Abstract
Salmonella Heidelberg (SH) is a highly invasive human pathogen for which turkeys can serve as reservoir hosts. Colonization of turkeys with SH may result in potential contamination and is a greater challenge to prevent in comminuted products. Antimicrobial efficacy of 3 GRAS-status plant-derived antimicrobials (PDAs), lemongrass essential oil (LG), citral (CIT), and trans-cinnamaldehyde (TC), against SH in ground turkey, a comminuted product implicated in several outbreaks, was evaluated in this study. Ground turkey samples inoculated with ∼3.50 log10 CFU/g of a three-strain SH cocktail were treated with either LG, CIT, or TC at either 0.5, 1, or 2% (vol/wt). Samples were stored at 4°C, and bacterial enumeration was performed on d 0, 1, 3, and 5. Appropriate controls were included alongside all treatments. Fluorescence microscopy was performed to evaluate the direct impact of the PDAs against SH in vitro. Appearance and aroma difference testing of raw patties was also performed for select treatments with trained sensory panelists. Treatment with 2% TC yielded a 2.5 log10 CFU/g reduction by d 1 and complete reduction by d 5 (P < 0.05). By d 3, 2% CIT and 2% LG resulted in SH reduction of at least 1.7 log10 CFU/g (P < 0.05). Addition of 1% TC resulted in reduction of at least 1.8 log10 CFU/g by d 3 (P < 0.05). Participants could distinguish PDA-treated raw patties by aroma. Most participants (7/11) could not distinguish patties treated with 0.5% TC based on appearance. Microscopic images indicate that all PDAs resulted in disruption of the SH membrane. Results of the present study indicate that the three tested PDAs, LG, CIT, and TC are effective against SH in ground turkey, indicating their potential use as interventions to mitigate Salmonella contamination in comminuted turkey products.
Key words: citral, lemongrass, trans-cinnamaldehyde, Salmonella, antibacterial
INTRODUCTION
Salmonella is the leading cause of foodborne illness in the United States, accounting for an estimated 1.35 million cases annually (CDC, 2020). Outbreaks of Salmonella have been linked to the consumption of contaminated poultry products. As consumer interest in high protein and low-fat diets increases, so has the projected demand for products like turkey meat. The U.S. Department of Agriculture (USDA) projected the increase of turkey meat production in 2020 by 1,561 million pounds (USDA ERS, 2020). Despite continued efforts to mitigate pathogens in poultry products, contamination with Salmonella continues to occur and has resulted in costly recalls (CDC, 2011, 2019a,b).
Among the serovars of Salmonella responsible for outbreaks and for which turkeys can serve as reservoir hosts, Salmonella Heidelberg is an invasive pathogen in humans. S. Heidelberg can colonize the turkey gastrointestinal tracts in a commensal-like manner, resulting in asymptomatic carriage (Bearson et al., 2017). Colonization of turkeys with S. Heidelberg increases the possibility of contamination during the processing stage. This is challenging to prevent, especially in comminuted products such as ground turkey, because a Salmonella-positive carcass may contaminate multiple batches of carcasses during processing.
The USDA's Food Safety and Inspection Services (FSIS) has increased the stringency of their Salmonella performance standards following cases of Salmonella outbreaks involving ground turkey products (FSIS, 2016). The 2016 FSIS performance standards allowed for a maximum of 7.1% of young turkey carcasses sampled to test positive for Salmonella (FSIS, 2016), whereas ground turkey samples are allowed a maximum of 13.5%. The threshold had been greater in 2011 when FSIS allowed up to 50% of sampled ground turkey to test positive for Salmonella. The higher threshold for ground turkey, compared to their whole carcasses counterparts is, in part, due to the greater challenge in controlling Salmonella in comminuted poultry products.
Although the prevalence of Salmonella from poultry products has decreased with additional control measures, Salmonella outbreaks associated with poultry products continue to occur (McEntire et al., 2014; Antunes et al., 2016). Therefore, interventions to mitigate the presence of S. Heidelberg in ground turkey are necessary to ensure a microbiologically safe product. Plant-derived extracts have garnered interest for food industry applications with the rise in popularity of the clean label among consumers. The antimicrobial properties of several plant-derived extracts and their components have previously been investigated. Cinnamon and lemongrass are commonly used in a variety of food products. Major components of cinnamon extract and lemongrass essential oil (LG) are trans-cinnamaldehyde (TC) and citral (CIT), respectively. These plant-derived antimicrobials are generally recognized as safe (GRAS) and approved for use in foods by the FDA (21 CFR § 182.20 and 182.60) (FDA, 2020a, b).
The antimicrobial properties of LG have previously been demonstrated against S. Heidelberg both in vitro, in water, and on chicken skin and meat (Peichel et al., 2019; Dewi et al., 2021). CIT is an acyclic monoterpene aldehyde that is the major component in LG, comprising up to 85% of the essential oil. Therefore, it is highly likely that most of the antimicrobial efficacy of LG could be contributed by CIT. Both compounds were included in this study to explore if there were any differences in their activities against S. Heidelberg. Similar to LG, studies have reported the antimicrobial activity of CIT against Salmonella and other Gram-negative foodborne pathogens, like Cronobacter sakazakii (Kim et al., 1995; Shi et al., 2016). Like CIT, TC is an aromatic aldehyde that is commonly utilized as a flavoring agent in food products. TC has demonstrated antimicrobial properties against Salmonella and enhanced the thermal destruction of Escherichia coli O157:H7 in ground beef (Amalaradjou et al., 2010; Kollanoor Johny et al., 2010). Therefore, the objective of this study was to evaluate the antimicrobial efficacy of the plant-derived antimicrobials (PDAs), LG, CIT, and TC, against S. Heidelberg in ground turkey during refrigerated storage and determine whether they changed the appearance or the aroma of the patties.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Three strains of S. Heidelberg were used in this study, including a strain from the 2011 outbreak in ground turkey (GT2011; Minnesota Department of Health), the 2014 outbreak in mechanically separated chicken (N13X001904; Tennessee Department of Health), and S. Heidelberg ATCC 8326. These strains were selected based on our previous studies (Nair and Kollanoor Johny, 2017; Nair et al., 2018; Dewi et al., 2021; Nair et al., 2021). Each strain was taken from a −80°C frozen stock and grown separately in 10 mL trypticase soy broth (TSB; catalog no. C7141, Criterion, Hardy Diagnostics, Santa Maria, CA) at 37°C for 24 h. All 3 strains were made resistant to 50 μg/mL nalidixic acid (NA; CAS. no. 3374-05-8, Alfa Aesar, Haverhill, MA) for selective enumeration and after 3 successive propagations were pelleted by centrifugation (3,600 × g for 15 min at 4°C; Allegra X-14R, Beckman Coulter, South Kraemer Boulevard, Brea, CA). The pellet was suspended in 10 mL sterile phosphate-buffered saline (PBS, pH 7.2) for the inoculum. The growth of Salmonella was determined by serial dilution and plating on xylose lysine deoxycholate agar (XLD; catalog no. C7322, Criterion, Hardy Diagnostics) incubated at 37°C for 24 h.
Plant-Derived Antimicrobial Agents
Lemongrass essential oil (LG; Natural, Food Grade; Catalog no. W262404-1KG-K), citral (CIT; Natural, Food Grade, FCC; Catalogue no. W230316-1KG-K), and trans-cinnamaldehyde (TC; Food Grade, FCC; Catalogue no. W228605-1KG-K) were purchased from Sigma Aldrich (St. Louis, MO).
Antimicrobial Efficacy of PDAs Against S. Heidelberg in Ground Turkey
S. Heidelberg Inoculation and PDA Treatment
Ground turkey (Commercial brand; 93% lean, 7% fat) purchased from a grocery store was divided into 15-gram samples. Each sample was then inoculated with 500 μL of the 3-strain cocktail of NA-resistant S. Heidelberg and hand mixed to achieve ∼3.50 log10 CFU S. Heidelberg per g of ground turkey. They were subsequently mixed with either LG, CIT, or TC, at either 0.5, 1, or 2% (vol/wt.) (Dewi et al., 2021; Manjankattil et al., 2021). Positive (PC; inoculated but not treated with PDAs) and negative (NC; not inoculated and not treated with PDAs) controls were included in each experiment. The samples were then formed into patties and stored in a sterile Whirl-Pak bag and refrigerated at 4°C before S. Heidelberg enumeration.
Microbiological Analysis
S. Heidelberg populations in the patties were enumerated on d 0 (30 min after inoculation), 1, 3, and 5 after inoculation. For enumeration, 35 mL of PBS was added to the Whirl-Pak bag containing the patty. The bags were subsequently homogenized for 1 min using a stomacher (100/125V, 50/60Hz; Neutec Group Inc., Farmingdale, NY). The samples were then serially diluted, and 100 µL from appropriate dilutions were plated onto XLD + 50 μg/mL NA plates. Plates were incubated at 37°C for 24 h before bacterial enumeration. Samples were enriched with selenite cystine broth (SCB; Hardy Diagnostics) and streaked on XLD+NA plates after 24 h of incubation to detect any surviving S. Heidelberg that was not observed with initial plating.
Visualization of Effect of PDAs on S. Heidelberg Using Fluorescence Microscopy
To examine the direct antimicrobial effect of the PDAs against S. Heidelberg, a fluorescence-based microscopy assay was performed. The 3 strains of S. Heidelberg were combined into a cocktail, pelleted by centrifugations, and suspended in PBS. Then, 0.1% (vol/vol) of either LG, CIT, or TC was added to the solution and incubated for 15 min. S. Heidelberg cocktail without either PDA was kept alongside the treatments as a control. The solutions were stained with equal volumes of SYTO 9 green-fluorescent nucleic acid stain and propidium iodide red-fluorescent nucleic acid stain from the LIVE/DEAD BacLight Bacterial Viability Kit (catalog no. L7012, Thermo Fisher Scientific, Waltham, MA). The SYTO 9 dye stains all cells, whereas the propidium iodide penetrates only those with compromised cell membranes, thus staining only damaged or dead bacterial cells. After 15-min incubations in the dark, 5 μL of the stained culture was placed on a slide and observed under a fluorescence microscope (Axio Scope.A1; Carl Zeiss MicroImaging GmbH, Pleasanton, CA). The SYTO 9 dye was visualized using the GFP fluorescence filter, whereas the propidium iodide dye was observed with the DsRED fluorescence filter.
Appearance and Aroma Difference Testing of Raw Patties
Ground Turkey Treatments
Ground turkey (93% lean, 7% fat) purchased from a local grocery store was divided into 15 g patties and treated with one of the following: no added treatment, 1% LG, 1% CIT, 0.5% TC or 1% TC (vol/wt). Only one set of untreated control were included along with treatments as the samples were not challenged with S. Heidelberg. The ground turkey samples were then kept refrigerated at 4°C for 5 d and taken out of refrigeration approximately 30 min before the sensory testing.
Panel Participants
Eleven members of the trained panel from the Sensory Center at the University of Minnesota, consisting of 2 males and 9 females, participated in this test. All were 6-n-propylthiouracil (PROP) tasters or supertasters. They were compensated for the one testing session. The University of Minnesota's Institutional Review Board approved all recruiting and experimental procedures (Study #6874).
Preparation of Samples for Appearance and Aroma Evaluation
Ground turkey samples for appearance evaluation were presented in the shape of patties with a diameter of approximately 5 cm. A single patty was placed in a Hefty Everyday Soak Proof 12 oz. foam bowl (Reynolds Consumer Products LLC., Lake Forest, IL) with a single layer of Reynolds Kitchens Quick Cut Plastic Wrap (Reynolds Consumer Products LLC.) over the top. Each bowl received an individual 3-digit blinding code. The plastic wrap was replaced once every hour throughout the 3 h of testing to maintain a clear view of the ground turkey and eliminate any condensation. For aroma evaluation, one teaspoon samples of raw ground turkey were served to each panelist in 4 oz. foam cups with opaque plastic lids (Dart Container Corporation, Mason, MI). Each cup was given a unique 3-digit blinding code.
Experimental Procedure
Panelists began either with the aroma or appearance part in approximately equal proportions to balance any order or carryover effects. Within both the aroma and the appearance parts, panelists observed 4 treatment sets in a balanced order to account for any potential order or carryover effects. Each treatment set consisted of 2 control samples and 3 samples of a specific treatment (or 3 control samples and 2 samples of a specific treatment). Panelists were given the instructions: “The 5 samples in this display comprise a group of 2 and a group of 3. Please sort the samples into the two groups based solely on their appearance or aroma. Write the sample codes for the two groups in the table below.”
For the appearance difference testing, 4 booths were set up with display samples of each of the 4 antimicrobial treatment sets for this test, allowing all 11 participants to make their judgments on the same samples. For the aroma difference testing, participants made evaluations on their own sets. After sorting the samples into their 2 groups (a group of 2 and a group of 3), participants were asked to “Please explain why you think these two sample groups differ” (open-ended question).
Statistical Analysis
Ground Turkey Challenge Studies
A completely randomized design with a 3 × 4 × 4 factorial treatment structure was followed for this study. The factors included 3 different PDAs at 4 concentrations and 4 d at which the patties were sampled across a 5-d storage period. The S. Heidelberg-challenge experiments were conducted with duplicates per treatment and repeated 3 times. Populations of S. Heidelberg were logarithmically transformed for analysis. Samples from which no S. Heidelberg was recovered after spread plating but detected after enrichment were assumed a value of 0.95 log10 CFU/g. The data was analyzed using the lmerTest package of R (R, version 3.6.1, R Core Team), and a significant difference was considered at P < 0.05.
Appearance and Aroma Analyses
Panelist responses were scored either as correct if they correctly sorted the 2 groups or incorrect. The number of correct responses out of 11 possible correct responses for the appearance and for the aroma sorting tasks for each treatment was tabulated. A binomial test was used to determine whether the panelists were able to discriminate between the untreated controls and each of the other treatments for appearance and for aroma.
RESULTS
PDA Against S. Heidelberg in Ground Turkey
The S. Heidelberg populations in the untreated patties remained at approximately 3.35 log10 CFU/g of ground turkey throughout the 5-d storage period. By contrast, a greater decrease in S. Heidelberg was observed over time in patties treated with the PDAs. The bacterial reductions observed were also proportional to the concentration of PDAs added to the patty, with higher concentrations yielding greater reductions in S. Heidelberg populations.
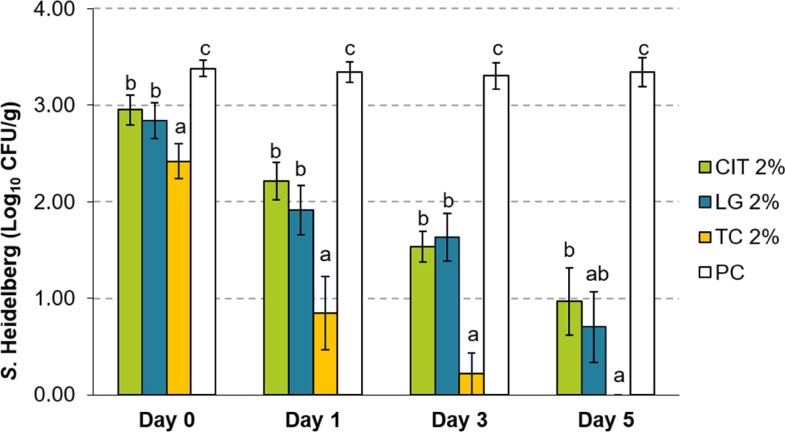
The addition of PDAs at 2% resulted in significant reductions of S. Heidelberg in ground turkey by d 1 (P < 0.05; Figure 1). The greatest reduction in S. Heidelberg was observed with 2% TC. At this concentration, TC resulted in 2.50- and 3.09-log10 CFU/g reductions compared to controls on d 1 and 3, respectively (P < 0.05). By d 5 of storage, a complete reduction was observed in TC-treated groups, with no S. Heidelberg detected in samples even after enrichment. A similar magnitude of reduction was observed between LG and CIT treated patties. LG yielded a reduction of 1.67 and 2.64 log10 CFU/g by d 3 and 5, and CIT yielded 1.77 and 2.38- log10 CFU/g (P < 0.05).
Figure 1.
Effect of 2% LG, CIT, or TC on Salmonella Heidelberg survival in ground turkey on d 0, 1, 3, and 5 of storage at 4°C (Means ± SEM; n = 6/treatment). a – c Treatments within each sampling time that lack common superscripts differ significantly from one another (P < 0.05). Abbreviations: CIT, Citral; LG, Lemongrass essential oil; PC, untreated control; TC, trans-Cinnamaldehyde.
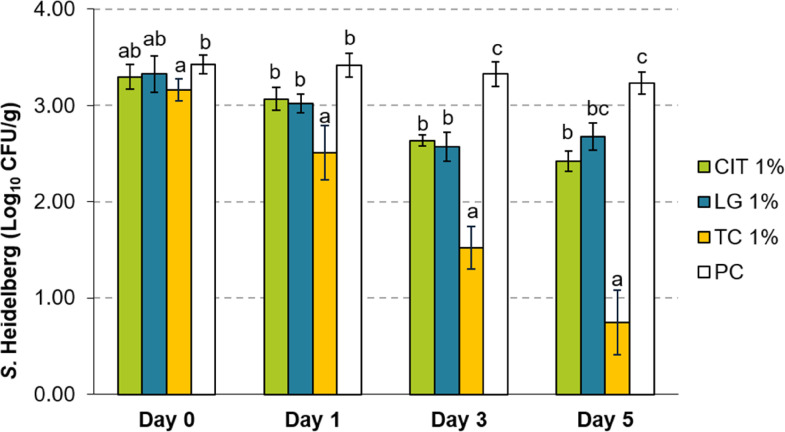
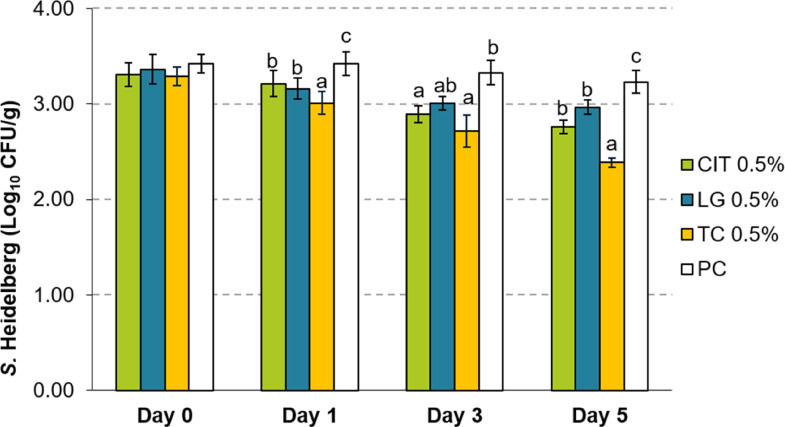
The PDAs exerted a similar time-dependent reduction at lower concentrations, albeit at a smaller magnitude. When added at 1%, TC yielded a reduction of 1.81 and 2.48 log10 CFU/g by d 3 and 5, respectively (Figure 2). At the same time, LG and CIT at this concentration yielded at most 0.55 and 0.81 log10 CFU/g reductions by d 5. Treatment with 0.5% PDA yielded at most 0.84 log10 CFU/g reduction with TC after 5 d of storage (Figure 3).
Figure 2.
Effect of 1% LG, CIT, or TC on Salmonella Heidelberg survival in ground turkey on d 0, 1, 3, and 5 of storage at 4°C (Means ± SEM; n = 6/treatment). a – c Treatments within each sampling time that lack common superscripts differ significantly from one another (P < 0.05). Abbreviations: CIT, Citral; LG, Lemongrass essential oil; PC, untreated control; TC, trans-Cinnamaldehyde.
Figure 3.
Effect of 0.5% LG, CIT, or TC on Salmonella Heidelberg survival in ground turkey on d 0, 1, 3, and 5 of storage at 4°C (Means ± SEM; n = 6/treatment). a – c Treatments within each sampling time that lack common superscripts differ significantly from one another (P < 0.05). Abbreviations: CIT, Citral; LG, Lemongrass essential oil; PC, untreated control; TC, trans-Cinnamaldehyde.
Visualization of Effect of PDAs on S. Heidelberg Using Fluorescence Microscopy
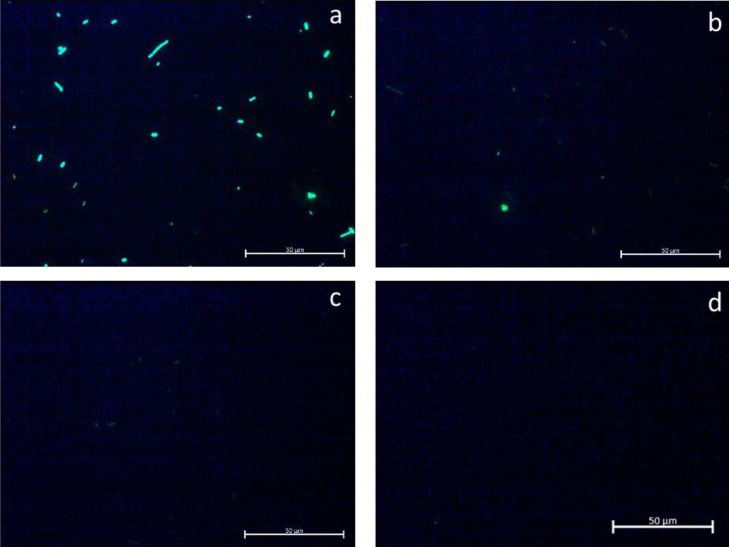
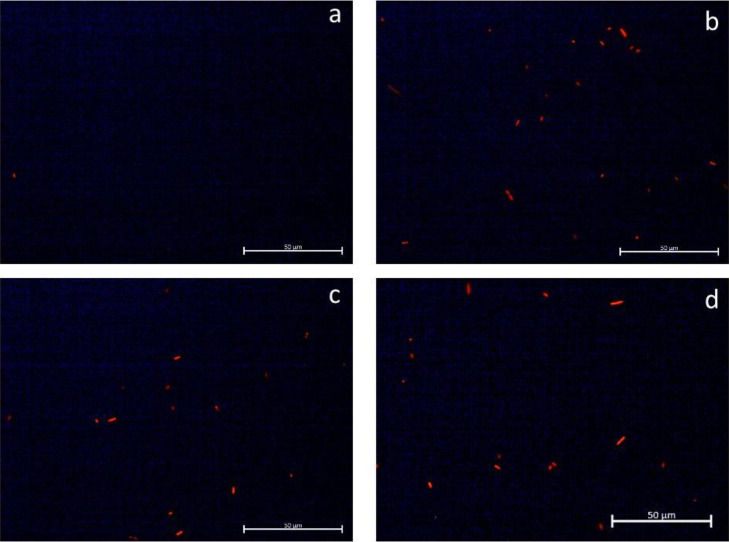
The effect of either LG, CIT, or PC on S. Heidelberg cell membrane integrity was investigated by staining with SYTO 9 dye (Figure 4) and propidium iodide (Figure 5). Images were taken of the same field using different filters to observe the 2 different stains. The SYTO 9 stain (green) was most prominently visible among the S. Heidelberg cells in the untreated control group (Figure 4A). Fewer cells were stained green among the cultures treated with the PDAs. Several green-stained cells were observed with LG (Figure 4B) and CIT (Figure 4C), but none were observed in TC-treated cultures (Figure 4D). By contrast, a greater number of cells stained with the propidium iodide dye were observed among the treatment groups compared to the control (Figure 5).
Figure 4.
Effect of 0.1% LG, CIT, or TC on Salmonella Heidelberg cell membrane integrity – Fluorescence microscopy of bacteria stained with SYTO 9: (A) untreated control, (B) lemongrass essential oil, (C) Citral, and (D) trans-Cinnamaldehyde. Abbreviations: CIT, Citral; LG, Lemongrass essential oil; TC, trans-Cinnamaldehyde.
Figure 5.
Effect of 0.1% LG, CIT, or TC on Salmonella Heidelberg cell membrane integrity – Fluorescence microscopy of bacteria stained with propidium iodide: (A) untreated control, (B) lemongrass essential oil, (C) Citral, and (D) trans-Cinnamaldehyde. Abbreviations: CIT, Citral; LG, Lemongrass essential oil; TC, trans-Cinnamaldehyde.
Appearance and Aroma Difference Testing of Raw Turkey Patties
The results for the appearance difference testing of the raw turkey patties are portrayed in Table 1. All 11 participants were able to detect differences in the appearance of the raw patties treated with 1% LG or CIT compared to the controls. The patties treated with LG or CIT were described to have a browner or yellower color compared to untreated controls. Ten participants correctly distinguished patties treated with 1% TC from controls. However, 7 of the 11 participants could not distinguish samples that were treated with 0.5% TC. TC-treated patties were less pink compared to untreated patties.
Table 1.
The number of correct responses out of 11 possible responses for the appearance sorting tasks for each treatment involving raw turkey patties directly added with respective plant-derived antimicrobial.
| Treatment (% vol/wt) | Correct appearance responses | P value |
|---|---|---|
| Citral (1%) | 11 | <0.001 |
| Lemongrass essential oil (1%) | 11 | <0.001 |
| Trans-cinnamaldehyde (1%) | 10 | <0.001 |
| Trans-cinnamaldehyde (0.5%) | 4 | 0.019 |
P values are from a binomial test.
Table 2 portrays the results for the aroma difference testing of the raw turkey patties. All participants detected the differences in the aroma of all treated patties compared to the untreated controls. Cinnamon was a common descriptor for the difference in aroma between the controls and groups treated with TC. Whereas citrus and lemon were common descriptors for the difference between the controls and the raw patties treated with LG and CIT.
Table 2.
The number of correct responses out of 11 possible responses for the aroma sorting tasks for each treatment involving raw turkey patties directly added with respective plant derived antimicrobial.
| Treatment (% vol/wt) | Correct aroma responses | P value |
|---|---|---|
| Citral (1%) | 11 | <0.001 |
| Lemongrass essential oil (1%) | 11 | <0.001 |
| Trans-cinnamaldehyde (1%) | 11 | <0.001 |
| Trans-cinnamaldehyde (0.5%) | 11 | <0.001 |
P values are from a binomial test.
DISCUSSION
Control of Salmonella spp. in comminuted poultry products such as ground turkey is especially challenging because a single contaminated carcass has the potential of disseminating the pathogen to a whole batch of ground product. Therefore, exploration of additional interventions is necessary to complement the control measures that are currently in place. The use of natural antimicrobials has garnered growing attention among processors, with the increasing popularity of the clean label trend among consumers and their beneficial effects, including antibacterial properties (Zink, 1997). Plant-derived extracts are among the naturally derived antimicrobials of interest for this purpose.
This study explored the direct antimicrobial properties of 3 PDAs, LG, CIT, and TC against S. Heidelberg in ground turkey. The study demonstrated the antibacterial efficacy of the 3 PDAs against S. Heidelberg in raw ground turkey during refrigerated storage, with greater pathogen reduction observed with increasing concentrations. TC exhibited the greatest antimicrobial activity among the 3 plant-derived antimicrobials explored. Furthermore, LG and CIT yielded comparable reductions in S. Heidelberg populations. This indicates that CIT may be the main component responsible for the antimicrobial properties as it makes up to 85% of LG. Other investigations exploring the use of the whole essential oil and CIT alone have reported similar findings (Adukwu et al., 2016). The results portrayed in this study reflect the antimicrobial effect of the plant-derived antimicrobials explored alone, without any additional pathogen intervention.
The SYTO 9 and propidium iodide nucleic acid stains differ in their ability to permeate the cell membrane. All cells, regardless of the integrity of their membrane, are stained by the SYTO 9 dye. Oppositely, the propidium iodide dye is unable to penetrate intact membranes and thus only stains cells that are damaged. Therefore, uptake of the propidium iodide dye indicates that the bacterial cells are either injured or dead based on the permeability of their outer membrane. This suggests that exposure to the antimicrobial extracts resulted in changes to the membrane integrity of S. Heidelberg that is indicative of dying or dead bacterial cells. Therefore, the antimicrobial activity of the PDAs may involve direct disruption to the S. Heidelberg cell membrane. The lipophilic nature of TC and CIT potentially facilitated their interaction with the membrane-forming lipids that are present in bacterial membranes (Wang et al., 2018). Similar findings were observed in studies exploring the effect of TC against the cell wall of other organisms (Huang et al., 2019). Transmission electron microscopy of Salmonella Enteritidis exposed to LG revealed structural damage to the cell wall and leakage of cellular contents (Raybaudi-Massilia et al., 2006). Similarly, part of CIT's antimicrobial properties was attributed to its impact on membrane function and integrity (Zheng et al., 2015; Shi et al., 2016).
Cinnamaldehyde and citral are flavor ingredients that are commonly used to add cinnamon and citrus flavors to food products, respectively (FDA, 2020b). We conducted the appearance and aroma testing of the raw patties exposed to TC and CIT using 11 trained panelists as a first step to determine if our approach of direct addition of the flavor compounds during patty preparation would result in appreciable changes in the 2 attributes. Results indicated that the trained panelists could detect a noticeable aroma of the flavor compounds in raw turkey patties. They were also able to differentiate the patties by appearance, though fewer panelists (4 of 11) were able to accurately distinguish patties treated with the lower concentration of TC from untreated controls. Neither the impact of this change on the patties’ appeal to or likeness of the participants nor the effect of cooking on their sensory detection was explored in the current study. The appearance of raw patties is a relevant factor that consumers consider before purchase. Also, the analysis of aroma changes may also be pertinent after heat treatment, as the cooking stage always precedes the consumption of turkey patties. Results of sensory analysis of products treated with rosemary oleoresin were reported to differ between raw and cooked ground products. Rosemary extract is used as an ingredient in some of the turkey products available in the market (Keokamnerd et al., 2008). Our current focus is to conduct the consumer testing of raw and cooked patties after a pre-grinding dip treatment of turkey meat with TC and CIT for shorter duration without compromising the microbiological safety.
Poultry products are always intended to be thoroughly cooked, unlike other meat cuts, which may be consumed at varying degrees of doneness. Improper preparation and handling of poultry products by consumers at home contribute to the cases of foodborne illnesses (Anderson et al., 2004). A national survey conducted among U.S. adult grocery shoppers found that 62% of participants owned a meat thermometer, 73% used it to determine the doneness of whole turkeys, but only 12% did the same for ground poultry (Kosa et al., 2017). This study demonstrated that the addition of PDAs in ground turkey patties reduced bacterial pathogen survival during refrigerated storage. The addition might also have contributed to increasing Salmonella susceptibility to heat during cooking. However, this was not explored in the current study. For example, Juneja et al. (2012) reported that the addition of TC to ground chicken increased the lethal effect of heat against Salmonella spp.
Other investigations had reported a more significant reduction when these PDAs were used in combination with other interventions. High-pressure processing (HPP) is also a non-thermal intervention that is currently used in processing facilities as pathogen interventions. This method is preferred as its antimicrobial action is derived solely from physical damage to the bacterial cells (Sheen et al., 2015). However, operating at high pressure where ideal pathogen reduction can be achieved may result in undesirable changes to sensory attributes. Results from this study indicate that the PDAs, TC, CIT, and LG damage the bacterial cell membrane, which may facilitate the antimicrobial effect of pressure on Salmonella organisms. Likewise, the HPP may enhance the antimicrobial properties of the PDAs by providing greater access of the compounds to the pathogen in the ground meat. Mathematical modeling has demonstrated that the combination of HPP with TC in ground chicken can exert a more significant impact on Salmonella spp. even at lower concentrations (Sheen et al., 2018; Chuang et al., 2021). Similarly, modeling found that the combination of HPP and CIT may obtain more than 5 log10 CFU/g reductions of E. coli O157: H7 in ground beef (Chien et al., 2017).
The results of the present study indicate that TC, CIT, and LG have inhibitory properties against S. Heidelberg in ground turkey, potentially due to their disruption of the S. Heidelberg cell membrane. These findings indicate their potential use to reduce the risk of Salmonella contamination in comminuted products. Additional studies exploring the combination of these PDAs with other interventions are warranted to determine how they could enhance the current pathogen intervention strategies against Salmonella.
ACKNOWLEDGMENTS
The authors would like to thank the Minnesota Turkey Research and Promotion Council (MTRPC) Grant (2018-02), the Minnesota Agricultural Experimentation Station (Projects MIN-16-120 and 18-138), and USDA NIFA Hatch Grant (Accession#1016910) for the financial support of this research. The authors would also like to acknowledge the Minnesota Discovery, Research and InnoVation Economy (MnDRIVE) graduate fellowship awarded to G. Dewi for professional training during her graduate research
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Adukwu E.C., Bowles M., Edwards-Jones V., Bone H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016;100:9619–9627. doi: 10.1007/s00253-016-7807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalaradjou M.A.R., Baskaran S.A., Ramanathan R., Johny A.K., Charles A.S., Valipe S.R., Mattson T., Schreiber D., Juneja V.K., Mancini R., Venkitanarayanan K. Enhancing the thermal destruction of Escherichia coli O157:H7 in ground beef patties by trans-cinnamaldehyde. Food Microbiol. 2010;27:841–844. doi: 10.1016/j.fm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Anderson J.B., Shuster T.A., Hansen K.E., Levy A.S., Volk A. A Camera's view of consumer food-handling behaviors. J. Am. Diet. Assoc. 2004;104:186–191. doi: 10.1016/j.jada.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Bearson B.L., Bearson S.M.D., Looft T., Cai G., Shippy D.C. Characterization of a multidrug-resistant Salmonella enterica Serovar Heidelberg outbreak strain in commercial turkeys: colonization, transmission, and host transcriptional response. Front. Vet. Sci. 2017;4:1–7. doi: 10.3389/fvets.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2011. Multistate outbreak of human Salmonella Heidelberg Infections linked to ground turkey (Final Update). 2010 through 2011 Outbreaks. (Accessed July 2019). https://www.cdc.gov/salmonella/2011/ground-turkey-11-10-2011.html.
- CDC. 2019a. Outbreak of Salmonella infections linked to butterball brand ground turkey. 2019 Outbreaks. (Accessed July 2019).https://www.cdc.gov/salmonella/schwarzengrund-03-19/index.html.
- CDC. 2019b. Outbreak of multidrug-resistant Salmonella infections linked to raw turkey products. 2018 Outbreaks. (Accessed July 2019). https://www.cdc.gov/salmonella/reading-07-18/index.html.
- CDC. 2020. Pathogen surveillance. FoodNet Fast. (Accessed Apr. 2020). https://wwwn.cdc.gov/foodnetfast/.
- Chien S.-Y., Sheen S., Sommers C., Sheen L.-Y. Modeling the inactivation of Escherichia coli O157:H7 and uropathogenic E. coli in ground beef by high pressure processing and citral. Food Control. 2017;73:672–680. [Google Scholar]
- Chuang S., Sheen S., Sommers C.H., Sheen L.-Y. Modeling the reduction of Salmonella and Listeria monocytogenes in ground chicken meat by high pressure processing and trans-cinnamaldehyde. LWT. 2021;139 [Google Scholar]
- Dewi G., Nair D.V.T., Peichel C., Johnson T.J., Noll S., Johny A.K. Effect of lemongrass essential oil against multidrug-resistant Salmonella Heidelberg and its attachment to chicken skin and meat. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2020a. Sec. 182.20 Essential oils, oleoresins (solvent-free), and natural extractives (including distillates). United States. Silver Spring, MD.
- FDA. 2020b. Sec. 182.60 Synthetic flavoring substances and adjuvants. United States. Silver Spring, MD.
- FSIS. 2016. New performance standards for Salmonella and Campylobacter in not-ready-to-eat comminuted chicken and turkey products and raw chicken parts and changes to related agency verification procedures: response to comments and announcement of implementation schedule. Pages 7285–7300 (16 pages) in Federal Register. Food Safety and Inspection Services, USDA, Washington, DC.
- Huang F., Kong J., Ju J., Zhang Y., Guo Y., Cheng Y., Qian H., Xie Y., Yao W. Membrane damage mechanism contributes to inhibition of trans-cinnamaldehyde on Penicillium italicum using surface-enhanced Raman spectroscopy (SERS) Sci. Rep. 2019;9:490. doi: 10.1038/s41598-018-36989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja V.K., Yadav A.S., Hwang C.-A., Sheen S., Mukhopadhyay S., Friedman M. Kinetics of thermal destruction of Salmonella in ground chicken containing trans-cinnamaldehyde and carvacrol. J. Food Prot. 2012;75:289–296. doi: 10.4315/0362-028X.JFP-11-307. [DOI] [PubMed] [Google Scholar]
- Keokamnerd T., Acton J.C., Han I.Y., Dawson P.L. Effect of commercial rosemary oleoresin preparations on ground chicken thigh meat quality packaged in a high-oxygen atmosphere. Poult. Sci. 2008;87:170–179. doi: 10.3382/ps.2007-00066. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Marshall M.R., Cornell J.A., Preston III J.F., Wei C.I. Antibacterial activity of carvacrol, citral, and geraniol against Salmonella Typhimurium in culture medium and on fish cubes. J. Food Sci. 1995;60:1364–1368. [Google Scholar]
- Kollanoor Johny A., Darre M.J., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010;19:237–244. [Google Scholar]
- Kosa K.M., Cates S.C., Godwin S., Chambers IV E. Barriers to using a food thermometer when cooking poultry at home: results from a national survey. Food Prot. Trends. 2017;37:116–125. [Google Scholar]
- Manjankattil S., Nair D.V.T., Peichel C., Noll S., Johnson T.J., Cox R.B., Donoghue A.M., Kollanoor Johny A. Effect of caprylic acid alone or in combination with peracetic acid against multidrug-resistant Salmonella Heidelberg on chicken drumsticks in a soft scalding temperature-time setup. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntire J., Acheson D., Siemens A., Eilertan S., Robach M. The public health value of reducing Salmonella levels in raw meat and poultry. Food Prot. Trends. 2014;34:386–392. [Google Scholar]
- Nair D.V.T., Johny A.Kollanoor. Effect of Propionibacterium freudenreichii on Salmonella multiplication, motility, and association with avian epithelial cells. Poult. Sci. 2017;96:1376–1386. doi: 10.3382/ps/pew367. [DOI] [PubMed] [Google Scholar]
- Nair D.V.T., Vazhakkattu Thomas J., Dewi G., Brannon J., Noll S.L., Johnson T.J., Cox R.B., Kollanoor Johny A. Propionibacterium freudenreichii B3523 reduces cecal colonization and internal organ dissemination of multidrug-resistant Salmonella Heidelberg in finishing turkeys. J. Appl. Poult. Res. 2021;30 [Google Scholar]
- Nair, D. V. T., J. Vazhakkattu Thomas, S. L. Noll, R. Porter Jr., and A. Kollanoor Johny. 2018. Effect of various inoculum levels of multidrug-resistant Salmonella enterica serovar Heidelberg (2011 ground turkey outbreak isolate) on cecal colonization, dissemination to internal organs, and deposition in skeletal muscles of commercial turkeys after experimental oral challenge. Front. Microbiol. 8:2680. [DOI] [PMC free article] [PubMed]
- Peichel C., Nair D.V.T., Dewi G., Donoghue A.M., Reed K.M., Kollanoor Johny A. Effect of lemongrass (Cymbopogon citratus) essential oil on the survival of multidrug-resistant Salmonella enterica serovar Heidelberg in contaminated poultry drinking water. J. Appl. Poult. Res. 2019;28:1121–1130. [Google Scholar]
- Raybaudi-Massilia R.M., Mosqueda-Melgar J., Martin-Belloso O. Antimicrobial activity of essential oils on Salmonella Enteritidis, Escherichia coli, and Listeria innocua in fruit juices. J. Food Prot. 2006;69:1579–1586. doi: 10.4315/0362-028x-69.7.1579. [DOI] [PubMed] [Google Scholar]
- Sheen S., Cassidy J., Scullen B., Uknalis J., Sommers C. Inactivation of Salmonella spp. in ground chicken using high pressure processing. Food Control. 2015;57:41–47. [Google Scholar]
- Sheen S., Huang C.-Y., Ramos R., Chien S.-Y., Scullen O.J., Sommers C. Lethality prediction for Escherichia coli O157:H7 and Uropathogenic E. coli in ground chicken treated with high pressure processing and trans-cinnamaldehyde. J. Food Sci. 2018;83:740–749. doi: 10.1111/1750-3841.14059. [DOI] [PubMed] [Google Scholar]
- Shi C., Song K., Zhang X., Sun Y., Sui Y., Chen Y., Jia Z., Sun H., Sun Z., Xia X. Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii (R Manganelli, Ed.) PLoS One. 2016;11 doi: 10.1371/journal.pone.0159006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA ERS. 2020. World agricultural supply and demand estimates at a glance. Commod. Outlook. (Accessed Aug. 2020).https://www.ers.usda.gov/topics/farm-economy/commodity-outlook/wasde-projections-at-a-glance/.
- Wang Y., Feng K., Yang H., Zhang Z., Yuan Y., Yue T. Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Jing G., Wang X., Ouyang Q., Jia L., Tao N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015;178:76–81. doi: 10.1016/j.foodchem.2015.01.077. [DOI] [PubMed] [Google Scholar]
- Zink D. The impact of consumer demands and trends on food processing. Emerg. Infect. Dis. 1997;3:467–469. doi: 10.3201/eid0304.970408. [DOI] [PMC free article] [PubMed] [Google Scholar]