Abstract
Objective
There is an unmet need for noninvasive continuous blood pressure (BP) monitoring technologies in various clinical settings. Continuous and noninvasive central aortic BP monitoring is technically not feasible currently, but if realized, would provide more accurate and real-time global hemodynamic information than any form of peripheral arterial BP monitoring in acute care setting. As part of our efforts to develop such, herein we examined the tracking correlation between noninvasively-derived peripheral arterial BP by Caretaker device against invasively measured central aortic BP.
Methods
Beat-to-beat BP by Caretaker was recorded simultaneously with central aortic BP measured in patients undergoing cardiac catheterization. Pearson’s correlation was also derived for systolic BP (SBP) and diastolic BP (DBP). A trend comparison analysis of the beat-to-beat BP change was performed using 4-quadrant plot analysis with the exclusion zones of 0.5 mmHg/second to determine concordance, (i.e. the direction of beat-to-beat changes in SBP and DBP).
Results
A total of 47 patients were included in the study. A total of 31,369 beats representing an average of 17.3 minutes of recording were used for analysis. The trend analysis yielded concordances of 84.4% and 83.5% for SBP and DBP, respectively. Respective correlations (Pearson’s r) for SBP and DBP trends were 0.87 and 0.86 (p < 0.01). Tracking of beat-to-beat BP by Caretaker showed excellent concordance and correlation in the direction and the degree of BP change with central aortic BP, respectively.
Conclusion
This study supports the satisfactory performance of the Caretaker device in continuous tracking of central aortic BP beat-to-beat BP and provides a basis to develop an algorithm for absolute central aortic BP estimation in the future.
Keywords: blood pressure, monitoring, validation
Introduction
Blood pressure (BP) monitoring constitutes the most important vital sign assessment in the acute care setting. There are many circumstances wherein a patient would benefit from more intensive continuous BP monitoring, but such is not possible due to invasive nature of the direct arterial catheter-derived BP monitoring [1, 2]. This limitation poses a great challenge in real-time hemodynamic assessment and timely clinical decision making. While limited options for noninvasive continuous BP monitoring technology have been available [3], their use is still rare in clinical practice for various reasons including lack of confidence about the accuracy, unfamiliarity of the technology by the users, and discomfort associated with the prolonged use of the finger cuff [4].
The Caretaker device is a recently developed continuous noninvasive physiological monitor (Caretaker Medical LLC, Charlottesville, Virginia) that is CE-cleared and FDA-cleared for the measurement of continuous noninvasive BP (FDA K151499), measurement of heart rate and respiratory rate, and self-calibration (FDA K163255). This device has been previously validated for continuous BP tracking against BP from radial artery catheter [5] and self-calibration [6] in full accordance with the ANSI/AAMI/ISO 81060–2:2019 [7].
The purpose of this study was to compare continuous beat-to-beat peripheral BP measured by Caretaker with invasively measured continuous beat-to-beat central aortic BP. Currently available central BP measuring devices employ a brachial cuff to estimate central aortic BP intermittently as opposed to the beat-to-beat measurement as in the Caretaker device. Caretaker BP has previously shown good correlation against invasively measured central aortic BP [8]. However, in that study, the observation period duration was brief (90 sec). As part of the effort to develop peripheral-central transfer function, this study was intended to examine the tracking correlation (i.e., correlation of the direction and the degree of peripheral BP change) between peripheral BP by Caretaker with invasively measured central aortic BP in a beat-to-beat fashion over a longer period of time.
Methods
Beat-to-beat BP monitoring technology
The Caretaker device uses a low pressure, pump-inflated, finger cuff that pneumatically couples arterial pulsations via a pressure line to a custom-designed piezo-electric pressure sensor for detection and analysis. The system and underlying approach have been described elsewhere [9, 10]. Briefly, the pulse decomposition analysis (PDA) approach is based on the concept that two central reflection sites are responsible for the shape of the pressure pulse envelope of the upper body. This concept has been illustrated in Figure 1. The first reflection site is the juncture between thoracic and abdominal aorta, which is marked by a significant decrease in diameter and a significant change in elasticity. The reflection coefficient of this juncture is highly sensitive to BP changes because of the pressure-dependent expansion of the diameter of the thoracic artery relative to that of the abdominal artery. The second reflection site arises from the juncture between abdominal aorta and the common iliac arteries. The two reflected arterial pressure pulses are referred to as component pulses and both counter-propagate with respect to the original pulse due to left ventricular contraction. Caretaker extracts these ‘reflected’ arterial pressure pulse wave forms (component pulses) from the peripheral site. The primary systolic pulse is termed P1. The reflected pulses, the renal reflection pulse (P2 i.e. second systolic pulse) and the iliac reflection pulse (P3 i.e. diastolic pulse as it arrives during diastole), arrive with distinct time delays. Quantification of the physiological parameters is accomplished by extracting these pertinent component pulse parameters. The ratio of the amplitude of the P2 to that of P1 tracks changes in central beat-by-beat systolic BP (SBP). The time difference between the arrival of P1 and P3, referred to as T13, tracks changes in arterial pulse pressure. This allows derivation of diastolic BP (DBP). Thus, Caretaker measures peripheral BP using central aortic pulse wave form reflected in the digital artery. As compared to other technologies based on volume clamping methods, the Caretaker’s finger cuff with typical coupling pressures of about 30–40 mmHg is readily tolerated by patients [11].
Figure 1.
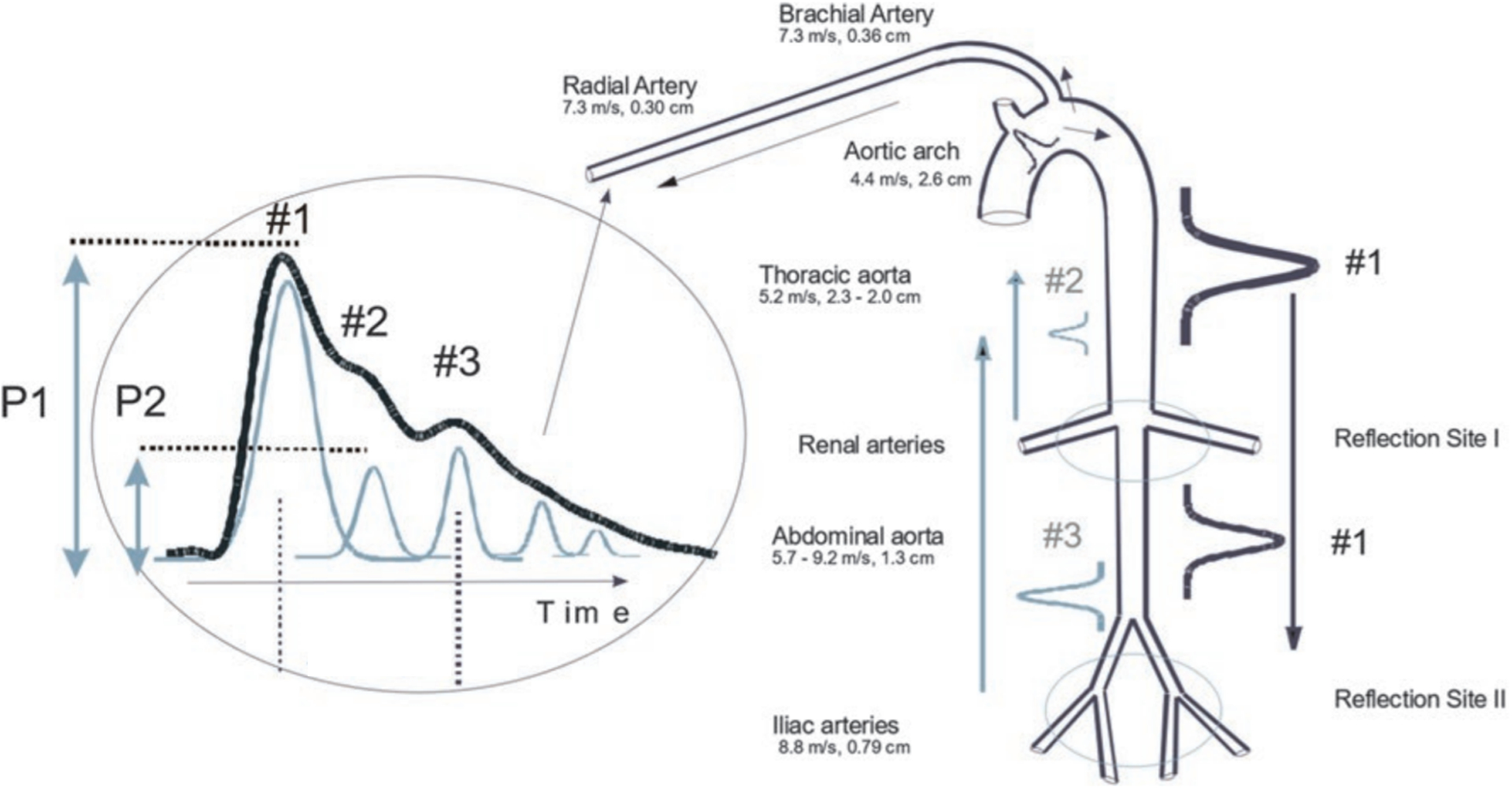
Sketch of the aorta/arm complex arterial system and its effect on the arterial pressure pulse line shape observed at the radial/digital artery. Two reflection sites, one at the height of the renal arteries, the other one in the vicinity of the iliac bifurcation, give rise to the reflected pulses (#2, #3, gray) that trail the primary left ventricular ejection (#1, black).
Patients and data collection
As part of the study approved by the University of Virginia Medical Center Review Board (IRB protocol 20826), patients undergoing clinically indicated cardiac catheterization and able to provide an informed consent at the University of Virginia Hospital were recruited. Those less than age 18 or undergoing emergency cardiac catheterization due to acute ST elevation myocardial infarction were not eligible for the study. Clinical indications for cardiac catheterization included evaluation of coronary heart disease, aortic valve disease, or preoperative evaluation. Enrolled patients had the Caretaker finger cuff applied to the proximal part of the middle phalanx of the left hand. With a patient resting in a supine position, a catheter was inserted into either radial or femoral artery and advanced toward the aortic root under fluoroscopy. The catheter size was either 5- or 6-French. All catheters were fluid-filled Judkins-type or Jacky angiographic catheters. Specific catheter size and choice was left to cardiac catheterization operator preference, and included the Medtronic (Minneapolis, MN, USA) Dxterity diagnostic cathether and Launcher guide catheter, as well as the Terumo (Tokyo, Japan) Optitorque diagnostic catheter. The catheter was zeroed prior to recording any pressure measurements. First, the phlebostatic axis was located at the fourth intercostal space at the mid-axillary line, which corresponds to the anatomical location of the right atrium. The pressure transducer was placed at the level of the phlebostatic axis, which eliminated the effect of hydrostatic pressure on readings. All catheters were flushed with heparinized saline prior to their use and underwent intermittent flushing with throughout their use with constant monitoring of the pressure contour. Rebalancing of the zero baseline was performed intermittently while the pressures were being collected. Invasive central aortic BP recordings (either at the tip of the catheter in the aortic root or engaged into coronary orifices) continued except at the time of contrast injection. Vasoactive medications were not utilized during the performance of the cardiac catheterization.
After the Caretaker system performed a self-calibration procedure, beat-to-beat BP was recorded simultaneously, and the recording continued throughout the entire procedure. The self-calibration procedure involves the pressure in the cuff’s bladder is ramping from 0 mmHg in steps of 10 mmHg, with the pulse signal being recorded between pressure steps. During the initial pressure rise the arterial walls of the two digital arteries are increasingly unloaded and the pulse signal accordingly increases until it reaches a peak, whereupon it declines as the arteries are increasingly clamped off. SBP and DBP are determined from the resulting pressure/signal profile. Real-time BP with waveform and heart rate were streamed wirelessly via Bluetooth from the wrist unit to a nearby tablet computer. Operators were blinded to Caretaker BP recordings.
Post processing and analysis of the data
Both data streams were time synchronized by first matching the recording’s computer time to the laboratory’s registered time and then matching the beat-to-beat inter-beat interval variability. The Caretaker’s SBP and DBP readings were then compared with respective BP recordings obtained directly from the catheter data tracings. Central aortic BP from the catheter were visually inspected and reviewed by two cardiologists involved in the study, but who were blinded to the Caretaker BP data. Intermittent portions of absent or incomplete central aortic BP tracings during contrast injection or percutaneous coronary intervention were excluded from the data. Segments containing dampened waveforms or motion artifacts, as well as segments with acute spontaneous deviation from baseline tracings that were felt to be non-physiologic response, were excluded. Only segments of at least greater than 100 beats of high-quality tracings were extracted and included in the analysis. For the Caretaker data, a custom signal/noise factor (SNF) was used to identify poor quality data sections for exclusion. The factor is based on the ratio of the variances of the physiological signal band to the noise band and obtained using Fourier spectral analysis over an 8-s window with 1s overlap [12]. The frequency range of the band associated with the physiological signal was set to 1–10 Hz, based on data by the authors and results by others [13], while the noise band was set to the 100–250 Hz frequency range, which is subject to ambient noise but contains no signal relevant to the base band phenomena of the arterial pressure pulse or its propagation characteristics. Data sections with an SNF below 80 were excluded from the analysis. All valid BP data points were included for BP analysis. The Caretaker and central aortic BP data were averaged over approximately 5 second time windows and matched rates, i.e. mmHg/sec, were calculated by obtaining the ratio of BP and time differences of adjacent SBP and DBP data points. Zero change rates were excluded. All beat-to-beat BP data from individual patients were combined. A trend comparison analysis of the beat-to-beat BP change was performed using 4-quadrant plot analysis with the exclusion zones of 0.5 mmHg/second to determine concordance, i.e. the direction of beat-to-beat changes in systole and diastole [14]. The 4-quadrant plot is a graphical tool to demonstrate the trending ability of measurement devices that allows for fast visual assessment of the characteristics of the studied technology and the reference technology. In this analysis, change in the same direction (concordant) will be shown as points in the right upper and left lower quadrant of the graph whereas change in the opposite direction (discordant) will be located in the right lower and left upper quadrant of the graph. Points that fall on the 45-degree diagonal line would imply perfect agreement. Points that fall above or below the 45-degree diagonal line would imply either overestimation or underestimation, respectively. Concordance rate in a four-quadrant plot was calculated by the ratio of the number of agreements to all data points [15]. Pearson’s correlation was also derived for SBP and DBP. The waveforms themselves were not compared.
Results
All 50 consecutive patients approached were enrolled. After excluding three patients whose data did not yield minimal quality central aortic BP data, 47 patients (Mean age 66.9 years, (female 42 %)) were included for the analysis. Patient characteristics were included in Table 1. For this set of patients and based on low SNF values in the Caretaker data, the mean total percentage of data excluded per patient data set was 6.3% (SD 2.85%). This mean value included 27% of data sets for which no data was excluded, i.e. 0%, while for the remainder of the data sets the maximum ranged up to 11.8%. Primary indications for cardiac catheterization procedures mostly consisted of coronary evaluation (37/47, 78%). A total of 31,369 beats (average 667.4 beats per patient) representing an average of 17.3 minutes of recording were used for analysis. Examples of central aortic and Caretaker BP tracings are illustrated in Figures 2a & 2b. All patients tolerated the Caretaker finger cuff throughout the procedure. Since the procedure was performed in hemodynamically stable patients, significant BP fluctuations during the recording were infrequent, though modest BP changes were observed in many patients.
Table 1.
Baseline Characteristics of Patients.
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age – mean (std. dev.) – yr. | 66.8 (9.8) |
| Body Mass Index – mean (std. dev.) – kg/m2 | 31.4 (7.3) |
| Male Sex – no. (%) | 27 (57.5) |
| Tobacco Use – no. (%) | 31 (66.0) |
| Hypertension – no. (%) | 40 (85.1) |
| Diabetes Mellitus – no. (%) | 22 (46.8) |
| Indication for Cardiac Catheterization – no. (%) | |
| Acute Coronary Syndrome, Stable Angina, or known Coronary Artery Disease | 28 (59.6) |
| Valvular Disease | 12 (25.5) |
| Other Indications | 7 (14.9) |
| Vascular Access Site – no. (%) | |
| Radial Artery | 26 (55.3) |
| Femoral Artery | 21 (44.7) |
Figure 2a.
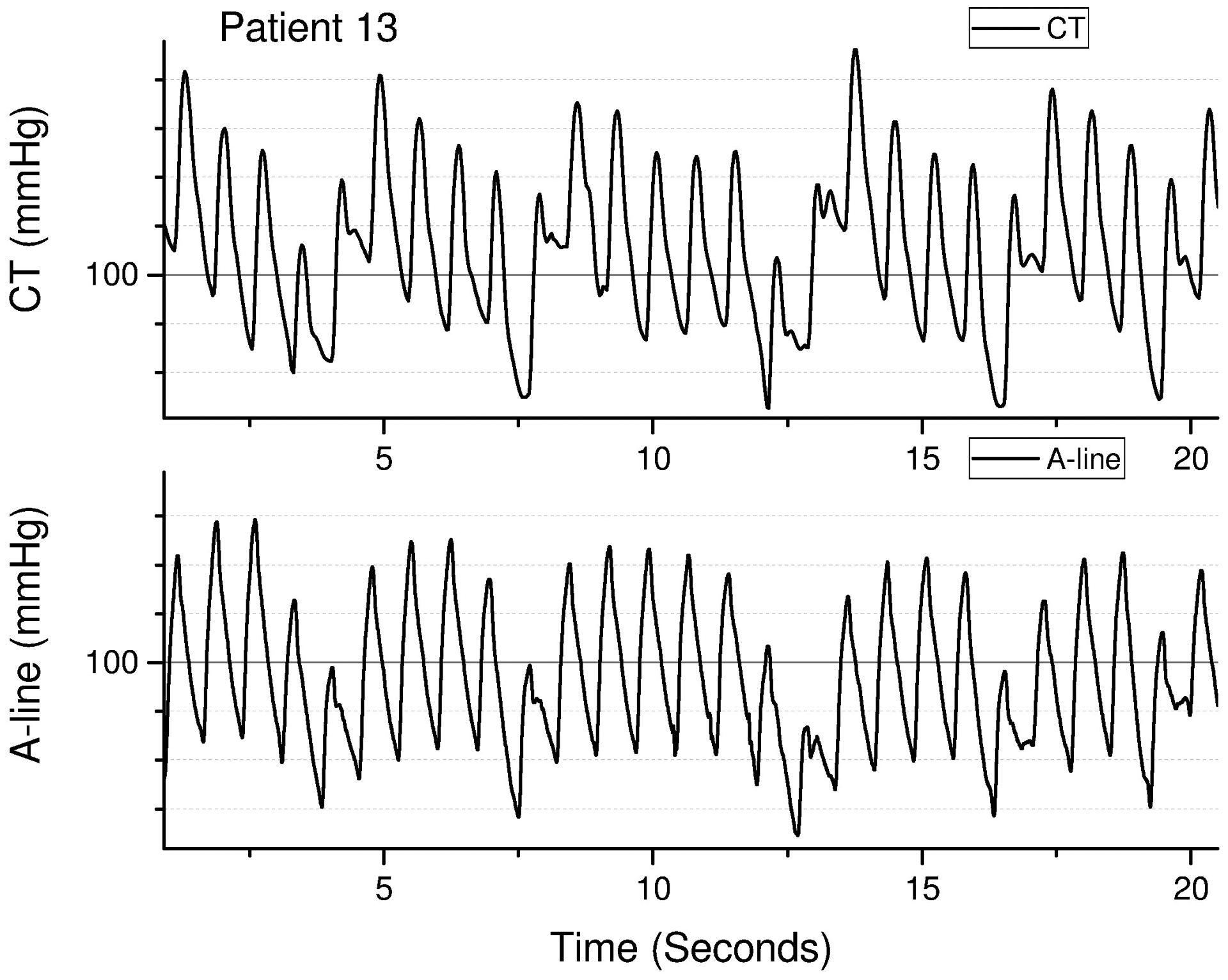
Example of pulse overlap between Caretaker (top) and aortic arterial catheter (bottom) for patient 13, a 58 year-old female with an indication for cardiac catherization of acute coronary syndrome.
Figure 2b.
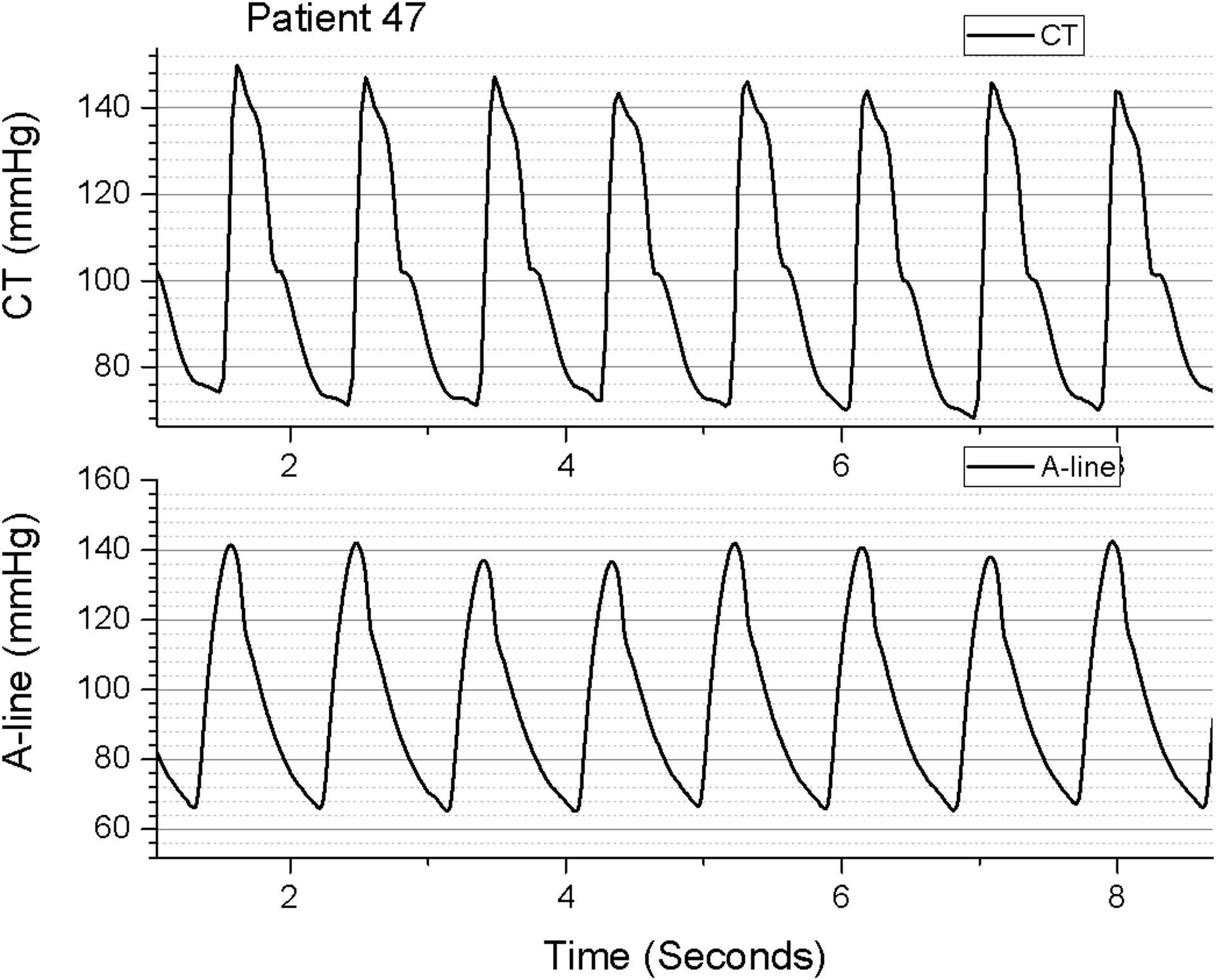
Example of pulse overlap between Caretaker (top) and aortic arterial catheter (bottom) for patient 47, a 75 year-old male with an indication for cardiac catherization of unstable angina.
The trend analysis yielded concordances of 84.4% and 83.5% for SBP and DBP, respectively (Figure 3). This implies that the direction of the beat-to-beat SBP and DBP changes (either increase or decrease) were concordant 84.4% and 83.5% of the time respectively between the Caretaker and invasive central aortic measurement. Respective correlations (Pearson’s r) for SBP and DBP trends were 0.87 and 0.86 (p < 0.01). Linear fits were performed as part of the trend analysis and are indicated in the respective plots of Figure 3. While for the systolic trend the angle between fitted line and unity slope, i.e. the 45-degree diagonal, was −15.3°, the corresponding angle for the diastolic trend was −12.2°. Figure 4 presents the distributions of the individual patient’s overall concordances for SBP and DBP.
Figure 3.
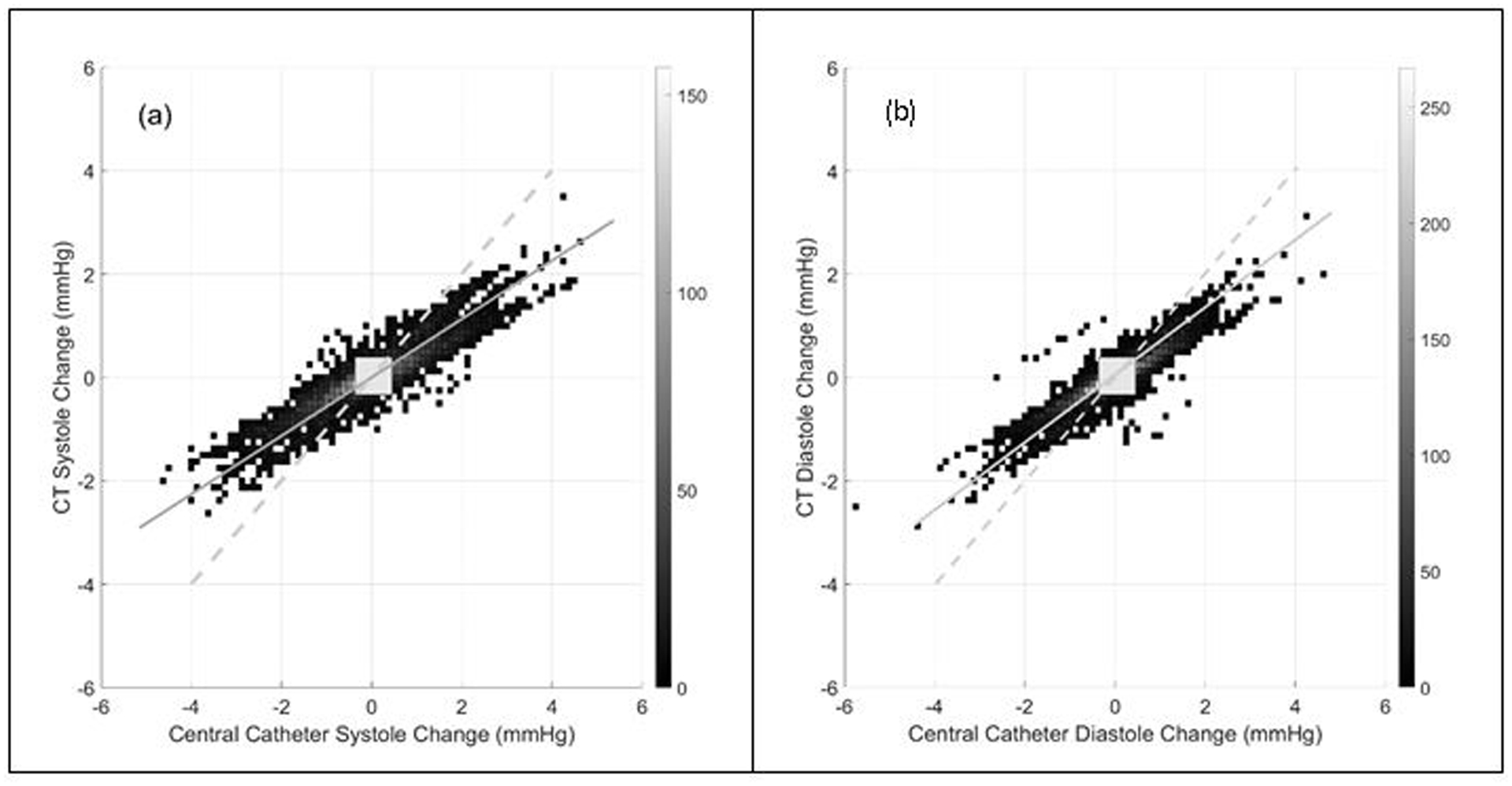
The surface plot of the three-dimensional histogram of beat-to-beat systolic (a) and diastolic (b) blood pressure change. The resolution of the histogram was 0.125 mmHg/second in both directions. The counts for each two-dimensional bin was represented as a grayscale intensity. Concordance rate for beat-to-beat systolic and diastolic blood pressure change were 0.844 and 0.835, respectively. The exclusion zones of 0.5 mmHg/second, or approximately 10% of the data range, are indicated as grey squares. Both plots also present linear fits to the distributions (solid gray lines) as well as unity slope (dashed gray) lines. For systole and diastole, respectively, the angular deviation from unity slope of the linear fit was −15.3° and −12.2°.
Figure 4.
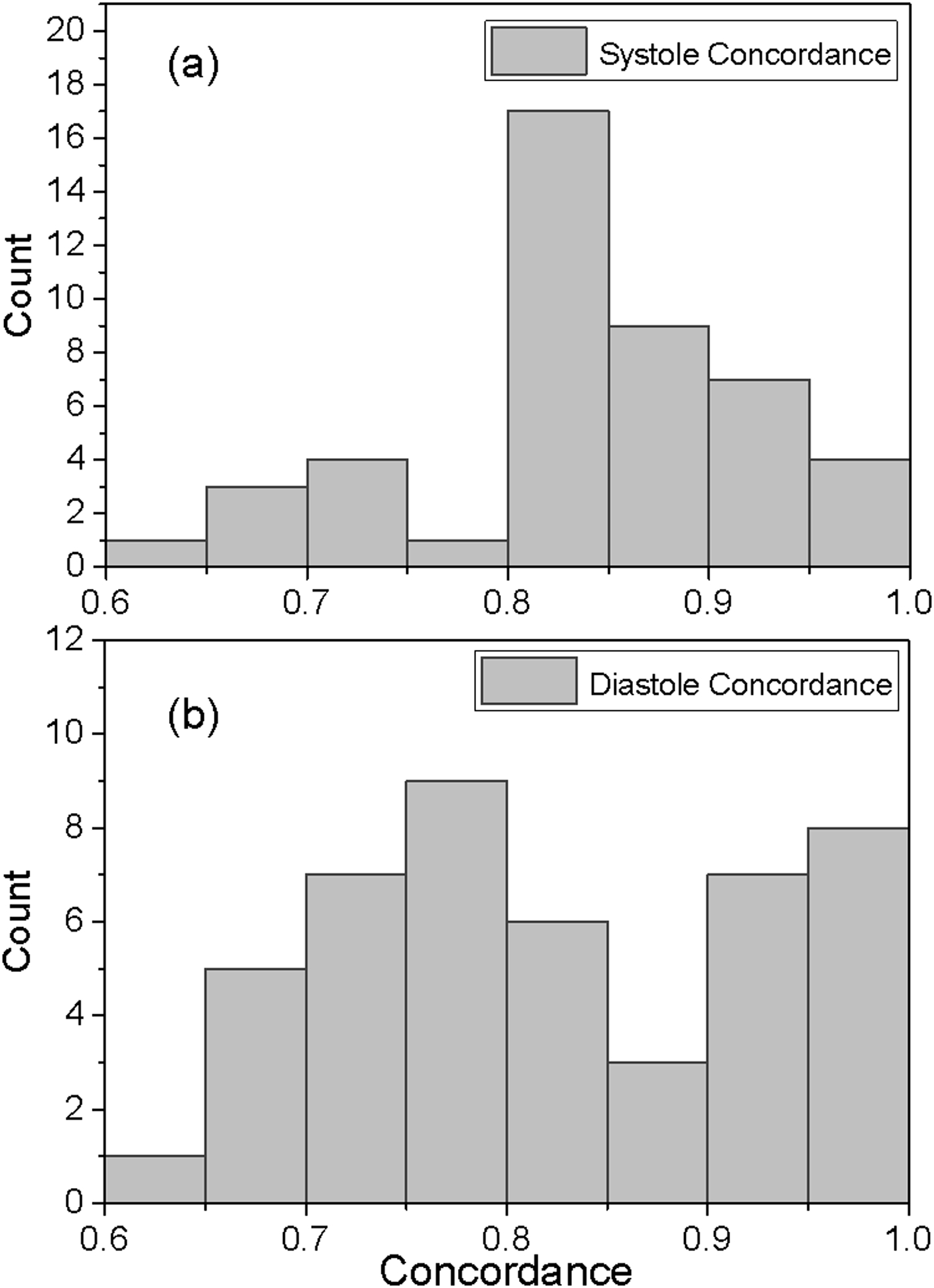
Distributions of the patient concordances for systole (a) and diastole (b). As an example, 17 patients had systolic concordances between 0.80 and 0.85, while only one patient had a systolic concordance between 0.75 and 0.80. The mean for the systolic concordance distribution is 0.83 (SD 0.08) while the corresponding measures for diastole are 0.82 (0.102).
Discussion
We demonstrated that the beat-to-beat BP measured by Caretaker highly tracked with invasively measured central aortic BP. In other words, there was excellent concordance in the direction and degree of BP change between the two methods [7]. We also observed that besides being convenient and of technical soundness, the use of the device did not interfere with workflow when used in the cardiac catheterization laboratory. The Caretaker finger cuff was well-tolerated by all participating patients and failures due to poor signal quality, principally due to motion artifacts, were rare. The demonstrated beat-to-beat BP tracking concordance results between Caretaker BP and central aortic BP show promising clinical implications in wide range of settings [16].
Central aortic BP has gained increasing attention, primarily in the context of hypertension and cardiovascular risk management. It is well known that central aortic BP differs from peripheral BP [17–19]. Somewhat tangentially, previous studies comparing simultaneous invasive BP monitoring of both central and peripheral sites have reported, albeit inconsistently, underestimation of SBP and mean BP by peripheral measurement. More importantly, central BP may be a better predicator of clinical outcome than peripheral BP. Specifically, the Conduit Artery Function Evaluation (CAFE) study, as well as other related studies, demonstrated significant reductions in coronary events, cardiovascular death, and stroke for BP-lowering drugs targeting central versus peripheral BP [20]. The prognostic value of central BP has also been reported in other studies [18, 21]. Recognition of the importance of central aortic BP has led to the development of noninvasive central BP measuring technologies, and as such, appropriate validation of these devices is becoming increasingly important [22]. However, those technologies that use either applanation tonometry or brachial cuff plethysmography are not meant to provide continuous beat-to-beat estimation of central aortic BP [23], but are limited to single spot-check measurements. Although it is not currently available, noninvasive continuous beat-to-beat central aortic BP monitoring, if realized, will provide unique hemodynamic information that may be beneficial in better understanding of the global hemodynamics in acute care setting. Therefore, the findings of this study, which focused on examining the behavior of peripheral beat-to-beat BP change measured by Caretaker in relation to central beat-to-beat aortic BP provide a basis for future transfer function development of central aortic BP estimation by Caretaker.
In our previous study including patients undergoing major abdominal surgery, Caretaker BP showed excellent agreement with invasively measured radial artery BP, where a direct comparison, as opposed to a tracking comparison of this current study, was possible [5]. It is important to note that the purpose of this study was not to validate or make a direct comparison with absolute central aortic BPs. While the Caretaker tracks central BP based on the predictions of the PDA model, through calibration process, it is referenced to peripheral BP derived from finger. In addition, there exists great subject variability in the relationship between central and peripheral BP [17–19, 24]. SBP generally amplifies as blood transits from the aorta through the brachial artery [25]. Therefore, estimation of central aortic BP by Caretaker would require more a complex transfer function.
Our 4-quadrant plot analysis showed excellent tracking of central aortic BP by Caretaker device as evidenced by narrow trend (change) distribution. However, some degree of negative angular difference from the unity slope (−15.3°, −12.2° for SBP and DBP, respectively) existed suggesting that the peripheral Caretaker BP data “underestimates” the degree of the central aortic BP beat-to-beat changes. This “dampening” of the peripheral trend response may be due to the digital arterial site where the Caretaker obtains its signal, since lower BP in the digits will correspondingly also attenuate BP trends. To our knowledge, this phenomenon has not been previously described in prior studies comparing central vs. peripheral BP [26–28]. While the trend response dampening we observed was clearly systemic, the divergence of peripheral and aortic blood pressure has been observed under certain circumstances [29]. However, it is also possible that the absence of larger dynamic swings skewed the trend results. Follow-up studies with larger patient cohorts will seek to illuminate this issue. The wide range of concordances between individuals is likely related to the overall magnitude of the individual patient’s BP swings. In fact, excessive BP change (as illustrated in Figure 2a) was rather uncommon, understandably as nearly all patients included in the study were hemodynamically stable. The wider distribution of the DBP concordances, quantitatively expressed by their SDs, can be explained by smaller DBP swings compared to SBP. Accurate assessment of the trend becomes more challenging with smaller BP variations because the systemic errors associated with assessing pulse parameters remain the same irrespective of BP and BP variations, which is primarily associated with the fidelity of the pulse signal.
Limitations of the study include motion artifact issues and time recordings of unequal lengths for different patients resulting in unequal weighing of patients’ data in the trend data. These were principally associated with the accommodations necessary to perform research within the clinical workflow of cardiac catheterization. Further, the age range of the included subjects skews towards patients of older age. This mirrors those at higher likelihood of undergoing coronary angiography, and also reflects the ages of the general intensive care unit population. Future studies should further examine the Caretaker’s usability in a broader clinical setting including in the intensive care unit over a longer time period. Moreover, it will be important to demonstrate its impact on workflow and outcomes. Future studies should also examine the robustness of the estimation in the setting of more extreme BP range and the use of vasoactive medications. In conclusion, BP by Caretaker yielded excellent concordance in the direction and the correlation of beat-to-beat BP change compared with invasively measured central aortic BP.
Acknowledgements:
This work has not been previously presented on the whole or in part.
Funding:
This work was partly supported by research grant provided by Caretaker Medical Inc. YK was supported by NIH R21 HL140432.
Footnotes
Conflicts of Interest: Martin Baruch is an employee of the Caretaker Medical Inc., which sponsored the study; Younghoon Kwon has received travel reimbursement for a company sponsored talk.
References
- [1].Stenglova A, Benes J. Continuous Non-Invasive Arterial Pressure Assessment during Surgery to Improve Outcome. Front Med [Internet]. 2017. November 17 [cited 2020 Nov 19];4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5698264/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saugel B, Hoppe P, Nicklas JY, Kouz K, Körner A, Hempel JC, et al. Continuous noninvasive pulse wave analysis using finger cuff technologies for arterial blood pressure and cardiac output monitoring in perioperative and intensive care medicine: a systematic review and meta-analysis. Br J Anaesth. 2020. July 1;125(1):25–37. [DOI] [PubMed] [Google Scholar]
- [3].Smolle K-H, Schmid M, Prettenthaler H, Weger C. The Accuracy of the CNAP® Device Compared with Invasive Radial Artery Measurements for Providing Continuous Noninvasive Arterial Blood Pressure Readings at a Medical Intensive Care Unit: A Method-Comparison Study. Anesth Analg. 2015. December;121(6):1508–16. [DOI] [PubMed] [Google Scholar]
- [4].Kwon Y, Stafford PL, Lim DC, Park S, Kim S-H, Berry RB, et al. Blood pressure monitoring in sleep: time to wake up. Blood Press Monit. 2020. April;25(2):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gratz I, Deal E, Spitz F, Baruch M, Allen IE, Seaman JE, et al. Continuous Non-invasive finger cuff CareTaker® comparable to invasive intra-arterial pressure in patients undergoing major intra-abdominal surgery. BMC Anesthesiol. 2017. 21;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Internal clinical study report based on which FDA 510k (K163255) clearance for self-calibration was obtained.
- [7].Association for the Advancement of Medical Instrumentation. Non-invasive Sphygmomanometers–Part 2: Clinical Investigation of Automated Measurement Type. ANSI/AAMI/ISO 81060–2/ANSI-AAMI. 2nd ed. Arlington, VA: AAMI; 2019. [Google Scholar]
- [8].Baruch MC, Kalantari K, Gerdt DW, Adkins CM. Validation of the pulse decomposition analysis algorithm using central arterial blood pressure. Biomed Eng OnLine. 2014. July 8;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Solà Josep, Delgado-Gonzalo R. In: The Handbook of cuffless blood pressure monitoring: a practical guide for clinicians, researchers, and engineers. Cham: Springer; 2019. p. 245. [Google Scholar]
- [10].Baruch MC, Warburton DE, Bredin SS, Cote A, Gerdt DW, Adkins CM. Pulse Decomposition Analysis of the digital arterial pulse during hemorrhage simulation. Nonlinear Biomed Phys. 2011. January 12;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Imholz BP, Langewouters GJ, van Montfrans GA, Parati G, van Goudoever J, Wesseling KH, et al. Feasibility of ambulatory, continuous 24-hour finger arterial pressure recording. Hypertens Dallas Tex 1979. 1993. January;21(1):65–73. [DOI] [PubMed] [Google Scholar]
- [12].Martina JR, Westerhof BE, van Goudoever J, de Beaumont EMFH, Truijen J, Kim Y-S, et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology. 2012. May;116(5):1092–103. [DOI] [PubMed] [Google Scholar]
- [13].Callaghan FJ, Babbs CF, Bourland JD, Geddes LA. The relationship between arterial pulse-wave velocity and pulse frequency at different pressures. J Med Eng Technol. 1984. January 1;8(1):15–8. [DOI] [PubMed] [Google Scholar]
- [14].Saugel B, Grothe O, Wagner JY. Tracking Changes in Cardiac Output: Statistical Considerations on the 4-Quadrant Plot and the Polar Plot Methodology. Anesth Analg. 2015. Aug;121(2):514–24. [DOI] [PubMed] [Google Scholar]
- [15].Perrino AC, O’Connor T, Luther M. Transtracheal Doppler cardiac output monitoring: comparison to thermodilution during noncardiac surgery. Anesth Analg. 1994. June;78(6):1060–6. [DOI] [PubMed] [Google Scholar]
- [16].Moharram MA, Wilson LC, Williams MJ, Coffey S. Beat-to-beat blood pressure measurement using a cuffless device does not accurately reflect invasive blood pressure. Int J Cardiol Hypertens. 2020. June 1;5:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shih Y-T, Cheng H-M, Sung S-H, Hu W-C, Chen C-H. Quantification of the Calibration Error in the Transfer Function-Derived Central Aortic Blood Pressures. Am J Hypertens. 2011. December 1;24(12):1312–7. [DOI] [PubMed] [Google Scholar]
- [18].Avolio Alberto P, Van Bortel Luc M, Boutouyrie Pierre, Cockcroft John R, McEniery Carmel M, Protogerou Athanase D, et al. Role of Pulse Pressure Amplification in Arterial Hypertension. Hypertension. 2009. August 1;54(2):375–83. [DOI] [PubMed] [Google Scholar]
- [19].Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res. 1955. November;3(6):623–32. [DOI] [PubMed] [Google Scholar]
- [20].The CAFE Investigators, CAFE Steering Committee and Writing Committee, Williams B, Lacy PS, Thom SM, Cruickshank K, et al. Differential Impact of Blood Pressure–Lowering Drugs on Central Aortic Pressure and Clinical Outcomes: Principal Results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation. 2006. March 7;113(9):1213–25. [DOI] [PubMed] [Google Scholar]
- [21].Huang C-M, Wang K-L, Cheng H-M, Chuang S-Y, Sung S-H, Yu W-C, et al. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens. 2011. March;29(3):454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sharman JE, Avolio AP, Baulmann J, Benetos A, Blacher J, Blizzard CL, et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017. 01;38(37):2805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng H-M, Chuang S-Y, Wang T-D, Kario K, Buranakitjaroen P, Chia Y-C, et al. Central blood pressure for the management of hypertension: Is it a practical clinical tool in current practice? J Clin Hypertens. 2020;22(3):391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A. Relationship between Radial and Central Arterial Pulse Wave and Evaluation of Central Aortic Pressure Using the Radial Arterial Pulse Wave. Hypertens Res. 2007;30(3):219–28. [DOI] [PubMed] [Google Scholar]
- [25].Cheng H-M, Chuang S-Y, Sung S-H, Yu W-C, Pearson A, Lakatta EG, et al. Derivation and Validation of Diagnostic Thresholds for Central Blood Pressure Measurements Based on Long-Term Cardiovascular Risks. J Am Coll Cardiol. 2013. November 5;62(19):1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hickson SS, Butlin M, Mir FA, Graggaber J, Cheriyan J, Khan F, et al. The accuracy of central SBP determined from the second systolic peak of the peripheral pressure waveform. J Hypertens. 2009. September;27(9):1784–1788. [DOI] [PubMed] [Google Scholar]
- [27].Szczepaniak-Chichel L, Markwitz W, Tykarski A. Difference between central and peripheral blood pressure in healthy and hypertension-complicated pregnancy. Blood Press Monit. 2016. April;21(2):103–110. [DOI] [PubMed] [Google Scholar]
- [28].Ryuzaki M, Morimoto S, Niiyama M, Seki Y, Yoshida N, Oshima Y, et al. The Relationships between the Differences in the Central Blood Pressure and Brachial Blood Pressure and Other Factors in Patients with Essential Hypertension. Intern Med. 2017;56(6):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Remington JW, Wood EH. Formation of peripheral pulse contour in man. J Appl Physiol. 1956. November;9(3):433–42. [DOI] [PubMed] [Google Scholar]


