Highlights
-
•
Selected studies are heterogeneous in terms of patient characteristics as well as type, duration and frequency of rehabilitative approach.
-
•
Neuromotor rehabilitation promotes neuroplasticity, as measured with MRI.
-
•
Advanced MRI techniques provide reliable markers of structural and functional connectivity that may potentially aid in helping to implement the most appropriate rehabilitation intervention.
Keywords: Stroke, MRI, Neuroplasticity, Rehabilitation, Connectivity, fMRI
Abstract
Background
Stroke-related disability is a major problem at individual and socio-economic levels. Neuromotor rehabilitation has a key role for its dual action on affected body segment and brain reorganization. Despite its known efficacy in clinical practice, the extent and type of effect at a brain level, mediated by neuroplasticity, are still under question.
Objective
To analyze studies applying MRI markers of functional and structural connectivity in patients affected with stroke undergoing motor rehabilitation, and to evaluate the effect of rehabilitation on brain reorganization.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria were applied to select studies applying quantitative non-conventional MRI techniques on patients undergoing motor rehabilitation, both physical and virtual (virtual reality, mental imagery). Literature search was conducted using MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE from inception to 30th June 2020.
Results
Forty-one out of 6983 papers were included in the current review. Selected studies are heterogeneous in terms of patient characteristics as well as type, duration and frequency of rehabilitative approach. Neuromotor rehabilitation promotes neuroplasticity, favoring functional recovery of the ipsilesional hemisphere and activation of anatomically and functionally related brain areas in both hemispheres, to compensate for damaged tissue.
Conclusions
The evidence derived from the analyzed studies supports the positive impact of rehabilitation on brain reorganization, despite the high data heterogeneity. Advanced MRI techniques provide reliable markers of structural and functional connectivity that may potentially aid in helping to implement the most appropriate rehabilitation intervention.
1. Introduction
Neurological diseases are the second leading death cause and the first of disability-adjusted life years (DALYs) according to the last available report of the Global Burden of Disease (GBD) (Collaborators GBDN). Among the non-communicable neurological diseases causing physical disability, stroke was the largest contributor to DALYs (Collaborators GBDN), and although age-standardized mortality rate has sharply decreased in the last two decades, the incidence did not show the same trend, resulting in an overall increased GBD for stroke survivors (Collaborators GBDS, 2019, Rajsic et al., 2019).
Motor rehabilitation impacts the clinical evolution of post-stroke phase both allowing functional recovery of the affected body segment and enhancing neuroplasticity (Dimyan and Cohen, 2011). Despite its advantages, there are some fundamental unanswered questions: is there an optimal time to start physiotherapy? What is more effective in terms of rehabilitation approach, frequency, duration, setting? Are there categories of patients more suitable for physiotherapy than others? Literature data in this regard are considerably heterogenous, and as such, not easily comparable. A recent review focused on motor rehabilitation in stroke analyzed 15 randomized controlled trials (RCTs), all but one yielding similar results in the intervention group and in the control group in terms of rehabilitation efficacy (Stinear et al., 2020). Conversely, several other smaller RCTs focusing on the same topic reported significantly positive results only in the active group (You et al., 2005, James et al., 2009, Whitall et al., 2011).
These apparently contradictory results might be related to the different rehabilitative treatments, but more likely are due to challenges in the design and realization of the clinical trials.
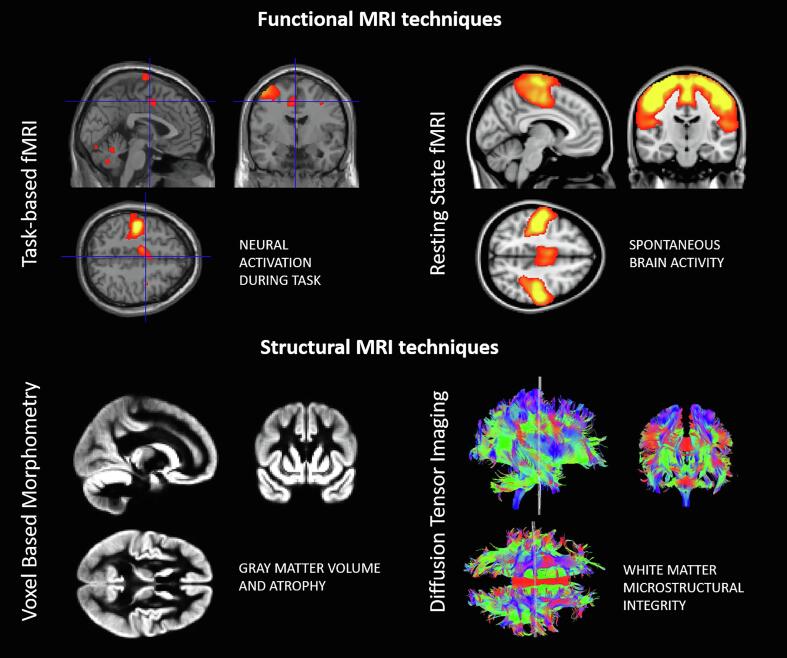
A better understanding of the underlying physiopathogenic mechanisms as well as the identification of biomarkers able to capture brain changes in response to rehabilitative treatment and to predict the clinical outcome in an early phase would help solve this quandary. Advanced MRI techniques might have a prominent role as they are non-invasive, reproducible, and allow to investigate both structural and functional aspects of the same pathogenic phenomenon. Indeed, the use of non-conventional MRI techniques have broadened the field’s knowledge about brain plasticity from a microstructural and functional point of view, and the derived quantitative markers have been recently included in studies evaluating the efficacy of rehabilitative treatments (Sun et al., 2020). Namely, functional MRI provides information about brain regional activity, based on blood oxygen level changes. This technique can provide both the level of activity during rest (resting state fMRI) and activation maps triggered by specific tasks (Fig. 1). On the other hand, structural changes can be measured both in terms of grey matter (GM) or white matter (WM) volume, through techniques such as voxel-based morphometry (VBM), or tissue integrity, evaluating the mobility and directionality of water molecules within the brain, using diffusion tensor imaging (DTI) (Fig. 1).
Fig. 1.
Examples of images derived from task-based fMRI (upper left), resting-state fMRI (upper right), voxel based-morphometry (lower left) and diffusion tensor imaging (lower right).
The advent of advanced MRI techniques has provided insight into how neuroplasticity occurs at a functional level, in terms of activation pattern or functional connectivity (FC) changes and at a structural level, which can translate in volumetric modifications or altered diffusivity profiles of the tissue. While some of these adaptive dynamic processes lead to an improvement of motor or cognitive performances, in some cases they are associated to a worse clinical outcome, a phenomenon called ‘maladaptive plasticity’ (Jang and Gordon, 2013, Lee et al., 2009).
In the last decades, a better understanding of disease-related pathogenic mechanisms, as well as the possibility to observe a more prolonged period of disease evolution in large cohorts of patients, have facilitated the development of a broader spectrum of rehabilitative approaches (Levard et al., 2021, Liu et al., 2020).
Despite these advances, the efficacy of rehabilitation on recovery is still under question, mostly due to the lack of robust markers, and the use of different variables (rehabilitative settings, type of treatment, inclusion criteria) that make literature studies difficult to compare.
With this background, the aim of this systematic review is to summarize and critically analyze the available data on MRI markers and motor rehabilitation applied to stroke and to describe the current state of the art with respect to the effects of neuromotor rehabilitation on brain plasticity.
2. Methods
Literature search, data selection and scientific writing were performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) criteria (Liberati et al., 2009). The protocol for the review was not registered before the literature search began.
2.1. PICOS eligibility criteria:
Participants: the only eligibility criterion was the recruitment of adult patients (≥18 years old) affected by ischemic or hemorrhagic stroke.
Interventions: studies applying quantitative non-conventional MRI techniques on patients undergoing motor rehabilitation, both physical (physical exercise, resistance training, aerobic exercise, endurance training, motor rehabilitation using robotic devices for upper or lower limbs) and virtual (virtual reality, motor imagery), were selected. Motor rehabilitation was defined as multiple training sessions of the selected physiotherapy approach. As such, studies reporting MRI results after single rehabilitative sessions or task training that was not part of rehabilitative treatments were excluded. Moreover, studies using exclusively brain stimulation to enhance brain plasticity, as well as studies on cognitive rehabilitation, or aiming at improving cognitive functions, were excluded.
Comparisons: Both studies with an active group of patients (i.e. patients undergoing motor rehabilitation of any sort) and a control group of patients not undergoing any treatment, studies comparing groups of patients undergoing different rehabilitative treatments and studies comparing patients undergoing rehabilitation and healthy subjects.
Outcomes: The outcome considered in the review was to evaluate the effect of motor rehabilitation on surrogate MRI markers representative of functional and/or tissue microstructure.
Study designs: Peer-reviewed Randomized and non-RCTs, including ≥ 5 subjects and case studies were included in the analysis. Conference proceedings, reviews, book chapters, case reports and editorials were excluded.
2.2. Information sources, search and study selection
Literature search was conducted using MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE from inception to 30th June 2020. The MeSH terms “stroke” AND (“rehabilitation” OR “physiotherapy” OR “exercise” OR “virtual reality” OR “robotics”) AND (“MRI” OR “brain plasticity” OR “connectivity”). Papers written in languages other than English were excluded. References from the selected articles were then screened for further records. Three researchers (E.T, A.P, M.C.) independently assessed the selected articles to evaluate their eligibility, and disagreements were solved by discussion.
2.3. Data extraction
For each study, the study design, number of subject, rehabilitative setting (i.e. inpatient, outpatient, home-based), MRI markers pre- and post-intervention were extracted and reported.
3. Results
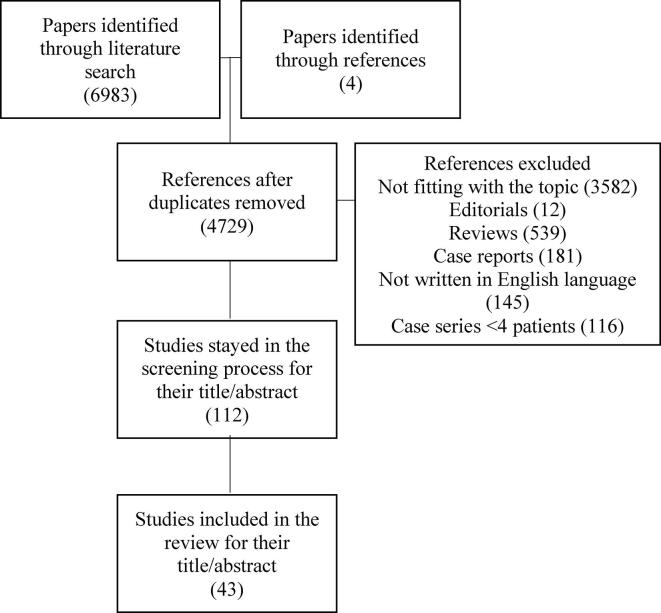
The literature search retrieved, as of June 30th, 2020, 6983 papers using the abovementioned MeSH terms, to which we added 4 papers retrieved from references. Six-thousand nine-hundred forty-four papers were eliminated for the following reasons: duplicates (2257), not fitting with the topic of the review after reading the title/abstract or the entire manuscript (3694), editorials (12), reviews (539), case reports (181), not written in English language (145), case series with less than 4 patients (116). A total of 43 papers were discussed in details in this review (Fig. 2).
Fig. 2.
Flow-chart of the papers selection process.
3.1. Characteristics of the studies
The key features of the studies are depicted in Table 1 for studies applying structural techniques and Table 2 for studies applying fMRI.
Table 1.
Clinical and MRI characteristics of the studies applying structural MRI techniques.
| Authors | Study design | Subjects | Training mode | Setting | Disease phenotype | Session duration (min) | Total duration | Imaging |
|---|---|---|---|---|---|---|---|---|
| Upper limb rehabilitation | ||||||||
| Fan et al. (2015) | CS | Tot = 10 | RAT | PT | Subacute | 90 | 4 weeks (5 d/w) | DTI |
| Gauthier et al. (2008) | RCT | Tot = 49 | CIMT | PT | Chronic | 180 | 10 days | VBM |
| Lower limb rehabilitation | ||||||||
| Yang et al. (2017) | Open label | Tot = 10 | RAT | PT | Subacute | 45 | 7 weeks (3d/w) | DTI |
| Kim et al. (2020) | RCT | Tot = 11 Group 1 = 5 Group 2 = 6 |
RAT | IP | Subacute | 45 | 4 weeks (5d/w) | DTI§ |
RCT-randomized controlled trial; CS-case series; PT-physiotherapist supervised; IN-inpatient; CIMT-constraint induced movement therapy; RAT-robot assisted therapy; VBM -voxel based morphometry; DTI-diffusion tensor imaging;
§Only in this study there were 2 MRI follow-ups
Table 2.
Clinical and MRI characteristics of studies applying functional MRI.
| Authors | Study design | Subjects with MRI results | Training mode | Setting | Disease phenotype | Session duration (min) | Total duration | Imaging |
|---|---|---|---|---|---|---|---|---|
| Upper limb rehabilitation | ||||||||
| Dechaumont-Palacin et al. (2008) | RCT | Tot = 13 Int = 7 Con = 6 |
Passive proprioceptive training vs CPT | PT | Subacute | NA | 4 weeks (5d/w) | Task fMRI |
| Askim et al. (2009) | CS | Tot = 12 | TSP | IP | Subacute | NA | NA | Task-fMRI |
| James et al. (2009) | CC | Tot = 8 Int = 5 Con = 3 |
TSP vs CPT | PT | Subacute | 120 | 3 weeks (5d/w) | Rs-fMRI |
| Murayama et al. (2011) | CC | Tot = 15 Int = 7 Con = 8 |
CIMT | IP | Subacute | 300 | 2 weeks (5d/w) | Task-fMRI§ |
| Pinter et al. (2013) | CS | Tot = 7 | RAT | PT | Subacute | 20 min | 3 weeks (5d/w) | Task-fMRI |
| Liu et al. (2014) | RCT | Tot = 15 Int = 10 Con = 5 |
MI + CPT vs CPT | PT | Subacute | 45 | 4 weeks (5d/w) | Task-fMRI |
| Fan et al. (2015) | CS | Tot = 10 | RAT | PT | Subacute | 90 | 4 weeks (5d/w) | Rs-fMRI |
| Horn et al. (2016) | CC | Tot = 26 Int = 12 Con = 14 |
CPT + AAT | IP | Subacute | 60 | 3 weeks (5 d/w) | Task-fMRI |
| Wu et al. (2019) | RCT | Tot = 25 Int = 14 Con = 11 |
BCI | IP | Subacute | 60 | 4 weeks (5d/w) | Rs-fMRI* |
| Johansen-Berg et al. (2002) | CS | Tot = 7 | CIMT | Home based | Chronic | 360 | 2 weeks | Task-MRI |
| Schaechter et al. (2002) | CC | Tot = 9 Int = 4 Con = 5 |
CIMT | PT | Chronic | 240 | 2 weeks (5d/w) | Task-fMRI§ |
| Luft et al. (2004) | RCT | Tot = 21 Int = 9 Con = 12 |
Bilateral arm training vs CPT | PT | Chronic | 60 | 6 weeks (3d/w) | Task-MRI |
| Jang et al. (2005) | RCT | Tot = 10 Int = 5 Con = 5 |
VR | PT | Chronic | 60 | 4 weeks (5d/w) | Task-fMRI |
| Hamzei et al. (2006) | CS | Tot = 6 | CIMT | PT | Chronic | 360 | NA | Rs-fMRI |
| Szaflarski et al. (2006) | CS | Tot = 14 Int = 4 Con = 10 |
mCIMT | PT | Chronic | 30 | 10 weeks (3d/w) | Task-fMRI |
| Carey et al. (2007) | RCT | Tot = 20 Group 1 = 10 Group 2 = 10 |
Finger tracking vs simple finger training | TR | Chronic | variable | 10 days | Task-fMRI |
| Dong et al. (2007) | CC | Tot = 16 Int = 4 Con = 12 |
CIMT | OP + home based | Chronic | 360 | 2 weeks | Task-fMRI** |
| Ertelt et al. (2007) | RCT | Tot = 7 Int = 6 Con = 13 |
AOT vs TSP | PT | Chronic | 90 | 3 weeks (5d/w) | Task-fMRI |
| Hamzei et al. (2008) | CS | Tot = 8 | CIMT | PT | Chronic | 180 | 4 weeks (5 d/w) | Task-fMRI |
| Takahashi et al. (2008) | semiRCT | Tot = 13 Group 1 = 6 Group 2 = 7 |
RAT | PT | Chronic | 90 | 3 weeks (5d/w) | Task-fMRI |
| Page et al. (2009) | CS | Tot = 10 | MI + TSP | PT | Chronic | 30 | 10 weeks (3d/w) | Task-fMRI |
| Lin et al. (2010) | RCT |
Tot = 13 Int = 5 Con = 8 |
CIMT | PT | Subacute/chronic | 120 | 3 weeks (5d/w) | Task-fMRI |
| Page et al. (2010) | CS | Tot = 8 | TSP with neuroprosthesis | OP + home based | Chronic | 30 | 8 weeks (5d/w) | Task-fMRI |
| Whitall et al. (2011) | RCT | Tot = 38 Int = 17 Con = 21 |
Bilateral vs monolateral arm training | OP | Chronic | 60 | 6 weeks (3d/w) | Task-fMRI |
| Kononen et al. (2012) | CS | Tot = 11 | CIMT | PT | Chronic | 360 | 3 weeks (5d/w) | Task-fMRI |
| Várkuti et al. (2013) | RCT | Tot = 9 Group 1 = 6 Group 2 = 3 |
MI-BCI vs RAT | PT | Subacute/ chronic |
NA | 4 weeks | rs-fMRI |
| Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019 | RCT | Tot = 32 Int = 16 Con = 16 |
BCI | OP + home-based | Chronic | 120 | 4 weeks | Task-fMRI§ |
| Sun et al. (2013) | RCT | Tot = 38 Int = 9 Con = 9 HS = 20 |
MI + CPT vs CPT | PT | Chronic | 240 | 4 weeks (5d/w) | Task-fMRI |
| Bajaj et al. (2015) | RCT | Tot = 13 Int = 6 Con = 7 |
MI vs MI + PT | PT | Subacute/ chronic |
240 | 3 weeks (5d/w) | Task-fMRI |
| Koganemaru et al. (2015) | CS | Tot = 11 | TMS + movement training | PT | Chronic | 15 | 6 weeks | Task-fMRI§ |
| Zheng et al. (2016) | CS | Tot = 24 Int = 12 Con = 12 |
MI + CPT vs CPT | OP + IP | Chronic | 240 | 4 weeks (5d/w) | Rs-fMRI |
| Saleh et al. (2017) | n-RCT | Tot = 19 Group 1 = 10 Group 2 = 9 |
RAVR vs TSP | PT | Chronic | 180 | 2 weeks (4d/w) | Task-fMRI |
| Wang et al. (2019) | RCT | Tot = 31 Int = 16 Con = 15 |
MI + CPT vs CPT | IP | Subacute/ chronic |
240 | 4 weeks (5d/w) | Rs-fMRI |
| Lower limb rehabilitation | ||||||||
| You et al. (2005) | RCT | Tot = 10 Int = 5 Con = 5 |
VR vs rest | PT | Chronic | 60 | 4 weeks (5d/w) | Task-fMRI |
| Luft et al. (2008) | RCT | Tot = 32 Int = 15 Con = 17 |
Treadmill training vs stretching | PT | Chronic | 40 | 6 months (3d/w) | Task-fMRI |
| Enzinger et al. (2009) | CS | Tot = 18 | Treadmill | PT | Chronic | 45 | 4 weeks (3d/w) | Task-fMRI |
| Deng et al. (2012) | RCT | Tot = 16 Group 1 = 8 Group 2 = 8 |
Complex vs simple ankle training | TR | Chronic | 60 | 20 days | Task -MRI |
| Landsmann et al. (2016) | CS | Tot = 24 Int = 8 Con = 16 |
CPT | PT | Chronic | 90 | 5 weeks (3d/w) | Task-fMRI |
CC-case control; RCT-randomized controlled trial; CS-case series; tot-total INT=intervention; CON-control; HS-healthy subjects; PT-physiotherapist supervised; OP-outpatient; TR-telerehabilitation; IN-inpatient; CPT-conventional physical therapy; AAT-arm ability training; MI-motor imagery; TSP-task specific training; BCI-brain computer interface; CIMT-constraint induced movement therapy; RAT-robot assisted therapy; RAVR-robot assisted virtual reality; TMS-transcranial magnetic stimulation; mins-minutes; rs-fMRI-resting state fMRI; VBM -voxel based morphometry; DTI-diffusion tensor imaging;
§Only in these studies there were 2 follow-ups for MRI
*Imaging was performed only in the intervention group
**In this study, 2 patients performed fMRI only before and after the rehabilitation treatment and 2 other patients had a longitudinal follow-up also 6 and 12 months after the rehabilitation treatment
The study designs were as follows: 20 RCTs (You et al., 2005, Whitall et al., 2011, Luft et al., 2004, Deng et al., 2012, Wu et al., 2019, Várkuti et al., 2013, Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019, Gauthier et al., 2008, Lin et al., 2010, Bajaj et al., 2015, Jang et al., 2005, Liu et al., 2014, Wang et al., 2019, Sun et al., 2013, Dechaumont-Palacin et al., 2008, Kim et al., 2020, Carey et al., 2007, Luft et al., 2008, Ertelt et al., 2007), 23 non-RCTs (1 semi-RCT (Takahashi et al., 2008), 5 case-control studies (CC) (James et al., 2009, Horn et al., 2016, Schaechter et al., 2002, Murayama et al., 2011, Dong et al., 2007), 15 case series (CS) (Johansen-Berg et al., 2002, Enzinger et al., 2009, Könönen et al., 2012, Page et al., 2009, Hamzei et al., 2008, Hamzei et al., 2006, Fan et al., 2015, Page et al., 2010, Szaflarski et al., 2006, Askim et al., 2009, Zheng et al., 2016, Fan et al., 2015, Pinter et al., 2013, Landsmann et al., 2016, Koganemaru et al., 2015), 1 open label study (Yang et al., 2017), 1 not specifying the study design other than non-RCT (Saleh et al., 2017).
With respect to patients’ characteristics, the diagnosis was either subacute (12 studies (James et al., 2009, Wu et al., 2019, Liu et al., 2014, Dechaumont-Palacin et al., 2008, Kim et al., 2020, Horn et al., 2016, Murayama et al., 2011, Fan et al., 2015, Askim et al., 2009, Fan et al., 2015, Pinter et al., 2013, Yang et al., 2017) or chronic (27 studies (You et al., 2005, Whitall et al., 2011, Luft et al., 2004, Deng et al., 2012, Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019, Gauthier et al., 2008, Jang et al., 2005, Sun et al., 2013, Carey et al., 2007, Luft et al., 2008, Ertelt et al., 2007, Takahashi et al., 2008, Schaechter et al., 2002, Dong et al., 2007, Johansen-Berg et al., 2002, Enzinger et al., 2009, Könönen et al., 2012, Page et al., 2009, Hamzei et al., 2008, Hamzei et al., 2006, Page et al., 2010, Szaflarski et al., 2006, Zheng et al., 2016, Landsmann et al., 2016, Koganemaru et al., 2015, Saleh et al., 2017) stroke, or both (4 studies (Várkuti et al., 2013, Lin et al., 2010, Bajaj et al., 2015, Wang et al., 2019), even though the definition of “subacute” and “chronic” varied among studies, with 3 or 6 months as temporal thresholds between the two stages.
With respect to the rehabilitative treatment, the setting was as follows: inpatient (IP, 6 (Wu et al., 2019, Wang et al., 2019, Kim et al., 2020, Horn et al., 2016, Murayama et al., 2011, Askim et al., 2009), outpatient (OP, 1 (Whitall et al., 2011), physiotherapist supervised without further information on the setting (28 (You et al., 2005, James et al., 2009, Luft et al., 2004, Várkuti et al., 2013, Gauthier et al., 2008, Lin et al., 2010, Bajaj et al., 2015, Jang et al., 2005, Liu et al., 2014, Sun et al., 2013, Dechaumont-Palacin et al., 2008, Luft et al., 2008, Ertelt et al., 2007, Takahashi et al., 2008, Schaechter et al., 2002, Enzinger et al., 2009, Könönen et al., 2012, Page et al., 2009, Hamzei et al., 2008, Hamzei et al., 2006, Fan et al., 2015, Szaflarski et al., 2006, Fan et al., 2015, Pinter et al., 2013, Landsmann et al., 2016, Koganemaru et al., 2015, Yang et al., 2017, Saleh et al., 2017), home-based (1 (Johansen-Berg et al., 2002), mixed (OP + home-based 4 (Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019, Dong et al., 2007, Page et al., 2010), OP + IP 1 (Zheng et al., 2016), telerehabilitation (TR, 2 (Deng et al., 2012, Carey et al., 2007). The mean duration of each rehabilitative session was 130.6 min (standard deviation 15–360 min), with constraint induced motor therapy (CIMT) sessions being the longest. The weekly frequency of the physiotherapy sessions ranged from 3 to 5 days/week, and the total duration of the rehabilitative cycle ranged from 10 days to 6 months.
MRI was always acquired both before and at the end of the rehabilitative cycle, whereas in 6 studies there was also a second MRI timepoint, either within the cycle (Kim et al., 2020) or after 2 weeks (Koganemaru et al., 2015), 3 months (Murayama et al., 2011), 4 months (Whitall et al., 2011), or 6 months (Ramos-Murguialday et al., 2019, Schaechter et al., 2002). Among the 43 selected studies, 4 applied structural MRI (3 diffusion tensor imaging (DTI) (Kim et al., 2020, Fan et al., 2015, Yang et al., 2017), 1 voxel-based morphometry (VBM (Gauthier et al., 2008), whereas the remaining 39 performed functional MRI (fMRI) (32 task-related fMRI (You et al., 2005, Whitall et al., 2011, Luft et al., 2004, Deng et al., 2012, Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019, Lin et al., 2010, Bajaj et al., 2015, Jang et al., 2005, Liu et al., 2014, Sun et al., 2013, Dechaumont-Palacin et al., 2008, Carey et al., 2007, Luft et al., 2008, Ertelt et al., 2007, Takahashi et al., 2008, Horn et al., 2016, Schaechter et al., 2002, Murayama et al., 2011, Dong et al., 2007, Johansen-Berg et al., 2002, Enzinger et al., 2009, Könönen et al., 2012, Page et al., 2009, Hamzei et al., 2008, Page et al., 2010, Szaflarski et al., 2006, Askim et al., 2009, Pinter et al., 2013, Landsmann et al., 2016, Koganemaru et al., 2015, Saleh et al., 2017), 7 resting state-fMRI (James et al., 2009, Wu et al., 2019, Várkuti et al., 2013, Wang et al., 2019, Hamzei et al., 2006, Zheng et al., 2016, Fan et al., 2015).
4. Discussion
In this review, the search for studies that incorporated MRI markers to analyze FC/tissue microstructure in stroke patients and monitor their evolution in response to rehabilitation led to 43 results, here discussed based on the type of MRI connectivity markers (Table 3, Table 4).
Table 3.
Main structural MRI results post-rehabilitation treatment.
| Authors | Disease phenotype | Imaging technique | Results |
|---|---|---|---|
| Upper limb rehabilitation | |||
| Fan et al. (2015) | Subacute | DTI | ↑FA ilCST, transcallosal motor fibers |
| Gauthier et al. (2008) | Chronic | VBM | ↑volume bl SMC, SMA and hippocampus |
| Lower limb rehabilitation | |||
| (Yang et al., 2017) | Subacute | DTI | ↑FA ilposterior cingulate cortex ↓FA ilinternal capsula, pediculopontine nucleus and substantia nigra ↑FA clsupramarginal gyrus and SMA |
| Kim et al. (2020) | Subacute | DTI | ↑FA cl superior temporal, cingulate and postcentral giri |
VBM-voxel based morphometry; DTI-diffusion tensor imaging; FA-fractional anisotropy; bl-bilateral; il-ipsilesional; cl-contralesional; SMC-sensorimotor cortex; SMA-supplemental motor area; CST-corticospinal tract.
Table 4.
Main functional MRI results post-rehabilitation treatment.
| Authors | Disease phenotype | Imaging | Results |
|---|---|---|---|
| Upper limb rehabilitation | |||
| Dechaumont-Palacin et al. (2008) | Subacute | Task fMRI | ↑ cl SMA, PMC, S2 |
| Askim et al. (2009) | Subacute | Task-fMRI | ↑il S1M1, bl S1, cl S2 |
| James et al. (2009) | Subacute | Rs-fMRI | ↑ EC il PMC → cl PMC |
| Murayama et al. (2011) | Subacute | Task-fMRI§ | ↑ il S1M1, cerebellum |
| Pinter et al. (2013) | Subacute | Task-fMRI | NS |
| Liu et al. (2014) | Subacute | Task-fMRI | ↑ il S1, cerebellum; ↓ cl M1 |
| Fan et al. (2015) | Subacute | Rs-fMRI | ↑ FC M1-M1 |
| Horn et al. (2016) | Subacute | Task-fMRI | ↑il PMC |
| Wu et al. (2019) | Subacute | Rs-fMRI* | ↑ diffuse bilateral activation |
| Johansen-Berg et al. (2002) | Chronic | Task-MRI | ↑ il PMC, S2 |
| Schaechter et al. (2002) | Chronic | Task-fMRI§ | ↑ cl PMC, SMA, M1 |
| Luft et al. (2004) | Chronic | Task-MRI | ↑ cl S1M1 |
| Hamzei et al. (2006) | Chronic | Rs-fMRI | Δ il S1M1* |
| Jang et al. (2005) | Chronic | Task-fMRI | ↑il S1M1 |
| Szaflarski et al. (2006) | Chronic | Task-fMRI | NS at a group level |
| Carey et al. (2007) | Chronic | Task-fMRI | NS at a group level |
| Dong et al. (2007) | Chronic | Task-fMRI** | ↓ il M1, cerebellum |
| Ertelt et al. (2007) | Chronic | Task-fMRI | ↑ bl ventral PMC, SMA, STG, IP areas |
| Hamzei et al. (2008) | Chronic | Task-fMRI | Δ il S1M1* |
| Takahashi et al. (2008) | Chronic | Task-fMRI | ↑ il S1M1 |
| Page et al. (2009) | Chronic | Task-fMRI | ↑ il PMC, M1, cl PMC, M1 |
| Lin et al. (2010) | Subacute / chronic | Task-fMRI | ↑ il PMC |
| Page et al. (2010) | Chronic | Task-fMRI | ↑ cl S1M1, IPL |
| Whitall et al. (2011) | Chronic | Task-fMRI | ↑il S1M1, SMA |
| Könönen et al. (2012) | Chronic | Task-fMRI | ↑ il S1M1 |
| Várkuti et al. (2013) | Subacute / chronic | Rs-fMRI | ↑ FC M1-M1, SMA, cerebellum |
| (Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019) | Chronic | Task-fMRI§ | ↑ il PMC, M1 |
| Sun et al. (2013) | Chronic | Task-fMRI | ↑il S1M1 (few pts showed ↓ il S1M1) |
| Bajaj et al. (2015) | Subacute / chronic | Task-fMRI | ↑ FC M1-SMA |
| Koganemaru et al. (2015) | Chronic | Task-fMRI§ | ↓ il S1M1, cl PMC |
| Zheng et al. (2016) | Chronic | Rs-fMRI | ↑ FC M1-M1, il M1- cl mSFG |
| Saleh et al. (2017) | Chronic | Task-fMRI | ↓ il S1M1 |
| Lower limb rehabilitation | |||
| You et al. (2005) | Chronic | Task-fMRI | ↑cl motor network |
| Luft et al. (2008) | Chronic | Task-fMRI | ↑ cerebellum, midbrain, cl IPL, S1, SFG |
| Enzinger et al. (2009) | Chronic | Task-fMRI | ↑ bl S1M1, il thalamus |
| Deng et al. (2012) | Chronic | Task -MRI | NS |
| Landsmann et al. (2016) | Chronic | Task-fMRI | ↑il M1, bl SFG |
il-ipsilesional; cl-contralesional; PMC-premotor cortex; SMA-supplemental motor area; M1-primary motor cortex, S1-primary somatosensory cortex; S2-secondary somatosensory cortex; SMC-somatosensory cortex; FC-functional connectivity; EC-effective connectivity; NS-not significant; pts-patients; IP-inferior parietal; IPL-inferior parietal lobule; msFG-medial superior frontal gyrus; STG-superior temporal gyrus.
*In this study, the directionality of ipsilesional SMC activation changed depending on the integrity of the corticospinal tract and M1 (when damaged, there was an increase of SMC activation).
In the present table results are reported only for main functional areas with ↑ indicating increased activation and ↓ indicating decreased activation. If the study design is a randomized clinical trial, results are described only for the active intervention group.
4.1. Tissue microstructure
Only 4 out of 43 studies applied structural MRI imaging (Gauthier et al., 2008, Kim et al., 2020, Fan et al., 2015, Yang et al., 2017). The paucity of structural data might be related to the short study duration, intrinsic to the nature of the studies itself, temporally linked to the few weeks of a rehabilitative cycle. Structural brain modifications have been described in response to motor tasks in healthy subjects, involving both GM and WM (Draganski et al., 2004, Scholz et al., 2009, Taubert et al., 2010), with plenty of histological data confirming it (Xu et al., 2009, Sampaio-Baptista et al., 2018). However, to detect such changes occurring over a brief period, in a population of older people in whom brain tissue is partially compromised by the ischemic insult, might present some challenges. Overall, the analyzed studies showed structural changes in treated patients (Gauthier et al., 2008, Kim et al., 2020, Fan et al., 2015, Yang et al., 2017), even though only 2 studies had a control group (Gauthier et al., 2008, Kim et al., 2020). Finally, future studies might benefit by utilizing graph theory-based analysis to better characterize structural connectivity, rather than just investigating tissue microstructure.
4.2. Lower limb rehabilitation
The 2 robot-assisted gait training studies showed higher fractional anisotropy (FA), a marker of tissue integrity, in the contralesional sensorimotor cortex (SMC) paralleled by improved locomotor function (Kim et al., 2020, Yang et al., 2017). Some evidence shows that increased functional and/or structural FC/SC of the contralesional hemisphere correlate with poorer recovery, as it might indicate that the ipsilesional hemisphere is beyond recovery for intensity and extent of damage (Enzinger et al., 2009, Cramer et al., 2007). On the contrary, gait function seems to be controlled by both hemispheres (MacKay-Lyons, 2002), and as such the dominance of the contralesional hemisphere might just represent a positive adaptive change in which functions of lesioned hemisphere are compensated by the activation of previously quiescent areas of the contralateral one.
4.3. Upper limb rehabilitation
The 2 studies that focused on the upper limb reported better clinical outcomes after rehabilitation, paralleled by higher FA in the ipsilesional cortico-spinal tract (CST) as well as in the transcallosal motor fibers in the bilateral arm training study (Fan et al., 2015), and increased GM volume in bilateral SMC and hippocampus in the constraint-induced (CI) therapy study (Gauthier et al., 2008). Interestingly, the former study performed on subacute stroke patients, showed an initial reduction of FA of both ipsilesional CST and transcallosal M1-M1 fibers in the early post-stroke phase (Kim et al., 2020).
Taken together, these results suggest that the clinical improvement of the affected limb/function is related to the restoration of an interhemispheric balance, represented by functional recovery of the ipsilesional hemisphere and activation of compensatory areas of the contralesional one.
4.4. Functional connectivity
Thirty-nine studies described FC changes after rehabilitation. Functional changes within different brain areas happen constantly as adaptive mechanisms to different stimuli or environmental situations. The abrupt onset of an imbalance between energy demand and blood supply caused by stroke leads to a functional rearrangement, with the aim to compensate for the damaged tissue, and often involves different brain areas in both hemispheres. The functional response to rehabilitation is complex and depends upon several factors, ranging from the lesion location, to the damage extent and also to the specific rehabilitation approach.
4.5. Lower limb rehabilitation
Among the 39 fMRI studies, only 5 were performed on lower limb-related rehabilitation (You et al., 2005, Deng et al., 2012, Luft et al., 2008, Enzinger et al., 2009, Landsmann et al., 2016). This might be due to technical challenges related to the motor task during MRI acquisition and to the abovementioned complex bihemispheric control on lower limb movements and gait function that would render challenging interpretation of results. Overall, these studies seem to confirm the previously mentioned structural data, showing both an increased activation of bilateral cortical areas and a functional recovery of the ipsilesional one, when possible, paralleling the clinical improvement of locomotor function (You et al., 2005, Deng et al., 2012, Enzinger et al., 2009, Landsmann et al., 2016). Notably, 2 studies highlighted the prominent role of brain structures, such as thalamus (Enzinger et al., 2009), cerebellum and midbrain (Luft et al., 2008), in gait function recovery. Indeed, increased activation of these structures was correlated with walking speed (Luft et al., 2008, Enzinger et al., 2009) and endurance (Enzinger et al., 2009) although it was only a statistical trend with respect to the thalamus.
4.6. Upper limb rehabilitation
Functional MRI changes in response to upper limb rehabilitation are very variable, ranging from an increased activation of ipsilesional sensorimotor areas (Whitall et al., 2011, Ramos-Murguialday et al., 2013, Jang et al., 2005, Sun et al., 2013, Dechaumont-Palacin et al., 2008, Takahashi et al., 2008, Dong et al., 2007, Johansen-Berg et al., 2002, Askim et al., 2009, Pinter et al., 2013, Saleh et al., 2017) or contralesional areas of the unaffected hemisphere (Luft et al., 2004, Lin et al., 2010, Schaechter et al., 2002, Page et al., 2010) or both (Ertelt et al., 2007, Page et al., 2009, Fan et al., 2015). Moreover, some studies reported a reduced activation of the ipsilesional hemisphere in the post-rehabilitation phase, usually associated with clinical improvement and as such interpreted as more efficient and focused activation of the interested areas (Sun et al., 2013, Zheng et al., 2016, Koganemaru et al., 2015, Ward et al., 2003).
An univocal interpretation of these heterogenous and apparently contradictory result is difficult, partly because many variables need to be taken into consideration, such as the individual disability level, the type of rehabilitative approach, the extent of tissue damage and the involvement of cortical and/or subcortical regions.
The type of rehabilitative approach adopted in the studies has a crucial role, determining what brain areas are functionally activated and potentially reorganized. In general, CIMT is associated with activation of SMC (Gauthier et al., 2008, Lin et al., 2010, Schaechter et al., 2002, Dong et al., 2007, Johansen-Berg et al., 2002, Hamzei et al., 2008, Hamzei et al., 2006, Szaflarski et al., 2006) and cerebellum (Murayama et al., 2011), bilateral limb training seems to facilitate the activation of bilateral areas (Whitall et al., 2011, Luft et al., 2004), action-observation therapy leads to an increased mirror neuron system (ventral PMC, inferior parietal areas)activation (Ertelt et al., 2007), passive proprioceptive training triggers the activation of secondary somatosensory areas (Dechaumont-Palacin et al., 2008), rehabilitation approaches using virtual reality, robotic therapy or brain-computer interface induce often the activation not only of motor areas but also of visuomotor or associative ones (Wu et al., 2019, Várkuti et al., 2013, Ramos-Murguialday et al., 2013, Ramos-Murguialday et al., 2019, Fan et al., 2015).
These latter rehabilitative treatments are not only associated with a widespread activation of different functional brain areas, but also to a more significant clinical improvement, when directly compared to other rehabilitative approaches (You et al., 2005, Wu et al., 2019, Takahashi et al., 2008, Saleh et al., 2017). Therefore, more cognitively challenging tasks, such as the ones involving the visuo-motor loop, in some patients promote neuroplastic changes more effectively, through the recruitment of motor as well as other areas involved in different functions such as motor learning, executive and visuospatial functions, as previously hypothesized (Gauthier et al., 2008, Landsmann et al., 2016, Pascual-Leone et al., 1995, Carey et al., 2005). Some studies have also tested the relationship between FC and the impact of MI, or mental practice, intended as the cognitive rehearsal of simple movements or more complex activities, usually part of the daily life activities (Bajaj et al., 2015, Liu et al., 2014, Wang et al., 2019, Sun et al., 2013, Page et al., 2009). Whereas a recent Cochrane review has reported no evidence of efficacy of such a rehabilitative approach (Silva et al., 2020), some studies show that it has an impact of brain reorganization, and seems to reinforce the effect of physical therapy (Bajaj et al., 2015, Liu et al., 2014).
The integrity of structures anatomically related to stroke areas is also relevant in the process of brain reorganization, as demonstrated by 2 studies on the relationship between ipsilesional SMC and the pyramidal tract (primary motor cortex and CST) (Hamzei et al., 2008, Hamzei et al., 2006). The integrity of the pyramidal tract is associated with a reduced, more focused activation of the ipsilesional SMC, likely due to increased synaptic efficiency. Conversely, damage to the former structure results in increased activation of the latter one, as a higher efficiency and number of activated neurons is necessary to maintain the same level of motor performance. Moreover, several studies have also described functional changes of thalamus (Askim et al., 2009, Fan et al., 2015) and cerebellum (Várkuti et al., 2013, Liu et al., 2014, Murayama et al., 2011, Askim et al., 2009, Fan et al., 2015) associated with functional gain of the affected body segment, as the first is the main relay center of cortico-subcortical pathways and the second plays a key role in motor learning and sensorimotor input/outputs integration.
Interestingly, few studies have analyzed not only FC (i.e., the strength of connectivity between regions) (Fan et al., 2015) but also effective connectivity (James et al., 2009, Saleh et al., 2017), intended as the directionality of functional interaction between brain regions. In both studies, rehabilitation strengthened the connectivity of the ipsilesional hemisphere, enhancing its influence on the contralateral one (James et al., 2009), as well as the influence of the ipsilesional primary somatosensory cortex on primary motor cortex (Saleh et al., 2017). In both studies, the rerouted connectivity was significantly associated with a clinical improvement.
Another important yet unanswered question concerns whether the clinical, structural and functional changes gained during rehabilitation last over time or fade away in the subsequent period. This is a relevant issue, as it allows to define whether the skills acquired during rehabilitation, as well as the functional and structural brain reorganization, are just a mere response to the intensive physical activity or they are consolidated acquisitions that the patient will be able to exploit in a real-life setting. Only 4 studies have included longer follow-ups and MRI results are quite heterogenous, ranging from a more focused activation of the ipsilesional motor cortex paralleling the functional gain (Dong et al., 2007), to maintenance of the gains observed immediately after training (Murayama et al., 2011), to a partial (Schaechter et al., 2002) or complete loss of the functional changes obtained with rehabilitation (Ramos-Murguialday et al., 2019).
Altogether, the findings of all the aforementioned studies suggest that although neuroplastic changes occur spontaneously after stroke, with the aim to functionally compensate the damaged area, rehabilitation has a crucial role in promoting and addressing functional and structural reorganization. The overall impact of rehabilitation on brain connectivity seems to be positive, reducing the risk of maladaptive changes and facilitating the recovery of an interhemispheric balance. This is usually reflected by a better quantitative and/or qualitative activation of the ipsilesional hemisphere, when possible, sometimes associated to the activation of other compensatory, functionally and anatomically related brain regions. Unfortunately though, the available data do not support a prolonged effect of rehabilitation over time, but further larger studies are needed to elucidate this question.
Notably, all the studies here analyzed underline the paramount importance of MRI markers in monitoring structural and functional brain response to rehabilitation and allowing to understand the pathophysiological mechanisms underlying neuroplasticity.
4.7. Adaptive vs maladaptive plasticity
As previously stated, the main aim of neuromotor rehabilitation is to promote functional recovery, and all the studies included in the current review describe a causal relationship between clinical improvement obtained through physiotherapy and pro-adaptive plastic brain reorganization. Maladaptive plasticity refers to aberrant brain changes resulting in limited clinical recovery and appearance of abnormal compensatory motor patterns (Jang and Gordon, 2013). There is some evidence of a relationship between poorer clinical outcome and a more widespread cortical activation (Lee et al., 2009), or a higher recruitment of the contralesional hemisphere (Schwerin et al., 2008, Calautti et al., 2007), leading to define these two latter compensatory mechanisms as “maladaptive”. Some studies indirectly support this theory, associating the improvement of clinical outcomes with reduced cortical activation, interpreted as increased neuronal efficiency (Sun et al., 2013, Dong et al., 2007, Zheng et al., 2016, Koganemaru et al., 2015, Ward et al., 2003). However, some other studies report an association between contralesional activation and clinical recovery (Luft et al., 2004, Lin et al., 2010, Schaechter et al., 2002, Page et al., 2010). This apparent discrepancy can be explained considering that the border between adaptive and maladaptive plasticity is thin and different pathophysiological mechanisms within the brain might underlie the same clinical symptom. Indeed, the lesion location and extent, as well as the degree of involvement of other components of the ipsilesional motor pathways are key factors addressing the neuroplastic changes able to effectively favor the functional recovery.
As a consequence, an accurate MRI assessment of patients eligible for neurorehabilitation with a functional and structural characterization of the lesion and the connected brain areas, can add valuable information and facilitate the choice of the most appropriate rehabilitation treatment.
4.8. Methodological considerations
Some methodological observations have to be considered, when interpreting studies results (Table 5). In general, the studies are difficult to compare because of an extreme heterogeneity in patient demographics, lesion size/location, time since stroke (even the temporal thresholds used to define “chronic” and “subacute” differ across studies), clinical scales applied to measure disability, rehabilitation duration and frequency, fMRI approach (region of interest vs global analysis), task. This aspect, together with the small sample size of most studies, renders it difficult to extrapolate and adapt results to the clinical practice, especially at an individual level.
Table 5.
Key points to improve interpretation and reproducibility of MRI results in future studies.
| Homogeneity of patient characteristics | Timing since stroke (subacute/chronic) |
| Stroke etiology (hemorrhagic/ischemic) | |
| Control group | Comparable exposure time to physical activity |
| MRI | Structural and functional characterization of lesion location (cortical/subcortical) ipsilesional motor pathway other brain regions anatomically and functionally related to stroke site Long term follow-up |
| Task-related fMRI | Standardization of task frequency and amplitude |
| Possible other confounding factors (head movements, mirror movements) |
4.9. Timing of rehabilitation treatment
The timing of the rehabilitative intervention with respect to stroke onset is highly relevant for different reasons. The brain undergoes spontaneous changes at a cellular and tissue level after stroke. Experimental and clinical evidence describes different reparative mechanisms including synaptogenesis, neurogenesis, neuroaxonal growth, angiogenesis, and rerouting of surviving networks (Alia et al., 2017, Dąbrowski et al., 2019). This network rewiring involves both hemispheres: on one hand, the small part of ipsilesional motor pathways residing in the contralesional hemisphere becomes active (Caramia et al., 2000), and on the other, the inhibitory influence of the lesioned hemisphere on the contralateral is significantly less effective (Askim et al., 2009, Vallone et al., 2016). Therefore, the contralesional hemisphere plays a dominant role in the first post-stroke phase, and the amount of its activation depends on lesion location and extension. However, whether the hyperactivation of contralesional brain areas is beneficial or detrimental is still controversial, as some studies associate it with clinical recovery (Lin et al., 2010, Schaechter et al., 2002, Page et al., 2010), others with maladaptive plasticity and a worse prognosis (Enzinger et al., 2008), and others have reported a positive relationship between lateralized activation towards the ipsilesional hemisphere and clinical improvement (Loubinoux et al., 2007). Environmental stimuli, such as life-related activities and rehabilitation, heavily influence brain reorganization, and consequently the timing of rehabilitative intervention is an important issue. Some animal studies have shown that earlier rehabilitation intervention is related to a greater extent of functional recovery, but whether this is applicable to humans remains a matter of debate (Lotze et al., 2019). Whereas it might be difficult to precisely quantify the contribution of rehabilitation in brain reorganization in the subacute stroke patients, as its effects overlap the spontaneous mechanisms of recovery, the potential efficacy of rehabilitative treatments might also be greater, as neuroplastic changes are still ongoing and more easily addressable than in the chronic phase.
4.10. Rehabilitation setting
Another aspect that needs to be taken into consideration concerns the rehabilitation setting. Only few studies have been carried out during hospitalization (Wu et al., 2019, Wang et al., 2019, Kim et al., 2020, Horn et al., 2016, Murayama et al., 2011, Askim et al., 2009, Zheng et al., 2016), whereas most of them have been performed in an outpatient setting, which is more economically sustainable and feasible. However, the results of these latter studies might be influenced by the global level of the patients’ physical activity that depends on disability, social/familial environment, working situation and educational level. Moreover, very frequently outpatient rehabilitation requires access to the rehabilitation center 3–5 times a week, with a possible selection bias of patients that have either lower disability or higher familial support. However, results derived by the few studies exploring the effects of home-based physiotherapy and telerehabilitation are generally positive and in support of it (Deng et al., 2012, Ramos-Murguialday et al., 2013, Johansen-Berg et al., 2002, Page et al., 2009). Considering the current widespread accessibility of technological devices and the significant reduction of related economic costs, telerehabilitation might be more widely exploited in the future, as a complementary approach able to strengthen the results obtained with therapist-supervised treatments.
4.11. Control group
Another possible confounding factor concerns the type of treatment performed by the control group. Sometimes, the control group is either “passive” (You et al., 2005, Jang et al., 2005) or receive the same kind of “conventional treatment” than the intervention group, but the latter is also performing the experimental activity (Gauthier et al., 2008, Bajaj et al., 2015, Liu et al., 2014, Kim et al., 2020). If this translates into more exposure time for the treatment group, the results might reflect the higher level of physical activity/motor stimulus instead of being specifically related to motor skills acquired during the rehabilitative treatment.
4.12. MRi
The last aspect worth discussing is related to the imaging techniques. First, structural data derived from diffusion imaging markers applied to GM, as well as functional data applied to cerebellum and brainstem, might need to be interpreted cautiously due to technical challenges of the MRI techniques in those specific brain regions (Sclocco et al., 2018). Second, motor tasks during fMRI acquisitions might present some issues related to the potential presence of head movements, mirror movements and the variability of motor task performance in terms of amplitude and frequency. Most of the studies describe effective ways to eliminate or control for these possible confounding factors. Resting state fMRI overcome task-related challenges as the use of a specific stimulus is not required and the patient simply remains still while being imaged.
4.13. Methodological considerations and bias assessment
Quantitative MRI typically requires a fair amount of post-processing to go from the original acquisition as it comes from the scanner to the final result. This naturally calls into question to what extent these measures are reproducible. One way to assess this is via scan-rescan experiments or by assessing how similar measures are across different scanning platforms. In this case, it has been shown in healthy individuals that measures of functional connectivity and DTI-derived indices are in fact reproducible (Huang et al., 2012, Prohl et al., 2019). However, this is typically the case only under carefully controlled conditions where acquisitions are kept homogenous and the post-processing is performed in the same manner. The fact that vastly different results can be obtained based on exactly how the post-processing was performed represents a potential source of bias in the studies that have been included in this systematic review of the literature. For example, several different software packages can be utilized, which in turn also allow for different processing options. It is often the case that authors do not extensively describe exactly what was done when describing the methodology utilized in a given study. Overall, these aspects render direct comparisons of data between studies quite difficult. One possible way to remediate this problem in the future is by encouraging authors to register their exact analysis plans ahead of time. Not only will this provide an accurate record of what will be done, it will also avoid the possibility of authors trying to analyze their data in many different ways and only picking the one that shows the “best” results. Although several of the RCTs included in our review were registered ahead of time (e.g., on clinicaltrials.gov), this is still not necessarily sufficient to overcome potential risks of bias as the detailed analysis plans were not also registered. As such, there is an unknown level of bias in nearly all of the studies that have been included in the current review.
5. Conclusions
In conclusion, all the studies analyzed in this review have provided useful information on the impact of different rehabilitation approaches on SC and FC in subacute and chronic stroke patients. The overall evidence suggests that rehabilitation-induced clinical improvement is paralleled by brain reorganization tending towards the recovery of an interhemispheric balance and of ipsilesional hemisphere activity, together with the activation of functionally related contralateral areas. Future clinical trials on the effects of neuromotor rehabilitation might be improved by the inclusion of more homogeneous populations, as well as MRI measures analyzing structural integrity of areas that are not directly damaged by the stroke but that are part of the motor pathways. Structural and functional MRI markers can be useful in capturing brain changes in response to rehabilitation, giving reliable and reproducible quantitative information useful to monitor treatment efficacy. However, there is still a clear gap in translating findings from group-based studies to the individual patient. In this regard, future work is needed to properly validate MRI markers derived from either functional connectivity and/or structural properties. It will only then be possible to incorporate MRI markers from rehabilitation-related research into a clinical setting. The advent of such studies that can better inform on neuroplasticity might then subsequently help lead to tailored rehabilitation strategies according to individual patient’s level of physical disability and entity of brain tissue damage. In turn, it could then potentially be possible to identify those patients that are more suitable for specific rehabilitative approaches.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Italian Ministry of Health (“Ricerca Corrente Program”). Eleonora Tavazzi disclosed receipt of the following financial support by Fondazione Crespi Spano.
References
- Alia C., Spalletti C., Lai S., Panarese A., Lamola G., Bertolucci F., Vallone F., Di Garbo A., Chisari C., Micera S., Caleo M. Neuroplastic Changes Following Brain Ischemia and their Contribution to Stroke Recovery: Novel Approaches in Neurorehabilitation. Front Cell Neurosci. 2017;11 doi: 10.3389/fncel.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askim T., Indredavik B., Vangberg T., Håberg A. Motor network changes associated with successful motor skill relearning after acute ischemic stroke: a longitudinal functional magnetic resonance imaging study. Neurorehabil Neural Repair. 2009;23(3):295–304. doi: 10.1177/1545968308322840. [DOI] [PubMed] [Google Scholar]
- Bajaj S., Butler A.J., Drake D., Dhamala M. Brain effective connectivity during motor-imagery and execution following stroke and rehabilitation. Neuroimage Clin. 2015;8:572–582. doi: 10.1016/j.nicl.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C., Naccarato M., Jones P.S., Sharma N., Day D.D., Carpenter A.T., Bullmore E.T., Warburton E.A., Baron J.-C. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34(1):322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Caramia M.D., Palmieri M.G., Giacomini P., Iani C., Dally L., Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol. 2000;111(11):1990–1996. doi: 10.1016/s1388-2457(00)00430-2. [DOI] [PubMed] [Google Scholar]
- Carey J.R., Bhatt E., Nagpal A. Neuroplasticity promoted by task complexity. Exerc Sport Sci Rev. 2005;33(1):24–31. [PubMed] [Google Scholar]
- Carey J.R., Durfee W.K., Bhatt E., Nagpal A., Weinstein S.A., Anderson K.M., Lewis S.M. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehabil Neural Repair. 2007;21(3):216–232. doi: 10.1177/1545968306292381. [DOI] [PubMed] [Google Scholar]
- Collaborators GBDN Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDS Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S.C., Parrish T.B., Levy R.M., Stebbins G.T., Ruland S.D., Lowry D.W., Trouard T.P., Squire S.W., Weinand M.E., Savage C.R., Wilkinson S.B., Juranek J., Leu S.-Y., Himes D.M. Predicting functional gains in a stroke trial. Stroke. 2007;38(7):2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- Dąbrowski J., Czajka A., Zielińska-Turek J., Jaroszyński J., Furtak-Niczyporuk M., Mela A., Poniatowski Ł.A., Drop B., Dorobek M., Barcikowska-Kotowicz M., Ziemba A. Brain Functional Reserve in the Context of Neuroplasticity after Stroke. Neural Plast. 2019;2019:1–10. doi: 10.1155/2019/9708905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechaumont-Palacin S., Marque P., De Boissezon X., Castel-Lacanal E., Carel C., Berry I., Pastor J., Albucher J.F., Chollet F., Loubinoux I. Neural correlates of proprioceptive integration in the contralesional hemisphere of very impaired patients shortly after a subcortical stroke: an FMRI study. Neurorehabil Neural Repair. 2008;22(2):154–165. doi: 10.1177/1545968307307118. [DOI] [PubMed] [Google Scholar]
- Deng H, Durfee WK, Nuckley DJ, et al. Complex versus simple ankle movement training in stroke using telerehabilitation: a randomized controlled trial. Phys Ther. 2012;92(2):197-209. [DOI] [PMC free article] [PubMed]
- Dimyan M.A., Cohen L.G. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7(2):76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Winstein C.J., Albistegui-DuBois R., Dobkin B.H. Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21(5):412–428. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Enzinger C., Johansen-Berg H., Dawes H., Bogdanovic M., Collett J., Guy C., Ropele S., Kischka U., Wade D., Fazekas F., Matthews P.M. Functional MRI correlates of lower limb function in stroke victims with gait impairment. Stroke. 2008;39(5):1507–1513. doi: 10.1161/STROKEAHA.107.501999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger C., Dawes H., Johansen-Berg H., Wade D., Bogdanovic M., Collett J., Guy C., Kischka U., Ropele S., Fazekas F., Matthews P.M. Brain activity changes associated with treadmill training after stroke. Stroke. 2009;40(7):2460–2467. doi: 10.1161/STROKEAHA.109.550053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt D., Small S., Solodkin A., Dettmers C., McNamara A., Binkofski F., Buccino G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36:T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Fan Y.-T., Lin K.-C., Liu H.-L., Chen Y.-L., Wu C.-y. Changes in structural integrity are correlated with motor and functional recovery after post-stroke rehabilitation. Restor Neurol Neurosci. 2015;33(6):835–844. doi: 10.3233/RNN-150523. [DOI] [PubMed] [Google Scholar]
- Fan Y.T., Wu C.Y., Liu H.L., Lin K.C., Wai Y.Y., Chen Y.L. Neuroplastic changes in resting-state functional connectivity after stroke rehabilitation. Front Hum Neurosci. 2015;9:546. doi: 10.3389/fnhum.2015.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L.V., Taub E., Perkins C., Ortmann M., Mark V.W., Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39(5):1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F., Liepert J., Dettmers C., Weiller C., Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31(2):710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Hamzei F., Dettmers C., Rijntjes M., Weiller C. The effect of cortico-spinal tract damage on primary sensorimotor cortex activation after rehabilitation therapy. Exp Brain Res. 2008;190(3):329–336. doi: 10.1007/s00221-008-1474-x. [DOI] [PubMed] [Google Scholar]
- Horn U., Roschka S., Eyme K., Walz A.-D., Platz T., Lotze M. Increased ventral premotor cortex recruitment after arm training in an fMRI study with subacute stroke patients. Behav Brain Res. 2016;308:152–159. doi: 10.1016/j.bbr.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Huang L., Wang X., Baliki M.N., Wang L., Apkarian A.V., Parrish T.B., Zuo X.-N. Reproducibility of structural, resting-state BOLD and DTI data between identical scanners. PLoS One. 2012;7(10):e47684. doi: 10.1371/journal.pone.0047684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G., Lu Z.-L., VanMeter J.W., Sathian K., Hu X.P., Butler A.J. Changes in resting state effective connectivity in the motor network following rehabilitation of upper extremity poststroke paresis. Top Stroke Rehabil. 2009;16(4):270–281. doi: 10.1310/tsr1604-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.H., Gordon W.A. Motor function-related maladaptive plasticity in stroke: a review. NeuroRehabilitation. 2013;32(2):311–316. doi: 10.3233/NRE-130849. [DOI] [PubMed] [Google Scholar]
- Jang S.H., You S.H., Hallett M., Cho Y.W., Park C.-M., Cho S.-H., Lee H.-Y., Kim T.-H. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: an experimenter-blind preliminary study. Arch Phys Med Rehabil. 2005;86(11):2218–2223. doi: 10.1016/j.apmr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Dawes H., Guy C., Smith S.M., Wade D.T., Matthews P.M. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125(Pt 12):2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Kang C.S., Kyeong S. Robot-assisted gait training promotes brain reorganization after stroke: A randomized controlled pilot study. NeuroRehabilitation. 2020;46(4):483–489. doi: 10.3233/NRE-203054. [DOI] [PubMed] [Google Scholar]
- Koganemaru S., Sawamoto N., Aso T., Sagara A., Ikkaku T., Shimada K., Kanematsu M., Takahashi R., Domen K., Fukuyama H., Mima T. Task-specific brain reorganization in motor recovery induced by a hybrid-rehabilitation combining training with brain stimulation after stroke. Neurosci Res. 2015;92:29–38. doi: 10.1016/j.neures.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Kononen M, Tarkka IM, Niskanen E, et al. Functional MRI and motor behavioral changes obtained with constraint-induced movement therapy in chronic stroke. Eur J Neurol. 2012;19(4):578-586. [DOI] [PubMed]
- Landsmann B., Pinter D., Pirker E., et al. An exploratory intervention study suggests clinical benefits of training in chronic stroke to be paralleled by changes in brain activity using repeated fMRI. Clin Interv Aging. 2016;11:97–103. doi: 10.2147/CIA.S95632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.Y., Park J.W., Park R.J., Hong J.H., Son S.M., Ahn S.H., Cho Y.W., Jang S.H. Cortical activation pattern of compensatory movement in stroke patients. NeuroRehabilitation. 2009;25(4):255–260. doi: 10.3233/NRE-2009-0523. [DOI] [PubMed] [Google Scholar]
- Levard D., Buendia I., Lanquetin A., Glavan M., Vivien D., Rubio M. Filling the gaps on stroke research: Focus on inflammation and immunity. Brain Behav Immun. 2021;91:649–667. doi: 10.1016/j.bbi.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KC, Chung HY, Wu CY, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehabil. 2010;89(3):177-185. [DOI] [PubMed]
- Liu F., Cheng X.i., Zhong S., Liu C., Jolkkonen J., Zhang X., Liang Y., Liu Z., Zhao C. Communications Between Peripheral and the Brain-Resident Immune System in Neuronal Regeneration After Stroke. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Song L., Zhang T. Changes in brain activation in stroke patients after mental practice and physical exercise: a functional MRI study. Neural Regen Res. 2014;9(15):1474–1484. doi: 10.4103/1673-5374.139465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M., Ladda A.M., Stephan K.M. Cerebral plasticity as the basis for upper limb recovery following brain damage. Neurosci Biobehav Rev. 2019;99:49–58. doi: 10.1016/j.neubiorev.2019.01.027. [DOI] [PubMed] [Google Scholar]
- Loubinoux I., Dechaumont-Palacin S., Castel-Lacanal E., De Boissezon X., Marque P., Pariente J., Albucher J.-F., Berry I., Chollet F. Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007;17(12):2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- Luft A.R., McCombe-Waller S., Whitall J., Forrester L.W., Macko R., Sorkin J.D., Schulz J.B., Goldberg A.P., Hanley D.F. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292(15):1853. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft A.R., Macko R.F., Forrester L.W., Villagra F., Ivey F., Sorkin J.D., Whitall J., McCombe-Waller S., Katzel L., Goldberg A.P., Hanley D.F. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39(12):3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys Ther. 2002;82(1):69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- Murayama T., Numata K., Kawakami T., Tosaka T., Oga M., Oka N., Katano M., Takasugi J., Shimizu E. Changes in the brain activation balance in motor-related areas after constraint-induced movement therapy; a longitudinal fMRI study. Brain Inj. 2011;25(11):1047–1057. doi: 10.3109/02699052.2011.607785. [DOI] [PubMed] [Google Scholar]
- Page S.J., Szaflarski J.P., Eliassen J.C., Pan H., Cramer S.C. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23(4):382–388. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S.J., Harnish S.M., Lamy M., Eliassen J.C., Szaflarski J.P. Affected arm use and cortical change in stroke patients exhibiting minimal hand movement. Neurorehabil Neural Repair. 2010;24(2):195–203. doi: 10.1177/1545968309360501. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Nguyet D., Cohen L.G., Brasil-Neto J.P., Cammarota A., Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74(3):1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pinter D., Pegritz S., Pargfrieder C., Reiter G., Wurm W., Gattringer T., Linderl-Madrutter R., Neuper C., Fazekas F., Grieshofer P., Enzinger C. Exploratory study on the effects of a robotic hand rehabilitation device on changes in grip strength and brain activity after stroke. Top Stroke Rehabil. 2013;20(4):308–316. doi: 10.1310/tsr2004-308. [DOI] [PubMed] [Google Scholar]
- Prohl A.K., Scherrer B., Tomas-Fernandez X., Filip-Dhima R., Kapur K., Velasco-Annis C., Clancy S., Carmody E., Dean M., Valle M., Prabhu S.P., Peters J.M., Bebin E.M., Krueger D.A., Northrup H., Wu J.Y., Sahin M., Warfield S.K. Reproducibility of Structural and Diffusion Tensor Imaging in the TACERN Multi-Center Study. Front Integr Neurosci. 2019;13 doi: 10.3389/fnint.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsic S., Gothe H., Borba H.H., Sroczynski G., Vujicic J., Toell T., Siebert U. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. 2019;20(1):107–134. doi: 10.1007/s10198-018-0984-0. [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A., Broetz D., Rea M., Läer L., Yilmaz Ö., Brasil F.L., Liberati G., Curado M.R., Garcia-Cossio E., Vyziotis A., Cho W., Agostini M., Soares E., Soekadar S., Caria A., Cohen L.G., Birbaumer N. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74(1):100–108. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Murguialday A., Curado M.R., Broetz D., Yilmaz Ö., Brasil F.L., Liberati G., Garcia-Cossio E., Cho W., Caria A., Cohen L.G., Birbaumer N. Brain-Machine Interface in Chronic Stroke: Randomized Trial Long-Term Follow-up. Neurorehabil Neural Repair. 2019;33(3):188–198. doi: 10.1177/1545968319827573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh S., Fluet G., Qiu Q., Merians A., Adamovich S.V., Tunik E. Neural Patterns of Reorganization after Intensive Robot-Assisted Virtual Reality Therapy and Repetitive Task Practice in Patients with Chronic Stroke. Front Neurol. 2017;8:452. doi: 10.3389/fneur.2017.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C., Sanders Z.-B., Johansen-Berg H. Structural Plasticity in Adulthood with Motor Learning and Stroke Rehabilitation. Annu Rev Neurosci. 2018;41(1):25–40. doi: 10.1146/annurev-neuro-080317-062015. [DOI] [PubMed] [Google Scholar]
- Schaechter J.D., Kraft E., Hilliard T.S., Dijkhuizen R.M., Benner T., Finklestein S.P., Rosen B.R., Cramer S.C. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16(4):326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E.J., Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerin S., Dewald J.P.A., Haztl M., Jovanovich S., Nickeas M., MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res. 2008;185(3):509–519. doi: 10.1007/s00221-007-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclocco R., Beissner F., Bianciardi M., Polimeni J.R., Napadow V. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage. 2018;168:412–426. doi: 10.1016/j.neuroimage.2017.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Borges LR, Santiago L, Lucena L, Lindquist AR, Ribeiro T. Motor imagery for gait rehabilitation after stroke. Cochrane Database Syst Rev. 2020;9:CD013019. [DOI] [PMC free article] [PubMed]
- Stinear C.M., Lang C.E., Zeiler S., Byblow W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19(4):348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
- Sun C., Liu X., Bao C., Wei F., Gong Y.i., Li Y., Liu J. Advanced non-invasive MRI of neuroplasticity in ischemic stroke: Techniques and applications. Life Sci. 2020;261:118365. doi: 10.1016/j.lfs.2020.118365. [DOI] [PubMed] [Google Scholar]
- Sun L., Yin D., Zhu Y., Fan M., Zang L., Wu Y.i., Jia J., Bai Y., Zhu B., Hu Y. Cortical reorganization after motor imagery training in chronic stroke patients with severe motor impairment: a longitudinal fMRI study. Neuroradiology. 2013;55(7):913–925. doi: 10.1007/s00234-013-1188-z. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Page S.J., Kissela B.M., Lee J.-H., Levine P., Strakowski S.M. Cortical reorganization following modified constraint-induced movement therapy: a study of 4 patients with chronic stroke. Arch Phys Med Rehabil. 2006;87(8):1052–1058. doi: 10.1016/j.apmr.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Takahashi C.D., Der-Yeghiaian L., Le V., Motiwala R.R., Cramer S.C. Robot-based hand motor therapy after stroke. Brain. 2008;131(2):425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- Taubert M., Draganski B., Anwander A., Muller K., Horstmann A., Villringer A., Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30(35):11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone F., Lai S., Spalletti C., Panarese A., Alia C., Micera S., Caleo M., Di Garbo A., Minnerup J. Post-Stroke Longitudinal Alterations of Inter-Hemispheric Correlation and Hemispheric Dominance in Mouse Pre-Motor Cortex. PLoS One. 2016;11(1):e0146858. doi: 10.1371/journal.pone.0146858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várkuti B., Guan C., Pan Y., Phua K.S., Ang K.K., Kuah C.W.K., Chua K., Ang B.T., Birbaumer N., Sitaram R. Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehabil Neural Repair. 2013;27(1):53–62. doi: 10.1177/1545968312445910. [DOI] [PubMed] [Google Scholar]
- Wang H., Xu G., Wang X.u., Sun C., Zhu B., Fan M., Jia J., Guo X., Sun L. The Reorganization of Resting-State Brain Networks Associated With Motor Imagery Training in Chronic Stroke Patients. IEEE Trans Neural Syst Rehabil Eng. 2019;27(10):2237–2245. doi: 10.1109/TNSRE.2019.2940980. [DOI] [PubMed] [Google Scholar]
- Ward N.S., Brown M.M., Thompson A.J., Frackowiak R.S. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(Pt 11):2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitall J., Waller S.M., Sorkin J.D., Forrester L.W., Macko R.F., Hanley D.F., Goldberg A.P., Luft A. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25(2):118–129. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Yue Z., Ge Y., Ma D.i., Yin H., Zhao H., Liu G., Wang J., Dou W., Pan Y.u. Brain Functional Networks Study of Subacute Stroke Patients With Upper Limb Dysfunction After Comprehensive Rehabilitation Including BCI Training. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Yu X., Perlik A.J., Tobin W.F., Zweig J.A., Tennant K., Jones T., Zuo Y.i. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462(7275):915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.E., Kyeong S., Lee S.H., Lee W.-J., Ha S.W., Kim S.M., Kang H., Lee W.M., Kang C.S., Kim D.H. Structural and functional improvements due to robot-assisted gait training in the stroke-injured brain. Neurosci Lett. 2017;637:114–119. doi: 10.1016/j.neulet.2016.11.039. [DOI] [PubMed] [Google Scholar]
- You S.H., Jang S.H., Kim Y.-H., Hallett M., Ahn S.H., Kwon Y.-H., Kim J.H., Lee M.Y. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter-blind randomized study. Stroke. 2005;36(6):1166–1171. doi: 10.1161/01.STR.0000162715.43417.91. [DOI] [PubMed] [Google Scholar]
- Zheng X., Sun L., Yin D., Jia J., Zhao Z., Jiang Y., Wang X., Wu J., Gong J., Fan M. The plasticity of intrinsic functional connectivity patterns associated with rehabilitation intervention in chronic stroke patients. Neuroradiology. 2016;58(4):417–427. doi: 10.1007/s00234-016-1647-4. [DOI] [PubMed] [Google Scholar]