was added into 293T cells for transfection. Myricetin was added at 12 h after transfection. At 48 h post transfection, 293T cells were collected and the cellular total proteins were extracted using NP-40 lysis buffer. The total extracted proteins (600 μL) were divided into two parts. Part of the proteins (100 μL) was denatured for input detection. The other part of the proteins (500 μL) was added with anti-Flag antibody and incubated with protein G beads at 4°C overnight for immunoprecipitation. The protein G bead was collected and denatured the protein adsorbed on the bead for western blot detection.
Western Blot
Protein samples were prepared as described above. Equivalent amounts of protein samples were subjected to SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 5% milk in tris buffered saline (TBS) buffer containing 0.1% Tween for 90 min at room temperature, the membranes were reacted with corresponding primary antibodies for 12 h at 4°C, and then followed by horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG at room temperature for 90 min. Finally, the blots membranes were subjected to develop with electrochemiluminescence (ECL) detection reagents.
Statistical Analysis
Statistical analysis was performed using IBM SPSS 21 software. Data were analyzed to establish their significance using the student's t test. The data were visualized using GraphPad Prism 7 software and the statistically significant differences were set at (*) P < 0.05, (**) P < 0.01, (***) P < 0.001.
RESULTS
Expression and Purification of IBV PLpro
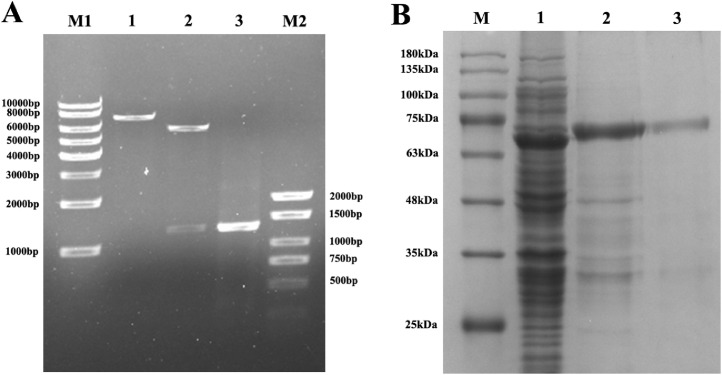
The result of identification for pET-32a-PLpro recombinant plasmid was shown in Figure 1A, which indicated that the IBV PLpro gene was successfully connected with pET-32a(+) and we succeed to construct the pET-32a-PLpro recombinant plasmid. Through protein prokaryotic expression in E. coil BL21, we successfully expressed and got the purified IBV PLpro (Figure 1B).
Figure 1.
Identification of pET-32a-PLpro recombinant plasmid and purification for IBV PLpro. (A) M1: 1kb DNA Marker; 1: Single restriction digestion identification of recombinant plasmid by SacI; 2: Double restriction digestion identification of recombinant plasmid by SacI and XhoI; 3: IBV-PLpro positive amplification products; M2: BM2000+ DNA Marker. (B) Purification for expression proteins of recombinant plasmid pET-32a-PLpro. M: Protein Marker; 1: Whole bacteria; 2: Bacterial breakage precipitation (inclusion bodies); 3: Purified IBV PLpro. Abbreviations: IBV, infectious bronchitis virus; PLpro, papain-like protease.
The Inhibitory Effects of Different Natural Products on the Deubiquitinating Activity of IBV PLpro
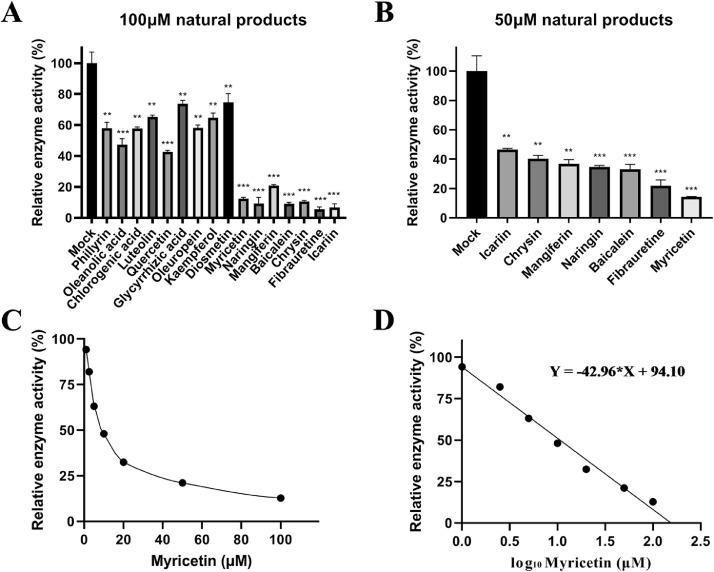
Under the concentration of 100 μM, myricetin, naringin, mangiferin, chrysin, baicalein, fibrauretine, and icariin had a significant inhibitory effect on the deubiquitinating activity of IBV PLpro (P < 0.001), which the relative enzymatic activity was less than 20% (Figure 2A). Therefore, we chose these natural products for further screening at 50 μM. The results indicated that myricetin exerted the strongest inhibitory effects to inhibit the deubiquitinating activity of IBV PLpro under the concentration of 50 μM (Figure 2B). Then, the concentration of myricetin was reduced to 20 μM, 10 μM, 5 μM, 2.5 μM, and 1 μM for further screening. The results showed that 10 μM myricetin still inhibited the deubiquitinating activity of IBV PLpro nearly 50% (Figure 2C).
Figure 2.
Inhibition of IBV PLpro deubiquitinating activity by different natural products. (A) and (B) The inhibitory rate of PLpro deubiquitinating enzyme activity under the concentration of 100 μM and 50 μM. The 100 μM drug was co-incubated with recombinant IBV PLpro for 30 min, and the substrate UB-AMC was added to initiate the enzymatic reaction. Kinetic method was used to measure the enzymatic reaction rate to reflect the changes of enzyme activity. (C) Inhibition rate of myricetin at different concentrations on PLpro deubiquitinating enzyme activity. (D) The regression equation fitting curve between the concentration of myricetin and the inhibition rate of PLpro deubiquitinating enzyme activity. Abbreviations: IBV, infectious bronchitis virus; PLpro, papain-like protease.
The software GraphPad Prism was used to convert the abscissimal concentration into a logarithmic function, and the fitting curve was drawn to calculate the IC50 of myricetin on IBV PLpro. The fitting curve was shown in Figure 2D. The IC50 of the myricetin on IBV PLpro was obtained by fitting curve: IC50 = 10.63 μM.
Cytotoxicity of Myricetin on CEK Cells
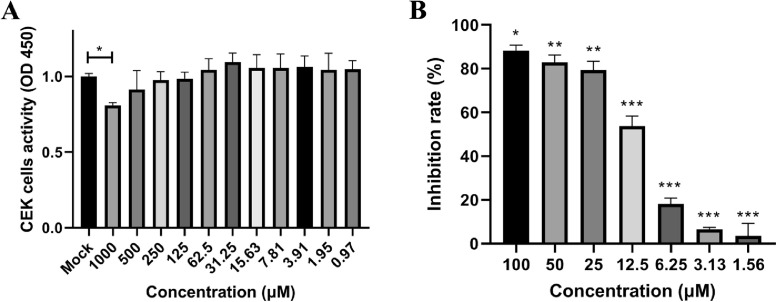
CCK-8 method was used to detect the cytotoxicity of myricetin on the primary CEK cells. Compared with the mock group, only 1,000 μM myricetin had significant inhibition on CEK cells (P < 0.05), and there was no significant toxicity of myricetin below 500 μM (Figure 3A).
Figure 3.
Cytotoxicity and antiviral activities of myricetin. (A) The cytotoxicity of myricetin on CEK cells (*: P < 0.05). (B) The inhibition rate of myricetin at different concentrations against IBV. The inhibition rate was determined as the percent of cell viability in treated cells compared with untreated cells. The different symbol on the columns represent the significant differences compared with blank control (*: P < 0.05; **: P < 0.01; ***: P < 0.001). Abbreviations: CEK, chicken embryo kidney; IBV, infectious bronchitis virus.
The Inhibitory Activity for Myricetin Against IBV Infection on CEK Cells
The CEK cells were infected with 0.1 MOI IBV and myricetin with different concentration was added for treatment. After 48 h, the viability of cells was tested by CCK-8 method. The results showed that myricetin with a concentration below 12.5 μM cannot effectively inhibit the IBV-induced cytopathic effect on CEK cells. In contrast, the inhibition rate of myricetin could reach 80% with the concentration of 25 μM above (Figure 3B). According to Reed-Muench formulation, the IC50 value for myricetin against IBV was 13.68 μM.
The Antiviral Activity for Myricetin Against IBV
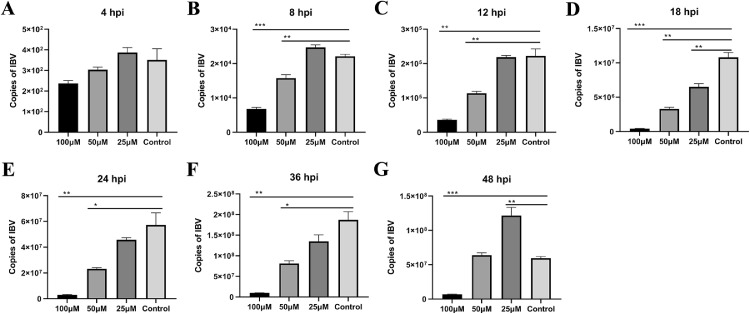
In order to further detect the antiviral activity of myricetin, we tested the copies of IBV (the transcription levels of IBV N gene) at 4 h, 8 h, 12 h, 18 h, 24 h, 36 h, and 48 h. In details, at 4 h postinfection, compared with the control group, there were no significant differences treating with 100 μM, 50 μM, and 25 μM myricetin (Figure 4A). In contrast, at 8 h (Figure 4B), 12 h (Figure 4C), 18 h (Figure 4D), 24 h (Figure 4E), and 36 h (Figure 4F) postinfection, the copies of IBV were significant reduced treating with 100 μM and 50 μM myricetin. Interestingly, the copies of IBV treating with 25 μM myricetin was higher than the copies of control group at 48 hpi (Figure 4G).
Figure 4.
The antiviral activity for myricetin against IBV. (A) Copies of IBV at 4 h postinfection. (B) Copies of IBV at 8 h postinfection. (C) Copies of IBV at 12 h postinfection. (D) Copies of IBV at 18 h postinfection. (E) Copies of IBV at 24 h postinfection. (F) Copies of IBV at 36 h postinfection. (G) Copies of IBV at 48 h postinfection. (*: P < 0.05; **: P < 0.01; ***: P < 0.001). Abbreviation: IBV, infectious bronchitis virus.
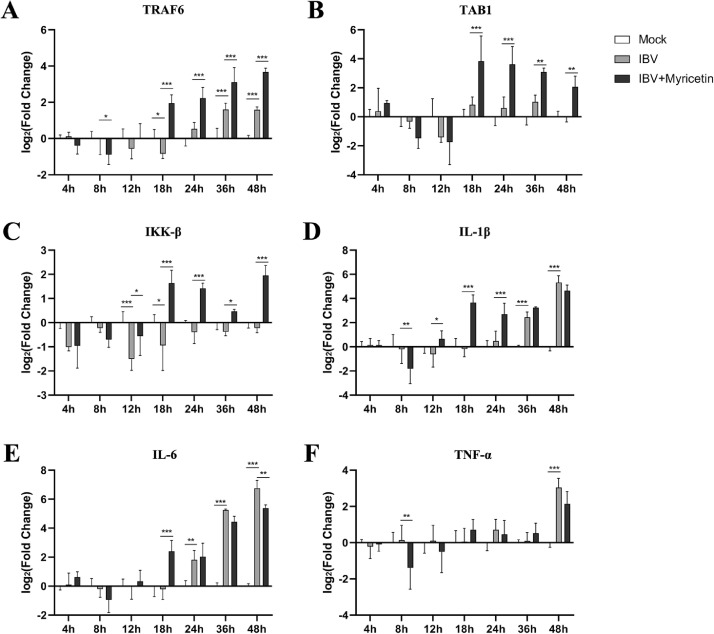
Myricetin Can Significantly Increase the mRNA Levels of NF-κB Pathway Proteins in IBV-Infected CEK Cells
In the nuclear factor kappa-B (NF-κB) pathway, we selected the tumor necrosis factor receptor-associated factor 6 (TRAF6), TGF-beta activated kinase 1 (TAB1), and inhibitor kappa B kinaseβ (IKK-β) protein genes that regulate the activation of NF-κB upstream of the pathway, and the proinflammatory cytokines interleukin-1 β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) proteins for detection.
Compared with the mock group, there was no significant difference in the mRNA expression levels on TAB1 (Figure 5B) and IKK-β (Figure 5C) proteins upstream of NF-κB pathway in the virus model group. The mRNA levels of IL-1β (Figure 5D), IL-6 (Figure 5E), TNF-α (Figure 5F), and TRAF6 (Figure 5A) did not change significantly before 24 h postinfection, but were significantly upregulated at 36 h and 48 h, respectively (P < 0.001).
Figure 5.
The transcript levels of NF-κB pathway proteins in CEK cells. The CEK cells were infected with 0.1 MOI of IBV and 100 μM myricetin was added to treat for 48 h. CEK cells were collected at 4, 8, 12, 18, 24, 36, and 48 h postinfection, and the total RNA of the cells was extracted and reverse transcribed into cDNA, and the relative expression of each target gene was detected by qRT-PCR. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Abbreviations: CEK, chicken embryo kidney; IBV, infectious bronchitis virus; NF- κB, nuclear factor kappa-B.
Compared with the virus model group, the mRNA expression level of TRAF6 (Figure 5A) in the myricetin treatment group was not significantly different from 4 h to 12 h, but was significantly upregulated from 18 h to 48 h (P < 0.001). Similar for TAB1 and IKK-β, the mRNA expression level of them did not change significantly during 4 h to 12 h, but the expression of TAB1 was extremely significantly upregulated at 18 h and 24 h (P < 0.001), while the expression of IKK-β was extremely significantly upregulated at 18 h, 24 h and 48 h (P < 0.001). Interestingly, for downstream proinflammatory cytokines, IL-1β (Figure 5D) mRNA expression level was significantly upregulated at 18 h and 24 h (P < 0.001), while the expression levels of IL-6 (Figure 5E) were extremely significantly upregulated at 18 h (P < 0.001).
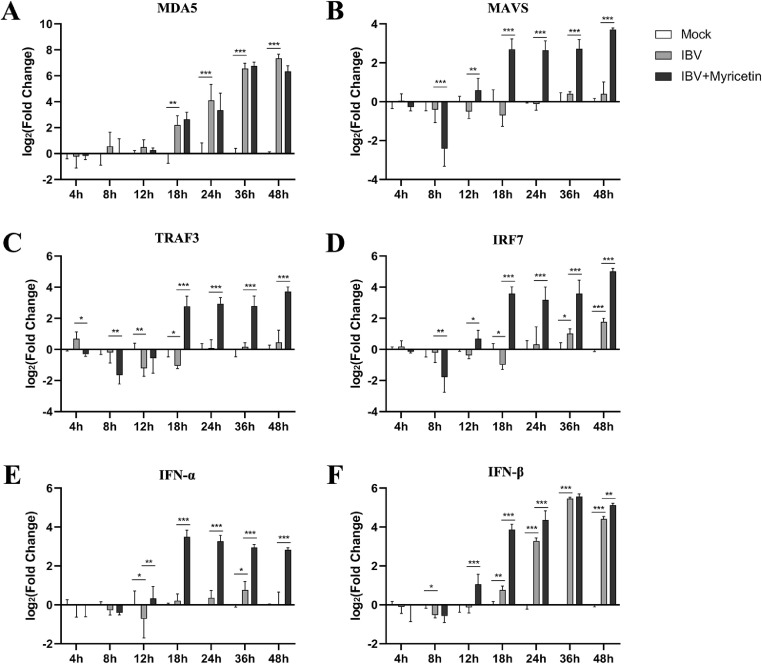
Myricetin Can Significantly Increase the mRNA Levels of MAVS/IRF7 Pathway Proteins in IBV-Infected CEK Cells
The IRF7 signal pathway is an important signal pathway to active interferon production. In this pathway, we selected the upstream signaling factors melanoma differentiation associated gene 5 (MDA5), mitochondrial antiviral signaling protein (MAVS), tumor necrosis factor receptor-associated factor 3 (TRAF3), interferon regulatory factor 7 (IRF7) and the downstream antiviral effector interferon alpha (IFN-α), interferon beta (IFN-β) for detection. The results were shown in Figure 6.
Figure 6.
The transcript levels of IRF7 pathway proteins in CEK cells. The CEK cells were infected with 0.1 MOI of IBV and 100 μM myricetin was added to treat for 48 h. CEK cells were collected at 4, 8, 12, 18, 24, 36, and 48 h postinfection, and the total RNA of the cells was extracted and reverse transcribed into cDNA, and the relative expression of each target gene was detected by qRT-PCR. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Abbreviations: CEK, chicken embryo kidney; IBV, infectious bronchitis virus; IRF7, interferon regulatory factor 7.
Compared with the mock group, the mRNA expression levels on MDA5 (Figure 6A) and IFN-β (Figure 6F) in the virus model group showed significantly upregulated from 18 h to 48 h (P < 0.001). The TRAF3 was significantly downregulated at 12 h and 18 h after IBV infection while the IRF7 was significantly down-regulated at 18h and upregulated at 36 and 48 h. Instead, the mRNA expression levels of MAVS and IFN-a had no significant changes from 4 to 48 h.
In the myricetin treatment group, compared with the virus model group, there was no significant difference in the mRNA expression levels of each protein during 4 h to 12 h. From 18 h to 48 h, the mRNA expression levels of MAVS (Figure 6B), TRAF3 (Figure 6C), IRF7 (Figure 6D), and IFN-α (Figure 6E) were significantly upregulated (P < 0.001). The MDA5 (Figure 6A) mRNA expression had no significant changes treating with myricetin.
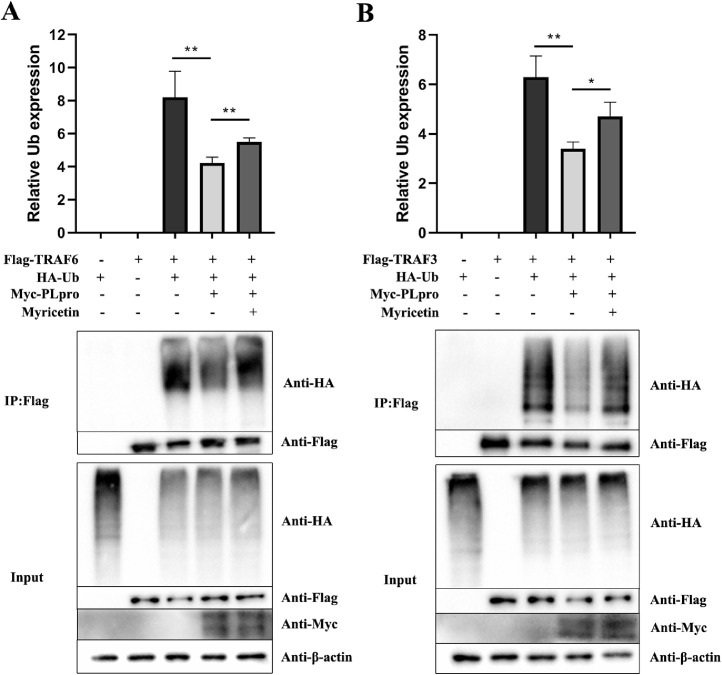
Myricetin Can Increase the Ubiquitin Modification Level on TRAF3 and TRAF6 Reduced by IBV PLpro
After transfection of the PLpro plasmid (Myc-PLpro), the levels of ubiquitin modification on the TRAF6 (Figure 7A) and TRAF3 (Figure 7B) were extremely reduced (P < 0.01), indicating that IBV PLpro had deubiquitinating activity and could remove ubiquitin molecules on TRAF6 and TRAF3. After treating with myricetin, the levels of ubiquitin modification on TRAF6 and TRAF3 were significantly upregulated (P < 0.01), which suggested that myricetin had a significant inhibitory effect on the deubiquitinating activity of IBV PLpro.
Figure 7.
Ubiquitin modification levels on TRAF6 and TRAF3. When the 293T cells reached to 70% to 90%, Flag-TRAF3 or Flag-TRAF6, Myc-PLP, HA-Ub plasmids were transfected, and the myricetin treated group was added with 100 μM myricetin 24 h after transfection. 48 h post-transfection, the cells of each sample were collected to extract total proteins. Part of the extracted total proteins was used for Input detection; the other part of the protein was incubated with anti-Flag antibody and Protein G overnight at 4°C, and centrifuged to collect magnetic beads to obtain protein precipitate for COIP detection. Abbreviations: TRAF3 and TRAF6, tumor necrosis factor receptor-associated factor 3 and 6.
DISCUSSION
The target-based drug development strategy has been used in drug discovery for decades. Compared with the traditional in-vivo drug screenings, there is no doubt that this strategy is an effective way for new drugs discovery. In this research, IBV PLpro was selected as the target to screen antiviral agents.
Due to the resistance of antiviral drugs, it has been prohibited to use antiviral drugs in food animals in China (Zhang et al., 2018). We turned our attention to natural products aimed to find antiviral active substances from natural products. In this study, 16 natural products reported with antiviral activity were selected to screen IBV PLpro inhibitors. In the initial screening test under the concentration of 100 μM, 7 natural products (myricetin, naringin, mangiferin, baicalein, chrysin, fibrauretine, and icariin) with strong inhibitory activity all belong to flavonoids, except for fibrauretine, which is an alkaloid. Among the studies on PLpro inhibitors of coronavirus reported so far, the inhibitors derived from natural products are also mostly flavonoids (Cho et al., 2013).
It is reported that PLpro belongs to the cysteine protease family and the core domain for catalytic reaction is a conserved catalytic triad consisting of Cys101, His264, and Asp275 (Kong et al., 2015). The sulfhydryl group (-SH) of cysteine is an unstable structure. Nucleophilic functional groups such as aldehydes, ketones, esters, carbon-carbon double bonds, and carbon-carbon triple bonds can bind to the sulfhydryl groups of cysteine, which are considered as potential structures to inhibit cysteine proteases.
The carbonyl group in the core structure of flavonoids may be a potential site that can bind to the cysteine sulfhydryl group. However, for flavonoids, only having this carbonyl group is not enough to inhibit the enzyme activity. Take kaempferol, quercetin, and myricetin as examples. All 3 are flavonoids and their chemical structure differs only in the number of phenolic hydroxyl groups on the 2-position phenyl group. However, the results showed that the three inhibitory effects on PLpro deubiquitinating activity are quite different. Among them, myricetin with 3 phenolic hydroxyl groups in the side chain has the strongest effect on inhibiting PLpro deubiquitinating activity, which may be related to the steric hindrance of myricetin.
After being infected with viruses, the host's innate immune response will be activated to play an antiviral effect in the early stage of virus infection (Kameka et al., 2014; Chhabra et al., 2016). Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs) are 2 important pattern recognition receptors (PRRs) to recognize the viruses in host cells (Liao et al., 2011). The former is located at cell membrane components and the latter at cytoplasm, so as to identify viral nucleic acids through different pathways and recruit specific adaptor proteins. The RIG-I-like receptors mainly consists of 2 members, retinoic-acid-inducible gene I (RIG-I) and melanoma differentiation-associated antigen 5 (MDA5), which can recognize the double-stranded RNA structure of viruses during cytoplasmic replication. Due to the lack of RIG-I in chicken RLRs receptors, therefore, MDA5 becomes the only model receptor for intracytoplasmic virus recognition in chicken cells (Kong et al., 2015; Ojha et al., 2021; Zhang et al., 2021).
After MDA5 recognizing the viral RNA structure, the signal will be transmitted to MAVS, and then the expression of type I interferon and pro-inflammatory cytokines will be initiated through IRF7 and NF-κB, respectively (Nanduri et al., 2013; Ojha et al., 2021). PLpro, as a crucial protease which is translated in the early stage of IBV infection, can remove the ubiquitin modification of proteins in antiviral signaling pathway through its deubiquitinating activity in the early stage of virus infection, thus blocking the innate immune response of cells (Nanduri et al., 2013; Yu et al., 2017; Ojha et al., 2021).
In order to verify whether the antiviral activity of myricetin is related to inhibiting deubiquitinating activity of PLpro, we firstly detected the mRNA expression levels of NF-κB and IRF7 signaling pathway. It is not difficult to find that myricetin can promote the activation of NF-κB and IRF7 signaling pathways compared with the virus model group. After treating with myricetin, the mRNA expression levels of IFN-α, IFN-β, IL-1β, and IL-6 at 12 h, 18 h, and 24 h were significantly higher than those in the virus model group. In other words, the expression time of antiviral effect factors in cells was earlier than the virus model group when treating with myricetin, which implied that myricetin may participate in inhibiting the immune evasion mechanism of IBV and enhancing the innate immune response pathways that inhibited by IBV to against IBV infection.
It is reported that the activation of NF-κB and IRF7 pathway is closely related to the regulation of ubiquitin modification (Yu et al., 2017; Zhang et al., 2021). Specifically, on the one hand, MAVS can interact with TRAF6 and make it ubiquitinated with k63-linked ubiquitin to recruit TAB2/TAB3/TAK1 complex, which will cause the phosphorylation of TAK1 and activate IKK kinase. Next, IκB will be activated and degraded to let NF-κB enter the nucleus and induce the expression of pro-inflammatory cytokines. On the other hand, MAVS can interact with TRAF3 and make it ubiquitinated to promote the activation of TBK1 and IKKε. Then, the downstream signal molecule IRF7 will be phosphorylated and enter the nucleus to stimulate the expression of type I interferon (Shaw et al., 2013; Ojha et al., 2021). All in all, in the conduction process of the NF-κB and IRF7 signal pathway, the ubiquitination process plays an important role in it (Nanduri et al., 2013).
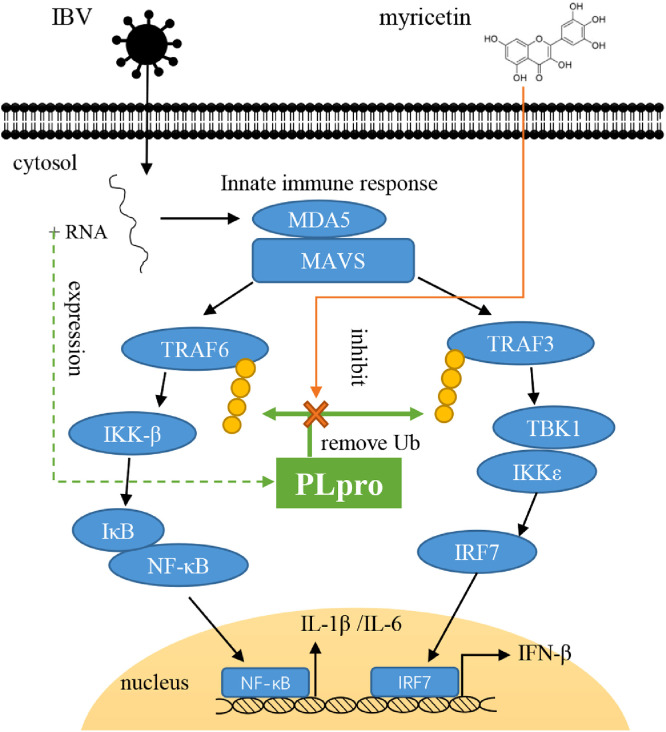
To further confirm whether the activation of NF-κB and IRF7 pathway by myricetin is related to inhibiting the deubiquitinating activity of PLpro, we selected the TRAF6 and TRAF3 proteins in the NF-κB and IRF7 pathway to study the effect of myricetin on PLpro deubiquitinating function in vitro. It showed that PLpro can remove the ubiquitin modification on TRAF6 and TRAF3 proteins, and after treating with myricetin, the ubiquitin modification levels of TRAF6 and TRAF3 can be significantly increased, indicating that myricetin can inhibit the deubiquitinating activity of PLpro and restore the ubiquitin modification levels of TRAF6 and TRAF3 to promote innate immune response (Figure 8).
Figure 8.
The mechanism for myricetin against IBV. Myricetin can inhibit the deubiquitinating activity of PLpro to block the IBV immune evasion function. Abbreviations: IBV, infectious bronchitis virus; PLpro, papain-like protease.
CONCLUSIONS
In conclusion, IBV PLpro can be an effective target for designing or selecting antiviral agents and myricetin has antiviral effects against IBV by inhibiting the deubiquitinating activity of PLpro.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the Program Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (SCCXTD-2020-18) and the Science and Technology Project of Sichuan Province (2021NZZJ0021)
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Armesto M., Cavanagh D., Britton P. The replicase gene of avian coronavirus infectious bronchitis virus is a determinant of pathogenicity. PLoS One. 2009;4:e7384. doi: 10.1371/journal.pone.0007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins S.L., Motaib S., Wiser L.C., Hopcraft S.E., Hardy P.B., Shackelford J., Foote P., Wade A.H., Damania B., Pagano J.S., Pearce K.H., Whitehurst C.B. Small molecule screening identifies inhibitors of the Epstein-Barr virus deubiquitinating enzyme, BPLF1. Antiviral Res. 2020;173 doi: 10.1016/j.antiviral.2019.104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Santos Y.M., St. John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014;10:24. doi: 10.1186/1746-6148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Muhammad I., Zhang Y., Ren Y., Zhang R., Huang X., Diao L., Liu H., Li X., Sun X., Abbas G., Li G. Antiviral activity against infectious bronchitis virus and bioactive components of Hypericum perforatum L. Front. Pharmacol. 2019;10:1272. doi: 10.3389/fphar.2019.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R., Kuchipudi S.V., Chantrey J., Ganapathy K. Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses. Virology. 2016;488:232–241. doi: 10.1016/j.virol.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.K., Curtis-Long M.J., Lee K.H., Kim D.W., Ryu H.W., Yuk H.J., Park K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorganic Med. Chem. 2013;21:3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit J.J.S., Cook J.K.A., van der Heijden H.M.J.F. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle N., Neuman B.W., Simpson J., Hawes P.C., Mantell J., Verkade P., Alrashedi H., Maier H.J. Infectious bronchitis virus nonstructural protein 4 alone induces membrane pairing. Viruses. 2018;10:477. doi: 10.3390/v10090477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenian E., Nandakumar D., Lari A., Ly M., Tucker J.M., Glaunsinger B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020;295:12910–12934. doi: 10.1074/jbc.REV120.013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. Vaccination against infectious bronchitis virus: a continuous challenge. Vet. Microbiol. 2017;206:137–143. doi: 10.1016/j.vetmic.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Kameka A.M., Haddadi S., Kim D.S., Cork S.C., Abdul-Careem M.F. Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology. 2014;450-451:114–121. doi: 10.1016/j.virol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Shaw N., Yan L., Lou Z., Rao Z. Structural view and substrate specificity of papain-like protease from avian infectious bronchitis virus. J. Biol. Chem. 2015;290:7160–7168. doi: 10.1074/jbc.M114.628636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wu J., Zhang Z., Ma Y., Liao F., Zhang Y., Wu G. Forsythoside A inhibits the avian infectious bronchitis virus in cell culture. Phyther. Res. 2011;25:338–342. doi: 10.1002/ptr.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Wang X., Huang M., Tam J.P., Liu D.X. Regulation of the p38 mitogen-activated protein kinase and dual-specificity phosphatase 1 feedback loop modulates the induction of interleukin 6 and 8 in cells infected with coronavirus infectious bronchitis virus. Virology. 2011;420:106–116. doi: 10.1016/j.virol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.P., Liu D.X. Characterization of the two overlapping papain-like proteinase domains encoded in gene 1 of the coronavirus infectious bronchitis virus and determination of the C-terminal cleavage site of an 87-kDa protein. Virology. 1998;245:303–312. doi: 10.1006/viro.1998.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.P., Ng L.F.P., Liu D.X. Identification of a novel cleavage activity of the first papain-like proteinase domain encoded by open reading frame 1a of the coronavirus avian infectious bronchitis virus and characterization of the cleavage products. J. Virol. 2000;74:1674–1685. doi: 10.1128/jvi.74.4.1674-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.Y., Chen H.W. Infectious bronchitis virus variants: molecular analysis and pathogenicity investigation. Int. J. Mol. Sci. 2017;18:2030. doi: 10.3390/ijms18102030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri B., Suvarnapunya A.E., Venkatesan M., Edelmann M.J. Deubiquitinating enzymes as promising drug targets for infectious diseases. Curr. Pharm. Des. 2013;19:3234–3247. doi: 10.2174/1381612811319180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha N.K., Liu J., Yu T., Fang C., Zhou J., Liao M. Interplay of the ubiquitin proteasome system and the innate immune response is essential for the replication of infectious bronchitis virus. Arch. Virol. 2021;166:2173–2185. doi: 10.1007/s00705-021-05073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.E., Jackwood M.W., McKinley E.T., Thor S.W., Hilt D.A., Acevedol N.D., Williams S.M., Kissinger J.C., Paterson A.H., Robertson J.S., Lemke C.Z. Changes in nonstructural protein 3 are associated with attenuation in avian coronavirus infectious bronchitis virus. Virus Genes. 2012;44:63–74. doi: 10.1007/s11262-011-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promkuntod N., van Eijndhoven R.E.W., de Vrieze G., Gröne A., Verheije M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology. 2014;448:26–32. doi: 10.1016/j.virol.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setz C., Friedrich M., Rauch P., Fraedrich K., Matthaei A., Traxdorf M., Schubert U. Inhibitors of deubiquitinating enzymes block HIV-1 replication and augment the presentation of gag-derived MHC-I epitopes. Viruses. 2017;9:222. doi: 10.3390/v9080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N., Ouyang S., Liu Z.J. Binding of bacterial secondary messenger molecule c di-GMP is a STING operation. Protein Cell. 2013;4:117–129. doi: 10.1007/s13238-012-2071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Zhang X., Wu T., Wang Y., Meng J., Liu Q., Niu X., Wu Y. The papain-like protease of avian infectious bronchitis virus has deubiquitinating activity. Arch. Virol. 2017;162:1943–1950. doi: 10.1007/s00705-017-3328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Liu X., Liu H., Wang W., Liu X., Li X., Wu X. Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication. Microb. Pathog. 2018;114:124–128. doi: 10.1016/j.micpath.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xu Z., Cao Y. Host antiviral responses against avian infectious bronchitis virus (Ibv): Focus on innate immunity. Viruses. 2021;13:1698. doi: 10.3390/v13091698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.W., Zhang J.Y., Zhou H.B., Guo Y.P., Ma Q.G., Ji C., Zhao L.H. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poult. Sci. 2020;99:5389–5398. doi: 10.1016/j.psj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]