Abstract
Aims:
Dipeptidyl peptidase-4 inhibitors (DPP4Is) may mitigate hypoglycemia-mediated declines in cognitive and physical functioning compared to sulfonylureas (SUs), yet comparative studies are unavailable among older adults, especially nursing home (NH) residents. We evaluated the effects of DPP4Is versus SUs on cognitive and physical functioning among NH residents.
Materials and Methods:
This new-user cohort study included long-stay NH residents aged ≥65 years from the 2007–2010 national US Minimum Data Set (MDS) clinical assessments and linked Medicare claims. We measured cognitive decline from the validated 6-point MDS Cognitive Performance Scale, functional decline from the validated 28-point MDS Activities of Daily Living scale, and hospitalizations or emergency department visits for altered mental status from Medicare claims. We compared 180-day outcomes in residents who initiated a DPP4I versus SU after 1:1 propensity score matching using Cox regression models.
Results:
The matched cohort (N=1,784) had a mean (SD) age of 80 (8) years and was 73% female. Approximately 46% had no or mild cognitive impairment and 35% had no or mild functional impairment before treatment initiation. Compared to SU users, DPP4I users had lower 180-day rates of cognitive decline (hazard ratio [HR]=0.61, 95%CI 0.31–1.19), altered mental status events (HR=0.71, 95%CI 0.39–1.27), and functional decline (HR=0.89, 95%CI 0.51–1.56), but estimates were imprecise.
Conclusions:
Rates of cognitive and functional decline may be reduced among older NH residents using DPP4Is compared to SUs, but larger studies with greater statistical power should resolve the remaining uncertainty by providing more precise effect estimates.
Keywords: Sulfonylurea Compounds, Dipeptidyl-Peptidase IV Inhibitors, Nursing Homes, Diabetes Mellitus, Frailty
INTRODUCTION
Type 2 diabetes mellitus (T2DM) increases the risk of cognitive1–4 and physical functional impairment5, 6 in older adults. Little is known about whether individual classes of glucose-lowering drugs affect the risks of adverse neuropsychological or physical functioning.1, 7 Understanding the effects of medications on such outcomes is especially important for frail older adults like nursing home (NH) residents, who are at higher risk of decline and who often have limited ability to regain cognitive or physical function. Furthermore, given their limited life expectancy, NH residents often prioritize preserving cognition, functional independence, and quality of life8 over disease-specific or longevity outcomes.9
Though the exact mechanisms responsible for T2DM-associated cognitive impairment are unknown, hyperinsulinemia, hyperglycemia, and hypoglycemia may cause cognitive impairment.10, 11 Hypoglycemia occurs frequently among NH residents and increases the risk of delirium, impaired cognition, fatigue, weakness, and in severe cases, seizures and unconsciousness.12–16 Hyperglycemia also frequently occurs in the NH population, and it can also lead to cognitive and functional impairments similar to those from hypoglycemia.17, 18 These symptoms of hypoglycemia and hyperglycemia may result in irreversible decline in cognitive functioning, physical functioning, and quality of life for frail and vulnerable older adults.19–22
Dipeptidyl peptidase-4 inhibitors (DPP4Is) and sulfonylureas (SUs) are two of the most commonly prescribed glucose-lowering drug classes for T2DM among NH residents.23–25 These oral glucose-lowering agents may improve cognition and the ability to perform activities of daily living by improving blood glucose control, potentially even reversing impaired neuro-motor function.2, 26–30 DPP4Is and SUs may differentially affect cognitive and physical functioning through their effects on hypoglycemia and hyperglycemia.28–31 SUs are associated with a greater risk of hypoglycemia than DPP4Is.25, 28–30 The use of DPP4Is may also reduce amyloid beta accumulation in the brain, which could further help to maintain or slow cognitive decline.32 However, hyperglycemia may occur more often with DPP4Is versus SUs. Studies that directly compare cognitive and physical functioning outcomes for DPP4I and SU users are scarce.26, 33
We estimated the effects of DPP4Is versus SUs on cognitive functioning, altered mental status events, and physical functioning among frail older NH residents. We hypothesized that DPP4I use would be associated with a lower rate of cognitive impairment, smaller number of altered mental status events, and better physical functioning than SU use because of the comparatively lower rate of hypoglycemia from DPP4Is.25
MATERIALS AND METHODS
Study Design and Data Source
This was a retrospective new-user cohort study that used the following linked national datasets for the years 2007 – 2010: 100% Medicare fee-for-service enrollment information, Part A inpatient claims, and Part B outpatient claims; a random 20% sample of Part D prescription drug claims; 100% Minimum Data Set (MDS) version 2.0 assessment records; and 100% Online Survey Certification and Reporting System (OSCAR) data.23–25, 34, 35 The MDS is a comprehensive, clinical assessment instrument used to document health status of NH residents, including functional status, psychological, and cognitive status information.36 MDS assessments occur at a minimum of every 3 months, and are conducted more frequently for residents with a major recent change in clinical status. OSCAR data provided NH-level information. The study was designed to mimic the hypothetical target trial detailed in Supplementary Table S1.37 This study was approved by the Brown University Institutional Review Board.
Study Population
The study population was adults aged ≥65 years who were long-stay NH residents (>100 days in the NH) on January 1, 2008, or who became a long-stay resident between January 1, 2008 and September 30, 2010. The index date (time zero) was the date of the first eligible dispensing of a DPP4I or SU between January 1, 2008 and September 30, 2010 after becoming a long-stay resident. Data from July 1, 2007 to December 31, 2007 was available to ascertain prior glucose-lowering treatment use. All individuals were required to have one year of continuous enrollment in Medicare fee-for-service Parts A, B, and D immediately preceding the index date. Cohort exclusions are shown in Supplementary Figure S1 and Table S1.
Exposures and Causal Contrast of Interest
Exposures of interest were new use of DPP4Is (saxagliptin, sitagliptin) or SUs (glimepiride, glipizide, glyburide) in NHs. New use was defined as the first Part D claim for a DPP4I or SU after 6 months without a dispensing for any glucose-lowering treatment other than metformin. As metformin is generally first-line therapy but is commonly contraindicated in the NH setting35, its use was permitted and adjusted for but not required for inclusion. The causal contrast of interest was defined as the effect of initiating DPP4Is versus SUs regardless of subsequent treatment discontinuation or switching (i.e., the observational study analog of the intention-to-treat [ITT] estimand).
Outcomes
The outcomes were decline in physical functioning, decline in cognitive functioning, and hospitalizations or emergency department (ED) visits for altered mental status. We defined physical functional decline as an increase of 3 points on a validated 28-point scale of independence in activities of daily living (ADLs) between the pre-initiation baseline and any available MDS assessment following initiation, up to 180 days after initiation.38 A 3-point increase corresponds to a major loss of independence in 1 ADL or incremental loss in 2 or more ADLs.39 Cognitive decline was defined as an increase of 1 point on the validated 6-point MDS Cognitive Performance Scale (CPS). A 1-point cognitive decline is considered clinically significant.40 Lastly, we defined a hospitalization or ED visit for altered mental status using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 780.0, 780.02, 780.09, or 780.97 in any position on a Part A inpatient hospital claim or a Part B ED claim, though inclusion of ED visits contributed only a small number of additional cases. While no validated algorithm exists for altered mental status events in claims, our definition is consistent with prior literature.41 We analyzed each outcome individually. We also explored a pre-specified composite outcome: time to functional or cognitive decline (3-point ADL or 1-point CPS).
Follow-Up
The start of follow-up (baseline or time zero) for each individual was the day of the first DPP4I or SU dispensing and continued until the earliest event of the following: Medicare disenrollment (from Parts A, B, or D), enrollment in a health maintenance organization (Medicare Advantage), death, an outcome (each evaluated separately), or administrative end of follow-up (September 30, 2010 or 180 days of follow-up). We chose a 180-day outcome period because it is long enough to be clinically meaningful, but short enough that many of these highly vulnerable residents have not yet died. Death is a prevalent competing event that complicates interpretation of longer-term outcomes in the NH setting.
Baseline characteristics
We identified 190 baseline characteristics that could potentially confound the relationship between receiving DPP4Is versus SUs and the outcomes (Supplementary Table S2). All variables were pre-specified, selected based on the prior literature and subject-matter knowledge, and measured using the most recent available record on or before the index date (time zero).23, 24, 35 These variables were obtained from Medicare claims and MDS v2.0 data, which has well-established reliability and validity for assessing the clinical status of NH residents. The MDS v2.0 also provided data on other patient characteristics including pre-treatment physical function38 and cognitive status, geriatric syndromes, nutrition, social characteristics, care preferences, and a mortality risk index: Changes in Health, End-stage Disease, and Symptoms Scale (CHESS) score.42 We used the OSCAR data to evaluate a variety of NH facility characteristics such as staffing, resident mix, and quality indicators (Supplementary Table S2).
Statistical Analyses
We adjusted for potential confounding by baseline covariates by estimating the propensity scores using a logistic regression that included 190 baseline characteristics to predict the initiation of DPP4Is versus SUs. We matched DPP4I to SU users on the propensity scores using a 1:1 greedy (nearest neighbor) 5-to-1 digit matching algorithm without replacement.43 Cox regression models with robust standard errors to account for clustering within the matched sets were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) comparing DPP4I versus SU users.44 No covariates were included in the model. Testing for violations of the proportional hazards assumption was unnecessary because we interpret the HRs as a weighted average of the true HRs over the entire follow-up period.45 We used SAS, version 9.4 (SAS Institute Inc) for data processing, and Stata version 14.0 (Stata Corp., College Station, TX) and R version 3.4.4 (The R Foundation) for data analyses.
Stability Analyses
We conducted several stability analyses to test the robustness of our treatment effect estimates to study design and analysis decisions. First, we evaluated more substantial declines by re-defining functional decline as a 4-point decrease and cognitive decline as a 2-point decrease along with a composite outcome of time to larger functional declines (4-point ADL or 2-point CPS). Second, to assess the impact of missing data, we performed multiple imputation with chained equations to impute missing covariate data for 582 residents excluded from the primary analysis. Third, we estimated the propensity scores using generalized boosted regression models to evaluate whether our parametric propensity score estimation model might have been misspecified. Fourth, we used Fine and Gray competing risks regression models to account for the potential competing risk of death. At the end of each follow-up period, subjects were classified as alive without one of the outcomes of interest, having had the outcome documented in the MDS or claims in that period, or having died without evidence of an outcome event. Fifth, because metformin is often the preferred first-line medication used to treat T2DM, we restricted our study cohort to individuals using metformin at baseline and performed the analyses in this subpopulation. Sixth, because analyzing the original scales as continuous outcomes might improve statistical power, we created new outcomes representing the differences in the CPS and ADL scores between baseline and the first MDS assessment during follow-up for each NH resident for the cognitive functioning and physical functioning outcomes, respectively. These continuous outcome variables were analyzed using ordinary least squares linear regression models to calculate the average difference in the changes in scores between the groups. Seventh, because using one-to-many fixed and variable matching ratios might improve precision, we employed these alternative propensity score matching approaches. Finally, we estimated the per-protocol estimand at as alternative to the ITT estimand.
RESULTS
Cohort
Before matching, the initial cohort included 903 new DPP4I users and 6,075 new SU users, and these groups had the same average baseline values of ADL (mean=16) and CPS scores (mean=3). DPP4I initiators had a higher medication burden, used other glucose-lowering treatments more frequently, and had more angiotensin receptor blocker, clopidogrel, fibrate, and omega-3 fatty acid medication use in the prior 12 months than new SU users (Table 1 and Supplementary Table S2). Additionally, DPP4I users were more likely to have abnormal laboratory results, ischemic heart disease, and a prior ED visit for hyperglycemia, but less likely to have a do not resuscitate order. The distributions of individual DPP4I and SU drugs initiated before and after propensity score matching is available in Supplementary Table S3.
Table 1.
Characteristics of Nursing Home Residents Initiating Dipeptidyl Peptidase-4 Inhibitors or Sulfonylureas Before and After Propensity Score Matching.
| Characteristic | n (%) | |||
|---|---|---|---|---|
|
| ||||
| Before matching | After matching | |||
|
| ||||
| DPP4I users (N=903) | SU users (N=6,075) | DPP4I users (N=892) | SU users (N=892) | |
|
| ||||
| Age, mean (SD) years | 81 (8) | 81 (8) | 81 (8) | 80 (8) |
|
| ||||
| Female sex | 652 (72) | 4,282 (71) | 644 (72) | 661 (74) |
|
| ||||
| Race | ||||
| White | 651 (72) | 4,593 (76) | 648 (73) | 628 (70) |
| Black | 145 (l6) | 963 (16) | 142 (16) | 155 (17) |
| Other | 107 (12) | 519 (8) | 102 (11) | 109 (13) |
|
| ||||
| Conditions | ||||
| Renal disease | 97 (11) | 572 (9) | 96 (11) | 96 (11) |
| Peripheral vascular disease | 157 (17) | 1,015 (17) | 156 (18) | 164 (18) |
| Heart failure | 284 (32) | 1,744 (29) | 281 (32) | 272 (31) |
| Ischemic heart disease | 162 (18) | 927 (15) | 159 (18) | 153 (17) |
| Depression | 509 (56) | 3,399 (56) | 504 (57) | 483 (54) |
| Chronic Obstructive Pulmonary Disease | 192 (21) | 1,223 (20) | 191 (21) | 202 (23) |
| Hypertension | 692 (77) | 4,681 (77) | 683 (77) | 710 (80) |
| Hip fracture | 15 (2) | 108 (2) | 15 (2) | 25 (3) |
|
| ||||
| Number of conditions, median (Q1, Q3) | 6 (4, 7) | 5 (4, 7) | 6 (4, 7) | 6 (4, 8) |
|
| ||||
| ADL score, mean (SD)† | 16 (8) | 16 (8) | 16 (8) | 17 (7) |
|
| ||||
| CPS score, mean (SD)‡ | 3 (2) | 3 (2) | 3 (2) | 3 (2) |
|
| ||||
| CHESS score, mean (SD)§ | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
|
| ||||
| Number of medications, mean (SD) | 14 (5) | 13 (5) | 14 (5) | 14 (5) |
|
| ||||
| Medication use | ||||
| Metformin | 330 (37) | 1,768 (29) | 325 (36) | 301 (34) |
| Statins | 401 (44) | 2,362 (39) | 394 (44) | 390 (44) |
| Clopidogrel | 168 (19) | 849 (14) | 163 (18) | 164 (18) |
| Warfarin | 141 (16) | 899 (15) | 139 (16) | 137 (15) |
| Antipsychotics | 283 (31) | 1,779 (29) | 281 (32) | 255 (29) |
| Steroids (oral) | 110 (12) | 751 (12) | 109 (12) | 122 (14) |
| Angiotensin-converting enzyme inhibitors | 371 (41) | 2,297 (38) | 368 (41) | 379 (43) |
| Angiotensin receptor blockers | 140 (16) | 682 (11) | 135 (15) | 136 (15) |
| Beta blockers | 455 (50) | 2,698 (44) | 449 (50) | 457 (51) |
|
| ||||
| Length of nursing home stay before treatment initiation, median (Q1, Q3) days | 623 (275, 1,260) | 586 (270, 1,177) | 628 (276, 1,263) | 604 (274, 1,248) |
|
| ||||
| Any physician visits in prior two weeks | 531 (59) | 3,518 (58) | 519 (58) | 536 (60) |
|
| ||||
| Number of physician order changes in prior two weeks, mean (SD) | 2 (2) | 2 (2) | 2 (2) | 2 (2) |
|
| ||||
| Any overnight hospitalizations in prior 90 days | 287 (32) | 1,792 (29) | 282 (32) | 295 (33) |
|
| ||||
| Any ED visits in prior 90 days | 71 (8) | 490 (8) | 70 (8) | 75 (8) |
|
| ||||
|
| ||||
| Nursing Home Facility Characteristics | ||||
|
| ||||
| Ownership | ||||
| For-profit | 657 (73) | 4,317 (71) | 648 (73) | 626 (70) |
| Non-profit | 193 (21) | 1,341 (22) | 192 (22) | 210 (24) |
| Government | 53 (6) | 417 (7) | 52 (6) | 56 (6) |
|
| ||||
| Quality indicators | ||||
| % of residents restrained, median (Q1, Q3) | 2 (0, 5) | 2 (0, 5) | 2 (0, 5) | 2 (0, 5) |
| No. of quality-of-life deficiencies, mean (SD) | 4 (6) | 4 (6) | 4 (6) | 4 (7) |
| % of residents with pressure sores, mean (SD) | 7 (4) | 7 (4) | 7 (4) | 7 (5) |
|
| ||||
| Staffing | ||||
| Direct care hours/resident/day, mean (SD) | 3 (1) | 3 (1) | 3 (1) | 3 (1) |
Abbreviations: DPP4I, dipeptidyl Peptidase-4 Inhibitors; SU, sulfonylureas; SD, standard deviation; Q1, first quartile; Q3, third quartile; ADL, Activities of Daily Living; CPS, Cognitive Performance Scale; CHESS, Changes in Health, End-stage disease and Symptoms and Signs Score; ED, emergency department.
Physical function was measured using activities of daily living (ADL) via the Minimum Data Set Morris 28-point ADL score. The ADL scores range from 0 to 28, with 0 indicating total independence and 28 indicating total dependence in all ADLs.
Cognitive status was measured using the Minimum Data Set Cognitive Performance Scale (CPS), where higher values indicate greater cognitive impairment. The CPS scores range from 0 to 6, with 0 indicating intact cognitive function and 6 indicating very severe cognitive impairment.
The Minimum Data Set Changes in Health, End-stage disease and Symptoms and Signs (CHESS) score is a composite measure addressing changes in health, end-stage disease, and symptoms and signs of medical problems. The CHESS scores range from 0 to 5, with 0 indicating no health instability and 5 indicating very high health instability.
Propensity score matching yielded a cohort of 892 new DPP4I users and an equal number of SU users (N=1,784; Table 1). The mean age (SD) was 80 (SD=8) years. Approximately 73% of the cohort was female and 72% was White race, and 46% had no or mild cognitive impairment (CPS score 0–2) and 35% had no or mild functional impairment (ADL score 0–14) before DPP4I or SU initiation. The matching procedure balanced covariates closely46 with all but 2 variables having absolute standardized mean differences of 0.06 or less (Supplementary Table S2). The propensity score distribution between new initiators of DPP4Is and of SUs overlapped substantially before and after matching (Supplementary Figure S2). Administrative end of follow-up and death were the predominant reasons for censoring during follow-up (Supplementary Table S4)
Effects on Outcomes
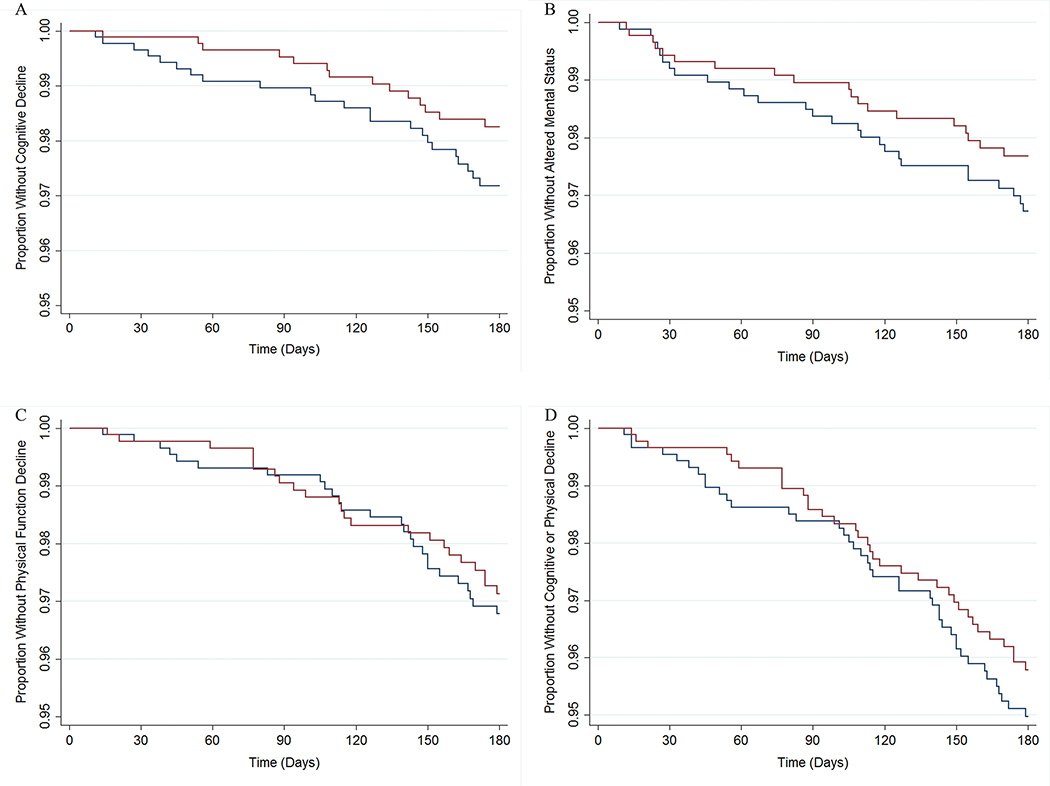
The rates of cognitive decline and altered mental status were not statistically different between DPP4I users and SU users (Table 2, Figure 1, Supplementary Table S5). The rate of cognitive decline among DPP4I users was 0.61 (95%CI 0.31–1.19) times that of SU users, and the rate of an altered mental status event was 0.71 (95%CI 0.39–1.27) times that of SU users (Table 2, Figure 2). DPP4I users were as likely as SU users to have a physical functional decline at 180 days after treatment initiation (HR=0.89, 95%CI 0.51–1.56). The rates of the composite outcome of cognitive or functional decline were not statistically different between DPP4I user and SU users (180 days, HR=0.83, 95%CI 0.53–1.31).
Table 2.
Effects of Dipeptidyl Peptidase-4 Inhibitors (n=892) versus Sulfonylureas (n=892) on 180-Day Outcomes Before and After Propensity Score Matching among Nursing Home Residents.
| Before Matching | After Matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Treatment | Events | PY | Rate† | HR (95% CI) | Events | PY | Rate† | HR (95% CI) |
| Cognitive Decline | SU | 97 | 2,746.3 | 35.3 | Ref | 23 | 404.0 | 56.9 | Ref |
| DPP4I | 14 | 407.1 | 34.4 | 0.98 (0.56–1.71) | 14 | 402.4 | 34.8 | 0.61 (0.31–1.19) | |
| Altered Mental Status Event | SU | 145 | 2,742.2 | 52.9 | Ref | 27 | 403.9 | 66.8 | Ref |
| DPP4I | 19 | 406.9 | 46.7 | 0.88 (0.55–1.42) | 19 | 402.2 | 47.2 | 0.71 (0.39–1.27) | |
| Functional Decline | SU | 135 | 2,742.5 | 49.2 | Ref | 26 | 404.3 | 64.3 | Ref |
| DPP4I | 23 | 407.0 | 56.5 | 1.15 (0.74–1.79) | 23 | 402.4 | 57.2 | 0.89 (0.51–1.56) | |
| Cognitive or Functional Decline Composite | SU | 205 | 2,731.8 | 75.0 | Ref | 41 | 401.3 | 102.2 | Ref |
| DPP4I | 34 | 405.2 | 83.9 | 1.12 (0.78–1.61) | 34 | 400.5 | 84.9 | 0.83 (0.53–1.31) | |
Abbreviations: PY, person-years of follow-up; HR, hazard ratio; CI, confidence interval; DPP4I, dipeptidyl peptidase-4 inhibitor; SU, sulfonylureas; Ref, reference.
Per 1,000 person-years of follow-up.
Figure 1. Survival Curves for Cognitive Decline, Altered Mental Status Event, Functional Decline, and Cognitive or Functional Decline Composite Outcomes over 180 Days of Follow-up Stratified by Dipeptidyl Peptidase-4 Inhibitor versus Sulfonylurea Use after Propensity Score Matching among Nursing Home Residents.
Panel A shows time to cognitive decline among DPP4I users and SU users. Panel B shows time to hospitalization or emergency department visit for altered mental status. Panel C shows time to functional decline. Panel D shows time to the first of either a cognitive or functional decline. Red lines are DPP4I users; blue lines are SU users. Please refer to Supplementary Table S5 for the corresponding risk table for each survival curve.
Figure 2. Effects of Dipeptidyl Peptidase-4 Inhibitor versus Sulfonylurea Use on 180-day Outcomes After Propensity Score Matching among Nursing Home Residents.
Note: Composite denotes a composite outcome of time to either cognitive or functional decline. Abbreviations: HR, hazard ratio; CI, confidence interval; DPP4I, dipeptidyl peptidase-4 inhibitor; SU, sulfonylurea.
Stability Analyses
Analyses re-defining cognitive and functional decline using thresholds representing larger changes (2-point increase in CPS score or 4-point increase in the Morris ADL scale) resulted in similar point estimates with decreased precision for the individual outcomes as well as the composite that reflected the most severe measures of cognitive or functional decline (Supplementary Table S6). The main results were generally similar when implementing multiple imputation of missing baseline covariate information (Supplementary Table S7), estimating the propensity score using generalized boosted regression models (Supplementary Table S8), employing Fine and Gray regression models to account for the competing risk of death (Supplementary Table S9), restricting to metformin users (Supplementary Table S10), using continuous forms of the cognitive functioning and physical functioning outcomes (Supplementary Table S11), employing one-to-many fixed and variable matching ratios (Supplementary Table S12), and estimating the per-protocol estimand (Supplementary Table S13).
DISCUSSION
In this national cohort study of NH residents, we observed statistically insignificant differences in rates of cognitive or functional decline and altered mental status among new users of DPP4Is compared to new users of sulfonylureas. However, the point estimates and lower 95% confidence bounds do not exclude the possibility that DPP4I use decreases the rate of functional decline, especially cognitive decline. Providers should not preferentially prescribe DPP4Is to affect change in cognition based on the insufficient evidence currently available, and should instead focus on other side effects for which there is more evidence, such as hypoglycemia.25
Few studies have examined the comparative effects of T2DM medications on cognition, physical functioning, or quality of life.16 Of the studies that have been conducted, major differences in the study designs and methods preclude any meaningful summative conclusions, especially because just one of those studies evaluated DPP4Is versus SUs.16, 26 Rizzo et al. evaluated the effect of DPP4Is versus SUs on changes in cognitive function in older Italian patients with mild cognitive impairment.26 The study was not conducted in NH residents and did not appear to explicitly adjust for confounding. The investigators concluded that DPP4I use improved cognitive function compared to SU use.
The closest comparable evidence on physical functioning is a large Veterans Affairs study of the relationship between glycemic control and functional decline (defined as a 2-point increase in the MDS ADL score) in NH residents.1 The investigators did not compare treatments, but conducted an analysis stratified by prevalent glucose lowering treatment use—insulin, sulfonylureas, or other glucose-lowering medications. They found that 238 (11.4%) of 2,094 users of glucose-lowering treatments other than sulfonylureas or insulin and 404 (14.1%) of 2,873 sulfonylurea users had a functional decline. However, their analysis did not include adjustment for differences in all relevant factors between these glucose lowering treatments.
Our study has several limitations. Because it is an observational study without randomization of treatments, we cannot rule out the possibility of residual confounding. Our study may have benefited from laboratory information on hemoglobin A1c and other measures of glycemic control that may have influenced prescribers’ decision-making, but direct measures of glycemic control were unavailable. Nonetheless, we obtained good balance on a wide array of baseline covariates between treatment groups, including variables that may be correlates of glycemic control. Moreover, DPP4Is and SUs have a similar place in therapy for treatment of T2DM, which reduces the likelihood of confounding by indication or T2DM severity.47
The data analyzed in thus study were generated between 2007 and 2010, but we believe the findings are still highly relevant to the present. Although the risk of hypoglycemia from sulfonylureas has been emphasized in evolving treatment guidelines, sulfonylureas are still commonly prescribed among older adults. Data from the U.S. Centers for Disease Control and Prevention’s National Ambulatory Medical Care Survey suggested that only a modest decrease in sulfonylurea use occurred between 2009 and 2015, and sulfonylureas were still prescribed in 39 percent of patients with T2DM aged 65 or older in 2015.48 Additional recent studies have documented sulfonylurea prescriptions in 25 percent and 23 percent of NH residents with T2DM in Germany and Canada, respectively.49, 50 Evolving treatment guidelines have also emphasized the use of higher hemoglobin A1c targets for older adults in recent years.51 The deintensification of T2DM treatment regimens resulting from higher targets can improve quality of life under circumstances where there is less concern about the slow multi-year progression of long-term T2DM complications.51, 52 For this reason, glycemic targets were not a major focus of NH prescribers in the past and are still not a major focus of prescribers in recent years.
Another important consideration is the size of our final study population and precision of our estimates. Functional status changes were somewhat rare over the follow-up period, and DPP4I use was not common during the study period, resulting in imprecise estimates of effects. These circumstances mandate a more nuanced interpretation of the range of plausible effect estimates, rather than simply seeking to reject or fail to reject the null hypothesis using p-values (i.e., rather than assessing whether estimates are or are not statistically significant). In addition to contributing to the imprecision of our estimates through a reduction in sample size, excluding individuals with a recent history of glucose-lowering treatment use other than metformin may have reduced the generalizability of our findings. Future work in a study population with a larger number of new DPP4I users appears to be merited; based on our observed results, sample sizes of between approximately 75,000 and 100,000 individuals may be necessary to achieve statistical power of at least 80% in future studies.
Lastly, there may be under-ascertainment of outcomes. It is possible that declines in cognitive or physical functioning are not noticed or documented by the NH staff between MDS assessments. However, provided that the under-ascertainment is non-differential by treatment group and the specificity of the MDS-based measures is high, relative effect measures are unlikely to be biased. Since the MDS requirements do not differ by glucose-lowering treatment status, differential ascertainment of declines in cognitive and physical functioning is unlikely.
Conclusion
In summary, the rates of cognitive decline, altered mental status events, and functional decline were not statistically different between DPP4I users and SU users, but the point estimates and lower 95% confidence bounds do no not rule out the possibility that DPP4Is result in reduced rates of cognitive decline among NH residents. Given that the evidence is currently insufficient, providers should not preferentially prescribe DPP4Is to influence cognition.53 Given the absence of clinical trials that include NH residents, additional studies using data from routine practice are needed to inform patient-centered treatment decisions for NH residents with T2DM. Because many NH residents, their families, their caregivers, and their clinicians value maintaining functioning and quality of life more than extending lifespan or minimizing traditional clinical adverse events, real world evidence could help guide the selection of T2DM treatments to achieve those goals.
Supplementary Material
ACKNOWLEDGEMENTS
Funding Sources: This study was supported by grants RF1AG061221, R01AG045441, R01AG065722, and R21AG061632 from the National Institute on Aging (NIA).
Sponsors’ Role: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest: Dr. Zullo is supported by grant funding from Sanofi Pasteur to Brown University for work on the epidemiology of infections and vaccinations among nursing home residents and infants. Dr. Berry previously received grant money from Amgen unrelated to the current project. Dr. Dore is an employee and stockholder of Exponent, Inc., an engineering and science firm that conducts unrelated work for manufacturers of medical products. All other authors have no relevant conflicts of interest to report.
Footnotes
Data Sharing and Accessibility: The data underlying the results presented in the study are available from the Centers for Medicare and Medicaid Services (CMS). These data are subject to a legally binding data use agreement with CMS and cannot be made publicly available to other researchers. However, researchers can establish their own data use agreement with CMS to obtain access to the raw data through the Research Data Assistance Center (ResDAC), a contractor that provides free assistance to researchers interested in using CMS data.
REFERENCES
- 1.Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. Journal of Alzheimer’s disease : JAD. 2011;24(3):485–93. doi: 10.3233/JAD-2011-101524 [DOI] [PubMed] [Google Scholar]
- 2.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of diabetes on cognitive function among older Latinos: a population-based cohort study. Journal of clinical epidemiology. July 2003;56(7):686–93. doi: 10.1016/s0895-4356(03)00077-5 [DOI] [PubMed] [Google Scholar]
- 3.Mattishent K, Loke YK. Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: a systematic review and meta-analysis. Diabetes, obesity & metabolism. February 2016;18(2):135–41. doi: 10.1111/dom.12587 [DOI] [PubMed] [Google Scholar]
- 4.Bordier L, Doucet J, Boudet J, Bauduceau B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes & metabolism. November 2014;40(5):331–7. doi: 10.1016/j.diabet.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Diabetes as a predictor of change in functional status among older Mexican Americans: a population-based cohort study. Diabetes care. February 2003;26(2):314–9. doi: 10.2337/diacare.26.2.314 [DOI] [PubMed] [Google Scholar]
- 6.Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. Journal of the American Medical Directors Association. August 2013;14(8):585–92. doi: 10.1016/j.jamda.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of antidiabetic medications on physical and cognitive functioning of older Mexican Americans with diabetes mellitus: a population-based cohort study. Annals of epidemiology. May 2003;13(5):369–76. doi: 10.1016/s1047-2797(02)00464-7 [DOI] [PubMed] [Google Scholar]
- 8.Gerety MB, Chiodo LK, Kanten DN, Tuley MR, Cornell JE. Medical treatment preferences of nursing home residents: relationship to function and concordance with surrogate decision-makers. Journal of the American Geriatrics Society. September 1993;41(9):953–60. [DOI] [PubMed] [Google Scholar]
- 9.Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. Journal of the American Geriatrics Society. October 2008;56(10):1839–44. doi: 10.1111/j.1532-5415.2008.01923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspar JM, Baptista FI, Macedo MP, Ambrosio AF. Inside the Diabetic Brain: Role of Different Players Involved in Cognitive Decline. ACS Chem Neurosci. February 17 2016;7(2):131–42. doi: 10.1021/acschemneuro.5b00240 [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Golden SH, Anderson C, et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes care. September 2015;38(9):1777–803. doi: 10.2337/dci15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes care. February 2006;29(2):345–51. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo MR, Marfella R, Barbieri M, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes care. October 2010;33(10):2169–74. doi: 10.2337/dc10-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. Bmj. July 29 2013;347:f4533. doi: 10.1136/bmj.f4533 [DOI] [PubMed] [Google Scholar]
- 15.Johnston SS, Conner C, Aagren M, Ruiz K, Bouchard J. Association between hypoglycaemic events and fall-related fractures in Medicare-covered patients with type 2 diabetes. Diabetes, obesity & metabolism. July 2012;14(7):634–43. doi: 10.1111/j.1463-1326.2012.01583.x [DOI] [PubMed] [Google Scholar]
- 16.Bolen S, Tseng E, Hutfless S, et al. Diabetes Medications for Adults With Type 2 Diabetes: An Update. Diabetes Medications for Adults With Type 2 Diabetes: An Update. 2016. AHRQ Comparative Effectiveness Reviews. [Google Scholar]
- 17.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. Journal of the American Geriatrics Society. December 2012;60(12):2342–56. doi: 10.1111/jgs.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pistrosch F, Natali A, Hanefeld M. Is hyperglycemia a cardiovascular risk factor? Diabetes care. May 2011;34 Suppl 2:S128–31. doi: 10.2337/dc11-s207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes care. February 2009;32(2):221–6. doi: 10.2337/dc08-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yau CK, Eng C, Cenzer IS, Boscardin WJ, Rice-Trumble K, Lee SJ. Glycosylated hemoglobin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus. Journal of the American Geriatrics Society. July 2012;60(7):1215–21. doi: 10.1111/j.1532-5415.2012.04041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. May 2010;6(3):134–43. [DOI] [PubMed] [Google Scholar]
- 22.Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. October 2013;1(2):106–14. doi: 10.1016/S2213-8587(13)70046-9 [DOI] [PubMed] [Google Scholar]
- 23.Zullo AR, Dore DD, Daiello L, et al. National Trends in Treatment Initiation for Nursing Home Residents With Diabetes Mellitus, 2008 to 2010. Journal of the American Medical Directors Association. July 1 2016;17(7):602–8. doi: 10.1016/j.jamda.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zullo AR, Dore DD, Gutman R, Mor V, Smith RJ. National Glucose-Lowering Treatment Complexity Is Greater in Nursing Home Residents than Community-Dwelling Adults. Journal of the American Geriatrics Society. November 2016;64(11):e233–e235. doi: 10.1111/jgs.14485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zullo AR, Smith RJ, Gutman R, et al. Comparative safety of dipeptidyl peptidase-4 inhibitors and sulfonylureas among frail older adults. Journal of the American Geriatrics Society. July 21 2021;doi: 10.1111/jgs.17371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo MR, Barbieri M, Boccardi V, Angellotti E, Marfella R, Paolisso G. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. The journals of gerontology Series A, Biological sciences and medical sciences. September 2014;69(9):1122–31. doi: 10.1093/gerona/glu032 [DOI] [PubMed] [Google Scholar]
- 27.Isik AT, Soysal P, Yay A, Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes research and clinical practice. January 2017;123:192–198. doi: 10.1016/j.diabres.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 28.Gazzaruso C, Coppola A, Luppi C, Giustina A, Solerte SB. Effect of different diabetes mellitus treatments on functional decline and death in elderly adults with diabetes mellitus. Journal of the American Geriatrics Society. April 2013;61(4):666–7. doi: 10.1111/jgs.12191 [DOI] [PubMed] [Google Scholar]
- 29.Abbatecola AM, Maggi S, Paolisso G. New approaches to treating type 2 diabetes mellitus in the elderly: role of incretin therapies. Drugs & aging. 2008;25(11):913–25. doi: 10.2165/0002512-200825110-00002 [DOI] [PubMed] [Google Scholar]
- 30.Shorr RI, de Rekeneire N, Resnick HE, et al. Glycemia and cognitive function in older adults using glucose-lowering drugs. J Nutr Health Aging. Jul-Aug 2006;10(4):297–301. [PubMed] [Google Scholar]
- 31.Farngren J, Persson M, Ahren B. Effects on the glucagon response to hypoglycaemia during DPP-4 inhibition in elderly subjects with type 2 diabetes: A randomized, placebo-controlled study. Diabetes, obesity & metabolism. August 2018;20(8):1911–1920. doi: 10.1111/dom.13316 [DOI] [PubMed] [Google Scholar]
- 32.Jeong SH, Kim HR, Kim J, et al. Association of Dipeptidyl Peptidase-4 Inhibitor Use and Amyloid Burden in Diabetic Patients With AD-Related Cognitive Impairment. Neurology. August 11 2021;doi: 10.1212/WNL.0000000000012534 [DOI] [PubMed] [Google Scholar]
- 33.Tasci I, Naharci MI, Bozoglu E, et al. Cognitive and functional influences of vildagliptin, a DPP-4 inhibitor, added to ongoing metformin therapy in elderly with type 2 diabetes. Endocr Metab Immune Disord Drug Targets. September 2013;13(3):256–63. [DOI] [PubMed] [Google Scholar]
- 34.Zullo AR, Hersey M, Lee Y, et al. Outcomes of “diabetes-friendly” vs “diabetes-unfriendly” beta-blockers in older nursing home residents with diabetes after acute myocardial infarction. Diabetes, obesity & metabolism. December 2018;20(12):2724–2732. doi: 10.1111/dom.13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zullo AR, Dore DD, Gutman R, Mor V, Alvarez CA, Smith RJ. Metformin Safety Warnings and Diabetes Drug Prescribing Patterns for Older Nursing Home Residents. Journal of the American Medical Directors Association. October 1 2017;18(10):879–884 e7. doi: 10.1016/j.jamda.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutchinson AM, Milke DL, Maisey S, et al. The Resident Assessment Instrument-Minimum Data Set 2.0 quality indicators: a systematic review. BMC health services research. June 16 2010;10:166. doi: 10.1186/1472-6963-10-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American journal of epidemiology. April 15 2016;183(8):758–64. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences. November 1999;54(11):M546–53. [DOI] [PubMed] [Google Scholar]
- 39.Steinman MA, Zullo AR, Lee Y, et al. Association of beta-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA internal medicine. February 1 2017;177(2):254–262. doi: 10.1001/jamainternmed.2016.7701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snowden M, McCormick W, Russo J, et al. Validity and responsiveness of the Minimum Data Set. Journal of the American Geriatrics Society. August 1999;47(8):1000–4. [DOI] [PubMed] [Google Scholar]
- 41.Romley JA, Gong C, Jena AB, Goldman DP, Williams B, Peters A. Association between use of warfarin with common sulfonylureas and serious hypoglycemic events: retrospective cohort analysis. Bmj. December 7 2015;351:h6223. doi: 10.1136/bmj.h6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: a new measure to predict mortality in institutionalized older people. Journal of the American Geriatrics Society. January 2003;51(1):96–100. [DOI] [PubMed] [Google Scholar]
- 43.Parsons L Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. 2001:
- 44.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. December 1989;84(408):1074–1078. doi:Doi 10.2307/2290085 [DOI] [Google Scholar]
- 45.Stensrud MJ, Hernan MA. Why Test for Proportional Hazards? JAMA : the journal of the American Medical Association. April 14 2020;323(14):1401–1402. doi: 10.1001/jama.2020.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical science : a review journal of the Institute of Mathematical Statistics. February 1 2010;25(1):1–21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. January 2009;52(1):17–30. doi: 10.1007/s00125-008-1157-y [DOI] [PubMed] [Google Scholar]
- 48.Kitten AK, Kamath M, Ryan L, Reveles KR. National ambulatory care non-insulin antidiabetic medication prescribing trends in the United States from 2009 to 2015. PloS one. 2019;14(8):e0221174. doi: 10.1371/journal.pone.0221174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kostev K, Rockel T, Jacob L. Prescription Patterns and Disease Control in Type 2 Diabetes Mellitus Patients in Nursing Home and Home Care Settings: A Retrospective Analysis in Germany. J Diabetes Sci Technol. January 2018;12(1):136–139. doi: 10.1177/1932296817710477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lega IC, Campitelli MA, Matlow J, et al. Glycemic Control and Use of High-risk Antihyperglycemic Agents Among Nursing Home Residents With Diabetes in Ontario, Canada. JAMA internal medicine. July 1 2021;181(7):992–994. doi: 10.1001/jamainternmed.2021.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munshi MN, Florez H, Huang ES, et al. Management of Diabetes in Long-term Care and Skilled Nursing Facilities: A Position Statement of the American Diabetes Association. Diabetes care. February 2016;39(2):308–18. doi: 10.2337/dc15-2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Liberating A1C goals in older adults may not protect against the risk of hypoglycemia. Journal of diabetes and its complications. July 2017;31(7):1197–1199. doi: 10.1016/j.jdiacomp.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 53.Murad MH, Chang SM, Fiordalisi CV, et al. Improving the utility of evidence synthesis for decision makers in the face of insufficient evidence. Journal of clinical epidemiology. July 2021;135:170–175. doi: 10.1016/j.jclinepi.2021.02.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.