Abstract
Objective
The prevalence of depression in oncological patients is 3, 4-fold compared to the general population. However, the specific risk factors for these prevalence rates are not fully understood.
Methods
A systematic literature review was conducted in nine electronic databases between 2005 and 2020. The quality of the eligible studies was appraised by two persons using the adapted 11-items Downs and Black checklist.
Results
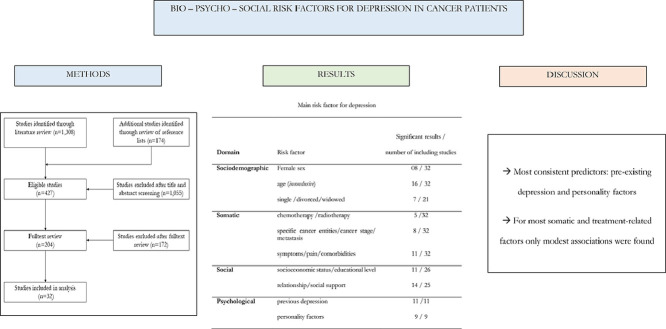
Among 2010 potentially relevant articles, 40 studies were eligible, with 27 studies of high quality and 13 studies of moderate quality. A total of 156 factors associated with depression were identified which were clustered into somatic, psychological, social and sociodemographic factors. Pre-existing depression and personality factors were the most consistent associated factors with depression in cancer patients, while for most somatic and treatment-related factors only modest associations were found.
Conclusions
Grouped as bio-psycho-social associated factors, somatic factors showed a modest influence, whereas social relationship (support) and previous depression are unequivocally significantly associated with depression.
Keywords: Cancer, Depression, Bio-psycho-social associations
Graphical abstract
Background
Depression and physical health multimorbidity are in a complex reciprocal relationship. Worldwide, depression is bidirectionally associated with higher physical multimorbidity [54]. The prevalence of clinical depression in oncological patients is 3, 4 fold of the depression risk in the general population [40,45].
Beyond the need to adjust to a life-threatening disease – resulting for many patients in distress and adjustment disorder – the specific risk factors for clinical depression are not fully understood [40]. Based on a review of studies of cancer and depression (first year after diagnosis) from 2005 to 2019 [45], we identified studies with information of risk factors and associated factors for depression. The aim of the present review was to present data on bio-psycho-social factors associated with depression across cancer entities.
Methods
Search strategy
This is a sub-analysis of data collected in a previously published systematic literature review [45]. The literature review is based on the PRISMA guidelines [34]. A systematic comprehensive search for eligible studies was conducted in nine electronic databases (MEDLINE, Cochrane Controlled Trials Register, PsycLIT, Social Science Citation Index, Science Citation Index, EMBASE, PsycINFO, PSYNDEX, PsycARTICLES). Primary aim of the literature review was to investigate prevalence rates of depression among different cancer samples. Peer-reviewed studies published in English or German between 2005 and 2019 were included in the analysis. A combination of nine search terms and according MESH-terms was used within each database. A manual search of the reference lists from retrieved papers and previous related reviews was conducted to identify further studies. The search strategy and study selection are described in more detail in Riedl and Schüssler [45]. Additionally, an update of the systematic comprehensive search was conducted for studies published between 2019 and 2020. In this present study, all studies with information on risk factors for depression were included.
Inclusion criteria
Studies were included for this analysis if (a) they included cancer patients with any kind of cancer aged 18 or older, (b) the studies investigated one or more risk factors for depression, (c) depression was assessed using questionnaires or based on a clinical diagnosis of depression (chart-based, ICD or DSM). Both cross-sectional and longitudinal studies were included. Studies with duplicate data, incomplete data, or unavailable full texts were excluded.
Quality assessment
A modified short version of the Downs and Black [16] checklist was used for quality assessment. The checklist has been cited in over 300 reviews and is usually used to evaluate randomized and non-randomized studies of healthcare interventions. After elimination of items that were not applicable for the current study, the modified quality checklist consisted of 11 items that could be scored with “Yes” (2), “partially” (1), or “No” (0). The items were added up to a total score ranging from 0 to 22 with higher values representing better study quality. Scores ≤ 15 indicated poor, 15–19 moderate and ≥ 20 high study quality. Since the subscales consisted of different numbers of items, mean values were calculated to enable a comparison between the quality subscales. Three different quality criteria were assessed: quality of reporting (7 items), external validity (1 item), and internal validity (3 Items). If no information was available for an item it was rated with 0 points.
Data synthesis
Due to the heterogeneity of the study characteristics and outcome variables, a quantitative meta-analysis of the extracted data was not feasible. Thus, a descriptive and qualitative analysis of the retrieved data was conducted. Main findings, as well as p-values and β-values, partial r-values, partial η2, odds ratios (OR), hazard ratios (HR), risk rations (RR) or standardized incidence rates (SIR) are presented if available. Based on a bio-psycho-social conception, risk factors were group as either ‘somatic’ (including type of anti-cancer therapy, cancer type, cancer symptoms, cancer stage, comorbidities, metastases, physical functioning or pain), ‘sociodemographic’ (age, sex, ethnicity), ‘social’ (socioeconomic status, relationship status, educational level, level of social support), or ‘psychological’ factors (pre-existing mental health problems, personality factors, disease awareness, health behavior, coping behavior). Studies may contain several different risk factors.
Results
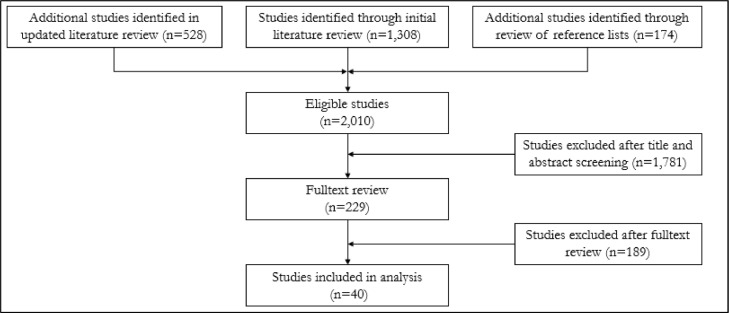
The initial database search resulted in a total number of 1308 studies, the updated search yielded another 528 potential studies and the manual search of the reference lists of these studies and previous reviews resulted in further 174 eligible studies. Of these 2010 studies, 1781 were excluded after title abstract screening. Thus, a total of 229 articles were then assessed by a full-text review and among these, 40 articles fulfilled the inclusion criteria and were included in this review. The selection process is illustrated in Fig. 1.
Fig. 1.
Flow-diagram study selection process.
Study characteristics
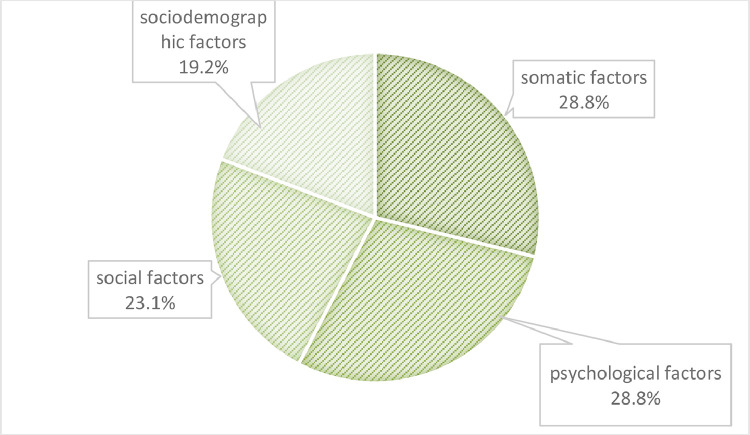
The included 40 studies consisted of 16 cross-sectional studies with a total of 34,436 patients and 24 longitudinal studies with a total of 445,241 patients. Of the cross-sectional studies, two studies were based on chart-diagnoses, eleven studies used questionnaire-based assessment two studies combined questionnaires and structured interviews and one study was based on structured interviews solely. The longitudinal studies included four studies with chart-based diagnoses, 19 studies using questionnaire data and one interview study. Generally, the most frequently used questionnaire was the HADS (n = 10; 33.3%), followed by the CES-D (7; 23.3%) and the BDI (n = 2; 6.7%). In three of four interview-based studies the SCID was applied and in one study the MINI. Overall, most studies were of high (n = 27; 67.5%) and moderate (n = 13; 31.5%) quality (Fig. 2).
Fig. 2.
Clustered percentage of assessed associated and risk factors with depression.
Risk factors
Across all included studies, a total of 156 different factors was described and were clustered into four bio-psycho-social domains: n = 45 factors (28.8%) belonged to the group of somatic, n = 45 (28.8%) to psychological, n = 36 (23.1%) to social and n = 30 (19.2%) to sociodemographic factors (Tables 1 and 2).
Table 1.
Risk of depression – cross sectional studies N = 16.
| Author | Cancer Types population | Study design | Assessment | Study quality | Risk of depression |
|---|---|---|---|---|---|
| Bektas et al. [3] | Gastrointestinal (n = 335) |
Cross sectional | HADS | moderate | female gender (p = .004) lower educational level (p = .003) single (p = .04) metastasis (p < .001) disease awareness (p = .006) |
| Bouras et al. [4] | Oesophageal-gastric (n = 1029) |
Cohort cross sectional After 2 years |
Chart based | High | younger age (OR=0.97) complications (OR=2.4; p < .001) psychiatric history (OR=6.7; p < .001) postoperative symptoms (OR=1.8; p = .008) |
| Chambers et al. [9] | Prostate (n = 189) |
Cohort study 4 months after diagnosis |
HADS QoL |
Moderate | younger age (β=−0.22; p < .05) urinary bother (β=−0.23; p < .01) bowel bother (β=0.20; p < .05) masculine self-esteem (β=−0.35; p < .001) |
| Choi and Park [13] | Mixed survivors (n = 1163) |
Cross sectional | Single item question | Moderate | female gender (OR=2.1) low monthly income (OR=1.8) smoking (OR=1.7) poor subjective health status (OR=3.6) chronic disease (OR=1.7) |
| Han et al. [21] | Esophageal (n = 330) |
Cohort Study (1–5 years after diagnosis) |
CES-D | High | hopelessness (β=0.97; p < .001) caregiver depression (β=0.46; p < .001) caregiver hopelessness (β=0.39; p < .001) stage of cancer (β=0.86; p = .03) disease awareness (β=0.77; p = .006) |
| Hartung et al. [23] | Mixed (n = 4020) |
14 months after diagnosis | PHQ | High | middle aged (p < .001) unemployed (p < .001) single patients (p < .001) patients in cancer rehabilitation (p < .001) chemotherapy (p < .001) metastasis and/or stage IV cancer (p < .001) ******* higher depression than general population (OR=5.4) highest prevalence: brain, thyroid, pancreas cancer |
| Hassan et al. [24] | Breast (n = 205) |
Cohort study | HADS | High | being single (OR=3.7; p = .01) lower financial status (OR=2.8; p < .001) |
| Hong and Tian [25] | Mixed (n = 1217) |
Two week after diagnosis | HADS | High | lower performance status (β=2.06; p < .001) pain (β=1.33; p < .001). age (β=0.07; p < .001) lower education (β=−0.814; p < .001) |
| Ladaninejad et al. [30] | Mixed (n = 200) |
Cross-sectional | GDS | Moderate | being widowed (p = .025) less contact with relatives (p = .05) lower income (p = .021) comorbidities (respiratory, diabetes) (p = .040 −0.044) pain (p = .001) cancer type (colon) (p = .007) |
| Lee et al. [33] | Head and neck (n = 113) Lung (n = 104) |
Cross sectional | MINI | Moderate |
Head & neck history of self-harm (OR=11.91; p = .020) lower education (OR=1.29; p = .002) Lung adverse life events (OR=2.78; p = .001) pre-existing anxiety (OR=1.18; p = .01) |
| Lima et al. [35] | Mixed (n = 1385) |
Cross sectional | SCID | Moderate | female gender (p < .001) previous psychiatric history (p < .001) previous psychological care (p = .004) |
| Rieke et al. [46] | Head and neck (n = 3533) |
Cross sectional | Medicare chart (ICD-9) | High | female gender (OR=1.6; p < .001) higher age (OR=1.5; p = .012) RT treatment (OR=1.9; p < .001) distant cancer stage (OR=1.7; p = .032) |
| Shahedah et al. [51] | Lung (n = 103) |
Cross-sectional | CES-D | Moderate | being single (η2=0.14; p = .001) lower physical functioning (η2=0.24; p = .001) reduced religiosity (η2=0.07; p = .023) |
| Tojal and Costa [55] | Breast (n = 150) |
Cohort study | BDI, Mini mental |
High | helplessness/hopelessness (β=0.30; p = .005) anxious preoccupation (β=0.37; p < .001) fighting spirit (β=−0.17; p = .052) cognitive avoidance (β=0.15; p = .04) |
| Walker et al. [57] | Mixed (n = 21,151) |
Cohort study | HADS SCID |
High | younger age (OR=1.03–1.3) female gender (OR=1.4–1.5) first year after diagnosis (OR=1.5) social deprivation (OR=2.2–11.0) **** 73% of depressed patients without psych. treatment |
| Wu et al. [59] | Lung (n = 194) |
Cohort Study After Diagnosis |
SDS GHQ |
Moderate | higher age (p = .04) female gender (p = .002) being single (p < .001) being religious (p = .041) |
HADS=Hospital Anxiety and Depression Scale; QoL=Quality of Life; PHQ=Patient Health Questionnaire; GDS=Geriatric depression scale;.
Table 2.
Risk of depression – longitudinal studies N = 24.
| Author | Cancer Types population | Study design longitudinal | Assessment | Study quality | Risk of depression |
|---|---|---|---|---|---|
| Avis et al. [2] | Breast (n = 653) |
Longitudinal after diagnosis up to 18 moths |
BDI | High | pain (p < .001) vasomotor symptoms (p = .01) higher social support (p = .005) spirituality (p < .001) passive coping (p < .001) illness intrusiveness (p < .001) financial difficulties (p < .001) |
| Buchmann et al. [5] | Head and neck (n = 89) |
Longitudinal 1–8 months |
DT | Moderate | emotional concerns/anxiety (OR=15.2; p = .01) history of depression (OR=8.3; p = .001) family problems (OR=4.0; p = .055) |
| Burgess et al. [7] | Breast (n = 170) |
Longitudinal 1–5 years |
SCID | High | history of depression/treatment (OR=1.9; p < .01) lack of relationship (OR=1.7; p < .01) stressful life (OR=1.5; p < .01) |
| Chang et al. [10] | Breast (n = 36,586) |
Longitudinal Up to 6 years |
Chart-based (ICD-9) |
High | all adjuvant therapies (chemo-radio-therapy, tamoxifen et al.) (OR=1.4 −1.5; p < .01) middle age (HR=1.3; p = .001) |
| Chen et al. [12] | Breast (n = 1389) |
Cohort study Longitudinal |
CES-D QoL |
High | higher age (p = .04) widowed/single (p = .006) lower income (p < .001) lower educational level (p = .006) menopausal symptoms (p < .001) comorbidities (p < .001) lower exercise (p = .009) lower QoL (p < .001) no radiotherapy (p = .004) |
| Den Oudsten et al. [15] | Breast (n = 223) |
Longitudinal 1–12 months |
CES-D, STAI Psychosocial scales |
High | previous depressive symptoms (β=0.52; p < .001) fatigue (β=0.49; p < .001) trait anxiety (β=0.33; p < .001) social support (β=−0.22; p = .008) neuroticism (β=0.22; p = .020) type of surgery (β=−0.21; p = .017) restfulness (β=−0.16; p = .032) agreeableness (β=−0.16; p = .047) |
| Enns et al. [18] | Mixed (n = 480) |
Longitudinal 0–12 months |
DT PSScan |
Moderate |
Continuous distress: female gender (OR=2.1; p < .05) chemotherapy (OR=1.9; p < .05) younger age (OR=1.8; p < .05) Higher depression: chemotherapy (OR=2.2; p < .05) |
| Erim et al. [19] | Prostate (n = 805) |
Longitudinal | SF-12 | High | African American race (OR=1.33; p < .05) unemployment (OR=1.02; p < .05) low income (OR=1.57; p < .05) past depressive episodes (OR=2.44 - 4.37; p < .01) comorbidities (OR=1.59; p < .01) treatment decisional regret (OR=3.31; p < .01) lower age (OR=1.02; p < .05) non-adherence with exercise recommendations (OR=1.49; p < .01) |
| Hulbert-Williams et al. [27] | Mixed (n = 160) |
Longitudinal 1–6 months |
HADS | High | previous depression (β=0.51 to 0.56; p < .01) mental HS (β=0.11; p < .05) neuroticism (β=0.09; p < .05) QoL (β=−0.08 to −0.09; p < .05) physical HS (β=−0.07; p < .05) age (β=0.06; p < .05) |
| Lam et al. [31] | Breast (n = 228) |
Longitudinal 1–12 months |
HADS | High | physical symptom distress (OR=12.3–41.1; p < .001) optimism (OR=1.5–2.3; p < .01) rumination (OR=1.2–1.4; p < .05) |
| Lee et al. [32] | Mixed (n = 302,488) |
Longitudinal 5 years |
Chart-based (ICD-9) |
High | lung Cancer older age (>60) (p < .05) female (p < .05) |
| Chen et al. [11] | Head and neck (n = 40) |
Longitudinal Pre-RT Follow-up |
HADS/ BDI |
Moderate | previous depression (p < .001) younger age (<55 years) (p = .03) single/divorced (p = .01) living alone (p < .01) being employed (p < .05) |
| Hao et al. [22] | Glioma (n = 190) |
Longitudinal 36 months |
HADS /SDS | lower education (OR=1.96; p = .042) being single, divorced, or widowed (OR=3.21; p = .001) comorbidities (OR=5.28; p = .028) female gender (OR=2.10; p = .038) |
|
| Lo-Fo-Wong et al. [36] | Breast (n = 746) |
Longitudinal 1–15 months |
DT | High | risk for chronic clinical distress: lack of muscle strength (OR=1.8; p < .05) lower life satisfaction (OR=1.3; p < .05) cancer worries (OR=1.4; p < .05) neuroticism (OR=1.1; p < .05) |
| Lu et al. [37] | Mixed (n = 1056) |
Longitudinal Up to 15 years |
CES-D | comorbidities (OR=2.00; p < .001) lower education (OR=1.93; p = .004) being single (OR=1.51; p = .013) female gender (OR=1.45; p = .005) |
|
| Manne et al. [39] | Gynecological (n = 113) |
Longitudinal 1–9 month |
BDI | High | younger age (part. r=−0.33; p = .001) white ethnicity (part. r = 0.26; p = .009) previous psych. problems (part. r = 0.34; p = .001) less social support (part. r = 0.20; p = .046) less emotional expressiveness (part.r=−0.29; p = .004) less positive reappraisal (part. r=−0.39; p < .001) physical impairment (part. r = 0.20; p = .034) physical disability (part. r = 0.45; p < .001) |
| Parajuli et al. [41] | Mixed (n = 1799) |
CES-D | comorbidities (b = 0.22; p < .001 functional disability (b = 0.23; p < .001) higher age (b = 0.01; p < .001) ethnicity other than Caucasian or African American (b = 0.53; p < .001) being single, divorced, or widowed (b = 0.07–0.24; p < .001) |
||
| Ravi et al. [42] | Prostate >65 years (n = 50,856) |
Longitudinal | Chart based (ICD-9) | High |
higher risk: urinary incontinence (HR=1.5; p < .001) older age (>75) (HR=1.3; p < .001) comorbidities (HR=1.2–1.6; p < .001) rural environment (HR=1.1; p < .001) being single, divorced or widowed (HR=1.1; p < .001) lower risk: black ethnicity (HR=0.8; p < .001) higher incomes status (HR=0.9; p < .001) definitive therapy (HR=0.9; p < .001) erectile dysfunction (HR=0.9; p < .001) |
| Recklitis et al. [43] | Prostate survivors (n = 693) |
Longitudinal 3–8 years after diagnosis |
GDS-15 BDI |
High |
Increased risk for suicidal ideation: disabled (OR=3.9; p < .05) frequent pain (OR=2.7; p < .05) lower subjective mental health (OR=1.1; p < .001) less hormone-related symptoms (OR=1.02; p < .005) |
| Robbertz et al. [47] | CLL (n = 106) |
Longitudinal | PHQ-9 | previous depression (β=0.33; p < .01) fatigue (β=0.30; p < .01) adverse life events (β=0.19; p = .02) |
|
| Saboonchi et al. [48] | Breast (n = 715) |
Longitudinal after surgery 1 year | HADS | High | previous depression (OR=7.8–11.6) adverse life events (OR=2.3–3.5) sickness absence (OR=1.1–2.1) post-OP chemotherapy (OR=1.1–1.6) |
| Stafford et al. [52] | Breast & Gynecological (n = 105) | Longitudinal 2 years |
HADS CES-D |
High | previous depression/anxiety (β=0.31; p < .01) neuroticism (β=0.27–0.31; p = .04–0.008) |
| Yang et al. [60] | Breast (n = 40,849 invasive) (n = 4402 in situ) |
Cohort Longitudinal 7,5 years |
Chart-based (ICD-10) | High | invasive breast cancer (SIR=1.6) younger age (HR=2.5–3.0) comorbidities (HR=1.4) positive lymph nodes (HR=1.3) ***** Development over time: 1st year invasive (SIR=1.8–2.5) 2nd year invasive (SIR=2.0) 2–5th year invasive (SIR=1.3) |
| Yu et al. [61] | Gastric (n = 300) |
Longitudinal 3 months - 4 years |
DSI | High | higher tumor stage (p < .001) operable tumor (p = .03) |
The different studies had different research goals - meaning that not all studies assessed all these bio-psycho-social factors. While sociodemographic factors and main somatic factors (cancer stage, treatment, main symptoms and comorbidities) were accounted for in all included studies, this was not done for pre-existing depression or more specific factors such as pain, socioeconomic strain, psychological predictors or aspects of social support. To give an accurate estimation of influential factors, the number of studies that included the specific predictor was used as numerator to calculate the percentage in which significant associations were found with depression. Pain for example was only specifically included in seven studies [2,5,15,25,30,33,43] of which four studies [2,25,30,43] reported a significant association, meaning that pain was identified as a significant predictor for depression in 57.1% (i.e., 4/7) of all studies that investigated this association. A summary of the main associated factors is presented in Table 3.
Table 3.
Factors associated with depression.
| Domain | Risk factor | No. investigated | + | ∼ | – |
|---|---|---|---|---|---|
| Sociodemographic factors | Ethnicity (Caucasian) | 11 / 40 | 2 | 7 | 2 |
| Gender (female) | 40 / 40 | 9 | 31 | 0 | |
| Age (older) | 36 / 40 | 8 | 19 | 7 | |
| Somatic factors | cancer treatment | 23 / 40 | 8 | 15 | 0 |
| cancer type | 16 / 40 | 3 | 13 | 0 | |
| cancer symptoms | 11 / 40 | 8 | 3 | 0 | |
| cancer stage | 23 / 40 | 4 | 19 | 0 | |
| comorbidities | 15 / 40 | 9 | 6 | 0 | |
| metastases | 8 / 40 | 4 | 4 | 0 | |
| pain | 7 / 40 | 4 | 3 | 0 | |
| physical functioning | 10 / 40 | 8 | 2 | 0 | |
| Social factors | educational level (lower) | 30 / 40 | 6 | 24 | 0 |
| relationship status (single / separated / widowed) | 29 / 40 | 12 | 17 | 0 | |
| socioeconomic status (lower) | 25 / 40 | 10 | 15 | 0 | |
| level of social support | 12 / 40 | 8 | 4 | 0 | |
| Psychological factors | previous depression | 13 / 40 | 13 | 0 | 0 |
| psychological / psychiatric history | 20 / 40 | 13 | 7 | 0 | |
| personality factors (introverted) | 10 / 40 | 9 | 0 | 1 | |
| disease awareness | 2 / 40 | 1 | 0 | 1 | |
| health behavior (worse) | 9 / 40 | 3 | 6 | 0 | |
| coping behavior (passive) | 5 / 40 | 4 | 1 | 0 |
+ positive association; ∼ no association; - negative association; reference value for risk factor in parentheses.
Sociodemographic factors
Age was investigated as a predictor for depression in all included studies. While 17 studies reported a significant association of age with depression, the results were inconclusive: seven studies indicated that younger cancer patients showed more depressive symptoms than older patients [4,9,11,18,39,57,60], two studies found middle aged patients to be most affected by depression [10,23] and another eight studies reported that older patients showed more depression than younger patients [12,25,27,32,41,42,46,59].
For gender on the other hand, the results were more clearly: all (9/40) studies that reported a significant association between sex and depression found women to be more prone to depression than men with odds ratios between 1.6 and 2.1 [3,13,18,22,32,35,46,57,59].
The patient's ethnicity was investigated in eleven studies. Four studies (36.4%) indicated a potential association of the patients’ ethnicity and depression. However, the results were ambiguous: While Manne et al. [39] found white patients to have higher depression scores than patients from other ethnicities and Ravi et al. [42] found black patients to be less at risk for depression (HR=0.79) than white patients, Erim et al. [19] reported a higher risk for depression for African American patients and Parajuli et al. [41] for ethnicities other than Caucasian or African American.
Somatic factors
Somatic factors associated with depression were investigated in all included studies. A total of 48 different somatic factors were identified (i.e., >1 risk factor for most studies), which included type of anti-cancer therapy, cancer type, cancer and treatment symptoms, cancer stage, comorbidities, metastases, pain and physical functioning.
Eight of 11 studies reported statistically significant associations of different cancer and treatment-related symptoms (e.g., fatigue, post-operative symptoms, erectile dysfunction, urinary incontinence) with higher levels of depression [2,4,9,12,15,42,43,47]. Patients with comorbidities and other chronic conditions were found to be consistently more at risk for depressions (up to 1.7-times) than patients without those other health issues in nine of fifteen studies [12,13,19,22,30,37,41,42,60]. Worse cancer stage and metastases were associated with higher rates of depression across different cancer types in five studies [3,21,23,46,61].
Similar results were found for presence of pain, which was associated with a 2.7-times increased risk for depression in four studies [2,25,30,43], whereas no significant association was found in three other studies. The association of type of cancer treatment and depression was investigated in 28 studies, of which eight studies found a significant association. Overall, Chang et al. [10] reported increased odds for depression associated with all types of adjuvant therapies. However, Rieke et al. [46] reported an almost two-fold increased risk for depression amongst patients with head-and-neck cancer, while Chen et al. [12] even reported lower depression scores associated with radiotherapy in a sample of breast cancer patients. Chemotherapy was associated with higher depression rates in two samples [18,48] and one study found an association with the type of surgery in breast cancer patients [15]. Regardless of specific type of therapy, “definitive” therapies were associated with lower levels of depression than patients undergoing watch and wait therapy [42]. Lower physical status and physical functioning was associated with depression in eight of ten studies [13,25,27,31,36,39,41,51].
Social factors
The influence of the socioeconomic status and educational level was investigated in a large body of studies (37/40). A lower educational level was consistently associated with depression in six of 30 studies [3,12,22,25,33,37], while socioeconomic factors were associated with depression in ten of 25 studies, including unemployment or sickness-related absenteeism [11,19,23,48] as well as financial difficulties and lower income [2,11,13,24,30,42].
The role of interpersonal relationships was investigated in 29 studies, of which 12 reported significant associations: patients without intimate partnership were consistently found to be more depression than patients in partnerships with an up to 4-times increased risk for depression [3,11,12,[22], [23], [24],30,37,41,42,51,59]. In accordance, social deprivation, lower social support and / or distressed caregivers and family were also consistently associated with depression and emotional distress [2,5,7,15,21,30,39,57].
Psychological factors
The psychological factors for depression could be clustered into five subdomains: pre-existing mental health problems, personality factors, disease awareness, health behavior and coping behavior.
A consistent body of literature linked previously existing mental health problems to increased levels of depression amongst all types of cancer patients. Pre-existing depression was identified as a significant predictor for depression in cancer patients in all thirteen studies which investigated the association with up to 6.7-times increased odds ratios [4,5,7,11,15,19,27,33,35,43,47,48,52]. Other psychopathological predictors included anxiety [5,15,52,55], feelings of hopelessness [21,55], reduced emotional functioning [12,27,43], stressful life events [7,48] or worries [36].
Also, all ten studies which assessed personality factors reported significant associations with patients’ depression: optimism [31], a fighting spirit [55], masculine self-esteem [9] and agreeableness [15] were associated with lower levels of depression. On the other hand, several studies found an association of neuroticism [15,27,36,52], or rumination [31] with increased levels of depression. As for spirituality, Avis et al. [2] and Shahedah et al. [51] found reduced levels of depression in cancer patients with higher spirituality, while Wu et al. [59] reported a higher prevalence of depression amongst patients with lung cancer who held a religious or spiritual understanding of life than participants with secular beliefs.
Significant associations of worse general health behavior (i.e., less exercising or smoking) with worse depression scores was found in three of nine studies [12,13,19]. Four of five studies that investigated coping behavior found a significant association with depression. While passive [2], avoidant [55], negative and less emotionally expressive [39] coping behaviors were associated with higher depression scores, restfulness [15] was associated with lower depression.
Disease awareness was significantly associated with depression in both studies which included this risk factor. However, results were inconsistent: while diseases awareness was positively correlated to the patients’ depression score and identified as the largest contributor to patients’ feelings of hopelessness in Chinese patients with esophageal cancer [21], in another study Turkish patients with gastrointestinal cancers who did not know their disease reported higher depression than patients who knew their disease [3].
Discussion
Depression is increasingly recognized as important comorbidity in the treatment of individuals with cancer [45]. International panels have indicated that screening for and treating depression should be integrated in cancer care (e.g. [1]). Since different diagnostic criteria for depression (ICD-10, DSM-IV) may lead to in differences in prevalence rates, the broader approach of a „depressive spectrum disorder“ [8] or „clinical depressive syndrome“ [45] would allow a better comparability of epidemiological data.
In this study, the diagnosis of „clinical depressive syndrome“ was based on chart diagnoses, standardized questionnaires (mainly HADS, CES-D and BDI) and interviews (mainly SCID). The pros and cons of different assessments are widely discussed [40]. The most problematic type of assessments are chart-based diagnoses, which may be prone to underreporting. In our review, the largest study on cancer mortality associated with depression [62], reported a prevalence of a comorbid depression in only 4.7% of the included cancer patients – a prevalence rate comparable to the general population [49]! Generally speaking, clinical interviews and questionnaire may be more valid and yield in more comparable results [45], if reliable and clinically relevant cut-offs are applied for the questionnaires.
Self-care (or neglect of it), maladaptive coping [50] and higher risk behavior (nicotine and drugs, life style) of individuals, but also problems in the medical systems play an important role in the emergence of depression [54] as with all severe and / or chronical physical diseases. In this review, we clustered the associated factors with depression in cancer into major domains according to the bio-psycho-social model of medicine [17].
Due to the heterogeneous nature of the studies included in this review (i.e., different forms of cancer and study design, assessment methods, statistical methods (non-) reported results) a quantitative meta-analysis was not applicable. Thus, a descriptive review for cross-sectional and longitudinal studies with all inherent limitations has been conducted. However, including a total number of 479,677 patients, the results of our review are still quite conclusive.
Generally speaking, most consistent associations with depression were found for previous (lifetime) depression, with an up to 6.7-times increased risk for depression in cancer patients. In a representative study, Mallet et al. [38] found that participants with a history of mental disorders were at higher risk to develop an emotional disorder after cancer diagnosis, while participants without previous mental disorders showed no elevated risks. However, in our sample only thirteen of the 40 included studies had investigated previous depression. The prevalence rates of the studies probably underreport the real dimension of previous depressive symptoms (about > 10% of cancer patient in the studies) due to methodological problems since some studies only accounted depression if pre-cancer anti-depressive drug therapy was given.
Another methodological challenge is the assessment of suicidal ideation as a substitute for depressive symptomatology. While suicidal ideation is often a symptom of depression, it is not solely found in depressive individuals. Therefore, the majority of studies concerning cancer and suicidal ideation were not included. A recent review on this matter identified pain, chronic conditions, depression and distress, socioeconomic status and marital status as risk factors for suicidal ideation [29].
Apart from depressive symptoms, other psychological factors show a clear association with depression too if taken into account. Personality factors can be risk factors (e.g., neuroticism) as well as protective factor (e.g., optimism, fighting spirit), or the other way round missing optimism was a risk factor for depression.
Generally, the methodological problems in investigating personality factors in cancer patients (reliability, validity) are paramount: What is state – what is trait?
In terms of the identified sociodemographic variables, contradicting results were found for age as an associated factor, with some studies indicating an increased risk for younger patients, while other studies found older patients to be more at risk for depression. This is in line with previous publications, which found no or at most small associations of age and depression in cancer patients [40,58]. Since age-dependent challenges are highly dependent of the varying age-related social contexts, the impact of cancer on the individual are unique and may lead to the inconsistent findings. Female gender on the other hand was consistently identified as an associated factor for depression amongst all studies. However, female gender is generally an unspecific risk factor for depression with 50% increased prevalence rates in the general population than for males [49]. Thus, this finding may be understood as confirmation on general gender difference in depression rather than cancer-specific findings.
For the somato-medical factors, only few cancer entities show a significant elevated risk for depression, especially head and neck cancer. Higher cancer stages (metastasis) bear a certain risk for depression, but not for the majority of the studies. Depression is not an invariable consequence of advanced cancer, but advanced cancer has an elevated risk. The importance of cancer stage and prognosis for depression, however, might be overstated [40].
The highest somatic association with depression are disease related comorbidities, cancer-related symptom burden and pain. These factors are well-known, and previous studies have shown a higher burden of symptoms (pain, fatigue) as a risk factor for depression in hospitalized patients with cancer [6]. This is in accordance with other chronic or life-threatening diseases [28,54]. On the other hand, oncological therapies (chemotherapy, radiotherapy and biological therapy) were not associated with an enhance risk for depression in the majority of studies. In a systematic review of predictors of emotional distress after cancer diagnoses, Cook et al. [14] found that only psychological factors (i.e., distress and neuroticism) consistently predicted long-form distress, whereas tumor characteristics and treatment forms did not.
Social factors and the socio-economic status (SES) play a prominent for all psychiatric diseases and have been discussed as a risk factor for all major diseases [26]. In our sample, eleven studies found the SES to be frequently and a significantly associated with depression. The absence of a (good) relationship and impaired social support are general risk factors. A supportive relationship is a fundamental beneficial health factor while missing relationships and lower social support are general risk factors [53,56]. We found that SES and social support (if accounted) are unequivocal significant factor associated with depression in cancer patients. According to Gariépy et al. [20], (missing) social support is a key element for depression. The interdependency of interpersonal (family-caregiver) relationship and depression is well known. Our findings are also in line with a previous review of risk factors for depression in a subgroup of cancer patients receiving chemotherapy [58], which reported that among 43 included studies, only social support, perceived stress and self-efficacy were constantly associated with depression. Disease- and treatment-related factors or physiological conditions on the other hand showed unequivocal associations with depression.
One limitation of the present study is that to our knowledge so far, no comprehensive overview of the risk factors for depression in cancer patients had been presented. Thus, we did not pre-specify risk factors of interest, but rather chose a broad and inclusive approach to avoid bias during data selection and interpretation. Also, it was not the primary aim of the initial literature research to identify risk factors, but rather prevalence rates of depression amongst cancer patients. However, due to the large body of studies we consider the results of our review as quite conclusive.
In conclusion: psychosocial factors are significantly associated with depression in cancer patients! Since depression may strongly influence the course of the cancer treatment and disease itself, the risk of depression should be evaluated in every patient: previous depression (lifetime) and social support should be an integral part of every medical (oncological) anamnesis as a base for doctor-patient relationship [44].
CRediT authorship contribution statement
David Riedl: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. Gerhard Schüßler: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
References
- 1.Andersen B.L., DeRubeis R.J., Berman B.S., Gruman J., Champion V.L., Massie M.J., Rowland J.H. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J. Clin. Oncol. 2014;32(15):1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avis N.E., Levine B., Naughton M.J., Case L.D., Naftalis E., Van Zee K.J. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res. Treat. 2013;139(1):199–206. doi: 10.1007/s10549-013-2513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bektas D.K., Demir S. Anxiety, depression levels and quality of life in patients with gastrointestinal cancer in Turkey. Asian Pac. J. Cancer Prev. 2016;17(2):723–731. doi: 10.7314/apjcp.2016.17.2.723. [DOI] [PubMed] [Google Scholar]
- 4.Bouras G., Markar S.R., Burns E.M., Huddy J.R., Bottle A., Athanasiou T., Hanna G.B. The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: a national linked database study. Eur. J. Surg. Oncol. 2017;43(2):454–460. doi: 10.1016/j.ejso.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Buchmann L., Conlee J., Hunt J., Agarwal J., White S. Psychosocial distress is prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424–1429. doi: 10.1002/lary.23886. [DOI] [PubMed] [Google Scholar]
- 6.Bukberg J., Penman D., Holland J.C. Depression in hospitalized cancer patients. Psychosom. Med. 1984;46(3):199–212. doi: 10.1097/00006842-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Burgess C., Cornelius V., Love S., Graham J., Richards M., Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruso R., GiuliaNanni M., Riba M.B., Sabato S., Grassi L. Depressive spectrum disorders in cancer: diagnostic issues and intervention. A critical review. Curr. Psychiatry Rep. 2017;19(6):33. doi: 10.1007/s11920-017-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers S.K., Schover L., Nielsen L., Halford K., Clutton S., Gardiner R.A., Occhipinti S. Couple distress after localised prostate cancer. Support. Care Cancer. 2013;21(11):2967–2976. doi: 10.1007/s00520-013-1868-6. [DOI] [PubMed] [Google Scholar]
- 10.Chang C.K., Hayes R.D., Broadbent M.T., Hotopf M., Davies E., Møller H., Stewart R. A cohort study on mental disorders, stage of cancer at diagnosis and subsequent survival. BMJ Open. 2014;4(1) doi: 10.1136/bmjopen-2013-004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A.M., Jennelle R.L., Grady V., Tovar A., Bowen K., Simonin P., Vijayakumar S. Prospective study of psychosocial distress among patients undergoing radiotherapy for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(1):187–193. doi: 10.1016/j.ijrobp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Lu W., Zheng Y., Gu K., Chen Z., Zheng W., Shu X.O. Exercise, tea consumption, and depression among breast cancer survivors. J. Clin. Oncol. 2010;28(6):991–998. doi: 10.1200/JCO.2009.23.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi K.H., Park S.M. Psychological status and associated factors among korean cancer survivors: a cross-sectional analysis of the fourth & fifth Korea national health and nutrition examination surveys. J. Korean Med. Sci. 2016;31(7):1105–1113. doi: 10.3346/jkms.2016.31.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook S.A., Salmon P., Hayes G., Byrne A., Fisher P.L. Predictors of emotional distress a year or more after diagnosis of cancer: a systematic review of the literature. Psychooncology. 2018;27(3):791–801. doi: 10.1002/pon.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Den Oudsten B.L., Van Heck G.L., Van der Steeg A.F., Roukema J.A., De Vries J. Predictors of depressive symptoms 12 months after surgical treatment of early-stage breast cancer. Psychooncology. 2009;18(11):1230–1237. doi: 10.1002/pon.1518. [DOI] [PubMed] [Google Scholar]
- 16.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel G.L. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 18.Enns A., Waller A., Groff S.L., Bultz B.D., Fung T., Carlson L.E. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J. Psychosoc. Oncol. 2013;31(5):489–506. doi: 10.1080/07347332.2013.822052. [DOI] [PubMed] [Google Scholar]
- 19.Erim D.O., Bensen J.T., Mohler J.L., Fontham E.T.H., Song L., Farnan L., Gaynes B.N. Prevalence and predictors of probable depression in prostate cancer survivors. Cancer. 2019;125(19):3418–3427. doi: 10.1002/cncr.32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gariépy G., Honkaniemi H., Quesnel-Vallée A. Social support and protection from depression: systematic review of current findings in Western countries. Br. J. Psychiatry. 2016;209(4):284–293. doi: 10.1192/bjp.bp.115.169094. [DOI] [PubMed] [Google Scholar]
- 21.Han Y., Yuan J., Luo Z., Zhao J., Wu J., Liu R., Lopez V. Determinants of hopelessness and depression among Chinese hospitalized esophageal cancer patients and their family caregivers. Psychooncology. 2013;22(11):2529–2536. doi: 10.1002/pon.3315. [DOI] [PubMed] [Google Scholar]
- 22.Hao A., Huang J., Xu X. Anxiety and depression in glioma patients: prevalence, risk factors, and their correlation with survival. Ir. J. Med. Sci. 2021;190(3):1155–1164. doi: 10.1007/s11845-020-02374-5. [DOI] [PubMed] [Google Scholar]
- 23.Hartung T.J., Brähler E., Faller H., Härter M., Hinz A., Johansen C., Mehnert A. The risk of being depressed is significantly higher in cancer patients than in the general population: prevalence and severity of depressive symptoms across major cancer types. Eur. J. Cancer. 2017;72:46–53. doi: 10.1016/j.ejca.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Hassan M.R., Shah S.A., Ghazi H.F., Mohd Mujar N.M., Samsuri M.F., Baharom N. Anxiety and depression among breast cancer patients in an urban setting in Malaysia. Asian Pac. J. Cancer Prev. 2015;16(9):4031–4035. doi: 10.7314/apjcp.2015.16.9.4031. [DOI] [PubMed] [Google Scholar]
- 25.Hong J.S., Tian J. Prevalence of anxiety and depression and their risk factors in Chinese cancer patients. Support. Care Cancer. 2014;22(2):453–459. doi: 10.1007/s00520-013-1997-y. [DOI] [PubMed] [Google Scholar]
- 26.House J.S., Landis K.R., Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 27.Hulbert-Williams N., Neal R., Morrison V., Hood K., Wilkinson C. Anxiety, depression and quality of life after cancer diagnosis: what psychosocial variables best predict how patients adjust? Psychooncology. 2012;21(8):857–867. doi: 10.1002/pon.1980. [DOI] [PubMed] [Google Scholar]
- 28.Katon W., Lin E.H., Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen. Hosp. Psychiatry. 2007;29(2):147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Kolva E., Hoffecker L., Cox-Martin E. Suicidal ideation in patients with cancer: a systematic review of prevalence, risk factors, intervention and assessment. Palliat. Support. Care. 2020;18(2):206–219. doi: 10.1017/S1478951519000610. [DOI] [PubMed] [Google Scholar]
- 30.Ladaninejad S., Ilali E., Mousavinasab N., Taraghi Z. The relationship between depressive symptoms and demographic-medical characteristics among elder people with cancer. Asia Pac. J. Oncol. Nurs. 2019;6(4):424–430. doi: 10.4103/apjon.apjon_13_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam W.W., Soong I., Yau T.K., Wong K.Y., Tsang J., Yeo W., Fielding R. The evolution of psychological distress trajectories in women diagnosed with advanced breast cancer: a longitudinal study. Psychooncology. 2013;22(12):2831–2839. doi: 10.1002/pon.3361. [DOI] [PubMed] [Google Scholar]
- 32.Lee B.O., Choi W.J., Sung N.Y., Lee S.K., Lee C.G., Kang J.I. Incidence and risk factors for psychiatric comorbidity among people newly diagnosed with cancer based on Korean national registry data. Psychooncology. 2015;24(12):1808–1814. doi: 10.1002/pon.3865. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y., Hung C.F., Chien C.Y., Lin P.Y., Lin M.C., Wang C.C., Wang L.J. Comparison of prevalence and associated factors of depressive disorder between patients with head and neck cancer and those with lung cancer at a tertiary hospital in Taiwan: a cross-sectional study. BMJ Open. 2020;10(6) doi: 10.1136/bmjopen-2020-037918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima M.P., Longatto-Filho A., Osório F.L. Predictor variables and screening protocol for depressive and anxiety disorders in cancer outpatients. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0149421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo-Fo-Wong D.N., de Haes H.C., Aaronson N.K., van Abbema D.L., den Boer M.D., van Hezewijk M., Sprangers M.A. Predictors of enduring clinical distress in women with breast cancer. Breast Cancer Res. Treat. 2016;158(3):563–572. doi: 10.1007/s10549-016-3896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu W., Pikhart H., Peasey A., Kubinova R., Pitman A., Bobak M. Risk of depressive symptoms before and after the first hospitalisation for cancer: evidence from a 16-year cohort study in the Czech Republic. J. Affect. Disord. 2020;276:76–83. doi: 10.1016/j.jad.2020.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallet J., Huillard O., Goldwasser F., Dubertret C., Le Strat Y. Mental disorders associated with recent cancer diagnosis: results from a nationally representative survey. Eur. J. Cancer. 2018;105:10–18. doi: 10.1016/j.ejca.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 39.Manne S., Rini C., Rubin S., Rosenblum N., Bergman C., Edelson M., Rocereto T. Long-term trajectories of psychological adaptation among women diagnosed with gynecological cancers. Psychosom. Med. 2008;70(6):677–687. doi: 10.1097/PSY.0b013e31817b935d. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell A.J., Chan M., Bhatti H., Halton M., Grassi L., Johansen C., Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 41.Parajuli J., Berish D., Valenti K.G., Jao Y.L. Prevalence and predictors of depressive symptoms in older adults with cancer. J. Geriatr. Oncol. 2021;12(4):618–622. doi: 10.1016/j.jgo.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Ravi P., Karakiewicz P.I., Roghmann F., Gandaglia G., Choueiri T.K., Menon M., Trinh Q.D. Mental health outcomes in elderly men with prostate cancer. Urol. Oncol. 2014;32(8):1333–1340. doi: 10.1016/j.urolonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Recklitis C.J., Zhou E.S., Zwemer E.K., Hu J.C., Kantoff P.W. Suicidal ideation in prostate cancer survivors: understanding the role of physical and psychological health outcomes. Cancer. 2014;120(21):3393–3400. doi: 10.1002/cncr.28880. [DOI] [PubMed] [Google Scholar]
- 44.Riedl D., Schüssler G. The influence of doctor-patient communication on health outcomes: a systematic review. Z Psychosom Med Psychother. 2017;63(2):131–150. doi: 10.13109/zptm.2017.63.2.131. [DOI] [PubMed] [Google Scholar]
- 45.Riedl D., Schüssler G. Prevalence of depression and cancer - a systematic review. Z. Psychosom. Med. Psychother. 2021;67 doi: 10.13109/zptm.2021.67.Oa11. [DOI] [PubMed] [Google Scholar]
- 46.Rieke K., Boilesen E., Lydiatt W., Schmid K.K., Houfek J., Watanabe-Galloway S. Population-based retrospective study to investigate preexisting and new depression diagnosis among head and neck cancer patients. Cancer Epidemiol. 2016;43:42–48. doi: 10.1016/j.canep.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Robbertz A.S., Weiss D.M., Awan F.T., Byrd J.C., Rogers K.A., Woyach J.A. Identifying risk factors for depression and anxiety symptoms in patients with chronic lymphocytic leukemia. Support. Care Cancer. 2020;28(4):1799–1807. doi: 10.1007/s00520-019-04991-y. [DOI] [PubMed] [Google Scholar]
- 48.Saboonchi F., Petersson L.M., Wennman-Larsen A., Alexanderson K., Brännström R., Vaez M. Changes in caseness of anxiety and depression in breast cancer patients during the first year following surgery: patterns of transiency and severity of the distress response. Eur. J. Oncol. Nurs. 2014;18(6):598–604. doi: 10.1016/j.ejon.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Substance Abuse and Mental Health Services Administration. Mental Health Annual Report 2017 . SAMHSA; Rockville, MD: 2019. [Google Scholar]
- 50.Schüssler G. Coping strategies and individual meanings of illness. Soc. Sci. Med. 1992;34(4):427–432. doi: 10.1016/0277-9536(92)90303-8. [DOI] [PubMed] [Google Scholar]
- 51.Shahedah K.K., How S.H., Jamalludin A.R., Mohd Faiz M.T., Kuan Y.C., Ong C.K. Depressive symptoms in newly diagnosed lung carcinoma: prevalence and associated risk factors. Tuberc. Respir. Dis. 2019;82(3):217–226. doi: 10.4046/trd.2018.0048. (Seoul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stafford L., Judd F., Gibson P., Komiti A., Mann G.B., Quinn M. Anxiety and depression symptoms in the 2 years following diagnosis of breast or gynaecologic cancer: prevalence, course and determinants of outcome. Support. Care Cancer. 2015;23(8):2215–2224. doi: 10.1007/s00520-014-2571-y. [DOI] [PubMed] [Google Scholar]
- 53.Streck B.P., Wardell D.W., LoBiondo-Wood G., Beauchamp J.E.S. Interdependence of physical and psychological morbidity among patients with cancer and family caregivers: review of the literature. Psychooncology. 2020;29(6):974–989. doi: 10.1002/pon.5382. [DOI] [PubMed] [Google Scholar]
- 54.Stubbs B., Vancampfort D., Veronese N., Kahl K.G., Mitchell A.J., Lin P.Y., Koyanagi A. Depression and physical health multimorbidity: primary data and country-wide meta-analysis of population data from 190 593 people across 43 low- and middle-income countries. Psychol. Med. 2017;47(12):2107–2117. doi: 10.1017/S0033291717000551. [DOI] [PubMed] [Google Scholar]
- 55.Tojal C., Costa R. Depressive symptoms and mental adjustment in women with breast cancer. Psychooncology. 2015;24(9):1060–1065. doi: 10.1002/pon.3765. [DOI] [PubMed] [Google Scholar]
- 56.Umberson D., Montez J.K. Social relationships and health: a flashpoint for health policy. J. Health Soc. Behav. 2010;51(Suppl):S54–S66. doi: 10.1177/0022146510383501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker J., Hansen C.H., Martin P., Symeonides S., Ramessur R., Murray G., Sharpe M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. 2014;1(5):343–350. doi: 10.1016/S2215-0366(14)70313-X. [DOI] [PubMed] [Google Scholar]
- 58.Wen S., Xiao H., Yang Y. The risk factors for depression in cancer patients undergoing chemotherapy: a systematic review. Support. Care Cancer. 2019;27(1):57–67. doi: 10.1007/s00520-018-4466-9. [DOI] [PubMed] [Google Scholar]
- 59.Wu X.N., Su D., Li H.P., Wang W.L., Wu W.Q., Yang Y.J., Zhang J.P. Relationship between the depression status of patients with resectable non-small cell lung cancer and their family members in China. Eur. J. Oncol. Nurs. 2013;17(5):668–672. doi: 10.1016/j.ejon.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Yang H., Brand J.S., Fang F., Chiesa F., Johansson A.L., Hall P., Czene K. Time-dependent risk of depression, anxiety, and stress-related disorders in patients with invasive and in situ breast cancer. Int. J. Cancer. 2017;140(4):841–852. doi: 10.1002/ijc.30514. [DOI] [PubMed] [Google Scholar]
- 61.Yu H., Wang Y., Ge X., Wu X., Mao X. Depression and survival in Chinese patients with gastric cancer: a prospective study. Asian Pac. J. Cancer Prev. 2012;13(1):391–394. doi: 10.7314/apjcp.2012.13.1.391. [DOI] [PubMed] [Google Scholar]
- 62.Zhu J., Fang F., Sjölander A., Fall K., Adami H.O., Valdimarsdóttir U. First-onset mental disorders after cancer diagnosis and cancer-specific mortality: a nationwide cohort study. Ann. Oncol. 2017;28(8):1964–1969. doi: 10.1093/annonc/mdx265. [DOI] [PubMed] [Google Scholar]