Abstract
Purpose
Cetirizine is a less sedative alternative to diphenhydramine for the prevention of infusion-related reactions (IRR) to paclitaxel. However, its use remains controversial. In this study, we assessed feasibility for a future definitive non-inferiority trial comparing cetirizine to diphenhydramine as premedication to prevent paclitaxel-related IRR.
Methods
This was a single-center randomized prospective feasibility study. Participants were paclitaxel-naive cancer patients scheduled to start paclitaxel chemotherapy. They were randomly assigned to receive either intravenous diphenhydramine 50 mg + oral placebo (control) or intravenous placebo + oral cetirizine 10 mg (intervention) for their first two paclitaxel treatments. The percentage of eligible patients completing a first paclitaxel treatment and the recruitment rate were assessed (feasibility outcomes). Drowsiness was measured at baseline and at selected time points using the Stanford Sleepiness Scale (SSS) (safety outcome). IRR events were also documented (efficacy outcome).
Results
Among 37 eligible patients, 27 were recruited and randomized (control 13; intervention 14) and 25 completed the study. The recruitment rate was 4.8 participants/month, meeting the primary feasibility target. Drowsiness was the main adverse effect associated with the premedication. The increase in drowsiness compared to baseline (ΔSSS) was greater in the diphenhydramine group compared to the cetirizine group (median ΔSSS 2 (IQR 3.25) vs median ΔSSS 0 (IQR 1), p < 0.01) when measured one hour after the premedication administration. One participant had an IRR and no unexpected serious adverse event occurred.
Conclusion
The trial methods were feasible in terms of recruitment, retention, and safety. Cetirizine was significantly less sedating than diphenhydramine. IRR were infrequent and a larger trial is warranted to confirm non-inferiority for IRR prevention.
Trial registration
ClinicalTrials.gov, NCT04237090 (22.01.2020).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-021-06734-4.
Keywords: Cetirizine, Diphenhydramine, Paclitaxel, Drug hypersensitivity, Premedication
Background
Infusion-related reactions (IRR) were very frequent with paclitaxel in phase I studies [1, 2]. In most cases, these reactions are thought to be triggered by Cremophor EL, the excipient used to solubilize paclitaxel, which can cause complement activation and the release of inflammatory mediators [3]. Premedication with a corticosteroid (dexamethasone), an H1 antihistamine (most commonly diphenhydramine), and an H2 antihistamine (most commonly ranitidine or famotidine) was empirically introduced in clinical practice and contributed to reduce the incidence of IRR to 4–10%, of which 1–2% are considered severe [4, 5].
First-generation H1 antihistamines, like diphenhydramine, are associated with central nervous system adverse effects such as drowsiness, dry mouth, and blurred vision [6]. In contrast, second-generation H1 antihistamines, like cetirizine, have a high selectivity for H1 receptors and do not readily cross the blood–brain barrier leading to minimal or no adverse effect [7]. Newer generation H1-antihistamines are now considered the first-line antihistamines for the treatment of allergic rhinitis and urticaria, because their safety profile makes them more advantageous with a similar efficacy profile [8–10]. Second-generation H1 antihistamines are safer as they cause less central nervous system impairment, less accidents, and less decreased cognitive performance compared to first-generation H1 antihistamines [11]. Drowsiness, but also anticholinergic toxicity of first-generation H1 antihistamines, can negatively impact patient experience at the hospital, their cooperation with healthcare professionals, and their ability to return home safely.
Very few studies have evaluated effectiveness of second-generation H1 antihistamines in preventing chemotherapy-associated IRR. Siderov et al. compared retrospectively oral loratadine with intravenous promethazine in 18 patients receiving either paclitaxel or rituximab [12]. Although the study was underpowered, no IRR occurred in patients receiving loratadine. More recently, Durham et al. compared oral cetirizine with intravenous or oral diphenhydramine as premedication for paclitaxel, rituximab, or cetuximab-based chemotherapy [13]. In patients receiving paclitaxel, IRR rates were comparable between cetirizine (used in 38 patients) and diphenhydramine (used in 62 patients) [13]. Although these results are promising, the retrospective design of these studies and the small sample sizes limit the generalizability of this practice.
In this study, we report the feasibility of a prospective non-inferiority clinical trial comparing oral cetirizine to intravenous diphenhydramine to prevent paclitaxel-associated IRR. Specifically, we aimed at estimating recruitment rates and acceptability of the trial, as well as providing preliminary safety outcomes in terms of drowsiness and IRR rates in paclitaxel-naive patients.
Methods
Study design
This prospective randomized controlled double-blind feasibility study was conducted in the outpatient oncology clinic of Hôpital Maisonneuve-Rosemont. Participants were recruited from February through September 2020. The study design is shown in Fig. 1.
Fig. 1.
Study design. a According to the chemotherapy protocol
The study protocol was approved by the local ethics committee and was conducted according to the Declaration of Helsinki and its amendments. Verbal and written informed consent were obtained for each participant before enrollment (ClinicalTrials.gov Identifier: NCT04237090).
Outcomes
The feasibility outcomes of the study were to determine (1) the recruitment rate to enroll 24 participants who received a first treatment of paclitaxel (primary), (2) the proportion of patients recruited, randomized, and who received the first treatment of paclitaxel following an assessment of their eligibility (co-primary), (3) the proportion of participants who completed the study and the reasons associated with loss to follow-up (exploratory), and (4) the proportion of oncology nurses and participants who accurately identified allocation despite the blinding procedures using a non-mandatory questionnaire (exploratory).
The safety outcomes of the study were to determine (1) the change from baseline in the level of drowsiness at different time points after premedication (primary), (2) the extent to which drowsiness was bothersome to participants (exploratory), and (3) the common adverse effects associated with H1 antihistamine premedication (exploratory).
The efficacy outcomes of the study were to assess (1) the proportion of participants who required discontinuation of the paclitaxel infusion and/or the use of rescue drugs because of an IRR (secondary) and (2) the grade of IRR according to the Common Terminology Criteria for Adverse Events (CTCAE) classification version 5 (secondary) [14] in participants who presented an IRR.
Participants
The target population of the study was adult individuals with cancer who were scheduled to start a paclitaxel chemotherapy.
Patients identified by oncology nurse navigators as well as administrative staff and oncology pharmacists were directed to the investigators. Eligible patients had at least 24 h to consider enrolling and could agree to participate in the study until the last day before their first paclitaxel treatment. In the context of the COVID-19 pandemic, recruitment procedures were allowed to be carried out by phone and consent forms could be sent by email, but written informed consent was secured before administration of the allocated study H1 antihistamine.
Cancer patients had to meet the following inclusion criteria: (1) intravenous chemotherapy treatments in the outpatient oncology clinic, (2) first lifetime exposure to paclitaxel (combination with other chemotherapeutic agents was permitted), (3) capable of providing informed consent, (4) aged 18 years and older, and (5) able to complete questionnaires. Patients were excluded if they (1) did not understand French or English, (2) were taking chronically an oral H1 antihistamine or a systemic corticosteroid, (3) had a contraindication related to the administration of cetirizine, diphenhydramine, placebo, or an ingredient in their formulation, (4) had already received a taxane chemotherapy agent in the past, (5) suffered from severe renal impairment (creatinine clearance < 10 ml/min), (6) were pregnant or breastfeeding, (7) received paclitaxel under a desensitization protocol, (8) had dysphagia or other pathophysiological condition preventing a tablet from being swallowed whole, (9) had drug/meal interactions preventing the full dose of oral cetirizine from being absorbed, and (10) participated in another clinical trial.
Drugs, blinding plan and randomization
Participants, oncology nurses, prescribing physicians, and investigators were blinded to the H1 antihistamine allocation.
Cetirizine 10 mg tablet (Apotex, Canada), diphenhydramine 50 mg (50 mg/ml solution, Frenesius-Kabi, Canada), and their respective placebo were conditioned by research support pharmacy staff. As an identical matching placebo was not available for cetirizine, the following procedure was used in an attempt to perform blinding: (1) tablets (cetirizine or lactose placebo) were conditioned into sealed opaque vials, (2) participants were asked to break the seal and take the tablet in the presence of an unblinded oncology pharmacist with a 180-ml glass of water without looking inside the opaque vial, and (3) the unblinded oncology pharmacist had to confirm that the tablet was effectively taken. Albeit unconventional, visual masking for cetirizine/placebo was considered practical, cost-saving, and easy to set up for a small-scale feasibility trial. A syringe filled with 1 ml of sodium chloride 0.9% was used as matching placebo for diphenhydramine. Treatment was allocated by an unblinded research pharmacist according to a 1:1 randomization in blocks of four.
In this feasibility study, we selected intravenous diphenhydramine 50 mg since it is the dosage used at our institution for all paclitaxel dosing schemes. It is also the dosage that is recommended in the monograph [4]. Oral cetirizine 10 mg was selected as a comparator namely for the advantageous pharmacokinetics and pharmacodynamics properties found at this dose and the real-life experience described by Durham et al. in more than 35 paclitaxel-naïve patients [13, 15, 16].
Prohibited drugs
The following drugs were prohibited: (1) first-generation H1 antihistamines within 72 h of the paclitaxel infusion, (2) second-generation H1 antihistamines within seven days of the paclitaxel infusion, (3) systemic corticosteroids within 72 h of the paclitaxel infusion, and (4) monoamine oxidase inhibitors within 14 days of the paclitaxel infusion.
Procedure
This study was conducted during the first two treatments of paclitaxel as IRR appear in 95% of cases during the first or second exposure [17]. Paclitaxel was administered according to local institutional chemotherapy infusion protocols. IRR were managed according to local procedures.
Participants were randomized and were planned to have the same premedication strategy for the first two cycles (see Fig. 1). They received oral cetirizine or placebo tablet at least 45 min before the start of the paclitaxel infusion since cetirizine onset of action occurs 0.7 h and its peak serum concentration one hour after oral intake [7, 18]. Participants were instructed to avoid food two hours before the oral premedication and at least 30 min after taking the oral premedication [15, 19]. However, participants who did not comply with this instruction were not excluded.
Other premedication agents (dexamethasone, H2 antihistamine, and diphenhydramine or sodium chloride 0.9%) were given at least 30 min prior to the start of the paclitaxel infusion, as per the standard operating procedures of the outpatient oncology clinic.
The Stanford Sleepiness Scale (SSS) [20] was used to assess the H1 antihistamine-associated drowsiness effect. The original questionnaire was used for English-speaking participants while a professionally translated version in Canadian French was proposed to French-speaking participants. Participants were told to fill the SSS questionnaire (1) before the administration of the oral premedication, (2) one hour after the administration of the intravenous premedication, (3) after returning back home, and (4) the morning after chemotherapy. A follow-up call was planned with participants the day after their paclitaxel infusion. Investigators documented any adverse effects reported by participants at the hospital or at home.
Participants reporting drowsiness one hour after the administration of the intravenous premedication (SSS score ≥ 2) were asked to determine how much this inconvenienced them using a 5-point Likert scale (not really, little, more or less, moderately, intensely).
At the end of the second paclitaxel treatment, participants were asked to determine to which H1 antihistamine they were allocated. If a participant had to withdraw before the second treatment, the procedure was performed at the end of the first treatment. Each participant’s oncology nurse who had administered the chemotherapy was asked to determine the participant’s allocation at each paclitaxel treatment using the same procedure.
Statistical methods
Data was summarized using descriptive statistics. Mean (SD) or median (IQR) were reported for continuous variables, medians (IQR) for ordinal variables, and percentages for categorical variables. All missing data for the SSS score and the 5-point item questionnaire were imputed by the median. The change from baseline on the drowsiness scale (ΔSSS) was calculated for each participant at each time point since it represented a more precise representation of the effect of H1 antihistamines. All SSS scores taken after premedication were adjusted for the score obtained before premedication (i.e., ΔSSS = SSS score after premedication—SSS score before premedication). Negative values were set to zero before performing statistical analysis. As ordinal data are not normally distributed, the statistical difference between each group’s ΔSSS at various time points was analyzed using non-parametric statistics (Mann–Whitney test). Overall blinding performance was analyzed using 2 × 2 contingency tables and the Fisher’s exact test. A p-value < 0.05 was considered as significant unblinding.
Sample size
Methods for estimating the sample size of a feasibility study vary [21–24]. The sample size was calculated to obtain a reasonable precision for the following feasibility outcome: proportion of patients recruited, randomized and who received the first treatment of paclitaxel following an assessment of their eligibility.
A target proportion of 60% with a range between 45 and 75% for a maximum period of eight months was considered acceptable. Thus, for a normal distribution, a 95% confidence interval of ± 15% and 60% of patients who received the first treatment of paclitaxel once their eligibility confirmed, a sample size of approximately 40 eligible patients would be required to obtain a minimum of 24 participants recruited over a maximum period of eight months. A minimum recruitment rate of 3 patients per month was considered sufficient to test the randomization procedure and effectively measure this feasibility outcome. The team had the option to stop the trial after recruiting 24 subjects or to pursue longer to collect more clinical information.
Criteria for pursuing a definitive clinical trial
Criteria were chosen according to security and feasibility considerations. As paclitaxel-related IRR could be life-threatening, the occurrence of unexpectedly frequent severe IRR, defined as having 2 consecutive participants with grade 3 or higher IRR within the first 10 participants, or 4 participants with grade 3 or higher IRR, was sufficient to stop the study or any further investigations.
Accrual rate and efficacy of recruitment milestones were made with respect of the study decision plan that considered sample size estimates and the projected length of a multicenter study (see Online Resource 1). Only primary feasibility outcomes were used for decision. The following had to be met to consider proceeding with a definitive trial: (1) an average recruitment rate ≥ 4 participants who received a first treatment of paclitaxel per month for the duration of the study within a single clinical trial site (this minimal criterion was arbitrary selected according to experience of public-funded successfully published clinical trials in the UK (range 4–10 participants/months) [25] and should take a maximum of 6 consecutive months) and (2) over 60% of patients assessed for eligibility consented to participate and were successfully recruited, randomized, and received the first treatment of paclitaxel over the whole trial (this milestone was selected to obtain an equivalent (or better) eligibility and consent performance than fifteen US sites funded by the National Cancer Institute [26]).
Results
Between February 3, 2020, and September 4, 2020, 119 patients were identified, of which 27 were recruited and randomized (Fig. 2). The first 24 participants were recruited within a period of 5 months.
Fig. 2.
Participant flowchart. a Insufficient time (less than 24 h) to give a free and informed consent or have already received the paclitaxel infusion. b One participant had an IRR on the first paclitaxel treatment in the cetirizine group. Thus, he could not continue the study for his second paclitaxel treatment as he developed the outcome
Among the 119 patients identified, 48 were excluded mainly because they were identified too late to assess eligibility. Consequently, 71 patients were evaluated for eligibility, of which 34 did not meet inclusion criteria (10 had received a taxane chemotherapy agent in the past, 8 were hospitalized (therefore did not receive their paclitaxel treatment in the outpatient oncology clinic), 5 spoke neither French nor English, 3 were taking chronically an oral H1 antihistamine or a systemic corticosteroid, 3 were transferred to another hospital, 2 had a contraindication related to the administration of cetirizine or diphenhydramine, 1 had dysphagia, 1 was pregnant, and 1 was unable to complete the questionnaires).
Participant characteristics are presented in Table 1 and treatment characteristics in Table 2. Our participants were predominantly Caucasian women of postmenopausal age with non-metastatic breast cancer receiving paclitaxel 80 mg/m2 each week. All participants received one dose of dexamethasone and one dose of an H2 antihistamine as part of their premedication. The majority received intravenous dexamethasone 10 mg with intravenous famotidine 20 mg. Paclitaxel infusion rates are presented in Online Resource 2.
Table 1.
Participant characteristics
| Diphenhydramine | Cetirizine | |
|---|---|---|
| n = 13 | n = 14 | |
| Gender (n (%) women) | 13 (100) | 13 (93) |
| Age (mean ± SD) | 59 ± 11 | 59 ± 8 |
| Weight in kg (mean ± SD) | 72.5 ± 22.7 | 71.5 ± 19.8 |
| Height in m (mean ± SD) | 1.61 ± 0.09 | 1.64 ± 0.08 |
| BMI (mean ± SD) | 27.8 ± 8.1 | 26.4 ± 6.3 |
| Ethnicity (n (%) Caucasians) | 12 (92) | 11 (79) |
| Degree of education (n (%) university) | 7 (54) | 7 (50) |
| Allergies (n (%)) a | 4 (31) | 6 (43) |
| Atopy (n (%)) b | 6 (46) | 2 (14) |
| Asthma and/or COPD (n (%)) | 2 (15) | 3 (21) |
| Menopause (n (%)) | 10 (77) | 11 (79) |
| ALT in U/l (mean ± SD) | 28 ± 29 | 28 ± 19 |
| AST in U/l (mean ± SD) | 31 ± 37 | 25 ± 13 |
| Total bilirubin in µmol/l (mean ± SD) | 7 ± 6 | 6 ± 3 |
| Creatinine clearance in ml/min (mean ± SD) | 76.1 ± 20.2 | 77.6 ± 18.9 |
| Type of cancer (n (%)) | ||
| Breast | 8 (61) | 10 (72) |
| Ovarian | 1 (8) | 1 (7) |
| Non-small cell lung cancer | 0 | 2 (14) |
| Endometrial | 2 (15) | 1 (7) |
| Thymus | 1 (8) | 0 |
| Vaginal | 1 (8) | 0 |
| Metastatic stage (n (%)) | 4 (31) | 2 (14) |
| Previous antineoplastic treatment (n (%)) | 6 (46) | 8 (57) |
| Dosing regimen (n (%)) | ||
| Every week | 8 (62) | 11 (79) |
| Every 3 weeks | 5 (38) | 3 (21) |
| Dose of paclitaxel (n (%)) | ||
| 45 mg/m2 | 0 | 1 (7) |
| 67 mg/m2 | 0 | 1 (7) |
| 80 mg/m2 | 8 (62) | 9 (64) |
| 175 mg/m2 | 5 (38) | 3 (22) |
| Other antineoplastic agents simultaneously (n (%)) | 8 (61) | 7 (50) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ALT, alanine transaminase; AST, aspartate transaminase
a Known hypersensitivity to a drug
b Allergic rhinitis and/or asthma and/or atopic dermatitis
Table 2.
Treatment characteristics
| Diphenhydramine | Cetirizine | |||
|---|---|---|---|---|
| Treatment 1 n = 13 |
Treatment 2 n = 11 |
Treatment 1 n = 14 |
Treatment 2 n = 13 |
|
| Dose of dexamethasone (n (%)) | ||||
| 10 mg | 8 (62) | 7 (64) | 11 (79) | 10 (77) |
| 20 mg | 5 (38) | 3 (27) | 3 (21) | 3 (23) |
| Other | 0 | 1 (9) a | 0 | 0 |
| H2 antihistamine (n (%)) | ||||
| Ranitidine 50 mg | 7 (54) | 3 (27) | 4 (29) | 5 (38) |
| Famotidine 20 mg | 6 (46) | 8 (73) | 10 (71) | 8 (62) |
| Time between end of H1 antihistamine administration and start of paclitaxel infusion in min (median (IQR)) b | 55 (15) | 58 (6) | 70 (18) | 71 (27) |
| Fasting 2 h before cetirizine administration (n (%)) | 14 (100) | 12 (92) | ||
| Fasting within 30 min of cetirizine administration (n (%)) | 13 (100) c | 11 (100) d | ||
| Duration of paclitaxel infusion in min (median (IQR)) e, f | ||||
| 45–80 mg/m2 | 82 (8) | 85 (9.5) | 85 (4) | 85 (10) |
| 175 mg/m2 | 195 (10) | 194 (10) | 195 (9.5) | 195 (8.5) |
a The participant received a 5-mg dose due to known adverse effects to corticosteroids
b The expected times were 30 min for diphenhydramine and 45 min for cetirizine
c One missing data (n = 13)
d Two missing data (n = 11)
e The expected times according to local institutional chemotherapy infusion protocols were 60 min for 45–80 mg/m2 and 180 min for 175 mg/m2
f Paclitaxel infusion rates are presented in Online Resource 2
Feasibility outcomes
Among the 37 eligible patients, 27 (73%) consented to participate and were recruited, randomized, and received the first treatment of paclitaxel. Two participants did not complete the study. One participant withdrew its consent due to chemotherapy-induced adverse effects (pancreatitis) and one was excluded for taking cetirizine in the last seven days to manage a skin rash with pruritus and blisters that followed the first paclitaxel treatment. Both participants were in the diphenhydramine group. Although the study could have stopped at 24 participants from a feasibility standpoint, there was interest in accruing exploratory data from a safety and efficacy perspective. The accrual rate was 4.8 participants per month for the first 24 participants and 3.9 participants per month for the full study.
Online Resource 3 shows the data on the allocation identification in contingency tables. The percentage of questionnaires completed was high for a non-mandatory exercise (98% nurses, 93% participants). Nurses and participants had a 50% chance of guessing correctly the drug they were assigned to (i.e., diphenhydramine or cetirizine). Participants correctly guessed their treatment allocation 48% of times, whereas nurses correctly guessed 64% of times. Despite the exploratory nature of this endpoint, we further addressed whether these proportions were significantly different from random guessing using the Fisher’s exact test. Our analysis showed that the proportion of participants or nurses who correctly guessed their allocated treatment were not significantly different from chance (p > 0.05). The most common reasons for identifying the H1 antihistamine allocation were (1) drowsiness (or lack thereof), (2) dizziness, and (3) irritation of the vein. No participant or oncology nurse mentioned the use of non-identical placebo as a reason for revealing the H1 antihistamine allocation.
Safety outcomes
A list of all reported adverse events is presented in Online Resource 4. Nervous system disorders, principally drowsiness, was reported by all participants in the diphenhydramine group (100%) compared to eight (57%) in the cetirizine group.
Drowsiness was statistically more prevalent in the diphenhydramine group compared to the cetirizine group (Fig. 3). The majority of participants in the diphenhydramine group reported a ΔSSS one hour after premedication ≥ 1 while about half of participants in the cetirizine group reported no increase in drowsiness (ΔSSS = 0). Increase in drowsiness was also more intense in the diphenhydramine group where 14 (58%) participants reported a ΔSSS ≥ 2 compared to one (4%) in the cetirizine group (Fig. 3).
Fig. 3.
Raincloud plot comparing the change in drowsiness (ΔSSS) one hour after the administration of the intravenous premedication a, b, c, d. a SSS: Stanford Sleepiness Scale.b ΔSSS = SSS score one hour after premedication — SSS score before premedication. A ΔSSS of 0 indicates no change in drowsiness when compared to baseline; increasing score indicates increased drowsiness. c Imputation with median SSS score results in each group was used for missing data.d A statistical difference was found between oral cetirizine 10 mg (median ΔSSS 0; IQR 1) and intravenous diphenhydramine 50 mg (median ΔSSS 2; IQR 3.25) when results from treatments 1 and 2 were combined (p < 0.01, Mann–Whitney test). Note that statistical significance was maintained when each paclitaxel treatment was analyzed separately (treatment 1, p < 0.01; treatment 2, p < 0.025)
No difference was found between ΔSSS once participants had returned home or the morning after chemotherapy (data not shown). No relationship was found between the dose of paclitaxel and the ΔSSS (data not shown).
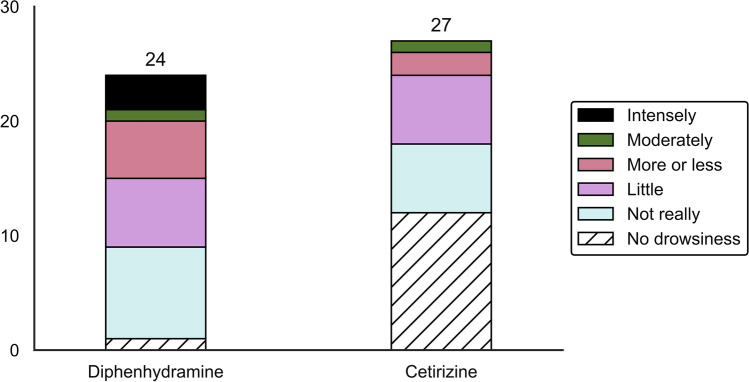
The majority of participants who experienced drowsiness appeared minimally inconvenienced by this adverse effect. Fourteen (61%) participants and 12 (80%) participants reported no to little discomfort for diphenhydramine and cetirizine, respectively. Intense discomfort was only described in the diphenhydramine group (three (13%) participants) (Fig. 4).
Fig. 4.
Level of inconvenience experienced by participants when drowsiness was reported at the hospital a, b, c. a No drowsiness: SSS score = 1. b Level of inconvenience is reported when SSS score ≥ 2. c Imputation with median inconvenience score results in each group was used for missing data
Efficacy outcomes
One participant suffered an IRR. The reaction appeared on the first paclitaxel treatment, five minutes after the start of the paclitaxel infusion. The participant had chest pain, hot flashes, and throat tightness. Symptoms lasted less than a minute after stopping the paclitaxel infusion. The use of rescue medication was not required and the symptoms did not recur after resuming the paclitaxel infusion. The IRR was classified as grade 2. The participant was in the cetirizine group.
Discussion
Our feasibility study met the criteria to proceed with a definitive trial, with accrual rate between 4.8 (first 24 participants) and 3.9 (full study) and a high percentage of patients receiving a first treatment of paclitaxel once their eligibility was confirmed (> 60%). The COVID-19 pandemic did not affect the recruitment rate as much as we anticipated. Our design, combined to a flexible consent model proposed by the research ethic board, allowed maintaining the recruitment rate. We believe that the latter flexible model, when applied wisely, could be used to accelerate research in oncology.
This was the first study comparing diphenhydramine to cetirizine in preventing chemotherapy-related IRR using a randomized double-blind prospective design. Although this feasibility trial was not powered to examine the efficacy at preventing IRR, the study was found to be safe. IRR were rare, with only one participant with grade 2 IRR in the cetirizine group. The observed rate of events confirms that a large multicenter trial would be needed to demonstrate non-inferiority between the two H1 antihistamines in preventing paclitaxel-associated IRR.
As expected, drowsiness was more common and disturbing in the diphenhydramine group compared to the cetirizine group [6, 15]. Despite the small sample size, the ΔSSS was sensitive enough to determine that the change in drowsiness reported with cetirizine was less intense when compared to diphenhydramine. Interestingly, only few participants reported being truly inconvenienced by drowsiness at the hospital. This information should be interpreted with caution since diphenhydramine users often experience a lack of awareness of a reduced level in functioning when compared to selective H1 antihistamines [27]. Future studies could explore the impact on cognitive performance with simple objective tools.
There are strengths and limitations to our design. Though oncology clinics may have already adopted different strategies to decrease side-effects from diphenhydramine based on experience or changes in practice, this prospective randomized trial design offers stronger internal validity compared with retrospective or observational designs [12, 13]. The performance of recruitment for eligible patients was higher than previously reported (73 vs 60%, respectively) [26]. The block randomization sequence effectively reduced the risk of bias by achieving a balance in the allocation [28]. In contrast, the paclitaxel group was unbalanced between diphenhydramine and cetirizine in the retrospective design of Durham et al. [13].
Among the limitations, although the study met its feasibility endpoints, we found that our recruitment strategy was highly dependent on the use of paclitaxel in various oncology care trajectories. For instance, breast cancer patients could be approached in advance which allowed some time to consider enrolling while only a few days were allowed for gynecologic cancer patients. Future strategies should aim at earlier identification of patients, especially for oncology specialties where the timing between consent and initiation of treatment is short. Our population consisted of a high proportion of university-educated individuals. The use of the SSS questionnaire may not be applicable for a population with a low level of literacy and other approaches may need to be considered [29]. Performance indicators such as chair time, overall cost savings, or patient satisfaction/quality of life should be taken into consideration for a larger clinical trial. Although most participants and nurses remained blind to treatment allocation throughout the study, blinding appeared more difficult to maintain for oncology nurses because of their knowledge of intravenous diphenhydramine adverse effects and this could have influenced their behavior during paclitaxel infusions. Interestingly, no participant or oncology nurse mentioned the use of non-identical placebo as a reason for revealing the H1 antihistamine allocation. Although visual masking with cetirizine/placebo was found cost-saving and practical in our hand, overencapsulation might represent a better alternative for a multicenter trial. In a larger setting, additional efforts should also be spent on robust mandatory blinding performance questionnaire and to mitigate the impact of nurse’s behaviors. Finally, this study did not provide a standardized protocol for the administration of paclitaxel, relying principally on clinical practice currently in place. Although this represents a real-life setting, important variables related to the administration of paclitaxel or its premedication were not fully controlled (e.g., rates of paclitaxel infusions). Since these variables as well as the paclitaxel dosing scheme (weekly vs every 3 week) could influence the incidence of IRR [1, 17], a definitive trial should consider stratification for those variables although it would require a larger sample size.
Conclusion
In this study, we demonstrated feasibility of a prospective controlled randomized trial comparing the efficacy of a second-generation H1 antihistamine with a first-generation H1 antihistamine in preventing chemotherapy-related IRR. Cetirizine produced less drowsiness when used as premedication than diphenhydramine. Given the infrequency of paclitaxel-related IRR found in our setting, especially severe events requiring medical intervention, consideration for a large multi-center non-inferiority trial using a predetermined non-inferiority margin is warranted. A complementary study is currently ongoing to determine the non-inferiority margin necessary to confirm whether the design will be practical considering sample size estimates of a non-inferiority trial.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to L. DeGuerke, C. Fillion, L. Sidéris, I. Hafiane, J.-C. Petit, and the oncology nurses and pharmacists for their contribution to the project development and implementation.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Johannie Beaucage-Charron, Laurence Gaudet, Sarah Lamothe, Cloé Pelletier, Anne-Sophie Pépin, and Valérie Roy. The first draft of the manuscript was written by Johannie Beaucage-Charron and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the Pharmacy Department of the Centre intégré universitaire de santé et de services sociaux de l’Est-de-l’Île-de-Montréal, Montréal, Canada.
Data availability
The datasets and material are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Comité d’Éthique de la Recherche de l’Hôpital Maisonneuve-Rosemont, Centre intégré universitaire de santé et de services sociaux de l’Est-de-l’Île-de-Montréal (17.01.2020, #2020–2110).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. Marie Lordkipanidzé was supported by the Fonds de recherche du Québec en Santé Junior 1 Research Scholarship (33048) and is a Canada Research Chair in Platelets as biomarkers and vectors (950–232706).
Footnotes
Philippe Bouchard and Matthieu Picard are joint senior authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/jco.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 2.Donehower RC, Rowinsky EK, Grochow LB, Longnecker SM, Ettinger DS. Phase I trial of taxol in patients with advanced cancer. Cancer Treat Rep. 1987;71:1171–1177. [PubMed] [Google Scholar]
- 3.Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:177–191. doi: 10.1007/s12016-014-8416-0. [DOI] [PubMed] [Google Scholar]
- 4.Pfizer Canada ULC (2020) Product monograph: paclitaxel for injection
- 5.Picard M. Management of hypersensitivity reactions to taxanes. Immunol Allergy Clin North Am. 2017;37:679–693. doi: 10.1016/j.iac.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Pharmaceutical Partners of Canada Inc (2012) Package insert: Diphenhydramine hydrochloride injection USP
- 7.Estelle R, Simons F, Akdis CA (2008) Histamine and H1 Antihistamines. In: Franklin Adkinson Jr N, Busse W, Bochner B, Holgate S, Estelle Simons F, Lemanske R (ed) Middleton’s Allergy: Principles and Practice, 7th edn. Elsevier Health Sciences, pp 1517–1548
- 8.Kalivas J, Breneman D, Tharp M, Bruce S, Bigby M. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy Clin Immunol. 1990;86:1014–1018. doi: 10.1016/s0091-6749(05)80246-5. [DOI] [PubMed] [Google Scholar]
- 9.Belaich S, Bruttmann G, DeGreef H, Lachapelle JM, Paul E, Pedrali P, et al. Comparative effects of loratadine and terfenadine in the treatment of chronic idiopathic urticaria. Ann Allergy. 1990;64:191–194. [PubMed] [Google Scholar]
- 10.Simons FE, McMillan JL, Simons KJ. A double-blind, single-dose, crossover comparison of cetirizine, terfenadine, loratadine, astemizole, and chlorpheniramine versus placebo: suppresive effects on histamine-induced wheals and flares during 24 hours in normal subjects. J Allergy Clin Immunol. 1990;86:540–547. doi: 10.1016/0091-6749(90)90091-H. [DOI] [PubMed] [Google Scholar]
- 11.Fein MN, Fischer DA, O’Keefe AW, Sussman GL. CSACI position statement: newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin Immunol. 2019;15:1–6. doi: 10.1186/s13223-019-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siderov J, Wendel N, Davis ID. Non-sedating antihistamines for premedication in ambulatory oncology patients. J Pharm Pract Res. 2002;32:108–109. doi: 10.1002/jppr2002322108. [DOI] [Google Scholar]
- 13.Durham CG, Thotakura D, Sager L, Foster J, Herrington JD. Cetirizine versus diphenhydramine in the prevention of chemotherapy-related hypersensitivity reactions. J Oncol Pharm Pract. 2019;25:1396–1401. doi: 10.1177/1078155218811505. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health, National Cancer Institute (2017) Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0
- 15.McNeil Consumer Healthcare (2017) Product monograph: reactine, reactine fast melt junior, reactine rapid dissolve, reactine allergy liquid gels
- 16.Petersen LJ, Church MK, Rihoux JP, Skov PS. Measurement of interstitial cetirizine concentrations in human skin: correlation of drug levels with inhibition of histamine-induced skin responses. Allergy. 1999;54:607–611. doi: 10.1034/j.1398-9995.1999.00038.x. [DOI] [PubMed] [Google Scholar]
- 17.Boulanger J, Boursiquot JN, Cournoyer G, Lemieux J, Masse MS, Almanric K, et al. Management of hypersensitivity to platinum- and taxane-based chemotherapy: CEPO review and clinical recommendations. Curr Oncol. 2014;21:e630–641. doi: 10.3747/co.21.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerji A, Long AA, Camargo CA. Diphenhydramine versus nonsedating antihistamines for acute allergic reactions: a literature review. Allergy Asthma Proc. 2007;28:418–426. doi: 10.2500/aap.2007.28.3015. [DOI] [PubMed] [Google Scholar]
- 19.Pasko P, Rodacki T, Domagala-Rodacka R, Palimonka K, Marcinkowska M, Owczarek D. Second generation H1 - antihistamines interaction with food and alcohol—a systematic review. Biomed Pharmacother. 2017;93:27–39. doi: 10.1016/j.biopha.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. doi: 10.1111/j.2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 22.Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol. 2013;13:104. doi: 10.1186/1471-2288-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julious S. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 24.Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS ONE. 2016;11:e0150205. doi: 10.1371/journal.pone.0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walters SJ, Dos Anjos B, Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trial funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7:e015276. doi: 10.1136/bmjopen-2016-015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avis N, Smith K, Link C, Hortobagyi GN, Rivera E. Factors associated with participation in breast cancer clinical trials. J Clin Oncol. 2006;24:1860–1867. doi: 10.1200/jco.2005.03.8976. [DOI] [PubMed] [Google Scholar]
- 27.Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105:s622–627. doi: 10.1067/mai.2000.106153. [DOI] [PubMed] [Google Scholar]
- 28.Efird J. Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health. 2011;8:15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldonado CC, Bentley AJ, Mitchell D. A pictorial sleepiness scale based on cartoon faces. Sleep. 2004;27:541–548. doi: 10.1093/sleep/27.3.541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and material are available from the corresponding author on reasonable request.
Not applicable.