Summary
Background
In 2012, Fiji introduced the 10-valent pneumococcal conjugate vaccine (PCV10). We assessed the impact of PCV10 on invasive pneumococcal disease (IPD), probable bacterial or pneumococcal meningitis (PBPM), meningitis and sepsis 3-5 years post-introduction.
Methods
Laboratory-confirmed IPD and PBPM cases were extracted from national laboratory records. ICD-10-AM coded all-cause meningitis and sepsis cases were extracted from national hospitalisation records. Incidence rate ratios were used to compare outcomes pre/post-PCV10, stratified by age groups: 1-23m, 2-4y, 5-9y, 10-19y, 20-54y, ≥55y. To account for different detection and serotyping methods in the pre-and post-PCV10 period, a Bayesian inference model estimated serotype-specific changes in IPD, using pneumococcal carriage and surveillance data.
Findings
There were 423 IPD, 1,029 PBPM, 1,391 all-cause meningitis and 7,611 all-cause sepsis cases. Five years post-PCV10 introduction, IPD declined by 60% (95%CI: 37%, 76%) in children 1-23m months old, and in age groups 2-4y, 5-9y, 10-19y although confidence intervals spanned zero. PBPM declined by 36% (95%CI: 21%, 48%) among children 1-23 months old, and in all other age groups, although some confidence intervals spanned zero. Among children <5y of age, PCV10-type IPD declined by 83% (95%CI; 70%, 90%) and with no evidence of change in non-PCV10-type IPD (9%, 95%CI; -69, 43%). There was no change in all-cause meningitis or sepsis. Post-PCV10, the most common serotypes in vaccine age-eligible and non-age eligible people were serotypes 8 and 23B, and 3 and 7F, respectively.
Interpretations
Our study demonstrates the effectiveness of PCV10 against IPD in a country in the Asia-Pacific of which there is a paucity of data.
Funding
This study was support by the Department of Foreign Affairs and Trade of the Australian Government and Fiji Health Sector Support Program (FHSSP). FHSSP is implemented by Abt JTA on behalf of the Australian Government.
Research in context.
Evidence before this study
We searched PubMed for evidence of PCV10 impact on invasive pneumococcal disease, meningitis or sepsis using the following terms in combination “pneumococcal” “conjugate vaccine” “PCV10” “invasive pneumococcal disease (IPD)” “meningitis” “sepsis” “impact” “effectiveness”. We screened 108 titles and identified nine longitudinal observational studies from five low-or middle-income countries (LMIC) which assessed the impact of PCV10 on all-type invasive pneumococcal disease, meningitis or sepsis in children and/or adults with data for a minimum of two years post-PCV introduction and settings with no prior use of PCV7. All studies used hospital admission data with the period of follow up post-PCV10 introduction ranging from 2-6 years. Brazil used a 3+1 schedule with a catch up campaign for children <2 years, Kenya used a 3+0 with a catch up campaign for children <5 years, Mozambique and Zambia used a 3+0 schedule without a catch up campaign and Chile used a 2+1 schedule without a catch up campaign. Results show reductions in all-type IPD between 25-68% in children <5 years from Brazil, Chile and Kenya, and 44% in Brazilian children 2-23m. For the outcome pneumococcal meningitis there was a 92-98% decline in children <5 years from Kenya and Mozambique and a 37-60% decline in Brazilian children <23m. One study assessed all-cause meningitis which declined by 62-72% in Zambian children <5 years of age. Only one study assessed PCV10 impact in adults which showed no decline in all-type IPD (IRR=0·63 (0·36–1·08) and 81% decline in PCV10-type-IPD among Kenyans aged >15yrs. No studies measured the impact on PBPM.
Added value of this study
We have evaluated the impact of the infant PCV10 vaccination program on hospitalisation rates of invasive disease in Fiji according to four outcomes: IPD, probable bacterial and pneumococcal meningitis (PBPM), all-cause meningitis and all-cause sepsis in young children and the indirect protection in unvaccinated age groups, >5 years of age, which may be useful outcomes for other LMIC assessing PCV impact. Our results demonstrate evidence of protection in the 1-23 months and evidence of a downward trend in disease in the other age groups, five years following the introduction of PCV10. These data build on the results from other LMICs in Africa and South America, however we provide data for the first time from the Asia-Pacific region. This region has been very slow in introducing PCV into their national immunisation schedules. In addition, our choice of invasive disease outcomes and methods used may augment standard approaches of measuring PCV impact and are suitable for similar settings where disease surveillance and serotyping are not complete or have been enhanced over the observation period. We demonstrate that combining IPD and carriage data can measure the impact of PCV10 on serotypes, and may be useful methods for other LMIC seeking to determine vaccine impact.
Implications of all the available evidence
The introduction of PCV10 into routine immunisation schedules using the traditional 3+0 schedule, without catch up, reduces childhood hospitalisations due to pneumococcal disease in LMIC. However there are sparse data from LMICs from the Asia-Pacific region, making our results the first to show PCV impact in this setting. As the immunogenicity of PCV10 is similar to PCV13, it is likely that either vaccine will be effective against invasive pneumococcal disease. Additionally, we found no evidence of serotype replacement five years following PCV10 introduction. Our findings provide evidence to support the national introduction of PCV in the Asia-Pacific region. This region has been very slow in introducing PCV into their national immunisation schedules, despite PCV being available for 20 years.
Alt-text: Unlabelled box
Background
There is a paucity of evidence of the impact of pneumococcal conjugate vaccine (PCV) on invasive pneumococcal disease (IPD) in Asia and the Pacific given that many Asian countries introduced PCV after countries in South America and Sub-Saharan Africa. Estimating the impact of PCV may be possible through the analysis of inpatient non-laboratory confirmed clinical diagnoses.1, 2, 3 In addition, pneumococcal carriage data can be used to predict reductions in vaccine-type and non-vaccine type IPD serotypes post-PCV introduction.4, 5, 6, 7, 8
Fiji was one of the first low or middle income country (LMIC) to introduce PCV. Prior to PCV introduction, it was estimated that 67% of IPD cases in Fijian children were due to PCV10 serotypes.9 These data supported the introduction of PCV10 in Fiji in October 2012 using a 3+0 schedule. In September 2013, Invasive Bacterial Vaccine Preventable Disease Surveillance (IB-VPD) was established. This included changes in laboratory processes to improve the sensitivity of pneumococcal detection.10,11 Pneumococcal carriage surveys conducted three years post-PCV10 introduction found reductions in PCV10-type carriage of 44-66% among infants too young to be vaccinated, toddlers, older children and their caregivers.12 In this study, we assess the impact of PCV10 in all ages in Fiji on the rates of various invasive disease outcomes, including IPD, probable bacterial or pneumococcal meningitis (PBPM), and meningitis and sepsis up to five years post-PCV10 introduction. A secondary aim is to estimate the changes in PCV10-type and non-PCV10-type IPD post-PCV10 introduction, using IPD and pneumococcal carriage survey data, whilst adjusting for the increased sensitivity in laboratory testing for pneumococci in the post-PCV10 era.
Methods
Study site
Fiji is an upper-middle income, South Pacific island country with a population estimate of 884,887 in 2017.13 Healthcare is free of charge at the point of use with good access to services. There are three tertiary hospitals, which capture 86% of national hospital admissions.14 Admission data are entered into a computerised database (PATIS) which has been demonstrated in a previous study to be 89% complete.14 Children with clinical danger signs (including children with meningitis, sepsis and severe pneumonia) are generally referred to a tertiary hospital, where a blood and/or cerebrospinal fluid (CSF) is taken, as clinically indicated.
In September 2013, IB-VPD surveillance was established, according to the World Health Organization protocols,15 at the main tertiary hospital, Colonial War Memorial Hospital (CWMH), which has a catchment of around 60% of the Fijian population. Surveillance protocols included changes in laboratory processes which may have increased the sensitivity of pneumococcal detection10,11 including improved blood culture media and the use of quantitative real-time PCR (qPCR) to detect pneumococcus from CSF.11 During periods where the improved culture media was not available the original culture media was used, therefore the type of media used was not consistent over the study period. In 2016, all microbiology laboratories, based at Lautoka and Labasa Hospitals, were included in the surveillance. Any patients whose blood or CSF samples yielded pneumococcus, meningococcus, or Haemophilus influenzae were enrolled in the surveillance.
Haemophilus influenzae type b (Hib) and PCV10 vaccines were introduced nationally in 1995 and October 2012, respectively, as a three-dose schedule at 6, 10, 14 weeks of age. National vaccine coverage estimates for three doses of both PCV10 and Hib were 91%, 92%, 91%, 85% and 82% in 2013, 2014, 2015, 2016 and 2017, respectively.16
Population denominators were sourced from the Fiji Government Bureau of Statistics, 2017 National Census and adjusted using annual population growth rate of 0·7%.13
Data collection
Clinical data on blood and CSF results for all ages between 2007 and 2017 were extracted from the microbiology registers at the three tertiary hospitals. For IPD data pre-PCV10, serotyping results for 2004-2006 were sourced from prospective surveillance at the main hospital (CWMH) from a previous study.17 Between 2007 and September 2013 serotyping of isolates was not performed prospectively. To obtain serotyping data for this period all available stored isolates were identified from -70°C freezer storage and serotyped retrospectively and included in the IB-VPD database. For IPD post-PCV10, serotyping data from the IB-VPD surveillance system were extracted. Between 2013 and 2015, pneumococcal isolates stored between 2007 and 2013 were serotyped at Murdoch Children's Research Institute, Melbourne using latex agglutination with final confirmation by the Quellung reaction.18,19 From 2015 onwards pneumococcal isolates were serotyped at the Microbiological Diagnostic Unit Public Health Laboratory in Melbourne by Quellung reaction or directly by qPCR (using the CDC protocol) on pneumococcal isolates.20
For all-cause meningitis and all-cause sepsis, hospital admission data were extracted from the national database between 2007 and 2017 according to ICD-10-AM codes. Laboratory or admission data from the same patient within 30 days of admission were considered as the same episode of illness.
Age groups were classified as follows: 1-23 months (m) 2-4 years (y), 5-9y, 10-19y, 20-54y, ≥55y. Neonates were excluded because there were major changes to admission criteria for this age group over the study period.
Data from four previously published annual cross-sectional nasopharyngeal carriage surveys conducted in 2012 (pre-PCV10) and 2013 to 2015 (post-PCV10) were used (n=8,109)12 to model the changes in PCV10-type IPD in the post-PCV10 period. Details of this study are published elsewhere.12
Pneumococcal surveillance data from a previulsy published research study conducted in 2004-2006 were used. Details of this study are published elsewhere.17
Case definitions
IPD cases and PBPM cases were laboratory-confirmed and classified according to the WHO case definition21 (further details are shown in the appendix). All-cause meningitis and all-cause sepsis cases were defined as hospital admissions with a primary clinical diagnosis of meningitis according to ICD-10-AM codes (further details are shown in the appendix).
PCV10 serotypes were 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F and 6A, for which PCV10 is cross protective.22 PCV10-type IPD cases were IPD cases caused by PCV10 serotypes. Non-PCV10-type IPD cases were cases caused by all other serotypes including non-typeable pneumococci.
Conditions not associated with pneumococcal disease were used to assess changes in admission criteria or hospital access unrelated to PCV10 introduction over the same time period. These were: all-cause admissions defined as admission to hospital for any cause of illness excluding meningitis, sepsis, respiratory, maternity and diarrhoea admissions (as rotavirus vaccine had been introduced in 2012) according to ICD-10-AM codes (further details are shown in the appendix); the number of blood cultures and CSF sample collected (for which data by age was not available), and for adults, diabetes admissions, as diabetes is a risk factor for adult pneumococcal disease.
Statistical analysis
Demographic characteristics of cases by each outcome were summarised. Age group specific incidence rates were calculated as the number of cases per age group divided by the age-specific population multiplied by 100,000 with exact Poisson confidence intervals. Incidence rates were stratified by period of vaccination (pre-PCV10, early post-PCV10, mid post-PCV10 or late post-PCV10). Three post-PCV10 time periods were used as a meningococcal outbreak in 2016-2017 would confound the non-specific outcomes we used. Further details on the time periods are described in the appendix.
Incidence rate ratios were calculated by dividing the pre-PCV10 incidence rates by the post-PCV10 incidence rates, with exact Poisson confidence intervals. Stata version 15 (Stata Corporation, College Station, TX) was used for analysis.
To determine the effect of PCV10 introduction on PCV10-type and non-PCV10-type IPD as well as individual serotypes, we jointly estimated these alongside the change in IPD detection sensitivity of the IB-VPD surveillance in the post-PCV10 period. This was achieved by coupling data from different sources; a research study collecting pneumococcal surveillance data in 2004-2006 and IB-VPD routine surveillance data collected between October 2012 and January 2018, combined with carriage data sourced from four annual carriage survyes conducted in 2012-2015.
Statistical modelling for carriage data
To infer the impact of PCV10 on IPD, we combined evidence on nasopharyngeal pneumococcal carriage (pre-PCV10 data are from one annual carriage survey conducted in 2012 and post-PCV10 data are from three annual carriage surveys conducted between 2013-2015) and IPD (pre-PCV10 data is extracted from research study surveillance data collected in 2004-2006 and post-PCV10 data is extracted from IB-VPD routine surveillance data collected between October 2012-January 2018) in a Bayesian estimation framework. Assuming that the serotype-specific case-to-carrier ratio is an inherent property of the capsule and hence unchanged by vaccination,4,5,7,8,23 further allowed estimation of a potential change in IPD reporting sensitivity in the pre- and post-PCV10 IPD surveillance periods where different systems were in place. We pooled both carriage survey data and IPD serotyping data from CWMH, respectively in two periods (pre- and post-PCV10 introduction) and two groups; children ≤5y old and caregivers aged 20 to 55y old. Serotype-specific IPD case counts and carriage prevalence were modelled as Poisson and binomially distributed variables, respectively. The model then estimated the serotype-specific true IPD incidence and carriage prevalence alongside a common change in reporting sensitivity of IPD in the post-PCV10 era.
Here, I is the serotype-specific number of IPD cases, C the number of carriage episodes detected, P the carriage prevalence, N the number of individuals tested for carriage, CCR the case to carrier ratio and r the change in reporting sensitivity. O and E indicate the observed data and the model estimates respectively. B and A the period before and after PCV10 introduction, respectively.
Impact estimates were derived as the rate ratio of estimated incidence accounting for the change in reporting sensitivity and the difference in duration of IPD surveillance and the number of individuals tested for pneumococcal carriage. Non-typeable pneumococci were classified as non-vaccine serotypes. Where the serotype of an isolate was missing, we used multiple imputation creating 1,000 resampled dataset and 1,000 corresponding model posterior estimates with 100 posterior samples each, creating a joint posterior sample of 100,000. We report the mode on 95% quantiles of this joint posterior estimates.
The model was programmed in R 3·4·424 interfacing with Just Another Gibbs Sampler25 and its code can be provided upon reasonable request to the authors. Ethics approval
Ethical approval for this study was given by Fiji National Health Review Ethics Committee ethics (approval number 2014·10·FNRETC·9·NW).
Results
Impact of PCV10 on IPD, meningitis and sepsis
Between 2008-2017, there were 423 IPD cases in patients aged >1m of age, and between 2007-2017 there were 1,029 PBPM cases, 1,391 cases of all-cause meningitis and 7,611 cases of all-cause sepsis in patients aged >1m (Table 1).
Table 1.
Demographic characteristics of patients aged above one month with invasive pneumococcal disease, probable bacterial or pneumococcal meningitis, all-cause meningitis or all-cause sepsis in Fiji between 2007-20171.
| Invasive pneumococcal disease1n=423 | Probable bacterial or pneumococcal meningitis1n= 1,029 | All-cause meningitis hospitalisations2n=1,391 | All-cause sepsis hospitalisations2n=7,611 | |
|---|---|---|---|---|
| Age group, n (%, col) | n=421 | n=1,026 | n=1,391 | n=7,611 |
| 1-23m | 134 (31) | 515 (50) | 725 (52) | 818 (11) |
| 2-4y | 40 (10) | 141 (14) | 153 (11) | 110 (1) |
| 5-9y | 27 (6) | 102 (10) | 128 (9) | 111 (1) |
| 10-19y | 37 (9) | 88 (9) | 185 (13) | 300 (4) |
| 20-54y | 120 (29) | 145 (14) | 161 (12) | 2,394 (32) |
| ≥55y | 63 (15) | 35 (3) | 39 (3) | 3,878 (51) |
| Total | 421 (100) | 1,026 (100) | 1,391 (100) | 7,611 (100) |
| Median age, y (IQR) | n=421 | n=1,026 | n=1,391 | n=7,611 |
| 12 (1-38) | 1 (0-11) | 1 (0-11) | 55 (32-67) | |
| Male, n (%) | n=422 | n=1,016 | n=1,391 | n=7,611 |
| 227 (54) | 401 (39) | 809 (58) | 3,650 (48) | |
| Ethnicity (%, col) | n=423 | n=1,016 | n=1,391 | n=7,611 |
| iTaukei | 362 (86) | 766 (75) | 1,075 (77) | 4,714 (62) |
| Fijians of Indian descent | 44 (10) | 194 (19) | 238 (17) | 2,537 (33) |
| Others | 17 (4) | 56 (6) | 78 (6) | 360 (5) |
| Median length of stay in hospital, days (IQR) | - | - | 10 (7-13) | 7 (3-12) |
| Deaths3 (Case fatality rate, %) |
n=83 | n=3,182 | ||
| 1-23m | - | - | 24 (3) | 90 (11) |
| 2-4y | - | - | 9 (6) | 25 (23) |
| 5-9y | - | - | 1 (1) | 13 (12) |
| 10-19y | - | - | 9 (5) | 51 (17) |
| 20-54y | - | - | 26 (16) | 870 (37) |
| ≥55y | - | - | 14 (36) | 2,133 (56) |
| Total | - | - | 83 (6) | 3, 182 (42) |
Cases identified using laboratory results from national microbiology laboratory records (2008-2017). Data on length of stay in hospital and deaths were not recorded in the laboratory records and therefore not available for analysis.
Cases identified using ICD10 code from national admission database from three tertiary hospitals.
Data on deaths not available for outcomes invasive pneumococcal disease, probable bacterial or pneumococcal meningitis outcomes.
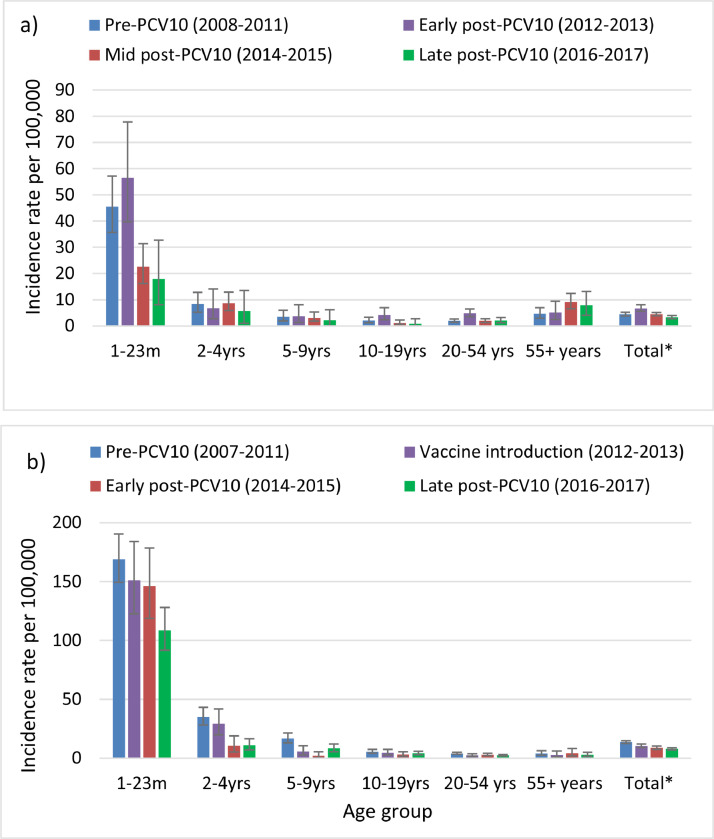
The incidence of IPD among children 1-23m of age initially increased during the early-PCV10 period (2012-2013) when the IB-VPD surveillance was introduced and then declined by 60% (95%CI: 37%, 76%) five years post-PCV (2014-2017) introduction (Table 2 and Figure 1). Among age groups 2-19y there was evidence of continued decline in IPD rates over time from the mid- to late-post-PCV10 period, however the confidence intervals crossed zero due to low case numbers (Figure 1 and Appendix Table 1).
Table 2.
Incidence rates and incidence rate ratios of invasive pneumococcal disease and probable bacterial or pneumococcal meningitis in the pre-PCV101 vs. the late post-PCV102 period.
| Age group | Incidence rate per 100,000 age-specific population (95% CI) |
IRR (95% CI) | P-value | |
|---|---|---|---|---|
| Pre-PCV101 | Late post-PCV102 | |||
| Invasive pneumococcal disease | ||||
| 1-23m | 45 (36, 57) | 18 (12, 27) | 0·40 (0·24, 0·63) | <0·001 |
| 2-4y | 8 (5, 13) | 6 (3, 10) | 0·69 (0·31, 1·46) | 0·30 |
| 5-9y | 4 (2, 6) | 2 (1, 4) | 0·60 (0·21, 1·59) | 0·28 |
| 10-19y | 2 (1, 3) | 1 (0, 2) | 0·42 (0·14, 1·13) | 0·06 |
| 20-54y | 2 (1, 3) | 2 (1, 3) | 1·03 (0·64, 1·65) | 0·89 |
| ≥55y | 5 (3, 7) | 8 (5, 11) | 1·69 (0·95, 3·07) | 0·06 |
| Total population excl. neonates | 4 (4, 5) | 3 (3, 4) | 0·74 (0·58, 0·93) | 0·01 |
| Probable bacterial or pneumococcal meningitis | ||||
| 1-23m | 169 (149, 190) | 109 (92, 128) | 0·64 (0·52, 0·79) | <0·001 |
| 2-4y | 35 (28, 43) | 11 (7, 17) | 0·31 (0·19, 0·50) | <0·001 |
| 5-9y | 17 (13, 21) | 8 (5, 12) | 0·49 (0·30, 0·78) | <0·001 |
| 10-19y | 5 (4, 7) | 4 (3, 6) | 0·75 (0·45, 1·23) | 0·23 |
| 20-54y | 4 (3, 5) | 2 (2, 3) | 0·57 (0·38, 0·84) | <0·001 |
| ≥55y | 4 (2, 6) | 3 (1, 5) | 0·70 (0·30, 1·54) | 0·34 |
| Total population excl. neonates | 14 (12, 15) | 8 (7, 9) | 0·57 (0·50, 0·67) | <0·001 |
Pre-PCV10 is defined as 2008-2011 for invasive pneumococcal disease, and 2007-2011 for probable bacterial or pneumococcal meningitis.
Late post-PCV10 is defined as 2014-2017.
Figure 1.
The incidence rate of disease by age group and outcome; a) invasive pneumococcal disease; b) probable bacterial or pneumococcal meningitis. Error bars indicate 95% confidence intervals.
*Total= Total population excluding neonates.
The incidence of PBPM followed a similar trend as IPD, with declines in both the mid- and late-post PCV10 periods among children under 10y of age (Figure 1 and Appendix Table 1). However, there was no increase in PBPM in the early-post-PCV10 period (2012-2013) as per IPD.
For all-cause meningitis hospitalisations, there was some evidence of an initial decline in incidence rates that was not sustained in the late-PCV10 period (Appendix Table 1). The incidence of all-cause sepsis hospitalisations increased in the majority of age groups in all post-PCV10 periods (Appendix Table 1). This coincided with an outbreak of Neisseria meningitidis serogroup C in Fiji in 2016-2018,26 and for the ≥55y age group, a slight increase in hospitalisation for diabetes (IRR; 1·05, 95%CI: 1·00, 1·10) and an increase in all-cause hospitalisations (Appendix Table 1).
All-cause admissions excluding conditions associated with pneumococcal disease, decreased in the early-post PCV10 period and then increased by 15-32% in age groups 2-23m, 2-4y, 5-9y and >55y during the late post-PCV10 (2014-2017) introduction (Appendix Table 1). The rate of CSF and blood samples processed in the microbiology laboratories for all ages increased by 23% (95%CI: 18, 28); There were 2,714 and 3,507 CSF and blood samples per 100,000 population were processed in the pre- and post-PCV10 period respectively.
Serotype distribution in the PCV10 era
There were 148 IPD cases in the IB-VPD surveillance (post-PCV10) period, of which 89 (60%) were serotyped. The remaining 40% of isolates were not available for serotyping due to either the sample being of insufficient volume or it not being stored. Of the 89 serotyped isolates, 19 (21%) were among vaccine age-eligible children and 70 (79%) were among children and adults who were not vaccine age-eligible. Figure 2 shows the number of IPD isolates by serotype and vaccine eligibility. Of the IPD isolates among both vaccine age-eligible and non-vaccine age-eligible cases, 27/89 (30%) were PCV10 serotypes (mainly serotypes 1 and 7F) and 62/89 (70%) were non-PCV10 serotypes (mainly serotypes 3, 8 and 23B). There were two PCV10-type IPD cases among PCV age-eligible children: one child with serotype 1 was unvaccinated; the other, a 19m old child, with serotype 14, whos clinical records indicated that they received three doses of Hib vaccine but not PCV10. This mismatch is likely to be a recording error for PCV10, as there were no known shortages of PCV10.
Figure 2.
Number of invasive pneumococcal disease serotypes/serogroups post-PCV10 (2013-2017) by vaccine type and PCV10 age-eligibility (n=89).
¹7=Serogroup 7 including serotypes 7A and 7F and has been classified as PVC10-type due to the rarity of 7A.
²18=Serogroup 18 including serotypes 18A, 18B, 18C and 18F.
³24=Serogroup 24 including serotypes 24A, 24B and 24F.
⁴NT=Non-typeable S. pneumoniae.
Serotype-specific impact
Using the modelled IPD data combined with carriage data, we found the model fitted the data well (Appendix Figure 1). We estimated that among children aged <5y, IPD reporting sensitivity in the post-PCV10 surveillance was 4·0 (95%CI; 2·3, 6·7) times higher than in the pre-PCV10 study. Among children under 5y, it was estimated that PCV10-type IPD declined by 83% (95%CI; 70%, 90%), there was no evidence for change in non-PCV10-type IPD (9%, 95%CI; -69, 43% and all IPD decreased by 43% (95%CI; 3%, 67%). We estimated a decrease in IPD incidence among all vaccine types, including 6A (Figure 3). No decrease in 19A was detected. The majority of the PCV impact on IPD incidence can be attributed to declines in serotypes 14, 6B and 7F. Some non-PCV10-types declined and some increased in the post-PCV10 era, compared with the pre-PCV10 era.
Figure 3.
Estimated reduction in invasive pneumococcal disease (IPD) in the post-PCV10 period compared to the pre-PCV10 period showing modelled results from the combination of IPD and carriage data; a) children under five years of age; b) Adult caregivers 20-55 years of age.
Among adults aged 20-55y, we estimated a 3·4 (95%CI; 1·4, 8·3) fold increase in IPD reporting sensitivity in the post-PCV10 period. Reductions in IPD following PCV10 use was 80% (95%CI; 51%, 92%) for PCV10 types, with no change in non-PCV10-types and all IPD; -12% (95%CI; -178%, 54%) and 36% (95%CI; -59%, 74%), respectively. There was a decline in IPD for almost all PCV10-types, with only serotypes 6A and 23F showing no change in point estimates (Figure 3). The majority of the impact can be attributed to a decline in IPD due to serotypes 1, 4 and 7F.
Discussion
There is a paucity of data on the impact of PCV10 on any disease outcome, and this study is among the first evaluations of the impact of PCV10 on IPD, meningitis and sepsis from a LMIC in the Asia-Pacific region, and one of a few studies from any LMIC. Our results demonstrate a decline in vaccine-type IPD and PBPM among children and adults following the introduction of PCV10 up to five years post-PCV10 introduction. We found little evidence for serotype replacement disease five years post introduction.
The incidence of all-type IPD in young children more than halved following the introduction of PCV10, while the incidence of PCV10-type IPD was reduced by >80%. Our results are consistent with other PCV10 impact studies among Kenyan, Chilean and Brazilian children with rates of all-type IPD declining between 42% and 80% and PCV10-type IPD declining between 79 and 92%.27, 28, 29 As expected the reduction in IPD was greatest in young children, due to the risk of pneumococcal infection being greatest in this age group as well as the fact that these children where vaccinated. In addition, our findings are likely to underestimate the benefit of PCV10 as the decline we observed was during a period of increased hospital admission rates, increased use of laboratory services and improvement in capacity to detect pneumococcus; specifically improved culture media (although the use was not consistent due to availability) and the introduction of molecular testing in late 2014. This increase in IPD early-post-PCV10 period (2012-2013) which was not seen in the PBPM outcome is likely to be due to these improvements in the detection of pneumococci in the diagnostic laboratory.
The most common non-PCV10-type causing IPD in the post-PCV10 period were serotypes 3, 7F, 8 and 23B. Serotype 3, a common cause of empyema, is contained within 13-valent PCV (PCV13) but has little efficacy against serotype 3.30 In our stuidy, we found no empyema cases in the IB-VPD surveillance. Studies in other settings show diversity in the common serotypes post-PCV, however, a review of 29 European countries showed serotype 8 to be the third most common post-PCV13 introduction.31 Our results show serotype 19A did not decline, instead it showed a slight increase. Serotype 19A has been seen to cause serotype replacement in other settings using PCV7, PCV10 and PCV13.32 Our study found four serotype 19A cases in the non-vaccine age eligible group and none among vaccine age eligible children. Our pneumococcal carriage study in Fiji found that 19A carriage increased among 5-8 weeks and 12-23m month old Fijian children following PCV10 introduction.12 Although most carriers are assymptmatic, pneumococcal carriage is a precurosor to disease, it is important to continue to monitor serotype replacement in Fiji.
The rates of PBPM declined among children age-eligible for vaccination. PBPM is a useful outcome to measure where the majority of suspected meningitis cases receive a lumbar puncture but there are challenges in laboratory assessment due to lack of reagents or human resource capacity to determine aetiology. We are not aware of any other published studies using PBPM as an outcome. However, other studies have used pneumococcal meningitis as an outcome which is a subgroup of IPD and PBPM. In our study, there were too few cases to undertake separate analysis on pneumococcal meningitis. Other studies have found that the incidence of pneumococcal meningitis among vaccinated children decreased by 20-70% following the introduction of PCV10 in Brazil, Madagascar, Kenya and Zimbabwe.27,33, 34, 35
Due to the inherent challenges of accurately diagnosing pneumococcal disease and therefore availability of quality laboratory confirmed study outcomes, we explored the non-laboratory confirmed outcomes of all-cause meningitis and sepsis hospitalisations using ICD-10 coded admissions. We observed a downward trend in incidence for all-cause meningitis in the mid post-PCV10 period. However, in the late post-PCV10 period there was an increase in all-cause meningitis, which coincided with a meningococcal C outbreak. All-cause meningitis as an outcome is relatively sensitive but not specific in relation to pneumococcal disease. Therefore, any impact of PCV on meningitis is likely to be ‘diluted’ by other causes of meningitis such as meningococcal meningitis and similar clinical presentations. For sepsis hospitalisations, we found a 29-30% increase among children aged 1m to 5y. This contrasts a study from Finland which found a 34% reduction in non-laboratory-confirmed IPD or unspecified sepsis among vaccine eligible children aged 3-42m.3 In our study, there was an increase in admissions due to clinically diagnosed sepsis (of any cause) among older age groups, particularly the elderly. The reasons for this are unclear and is in contrast to the decreasing global trend seen.36 The increase in admissions among the elderly was also seen in all-cause admissions and ICD-10 coded all-casue pneumonia (unpublished) which suggests the reason is not specific to sepsis. Increased rates of type-two diabetes in Fiji, a risk factor for pneumococcal disease and sepsis37 or Staphylococcus aureus infections may play a role and needs further investigation including including additional microbiological data.
We found some evidence of indirect effects of PCV10 in adults, with declines in PCV10-type IPD and PBPM in older unvaccinated children and adults. This is consistent with the evidence of indirect effects we found in our previous carriage surveys,12 as pneumococcal carriage is a precursor for invasive disease. A number of studies have documented indirect effects of PCV on IPD in older children and adults.27 A meta-analysis showed indirect protection resulted in a 90% reduction in vaccine-type IPD among adults within a decade of establishing a childhood programme.38
The modelled results using pneumococcal carriage surveys compared to the observed data showed similar declines in all-type IPD compared to estimates using observed data; 43% vs 39-60% decline among children <5y of age, respectively. Finding similar point estimates for PCV10 impact in caretakers and children, indicates potentially substantial indirect effects, however, the confidence intervals are wide. Although there was little evidence for indirect effects among adults in the IPD data alone, caregivers may be more like to experience indirection protection due to their close contact with immunised children. The model was validated using a test dataset with pre-specified vaccine efficacies and reporting sensitivity for which the model was able to back-infer correctly. The assumption of a fixed case-to-carrier ratio has been used several times before and has shown to be key in understanding and predicting changes in disease incidence in the post-PCV era.4, 5, 6, 7, 8 However, there are limitations, it ignores the direct effect PCV has on preventing progression from carriage to disease; hence in largely vaccinated cohorts, i.e. children in this case, we may slightly underestimate the true PCV10 impact on PCV10-type IPD. In addition, co-infection with other pathogens may alter the case to carrier ratio over time.39 There were assumptions including linking carriage and disease data from different years in the pre-PCV10 period and pooling the post-PCV10 period despite some likely ongoing dynamic changes. Another limitation was that the carriage study enrolled caregivers, who may have different demographic characteristics compared with adults hospitalised with IPD; caregivers were more likely to be younger and female, and less likely to have underlying risk factors.12
Our study has additional limitations. Firstly, limitations related to observational studies does not allow controlling for all confounders and causation cannot be implied. Secondly, administrative data is subject to under-reporting. An audit of the national hospital admission database and found that 89% of admissions at the tertiary hospitals were complete and there was no evidence of changes in diagnostic coding or reporting of admissions over time.14 The dependence of the study on hospital admission data makes the study sensitive to changes in health information systems, access to care, admission practice, population changes and other health interventions. Assessment of ‘all-cause admissions’ suggested a slight increase over the study period that might have resulted in an underestimation of the true vaccine effect. The poor availability of IPD serotyping data pre-PCV10 introduction limited direct comparison of PCV impact on specific serotypes. This study has a number of strengths, including the long period of analysis; 11 years of hospital admission and microbiological data. Fiji has good access to healthcare as well as diagnostics which often lacking in LMIC which makes assessment with outcome IPD challenging. Although IPD serotype data is incomplete the availability of some data as well as and the pneumococcal carriage data has allowed assessment of PCV10 impact on invasive serotypes.
In conclusion, our results found declines in PCV10 serotypes, and all-type IPD and PBPM among children <2y of age and supportive evidence of indirect effects on PBPM among older children in Fiji, five years after PCV10 introduction. There was a decline in PCV10-type IPD in children and adults. There is little evidence for clinically meaningful serotype replacement at this stage. Other clinical outcomes, all-cause meningitis and sepsis hospitalisations were not helpful in this setting as our evaluation coincided with a meningococcal outbreak, which shares these same clinical diagnoses, but should be further explored in outher settings. These results provide additional evidence of the benefits of PCV10 in Fiji and will guide public health policy on the use of PCV in the Asia-Pacific region.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationshipsthat could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge the major contribution from the Ministry of Health and Medical Services in particular: the Minister for Health, the Permanent Secretary for Health, Head of Health Protection, and the National Advisor for Family Health; the staff of the microbiology laboratories, the CWMH paediatric department, and the Research, Innovation, Digital Health and Data Analysis Management and in particular the Data Analysis Management Unit, and the paediatric records clerks. The staff of the New Vaccines and the Asia-Pacific Health Groups and the microbiology team at the Murdoch Children's Research Institute. The staff of the WHO Invasive Bacterial Vaccine Preventable Diseases Regional Reference Laboratory, Microbiological Diagnostic Unit Public Health laboratory, The University of Melbourne at the Doherty Institute. This study was support by the Department of Foreign Affairs and Trade of the Australian Government and Fiji Health Sector Support Program (FHSSP). FHSSP is implemented by Abt JTA on behalf of the Australian Government. In particular, we would like to thank the staff of the FHSSP. Murdoch Children's Research Institute is supported by the Victorian Government's Operational Infrastructure Support Program. FR and CS are supported by NHMRC Fellowships. CS was supported by an Australian National Health Medical Research Council Career Development Fellowship (1087957) and a Veski Inspiring Women Fellowship. SF is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant number 208812/Z/17/Z).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2021.100352.
Appendix. Supplementary materials
References
- 1.Gidding HF, Sheridan S, Fathima P, et al. Impact of childhood pneumococcal conjugate vaccine on nonnotified clinically suspected invasive pneumococcal disease in Australia. Pediatr Infect Dis J. 2019;38:860–865. doi: 10.1097/INF.0000000000002314. [DOI] [PubMed] [Google Scholar]
- 2.De Oliveira LH, Camacho LAB, Coutinho ESFF, et al. Public Library of Science; 2016. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American countries: A systematic review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmu AA, Kilpi TM, Rinta-Kokko H, et al. Pneumococcal conjugate vaccine and clinically suspected invasive pneumococcal disease. Pediatrics. 2015;136:e22–e27. doi: 10.1542/peds.2015-0458. [DOI] [PubMed] [Google Scholar]
- 4.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flasche S, Givon-Lavi N, Dagan R. Using pneumococcal carriage data to monitor postvaccination changes in the incidence of pneumococcal otitis media. Am J Epidemiol. 2016;184:652–659. doi: 10.1093/aje/kww012. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger DM, Bruden DT, Grant LR, et al. Using pneumococcal carriage data to monitor postvaccination changes in invasive disease. 2013;178:1488–1495. doi: 10.1093/aje/kwt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flasche S, Le Polain de Waroux O, O'Brien KL, Edmunds WJ. The serotype distribution among healthy carriers before vaccination is essential for predicting the impact of pneumococcal conjugate vaccine on invasive disease. PLOS Comput Biol. 2015;11 doi: 10.1371/journal.pcbi.1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurhonen M, Auranen K. Optimal serotype compositions for pneumococcal conjugate vaccination under serotype replacement. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell FM, Carapetis JR, Tikoduadua L, et al. Invasive pneumococcal disease in Fiji. Pediatr Infect Dis J. 2010;29:870–872. doi: 10.1097/INF.0b013e3181ec7ae2. [DOI] [PubMed] [Google Scholar]
- 10.Russell FM, Biribo SSN, Selvaraj G, et al. As a bacterial culture medium, citrated sheep blood agar is a practical alternative to citrated human blood agar in laboratories of developing countries. J Clin Microbiol. 2006;44:3346–3351. doi: 10.1128/JCM.02631-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne EM, Mantanitobua S, Singh SP, et al. Real-time qPCR improves meningitis pathogen detection in invasive bacterial-vaccine preventable disease surveillance in Fiji. Sci Rep. 2016;6 doi: 10.1038/srep39784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne EM, Satzke C, Ratu FT, et al. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: results from four annual cross-sectional carriage surveys. Lancet Glob Heal. 2018;6:e1375–e1385. doi: 10.1016/S2214-109X(18)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Government of Fiji. Census 2017 - Release 1 - Fiji Bureau of Statistics. 2017 https://www.statsfiji.gov.fj/index.php/census-2017/census-2017-release-1 (accessed April 16, 2020).
- 14.Reyburn R, Nand D, Nguyen C, et al. Validation of administrative data to estimate vaccine impact: Audit of the Fiji hospital admissions electronic database, 2007–2011 & 2014–2015. Vaccine. 2017;35:6416–6421. doi: 10.1016/j.vaccine.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO). Invasive bacterial vaccine preventable diseases (IB-VPD) surveillance case definitions. 2012. https://www.who.int/immunization/research/development/WHO_IVB_11.09_eng.pdf?ua=1.
- 16.World Health Organization (WHO). WPRO | National immunization data - EPI summaries by country. http://www.wpro.who.int/immunization/documents/national_immunization_data/en/(accessed Jan 10, 2016).
- 17.Russell FM, Carapetis JR, Tikoduadua L, et al. Invasive pneumococcal disease in Fiji; Clinical syndromes, epidemiology, and the potential impact of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:870–872. doi: 10.1097/INF.0b013e3181ec7ae2. [DOI] [PubMed] [Google Scholar]
- 18.Porter BD, Ortika BD, Satzke C. Capsular serotyping of Streptococcus pneumoniae by latex agglutination. J Vis Exp. 2014:51747. doi: 10.3791/51747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habib M, Porter BD, Satzke C. Capsular serotyping of Streptococcus pneumoniae using the Quellung reaction. J Vis Exp. 2014:e51208. doi: 10.3791/51208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) and United States Center for Disease Control and Prevention (US-CDC). Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. 2011; 2: 1–14.
- 21.WHO WHO – recommended standards for surveillance of selected vaccine-preventable diseases. Bull World Heal Organ. 2003;03:1–51. [Google Scholar]
- 22.Rinta-Kokko H, Palmu AA, Auranen K, et al. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36:1934–1940. doi: 10.1016/j.vaccine.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger D. Using administrative data to evaluate PCV impacts against pneumonia: challenges and solutions.
- 24.R Core Team . 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 25.Plummer M. 3rd International Workshop on Distributed Statistical Computing. 2003. JAGS: A program for analysis of bayesian graphical models using gibbs sampling. [Google Scholar]
- 26.The Government of Fiji. Meningococcal disease. http://www.health.gov.fj/your-health-2/meningococcal/(accessed June 29, 2020).
- 27.Hammitt LL, Etyang AO, Morpeth SC, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet. 2019;393:2146–2154. doi: 10.1016/S0140-6736(18)33005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreira M, Cintra O, Harriague J, Hausdorff WP, Hoet B. Impact of the introduction of the pneumococcal conjugate vaccine in the Brazilian routine childhood national immunization program. Vaccine. 2016;34:2766–2778. doi: 10.1016/j.vaccine.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Alvarado S, Cavada G, Villena R, et al. Efecto de la vacuna antineumocócica conjugada 10-valente en el área sur de Santiago de Chile, 2009-2015. Rev Panam Salud Pública. 2018;42:1–7. doi: 10.26633/RPSP.2018.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sings HL, De Wals, Philippe Gessner BD, Isturiz, Raul Laferriere C, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: A systematic review and meta-analysis of observational studies. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6541704/(accessed May 15, 2020). [DOI] [PMC free article] [PubMed]
- 31.Levy C, Ouldali N, Caeymaex L, Ois Angoulvant F, Varon E, Cohen R. Diversity of serotype replacement after pneumococcal conjugate vaccine implementation in Europe. 2019. DOI:10.1016/j.jpeds.2019.07.057. [DOI] [PubMed]
- 32.Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert R-R, Jodar L. Streptococcus pneumoniae serotype 19A: Worldwide epidemiology. Expert Rev Vaccines. 2017;16:1007–1027. doi: 10.1080/14760584.2017.1362339. [DOI] [PubMed] [Google Scholar]
- 33.Grando IM, Moraes C de, Flannery B, et al. Impact of 10-valent pneumococcal conjugate vaccine on pneumococcal meningitis in children up to two years of age in Brazil. Cad Saude Publica. 2015;31:276–284. doi: 10.1590/0102-311x00169913. [DOI] [PubMed] [Google Scholar]
- 34.Andriatahirintsoa EJPR, Raboba JL, Rahajamanana VL, et al. Impact of 10-Valent pneumococcal conjugate vaccine on bacterial meningitis in Madagascar. Clin Infect Dis. 2019;69:S121–S125. doi: 10.1093/cid/ciz504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mpabalwani EM, Lukwesa-Musyani C, Imamba A, et al. Declines in pneumonia and meningitis hospitalizations in children under 5 years of age after introduction of 10-valent pneumococcal conjugate vaccine in Zambia, 2010–2016. Clin Infect Dis. 2019;69:S58–S65. doi: 10.1093/cid/ciz456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrell S, Lin S, Tukana I, et al. Diabetes incidence and projections from prevalence surveys in Fiji. Popul Health Metr. 2016;14:45. doi: 10.1186/s12963-016-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiri T, Datta S, Madan J, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Heal. 2017;5:e51–e59. doi: 10.1016/S2214-109X(16)30306-0. [DOI] [PubMed] [Google Scholar]
- 39.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16:355. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.