Abstract
Human skeletal muscle fiber is heterogenous due to its diversity of slow- and fast-twitch fibers. In human, slow-twitched fiber gene expression is correlated to MOTS-c, a mitochondria-derived peptide that has been characterized as an exercise mimetic. Within the MOTS-c open reading frame, there is an East Asian-specific m.1382A>C polymorphism (rs111033358) that changes the 14th amino acid of MOTS-c (i.e., K14Q), a variant of MOTS-c that has less biological activity. Here, we examined the influence of the m.1382A>C polymorphism causing MOTS-c K14Q on skeletal muscle fiber composition and physical performance. The myosin heavy chain (MHC) isoforms (MHC-I, MHC-IIa, and MHC-IIx) as an indicator of muscle fiber composition were assessed in 211 Japanese healthy individuals (102 men and 109 women). Muscular strength was measured in 86 physically active young Japanese men by using an isokinetic dynamometer. The allele frequency of the m.1382A>C polymorphism was assessed in 721 Japanese athletes and 873 ethnicity-matched controls. The m.1382A>C polymorphism genotype was analyzed by TaqMan SNP Genotyping Assay. Individuals with the C allele of the m.1382A>C exhibited a higher proportion of MHC-IIx, an index of fast-twitched fiber, than the A allele carriers. Men with the C allele of m.1382A>C exhibited significantly higher peak torques of leg flexion and extension. Furthermore, the C allele frequency was higher in the order of sprint/power athletes (6.5%), controls (5.1%), and endurance athletes (2.9%). Additionally, young male mice were injected with the MOTS-c neutralizing antibody once a week for four weeks to mimic the C allele of the m.1382A>C and assessed for protein expression levels of MHC-fast and MHC-slow. Mice injected with MOTS-c neutralizing antibody showed a higher expression of MHC-fast than the control mice. These results suggest that the C allele of the East Asian-specific m.1382A>C polymorphism leads to the MOTS-c K14Q contributes to the sprint/power performance through regulating skeletal muscle fiber composition.
Keywords: MOTS-c, genetic polymorphism, muscle fiber composition, muscular strength, sprint/power performance
INTRODUCTION
Mitochondrial-derived peptides (MDPs) are biologically active peptides translated from small open reading frames in the mitochondrial DNA (mtDNA) [1–4]. MOTS-c (mitochondrial open-reading-frame of the twelve S rRNA – type c), a 16-amino acid peptide, is one of the MDPs encoded within the mitochondrial 12S rRNA and expressed in multiple tissues, including skeletal muscle in mice and human [5–7]. MOTS-c regulates insulin sensitivity in the skeletal muscle and prevents high-fat diet-induced weight gain [5] and exercise increased MOTS-c levels in the plasma and skeletal muscle [6, 8]. The East Asian-specific mtDNA polymorphism in the MOTS-c cording region, m.1382A>C (rs111033358), causes an amino acid replacement from Lys (K) to Gln (Q) at the 14th amino-acid residue [9]. Recently, it has been demonstrated that men with the C allele of m.1382A>C exhibited a significantly higher risk of type 2 diabetes mellitus (T2DM) in Japanese sedentary individuals [10]. In vitro and in vivo studies showed that the MOTS-c K14Q is a partially bioinactive form of MOTS-c peptide [10]. Therefore, the m.1382A>C polymorphism may have a role in the regulation of MOTS-c activity.
Human skeletal muscle fibers are divided into types I, IIa, and IIx [11], and fiber type composition is associated with metabolic disorders [12]. Type I fibers contain high levels of mitochondrial oxidative enzymes and low levels of glycolytic enzymes, whereas type IIx fibers contain high levels of glycolytic enzymes and low levels of mitochondrial oxidative enzymes. An intermediate type of fiber between types I and IIx is the type IIa fiber [13]. The proportion of type I fiber and type IIx are positively and negatively correlated to whole body glucose uptake, respectively [14, 15]. On the other hand, fiber type composition is also associated with physical performance and its related phenotypes. Type I fibers show high resistance to fatigue and are suitable for endurance performance, while type IIx fibers are suitable for short bursts of strength and speed performance [16]. Therefore, muscle fiber type composition is one of the determinants of exercise performance as well as metabolic disorders. Interestingly, the expression levels of the MOTS-c protein are positively correlated with the gene expression levels of type I fiber in the human skeletal muscle [5–7]. Given that Q form of the MOTS-c K14Q is suggested to be a bioinactive of MOTS-c peptide, we hypothesized that m.1382A>C polymorphism causing MOTS-c K14Q could have an effect on muscle fiber composition and physical performance. However, the possible associations are not clarified yet.
Here, we investigate the influence of m.1382A>C polymorphism on skeletal muscle fiber composition and physical performance.
METHODS
Myosin heavy chain (MHC) isoform
A total of 211 Japanese men and women were analyzed as we have reported previously [17]. Briefly, the subjects were recruited form Juntendo University and Fukuoka University. Written informed consent was obtained from each subject in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committees of Juntendo University. Under sterile conditions and local anesthesia (1% lidocaine), muscle samples were taken from the belly of the vastus lateralis by using a disposal needle biopsy instrument (Max Core or MAGNUM; C.R. Bard, Covington, GA, USA). The obtained muscle specimens were frozen immediately in liquid nitrogen and stored at −80°C until analysis. Frozen muscle tissues were homogenized in ice-cold lysis buffer with phosphatase (Phosstop tablet; Roche Diagnostics Corp., Indianapolis, IN, USA) and protease inhibitors (Complete tablet; Roche Diagnostics Corp.), then centrifuged at 10,000×g for 10 min or 15,000×g for 60 min at 4 °C. The insoluble pellet after homogenization was suspended in a sufficient volume of SDS sample buffer (30% glycerol, 5% β-mercaptoethanol, 2.3% SDS, 0.05% bromophenol blue, and 62.5 mM Tris-HCl, pH 6.8) and boiled at 95°C for 5 min. The MHC composition was determined using glycerol SDS-PAGE according to previously described methods [18] with modifications. Briefly, the protein samples were separated by glycerol SDS-PAGE (stacking gel: 4% acrylamide, 34.7% glycerol, 125mM Tris-HCl pH6.8; separating gel: 8% acrylamide, 33.3% glycerol, 375mM Tris-HCl pH8.3). Electrophoresis was started at 60 V and 8°C until the tracking dye exited the stacking gel and completely entered the separating gel. Then, the voltage was set to 150 V, and electrophoresis was continued for 18 h at 8°C. Gels were stained with Coomassie brilliant blue (Biosafe G250; Bio-Rad), followed by repeated rinses with water. Each gel was scanned using a calibrated densitometer (ChemiDoc™ Touch Imaging System; Bio-Rad). The relative contents of MHC isoforms were determined using an analytical software (Image Lab™ software version 5.2.1; Bio-Rad).
Animal experiment
Male C57BL/6 mice at 10 weeks of age (n = 16) were fed a normal diet. Mice were randomly assigned to control (n = 8) and MOTS-c antibody (n = 8) groups and received once a week intraperitoneal (IP) injection of MOTS-c antibody or normal rabbit IgG (1 mg/kg/week for 4 weeks). After four weeks of experimental period, skeletal muscle samples (gastrocnemius muscle) were harvested. These samples were flash-frozen in liquid nitrogen and stored at −80°C. Skeletal muscle tissue was lysed with RIPA buffer containing 20 mM Tris-HCl, pH 7.8, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM EDTA, 1% Triton X-100, 10% Glycerol, and the Halt protease & phosphatase inhibitor cocktail (ThermoFisher Scientific) and homogenized followed by sonication. The supernatant was collected by centrifugation at 15,000 × g for 15 min at 4 °C. Protein content in the lysates was quantified using the Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific). Predetermined amounts of proteins (30 μg) were separated on 4–20% SDS-PAGE gels and blotted onto PVDF membranes (Biorad, Hercules, CA, USA). Membranes were blocked with 5% bovine serum albumin (MHC fast and slow) or nonfat dried milk (GAPDH) Tris-buffered saline with 0.1% Tween 20 and incubated with diluted primary antibodies for MHC fast (1:1000, Sigma), MHC slow (1:1000, Sigma), and GAPDH (1:2000, abcam) at 4 °C overnight according to the manufacturer’s instructions. After several washes with Tris-buffered saline containing 0.1% Tween-20, membranes were incubated at room temperature for 1 hr with the appropriate HRP-conjugated secondary antibody. Clarity™ Western ECL substrate (Biorad) was used for detecting specific bands. Membranes were imaged on a Bio-Rad ChemiDoc XRS+ imager and Relative intensities of the bands were quantified using Image Lab software (Biorad). The study was approved by the Animal Ethics Committees of Juntendo University.
Muscular strength
The knee flexor and extensor muscle strength were assessed in 86 young physical active Japanese males. An isokinetic dynamometer (BIODEX System 4; Biodex Medical Systems, Shirley, NY) was used to measure muscle strength. After at least a 15 minutes rest, participants were seated on the chair with hip joint angle positioned at 85° (full hip flexion, 0°). The center of rotation of the knee joint was visually aligned with the axis of the dynamometer lever arm, and the ankle was firmly strapped to the distal pad of the lever arm. A knee joint angle of 0° corresponded to full knee extension. Several warm-up contractions were performed before testing. Participants were instructed to perform maximal isokinetic knee extension and flexion from 0° to 90° at 60°/s, 180°/s, and 300°/s. Three maximal efforts for each isokinetic measurement were performed, and each peak torque was used in data analysis. Written informed consent was obtained from each subject in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committees of Juntendo University.
Japanese athletes
This analysis included 721 Japanese athletes (495 men and 226 women) and 873 ethnicity-matched controls (222 men and 651 women). The athlete group consisted of track and field athletes and swimmers with regional, national, and international levels. Athletes were classified into the sprint/power and endurance groups. The sprint/power group included sprinters competing in events of ≤ 400 m, jumpers, throwers, and swimmers competing in events of ≤ 100 m. The endurance group included middle- and long-distance runners and swimmers competing > 100m. Written informed consent was obtained from each subject in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committees of Juntendo University.
Genotyping
Total DNA was isolated from saliva or venous blood with an Oragene® DNA collection kit (DNA Genotek, ON, Canada) or QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions, respectively. The concentration of DNA was quantified using a NanoDrop 8000 UV-Vis spectrophotometer (Thermo Fisher Scientific, DE, USA). DNA samples were stored at 4°C until use. The m.1382A>C polymorphism (rs111033358) was genotyped using a real-time thermocycler in the endpoint analysis mode (LightCycler 480, Roche Applied Science, Mannheim, Germany) using custom TaqMan® SNP Genotyping Assay. A genotyping mixture (final volume of 5 μl) containing 2.5 μl TaqMan® GTXpress™ Master Mix (2×), 0.0625 μl TaqMan® SNP Genotyping Assay Mix (40×), 1.4375 μl sterilized water, and 1 μl genomic DNA (10 ng/μl) was used. Two to four negative controls were included on each plate. Genotypes were determined on the basis of the TaqMan® assay results using LightCycler® 480 SW, version 1.5 (Roche Molecular Systems).
Statistical analysis
All data are expressed as the mean ± standard error (SE) or frequency counts. The Shapiro-Wilk test was used to assess the normality of all parameters. Unpaired t-test or Mann-Whitney U test were applied for continuous variables, while the chi-squared test was applied for nominal variables. Independent correlates of each muscle fiber composition were examined by performing multiple linear regression analysis. Statistical significance was set at P < 0.05. Statistical analyses were performed using JMP Pro version 12 (SAS Institute).
RESULTS
MOTS-c regulates MHC composition in the skeletal muscle
m.1382A>C, a SNP located in the MOTS-c coding region, is associated with MHC composition differences. Specifically, the m.1382A>C alternative allele carriers (i.e., C allele) exhibited a trend of lower MHC-I isoform compared to reference allele carriers (i.e., A-allele) (45.6 ± 3.2 % vs. 40.2 ± 0.8 %, P = 0.098). In addition, the m.1382A>C alternative allele carriers showed significantly higher MHC-IIx composition than the A allele carriers (26.3 ± 2.5 % vs. 21.1 ± 0.6 %, P < 0.05) after adjusting for age and sex (Figure 1A).
Figure 1. Association of skeletal muscle myosin heavy chain (MHC) composition on MOTS-c variation and activity.
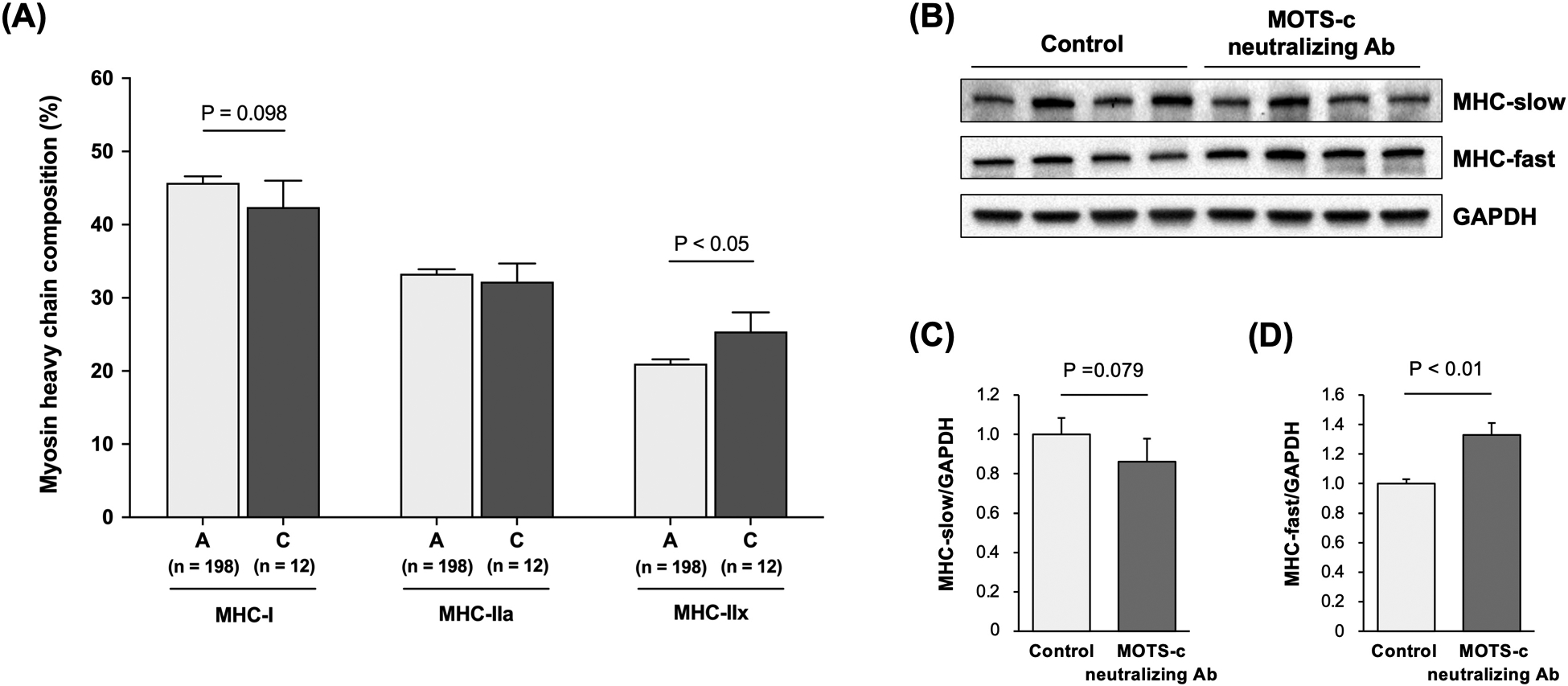
(A) Skeletal muscle MHC composition in Japanese with the A allele and C allele of the m.1382A>C polymorphism. P-values are adjusted by age and sex. The genotyping success rate was 99.5% for the m.1382A>C polymorphism. (B) Expression levels of the MHC-slow (C) and MHC-fast (D) in the gastrocnemius muscle in mice injected with MOTS-c neutralizing antibody (Ab) (n = 8) or rabbit IgG as a control group (n = 8). Mice received once a week intraperitoneal injection of MOTS-c antibody or normal rabbit IgG (1 mg/kg/week for 4 weeks). Data are expressed as the mean ± standard error.
Because the MOTS-c K14Q is suggested to be a bioinactive form of MOTS-c peptide, we injected MOTS-c neutralizing antibody to suppress MOTS-c action in young mice. The MOTS-c antibody injection group exhibited approximately 15% lower (P = 0.079) and 30% higher (P < 0.01) protein expression of MHC-slow and MHC-fast in the skeletal muscle, respectively (Figure 1B–D).
The m.1382A>C polymorphism is associated with muscle strength
Markers of muscle strength were measured by MOTS-c genotype. Characteristics of the studied young male subjects for muscle strength are shown in Table 1. There were no differences in age, height, and weight between the A and C allele carriers of m.1382A>C polymorphism (Table 1). Men with the C allele of m.1382A>C exhibited significantly higher peak torque of leg extension and flexion at 60°/s (leg extension; 245 ± 14 NM vs. 204 ± 4 NM, P < 0.01, leg flexion; 122 ± 8 NM vs. 102 ± 3 NM, P < 0.05), 180°/s (leg extension; 166 ± 10 NM vs. 144 ± 3 NM, P < 0.05, leg flexion; 94 ± 6 NM vs. 77 ± 2 NM, P < 0.05), and 300°/s (leg extension; 130 ± 8 NM vs. 113 ± 3 NM, P < 0.05, leg flexion; 80 ± 6 NM vs. 64 ± 2 NM, P < 0.05) than the A allele carriers (Figure 2-A and B).
Table 1.
Characteristics of the subjects (n = 86)
| A allele | C allele | P value | |
|---|---|---|---|
| n = 78 | n = 8 | - | |
| Age, year | 20.1 ± 0.2 | 20.1 ± 0.7 | n.s. |
| Height, cm | 175.8 ± 0.7 | 176.5 ± 2.3 | n.s. |
| Weight, kg | 68.2 ± 0.9 | 71.6 ± 2.9 | n.s. |
Data are shown as the mean ± SE.
Figure 2. Muscular strength in the m.1382A>C polymorphism carriers.
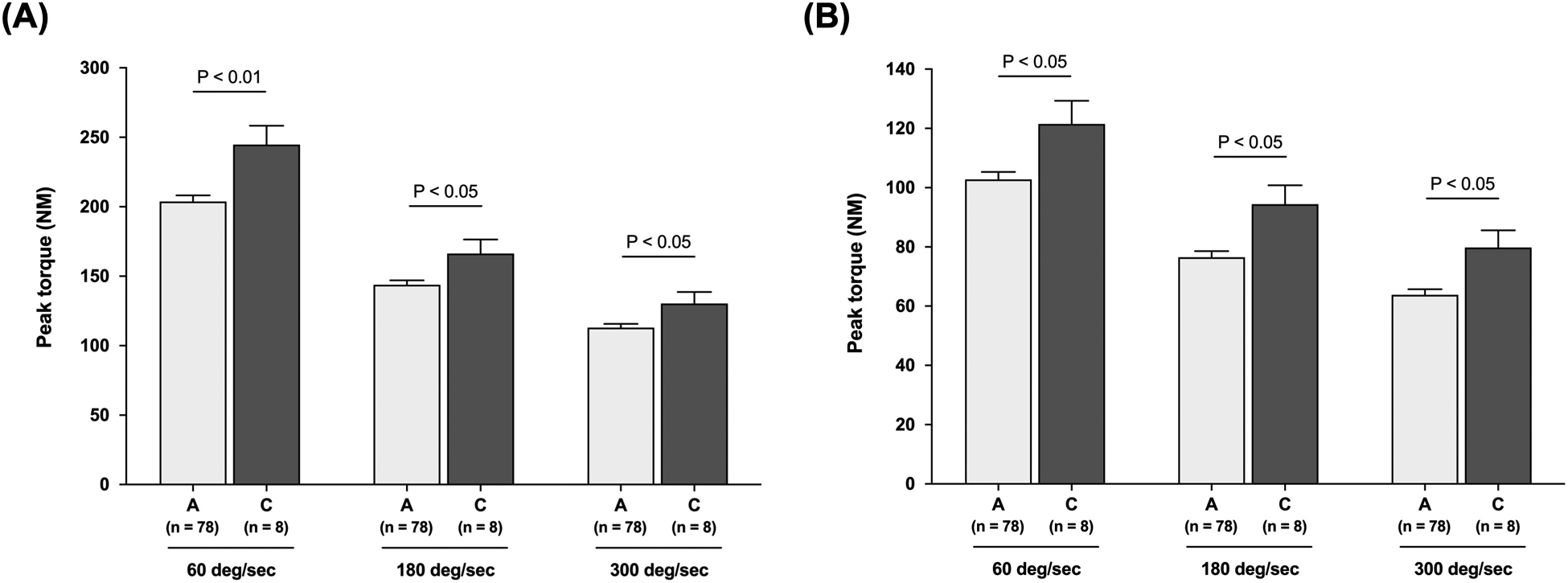
Peak torque of leg extension (A) and flexion (B) at 60°/s, 180°/s, and 300°/s in young Japanese men. The genotyping success rate was 100% for the m.1382A>C polymorphism. Data are expressed as the mean ± standard error.
The C allele of m.1382A>C is overrepresented in the sprint/power athletes
Table 2 shows the characteristics of studied Japanese athletes. Sprint/power athletes were taller and heavier than endurance athletes (Table 2). In athletes, the C allele frequency was higher in the order of sprint/power athletes (6.5%), controls (5.1%), and endurance athletes (2.9%) (Figure 3, Chi-square test: P = 0.062, Trend test: P < 0.05). A similar finding was observed in men, namely, sprint/power athletes (7.9%) > control (5.4%) > endurance athletes (3.8%) (Chi-square test: P = 0.135, Trend test: P < 0.05). In contrast, there were no significant differences among groups in women.
Table 2.
Characteristics of Japanese athletes
| Endurance | Sprint/power | P value | |
|---|---|---|---|
| n = 413 | n = 308 | - | |
| Female (%) | 146 (35.4) | 80 (26.0) | < 0.01 |
| Age, year | 22.7 ± 0.3 | 21.7 ± 0.3 | < 0.05 |
| Height, cm | 168.3 ± 0.4 | 171.9 ± 0.5 | < 0.001 |
| Weight, kg | 55.2 ± 0.5 | 64.6 ± 0.6 | < 0.001 |
Data are shown as the mean ± SE.
Figure 3. Frequency of the C allele in the m.1382A>C polymorphism in Japanese athletes.
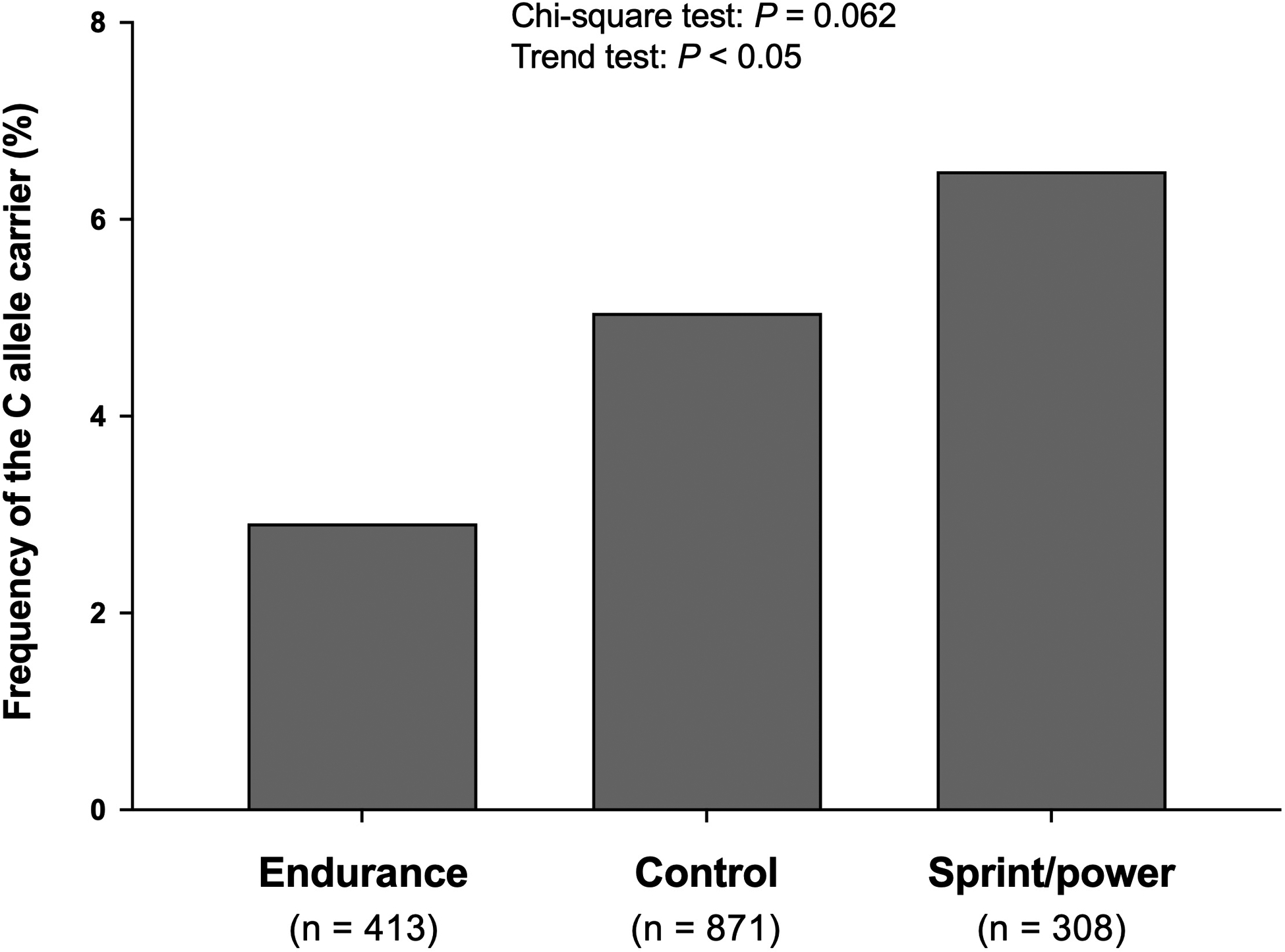
The genotyping success rate was 99.9% for the m.1382A >C polymorphism.
DISCUSSION
Here, we report that the East Asian-specific m.1382A>C polymorphism causing MOTS-c K14Q is associated with skeletal muscle fiber composition. Alternative allele carriers of m.1382A>C exhibited higher and lower percentages of MHC-IIx and MHC-I than the reference A allele carriers. Additionally, MOTS-c neutralizing antibody injected mice – mimicking the C allele of m.1382A>C polymorphism – showed significantly higher MHC-fast and trended lower in MHC-slow expression than the control group. Complementing in vivo findings, young men with the alternative allele of m.1382A>C exhibited higher muscular strength, and the frequency of the alternative allele carriers were enriched in the male sprint/power athlete group compared with the reference allele. These findings suggest that the m.1382A>C polymorphism casing MOTS-c K14Q modifies physical performance by inactivating MOTS-c and thereby regulating skeletal muscle fiber composition.
In past studies, MOTS-c administration increased exercise endurance in young, middle-aged, and old mice [6]. Additionally, the expression levels of the MOTS-c are positively and negatively correlated with the gene expression levels of slow- and fast-twitched fiber, respectively, in the human skeletal muscle [7]. These studies suggest that MOTS-c influences physical performance through regulating skeletal muscle fiber composition. We have recently shown that the MOTS-c K14Q was bioinactive and partially lost the function of the MOTS-c [10].
We administrated a MOTS-c neutralizing antibody to the mice and assessed the MHC expression in the mice. Mice injected with the MOTS-c neutralizing antibody exhibited higher and lower expression levels of MHC-fast and MHC-slow than the control group, respectively. These results support the finding that the C allele carriers of the m.1382A>C polymorphism exhibited a higher percentage of fast-twitched fibers than the A allele carriers. On the other hand, this study also showed that administration of the MOTS-c neutralizing could be a possible way to knock down MOTS-c in vivo. Although we administrated K14Q MOTS-c into mice to mimic the C allele carriers of m.1382A>C polymorphism in the previous study [10], administrating the MOTS-c neutralizing antibody could be an alternative way to imitate the C allele carriers of m.1382A>C polymorphism.
We recently demonstrated that men with the C allele of the m.1382A>C polymorphism in the MOTS-c exhibited a higher risk for T2DM than the A allele carriers in the Japanese population [10]. Additionally, the present study demonstrated that the frequency of the C allele of the m.1382A>C in the MOTS-c polymorphism overrepresented in the sprint/power athlete in the Japanese population. These findings imply that the sprint/power athlete-related mtDNA polymorphisms could become a risk for T2DM. Interestingly, we previously demonstrated a similar finding that the frequency of mitochondrial haplogroup F was higher in both sprint/power Olympic athletes and T2DM subjects than controls [19, 20]. One of the possible mechanisms of these observations is skeletal muscle fiber composition. The fast-twitched fibers are suitable for short bursts of strength and speed [16], while the proportion of fast-twitched fiber is negatively correlated to glucose uptake [14, 15]. Indeed, it has been reported that skeletal muscle fiber composition is associated with metabolic syndrome [12]. Although we did not assess differences in the glucose metabolism between the A allele and C allele carriers, these muscle fiber type-specific characteristics could affect phenotypes with shared biological processes to muscle fibers.
MOTS-c might influence skeletal muscle fiber composition through regulating signaling pathways in skeletal muscle. Lee et al. firstly reported that MOTS-c activated AMPK and prevented aging- and high fat diet-induced insulin resistance [5]. Additionally, a recent study demonstrated that MOTS-c treatment activated AMPK and increased PGC-1α expression in C2C12 myotubes [8]. Overexpression of PGC-1α in mice increases slow-twitched fibers [21], whereas PGC-1α knock-out mice showed increased fast-twitched fibers [22]. These studies suggest that MOTS-c may regulate muscle fiber composition through the AMPK/ PGC-1α signaling pathway. The transcription factor forkhead box protein O1 (FoxO1) signaling may also mediate the association between MOTS-c and muscle fiber type composition. A previous study demonstrated that overexpression of the FoxO1 in skeletal muscle decreased expression levels of slow-twitched fiber-related genes [23], suggesting that FoxO1 expression levels are associated with skeletal muscle fiber composition. We previously demonstrated that MOTS-c administration decreased FoxO1 protein levels in the skeletal muscle in high-fat-fed mice [24]. Thus, decreased FoxO1 proteins by MOTS-c may upregulate the proportion of slow-twitched fiber in the skeletal muscle. Taken together, these signaling pathways in the skeletal muscle could be involved in the association between MOTS-c and skeletal muscle fiber composition.
In summary, the C allele of the East Asian-specific m.1382A>C polymorphism, which causes MOTS-c K14Q, associated with sprint/power performance and regulated skeletal muscle fiber composition. These observations suggest that the m.1382A>C polymorphism in MOTS-c can modify physical performance and risks for metabolic disorders via skeletal muscle fiber regulation.
Acknowledgments
The authors thank all staff members who supported the data collection and analyses.
Source of Funding
This research was supported in part by JSPS KAKENHI Scientific Research (B) (18H03155 to N.F.), Young Scientists (A) (17H04752 to E.M.), Young Scientists (18K17863 to H.K.), and by the Institute of Health and Sports Science & Medicine, Juntendo University. This study was also supported in part by grants P01AG034906 and R01AG069698 to P.C.
Footnotes
Declaration of Competing Interest
Pinchas Cohen is a consultant and stockholder of CohBar Inc.
References
- [1].Kim S-J, Miller B, Kumagai H, Silverstein AR, Flores M, Yen K, Mitochondrial-derived peptides in aging and age-related diseases, GeroScience, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Miller B, Kim SJ, Kumagai H, Mehta HH, Xiang W, Liu J, Yen K, Cohen P, Peptides derived from small mitochondrial open reading frames: Genomic, biological, and therapeutic implications, Exp Cell Res, (2020) 112056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Merry TL, Chan A, Woodhead JST, Reynolds JC, Kumaga H, Kim SJ, Lee C, Mitochondrial-derived peptides in energy metabolism, Am J Physiol Endocrinol Metab, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim SJ, Xiao J, Wan J, Cohen P, Yen K, Mitochondrially derived peptides as novel regulators of metabolism, J Physiol, 595 (2017) 6613–6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P, The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance, Cell Metab, 21 (2015) 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, Lu R, Cohen P, Graham NA, Benayoun BA, Merry TL, Lee C, MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis, Nature Communications, 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].D’Souza RF, Woodhead JST, Hedges CP, Zeng N, Wan J, Kumagai H, Lee C, Cohen P, Cameron-Smith D, Mitchell CJ, Merry TL, Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition, Aging (Albany NY), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang B, Yu Q, Chang B, Guo Q, Xu S, Yi X, Cao S, MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1867 (2021) 166126. [DOI] [PubMed] [Google Scholar]
- [9].Fuku N, Pareja-Galeano H, Zempo H, Alis R, Arai Y, Lucia A, Hirose N, The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity?, Aging Cell, 14 (2015) 921–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zempo H, Kim SJ, Fuku N, Nishida Y, Higaki Y, Wan J, Yen K, Miller B, Vicinanza R, Miyamoto-Mikami E, Kumagai H, Naito H, Xiao J, Mehta HH, Lee C, Hara M, Patel YM, Setiawan VW, Moore TM, Hevener AL, Sutoh Y, Shimizu A, Kojima K, Kinoshita K, Arai Y, Hirose N, Maeda S, Tanaka K, Cohen P, A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c, Aging (Albany NY), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brooke MH, Kaiser KK, Muscle Fiber Types: How Many and What Kind?, Archives of Neurology, 23 (1970) 369–379. [DOI] [PubMed] [Google Scholar]
- [12].Stuart CA, McCurry MP, Marino A, South MA, Howell MEA, Layne AS, Ramsey MW, Stone MH, Slow-Twitch Fiber Proportion in Skeletal Muscle Correlates With Insulin Responsiveness, The Journal of Clinical Endocrinology & Metabolism, 98 (2013) 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B, Metabolic characteristics of fibre types in human skeletal muscle, Acta Physiol Scand, 95 (1975) 153–165. [DOI] [PubMed] [Google Scholar]
- [14].Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C, Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man, J Clin Invest, 80 (1987) 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fisher G, Windham ST, Griffin P, Warren JL, Gower BA, Hunter GR, Associations of human skeletal muscle fiber type and insulin sensitivity, blood lipids, and vascular hemodynamics in a cohort of premenopausal women, Eur J Appl Physiol, 117 (2017) 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gollnick PD, Matoba H, The muscle fiber composition of skeletal muscle as a predictor of athletic success, The American Journal of Sports Medicine, 12 (1984) 212–217. [DOI] [PubMed] [Google Scholar]
- [17].Kumagai H, Tobina T, Ichinoseki-Sekine N, Kakigi R, Tsuzuki T, Zempo H, Shiose K, Yoshimura E, Kumahara H, Ayabe M, Higaki Y, Yamada R, Kobayashi H, Kiyonaga A, Naito H, Tanaka H, Fuku N, Role of selected polymorphisms in determining muscle fiber composition in Japanese men and women, J Appl Physiol (1985), 124 (2018) 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sugiura T, Morita S, Morimoto A, Murakami N, Regional differences in myosin heavy chain isoforms and enzyme activities of the rat diaphragm, J Appl Physiol (1985), 73 (1992) 506–509. [DOI] [PubMed] [Google Scholar]
- [19].Mikami E, Fuku N, Takahashi H, Ohiwa N, Scott RA, Pitsiladis YP, Higuchi M, Kawahara T, Tanaka M, Mitochondrial haplogroups associated with elite Japanese athlete status, British Journal of Sports Medicine, 45 (2011) 1179–1183. [DOI] [PubMed] [Google Scholar]
- [20].Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, Segawa T, Watanabe S, Kato K, Yokoi K, Nozawa Y, Lee HK, Tanaka M, Mitochondrial Haplogroup N9a Confers Resistance against Type 2 Diabetes in Asians, The American Journal of Human Genetics, 80 (2007) 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin J, Wu H, Tarr PT, Zhang C-Y, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM, Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres, Nature, 418 (2002) 797–801. [DOI] [PubMed] [Google Scholar]
- [22].Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM, Skeletal Muscle Fiber-type Switching, Exercise Intolerance, and Myopathy in PGC-1α Muscle-specific Knock-out Animals, Journal of Biological Chemistry, 282 (2007) 30014–30021. [DOI] [PubMed] [Google Scholar]
- [23].Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O, Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control, J Biol Chem, 279 (2004) 41114–41123. [DOI] [PubMed] [Google Scholar]
- [24].Kumagai H, Coelho AR, Wan J, Mehta H, Yen K, Huang A, Zempo H, Fuku N, Maeda S, Oliveira PJ, Cohen P, Kim SJ, MOTS-c reduces myostatin and muscle atrophy signaling, Am J Physiol Endocrinol Metab, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


