Abstract
Grasslands cover up to 40.5% of the world's landmass and store 30% terrestrial carbon (C). Various practices, including mineral fertilization and liming, are used to manage these ecosystems with potential long-term effects on the size and distribution of soil aggregates and inevitably carbon dynamics. The objective of this study was to examine the long-term effects of nitrogen fertilization and liming on soil carbon storage and its dynamics in water-stable aggregates of a semi-arid grassland. Soil samples (0–10 cm) were collected from Ukulinga long-term grassland trial in Pietermaritzburg, South Africa where nitrogen fertilizers have been applied annually and lime every five years for 70 years. Ten treatments were studied: the control (0 kgN/ha and unlimited), lime at 2250 kg/ha (L), ammonium sulphate at 70 kg/ha (AS70) and 211 kg/ha (AS211); ammonium nitrate at 70 kg/ha (AN70) and 211 kg/ha (AN211); AS70 + lime (AS70L); AS211 + lime (AS211L); AN70 + lime (AN70L) and AN211 + lime (AN211L).
Nitrogen fertilizers significantly reduced soil pH and increased total soil N. Liming increased soil pH with no effect on total soil N. Lime and lime + N fertilizer treatments had no effect on mean weight diameter (MWD) while separate N application decreased MWD and large macro-aggregates (LMA). Lime only treatment had no effect on water stable aggregate (WSA) fractions. Nitrogen fertilization and liming (separately or in combination) did not affect total C concentration and stocks. Overall, soils had very high total soil organic carbon ranging from 49.7 – 57.6 g/kg across treatments. Nitrogen fertilization decreased organic carbon in LMA in AS70 (1.52%) and AN211 (1.67%) treatments compared to the control (3.40%) which was in concert with increases in C associated with small macro-aggregates (SMA) and micro-aggregates (MiA and SCA). Organic carbon in SMA was 2.67 % (AS70); AS211 (2.62 %); AN70 (2.02 %); AN211 (2.49 %) compared to 1.26 % in the control. Lime + N fertilizer treatments increased C storage in all aggregate fractions compared to N fertilizer only treatments. The lack of response in total SOC to 70 years of N fertilization and liming suggests possible C saturation given the high soil C concentration. Changes in C associated with WSA fractions suggests their importance as diagnostic indicators of N fertilization and liming induced changes in SOC. Findings also show that ammonium-based N fertilization is associated with soil acidification, dispersion of LMA resulting in an increase of microaggregates and C stored in them. Liming can counteracts acidifying and the dispersive effect on NH4+ associated with ammonium-based fertilizers thus restoring macro-aggregation in N fertilized grasslands. These findings suggests that long-term N addition may result in poor soil physical condition and possible stabilization of C in stable fractions.
Keywords: Carbon storage, Mean weight diameter, Lime, Ammonium sulphate, Ammonium nitrate, Soil aggregates
Highlights
-
•
Long-term N addition and liming did not affect bulk SOC content and stocks but lead to redistribution of C into water stable aggregates.
-
•
Long-term N fertilization caused soil acidification and reduced mean weight diameter.
-
•
Long-term N fertilization increased micro-aggregates and SOC stored in them.
-
•
Long-term liming did not affect WSA, and C stored in them.
-
•
Liming improved aggregation and increased C associated with large macro aggregates when co-applied with N fertilizers.
Carbon storage; Mean weight diameter; Lime; Ammonium sulphate; Ammonium nitrate; Soil aggregates.
1. Introduction
Grasslands are considered among the worlds’ largest ecosystems consisting of rangelands, shrublands, pastureland and croplands sown with pasture and fodder crops (Rumpel et al., 2015). In total, these ecosystems are estimated to cover up to 52.5 million square kilometres (Suttie et al., 2005), which make up to 40.5 % of the terrestrial area. Grasslands store about 30 % of terrestrial C (Cenini et al., 2015) and contribute significantly to carbon and nutrient cycling. Management practices such as liming and nitrogen fertilization, widely used on often N-limited grasslands, affect soil physicochemical properties of this ecosystem. Nitrogen (N) fertilization increases N availability and plant growth thus increasing above-ground C storage. Nitrogen availability has a major control on soil C cycling and storage (Averill and Waring, 2018).
Inconsistent results have been reported with regards to total SOC in response to N addition with some studies reporting no response (Hassink, 1994; Zeglin et al., 2007; Lu et al., 2011), decrease (Luo et al., 2019) and increase (Du et al., 2014; Cenini et al., 2015; Eze et al., 2018; Crème et al., 2020; Glendining et al., 1996). No response of total SOC has been attributed to decoupling of above-ground and below-ground C dynamics due to complex C related processes associated with N addition causing lack of soil C storage (Lu et al., 2021). High above-ground C storage and organic inputs to soils does not always result in increased soil C storage suggesting a divergent below-ground C process (Lu et al., 2011). In addition, some soils have been found to be carbon saturated with respect to C inputs (Stewart et al., 2007). High C inputs and/or high soil C levels have been associated with decreased bulk soil C stabilization in long-term agroecosystem experiments (Nyborg et al., 1995; Reicosky et al., 2002; Gulde et al., 2008) suggesting a saturation level for soil C (Six et al., 2002). Nitrogen availability together with high plant tissue N due to N addition accelerates soil organic matter decomposition explaining a decrease in soil C observed in some studies. On the other hand, N addition can slow microbial activity and litter decomposition resulting in enhanced C input and reduced C output resulting in increased C storage (Buchkowski et al., 2015; Chen et al., 2020). Size and turnover of labile and recalcitrant C pools are largely influenced by differences in soil management, ecosystems and soil properties making it difficult to predict the net effect of N enrichment on soil C storage (Neff et al., 2002). According to Averill and Waring (2018) interaction among plants, microbes and soil properties as well as control by microbial physiology, soil mineralogy and acidity can explain observed contrasting effects of N addition on soil C dynamics. Moreover, Schmidt et al. (2011) showed the persistence of organic carbon in ecosystems as being a function of complex interactions between organic matter and its environment.
Grasslands and pastures are subjected to acidic conditions due to fertilizer application or extensive cation leaching (Paradelo et al., 2015). Application of ammonium-based fertilizers are particularly responsible for significant soil acidification in human-managed pastured. The N-induced acidity results in loss of basic cations and high aluminum toxicity. Loss of Ca due to N-induced acidity interferes with the bridging of clays and organic materials thus decreasing stability of water stable aggregates (Oades, 1984). Exchangeable Ca contributes significantly to aggregation through flocculation of clay particles (Rengasamy and Marchuk, 2011). Moreover, fertilization-induced release of NH4+ is associated with reduced aggregate stability (Haynes and Naidu, 1998). Adsorption of NH4+ (low hydration energy) in place of cations with high hydration energy (e.g. Mg2+ and Ca2+) results in the collapse of the interlayer (Rigol et al., 1999) due to NH4+-induced dispersion (Blanco-Canqui, 2020). Liming is employed to remediate the effect of acidity on soil's physical and chemical properties. A review by Paradelo et al. (2015) showed that several factors determine the net effect of liming on SOC. For example, (a) liming can increase microbial activity by creating more favourable pH conditions, which will enhance OM mineralization, thus decreasing SOC stocks (b) favourable pH condition increases plant productivity and net primary production (NPP) resulting in more significant OM inputs in the form of dead roots and decaying crop residues consequently increasing SOC and (c) liming is known to ameliorate soil structure thus increasing clay assemblages and clay-organic matter bonds creating physical and physico-chemical protection of SOC.
Stabilization of organic matter in soil aggregates is the principal mechanism for long-term soil C sequestration (Verchot et al., 2011; Mosquera-Losada et al., 2015). Soil aggregation is one of the main factors that encourage the persistence of SOC by forming a protecting barrier that impedes microbial access and increases water filtration, thus reducing water runoff and C losses through erosion (Bai et al., 2021). Generally, increases in SOM are associated with C-rich macro-aggregates but long-term C sequestration depends on stabilization of carbon in micro-aggregates (Tisdall and OADES, 1982; Six et al., 2000a, Six et al., 2000b). According to Wu et al. (2004) soil organic carbon (SOC) in different size aggregates characterizes the relationship between organic matter balance and mineralization rate with dual significance in soil fertility and carbon sink. Organic C stored in differently sized stable aggregates has shown a strong response to management compared to bulk SOC (Denef et al., 2007; Wang et al., 2015). For example, Chen et al. (2020) reported a significant increase in mineral associated C while no response was observed for bulk SOC following N addition to B. platyphylla forest. According to Lu et al. (2021) the combined effects of individual aggregate C drives the general response of soil C pools to N enrichment. This suggests that separating bulk soil into different functional C pools may be used as a diagnostic indicator for potential changes and reveal observed complex responses of SOC to N addition. Investigating carbon pools in soil aggregates thus provides an understanding of the dynamics of carbon sequestration and mineralization in aggregates (Whalen and Chang, 2002).
Despite many studies on the effect of N enrichment on bulk soil C dynamics, there is lack of evidence on the mechanisms explaining the response of soil aggregates (Lu et al., 2021). In addition, most long-term studies on grassland management focus on separate effects of liming and nitrogen on SOC, C in aggregates and aggregates stability while little attention has been given to their interaction effects on these parameters. For example, Johnson et al. (2005) has shown that a combination of lime and N affect soil microbial biomass, soil pH, and total N in a short-term upland grassland experiment in Scotland but with inconsistent results due to differences in climate, soil properties, and type of fertilizer. Moreover, similar studies from Ukulinga veld fertilization experiment in South Africa, focused on plant productivity (Le Roux and Mentis, 1986), plant diversity (Grunow et al., 1970; Scott and Rabie, 1956), soil respiration (Ward et al., 2017b), and soil biology (Zeglin et al., 2007) as affected by fertilizer and lime addition. Soil properties such as SOC, MWD, and aggregate stability are reported as essential soil characteristics and not profoundly examined on the possible processes involved. This paper aims to examine the long-term effects of nitrogen fertilization and liming on water-stable aggregates (WSA) fractions and carbon stored in them. We hypothesize that; (1) Nitrogen application will increase carbon concentration in bulk soil and decrease WSA because of N-induced effects on soil pH and aggregation; (2) Liming will increase the WSA, MWD, and increase C concentration in macro-aggregates.
2. Material and methods
2.1. Study site
The study was conducted in a long-term grassland trial located at the University of KwaZulu Natal Ukulinga research farm, in Pietermaritzburg, South Africa (29° 24′E, 30° 24′S) (Figure 1). The area is semi-arid with mean annual precipitation of 790 mm and located on a plateau at 838 m a.s.l. (Ward et al., 2017b, 2020). The vegetation of the area is southern tall grass veld or, at a larger spatial scale, KwaZulu-Natal hinterland thornveld, which is an open savanna of Acacia Vachellia sieberiana with patches of Hyparrhenia hirta L. and other herbaceous species (Ward et al., 2017d). The soil is a Westleigh form, derived from localized dolerite intrusions into a shale parent material (Rutherford et al., 2006).
Figure 1.
Location of the long-term grassland study site in South Africa. (Abdalla et al., 2016).
2.2. Experimental design
The experiment was established in 1951 with the application of nitrogen, phosphorus, and lime as the main treatments and had the average pH of 5.7 (Scott and Rabie., 1956). This study focused on nitrogen, applied annually as ammonium nitrate and ammonium sulphate at 70 and 211 kg N/ha and dolomite lime applied at 2250 kg/ha every five years. The experiment was laid out in a randomized block design with 9.0 × 2.7 m size plots replicated three times (Morris and Fynn, 2001; Ward et al., 2017d). The following treatments were selected: 1) Control (0 kg lime or N fertilizer/ha); 2) lime at 2250 kg/ha (L); 3) ammonium sulphate at 70 kg/ha (AS70); 4) ammonium sulphate at 211 kg/ha (AS211); 5) ammonium nitrate at 70 kg/ha (AN70); 6) ammonium nitrate at 211 kg/ha (AN211); 7) ammonium sulphate at 70 kg/ha + lime (AS70L); 8) ammonium sulphate at 211 kg/ha + lime (AS211L); 9) ammonium nitrate at 70 kg/ha + lime (AN70L); and 10) ammonium nitrate at 211 kg/ha + lime (AN211L).
2.3. Soil sampling and analysis
Soil samples were taken at the fall of 2020 where five subsamples were collected from 0-10 cm depth of each plot using an auger and mixed to make a composite sample. The samples were air-dried for five days, sieved (<2 mm) and stored in plastic jars for further analysis. Soil bulk density was determined using the core method with the samples collected from the middle of the plots using a steel core sampler with 7.5 cm diameter 5 cm height (Blake, 1965).
Soil samples were analyzed for exchangeable bases, total N, and pH using method described by Non-Affiliated Soil Analysis Committee (1990). Ammonium acetate (pH 7) was used for the extraction of exchangeable bases which were then quantified with the atomic adsorption spectrophotometry (Varian 2600). Soil pH was measured using a 1:2.5 ratio of soil: 1M KCL. Total N was measured using the LECO Trumac CNS Autoanalyser (2012).
Aggregate stability was determined using the wet sieving method as described by (Six et al., 2000a). Air-dried samples were sieved through an 8 mm sieve. An 80 g of 8 mm sieved soil sample was placed on a 2 mm sieve and submerged in a bowl with water for 5 min, followed by sieving for 2 min by moving the sieve up and down 50 times. The material remaining on the 2 mm sieve was transferred by backwashing into a pre-weighed pan and dried for 48 h at 60 °C. By repeating wet sieving using 250 μm, 53 μm sieves, four aggregate fractions were obtained. The water-stable soil aggregates (WSA) classes were separated into large macro-aggregates (LMA; > 2 mm), small macro-aggregates (MMA; > 250 μm–2 mm), micro-aggregates (MiA; 53–250 μm) and silt + clay fractions (SCA; < 53 μm). Mean weight diameter for WSA was used as a measure of aggregate stability. The MWD was calculated using the equation:
| (1) |
(Abdalla et al., 2016) where Xi is the mean diameter for each fraction size, Wi is the proportional weight of the fraction from the total dry weight of soil used, and n is the number of aggregate classes separated. Organic C in water-stable soil aggregate fractions and bulk soil was analyzed using the Walkley- Black dichromate oxidation method (Walkley and Black, 1934). Carbon measured in each pool was then expressed as a percent of total SOC of bulk soil which allowed for the calculation of the percent recovery of soil C. The recovery of total SOC after fractionation was 87.7–99.5% across all treatments. Soil carbon stocks in bulk soil were calculated using the formula
| (2) |
(Don et al., 2007) where SOCstocks = soil organic carbon stocks (t.ha−1), BD = Bulk density (g.cm−3), SOCconc = soil organic carbon concentration (g/kg) and depthvolume is the sampling depth (cm).
2.4. Statistical analysis
Statistical analysis was done using GENSTAT 18th edition. One-way analysis of variance (ANOVA) was used to compare effects of different treatments on studied soil properties. Treatment means were compared using Turkey's multiple comparisons with a significant difference at p < 0.05.
3. Results
3.1. Soil pH, organic carbon, nitrogen and aggregate stability
Long-term nitrogen addition and liming significantly (p < 0.05) altered pH, total N and MWD. The addition of N fertilization as ammonium sulphate significantly decreased soil pH irrespective of rate (Table 1). Only the higher application rate of ammonium nitrate (AN211) significantly decreased soil pH compared to the control. Lime (L) and all lime and nitrogen fertilizer combinations, except AS211L, significantly increased soil pH (p < 0.001) compared to the control (Table 1). Soil organic C content was not significantly altered by liming and N addition when compared with the control. Carbon stocks ranged from 43.4 to 50.6 tons C/ha across all treatments. Total soil N in AN211 (4.15 g N/kg) was comparable to AS70 and AS211 and all were significantly higher than AN70 and control (Table 1).
Table 1.
Analysis of variance for different N and liming application on pH, exchangeable bases, organic carbon, total nitrogen and mean weight diameter.
| Treatment | pH | Exch. Ca2+ |
Exch. Mg2+ |
Exch. K+ |
SOC |
C stocks |
Total N |
MWD |
|---|---|---|---|---|---|---|---|---|
| (cmol+/kg) | (g/kg) | (t/ha) | (g/kg) | (mm) | ||||
| Control | 4.31b | 7.71c | 4.00bc | 0.56 | 49.7ab | 43.34 | 3.11a | 3.66bc |
| L | 6.56d | 17.04d | 4.90c | 1.09 | 50.4ab | 46.8 | 3.39abc | 4.03c |
| AS70 | 3.54a | 2.27ab | 0.83a | 0.77 | 57.2b | 50.6 | 3.98cd | 2.01a |
| AS211 | 3.27a | 1.03a | 0.28a | 0.74 | 56.1b | 47.8 | 3.99cd | 3.01abc |
| AN70 | 4.11b | 6.36bc | 3.32b | 1.00 | 46.6a | 44.5 | 3.57ab | 2.67ab |
| AN211 | 3.61a | 3.07ab | 1.52a | 0.73 | 57.6b | 46.5 | 4.15d | 2.27a |
| AS70L | 6.05c | 14.52d | 4.82c | 0.87 | 50.2ab | 47.7 | 3.57abcd | 3.51bc |
| AS211L | 3.52a | 5.38bc | 1.31a | 0.51 | 53.3ab | 45.5 | 3.89bcd | 2.84ab |
| AN70L | 6.38 cd | 14.72d | 4.58bc | 0.85 | 49.1ab | 44.9 | 3.42abc | 3.61bc |
| AN211L | 5.97c | 15.30d | 4.74bc | 0.57 | 50.6ab | 46.6 | 3.83bcd | 3.58bc |
| P value | <.001 | <.001 | <.001 | 0.556 | 0.003 | 0.976 | <.001 | <.001 |
Values followed by a different letter in each column are significantly different (p < 0.05) according to Tukey's LSD procedure. C = control, L = lime (2250 kg ha−1), AS70 = ammonium sulphate at 70 kg ha−1; AS211 = ammonium sulphate at 211 kg ha−1; AN70 = ammonium nitrate at 70 kg ha−1; AN211 = ammonium nitrate at 211 kg ha−1; AS70L = ammonium sulphate at 70 kg ha−1 + lime; AS211L = ammonium sulphate at 211 kg ha−1 + lime; AN70L = ammonium nitrate at 70 kg ha−1 + lime and AN211L = ammonium nitrate at 211 kg ha−1 + lime. Exch.- exchangeable.
Mean weight diameter was only significantly decreased by the AS70 and AN211 treatments compared to the control (Table 1). While liming did not significantly affect MWD when compared to the control, the MWD in the L treatment (4.03) was significantly higher than in the AS70 (2.01), AN70 (2.67), AN211 (2.27), and AS211L (2.84).
Exchangeable Ca2+ and Mg2+ concentration were affected by lime and N addition (p < .001). The Ca2+ concentration ranged from 1.03 to 17.04 cmol+/kg across the treatments, while Mg2+ ranged from 0.28 to 4.90 cmol+/kg. Limed treatments significantly increased the concentration of exchangeable Ca2+ except AS211L compared to the control, while AS70 (2.27 cmol+/kg), AS211 (1.03 cmol+/kg) and AN211 (3.07 cmol+/kg) significantly decreased exchangeable Ca2+ when compared to the control (7.71 cmol+/kg) (Table 1). Exchangeable Mg2+ was lowered by AS70 (0.83 cmol+/kg), AS211 (0.28 cmol+/kg), AN211 (1.52 cmol+/kg) and AS211L (1.31 cmol+/kg) treatments (Table 1). Only the Lime (4.90 cmol+/kg) and AS70L (4.82 cmol+/kg) treatments had higher exchangeable Mg2+ concentration than the control. We found that neither long-term N addition nor liming affected exchangeable K+. Exchangeable bases followed the order Ca2+ > Mg2+ > K+ in all treatments.
3.2. Weight of water-stable aggregate fractions
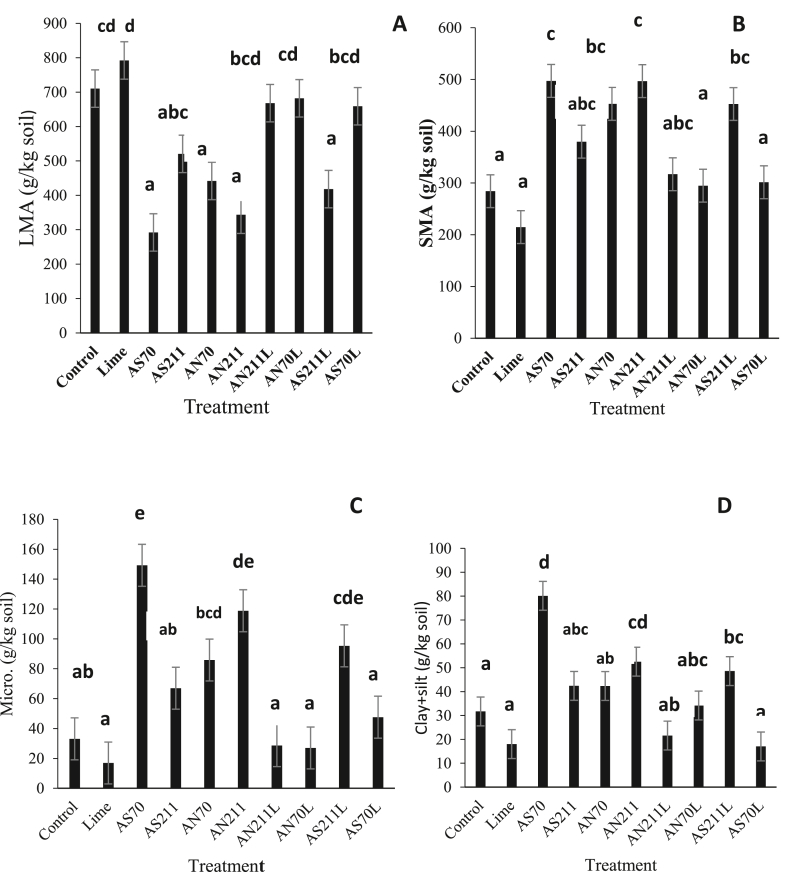
Long-term nitrogen fertilizer and liming application affected water-stable aggregates (Figure 2). Except for AS211, all nitrogen fertilizer treatments had significantly lower large-macro-aggregates (LMA; >2 mm) fractions compared to the control (Figure 2A). Separate lime application did not affect water stable aggregates. Except for the AS211L, the combination of lime with nitrogen fertilizer treatments had higher large macro-aggregates when compared with the N fertilizer treatments without lime. The fertilizer treatments without lime, except the AS211, increased SMA (>250 μm–2 mm) when compared with the control (Figure 2B). The results of micro-aggregates (MiA; 53–250 μm) (Figure 1C) and clay + silt fractions (Figure 2D) followed the same trend as that of SMA (Figure 2B). Both AS70 and AN211 had higher MiA and clay + silt fractions compared to the control. Only MiA fraction was higher than the control in AN70 treatment. The addition of lime to the fertilizer treatments decreased the SMA and MiA fractions except in the AS211L wherein both fractions increased. Similarly, the combination of lime with AS70 and AN211 decreased the clay + silt fractions, while liming the AS11 treatment increased this fraction compared to the same treatments without lime. The WSA were in the order; (LMA; >2 mm) > (SMA; >250 μm–2 mm) > (SCA; < 53 μm) > (MiA; 53–250 μm), with the macroaggregate (>250 μm) fractions making up >50% of the total soil weight in all treatments.
Figure 2.
Masses of large macro-aggregates (A) (LMA; >2 mm), (B) Small macro-aggregates (C) micro-aggregates, and (D) silt + clay fractions for different treatments. Error bars represent standard error (n = 3).
3.3. Organic carbon in water-stable aggregates
The SOC content in the AS70 and AN211 treatments was significantly lower in the LMA fraction and higher in the SMA when compared to the control (Table 2). Lime and lime+ N fertilizer treatments did not significantly change carbon concentration in differently sized aggregates compared to the control (Table 2). However, lime and N fertilizer combination treatments had significantly higher C in all aggregate fractions compared to the same treatments without lime. Overall the control, L, AS70L, and AN70L, and AN211L had >3% C stored in the LMA fraction (Table 2). In all treatments, more than 60 % of SOC was stored in large macro-aggregates except for AS70, AN70, AN211, and AS211L where more carbon was stored in small macro-aggregates (>45 % of SOC). The results of SOC in the other fractions followed an inverse trend to that in the LMA, where the AS211L, AN211, and AS70 treatments significantly increased C in micro aggregate (53–250 μm) fraction in the order; AS211L < AN211 < AS70. Limed plot had lowest C content in MiA; 53–250 μm (0.09 %) compared to AS70 (2.67%), AN211 (2.49%) and AS211L (2.31%). Clay and silt had the least carbon content of all the aggregate fractions of which only AS70 had a significantly higher concentration compared to control (Table 2). Both micro aggregates and clay and silt fraction (<250 μm) stored less than 20% of the total C. There was a significant difference between L and nitrogen fertilized plots, where nitrogen fertilized plots increased C with an increase in N level in clay + silt compared to limed plots (Table 2).
Table 2.
Organic carbon in water-stable aggregates.
| Treatment | % Organic C in WSA |
|||
|---|---|---|---|---|
| LMA; >2 mm | SMA; >250 μm–2 mm | MiA; 53–250 μm | SCA; < 53 μm | |
| Control | 3.40cd | 1.26ab | 0.17abc | 0.13abc |
| Lime | 3.65d | 0.96a | 0.09a | 0.079ab |
| AS70 | 1.52a | 2.67c | 0.86e | 0.37d |
| AS211 | 2.58abcd | 2.02abc | 0.47cd | 0.24cd |
| AN70 | 1.91abc | 2.02abc | 0.39bcd | 0.15abc |
| AN211 | 1.67ab | 2.49c | 0.65de | 0.25cd |
| AS70L | 3.41cd | 1.35ab | 0.22abc | 0.064a |
| AS211L | 2.08abcd | 2.31bc | 0.53d | 0.22bc |
| AN70L | 3.34bcd | 1.58abc | 0.15ab | 0.15abc |
| AN211L | 3.07abcd | 1.48abc | 0.12ab | 0.067ab |
| p value | <.001 | <.001 | <.001 | <.001 |
Values followed by a different lowercase letter in the same column are significantly different (p < 0.05) according to Tukey's LSD procedure. C = control (0 kg/ha), L = lime (2250 kg ha−1), AS70 = ammonium sulphate at 70 kg ha−1; AS211 = ammonium sulphate at 211 kg ha−1; AN70 = ammonium nitrate at 70 kg ha−1; AN211 = ammonium nitrate at 211 kg ha−1; AS70L = ammonium sulphate at 70 kg ha−1 + lime; AS211L = ammonium sulphate at 211 kg ha−1 + lime; AN70L = ammonium nitrate at 70 kg ha−1 + lime and AN211L = ammonium nitrate at 211 kg ha−1 + lime.
4. Discussion
Long-term nitrogen fertilizer addition resulted in significant soil acidification as pH ranged from 3.27-3.61 in N fertilized plots compared to 4.11 in the control. Similar results have been reported in several studies on the effect of long-term application of ammonium-based fertilizer (Malhi et al., 1998; Rasmussen and Rohde, 1989; Xu et al., 2020). Long-term application of nitrogen decreased soil pH in 0–10 cm depth by >16%. Ammonium based fertilizers are known to acidify the soil due to nitrification of NH4+ which releases H+ ions, further reducing the pH of this acidic soil (Are et al., 2018; Bouman et al., 1995). The greater acidifying effect of ammonium sulphate than of ammonium nitrate was due to the higher ammonium added in former than the latter at a given application rate. This has serious implications for grassland species composition and diversity which has been shown to decrease under nitrogen addition (Fang et al., 2012; Midolo et al., 2019; Ward et al., 2017c).
High total N concentration may be explained by significant amounts of N added (70 kg N/ha and 211 kg N/ha) annually via fertilization and reduced nitrification due to N-induced acidity. The recorded increase in soil N with increasing N rates for both N fertilizers is coherent with earlier findings (Ward et al., 2017a). Nitrification process is sensitive to acidic soils and limited by high Al and Mn or H+ concentrations (Robson and Abbott, 1989).
While some authors reported decline (Luo et al., 2019), increase (Eze et al., 2018; Fornara and Tilman, 2012; Xu et al., 2020) in SOC concentration and C stocks in grasslands with N addition and liming, SOC was not significantly affected by these treatments in this study. Current findings are consistent with earlier results from Ukulinga grassland experiment (Zeglin et al., 2007). Lack of SOC response may be attributed to possible carbon saturation. Studied soils have very high C, ranging from 49.7 g/kg to 57.6 g/kg across treatments, which may have decreased bulk soil C stabilization (Six et al., 2006). Carbon saturation prevents addition of new SOC even in fertilized plots with high C inputs. High soil carbon stocks in the surface layer of most grassland soils cause carbon saturation deficit to be relatively low therefore limiting the increase in soil carbon due to carbon inputs from residues (Whitehead et al., 2018). Angers et al. (2011) showed that soil pH and clay and silt particles are strongly related to carbon saturation deficit. Our soils were acidic and had a moderate proportion of clay and silt, which could also support carbon saturation (Angers et al., 2011). While Are et al. (2018) showed a positive correlation between SOC and aggregate stability, the relationship of SOC with aggregate stability was not significant in this study. However, aggregate size distribution and the SOC stored in the differently sized aggregate fractions were affected by N fertilizer application.
Nitrogen addition decreased the proportion of large macro-aggregates and increased micro-aggregates fractions which reduced mean weight diameter. This was due to the acidifying effect of N which led to rapid depletion of Ca2+ and Mg2+ (Table 1) cations playing an important role in aggregation through flocculation of clay particles (Rengasamy and Marchuk, 2011). Furthermore, ammonium-based fertilizers release NH4+ (with low hydration energy) to the soil which is adsorbed in place of high hydration energy cations (e.g. Ca2+ and Mg2+) causing dispersion of soil aggregates as the interlayer collapses (Rigol et al., 1999). N-induced acidity may have reduced nitrification making NH4+ a predominate form of N in studied plots. As such, NH4+-induced dispersion may also explain reduced fraction of large macroaggregates and MWD under separate N fertilization (Table 1). Similarly, Blanco-Canqui and Schlegel (2013) reported a decrease in aggregate stability upon addition of N at >100 kg N/ha/yr. Bai et al. (2020) found a strong correlation between MWD and exchangeable Mg2+ and fungal biomass. The acidity caused by increased N fertilizer application rate has been shown to reduce fungal biomass, root growth and length responsible for excreting binding agents.
Conversely, liming increased the soil pH towards neutral (pH 6.56). However, its effect was less significant when combined with the higher application rate of ammonium sulphate fertilizer due to the H+ released through nitrification. Liming also increased basic cations (Table 1) thus encouraging the formation of cation bridging between negatively charged clay surfaces and organic compounds (Bai et al., 2020). However, the effect of lime on WSA was only evident when applied in combination with N fertilizers. This suggests that an increase in soil pH when lime was led to improved conditions for nitrification thus decreasing NH4+ concentration. This may have counteracted the N-induced effects on soil aggregation, especially where lime was combined with AS70 and AN211. These results imply that liming helps maintain a stable soil structure while N addition can make the soil susceptible to erosion and degradation. Interestingly, AS211L did not increase MWD as expected with lime addition, instead, it reduced LMA, possibly due to a greater acidifying effect than the other N fertilizer treatments.
Several studies recorded higher SOC in macro-aggregates than in micro-aggregates after long-term application of mineral and/or organic fertilizers (Bhattacharyya et al., 2010; Tripathi et al., 2014). Generally, 80% of the total C is associated with macro-aggregates (>250 μm) in the upper soil layers of grasslands (Chen et al., 2019; Schwendenmann and Pendall, 2006). Although SOC in bulk soil was not affected by treatments, it can be said that SOC was redistributed into smaller fractions upon the breakdown of aggregates rather than being lost by decomposition suggesting that C dynamics were driven more by fertilizer and liming induced effect on aggregates. Applying N fertilizer without lime led to more carbon in SMA, MiA and SCA (Table 2) possibly due N-induced acidity and oxides rather than organic material being the primary binding agents. This high C in micro-aggregates suggests long-term storage of C as it is generally protected from microbial attack and thus have longer resident time compared to that stored in macro-aggregates (Six et al., 2000b).
Lime and control treatments had significantly higher mean values of OC in the LMA while their SMA and micro-aggregate fractions had lower OC compared to N fertilized plots. These results are consistent with the concept of aggregate hierarchy, which states that increasing aggregate class size results in an increase in C concentration in which micro-aggregates are bound together into macro-aggregates by binding agents (Six et al., 2000b; Tisdall and Oades, 1982). It seems that liming was effective as a control of N-induced acidity and NH4+ toxicity as no effects were observed when lime was applied separately.
5. Conclusion
The findings of this study showed that long-term N addition resulted in soil acidification and redistribution of organic C in water-stable aggregates at Ukulinga grassland. Nitrogen fertilizer, particularly ammonium sulphate, resulted in increased acidity and total soil N, reduction in both aggregate stability and exchangeable Ca and Mg. Liming had a reverse effect on soil pH and exchangeable bases but no effect on total soil N and MWD (except when applied in combination with N fertilizers). Total SOC did not respond to N fertilization and liming suggesting that studied soils may be carbon saturated thus limiting addition of new SOC from residue inputs. Separate application of N fertilizers decreased large macro-aggregates and increased micro-aggregates while separate liming had no effect on WSA. Despite lack of response of bulk soil C, N addition and liming resulted in redistribution of SOC in differently sized aggregate fractions. Nitrogen addition had a strong influence on labile organic carbon which was shifted to stable soil fractions confirmed by high C in SMA and micro-aggregates. Liming alone did not affect C associated with water stable aggregates while combination of lime with N fertilizers increased C associated with labile fractions and lowered it in stable fractions compared to separate N fertilization. This suggests that induced soil pH changes were the main driver of SOC redistribution and shift in WSA distribution due N fertilization and liming at Ukulinga grassland experiment. Carbon associated with differently sized aggregates may thus be used as diagnostic indicators of management induced changes in SOC. Liming may be used to ameliorate N-induced effects on soil pH and structure when N fertilizers are applied on grasslands. This will ensure that while the goal is mainly to improve grassland productivity using N fertilizers, caution is also taken to maintain good soil condition.
Declarations
Author contribution statement
Nkosinomusa Buthelezi-Dube: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kwenama Buthelezi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by National Research Foundation (122198).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge the valuable assistance of soil science technical staff at the university of KwaZulu-Natal.
References
- Abdalla K., Chivenge P., Everson C., Mathieu O., Thevenot M., Chaplot V. Long-term annual burning of grassland increases CO2 emissions from soils. J. Geoderma. 2016;282:80–86. [Google Scholar]
- Angers D., Arrouays D., Saby N., Walter C. Estimating and mapping the carbon saturation deficit of French agricultural topsoils. Soil Use Manag. 2011;27:448–452. [Google Scholar]
- Are M., Kaart T., Selge A., Astover A., Reintam E. The interaction of soil aggregate stability with other soil properties as influenced by manure and nitrogen fertilization. Zemdirbyste-Agriculture. 2018;105 [Google Scholar]
- Averill C., Waring B. Nitrogen limitation decomposition and decay:How can it occur? Glob. Change Biol. 2018;24(4):1417–1427. doi: 10.1111/gcb.13980. [DOI] [PubMed] [Google Scholar]
- Bai T., Wang P., Hall S.J., Wang F., Ye C., Li Z., Li S., Zhou L., Qiu Y., Guo J. Interactive global change factors mitigate soil aggregation and carbon change in a semi-arid grassland. Glob. Change Biol. 2020;26:5320–5332. doi: 10.1111/gcb.15220. [DOI] [PubMed] [Google Scholar]
- Bai T., Wang P., Ye C., Hu S. Form of nitrogen input dominates N effects on root growth and soil aggregation: a meta-analysis. Soil Biol. Biochem. 2021:108251. [Google Scholar]
- Bhattacharyya R., Prakash V., Kundu S., Srivastva A., Gupta H., Mitra S. Long term effects of fertilization on carbon and nitrogen sequestration and aggregate associated carbon and nitrogen in the Indian sub-Himalayas. Nutr. Cycl. Agroecosyst. 2010;86:1–16. [Google Scholar]
- Blake G. Bulk density. Method Soil Anal.: Part 1 Phys. Mineral. Propert. Includ. Stat. Measure. Sampl. 1965;9:374–390. [Google Scholar]
- Blanco-Canqui H. In: No-till Farming Systems for Sustainable Agriculture: Challenges and Opportunities. Dang Y.P., Dalal R., Menzies N.W., editors. Springer; Cham: 2020. Enhancing soil aggregation in no-till farming systes; pp. 233–250. [Google Scholar]
- Blanco-Canqui H., Schlegel A.J. Implications of inorganic fertilization of irrigates corn onsoil properties:Lessons learned after 50 years. J. Environ. Qual. 2013;42(3):861–871. doi: 10.2134/jeq2012.0451. [DOI] [PubMed] [Google Scholar]
- Bouman O., Curtin D., Campbell C., Biederbeck V., Ukrainetz H. Soil acidification from long-term use of anhydrous ammonia and urea. Soil Sci. Soc. Am. J. 1995;59:1488–1494. [Google Scholar]
- Buchkowski R.W., Schmitz O.J., Bradford M.A. Microbial stoichiometry overrides biomass as a regulator of soil carbon and nitrogen cycling. Ecology. 2015;96(4):1139–1149. doi: 10.1890/14-1327.1. [DOI] [PubMed] [Google Scholar]
- Cenini V.L., Fornara D.A., McMullan G., Ternan N., Lajtha K., Crawley M.J. Chronic nitrogen fertilization and carbon sequestration in grassland soils: evidence of a microbial enzyme link. Biogeochemistry. 2015;126:301–313. [Google Scholar]
- Chen Z., Zhou X., Geng S., Miao Y., Cao Y., Chen Z., Zhang J., Han S. Interactive effect of nitrogen addition and throughfall reduction decreases soil aggregate stability through reducing biological binding agents. For. Ecol. Manag. 2019;445:13–19. [Google Scholar]
- Chen J., Ji C., Fang J., He H., Zhu B. Dynamics of microbial residues control the responses of mineral-associated soil organic carbon to N addition in two temperate forests. Sci. Total Environ. 2020;748:141318. doi: 10.1016/j.scitotenv.2020.141318. [DOI] [PubMed] [Google Scholar]
- Crème A., Rumpel C., Malone S.L., Saby N.P.A., Vaudour E., Decau M., Chabbi A. Monitoring grassland management effects on soil organic carbon- A matter of scale. Agronomy. 2020:10122016. [Google Scholar]
- Denef K., Zotarelli L., Boddey R.M., Six J. Microaggregate-associated carbon as a diagnostic fraction for management-induced changes in soil organic carbon in two Oxisols. Soil Biol. Biochem. 2007 [Google Scholar]
- Don A., Schumacher J., Scherer-Lorenzen M., Scholten T., Schulze E.-D. Spatial and vertical variation of soil carbon at two grassland sites—implications for measuring soil carbon stocks. Geoderma. 2007;141:272–282. [Google Scholar]
- Du Z., Xie Y., Hu L., Hu L., Xu S., Li D., Wang G., Fu J. Effects of fertilization and clipping on carbon, nitrogen storage, and soil microbial activity in a natural grassland in southern China. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze S., Palmer S.M., Chapman P.J. Soil organic carbon stock in grasslands: effects of inorganic fertilizers, liming and grazing in different climate settings. J. Environ. Manag. 2018;223:74–84. doi: 10.1016/j.jenvman.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Fang Y., Xun F., Bai W., Zhang W., Li L. 2012. Long-term Nitrogen Addition Leads to Loss of Species Richness Due to Litter Accumulation and Soil Acidification in a Temperate Steppe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornara D.A., Tilman D. Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology. 2012;93:2030–2036. doi: 10.1890/12-0292.1. [DOI] [PubMed] [Google Scholar]
- Glendining M., Powlson D., Poulton P., Bradbury N., Palazzo D., Ll X. The effects of long-term applications of inorganic nitrogen fertilizer on soil nitrogen in the Broadbalk Wheat Experiment. J. Agric. Sci. 1996;127:347–363. [Google Scholar]
- Gulde S., Chung H., Amelung W., Chang C., Six J. Soil carbon saturation controls labile and stable carbon pool dynamics. Soil Sci. Soc. Am. J. 2008;72:605–612. [Google Scholar]
- Grunow J., Pienaar A., Breytenbach C. Long term nitrogen application to veld in South Africa. Proceed. Ann. Cong. Grassland Soc. South. Afr. 1970;5:75–90. [Google Scholar]
- Hassink J. Effects of soil texture and grassland managament on soil organic C and N and rates of C and N mineralization. Soil Biol. Biochem. 1994;26(9):1221–1231. [Google Scholar]
- Haynes R.J., Naidu R. Influence of lime, fertilizer and manure application on soil organic matter content and soil physical conditions: a review. Nutr. Cycl. Agroecosyst. 1998;51:123–137. [Google Scholar]
- Johnson D., Leake J., Read D. Liming and nitrogen fertilization affects phosphatase activities, microbial biomass and mycorrhizal colonisation in upland grassland. Plant Soil Biol. 2005;271:157–164. [Google Scholar]
- Le Roux N., Mentis M. Veld compositional response to fertilization in the tall grassveld of Natal. South Afr. J. Plant Soil Biol. 1986;3:1–10. https://hdl.handle.net/10520/AJA02571862_70 [Google Scholar]
- LECO Corporation . 2012. Trumac CNS/NS Detrminators. St Joseph. [Google Scholar]
- Lu M., Zhou X., Luo Y., Yang Y., Fang C., Chen J., Li B. Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agricul. Ecosyst. Environ. Monit. 2011;140:234–244. [Google Scholar]
- Lu X., Hou E., Guo J., Gilliam F.S., Li J., Tang S., Kuang Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: a meta-analysis. Global Change Biol. 2021 doi: 10.1111/gcb.15604. [DOI] [PubMed] [Google Scholar]
- Luo R., Fan J., Wang W., Luo J., Kuzyakov Y., He J.-S., Chu H., Ding W. Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2019;650:303–312. doi: 10.1016/j.scitotenv.2018.09.038. [DOI] [PubMed] [Google Scholar]
- Malhi S., Nyborg M., Harapiak J. Effects of long-term N fertilizer-induced acidification and liming on micronutrients in soil and in bromegrass hay. Soil Tillage Res. 1998;48:91–101. [Google Scholar]
- Midolo G., Alkemade R., Schipper A.M., Benítez-López A., Perring M.P., De Vries W. Impacts of nitrogen addition on plant species richness and abundance: a global meta-analysis. Glob. Ecol. Biogeogr. 2019;28:398–413. [Google Scholar]
- Morris C., Fynn R. The Ukulinga long-term grassland trials: reaping the fruits of meticulous, patient research. Bull. Grassland Soc. South. Afr. 2001;11:7–22. [Google Scholar]
- Mosquera-Losada M., Rigueiro-Rodríguez A., Ferreiro-Domínguez N. Effect of liming and organic and inorganic fertilization on soil carbon sequestered in macro-and microaggregates in a 17-year old Pinus radiata silvopastoral system. J. Environ. Manag. 2015;150:28–38. doi: 10.1016/j.jenvman.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Neff J.C., Gleixner G., Townsend A.R. Variable effects of nitrogen addition on the stability and turnover of soil carbon. Nature. 2002;419:915–917. doi: 10.1038/nature01136. [DOI] [PubMed] [Google Scholar]
- Non-Affliliated Soil Analysis Work Committee . 1990. Handbook of Standard Soil Testing Methods for Advisory Purposes. Pretoria, South Africa. [Google Scholar]
- Nyborg M., Solberg E.D., Malhi S.S., Izaurralde R.C. In: Advances in Soil Science: Soil Management and Greenhouse Effect. Lal R., Kimble J., Levine E., Stewart B.A., editors. CRC Press Inc.; Boca Ration, FL: 1995. Fertilizer N, crop residue, and tillage alter soil C and N content in a decade; pp. 93–100. [Google Scholar]
- Oades J.M. Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil. 1984;76(1):319–337. [Google Scholar]
- Paradelo R., Virto I., Chenu C. Net effect of liming on soil organic carbon stocks: a review. Agric. Ecosyst. Environ. 2015;202:98–107. [Google Scholar]
- Rasmussen P.E., Rohde C. Soil acidification from ammonium-nitrogen fertilization in moldboard plow and stubble-mulch wheat-fallow tillage. Soil Sci. Soc. Am. J. 1989;53:119–122. [Google Scholar]
- Reicosky D.C., Evans S.D., Cambardella C.A., Armaras R.R., Wilts A.R., Huggins D.R. Continuous corn with moldboard tillage: residue and fertility effects on soil carbon. J. Soil Water Conserv. 2002;57:277–284. [Google Scholar]
- Rengasamy P., Marchuk A. Cation ratio of soil structural stability (CROSS) Soil Res. 2011;49:280–285. [Google Scholar]
- Rigol A., Vidal R., Rauret G. Effect of the ionic status and drying on radiocesium adsorption and desorption in organic soils. Environ. Sci. Technol. 1999;33:3788–3794. [Google Scholar]
- Robson A., Abbott L. The effect of soil acidity on microbial activity in soils. Soil Acid. Plant Growth. 1989:139–165. [Google Scholar]
- Rumpel C., Crème A., Ngo P., Velásquez G., Mora M., Chabbi A. The impact of grassland management on biogeochemical cycles involving carbon, nitrogen and phosphorus. J. Soil Sci. Plant Nutr. 2015;15:353–371. [Google Scholar]
- Rutherford M.C., Mucina L., Powrie L.W. Biomes and bioregions of Southern Africa. Veget. South Afr. Lesotho Swaziland. 2006;19:30–51. [Google Scholar]
- Schmidt M.W.I., Torn M.S., Abiven S., Dittmar T., Guggenberger G., Janssens I.A., Kleber M., Kögel-Knabner I., Lehmann J., Manning D.A.C., Nannipieri P., Rasse D.P., Weiner S., Trumbore S.E. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- Schwendenmann L., Pendall E. Effects of forest conversion into grassland on soil aggregate structure and carbon storage in Panama: evidence from soil carbon fractionation and stable isotopes. Plant Soil Acidity Plant Growth. 2006;288:217–232. [Google Scholar]
- Scott J., Rabie J. Effects of certain fertilizers on veld at Ukulinga. South Afr. J. Sci. 1956;52:240–243. [Google Scholar]
- Stewart C.E., Paustian K., Conant R.T., Plant A.F., Six J. Soil carbon saturation: concept, evidence and evaluation. Biogeochemistry. 2007;86:19–31. [Google Scholar]
- Six J., Conant R.T., Paul E.A., Paustian K., et al. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil. 2002;241(2):155–176. [Google Scholar]
- Six J., Elliott E., Paustian K. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000;32:2099–2103. [Google Scholar]
- Six J., Frey S.D., Thiet R.K., Batten K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006;70:555–569. [Google Scholar]
- Six J., Paustian K., Elliott E.T., Combrink C. Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000;64:681–689. [Google Scholar]
- Suttie J.M., Reynolds S.G., Batello C. Food & Agriculture Org; 2005. Grasslands of the World. [Google Scholar]
- Tisdall J.M., Oades J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982;33:141–163. [Google Scholar]
- Tripathi R., Nayak A., Bhattacharyya P., Shukla A., Shahid M., Raja R., Panda B., Mohanty S., Kumar A., Thilagam V. Soil aggregation and distribution of carbon and nitrogen in different fractions after 41 years long-term fertilizer experiment in tropical rice–rice system. Geoderma. 2014;213:280–286. [Google Scholar]
- Verchot A.L., Dutaur L., Shepherd K.D., Albrecht A. Organic matter stabilization in aggregates: understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma. 2011;161:182–193. [Google Scholar]
- Walkley A., Black I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. [Google Scholar]
- Wang Q., Wang D., Wen X., Yu G., He N., Wang R. Differences in SOM decomposition and temperature sensitivity among soil aggregate size classes in a temperate grasslands. PLoS One. 2015;10(2):E117033. doi: 10.1371/journal.pone.0117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D., Kirkman K., Hagenah N., Tsvuura Z. Soil respiration declines with increasing nitrogen fertilization and is not related to productivity in long-term grassland experiments. Soil Biol. Biochem. 2017;115:415–422. [Google Scholar]
- Ward D., Kirkman K., Hagenah N., Tsvuura Z. Soil respiration declines with increasing nitrogen fertilization and is not related to productivity in long-term grassland experiments. Soil Biol. Biochem. 2017;115:415–422. [Google Scholar]
- Ward D., Kirkman K., Tsvuura Z. An African grassland responds similarly to long-term fertilization to the Park Grass experiment. Soil Biol. Biochem. 2017;12 doi: 10.1371/journal.pone.0177208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D., Kirkman K., Tsvuura Z. An African grassland responds similarly to long-term fertilization to the Park Grass experiment. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D., Kirkman K.P., Tsvuura Z., Morris C., Fynn R.W. Are there common assembly rules for different grasslands? Comparisons of long-term data from a subtropical grassland with temperate grasslands. J. Veg. Sci. 2020;31:780–791. [Google Scholar]
- Whalen J.K., Chang C. Macroaggregate characteristics in cultivated soils after 25 annual manure applications. Soil Sci. Soc. Am. J. 2002;66(5):1637–1647. [Google Scholar]
- Whitehead D., Schipper L.A., Pronger J., Moinet G.Y., Mudge P.L., Pereira R.C., Kirschbaum M.U., McNally S.R., Beare M.H., Camps-Arbestain M. Management practices to reduce losses or increase soil carbon stocks in temperate grazed grasslands: New Zealand as a case study. Agricul. Ecosyst. Environ. Monit. 2018;265:432–443. [Google Scholar]
- Wu J.G., Zhang X.Q., Xu D.Y., et al. Changes in soil labile organic carbon under different land use in the Liupan Mountain forest zone. Chin. J. Plant Ecol. 2004;28(5):657. [Google Scholar]
- Xu D., Carswell A., Zhu Q., Zhang F., de Vries W. Modelling long-term impacts of fertilization and liming on soil acidification at Rothamsted experimental station. Sci. Total Environ. 2020;713:136249. doi: 10.1016/j.scitotenv.2019.136249. [DOI] [PubMed] [Google Scholar]
- Zeglin L.H., Stursova M., Sinsabaugh R.L., Collins S.L. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia. 2007;154:349–359. doi: 10.1007/s00442-007-0836-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.