Highlights
-
•
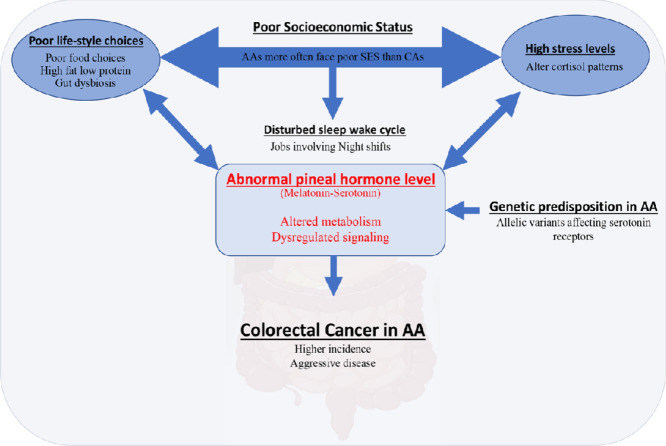
Poor socioeconomic status in african american leads to dysregulation of the melatonin-serotonin signaling which contributes to disparity in colorectal cancer risk.
-
•
Unhealthy dietary choice impacts gut microbiome, which contributes to disparity in colorectal cancer risk.
-
•
High cortisol due to poor socioeconomic status and vitamin D deficiency in african americans mount melatonin-serotonin signaling anomalies and contribute to colorectal cancer.
-
•
Clinical outcome of colorectal cancer in african americans can be improved by addressing the disturbed melatonin-serotonin axis.
Keywords: Colorectal cancer, Racial disparity, SES, Melatonin, Serotonin
Abstract
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths in the United States. Despite increased screening options and state-of-art treatments offered in clinics, racial differences remain in CRC. African Americans (AAs) are disproportionately affected by the disease; the incidence and mortality are higher in AAs than Caucasian Americans (CAs). At the time of diagnosis, AAs more often present with advanced stages and aggressive CRCs, primarily accounting for the racial differences in therapeutic outcomes and mortality. The early incidence of CRC in AAs could be attributed to race-specific gene polymorphisms and lifestyle choices associated with socioeconomic status (SES). Altered melatonin-serotonin signaling, besides the established CRC risk factors (age, diet, obesity, alcoholism, and tobacco use), steered by SES, glucocorticoid, and Vitamin D status in AAs could also account for the early incidence in this racial group. This review focuses on how the lifestyle factors, diet, allelic variants, and altered expression of specific genes could lead to atypical serotonin and melatonin signaling by modulating the synthesis, secretion, and signaling of these pineal hormones in AAs and predisposing them to develop more aggressive CRC earlier than CAs. Crosstalk between gut microbiota and pineal hormones and its impact on CRC pathobiology is addressed from a race-specific perspective. Lastly, the status of melatonin-focused CRC treatments, the need to better understand the perturbed melatonin signaling, and the potential of pineal hormone-directed therapeutic interventions to reduce CRC-associated disparity are discussed.
Graphical abstract
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the United States, regardless of gender [87]. The American Cancer Society estimates that, in 2021, there will be about 149,500 new colorectal cancer cases and 52,980 deaths in the United States. The lifetime risk of developing CRC is 1 in 23 (4.3%) for men and 1 in 25 (4.0%) for women. Theoverall death rate from CRC continues to drop due to early diagnosis and state-of-art interventions enabled by continued research studying the disease pathogenesis.We have shown the significance of optimum acetaldehyde dehydrogenase expression in maintaining gut epithelial integrity [15] and association of CCR6-CCL20 chemokine axis in CRC pathogenesis [44]. However, the incidence and mortality rates for African Americans (AAs) have not decreased to the same extent relative to non-Hispanic Caucasian Americans (CAs) [2,4,41,87]. The racial discrepancy in mortality rates reflects differential CRC incidence and patient survival. The five-year relative survival is lower for AAs than CAs irrespective of disease stage (i.e., localized, regional metastasis, distant metastasis, and all stages combined) [31,87]. Racial differences in relative survival may be related to barriers to CRC screening; however, the gap persists even after normalizing the socioeconomic status (SES) and differences in access to treatment [19,64,68]. Notably, the effect that low SES-induced chronic stress has on the pathobiology of CRC and the racial disparity is not explored adequately. Hence, the purpose of this review is to evaluate the association of the earlier onset, higher incidence, and poor outcomes of CRC in AA patients with SES driven chronic stress and chronic stress-induced changes in biochemical signaling, specifically that of pineal hormones.
Association of modifiable and non-modifiable risk factors with CRC disparity
Modifiable risk factors, such as environmental factors, obesity, physical activity, nutrition/diet, smoking, and alcohol consumption, are well defined [39,86,94] and manageable [42]. A consistent body of evidence indicates that geographical location, overweight, and obesity are positively associated with the lifetime risk of developing CRC [51,94]. In the United States, AAs have higher obesity prevalence rates than other racial groups. Dietary habits independently influence the risk of CRC, in addition to contributing to obesity [92]. Avoiding high fat and red meat consumption reduces CRC risk [43,50]. Animal fat increases the conversion of bile salts to potential carcinogens by the gut microbiome [50]. Studies published by our group suggest that natural products like emodin and cinnamtannin B1 reduce CRC progression [13,84] while glutamine supplementation improves epithelial integrity of the gut [16]. Besides the dietary ingredients, high-temperature food preparation methods also increase CRC risk by generating carcinogenic heterocyclic amines and polycyclic aromatic hydrocarbons [82,88]. Further, physical activity reduces CRC risk by increasing metabolic activity, oxygen uptake, and gut motility while reducing body weight [7,18,94]. Long-term exercise reduces CRC risk also by reducing insulin resistance [52]. In this regard, AAs who often belong to low socioeconomic status (SES), a multidimensional concept measured at the individual level (education, income, and occupation); the household level (poverty, family income, and wealth); and neighborhood level (i.e., community structural characteristics, neighborhood, and crime), [48,93] are forced to make poor lifestyle and food choices (i.e., processed food and high fat intake), which results in high BMI, increasing their risk of CRC. In addition, individuals with low SES exhibit abnormally high p53 [90], which contributes to an immunosuppressive environment conducive to cancer immune evasion and tumor tolerance- two critical factors in the development and progression of CRC and amplifying racial disparity.
Non-modifiable factors such as genetic background, history of inflammatory bowel disease (IBD) and adenomatous polyps, and age are associated with a higher risk of CRC. Family history increases CRC risk by 20%, while ∼5–10% of CRCs are hereditary [38]. Interestingly, AAs and CAs do not show any differences in the CRC risk factor, IBD [1,63]. Adenomatous polyps, the pre-cancerous lesions contributing to about 95% of sporadic CRCs [39], take 5–10 years to develop tumors[18,27]. Hence, early removal of adenomatous polyps reduces the risk of developing CRC. However, this intervention is not readily available for AAs with low SES due to lack of awareness and poor access to care.
Moreover, although aging increases the risk of CRC irrespective of ethnicity [87], CRC presents at an earlier age in AAs compared to CAs. Considering these factors, AAs are generally at a higher risk of developing CRC at an earlier age than their CA counterparts. Moreover, AAs with a history of other malignancies are more prone to develop CRC. Hence, for this ethnic population, lack of timely screening, poor access to care, and poor food choice due to low SES contribute to the observed disparity in incidence, prognosis, and mortality. It is, therefore, imperative to identify race-specific risk factors and biomarkers to improve the therapeutic outcome in AAs.
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and melatonin in African Americans and their effects on CRC risk
Poor SES is associated with increased stress that is evident from variations in diurnal rhythms of cortisol [17]. AAs have a more blunted diurnal cortisol slope than CAs as assayed using salivary samples [30], whereas hair cortisol levels are higher in AAs than CAs [96]. Besides, irregular sleep schedule, which is often found in AAs, is associated with significantly higher levels of salivary cortisol in AAs compared to CAs, in addition to smoking [99]. These deviations reflect dysregulation of the HPA axis [47,83] in AAs. Regulation of the HPA axis and the pineal gland is closely related [91] and interestingly, glucocorticoids and melatonin have contrasting effects. Glucocorticoids negatively regulate melatonin synthesis by the pineal gland [73], and extra-pineal melatonin produced after food intake affects cortisol rhythms [10]. This is noteworthy because many of the CRC risk factors could be related to melatonin's atypical or anomalous status [10,11]. Like CRC risk, melatonin production varies by race, gender, and age [45,74,81]. This hormone acts as a free radical scavenger, regulates food digestion, gut motility, and bowel movement through specific receptor signaling [10]. In addition to this, melatonin affects obesity risk by controlling food intake [11] and regulating mucosal immunity [62]; both factors associate with CRC and are frequently compromised in AAs [46].
Remarkably, several investigators have reported differences in melatonin levels in people of different ancestries. One report asserts that the highest melatonin levels are found in AAs, and within the racial groups, levels are highest among young AAs (ages 30–50) and older CAs (ages 60–90) [45]. Another study found that AAs have lower melatonin levels relative to CAs [40]. The difference could be attributed to the fact that melatonin levels are affected by the circadian clock [37] and the time of sample collection was different in both studies. Nevertheless, a variance of melatonin levels by race is apparent and may lead to a disturbed sleep-wake cycle for AAs, predisposing them to develop CRC. This possibility is supported by studies demonstrating a higher risk for rectal cancer in night shift workers due to changed melatonin rhythms [77] and the fact that a higher percentage of AAs are night shift workers. Nonetheless, these findings warrant studies of melatonin profiles to test its potential as a race-specific biomarker to better predict CRC risk in AAs.
Impaired serotonin signaling in African Americans and CRC risk
Melatonin disturbances in the body reflect a more complex molecular issue since the metabolism and signaling of melatonin are tightly coordinated with serotonin (5-hydroxytryptamine, 5HT) signaling [11]. Both melatonin and serotonin are brain-derived and locally generated in the gut. Enterochromaffin cells lining the gut are the primary producers of serotonin. Tryptophan hydroxylase, coded by TPH1, catalyzes serotonin synthesis from dietary tryptophan. A small amount of serotonin is also produced by the TPH2-coded enzyme of enteric neurons. Serotonin regulates gut motility, secretion, and sensation via serotonin receptors (5-HTRs). Of the 5-HTR family, five components (5-HTR1–4 and 5-HTR7) are expressed in the gut. The receptor-mediated signaling is controlled mainly by the serotonin transporter (SERT) facilitated serotonin reuptake and its consequent degradation by monoamine oxidase A [67].
Intriguingly, there is a high frequency of race-specific polymorphism in genes involved in serotonin synthesis, reuptake, and signaling. Specifically, there are polymorphisms in genes (Table 1) coding for tryptophan hydroxylases (TPH1 and 2) and those coding for enzymes converting serotonin to melatonin, namely acetyltransferases (NAT1 and 2), coding for N-acetyltransferases 1 and 2, and ASMT, coding for acetyl serotonin O-methyltransferase. Notably, a variance in NAT2 is associated with prostate cancer susceptibility for AAs [35]. However, the only evidence for its clinical significance in CRC comes from the human protein atlas database, which indicates NAT2 as a favorable marker for CRC. A higher percentage of African descendants exhibit SLC6A4 (L)-allele ( serotonin transporter coding gene) relative to those of European ancestry [24,75]. The L-allele is associated with elevated transcription of the serotonin transporter, meaning that reduced extracellular serotonin levels will be available for signaling to surrounding cells in these individuals [55]. AAs also carry mutations in serotonin receptor 1F (HTR1F) [12,29]. At present, not much is known about this G-protein-coupled receptor, other than its association with migraines [36]. In-depth studies will help determine if polymorphism(s) in these genes correlate with CRC pathogenesis.
Table 1.
Polymorphisms associated with serotonin and melatonin signaling and their predicted effects.
| Gene | Product | Polymorphism | Effect |
|---|---|---|---|
| SLC6A4 | Serotonin transporter | L (long) and S (short) allele | L allele is associated with elevated transcription of the gene and reduced serotonin signaling. |
| HTR1F | Serotonin receptor | Associated with migraines. | |
| NAT2 | N-acetyl transferase 2 | Associated with prostate cancer susceptibility of AAs. | |
| TPH1 | Tryptophan hydroxylase (Gut) | A218C | A allele is associated with borderline personality disorder due to serotonergic dysfunction [51]. |
| TPH2 | Tryptophan hydroxylase (Enteric neurons) | rs4290270; rs4570625; rs41317118; rs11178998; rs7305115; rs17110747; rs10748185; rs18438099; rs11316791; rs1386493; rs1386494 | rs4290270 (A/T allele) could be associated with major depressive disorder [52]. |
High cortisol and vitamin D deficiency in African Americans mount melatonin-serotonin signaling anomalies
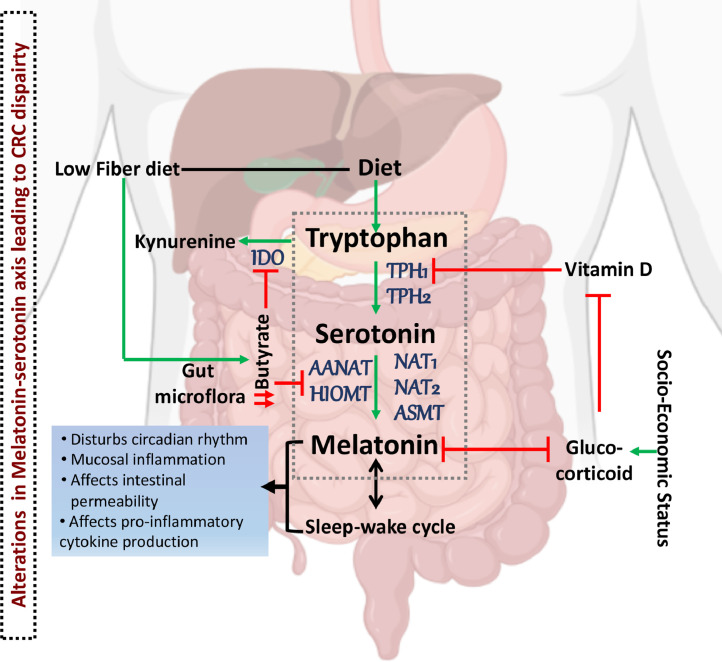
As found in AAs, excess cortisol negatively regulates vitamin D receptor expression and vitamin D synthesis [28,89]. Consequently, AAs suffer chronic vitamin D deficiency more frequently than CAs [32,33,61]. Plasma vitamin D levels relate to CRC risk [21,26,95], and deficiencies may contribute to high CRC-related mortality [22,28]. One of the pleiotropic effects of vitamin D is its influence on sleep quality and duration [8,66] by its action on serotonin-melatonin signaling. A heterodimeric complex of vitamin D bound receptor and retinoid X receptor regulates serotonin and melatonin synthesis via its effect on the vitamin D responsive element (VDRE) on TPH1 and TPH2 genes [71,79]. Low vitamin D leads to elevated TPH1 expression [79] and gut serotonin production, resulting in inflammatory conditions [54,56]. Thus, glucocorticoids could affect melatonin synthesis and circadian rhythm by regulating vitamin D (Fig. 1).
Fig. 1.
Factors affecting the melatonin-serotonin axis and CRC risk. Increased socioeconomic stress leads to an increase in glucocorticoids, which have a negative impact on melatonin and vitamin D. Vitamin D downregulates expression of tryptophan hydroxylase 1, the gene product of which generates serotonin. A diet rich in fat reduces butyrate-producing species of bacteria in the gut. Low butyrate levels remove the inhibitory effect on indole dioxygenase and acetyl and methyl transferases, thereby affecting melatonin production in the gut. African Americans (AAs) have high levels of glucocorticoids due to lower SES and vitamin D deficiency. They also tend to have a high-fat, low-fiber diet. Further, AAs express allelic variants of genes encoding the serotonin receptor and reuptake proteins, and enzymes converting serotonin to melatonin. These factors affect the conversion of dietary tryptophan to serotonin, changing melatonin production. Changes in melatonin production affect the sleep-wake cycle, and together these two altered factors modulate circadian rhythm, intestinal permeability, cytokine production, and mucosal immunity. The dotted gray box indicates the serotonin-melatonin metabolism that is prone to be affected in AAs to increase their CRC risk.
Based on the current body of knowledge, it is difficult to determine which of these defects- high glucocorticoid levels, vitamin D deficiency, and deregulated serotonin-melatonin signaling, is the primary causative factor leading to elevated CRC risk in AAs. It is also possible that vitamin D deficiency, SLC6A4 (L) allele expression or NAT variants present in AAs are merely compensatory alterations. Furthermore, which of these variations is more significant in regulating extracellular serotonin levels and melatonin synthesis is also unclear. A detailed understanding of HPA-pineal regulation on CRC pathogenesis is required to answer these questions.
Crosstalk between the diet-influenced gut microbiome and the serotonin-melatonin axis affecting CRC risk in African Americans
Gut flora responsive to dietary intake, cortisol, and melatonin, can vary CRC risk by a constant dialog with the intestinal epithelia. A fiber-rich diet reduces CRC risk by increasing the butyrate and other short-chain (SCFAs, such as propionate and acetate) fatty acids-producing commensal bacteria that are protective against carcinogenesis while the economical food choices made due to poor SES have high-fat, low-fiber content which reduce the beneficial microbes by promoting gut dysbiosis. The beneficial effects of SCFA-producing microbes could be attributed to the repression of IDO-1 (indole dioxygenase-1) and induction of AANAT (aralkylamine N-acetyltransferase) and HIOMT (hydroxyindole-O-methyltransferase) in the intestinal epithelial cells by butyrate [3,65] (Fig. 1). IDO-1 catalyzes the step that commits the bulk of tryptophan to the kynurenine pathway, and aralkylamine N-acetyltransferase and acetyl serotonin O-methyltransferase, the respective gene products of AANAT and HIOMT, convert serotonin to melatonin. Therefore, butyrate directly influences tryptophan metabolism and diverts the flux towards melatonin synthesis. However, differences in intestinal flora caused only due to the dietary choices have been associated with higher CRC incidence and outcomes in AAs [76]. The gut microbiome of AAs is low in butyrate-producing microbes; instead, it is dominated by potentially pathogenic Escherichia and Acinetobacter species. However, as stated earlier, this difference is due to a high-fat and low-fiber diet [76] and is not associated with racial ethnicity per se [100].
Gut microbes also generate 5-HT and indole derivatives from tryptophan [9]. The crosstalk is bidirectional since melatonin affects the microbiome as well [80]. More research with data stratification based on age, physical activity, and diet is required to determine if there are any racial disparities in the microbiome and the gut epithelial tryptophan-serotonin-melatonin axis.
Prospective race-specific CRC biomarkers associated with serotonin-melatonin signaling
In AAs, genes encoding the serotonin receptor (5-hydroxytryptamine receptor 1F, HTR1F) are specifically mutated [12,29]. HTR1F is the transducer of intracellular serotonin signaling [36], which could be used as a potential race-specific biomarker considering the above discussed findings implying disturbed melatonin-serotonin signaling axis in AAs. However, further research is needed to establish a more specific function of HTR1F in CRC, particularly in AAs. Whole-exome sequencing of CRC tissues from AAs and CAs shows fifteen preferentially mutated genes in AAs [29]. Mutations in these genes accounted for 41% of all AA CRCs but only 15% of CA CRCs. In addition to HTR1F, mutations in epha6 (ephrin type-A receptor 6) and flcn (folliculin) were detected exclusively in AA CRCs. Mutations in flcn (a tumor suppressor gene) are also associated with Birt-Hogg-Dubé syndrome, kidney cancer, and lung complications [12,29,72]. Mutation frequencies of htr1f and flcn are low, and the association with AAs is not statistically significant (P = 0.086 for each gene). However, lack of statistically significant association could be attributed to the small sample size (AA = 103 & CA = 129) and lack of ancestry genotyping. More research is necessary to define and validate FLCN and HTR1F as prognostic tools for AA CRCs. Further, studies focusing on factors regulating serotonin and melatonin signaling, as discussed above, should be undertaken, as these could be potential biomarkers for predicting CRC risk for AAs. It is also likely that some of these factors could cumulatively work as CRC predictors.
Clinical relevance of the melatonin-serotonin axis in CRC outcome
Investigating differences in melatonin and serotonin among different races may be clinically significant given the above background. Recently, melatonin has gained considerable attention in the field of chemotherapeutic intervention [49] due to its onco-static effects [20,25,69]. Melatonin imposes its anti-CRC effects by targeting the processes that support the hallmarks of cancer [85]. It reestablishes the balance between cellular apoptosis and survival by downregulating survival factors [53] and by inducing cell cycle arrest and apoptosis [34]. It also reduces the metastatic potential of cancer cells by impeding the epithelial to mesenchymal transition [97] and by inhibiting migration [98]. Further, the combination of melatonin and 5-fluorouracil (5-FU) is more effective than 5-FU alone in controlling CRC progression in murine model [23,78]. Colon cancer growth also reduces after the administration of melatonin in combination with irinotecan metabolite and curcumin analog. Combining melatonin with somatostatin hampers colon cancer growth more efficiently as well. The combination treatments worked by reducing cellular superoxide/hydrogen peroxide ratio and cellular proliferation/apoptotic index ratio, respectively [5,70]. Thus, melatonin clearly shows anti-cancer effects in vitro and in vivo experimental models of CRC. However, clinical data supporting its therapeutic potential in cancer is less promising. Melatonin treatment in combination with interleukin-2 shows a partial response in CRC patients [59]. It improves the performance status of cancer patients who are non-responsive to standard therapies [60], like those with metastatic CRCs resistant to 5-FU [6]. In similar other studies, the efficacy enhancing effect of melatonin when administered in combination with irinotecan (CPT11) as a second line of treatment to metastatic CRC patients are also studied [14,57,58]. Melatonin improves the quality of life of patients receiving radio/chemotherapy irrespective of stages [25]. Thus, though melatonin treatment is beneficial, the results are not as favorable as projected based on the in vitro and preclinical studies. Race-specific differences in the melatonin-serotonin signaling axis and inadequate participation of the AA population in clinical trials may be leading to such ambiguous clinical output. Hence, studying the race-specific impact of serotonin-melatonin targeted CRC therapy might give more assuring results and discover new therapeutic targets opening novel avenues for designing racially tailored treatments for CRC.
Conclusion
The racial gap in CRC incidence, mortality, and therapeutic outcome could be attributed to a combination of late-stage diagnosis due to early incidence, increased aggressiveness of the disease, and poor SES-induced poor access to care in the AAs compared to CAs. The influence of the genetic predisposition of AAs and their SES on the interplay of the neuronal system and pineal hormone signaling and, in turn, its effect on CRC biology and contribution to disease aggressiveness is a crucial aspect that needs urgent consideration. Namely, the changes in pineal hormone profile support CRC promoting cellular phenotypes while creating a tolerogenic immune environment resulting in inadequate therapeutic response to standard care in AA CRC patients. Therefore, targeting the pineal hormonal changes, addressing social and economic factors of individual patients, and following race-specific screening protocols may improve the therapeutic outcome and overall survival in AAs with CRC and promise health equity.
CRediT authorship contribution statement
Talaijha Haynes: Writing – original draft. Gabriela Oprea-Ilies: Writing – review & editing. Upender Manne: Writing – review & editing. Rajesh Singh: Writing – review & editing. Shailesh Singh: Writing – review & editing. Hina Mir: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported in part by the funds from the National Cancer Institute (U54 CA118638). T.H. was supported by the U54 funds during the Summer Cancer Research Education Program (SCREP) to write this manuscript. Also, the authors thank Dr. Donald Hill for editing the manuscript. The content is solely the authors' responsibility and does not necessarily represent the official views of the NIH.
References
- 1.Afzali A., Cross R.K. Racial and ethnic minorities with inflammatory bowel disease in the United States: a systematic review of disease characteristics and differences. Inflamm. Bowel Dis. 2016;22(8):2023–2040. doi: 10.1097/MIB.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 2.Al-Husseini M.J., Saad A.M., Jazieh K.A., Elmatboly A.M., Rachid A., Gad M.M., Ruhban I.A., Hilal T. Outcome disparities in colorectal cancer: a SEER-based comparative analysis of racial subgroups. Int. J. Colorectal Dis. 2019;34(2):285–292. doi: 10.1007/s00384-018-3195-3. [DOI] [PubMed] [Google Scholar]
- 3.Anderson G. Mitochondria and the Gut as crucial hubs for the interactions of melatonin with sirtuins, inflammation, butyrate, tryptophan metabolites, and alpha 7 nicotinic receptor across a host of medical conditions. Melatonin Res. 2019;2(2):70–85. [Google Scholar]
- 4.Ashktorab H., Kupfer S.S., Brim H., Carethers J.M. Racial disparity in gastrointestinal cancer risk. Gastroenterology. 2017;153(4):910–923. doi: 10.1053/j.gastro.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakalova R., Zhelev Z., Shibata S., Nikolova B., Aoki I., Higashi T. Impressive suppression of colon cancer growth by triple combination SN38/EF24/melatonin: "Oncogenic" versus "Onco-suppressive" reactive oxygen species. Anticancer Res. 2017;37(10):5449–5458. doi: 10.21873/anticanres.11973. [DOI] [PubMed] [Google Scholar]
- 6.Barni S., Lissoni P., Paolorossi F., Crispino S., Archili C., Tancini G. A study of the pineal hormone melatonin as a second line therapy in metastatic colorectal cancer resistant to fluorouracil plus folates. Tumori. 1990;76(1):58–60. doi: 10.1177/030089169007600115. [DOI] [PubMed] [Google Scholar]
- 7.Bazensky I., Shoobridge-Moran C., Yoder L.H. Colorectal cancer: an overview of the epidemiology, risk factors, symptoms, and screening guidelines. Medsurg Nurs. 2007;16(1):46–51. quiz 52. [PubMed] [Google Scholar]
- 8.Bertisch S.M., Sillau S., de Boer I.H., Szklo M., Redline S. 25-Hydroxyvitamin D concentration and sleep duration and continuity: multi-ethnic study of atherosclerosis. Sleep. 2015;38(8):1305–1311. doi: 10.5665/sleep.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosi A., Banfi D., Bistoletti M., Giaroni C., Baj A. Tryptophan metabolites along the Microbiota-gut-brain axis: an interkingdom communication system influencing the gut in health and disease. Int. J. Tryptophan Res. 2020;13 doi: 10.1177/1178646920928984. 1178646920928984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubenik G.A. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci. 2002;47(10):2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 11.Bubenik G.A., Pang S.F. The role of serotonin and melatonin in gastrointestinal physiology: ontogeny, regulation of food intake, and mutual serotonin-melatonin feedback. J. Pineal Res. 1994;16(2):91–99. doi: 10.1111/j.1600-079x.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 12.Carethers J.M. clinical and genetic factors to inform reducing colorectal cancer disparitites in African Americans. Front. Oncol. 2018;8:531. doi: 10.3389/fonc.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriere P.P., Kapur N., Mir H., Ward A.B., Singh S. Cinnamtannin B-1 inhibits cell survival molecules and induces apoptosis in colon cancer. Int. J. Oncol. 2018;53(4):1442–1454. doi: 10.3892/ijo.2018.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerea G., Vaghi M., Ardizzoia A., Villa S., Bucovec R., Mengo S., Gardani G., Tancini G., Lissoni P. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer Res. 2003;23(2c):1951–1954. [PubMed] [Google Scholar]
- 15.Chaudhry K.K., Samak G., Shukla P.K., Mir H., Gangwar R., Manda B., Isse T., Kawamoto T., Salaspuro M., Kaihovaara P., Dietrich P., Dragatsis I., Nagy L.E., Rao R.K. ALDH2 deficiency promotes ethanol-induced gut barrier dysfunction and fatty liver in mice. Alcohol Clin. Exp. Res. 2015;39(8):1465–1475. doi: 10.1111/acer.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhry K.K., Shukla P.K., Mir H., Manda B., Gangwar R., Yadav N., McMullen M., Nagy L.E., Rao R. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J Nutr. Biochem. 2016;27:16–26. doi: 10.1016/j.jnutbio.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S., Doyle W.J., Baum A. Socioeconomic status is associated with stress hormones. Psychosom. Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 18.de Jong A.E., Morreau H., Nagengast F.M., Mathus-Vliegen E.M., Kleibeuker J.H., Griffioen G., Cats A., Vasen H.F. Prevalence of adenomas among young individuals at average risk for colorectal cancer. Am. J. Gastroenterol. 2005;100(1):139–143. doi: 10.1111/j.1572-0241.2005.41000.x. [DOI] [PubMed] [Google Scholar]
- 19.Dominitz J.A., Samsa G.P., Landsman P., Provenzale D. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82(12):2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Favero G., Moretti E., Bonomini F., Reiter R.J., Rodella L.F., Rezzani R. Promising antineoplastic actions of melatonin. Front. Pharmacol. 2018;9:1086. doi: 10.3389/fphar.2018.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fichera A., Little N., Dougherty U., Mustafi R., Cerda S., Li Y.C., Delgado J., Arora A., Campbell L.K., Joseph L., Hart J., Noffsinger A., Bissonnette M. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J. Surg. Res. 2007;142(2):239–245. doi: 10.1016/j.jss.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 22.Fiscella K., Winters P., Tancredi D., Hendren S., Franks P. Racial disparity in death from colorectal cancer: does vitamin D deficiency contribute? Cancer. 2011;117(5):1061–1069. doi: 10.1002/cncr.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y., Xiao X., Zhang C., Yu W., Guo W., Zhang Z., Li Z., Feng X., Hao J., Zhang K., Xiao B., Chen M., Huang W., Xiong S., Wu X., Deng W. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J. Pineal Res. 2017;62(2) doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 24.Gelernter J., Cubells J.F., Kidd J.R., Pakstis A.J., Kidd K.K. Population studies of polymorphisms of the serotonin transporter protein gene. Am. J. Med. Genet. 1999;88(1):61–66. [PubMed] [Google Scholar]
- 25.Gil-Martin E., Egea J., Reiter R.J., Romero A. The emergence of melatonin in oncology: focus on colorectal cancer. Med. Res. Rev. 2019;39(6):2239–2285. doi: 10.1002/med.21582. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann. Epidemiol. 2009;19(2):84–88. doi: 10.1016/j.annepidem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Grande M., Milito G., Attina G.M., Cadeddu F., Muzi M.G., Nigro C., Rulli F., Farinon A.M. Evaluation of clinical, laboratory and morphologic prognostic factors in colon cancer. World J. Surg. Oncol. 2008;6:98. doi: 10.1186/1477-7819-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant W.B., Peiris A.N. Differences in vitamin D status may account for unexplained disparities in cancer survival rates between African and white Americans. Dermatoendocrinol. 2012;4(2):85–94. doi: 10.4161/derm.19667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guda K., Veigl M.L., Varadan V., Nosrati A., Ravi L., Lutterbaugh J., Beard L., Willson J.K., Sedwick W.D., Wang Z.J., Molyneaux N., Miron A., Adams M.D., Elston R.C., Markowitz S.D., Willis J.E. Novel recurrently mutated genes in African American colon cancers. Proc. Natl. Acad. Sci. U. S. A. 2015;112(4):1149–1154. doi: 10.1073/pnas.1417064112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajat A., Diez-Roux A., Franklin T.G., Seeman T., Shrager S., Ranjit N., Castro C., Watson K., Sanchez B., Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall S.A., Kaufman J.S. Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma. Cancer. 2004;101(12):2899. doi: 10.1002/cncr.20702. author reply 2900. [DOI] [PubMed] [Google Scholar]
- 32.Harris S.S., Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am. J. Clin. Nutr. 1998;67(6):1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 33.Harris S.S., Soteriades E., Coolidge J.A., Mudgal S., Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J. Clin. Endocrinol. Metab. 2000;85(11):4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y., Won J., Lee Y., Lee S., Park K., Chang K.T., Hong Y. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J. Pineal Res. 2014;56(3):264–274. doi: 10.1111/jpi.12119. [DOI] [PubMed] [Google Scholar]
- 35.Hooker S., Bonilla C., Akereyeni F., Ahaghotu C., Kittles R.A. NAT2 and NER genetic variants and sporadic prostate cancer susceptibility in African Americans. Prostate Cancer Prostatic Dis. 2008;11(4):349–356. doi: 10.1038/sj.pcan.4501027. [DOI] [PubMed] [Google Scholar]
- 36.Hou M., Xing H., Li C., Wang X., Deng D., Li J., Zhang P., Chen J. Short-term efficacy and safety of lasmiditan, a novel 5-HT(1F) receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J. Headache Pain. 2020;21(1):66. doi: 10.1186/s10194-020-01138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsing A.W., Meyer T.E., Niwa S., Quraishi S.M., Chu L.W. Measuring serum melatonin in epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 2010;19(4):932–937. doi: 10.1158/1055-9965.EPI-10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson-Thompson J., Ahmed F., German R.R., Lai S.M., Friedman C. Descriptive epidemiology of colorectal cancer in the United States, 1998-2001. Cancer. 2006;107(5 Suppl):1103–1111. doi: 10.1002/cncr.22007. [DOI] [PubMed] [Google Scholar]
- 39.Janout V., Kollarova H. Epidemiology of colorectal cancer. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2001;145(1):5–10. doi: 10.5507/bp.2001.001. [DOI] [PubMed] [Google Scholar]
- 40.Jeong J., Zhu H., Harris R.A., Dong Y., Su S., Tingen M.S., Kapuku G., Pollock J.S., Pollock D.M., Harshfield G.A., Wang X. Ethnic differences in nighttime melatonin and nighttime blood pressure: a study in European Americans and African Americans. Am. J. Hypertens. 2019;32(10):968–974. doi: 10.1093/ajh/hpz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jinjuvadia R., Jinjuvadia K., Liangpunsakul S. Racial disparities in gastrointestinal cancers-related mortality in the U.S. population. Dig. Dis. Sci. 2013;58(1):236–243. doi: 10.1007/s10620-012-2312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson I.T., Lund E.K. Review article: nutrition, obesity and colorectal cancer. Aliment. Pharmacol. Ther. 2007;26(2):161–181. doi: 10.1111/j.1365-2036.2007.03371.x. [DOI] [PubMed] [Google Scholar]
- 43.Kabat G.C., Miller A.B., Jain M., Rohan T.E. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. Br. J. Cancer. 2007;97(1):118–122. doi: 10.1038/sj.bjc.6603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapur N., Mir H., Clark Iii C.E., Krishnamurti U., Beech D.J., Lillard J.W., Singh S. CCR6 expression in colon cancer is associated with advanced disease and supports epithelial-to-mesenchymal transition. Br. J. Cancer. 2016;114(12):1343–1351. doi: 10.1038/bjc.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim T.K., Lin Z., Tidwell W.J., Li W., Slominski A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015;404:1–8. doi: 10.1016/j.mce.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King Thomas J., Mir H., Kapur N., Singh S. Racial differences in immunological landscape modifiers contributing to disparity in prostate cancer. Cancers. 2019;11(12):1–22. doi: 10.3390/cancers11121857. (Basel) 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koss K.J., Gunnar M.R. Annual Research Review: early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. J. Child Psychol. Psychiatry. 2018;59(4):327–346. doi: 10.1111/jcpp.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krieger N., Williams D.R., Moss N.E. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu. Rev. Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 49.Kvietkauskas M., Zitkute V., Leber B., Strupas K., Stiegler P., Schemmer P. The role of melatonin in colorectal cancer treatment: a comprehensive review. Ther. Adv. Med. Oncol. 2020;12:1–14. doi: 10.1177/1758835920931714. 1758835920931714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsson S.C., Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int. J. Cancer. 2006;119(11):2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 51.Wilson S.T., Stanley B., Brent D.A., Oquendo M.A., Huang Y.Y., Mann J.J. The tryptophan hydroxylase-1 A218C polymorphism is associated with diagnosis, but not suicidal behavior, in borderline personality disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(2):202–208. doi: 10.1002/ajmg.b.30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao S., Chattun M.R., Yan R., Geng J., Zhu R., Shao J., Lu Q., Yao Z. TPH-2 Gene Polymorphism in Major Depressive Disorder Patients With Early-Wakening Symptom. Front Neurosci. 2018;12(827):1–10. doi: 10.3389/fnins.2018.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.León J., Casado J., Jiménez Ruiz S.M., Zurita M.S., González-Puga C., Rejón J.D., Gila A., Muñoz de Rueda P., Pavón E.J., Reiter R.J., Ruiz-Extremera A., Salmerón J. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-κβ. J. Pineal Res. 2014;56(4):415–426. doi: 10.1111/jpi.12131. [DOI] [PubMed] [Google Scholar]
- 54.León-Ponte M., Ahern G.P., O'Connell P.J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109(8):3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesch K.P., Bengel D., Heils A., Sabol S.Z., Greenberg B.D., Petri S., Benjamin J., Müller C.R., Hamer D.H., Murphy D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 56.Li N., Ghia J.E., Wang H., McClemens J., Cote F., Suehiro Y., Mallet J., Khan W.I. Serotonin activates dendritic cell function in the context of gut inflammation. Am. J. Pathol. 2011;178(2):662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lissoni P. Is there a role for melatonin in supportive care? Support. Care Cancer. 2002;10(2):110–116. doi: 10.1007/s005200100281. [DOI] [PubMed] [Google Scholar]
- 58.Lissoni P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol. Biol. 2007;55(3–4):201–204. doi: 10.1016/j.patbio.2006.12.025. (Paris) [DOI] [PubMed] [Google Scholar]
- 59.Lissoni P., Barni S., Tancini G., Ardizzoia A., Rovelli F., Cazzaniga M., Brivio F., Piperno A., Aldeghi R., Fossati D., et al. Immunotherapy with subcutaneous low-dose interleukin-2 and the pineal indole melatonin as a new effective therapy in advanced cancers of the digestive tract. Br. J. Cancer. 1993;67(6):1404–1407. doi: 10.1038/bjc.1993.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lissoni P., Barni S., Tancini G., Crispino S., Paolorossi F., Lucini V., Mariani M., Cattaneo G., Esposti D., Esposti G., et al. Clinical study of melatonin in untreatable advanced cancer patients. Tumori. 1987;73(5):475–480. doi: 10.1177/030089168707300508. [DOI] [PubMed] [Google Scholar]
- 61.Looker A.C., Dawson-Hughes B., Calvo M.S., Gunter E.W., Sahyoun N.R. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 62.Ma N., Zhang J., Reiter R.J., Ma X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: a therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020;40(2):606–632. doi: 10.1002/med.21628. [DOI] [PubMed] [Google Scholar]
- 63.Mahid S.S., Mulhall A.M., Gholson R.D., Eichenberger M.R., Galandiuk S. Inflammatory bowel disease and African Americans: a systematic review. Inflamm. Bowel Dis. 2008;14(7):960–967. doi: 10.1002/ibd.20389. [DOI] [PubMed] [Google Scholar]
- 64.Marcella S., Miller J.E. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J. Clin. Epidemiol. 2001;54(4):359–366. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 65.Martin-Gallausiaux C., Larraufie P., Jarry A., Béguet-Crespel F., Marinelli L., Ledue F., Reimann F., Blottière H.M., Lapaque N. Butyrate produced by commensal bacteria down-regulates indolamine 2,3-dioxygenase 1 (IDO-1) expression via a dual mechanism in human intestinal epithelial cells. Front. Immunol. 2018;9:2838. doi: 10.3389/fimmu.2018.02838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massa J., Stone K.L., Wei E.K., Harrison S.L., Barrett-Connor E., Lane N.E., Paudel M., Redline S., Ancoli-Israel S., Orwoll E., Schernhammer E. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: the MrOS sleep study. Sleep. 2015;38(2):251–257. doi: 10.5665/sleep.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mawe G.M., Hoffman J.M. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mayberry R.M., Coates R.J., Hill H.A., Click L.A., Chen V.W., Austin D.F., Redmond C.K., Fenoglio-Preiser C.M., Hunter C.P., Haynes M.A., et al. Determinants of black/white differences in colon cancer survival. J. Natl. Cancer Inst. 1995;87(22):1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 69.Mediavilla M.D., Sanchez-Barcelo E.J., Tan D.X., Manchester L., Reiter R.J. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr. Med. Chem. 2010;17(36):4462–4481. doi: 10.2174/092986710794183015. [DOI] [PubMed] [Google Scholar]
- 70.Mełen-Mucha G., Winczyk K., Pawlikowski M. Somatostatin analogue octreotide and melatonin inhibit bromodeoxyuridine incorporation into cell nuclei and enhance apoptosis in the transplantable murine colon 38 cancer. Anticancer Res. 1998;18(5a):3615–3619. [PubMed] [Google Scholar]
- 71.Muscogiuri G., Barrea L., Scannapieco M., Di Somma C., Scacchi M., Aimaretti G., Savastano S., Colao A., Marzullo P. The lullaby of the sun: the role of vitamin D in sleep disturbance. Sleep Med. 2019;54:262–265. doi: 10.1016/j.sleep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 72.Nickerson M.L., Warren M.B., Toro J.R., Matrosova V., Glenn G., Turner M.L., Duray P., Merino M., Choyke P., Pavlovich C.P., Sharma N., Walther M., Munroe D., Hill R., Maher E., Greenberg C., Lerman M.I., Linehan W.M., Zbar B., Schmidt L.S. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2(2):157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 73.Nikaido Y., Aluru N., McGuire A., Park Y.J., Vijayan M.M., Takemura A. Effect of cortisol on melatonin production by the pineal organ of tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010;155(1):84–90. doi: 10.1016/j.cbpa.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Obayashi K., Saeki K., Tone N., Iwamoto J., Miyata K., Ikada Y., Kurumatani N. Lower melatonin secretion in older females: gender differences independent of light exposure profiles. J. Epidemiol. 2015;25(1):38–43. doi: 10.2188/jea.JE20140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Odgerel Z., Talati A., Hamilton S.P., Levinson D.F., Weissman M.M. Genotyping serotonin transporter polymorphisms 5-HTTLPR and rs25531 in European- and African-American subjects from the national institute of mental health's collaborative center for genomic studies. Transl. Psychiatry. 2013;3(9):e307. doi: 10.1038/tp.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ou J., Carbonero F., Zoetendal E.G., DeLany J.P., Wang M., Newton K., Gaskins H.R., O'Keefe S.J. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013;98(1):111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papantoniou K., Devore E.E., Massa J., Strohmaier S., Vetter C., Yang L., Shi Y., Giovannucci E., Speizer F., Schernhammer E.S. Rotating night shift work and colorectal cancer risk in the nurses' health studies. Int. J. Cancer. 2018;143(11):2709–2717. doi: 10.1002/ijc.31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pariente R., Bejarano I., Rodríguez A.B., Pariente J.A., Espino J. Melatonin increases the effect of 5-fluorouracil-based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol. Cell. Biochem. 2018;440(1–2):43–51. doi: 10.1007/s11010-017-3154-2. [DOI] [PubMed] [Google Scholar]
- 79.Patrick R.P., Ames B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28(6):2398–2413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 80.Paulose J.K., Wright J.M., Patel A.G., Cassone V.M. Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reiter R.J., Guerrero J.M., Garcia J.J., Acuna-Castroviejo D. Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin. Ann. N. Y. Acad. Sci. 1998;854:410–424. doi: 10.1111/j.1749-6632.1998.tb09920.x. [DOI] [PubMed] [Google Scholar]
- 82.Santarelli R.L., Pierre F., Corpet D.E. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr. Cancer. 2008;60(2):131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sapolsky R.M. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp. Gerontol. 1999;34(6):721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 84.Saunders I.T., Mir H., Kapur N., Singh S. Emodin inhibits colon cancer by altering BCL-2 family proteins and cell survival pathways. Cancer Cell Int. 2019;19:98. doi: 10.1186/s12935-019-0820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shafabakhsh R., Reiter R.J., Davoodabadi A., Asemi Z. Melatonin as a potential inhibitor of colorectal cancer: molecular mechanisms. J. Cell. Biochem. 2019;120(8):12216–12223. doi: 10.1002/jcb.28833. [DOI] [PubMed] [Google Scholar]
- 86.Shukla P.K., Chaudhry K.K., Mir H., Gangwar R., Yadav N., Manda B., Meena A.S., Rao R. Chronic ethanol feeding promotes azoxymethane and dextran sulfate sodium-induced colonic tumorigenesis potentially by enhancing mucosal inflammation. BMC Cancer. 2016;16:189. doi: 10.1186/s12885-016-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 88.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat. Res. 2002;506-507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 89.Skversky A.L., Kumar J., Abramowitz M.K., Kaskel F.J., Melamed M.L. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the national health and nutrition examination survey (NHANES): 2001-2006. J. Clin. Endocrinol. Metab. 2011;96(12):3838–3845. doi: 10.1210/jc.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vogtmann E., Shanmugam C., Katkoori V.R., Waterbor J., Manne U. Socioeconomic status, p53 abnormalities, and colorectal cancer. J Gastrointest. Oncol. 2013;4(1):40–44. doi: 10.3978/j.issn.2078-6891.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wetterberg L. The relationship between the pineal gland and the pituitary–adrenal axis in health, endocrine and psychiatric conditions. Psychoneuroendocrinology. 1983;8(1):75–80. doi: 10.1016/0306-4530(83)90042-2. [DOI] [PubMed] [Google Scholar]
- 92.Willett W.C. Diet and cancer: an evolving picture. JAMA. 2005;293(2):233–234. doi: 10.1001/jama.293.2.233. [DOI] [PubMed] [Google Scholar]
- 93.Williams D.R., Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annu. Rev. Sociol. 1995;21(1):349–386. [Google Scholar]
- 94.Wiseman M. The second world cancer research fund/American institute for cancer research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc. Nutr. Soc. 2008;67(3):253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 95.Woolcott C.G., Wilkens L.R., Nomura A.M., Horst R.L., Goodman M.T., Murphy S.P., Henderson B.E., Kolonel L.N., Marchand L.Le. Plasma 25-hydroxyvitamin D levels and the risk of colorectal cancer: the multiethnic cohort study. Cancer Epidemiol. Biomark. Prev. 2010;19(1):130–134. doi: 10.1158/1055-9965.EPI-09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wosu A.C., Gelaye B., Valdimarsdottir U., Kirschbaum C., Stalder T., Shields A.E., Williams M.A. Hair cortisol in relation to sociodemographic and lifestyle characteristics in a multiethnic US sample. Ann. Epidemiol. 2015;25(2):90–95. doi: 10.1016/j.annepidem.2014.11.022. 95 e91-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Y., Zhou R., Park S.Y., Back K., Bae W.K., Kim K.K., Kim H. 2-Hydroxymelatonin, a predominant hydroxylated melatonin metabolite in plants, shows antitumor activity against human colorectal cancer cells. Molecules. 2017;22(3) doi: 10.3390/molecules22030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zou D.B., Wei X., Hu R.L., Yang X.P., Zuo L., Zhang S.M., Zhu H.Q., Zhou Q., Gui S.Y., Wang Y. Melatonin inhibits the migration of colon cancer RKO cells by down-regulating myosin light chain kinase expression through cross-talk with p38 MAPK. Asian Pac. J. Cancer Prev. 2015;16(14):5835–5842. doi: 10.7314/apjcp.2015.16.14.5835. [DOI] [PubMed] [Google Scholar]
- 99.Allen J.O., et al. Cortisol and Racial Health Disparities Affecting Black Men in Later Life: Evidence From MIDUS II. Am J Mens Health. 2019;13(4) doi: 10.1177/1557988319870969. 1557988319870969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ou J., DeLany J.P., Zhang M., Sharma S., O'Keefe S.J. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr Cancer. 2012;64(1):34–40. doi: 10.1080/01635581.2012.630164. [DOI] [PMC free article] [PubMed] [Google Scholar]