Abstract
Background
To observe whether guideline non-adherence in initial palliative treatment choices for premenopausal hormone receptor-positive (HR+), HER2-negative metastatic breast cancer (MBC) patients result in worse clinical outcomes in the Chinese population.
Methods
The China National Cancer Center database was used to identify 2194 patients diagnosed between 2004 and 2015. A total of 451 premenopausal patients with HR + HER2- MBC were included. Clinicopathological features and survival information were extracted. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method and compared using log-rank test.
Results
The number of patients receiving initial chemotherapy, endocrine therapy and chemo-endocrine therapy were 222 (49.2%), 80 (17.7%), and 149 (33.0%), respectively. Patients receiving initial chemotherapy were more likely to be luminal B subtype, had more de novo stage IV disease and more liver metastasis, compared with patients receiving initial endocrine therapy. Both PFS and OS were significantly longer for chemo-endocrine therapy group (median PFS 18.9 months and OS 75.0 months), than for endocrine therapy group (median PFS 11.7 months and OS 53.5 months), and chemotherapy group (median PFS 7.1 months and OS 43.9 months). In multivariate analysis, none of the three treatment strategies were independently associated with PFS or OS.
Conclusion
In real world, a high percentage of premenopausal patients with HR + HER2- disease received chemotherapy as initial palliative treatment in China, which was not associated with worsened survival. Further studies with larger sample size across China are needed to explore the relationship between this guideline non-adherence and clinical outcomes.
Keywords: Hormone receptor positive, Premenopausal, Metastatic breast cancer, Real-world
Highlights
-
•
Large retrospective analysis focusing on premenopausal patients with HR + HER2-metastatic breast cancer in China.
-
•
A high percentage of the study patients received chemotherapy as initial palliative treatment.
-
•
No differences in survival were observed on first-line chemotherapy or endocrine therapy.
-
•
Further studies across China are needed to explore the relationship between guideline non-adherence and clinical outcomes.
1. Introduction
Breast cancer is the most common diagnosed cancer and the fourth leading cause of cancer death in female population in China [1]. Despite curative aim of treatment at early stage, 20–50% of patients developed metastatic disease and 6–10% were newly diagnosed metastatic breast cancer (MBC) cases [2].Once metastasized, the disease was generally considered incurable, with 5-year survival rate of only 28% [3].
Hormone receptor positive (HR+) subtype accounts for two thirds of breast cancer patients [4]. For patients with HR+, human epidermal growth factor receptor 2 negative (HER2-) metastatic disease, endocrine therapy is the recommended initial treatment by current guidelines, even in the presence of visceral metastases [[5], [6], [7]]. However, previous real-world studies have shown that chemotherapy is still prescribed as first-line treatment to a significant portion of patients rather than endocrine therapy, with inconsistent results in survival outcomes.
Breast cancer patients in Asian population show distinct epidemiological and biological features as compared to patients in non-Asian population, with peak incidence almost two decades younger than western counterparts [8]. Premenopausal patients make up about half of the whole breast cancer patients in Asian countries [9]. In China, approximately two-thirds of patients are premenopausal at breast cancer diagnosis. Of note, premenopausal breast cancer is associated with more aggressive behavior and poorer prognosis, thus more intensive treatment are often prescribed in clinical practice according to our experience [10]. Currently, robust studies focusing on first-line treatment choices and outcomes of premenopausal patients with HR + HER2- MBC in China are lacking, and whether guideline non-adherence in these patients result in worse clinical outcomes remains unknown. In this real-world experience, we aimed to observe the initial palliative treatment patterns of HR + HER2- premenopausal patients with metastatic breast cancer and determine if this discrepancy between guidelines and real life practice resulted in worse clinical outcomes in the Chinese population.
2. Material and methods
2.1. Patients
Medical records of breast cancer patients treated at the China National Cancer Center were retrospectively reviewed. The China National Cancer Center database was used to identify metastatic breast cancer patients diagnosed between January 2004 to October 2015. Patients were included if they met the following criteria: (1) Histologically confirmed breast cancer with reliable estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) status, reviewed and reported by two independent breast cancer pathologists from the pathology department of the China National Cancer Center. ER/PgR positivity were defined as ≥ 10% positive tumor cells with nuclear staining by immunohistochemistry (IHC) and then ≥1% after April 2010, according to the new College of American Pathologists guidelines. HER2 status was assessed by IHC and/or fluorescence in situ hybridization (FISH). HER2 negativity was defined as IHC scoring 0–1+ or IHC 2+, but without FISH amplified based on the American Society of Clinical Oncology (ASCO) guidelines [11]. (2) Recurrent or metastatic breast cancer. (3) Premenopausal breast cancer. Premenopausal status was defined by last menstrual period within one year or FSH levels below 40 mIU/ml at MBC diagnosis. We excluded patients who were with more than one primary cancer except excised basal cell skin carcinoma and cervical carcinoma in situ in the past five years, lack of first-line treatment information, male breast cancer and unknown menopausal status. Demographics of patients, clinical and pathological features, sites of disease recurrence, imaging results, first-line treatment strategies and survival information were extracted. The following breast cancer subtypes were defined according to the St. Gallen International Expert Consensus 2013: “luminal A” (HER2-negative, with PgR≥20%, or Ki-67 < 14%), “luminal B” (HER2-negative, with PgR <20%, or Ki-67 ≥ 14%) [12]. First-line treatment strategies were classified as: chemotherapy, endocrine therapy, or chemotherapy-endocrine therapy group. The chemotherapy-endocrine therapy group was defined as receiving initial palliative chemotherapy and maintained by endocrine therapy without disease progression. First-line progression-free survival (PFS) was defined as the time from first palliative systemic therapy to the date of disease progression or last follow-up. Overall survival (OS) was defined as the time from initial metastatic diagnosis to the date of death from any cause or last follow-up.
2.2. Statistical analysis
Clinical and pathological features of patients were summarized and stratified by first-line treatment strategies compared across groups using chi square test. Multivariable logistic regression model was utilized to determine factors that could predict for the choices of first-line treatment strategies. Survival analyses were estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Prognostic factors associated with OS were analyzed using Cox regression model with 95% confidence interval (95%C.I.). All statistical analyses were performed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient and data collection
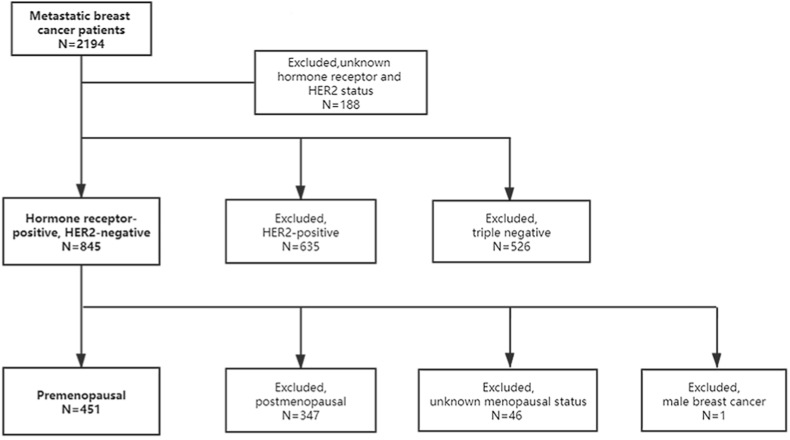
We identified 2194 metastatic breast cancer patients diagnosed between January 2004 and October 2015 at National Cancer Center, China. Of them, 188 patients were excluded due to unknown hormone receptor or HER2 status, or double primary cancer. In the remaining 2006 patients, HER2-positive (n = 635) and triple negative breast cancer patients (n = 526) were excluded to obtain 845 HR + HER2- MBC patients. We further excluded postmenopausal patients (n = 347), patients with unknown menopausal status (n = 46) and male breast cancer patients (n = 1) (Fig. 1).
Fig. 1.
Study flow chart. HER2, human epidermal growth factor receptor 2.
In total, 451 HR + HER2- premenopausal MBC patients were included in our study. First-line treatment strategies were classified into three groups as described in the methods section. Chemotherapy alone was performed in 222 patients (49.2%), while initial endocrine therapy was given in 80 patients (17.7%). The remaining 149 patients (33.0%) received chemotherapy followed by endocrine therapy as first-line treatment.
3.2. Baseline patient characteristics
Baseline patient demographics and tumor characteristics stratified by first-line treatment strategies are summarized in Table 1. The median age was 44 years (range 22–53 years) for the whole cohort. The majority of patients (400/451, 88.7%) had recurrent disease after curative surgery and adjuvant therapy. Among them, 347 patients (347/400, 86.8%) had disease relapse within one year after completing adjuvant endocrine therapy, which was considered as a hormone-resistant population. Patients with potentially more favorable characteristics such as luminal A subtype, recurrent diseases and less liver metastases received more endocrine therapy than chemotherapy. On multivariate analysis, bone and soft tissue metastases was the only factor that associated with more frequent use of endocrine therapy as first-line treatment strategy (OR 3.09, 95%C.I. 1.35–7.06, P = 0.007) (Table 2).
Table 1.
Baseline patient characteristics.
| Total (n = 451) | Chemotherapy (n = 222) | Endocrine therapy (n = 80) | Chemotherapy-endocrine therapy (n = 149) | P Valueb | |
|---|---|---|---|---|---|
| Age (median) | 44 | 44 | 45 | 44 | |
| Performance status | |||||
| 0–1 | 442 (98.0%) | 218 (98.2%) | 78 (97.5%) | 146 (98.0%) | – |
| ≥2 | 9 (2.0%) | 4 (1.8%) | 2 (2.5%) | 3 (2.0%) | |
| Subtypea | |||||
| Luminal A | 118 (26.2%) | 43 (19.4%) | 23 (28.8%) | 52 (34.9%) | 0.039 |
| Luminal B | 199 (44.1%) | 98 (44.1%) | 40 (50.0%) | 61 (40.9%) | |
| Disease status | <0.001 | ||||
| De novo | 51 (11.3%) | 21 (9.5%) | 1 (1.3%) | 29 (19.5%) | |
| Recurrent | 400 (88.7%) | 201 (90.5%) | 79 (98.8%) | 120 (80.5%) | |
| Disease-free interval in recurrent population (n = 400) | |||||
| ≤24 months | 119 (26.4%) | 66 (29.7%) | 19 (23.8%) | 34 (22.8%) | 0.30 |
| >24 months | 281 (62.3%) | 134 (60.4%) | 60 (75.0%) | 87 (58.4%) | |
| Initial metastatic sites | |||||
| Bone and soft tissue only | 247 (54.8%) | 116 (52.3%) | 47 (58.8%) | 84 (56.4%) | 0.55 |
| Visceral | 196 (43.5%) | 103 (46.4%) | 29 (36.3%) | 64 (43.0%) | 0.29 |
| Liver | 100 (22.2%) | 60 (27.0%) | 11 (13.8%) | 29 (19.5%) | 0.03 |
| Lung | 129 (28.6%) | 64 (28.8%) | 21 (26.3%) | 44 (29.5%) | 0.91 |
| Brain | 17 (3.8%) | 10 (4.5%) | 5 (6.3%) | 2 (1.3%) | 0.12 |
| Number of sites | 0.30 | ||||
| <3 | 369 (81.8%) | 177 (79.7%) | 70 (87.5%) | 122 (81.9%) | |
| ≥3 | 82 (18.2%) | 45 (20.3%) | 10 (12.5%) | 27 (18.1%) | |
| Neo/Adjuvant therapy | 0.85 | ||||
| Endocrine therapy | 305 (67.6%) | 153 (68.9%) | 66 (82.5%) | 86 (57.7%) | |
| Chemotherapy | 373 (82.7%) | 192 (86.5%) | 74 (92.5%) | 107 (71.8%) |
The subtypes of 134 cases were not available.
Bold values indicate statistically significant results.
Table 2.
First-line treatment choice by tumor and patient characteristics (endocrine therapy vs. chemotherapy).
| OR | 95%CI | P valuea | |
|---|---|---|---|
| Age ≤35 | 0.83 | 0.36–1.89 | 0.66 |
| DFS ≤2 years | 0.72 | 0.34–1.54 | 0.39 |
| ECOG >1 | 1.70 | 0.27–10.72 | 0.57 |
| Luminal B subtype | 1.13 | 0.61–2.12 | 0.70 |
| Bone and soft tissue metastasis only | 3.09 | 1.35–7.06 | 0.007 |
| Liver metastasis | 0.33 | 0.06–1.68 | 0.18 |
| Lung metastasis | 0.73 | 0.13–4.24 | 0.73 |
| Brain metastasis | 3.75 | 0.73–19.25 | 0.11 |
| Viseral metastasis | 3.19 | 0.48–21.00 | 0.23 |
| Metastatic sites ≥3 | 0.68 | 0.27–1.71 | 0.41 |
| De novo stage IV | – | – | – |
| Neo/adjuvant CT | 0.85 | 0.25–2.91 | 0.80 |
| Neo/adjuvant ET | 1.36 | 0.60–3.09 | 0.46 |
OR = odds ratio; 95% CI = 95% confidence interval; DFS = disease free survival; ECOG = Eastern Cooperative Oncology Group; CT = chemotherapy; ET = endocrine therapy.
Bold values indicate statistically significant results.
3.3. First-line treatment regimens
The first-line treatment regimens in different treatment groups are detailed in Tables S1–3. In the chemotherapy group, taxanes were most frequently used (65.8%), followed by capecitabine (39.2%), anthracyclines (25.7%) and other cytotoxic agents. In the endocrine therapy group, aromatase inhibitors (AI) was predominantly used (75.1%). Similar patterns were observed in the chemotherapy-endocrine therapy group. The median treatment duration was 7.0 months (1.4–89.3 months), 11.6 months (2.1–66.1 months) and 19.0 months (3.3–144.0 months) for chemotherapy group, endocrine therapy group, and chemotherapy-endocrine therapy group, respectively.
3.4. Outcomes according to first-line treatment choices
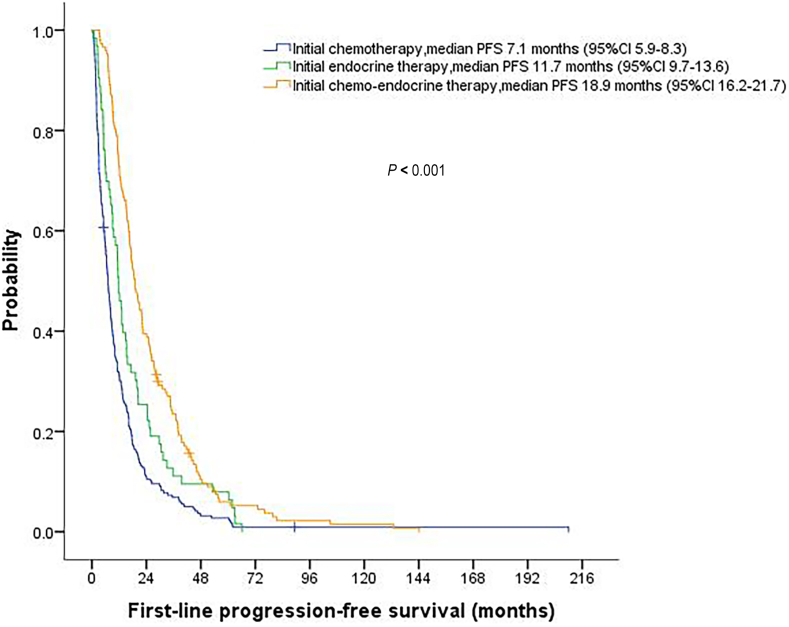
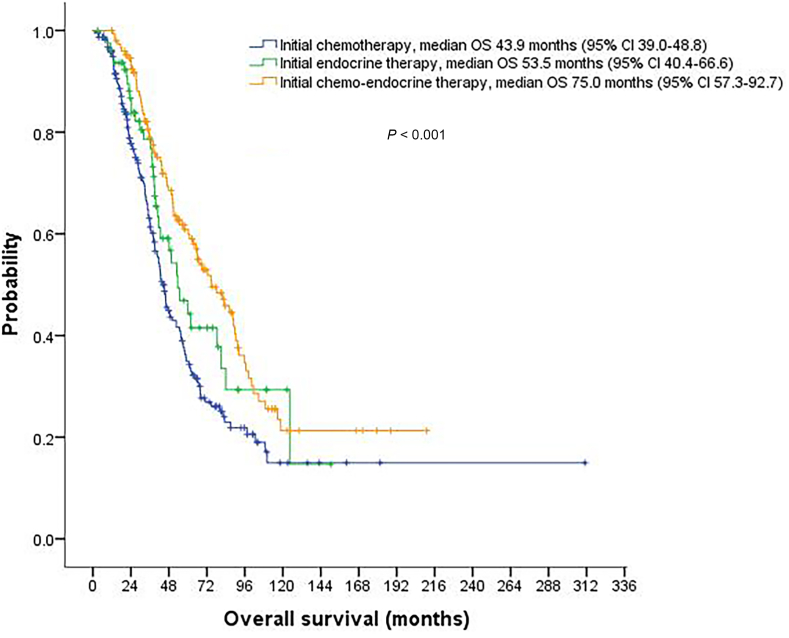
Median follow-up time was 77.7 months (95%CI, 70.2–85.2) for the entire cohort. The median first-line PFS and OS were 11.4 months (95%CI, 9.9–12.8) and 54.9 months (95%CI, 48.4–61.4), respectively. Among the three treatment groups, both first-line PFS and OS were significantly longer for the chemotherapy-endocrine therapy group compared with the other two groups (P < 0.001) (Fig. 2, Fig. 3). Comparisons of PFS and OS between different treatment groups are summarized in Table 3. For response evaluation, we observed a significantly higher objective response rate for chemotherapy-endocrine therapy group (53.7%) than for chemotherapy group (36.0%) and endocrine group (18.8%) (P < 0.001) (Table 3).
Fig. 2.
Kaplan-Meier curve for progression-free survival (PFS) stratified by initial palliative treatment strategies.
Fig. 3.
Kaplan-Meier curve for overall survival (OS) stratified by initial palliative treatment strategies.
Table 3.
Response evaluation and treatment outcomes stratified by first-line treatment strategy.
| Chemotherapy (n = 222) | Endocrine therapy (n = 80) | Chemotherapy-endocrine therapy (n = 149) | P Valuea | |
|---|---|---|---|---|
| Best response | <0.001 | |||
| Complete response | 8 (3.6%) | 6 (7.5%) | 9 (6.0%) | |
| Partial response | 72 (32.4%) | 9 (11.3%) | 71 (47.7%) | |
| Stable disease | 53 (23.9%) | 36 (45.0%) | 53 (35.6%) | |
| Progressive disease | 49 (22.1%) | 13 (16.3%) | 1 (0.7%) | |
| Objective response rate | 36.0% | 18.8% | 53.7% | <0.001 |
| Progression-free survival median (95% CI) | 7.1 (5.9–8.3) | 11.7 (9.7–13.6) | 18.9 (16.2–21.7) | <0.001 |
| Overall survival median (95% CI) | 43.9 (39.0–48.8) | 53.5 (40.4–66.6) | 75.0 (57.3–92.7) | <0.001 |
Bold values indicate statistically significant results.
In multivariate analysis, worse performance status (HR 3.49, 95%CI, 1.07–11.38, P = 0.038) and more metastatic sites (≥3) (HR 1.98, 95%CI, 1.19–3.29, P = 0.009) were associated with shorter PFS. The worsened outcome of patients with worse performance status also extended to OS (Table 4). Of note, first-line endocrine therapy or chemotherapy followed by endocrine therapy were not independent prognostic factors for PFS or OS (Table 4).
Table 4.
Multivariable survival analysis of premenopausal hormone receptor-positive, HER2-negative metastatic breast cancer patients.
| Progression-free survival (PFS) |
Overall survival (OS) |
|||
|---|---|---|---|---|
| HR (95%CI) | P Valuea | HR (95%CI) | P Valuea | |
| Age ≤35 | 1.37 (0.74–2.55) | 0.32 | 1.00 (0.63–1.58) | 0.99 |
| ECOG >1 | 3.49 (1.07–11.38) | 0.038 | 4.47 (1.08–18.48) | 0.039 |
| Metastatic sites ≥3 | 1.98 (1.19–3.29) | 0.009 | 1.33 (0.91–1.96) | 0.15 |
| De novo stage IV | 0.69 (0.36–1.31) | 0.26 | 0.73 (0.48–1.11) | 0.14 |
| Luminal B subtype | 1.03 (0.66–1.61) | 0.89 | 1.28 (0.93–1.76) | 0.13 |
| Initial endocrine therapy | 1.07 (0.72–1.58) | 0.75 | 1.06 (0.68–1.66) | 0.79 |
| Chemotherapy-endocrine therapy | 1.01 (0.76–1.34) | 0.94 | 1.11 (0.80–1.55) | 0.53 |
HER2 = human epidermal growth factor receptor 2; HR = hazard ratio; 95% CI = 95% confidence interval; ECOG = Eastern Cooperative Oncology Group.
Bold values indicate statistically significant results.
4. Discussion
In this study, we presented data on the discrepancy between guidelines and real-world daily practice for premenopausal patients with HR + HER2- metastatic breast cancer. To our knowledge, this is the first real-world experience focusing on the initial palliative systemic treatment strategies for premenopausal HR + MBC patients in China. Our work provides useful information to interpret difference between guideline adherence and patterns of care.
Although endocrine therapy is preferred by major guidelines for HR + HER2-MBC, even with visceral disease (unless there is visceral crisis or concern of endocrine resistance) [5,7], our experience has been that chemotherapy is frequently administered in premenopausal patients even without visceral crisis in daily practice, which has been confirmed in this study. We showed that chemotherapy alone was given in nearly 50% of HR + HER2- premenopausal MBC patients as initial palliative therapy, and another one third of HR + HER2- premenopausal patients received induction chemotherapy followed by maintenance endocrine therapy. In total, approximately 80% of premenopausal HR + HER2- MBC patients received chemotherapy during first-line treatment, which was remarkably high and unexpected.
This guideline-non-adherence has also been reported previously. Descriptive studies demonstrated that 46–49% of European patients received chemotherapy in first-line treatment, and this proportion was slightly lower in the United States (40–47%) [13,14]. In a study focusing on premenopausal patients from South Korea, 49.7% of patients received chemotherapy as first-line treatment [15]. The higher percentage of initial chemotherapy in our real-life experience might be explained by the following reasons:
Firstly, a larger proportion of MBC patients in China are young and premenopausal, which has been supported by the present study. Indeed, the epidemiology of breast cancer in young women differs manifestly between Asians and non-Asians, with incidence peaks at 40–50 in Chinese, but∼70 years in the United States [16]. According to breast cancer in China reported in Lancet Oncology, 57.4% of women in China diagnosed with breast cancer before age 50, and 62.9% were premenopausal at breast cancer diagnosis [17]. Premenopausal breast cancer has been reported to be more aggressive and had poorer prognosis, thus more intensive treatment might be needed. In fact, the Epidemiological Strategy and Medical Economics (ESME) breast cohort previously reported that chemotherapy was preferentially prescribed to younger patients with visceral or brain metastases [18], which was in accordance with our findings that bone and soft tissue metastases was the only factor that associated with first-line choice of endocrine therapy.
In addition, given distinctive features of breast cancer patients in China, we are concerned that whether the current major treatment guidelines are universally generalizable. On one hand, both premenopausal women and Chinese or Asian population were understudied in global clinical trials, and recommendations were mainly based on evidence from Caucasian women. On the other hand, guidelines recommended endocrine treatment of premenopausal women to refer to postmenopausal women with addition of ovarian suppression. However, ovarian suppression has not been evaluated in efficacy versus chemotherapy in the premenopausal MBC population during our study period (2004–2015), and that might explain why chemotherapy continues to be prescribed to a large portion of HR + HER2- premenopausal patients in China.
Furthermore, among multiple reasons of choosing chemotherapy as first-line treatment, such as high tumor burden, aggressive tumor behavior and so on, one important consideration is endocrine resistance. In fact, 86.8% of patients in our study experienced disease relapse within one year after completing adjuvant endocrine therapy, which were considered as secondary endocrine resistance, and about one third of patients had recurrent disease within two years of adjuvant endocrine therapy, which were considered as primary endocrine resistance. This high proportion of endocrine resistance population also contributed to the wide use of chemotherapy in our cohort.
Last but not least, cytotoxic chemotherapy might be a reasonable option for HR + HER2-MBC patients. Chemotherapy is associated with more side effects, like nausea, vomiting, neutropenia, liver toxicity and more intravenous infusion than endocrine therapy, however, premenopausal patients without visceral crisis were generally at good performance status, and oral agents like capecitabine might be well-tolerated. On the other hand, chemotherapy has advantage in terms of higher response rate than endocrine therapy. In our study, the majority of patients received cytotoxic agents with relatively less burden of side effects, like taxanes and oral capecitabine as first-line chemotherapy regimens. In addition, the gonadotropin-releasing hormone agonists (GnRHa) like goserelin were not covered by the national health insurance until 2009, and chemotherapy has the additional advantage of costing less, which might be another important reason for the high percentage of chemotherapy use in real life.
In our study, endocrine therapy alone and chemo-endocrine therapy were associated with improved PFS over chemotherapy alone, however, the OS was not significantly different between endocrine therapy and chemotherapy, or between chemo-endocrine therapy and endocrine therapy groups. After adjusting for other prognostic factors, neither endocrine therapy alone nor chemo-endocrine therapy was independently associated with overall survival. This guideline non-adherence and real-world outcomes of patients has recently been evaluated by several studies with varying results. The Southern Netherlands Breast Cancer Consortium evaluated 482 HR + HER2- MBC patients and found that chemotherapy resulted in worse clinical outcomes in terms of both PFS (HR 2.33, P < 0.0001) and OS (HR 2.24, P < 0.0001), despite the fact that chemotherapy group was about ten years younger and had lower frequency of comorbidities [19]. Other two studies from Italy and the ESME programme all showed that no differences in survival were observed on first-line chemotherapy or endocrine therapy in HR + HER2- MBC patients [18,20]. However, these studies included both pre-and postmenopausal patients from the Caucasian population and the results might not be that comparable in the Chinese population. One study from South Korea investigated premenopausal HR + HER2- MBC patients in the Korean population, and found that chemo-endocrine therapy was independently associated with improved overall survival [15]. The inconsistency of the outcomes of guideline non-adherence between the Korean and Dutch study might result from the inconsistent inclusion criteria, the different ethics population, and the retrospective nature of the two studies. The authors from the Korean study suggested that there might be a distinct group among ER + premenopausal populations who could benefit from chemo-endocrine therapy in the Korean population, as a multi-omics study showed that Korean breast cancer was independently associated with increased tumor-infiltrating lymphocyte (TIL) and decreased transforming growth factor (TGF)-signaling expression signatures, suggesting that Korean breast tumors may harbor a different biology [21]. Our results were inconsistent with the Korean study by showing that chemo-endocrine therapy was not an independent prognostic factor for OS. This inconsistency might results from a combination of possible factors: the limited sample size, the retrospective nature, the different ethics population and the bias for patients selection of the two studies. So far, to our knowledge, no study in China has looked at the biological features of premenopausal women from a multi-omics point of view, and whether there was a distinct biology of Chinese breast cancer among Asian tumors which could guide first-line treatment choices needs further investigation.
Of note, our findings may not apply to new therapeutic strategies. Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors have changed the treatment landscape of HR + MBC patients during the past few years. The combination of AI and CDK4/6 inhibitors significantly improved the outcomes of endocrine therapy treatment, with a median PFS exceeding 2 years [22]. The Young-PEARL study showed a direct survival benefit of AI combined with CDK4/6 inhibitors over chemotherapy in premenopausal HR + MBC patients (median PFS 20.1 months vs.14.4 months, HR = 0.66; 95%CI 0.44–0.99, P = 0.0469) [23]. Although the PEARL study failed to show the same survival benefit of endocrine therapy over chemotherapy in AI-progressed patients, we might anticipate that such combination may change the natural history of premenopausal HR + MBC patients [24].
Our study have several limitations. First, this was a single institution study, and some referral bias might exist. Second, other confounding factors in making treatment decisions such as laboratory results, patient preference and comorbidities were not included. Third, the molecular subtypes were diagnosed on the primary tumor. Re-biopsy of metastatic lesions was not performed in the majority of cases, and the discordance of molecular subtypes could not be ruled out.
5. Conclusions
In conclusion, our real-life experience provides valuable information of guideline adherence, patterns of care and outcomes of premenopausal HR + HER2- MBC patients in China. The high percentage of initial chemotherapy is an important finding that could not only raise awareness of physicians, but also reveals the unmet need of establishing our own treatment consensus or guidelines for premenopausal HR + breast cancer patients in China. Chemotherapy was not inferior to endocrine therapy in the current study. Future clinical trials involving premenopausal women across Asian population are warranted, thus facilitating the guideline implementation and thereby improving the quality of care and optimizing treatment strategies and outcomes of HR + premenopausal patients in the Asian population.
Ethics approval and consent to participate
The study was reviewed and approved by the Institutional Review Board of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The informed consent of the participants was exempted by the review board due to the retrospective design of this study. All procedures were carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Li Yiqun: manuscript writing, data collection and analysis; Mo Hongnan, Guan Xiuwen, Lin Shaoyan, Wang Zijing, Chen Yimeng: data extraction; Li Qiao, Chen Shanshan, Cai Ruigang, Wang Jiayu, Luo Yang, Fan Ying, Yuan Peng, Zhang Pin, Li Qing, Ma Fei: management of patients and data collection; Xu Binghe: study design and supervision. All authors have read and approved this manuscript, and agree to its submittal to this journal.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author on reasonable request.
Declaration of competing interest
None.
Acknowledgements
We would like to thank Zheng Quan for data analysis assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.12.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhang S.W.S.K., Zheng R.S., Zeng H.M., Wang S.M., Chen R., Wei W.Q., He J. Cancer incidence and mortality in China, 2015. J Natl Cancer Cent. 2020;1(1):2–11. doi: 10.1016/j.jncc.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J., Steeg P.S., Price J.E., Krishnamurthy S., Mani S.A., Reuben J., Cristofanilli M., Dontu G., Bidaut L., Valero V., et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69(12):4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA A Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Li C.I., Daling J.R., Malone K.E. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., Andre F., Barrios C.H., Bergh J., Bhattacharyya G.S., Biganzoli L., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano S.H., Temin S., Chandarlapaty S., Crews J.R., Esteva F.J., Kirshner J.J., Krop I.E., Levinson J., Lin N.U., Modi S., et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(26):2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) clinical practice guidelines in Oncology. Breast Cancer. 2021 https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Version 4. [Google Scholar]
- 8.Leong S.P., Shen Z.Z., Liu T.J., Agarwal G., Tajima T., Paik N.S., Sandelin K., Derossis A., Cody H., Foulkes W.D. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34(10):2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y.C., Chang C.J., Hsu C., Cheng C.C., Chiu C.F., Cheng A.L. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: implications from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1986–1990. doi: 10.1158/1055-9965.EPI-04-0932. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm H., Eaker S., Frisell J., Holmberg L., Fredriksson I., Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4(11) doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., Dowsett M., Fitzgibbons P.L., Hanna W.M., Langer A., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thurlimann B., Senn H.J. Panel m: personalizing the treatment of women with early breast cancer: highlights of the St Gallen international Expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldeira R., Scazafave M. Real-world treatment patterns for hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in Europe and the United States. Oncol Ther. 2016;4(2):189–197. doi: 10.1007/s40487-016-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swallow E., Zhang J., Thomason D., Tan R.D., Kageleiry A., Signorovitch J. Real-world patterns of endocrine therapy for metastatic hormone-receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) breast cancer patients in the United States: 2002-2012. Curr Med Res Opin. 2014;30(8):1537–1545. doi: 10.1185/03007995.2014.908829. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.K., Lee S.H., Kim Y.J., Park S.E., Lee H.S., Lim S.W., Cho J.H., Kim J.Y., Ahn J.S., Im Y.H., et al. Does guideline non-adherence result in worse clinical outcomes for hormone receptor-positive and HER2-negative metastatic breast cancer in premenopausal women?: result of an institution database from South Korea. BMC Cancer. 2019;19(1):84. doi: 10.1186/s12885-018-5258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo W., Ueno T., Lin C.H., Liu Q., Lee K.H., Leung R., Naito Y., Park Y.H., Im S.A., Li H., et al. Treating HR+/HER2- breast cancer in premenopausal Asian women: Asian breast cancer cooperative group 2019 consensus and position on ovarian suppression. Breast Cancer Res Treat. 2019;177(3):549–559. doi: 10.1007/s10549-019-05318-5. [DOI] [PubMed] [Google Scholar]
- 17.Fan L., Strasser-Weippl K., Li J.J., St Louis J., Finkelstein D.M., Yu K.D., Chen W.Q., Shao Z.M., Goss P.E. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacquet E., Lardy-Cleaud A., Pistilli B., Franck S., Cottu P., Delaloge S., Debled M., Vanlemmens L., Leheurteur M., Guizard A.V., et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2-negative metastatic breast cancer patients. Eur J Cancer. 2018;95:93–101. doi: 10.1016/j.ejca.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Lobbezoo D.J., van Kampen R.J., Voogd A.C., Dercksen M.W., van den Berkmortel F., Smilde T.J., van de Wouw A.J., Peters F.P., van Riel J.M., Peters N.A., et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol. 2016;27(2):256–262. doi: 10.1093/annonc/mdv544. [DOI] [PubMed] [Google Scholar]
- 20.Bonotto M., Gerratana L., Di Maio M., De Angelis C., Cinausero M., Moroso S., Milano M., Stanzione B., Gargiulo P., Iacono D., et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast. 2017;31:114–120. doi: 10.1016/j.breast.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Kan Z., Ding Y., Kim J., Jung H.H., Chung W., Lal S., Cho S., Fernandez-Banet J., Lee S.K., Kim S.W., et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rugo H.S., Finn R.S., Dieras V., Ettl J., Lipatov O., Joy A.A., Harbeck N., Castrellon A., Iyer S., Lu D.R., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park Y.H., Kim T.Y., Kim G.M., Kang S.Y., Park I.H., Kim J.H., Lee K.E., Ahn H.K., Lee M.H., Kim H.J., et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20(12):1750–1759. doi: 10.1016/S1470-2045(19)30565-0. [DOI] [PubMed] [Google Scholar]
- 24.Martin M., Zielinski C., Ruiz-Borrego M., Carrasco E., Turner N., Ciruelos E.M., Munoz M., Bermejo B., Margeli M., Anton A., et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021;32(4):488–499. doi: 10.1016/j.annonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on reasonable request.