Abstract
Major extremity hemorrhage is a surgical emergency, and the physical examination is essential to help dictate appropriate clinical decision making. Hard signs that require immediate surgical intervention include ongoing bleeding, expanding hematoma, ischemic limb, as well as partial/complete amputation. Packing, compression, balloon tamponade, and tourniquets are very helpful to temporize major hemorrhage. Mangled extremities are very challenging to manage and require a multidisciplinary approach. Temporary vascular shunts are excellent tools for vascular/orthopedic damage control and for temporary stabilization prior to transport for definitive care.
Although the incidence of major vascular injury in trauma patients is relatively low (0.2%–3.7%), the extremities are the most common site for such injuries [1]. These vascular injuries are not surprisingly more common with penetrating trauma extremity trauma and associated venous, nerve, and orthopedic injuries may occur in up to one third of all extremity arterial injuries [1]. The femoral artery is the most commonly injured artery followed by the brachial artery.
TRAUMA ALERT FOR PATIENT WITH MASSIVE BLEEDING FROM LOWER EXTREMITY: WHAT NOW?
Initial assessment of extremity injuries should follow ATLS protocol with identification of life-threatening injuries taking precedence over limb-saving injuries; however, in the case of exsanguinating lower extremity injuries, they may be one of the same. As always, strong leadership during resuscitation, as well as in the operating room, is essential to keep the team focused on resuscitation of the complex, potentially multiple-injured trauma patient. It is often helpful to have two surgical teams available: one for the exsanguinating extremity and the other to deal with the other “life-threatening” injuries.
Major hemorrhage, or “circulation”, is the priority for major extremity injuries, and basic techniques, such as those taught in Stop the Bleed (www.stopthebleed.org), are paramount [2]. Realignment of the extremity as well as proper exposure with packing and direct pressure can control most bleeding. If bleeding persists, a proximal tourniquet should be used (Fig 1). Balloon tamponade (most commonly with either Fogarty or Foley catheter with 5–30 mL balloons) is also an excellent modality to arrest hemorrhage for deeper wounds [3].
Fig 1.
Traumatic amputation of lower extremity with tourniquet.
TOURNIQUETS ARE YOUR FRIEND—WHEN PROPERLY APPLIED
As with many advancements in trauma care, lessons learned from the military environment have been translated to the civilian setting, and the same can be said about tourniquet use. The windlass combat-type application tourniquet is most commonly used, but it does require formal training to be effective [4]. Tourniquets can be applied in the prehospital setting, the emergency department, or the operating theater, and time of application must be recorded.
Prehospital tourniquet application has increased significantly in the last decade, as many emergency medical services (EMS) now routinely carry commercial tourniquets. This has proven to be safe with low rate of complications, and data also suggest that there are associated increased survival benefit, decreased blood loss and decreased limb specific complications [[5], [6], [7], [8], [9]]. Although the use of tourniquets for pediatric vascular extremity trauma is less well studied, the Pediatric Trauma Society does support its use in the prehospital setting and during the resuscitation of children with exsanguinating hemorrhage from severe extremity trauma [10].
After extremity hemorrhage has been controlled, a rapid clinical examination to assess for associated injuries is warranted. After examining for distal pulses, a detailed physical examination of bony, nervous, and soft tissue structures is essential. If the patient is awake and cooperative, evaluation for compartment syndrome and a detailed neurologic examination of the injured limb are crucial. Liberal use of plain film radiography is encouraged, and wound markers should be used to delineate penetrating wounds. It should be noted that palpable pulses in the affected extremity do not exclude a vascular injury [11,12].
ANGIOGRAPHY OR STRAIGHT TO THE OPERATING THEATER?
The ankle–brachial index (ABI), the brachial–brachial index (BBI), and the arterial pressure index (API) are key tools to evaluate the vascular status of the injured limb. The ABI and BBI are calculated by using the systolic blood pressure of the injured extremity below the injury over the systolic pressure in the uninjured brachial artery. The API follows the same principle but uses the systolic Doppler pressure. An ABI/BBI/API of 0.9 or above is assumed to have a normal artery or at worst a small nonocclusive lesion (intimal tear, intramural hematoma of false aneurysm) that does not require emergent investigations [13]. An ABI/BBI/API of less than 0.9 mandates angiography with distal runoff (Table 1) [14].
Table 1.
Indication for angiogram with injured extremity
| Yes | Maybe | No |
|---|---|---|
| ABI < 0.90 | Pulseless | Ongoing hemorrhage |
| Bruit | Multilevel injuries | Partial or complete amputation |
| Thrill | Proximity wounds | |
| Shotgun wounds |
Indications for immediate surgical intervention include the traditional “hard signs” of ongoing hemorrhage/expanding hematoma, pulseless/cool limb, as well as partial/complete amputation, and indications for immediate investigation include palpable thrill, audible bruit, or potential multisegment injury (eg, shotgun injuries) [15,16]. CT angiography with distal runoff has become the imaging modality of choice at most modern trauma centers [17]. It has largely supplanted emergent conventional digital subtraction angiography, and bedside surgeon-performed angiograms for proximity wounds are now rarely performed. It should be remembered that for complex, life-threatening injuries, it may be more expedient to perform the angiogram in the operating theater.
If there is history of major bleeding at the scene (“soft sign”) and arterial and orthopedic injuries have been excluded, one must rule out a major venous injury. It is imperative to ambulate these patients in the emergency department/trauma bay to increase their venous return. If this does not induce significant hemorrhage, the patient may be safely discharged. There is no role for urgent venography, but these patients should be followed to rule out an arteriovenous fistula.
THE LIMB THAT IS BARELY HANGING ON
A mangled extremity is defined as having significant injuries to 3 of the 4 functional components (vessels, soft tissue, bones, and nerves) that result in questionable limb viability. Although these injuries may not be initially life threatening, they generally pose very difficult, complex management challenges (Fig 2). There are several scoring indices available to help predict limb salvage and adverse outcomes, but they have been met with lack of external validity and poor prediction of long-term function (Table 2) [[18], [19], [20], [21], [22], [23]]. The management of such injuries requires a multidisciplinary approach that must weigh the complex physiologic interactions between systemic effects of the injured extremity and limb specific factors to decide whether one should proceed with limb salvage or amputation. Limb salvage is multifaceted, is resource intensive, and requires ongoing specialized care. Should the decision be made to proceed with amputation, every attempt should be made to have at least 2 surgical opinions especially for upper extremities. The Western Trauma Association has a comprehensive, practical, well laid out algorithm for management of a mangled extremity [18].
Fig 2.
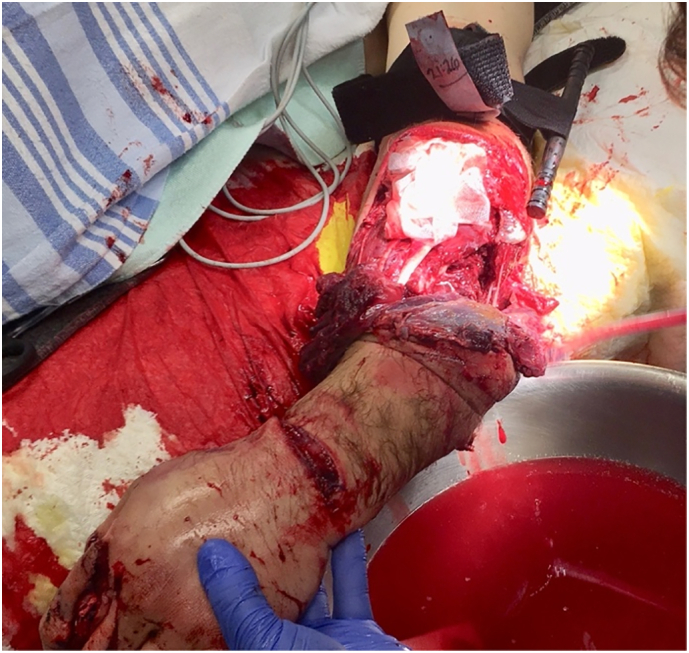
Mangled upper extremity with tourniquet.
Table 2.
Mangled extremity predictive scoring systems
| MESS (Mangled Extremity Severity Score) | |
| PSI (Predictive Salvage Index) | |
| NISSA (Nerve injury, Ischemia, Soft tissue, Skeletal injury, Shock, Age) | |
| LSI (Limb Salvage Index) | |
| HFS (Hanover Fracture Scale) |
However, traumatic amputation is an entirely different situation, as the “major” decision has already been made. Although intuitive, the level of debridement and subsequent amputation should occur in the most distal area with adequate perfusion. Guillotine amputation should be avoided because it sacrifices viable tissue that will certainly compromise proper closure. The focus at the initial operation is suture ligation of transected vessels for hemorrhage control, irrigation (not high pressure), and judicious debridement of devitalized tissue leaving as much healthy tissue and bone behind as possible. In the acute setting, it is best to pack the wound open and bring the patient back to the operating theater once physiology has been restored and the wound can be more adequately assessed.
TIME IS NOT ALWAYS ON YOUR SIDE
Time is of the essence with arterial injuries. The quicker one can restore reperfusion to the injured limb is paramount to improving long-term function, and anything beyond 6 hours of ischemia is unlikely going to be successful [24]. Although the specific surgical management for extremity vascular injuries is beyond the scope of this paper, it is important to highlight a few points and discuss several damage control techniques.
FINALLY IN THE OPERATING THEATER
Prep widely. This should include the chest to the contralateral nipple to the ipsilateral fingertips for the upper extremity and up to umbilicus down to toes for the lower extremity. Be sure to always prep the uninjured lower extremity. If manual pressure is required to control major hemorrhage, the assistant can place sterile gloves and apply direct pressure to the wound as the team preps the patient. After prepping, proximal control of the vessel can be obtained through a separate incision before the assistant removes his/her hand. With small wounds, balloon tamponade (5–30 mL balloon) works very well to control hemorrhage while prepping and even during the operation. It is entirely reasonable to perform separate small incisions to obtain proximal and/or distal control rather than making one large incision that includes the wound or pulsatile hematoma [12].
CAN I LIGATE THAT?
Ligation of named major arteries proximal to the knee or elbow, with plans for delayed reconstruction and or/anatomic bypass, should only be considered if the patient is in extremis because it carries a significantly increased risk of amputation and mortality. In this situation, a temporary intravascular shunt (TIVS) is a much better option (see below). However, the brachial artery can be ligated distal to the profunda brachii artery. Smaller distal vessels such as the ulnar, radial, or tibial arteries can be ligated as long as one in that limb remains patent.
Although almost any major peripheral vein can be ligated without fear of limb loss, it is not without potential complications. Immediate effects of ligation may result in increased venous hemorrhage from proximal wounds; decreased arterial patency with a concomitant, ipsilateral arterial repair; and increased need for fasciotomies. The latter two are particularly concerning with popliteal vein ligation, and as such, it is more the exception to the venous ligation rule and a reasonable attempt should be made to repair it. In the stable patient, primary venous repair is a reasonable choice. Although complex repairs are generally performed with a contralateral saphenous vein graft, externally supported PTFE in larger veins has been shown to have acceptable patency rates [25]. Long-term effects of venous ligation may include sequelae of venous hypertension.
TO SHUNT OR NOT TO SHUNT
Temporary intravascular shunts are most commonly indicated for damage control in the patient with near exsanguination. They are also indicated for repair of open fractures with associated soft tissue loss and arterial injury (Gustillo IIIC), distal perfusion during complex revascularization procedures, perfusion of a near-amputated part of the upper extremity prior to reimplantation, and temporary stabilization before transfer to definitive care (Table 3) [12,[26], [27], [28], [29]]. Although many options exist for TIVS (chest tubes, Pruitt-Inahara, Javid), Argyle shunts are the most commonly used with average dwell times up to 48 hours [26].
Table 3.
Indications for temporary intravascular shunts
| Vascular damage control | |
| Gustillo IIIC fractures | |
| Temporize during complex vascular repairs | |
| Perfusion of near amputated extremity prior to re-implantation | |
| Temporary stabilization prior to transfer to definitive care |
There are several important technical points to consider when inserting a TIVS [12,28,30]. The largest possible shunt that the vessel can accommodate should be used. It should be cut at least 4 cm longer than the gap between the 2 vessels so that 1.5–2.0 cm can be inserted into each lumen of the transected artery. Any kink in the TIVS or native vessel should be avoided, and the TIVS should be placed and kept in line with the native vessel. A 2-0 tie is placed in the midpoint of the shunt, and an occluding hemostat should be closed on the same spot as the tie. Fogarty catheter (balloon) thrombectomy should be performed proximally and distally before inserting the shunt. The proximal end is inserted first and secured down to the native vessel approximately 0.5–1.0 cm from the transected end with a free tie. The occlusive hemostat is then temporarily released to evaluate pulsatile flow. The distal end of the TIVS is inserted and tied down in similar fashion. The hemostat is released, and pulsatile flow can be confirmed by palpation and/or Doppler signal. The ties on the vessels can be left long so that they may be tied together to further stabilize the TIVS and prevent dislodgement or migration. Local heparinization prior to securing the TIVS is important, but systemic heparinization is not necessary intraoperatively or postoperatively. Associated major venous injuries should be shunted or repaired to decrease the risk of arterial thrombosis (Fig 3).
Fig 3.
Temporary intravascular shunts in the superficial femoral artery (SFA) and superficial femoral vein (SFV).
THAT LIMB LOOKS TIGHT
These types of patients are at high risk of developing extremity compartment syndrome especially if they have been hypotensive with prolonged ischemic time, have combined arterial and venous injuries, have the need for ligation of a named vessel (venous or especially arterial), and/or have combined orthopedic injuries. Preoperative assessment for the 6 “P's” (pulselessness, pain, pallor poikilothermia, paresthesia, and paralysis) is important, but most are very late signs. With an awake cooperative patient, excessive pain particularly with passive stretch at the ankle or wrist is often the earliest sign and must prompt investigation or intervention. Compartment pressures can be easily measured with a commercial handheld device or with a long needle attached to bedside pressure transducer (as typically used for arterial lines). Any pressure over 30 mm Hg and a risk factor listed above should be considered for extremity fasciotomy. Surgical techniques to describe fasciotomies have been well described [30]. Briefly, a 2-incision approach can be used to open all four compartments of the lower extremity (below knee), and a single volar incision can be used to decompress the 3 main compartments of the upper extremity (below elbow).
Thankfully, massive extremity hemorrhage can generally be temporarily controlled with direct pressure, balloon tamponade, or a properly applied tourniquet which can allow for safe transport to definitive care. With the multiple-injured patient, it is often very helpful to have 2 surgical teams available when evaluating/treating massive extremity hemorrhage. Management of the mangled extremity continues to be very challenging and is best accomplished with a multidisciplinary approach. TIVSs have been shown to be very effective and safe. Knowing when and how to use them is critical in the management of massive traumatic lower extremity hemorrhage.
Conflict of Interest
The author has no conflicts of interest to declare.
Funding Sources
No funding sources were used in the preparation of this manuscript.
References
- 1.Frykberg E.R., Schinco M.A., Feliciano D.V., Mattox K.L., Moore E.E. Trauma. 6th ed. McGraw-Hill Medical; 2008. Peripheral vascular injury; pp. 941–971. [Google Scholar]
- 2.Butler F.K. Stop the bleed. Strategies to enhance survival in active shooter and intentional mass casualty events. The Hartford consensus. A major step forward in translating battlefield trauma care advances to the civilian sector. J Spec Oper Med. 2015;15(4):133–135. [PubMed] [Google Scholar]
- 3.Ball C.G., Wyrzykowski A.D., Nicholas J.M., Rozycki G.S., Feliciano D.V. A decade’s experience with balloon catheter tamponade for emergency control of hemorrhage. J Trauma. 2011;70:330–333. doi: 10.1097/TA.0b013e318203285c. [DOI] [PubMed] [Google Scholar]
- 4.Dennis A., Bajani F., Schlanser V., Tatebe L.C., Impens A., Ivkovic K., et al. Missing expectations: windlass tourniquet use without formal training yields poor results. J Trauma Acute Care Surg. 2019;87(5):1096–1103. doi: 10.1097/TA.0000000000002431. [DOI] [PubMed] [Google Scholar]
- 5.Inaba K., Siboni S., Resnick S., Zhu J., Wong M.D., Haltmeier T., et al. Tourniquet use for civilian extremity trauma. J Trauma Acute Care Surg. 2015;79(2):232–237. doi: 10.1097/TA.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 6.Smith A.A., Ochoa J.E., Wong S., Beatty S., Elder J., Guidry C., et al. Prehospital tourniquet use in penetrating extremity trauma: decreased blood transfusions and limb complications. J Trauma Acute Care Surg. 2019;86(1):43–51. doi: 10.1097/TA.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 7.Benitez C.Y., Ottolino P., Pereira B.M., lima D.S., Guemes A., Khan M., et al. MAF. Tourniquet use for civilian extremity hemorrhage: systematic review of the literature. Rev Col Bras Cir. 2021;13(48) doi: 10.1590/0100-6991e-20202783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira P.G.R., Brown C.V.R., Emigh B., Long M., Foreman M., Eastridge B., et al. Texas Tourniquet Study Group. Civilian prehospital tourniquet use is associated with improved survival in patients with peripheral vascular injury. J Am Coll Surg. 2018;226(5):769–776. doi: 10.1016/j.jamcollsurg.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Passos E., Dingley B., Smith A., Engels P.T., Ball C.G., Faidi S., et al. Canadian Trauma Trials Collaborartive. Tourniquet use for peripheral vascular injuries in the civilian setting. Injury. 2014;45(3):573–577. doi: 10.1016/j.injury.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham A., Auerbach M., Cicero M., Jafri M. Tourniquet usage in prehospital care and resuscitation of pediatric trauma patients—Pediatric Trauma Society position statement. J Trauma Acute Care Surg. 2018;85(4):665–667. doi: 10.1097/TA.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 11.Ball C.G. Penetrating non-torso trauma: the extremities. Can J Surg. 2015;58(4):286–288. doi: 10.1503/cjs.005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feliciano D.V. Pitfalls in the management of peripheral vascular injuries. Trauma Surg Acute Care Open. 2017;2:1–8. doi: 10.1136/tsaco-2017-000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen K., Lynch K., Paun M., Copass M. Non-invasive vascular tests reliably exclude occult arterial trauma in injured extremities. J Trauma. 1991;31:515–522. [PubMed] [Google Scholar]
- 14.Lynch K., Johansen K. Can Doppler pressure measurement replace “exclusion” arteriography in the diagnosis of occult extremity arterial trauma? Ann Surg. 1991;214:737–742. doi: 10.1097/00000658-199112000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feliciano D.V., Moore F.A., Moore E.E., West M.A., Davis J.W., Cocanour C.S., et al. Evaluation and management of peripheral vascular injury. Part 1. Western Trauma Association/critical decisions in trauma. J Trauma. 2011;70(6):1551–1556. doi: 10.1097/TA.0b013e31821b5bdd. [DOI] [PubMed] [Google Scholar]
- 16.Feliciano D.V., Moore E.E., West M.A., Moore F.A., Davis J.W., Cocanour C.S., et al. Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part II. J Trauma Acute Care Surg. 2013;75(3):391–397. doi: 10.1097/TA.0b013e3182994b48. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K., Branco B.C., Reddy S., Park J.P., Green D., Plurad D., et al. Prospective evaluation of multidetector computed tomography for extremity vascular trauma. J Trauma. 2011;70(4):808–815. doi: 10.1097/TA.0b013e3182118384. [DOI] [PubMed] [Google Scholar]
- 18.Scalea T.M., DuBose J., Moore E.E., West M., Moore F.A., McIntyre R., et al. Western Trauma Association critical decisions in trauma: management of the mangled extremity. J Trauma Acute Care Surg. 2012;72(1):86–93. doi: 10.1097/TA.0b013e318241ed70. [DOI] [PubMed] [Google Scholar]
- 19.McNamara M.G., Heckman J.D., Corley F.G. Severe open fractures of the lower extremity: a retrospective evaluation of the Mangled Extremity Severity Score (MESS) J Orthop Trauma. 1994;8:81–87. doi: 10.1097/00005131-199404000-00001. NISSA. [DOI] [PubMed] [Google Scholar]
- 20.Howe H.R., Jr., Poole G.V., Jr., Hansen K.J., et al. Salvage of lower extremities following combined orthopedic and vascular trauma. A predictive salvage index. Am Surg. 1987;53:205–208. PSI. [PubMed] [Google Scholar]
- 21.Johansen K., Daines M., Howey T., Helfet D., Hansen S.T., Jr. Objective criteria accurately predict amputation following lower extremity trauma. J Trauma. 1990;30:568–572. doi: 10.1097/00005373-199005000-00007. [discussion 572–573.(MESS)] [DOI] [PubMed] [Google Scholar]
- 22.Russell W.L., Sailors D.M., Whittle T.B., Fisher D.F., Jr., Burns R.P. Limb salvage versus traumatic amputation. A decision based on a sevenpart predictive index. Ann Surg. 1991;213:473–480. doi: 10.1097/00000658-199105000-00013. [discussion 480–481. (LSI)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tscherne H., Oestern J.H. A new classification of soft-tissue damage in open and closed fractures. Unfallheilkunde. 1982;85:111–115. HFS. [PubMed] [Google Scholar]
- 24.Burkhardt G.E., Gifford S.M., Propper B., Spencer J.R., Williams K., Jones L., et al. The impact of ischemic intervals on neuromuscular recovery in a porcine (Sus scrofa) survival model of extremity vascular injury. J Vasc Surg. 2011;53(1):165–173. doi: 10.1016/j.jvs.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Parry N.G., Felciano D.V., Burke R.M., Cava R., Nicholas J.M., Dente C.J., et al. Management and short-term patency of lower extremity venous injuries with various repairs. Am J Surg. 2003;186(6):631–635. doi: 10.1016/j.amjsurg.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Inaba K., Aksoy H., Seamon M.J., Marks J.A., Duchesne J., Schroll R., et al. Multicenter Shunt Study Group. Multicenter evaluation of temporary intravascular shunt use in vascular trauma. J Trauma and Acute Care Surg. 2016;80(3):359–365. doi: 10.1097/TA.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian A., Vercruysse G., Dente C., Wyrzykowski A., King E., Feliciano D.V. A decade’s experience with temporary intravascular shunts at a civilian level I trauma center. J Trauma. 2008;65(2):316–324. doi: 10.1097/TA.0b013e31817e5132. (discussion 324–6) [DOI] [PubMed] [Google Scholar]
- 28.Ball C.G., Feliciano D.V. Damage control techniques for common and external iliac artery injuries: have temporary intravascular shunts replaced the need for ligation? J Trauma. 2010;68(5):1117–1120. doi: 10.1097/TA.0b013e3181d865c0. [DOI] [PubMed] [Google Scholar]
- 29.Ball C.G., Kirkpatrick A.W., Rajani R.R., Wyrzykowski A.D., Dente C.J., Vercruysse G.A., et al. Temporary intravascular shunts: when are we really using them according to the NTDB. Am Surg. 2009;75(7):605–607. [PubMed] [Google Scholar]
- 30.Parry N.G., Bowyer M.W., Kuncir E.J. 2nd ed. American College of Surgeons; Chicago (IL): 2020. Advanced surgical skills for exposure in trauma: exposure techniques when time matters. [Google Scholar]




