Abstract
Kidney fibrosis is associated with the progression of acute kidney injury to chronic kidney disease. MG53, a cell membrane repair protein, has been shown to protect against injury to kidney epithelial cells and acute kidney injury. Here, we evaluated the role of MG53 in modulation of kidney fibrosis in aging mice and in mice with unilateral ureteral obstruction (UUO) a known model of progressive kidney fibrosis. Mice with ablation of MG53 developed more interstitial fibrosis with age than MG53-intact mice of the same age. Similarly, in the absence of MG53, kidney fibrosis was exaggerated compared to mice with intact MG53 in the obstructed kidney compared to the contralateral unobstructed kidney or the kidneys of sham operated mice. The ureteral obstructed kidneys from MG53 deficient mice also showed significantly more inflammation than ureteral obstructed kidneys from MG53 intact mice. In vitro experiments demonstrated that MG53 could enter the nuclei of proximal tubular epithelial cells and directly interact with the p65 component of transcription factor NF-κB, providing a possible explanation of enhanced inflammation in the absence of MG53. To test this, enhanced MG53 expression through engineered cells or direct recombinant protein delivery was given to mice subject to UUO. This reduced NF-κB activation, inflammation and attenuated kidney fibrosis. Thus, MG53 may have a therapeutic role in treating chronic kidney inflammation and thereby provide protection against fibrosis that leads to the chronic kidney disease phenotype.
Keywords: TRIM72, inflammation, myokine, cytokine, chronic kidney disease
Graphical Abstract

INTRODUCTION
Kidney dysfunction, which can be classified as acute kidney injury (AKI) or chronic kidney disease (CKD), has grown into an epidemic in older populations. In 2017, the prevalence of CKD reached 14.5% of people aged 65 and over, resulting in Medicare expenditures of over $84 billion for treatment.1, 2 AKI and CKD are more common in older individuals, mainly due to increased susceptibility to injury plus a diminished ability to repair the aging kidney. Clinical studies have shown that CKD is a major risk factor for AKI and conversely, episodes of AKI can accelerate the progression of CKD toward end-stage kidney disease (ESKD). Currently, the treatment of AKI is mainly supportive, and the treatment of established CKD is focused on slowing progression by through inhibition of the renin-angiotensin-aldosterone system, and now possibly through sodium-glucose co-transporter-2 (SGLT2) inhibition.3, 4
Depending on the severity of injury, proximal tubular cells, the major target of acute injury, may undergo changes in cell cycle progression,5, 6 metabolism,7 secretion of pro-inflammatory and profibrotic cytokines, or partial epithelial-mesenchymal transition (EMT),8 which will determine whether remodeling/repair will be successful, or maladaptive and result in kidney fibrosis, a hallmark of CKD.9, 10 This remodeling generally occurs on a background of renal inflammation, diminished vascular supply and production of extracellular matrix (ECM) proteins, and includes loss of epithelial cells and accumulation of collagens, α-smooth muscle actin (SMA) and fibronectin, and has a high correlation with deterioration of kidney function.
Chronic inflammation leads to fibrotic remodeling that may also underlie the transition from AKI to CKD.11–13 Cumulative research suggests the involvement of the NF-κB transcription factor in the pathogenesis of renal inflammation caused by infection, injury, or transplantation.11,12, 14 Activation of the canonical NF-κB pathway starts with activation of IκB kinase (IKK) which leads to phosphorylation and degradation of IκBα and the nuclear translocation of NF-κB heterodimers.15 Activation of NF- κB signaling in kidney epithelial cells and infiltrating immune cells can be elicited by pathophysiological triggers such as exposure to lipopolysaccharides (LPS) or ischemia-reperfusion injury (IRI).16 Molecular profiling studies reveal nfkb1 as a major driver of renal fibrosis.17 In addition to renal tubular cells, innate immune cells such as macrophages and dendritic cells also contribute to renal injury, inflammation and fibrotic remodeling.13, 18–20
Growing evidence suggests that muscle-derived secretory factors, myokines, modulate systemic physiology via tissue crosstalk to influence the progression of kidney diseases.21 MG53 (also named TRIM72) is a muscle-enriched tripartite motif (TRIM) protein with a critical function in cell membrane repair.22, 23 TRIM family proteins have diverse functions ranging from regulation of immune signaling to tissue repair.24 MG53-mediated membrane repair is involved in mitigating acute injury to skeletal muscle,25 kidney,26 heart,27 lung,28 brain,29, 30 and skin.31 Our previous studies identified a low level of MG53 expression in the kidney, which is a key component in AKI protection, and mice deficient in MG53 (mg53−/−) were more susceptible to stress-induced AKI26. We also recently demonstrated that sustained elevation of MG53 in the circulation enhances tissue injury-repair and regeneration.32 The relative roles of kidney-specific and extrinsic circulating MG53 in modulating renal fibrosis during progressive CKD are still unknown.
Recently, an emerging role of MG53 in modulating tissue protection through inhibition of inflammation, especially via modulation of NF-κB signaling has evolved. As examples, MG53 attenuates LPS-induced neurotoxicity and neuroinflammation by inhibiting the TLR4/NF-κB pathway33 and cardiac MG53 regulates KChIP2 expression to control electrophysiological remodeling during cardio-hypertrophy.34 In addition, we demonstrated a dual function of MG53 in preventing a maladaptive hyper-inflammatory response during a sub-lethal influenza A virus (IAV) infection, characterized by excessive IFNβ production, via suppression of IRF3/NF-κB activation, and inhibition of aberrant intracellular Ca2+ signaling in macrophages.35 Furthermore, upon a lethal dose of IAV infection, MG53 was shown to prevent mortality and lung damage, not by directly affecting viral titers per se, but by mitigating cytokine storm (IFNβ, IL-6, and IL-1β) through negative regulation of the NLRP3 inflammasome and prevention of pyroptosis of the lung.36 Moreover, high dose chronic treatment of exogenous MG53 was linked to suppression of NF-κB-mediated inflammation in the aged heart.37 These studies suggest MG53 can modulate aberrant NF-κB signaling during inflammation, but whether this occurs in the context of kidney inflammation has not been studied.
We postulated that MG53 regulates kidney inflammation by modulating the transcriptional activity of NF-κB, and in so doing can attenuate the kidney fibrosis that occurs as a consequence of chronic inflammation. This study was undertaken to address this hypothesis.
METHODS
Animals Studies
All experiments with animals adhered to the NIH Guide for the Care and Use of Laboratory Animals, and followed protocols approved by the Ohio State University IACUC. Age-matched wild type (wt) or mg53−/− non-littermates of the 129S1/SvImJ strain were used22 to establish the role of MG53 in modulation of age-dependent and unilateral ureteric obstruction (UUO)-induced kidney fibrosis. C57B6/J mice (9–10 weeks) were purchased from Jackson Labs.
For kidney injury studies, mice (9–12 weeks) were subjected to UUO to induce kidney fibrosis. Mice were anesthetized via isoflurane (1.5 – 2.0%) to ensure a deep plane of anesthesia. The UUO procedure was performed by ligating the left ureter with 5–0 surgical silk twice according to published protocols.38 Sham mice only had an abdominal skin incision. To evaluate the effect of rhMG53 on obstructive kidney injury, one group of UUO mice received rhMG53 (2 mg/kg) via tail vein injection beginning immediately after UUO surgery (day 0) and then daily for 7 days, with two additional doses on days 9 and 11. A control group of UUO mice received saline injections according to the same schedule. Mice were sacrificed on day 12 post UUO surgery, kidneys were perfused in situ with PBS, and tissue samples from the UUO kidney and contralateral kidney (CLK) were collected for histology and western blot analysis.
In separate studies, wt and mg53−/− mice underwent UUO or sham surgery and were sacrificed day 7 post-operation. MG53 was given to mice using a cell-based therapy approach.39 RAW 264.7 macrophages were infected with doxycycline (DOX)-dependent Ad-tPA-MG53-mcherry viral particles for 24 hr. The infected cells (at a concentration of 2 × 10 ^6/100 ul saline per mouse) were tail-vein-injected into mice immediately after surgery (day 0 injection). Mice received four injections of Ad-tPA-MG53 transduced RAW cells on days 0, 1, 3 and 6. Immediately after the day-0 cell injection, mice were randomized into a group that received and a group that did not receive DOX (2 mg/ml in 5% sucrose solution) in their drinking water for 7 days and then sacrificed. Tissue samples from the UUO kidney and contralateral kidney (CLK) were collected for histology, RNA and protein analyses.
Statistical Analyses
Data are expressed as means ± SD. Comparison within groups was made by Student’s t-test when comparing two experimental groups and by one-way analysis of variance (ANOVA) for more than two groups (Graphpad Prism 8.2), followed by ad-hoc either Bonferroni’s or Holm-Sidak’s multiple comparison test. A value of P < 0.05 was considered statistically significant.
Supplementary Methods
Full methods including histologic evaluation, serum creatinine (Scr) measurements, plasmid constructs, cell culture and primary tubular epithelial cell isolation, adenovirus preparation and cell infection, NF-κB p65 nuclear translocation assay, immunohistochemistry and confocal images, NF-κB Luciferase Reporter Assay, cytokines ELISA, tissue fractionation, immunoblotting, co-immunoprecipitation (IP), quantitative real-time PCR (qRT-PCR), rhMG53 production are in the Supplementary Methods.
RESULTS
Mice with ablation of MG53 develop age-dependent renal fibrosis
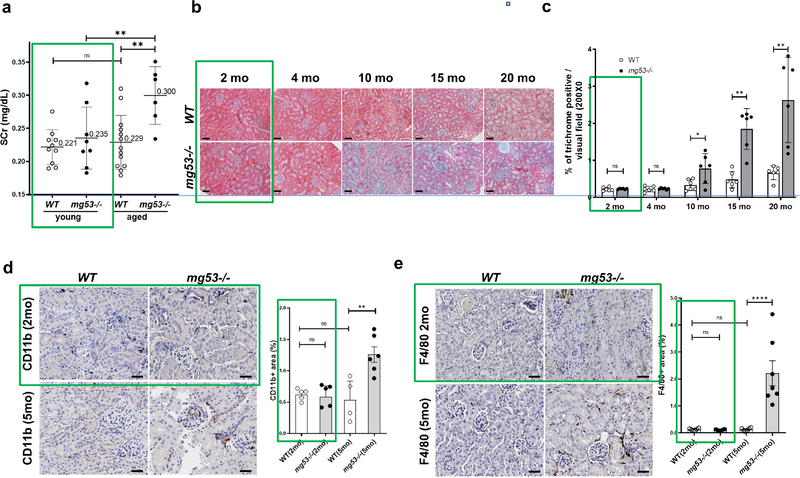
We compared the effect of aging on kidney function and fibrosis in mg53−/− and wild type (wt) mice. We confirmed our previous observation that while Scr levels of young mice (2-month) with and without MG53 were not significantly different, older (16–20 month) mg53−/− mice exhibited a significantly higher Scr than age-matched wild-type controls (Figure 1a). Based on trichrome staining, we found that mg53−/− mice had more renal fibrosis compared with the wt mice, starting at the age of 5-months and continuing through the age of 20 months (Figure 1b, 1c). Immunohistochemical staining was used to evaluate the impact of MG53 ablation on infiltration of leukocytes, monocytes and macrophages in 2-month and 5-month old mouse kidneys (Figure 1d, e). Kidneys from young wild-type and mg53−/− mice had similar levels of intra-renal leukocytes, but significantly more inflammatory cells were found in mg53−/− kidneys than wt kidneys in 5-month old mice.
Figure 1. The effect of the absence of MG53 on kidney function and histology in aged mice.
(a) Compared to young (2-month) mice, Scr concentrations from aged mice (16–20 month) are significantly higher in the absence of MG53. (b) Masson’s trichrome stain for renal fibrosis in kidneys from WT and mg53−/− mice of different ages (2–20 month). Scale bars, 50 μm. (c) Quantification of renal fibrosis in aging WT and mg53−/− mice (n=6). Data are presented as mean ± SD. Data were compared by two-way ANOVA test, followed by Bonferroni’s correction for multiple comparison. ns, not significant; *P <0.05, ** P <0.01. (d and e) Immunostaining of kidney sections from WT (left) or mg53−/− (right) (2 or 5 months of age) with anti-CD11b (d) or anti-F4/80 (e). Representative image of cortex and outer medulla are shown. Scale bars, 100 μm. (e). Quantification of renal inflammation in WT and mg53−/− mice (n=4). Values are given as means ± SD, Student’s t-test. ** P <0.01.
Moreover, immunohistochemistry for α-SMA and fibronectin, two extracellular matrix proteins commonly found in areas of kidney fibrosis, showed enhanced (3–8 fold) staining in the glomeruli and tubulointerstitium of kidneys from 10-month old mg53−/− mice compared to 10-month old wt mice (Supplementary Figure S1).
UUO induces aggravated renal inflammation and fibrosis in mg53−/− mice
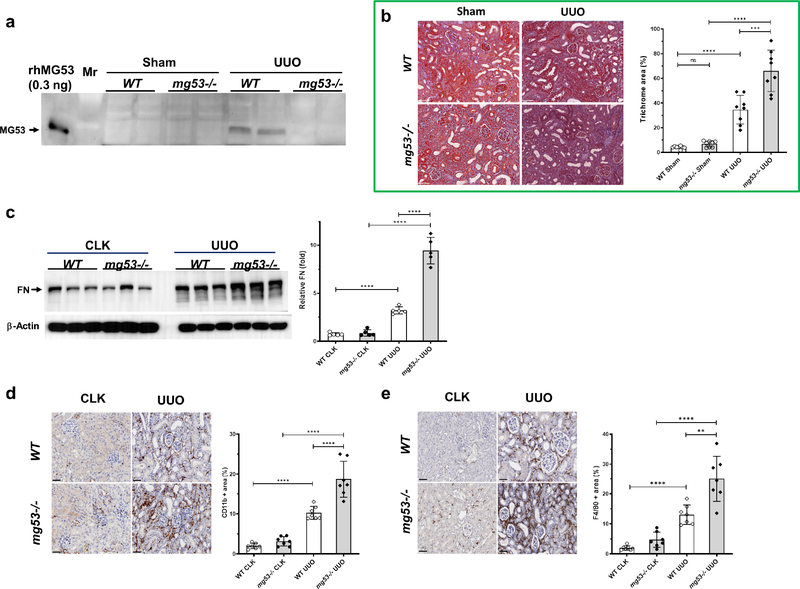
Kidney MG53 levels detected by immunoblotting significantly increased 7-days after UUO (Figure 2a). The mg53−/− UUO kidneys displayed exaggerated fibrosis, with 30% additional trichrome-stained area, than wt UUO kidneys (Figure 2b). Fibronectin was also significantly increased in UUO kidneys (Figure 2c). Kidney infiltration by leukocytes and macrophages was significantly increased after UUO in wt and mg53−/− mice, but was further increased in mg53 deficient animals (Figure 2d and 2e).
Figure 2. Loss of MG53 exacerbates renal fibrosis and immune cell accumulation after UUO.
(a) Kidney MG53 expression increases 7 days post-UUO. Total kidney lysates (50 μg) from Sham or UUO kidneys were examined by Western blotting with a custom made anti-MG53 antibody. rhMG53 (0.3 ng) was loaded in lane 1 as a positive control. (b) Representative pairwise trichrome stains showed Sham (left) and UUO (right) kidney from WT (top) or mg53−/− (bottom) mice and quantified with Masson’s Trichrome fibrosis analysis (n= 6–8). Bar= 50 μm. Values are given as means ± SD, Student’s t-test. ***P <0.001, ****P <0.0001. (c) Western blotting and quantification for fibronectin (FN) from WT and mg53−/− contralateral (CLK) and UUO kidney lysates. Data are presented as mean ± SD. (d and e) Immunostaining of the CLK or obstructed kidney after UUO in WT or mg53−/− mice with either anti-CD11b (d) or anti-F4/80 (e). Representative image of cortex and outer medulla are shown. Scale bars, 100 μm. n= 6 WT and mg53−/− mice. Data are presented as means ± S.D, **P < 0.01, ***P < 0.001, ****P<0.0001 (One way ANOVA followed by Bonferroni’s test).
MG53 modulates NF-κB activation and p65 nuclear localization
The activity of NF-κB (p65) was assessed in the UUO model. The amount of total p65 (t-p65) was significantly increased in UUO kidneys from wt and mg53−/− mice, but NF-κB activation, assessed as phosphorylation of p65, was greater (16-fold) in the mg53−/− UUO kidney than the wt UUO kidney (2-fold) compared to sham-operated animals (Figure 3a). We analyzed the relative RNA levels of TGF-β1, PAI-1 and Col1a1 in the obstructed kidney.40 We detected low levels of gene expression in sham animals, and similar induction (TGF-β1~10 fold; PAI-1 ~40 fold; Col1a1 ~40 fold) in UUO kidney from wt and mg53−/− (Figure 3b). To examine the spatial distribution of MG53 in the kidney cortex under normal conditions, renal tissue fractions were analyzed. We regularly obtained 10 fold more total cytosol protein than the total extracted nuclear protein. The t-p65 levels were comparable in the cytosolic fraction between wt and mg53−/− mice, but was greater in the nuclear fraction of mg53−/− mice (Figure 3c). MG53 was detected in the cytosol fraction (with tubulin as cytosol marker). Unexpectedly, a significant amount of MG53 was detected at the nuclear fraction (with Histone H3 as nuclear fraction marker).
Figure 3. NF-κB signaling is activated in the obstructed kidney of mg53−/− mice.
(a) Western blot of phospho (p)-p65 and total (t)-p65 levels from kidney cortex lysates prepared from sham, contralateral (CLK) and UUO kidneys. GAPDH was used as loading control. (N = 6 for each group). Analysis of the p-p65 to t-p65 ratio. Values are given as means ± SD. Student’s t-test. ns, not significant; **** P <0.0001 (b) Quantitative RT-PCR showed the relative abundance of TGF-β1, PAI-1 and Col1a1 mRNA in different groups as indicated. Values represent the means ± SD. ns, not significant; (c) Western blot analysis for t-p65 and MG53 from the cytosol and nuclear fractions (40 μg) of kidney cortex. Histone H3 was used as a nuclear marker and tubulin as a cytosol marker. The relative p65 N/C ratio was calculated with nuclear p65 (normalized to Histone H3) to cytosol p65 (normalized to tubulin) and factored in 10 to represent the 10 fold more of total cytosol protein extracted than that from the total nuclear protein. (n=6 per group) Values are given as means ± SD, Student’s t-test. **P <0.01.
To confirm this finding, we also examined MG53 localization in skeletal muscle fractions (Supplementary Figure S2). We reproduced our previous studies that MG53 localized to cytosol and sarcolemmal membrane (Na-K-ATPase as marker of membrane fraction),22, 23, 25 but also detected enriched nuclear MG53 from skeletal muscle.
MG53 interacts with p65 to control TNFα-induced transcriptional activity
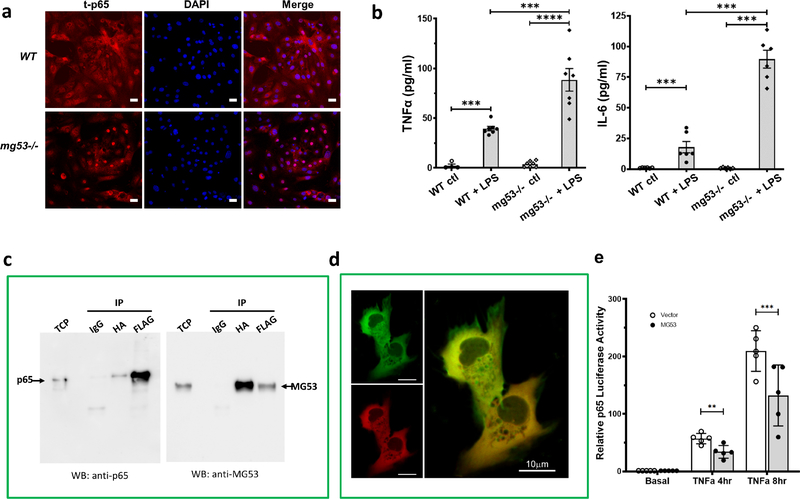
To confirm our tissue fractionation results, we conducted the following immunohistochemical studies. In cultured wt proximal tubular epithelial cells (PTEC), p65 was found mainly in the cytosol, but localized to nuclei in the absence of MG53 (Figure 4a). When these cells were treated with LPS (5μg) for 8 hours, pro-inflammatory cytokines secretion was significantly greater in mg53−/− cells than wt cells (Figure 4b).
Figure 4. MG53 interacts with p65 to control TNFα-induced transcriptional activity.
(a) Primary proximal tubule epithelial cells (PTEC) were isolated from WT and mg53−/− kidneys and stained for total p65 (t-p65, left panels), nuclei (DAPI, middle panels) and merged images (right panels). Representative images shown from n = 3 experiments. Scale bar = 25 μm. (b) PTEC secretion of TNFα (left) or IL-6 (right) 8 hours post LPS (5 μg/ml) stimulation. Values are the means ± SD of 3 independent experiments, One way ANOVA test followed by Bonferroni’s test. ***P <0.001, ****P <0.0001. (c) Co-immunoprecpitation assay using HEK293 cells co-transfected with Flag-p65 and HA-MG53 plasmids as indicated. Total cell proteins (TCP, 2%) were loaded as input. Lysates were immune-precipitated with either anti-mouse IgG control, or anti-HA magnetic beads or anti-Flag agarose beads. The immunoprecipitated proteins were loaded on gels and blots were probed with anti-p65 (left) or anti-MG53 (right). (d) HKC-8 cells were co-transfected with GFP-p65 and RFP-MG53 and analyzed with confocal microscope. Scale bar = 10 μm. (e) Quantification of MG53 effects on NF-κB/(p65) transcriptional activity. An NF-κB/(p65) luciferase reporter activity was measured with a bioluminescence luciferase assay under control conditions or after 4 hours, 8 hours of TNFα stimulation. Values are represented means ± SD from n=5 independent experiments from different days, **P < 0.01, ***P < 0.001 (Two way ANOVA followed by Bonferroni’s test).
As these data suggested MG53 may interact with p65 to regulate inflammatory cytokine expression, a direct interaction between p65 and MG53 was assessed by co-IP assays using HEK293 cells co-transfected with Flag-p65 and HA-MG53. We demonstrated weak interaction (Figure 4c). Co-transfection of GFP-p65 and RFP-MG53 plasmids and confocal analysis confirmed colocalization of p65 and MG53 (Figure 4d). We then quantified whether MG53 affects NF-κB (p65) transcriptional activity after TNFα stimulation by measuring the luciferase activity from a stable NF-κB/293-GFP-Luc transcriptional reporter cell line that was transiently transfected with either pHM6 vector or HA-MG53. The reporter cells contain a transduced lentivirus expression Firefly Luciferase Reporter driven by a minimal cytomegalovirus (mCMV) promoter in conjunction with four copies of consensus NF-κB transcriptional response elements (κB site) upstream. In the absence of TNFα there was very low intrinsic luciferase activity detected in the reporter cells expressing MG53 proteins. MG53 suppressed TNFα-elicited NF-κB (p65) transcriptional activity by about 40%. (Figure 4e).
Overexpression of MG53 prevented NF-κB p65 nuclear translocation upon TNFα treatment
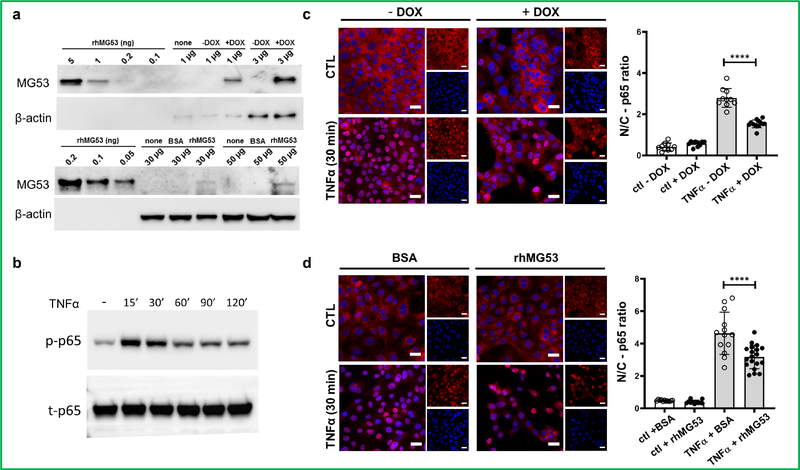
NF-κB p65 translocated to nucleus upon TNFα treatment in kidney PTE41. To explore whether treatment with MG53 could atteuate NF-κB p65 nuclear translocation upon TNFα stimulation, we overexpressed MG53 in HKC-8 cells either via DOX-dependent Ad-tPA-MG53 viral transduction or through treatment with recombinant human MG53 (rhMG53). Using an MG53-specific mAb, after DOX-dependent induction MG53 was detected by immunoblotting. MG53 was present at a very high level in Ad-tPA-MG53 transduced HKC-8 cells, equivalent to 1 ng rhMG53/μg cellular lysate. In contrast, HKC-8 took up and expressed a much lesser amount of rhMG53 (Figure 5a). Uptake of fluorescent-labeled rhMG53 by HKC-8 was confirmed by confocal microscopy (Supplementary Figure S3). After TNFα treatment time to peak p65 activation was 15 – 30 min (Figure 5b). Under basal conditions, NF-κB p65 resided mainly in the cytosol, and overexpression of MG53 did not change this (Figures 5c and d). However, after TNFα stimulation, p65 translocated to the nuclei in HKC-8 cells not over-expressing MG53. In comparison, nuclear translocation of p65 fell by 33% (cells given rhMG53) to 50% (transduced cells given DOX) in cells overexpressing MG53 (Figures 5c and d).
Figure 5. MG53 prevents p65 nuclear translocation upon TNFα treatment.
(a) Western blot showing MG53 overexpression either by Ad-tPA-MG53 viral transduction (upper panel) or rhMG53 uptake (lower panel) in HKC-8 cells. Various quantities of rhMG53 were loaded as standards. Exposure time was adjusted as needed. (b) time-course of TNFα treatment and expression of p-p65 and t-p65. (c and d) Confocal analysis the effect of HKC-8 cells expressing Ad-tPA-MG53 (c) or uptake rhMG53 (d) on nuclear translocation of NF-κB p65 upon TNFα treatment. Values represent the means ± SD from 3 independent experiments. ****P < 0.0001 (One way ANOVA followed by Turkey’s multiple comparison test).
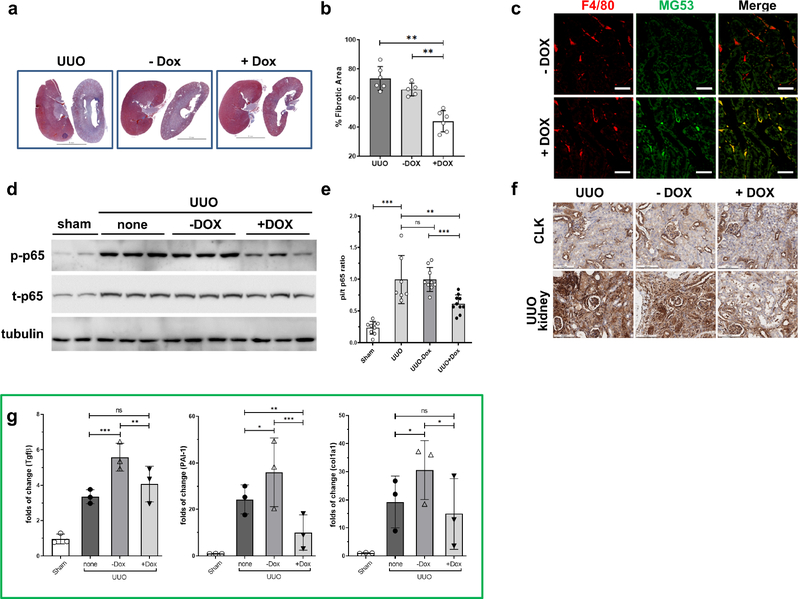
Doxycycline-inducible expression and secretion of MG53 from RAW 264.7 cells ameliorates UUO-induced kidney fibrosis in mice
To understand whether treatment with MG53 could attenuate fibrosis, MG53 was increased in the UUO model via engineered RAW 264.7 macrophages (RAW). We engineered RAW via Dox-dependent induction of Ad-tPA-MG53 expression. DOX-dependent induction of tPA-MG53 expression was confirmed by confocal immuostaining of RAW with an MG53-specific mAb (Supplementary Figure S4a), immunoblotting RAW cell lysates after viral transduction (Supplementary Figure S4b), and immunoblotting concentrated culture medium of transduced RAW (Supplementary Figure S4c).
Adoptive transfer of engineered RAW cells into the UUO mouse model was done with 1 × 106 cells administered through tail vein injection immediately after UUO-surgery. Mice were randomly divided into a group that received normal drinking water (−DOX) and a group that received DOX (2 mg/ml) in drinking water. UUO mice received RAW every other day for a total of 4 injections. Kidneys were harvested 7-days post-surgery.
Mice infused with engineered RAW followed by DOX induction displayed significantly reduced (by 30%) kidney fibrosis compared to those who received no cells, or those infused with engineered RAW cells but without Dox induction (66% fibrotic area) (Figure 6a and 6b). Only a small RAW/Ad-tPA-MG53 population survived the surveillance of reticuloendothelial system by the end of the experiments. IHC staining confirmed that Dox-treatment induced RAW-mediated MG53 expression in the kidney (Figure 6c). Interestingly, UUO kidneys from RAW infused mice and DOX-induced MG53 exhibited a lower p-65/t-p65 ratio (by about 40%) than those who either did not receive RAW, or those given RAW without DOX (Figure 6d and 6e). The reduced p-p65 level was confirmed with p-p65 (S536) IHC staining of UUO kidneys (Figure 6f). We think circulating MG53 delivered by Raw/Ad-tPA-MG53/+DOX and uptake by kidney proximal tubule cells could participate in reducing p-p65 (S536) and decrease UUO kidney inflammation. Compared to UUO-only kidneys, TGF-β1, PAI-1 and Col1a1 expression were increased significantly in UUO kidneys from mice infused with RAW without DOX (no MG53 induction). Elevation of these pro-fibrotic genes could be attributed to induction of a strong allograft reaction from infused cells into immunocompetent mice. However, UUO kidneys from mice infused with RAW and given DOX (MG53 induction) expressed significantly lower (60 – 72% reduction) PAI-1, and relatively lower levels of TGF-β1 and Col1a1 than mice without Dox treatment (Figure 6g).
Figure 6. MG53 treatment of UUO mice blocks kidney fibrosis.
(a) Representative pairwise trichrome stains showed CLK (left) and UUO (right) kidney from UUO alone (UUO, left, without RAW cells xenotransplantation), −DOX (middle, UUO mice injected with virally transduced RAW cells, no DOX in drinking water), and + DOX (right, UUO mice injected with viral transduced RAW cells, with DOX in drinking water). Scale bars, 5 mm. (b) Quantification of Masson’s Trichrome fibrosis analysis from (a). Average of 2 independent experiments, N = 5–6 from each group. Data are the means ± SD. **P < 0.01. (One way ANOVA followed by Bonferroni’s test). (c) Immunofluorescence to show kidney expression of MG53 (anti-MG53, green) from xenotransplantation of virally-transduced RAW cells labeled with F4/80 (red fluorescence). Scale bars, 25 μm. (d) Western blot analysis of p-p65 and t-p65 from renal cortical lysates from sham and UUO mice, 7 days post-UUO, with or without RAW (Ad-tPA-MG53) xenotransplantation. Tubulin served as the loading control. (e) Relative p-p65/t-p65 ratios were analyzed. Values represent the means ± SD from 3 independent experiments. N= 9–10 mice per group. ns, not significant, **P < 0.01; ***P < 0.001 (One way ANOVA followed by Bonferroni’s test). (f) Immunohistochemistry of CLK and UUO kidneys stained with p-p65 (S536) antibody. Scale bars = 100 μm. (g) Quantitative RT-PCR showing the relative abundance of TGF-β1, PAI-1 and Col1a1 mRNA in the indicated groups. Values represent the means ± SD. ns, not significant; *P <0.05, ** P <0.01. *** P <0.001.
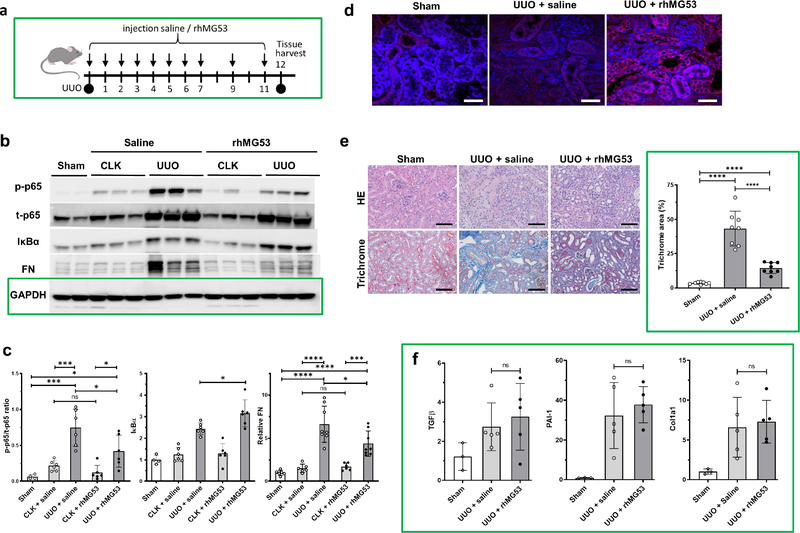
Systemic administration of rhMG53 mitigates NF-κB activation and fibrosis development in mice subjected to UUO
We tested whether rhMG53 displayed a similar anti-fibrotic effect as the engineered RAW cell therapy described above. Our prior pharmacokinetic data indicated the short, circulating half-life (about 90 minutes in rodents) of rhMG53.42 Mice tolerated chronic administration of 2–6 mg/kg rhMG5326, 37 well, so the administration strategy shown in Figure 7a was used. UUO mice were randomly given either vehicle (saline) or rhMG53 (2 mg/kg) daily for the first 7 days post-surgery, then every other day until they were euthanized on day 12. Sham and CLK kidneys exhibited roughly equivalent t-p65 and fibronectin expression levels, with the p-p65 levels slightly increased in CLK kidneys compared to sham kidneys. After UUO, renal t-p65 increased. Compared to saline-treated animals, the p-65/t-p65 ratio in UUO kidneys of rhMG53-treated animals was reduced by about 37%, while IκBα increased by about 27% (Fig. 7b, c). Additionally, fibronectin fell by about 33% (Fig. 7b, c). IHC confirmed rhMG53 was present in UUO kidneys after treatment (Figure 7d).Total fibrosis was reduced in rhMG53-treated UUO kidneys (Figure 7e). However, RNA expression of TGF-β1, PAI-1 and Col1a1 was similar in mice treated with either saline or rhMG53 (Figure 7f).
Figure 7. rhMG53 modulates p65 signaling to control renal fibrosis.
(a) rhMG53 administration scheme. (b) Kidney cortex lysates were prepared on day 12 post-surgery from sham or UUO mice that had received either saline or rhMG53 treatment. Lysates were immunoblotted for p-p65, t-p65, t-IκBα and fibronectin (FN). GAPDH was used as a loading control. (c) Quantitative analyses of the blots. All values represent means ± SD from 3 independent experiments. N= 6–10 mice per group. ns, not significant, *P < 0.05, ***P < 0.001, ****P < 0.0001. (One way ANOVA followed by Holm-Sidak’s test). (d) Immunofluorescence of anti-MG53 staining (red) on kidney samples from sham (left), UUO + saline (center) or UUO with rhMG53 treatment (right). DAPI (blue) staining. Scale bars, 25 μm. (e) Hematoxylin and eosin and trichrome images and analysis of the kidneys as described above. Scale bar =100 μm. Values represent the means ± SD from 3 independent experiments. N= 4 mice per group. ***P < 0.001 (One way ANOVA followed by Bonferroni’s test). (f) Quantitative RT-PCR showed the relative abundance of TGF-β1, PAI-1 and Col1a1 mRNA in different groups as indicated. Values represent the means ± SD. ns, not significant.
DISCUSSION
MG53 is a muscle-enriched TRIM protein initially identified as a prominent component of plasma membrane repair machinery.22, 26, 42, 43 However, the actions of MG53 are not confined to skeletal muscle, and MG53 can be released into the circulation as a myokine.32 We have previously shown MG53 can control inflammation during tissue damage in remote organs.27, 29, 33, 35, 36, 37 The present investigation extends the scope of MG53-mediated organ protection to the kidney. We conclude that MG53 can attenuate intra-renal inflammation and kidney fibrosis by blocking cytokine-induced activation of the pro-inflammatory and pro-fibrotic transcription factor NF-κB. This conclusion is based on the following observations: i) Genetic deletion of MG53 in normal mice resulted in a decline in kidney function as the mice aged that was accompanied by an increase in kidney fibrosis and infiltration of the kidney by leukocytes and macrophages. ii) In a murine UUO kidney injury model, the obstructed kidneys of MG53-deficient mice exhibited more fibrosis and inflammation than MG53-replete mice. iii) TNFα and interleukin-6 production by lipopolysaccharide-treated PTECs was attenuated in the presence of MG53. iv) Treatment with, or over-expression of MG53 in a PTEC cell-line diminished TNFα-induced NF-κB activation by blocking the nuclear translocation of the p65 component of NF-κB. MG53 was found to bind directly to p65, albeit weakly. v) Phosphorylation of p65, a necessary step in activation of NF-κB was decreased in the obstructed kidneys of UUO mice treated with recombinant MG53 or induced to over-express MG53 in macrophages. Furthermore, the inhibitor of NF-κB activation, IκBα, was increased in the obstructed kidneys of UUO mice injected with recombinant MG53.
Genetic deletion of MG53 in normal mice was permissive to the adverse effects of aging on the kidneys. This suggests that the presence of endogenous MG53 provides a baseline level of protection during aging. Similarly, loss of endogenous MG53 made acute kidney injury due to obstruction worse. The UUO kidney accumulated MG53, possibly reflecting remote myokine activity from skeletal muscle. It remains unclear how muscle MG53 may be recruited to the kidney during injury, however a damaged kidney often develops hypoxia and undergoes oxidative stress,44, 45 signals that could serve to recruit MG53. If so, this “muscle-kidney-immune cell” axis could be leveraged to attenuate kidney damage.46 Alternatively, kidney cells do express low levels of MG53 at baseline,26 and during acute or chronic injury MG53 production could be upregulated.
NF-κB is a prominent transcriptional driver of inflammation and fibrosis in kidney injury and aging.14, 17, 47, 48 We were prompted to investigate whether the effects of MG53 could be mediated via NF-κB becasue the presence of MG53 in the nuclear compartment of kidney homogenates suggested the potential for transcriptional interference. Nuclear localization of MG53 is not without precedent. In a transgenic model overexpressing myocardial MG53, nuclear MG53 regulated the expression of peroxisome proliferation-activated receptor alpha (PPARα) at the transcriptional level and affected cardiac lipid metabolism.49 Moreover, we recently demonstrated rhMG53 suppressed NF-κB activation to mitigate age-related heart failure through reducing pro-inflammatory cytokines, apoptosis and oxidative stress in aged cardiomyocytes.37
The molecular mechanism of how MG53 modulates p65 nuclear/cytoplasmic mobilization remains unclear. MG53 contains no nuclear localization signal (NLS), but it contains 3 putative N-terminal leucine-rich nuclear export signals (NES), a motif recognized by nuclear export protein exportin (CRM1).50 The direct interaction between MG53 and p65 was rather weak as the only or main disruptive effect of MG53 on NF-κB activation. Although MG53 appears to affect the dynamic nucleo-cytoplasmic shuttling of NF-κB critical components, the exact mechanisms remain to be determined.
The critical outcome of MG53-mediated renoprotection appears to be to decrease fibrotic remodeling through attenuation of NF-κB-mediated inflammation. Kidney fibrosis is a complex process involving pathologic deposition of various ECM proteins.51 MG53 seemed to have clear effects on the ECM protein fibronectin. Fibronectin increased in UUO kidneys deficient in MG53 and decreased in UUO mice treated with MG53. The expression of other ECM proteins in the presence and absence of MG53 is more difficult to understand. We previously demonstrated that rhMG53 negatively regulates TGF-β1/Smad signaling and suppresses the synthesis of ECM proteins in an in vitro study.31 In vivo, TGF-β1, PAI-1, and Col1a1 transcripts did not increase in UUO kidneys from mg53−/− deficient animals. These transcripts did decrease in UUO animals induced to over express MG53 through administration of virally-induced mouse macrophages, but when recombinant MG53 was given, no effect was seen. This may be simply a case of sufficient dosing, as the intra-renal MG53 levels achieved were far greater in animals given the macrophage vector as opposed to recombinant MG53. Additionally, protein levels were not measured, and post-transcripitonal events could modify the ECM in UUO. Further research is needed to elucidate the emerging role of MG53 in modulating specific ECM proteins.
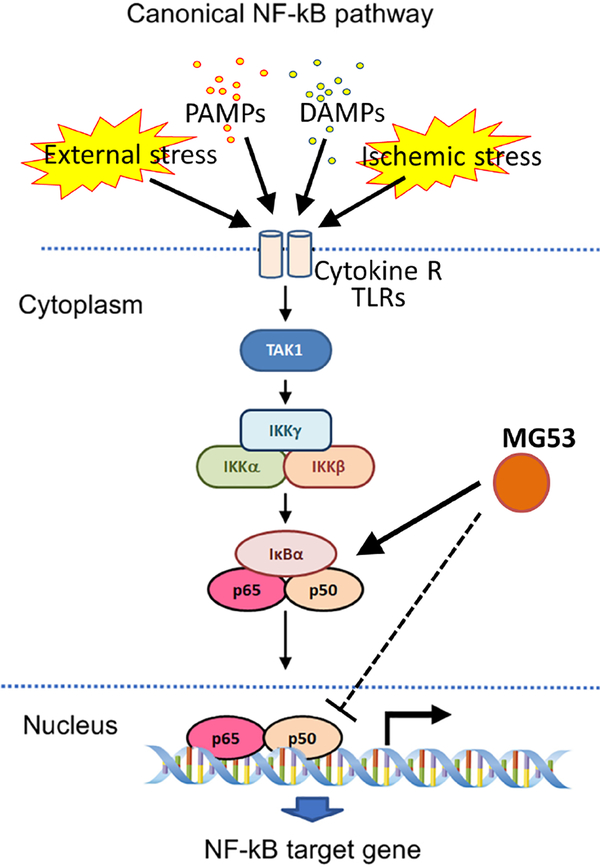
In conclusion, this study has demonstrated that a myokine may help regulate baseline inflammation in a remote organ like the kidney, and in the setting of kidney injury may help to attenuate and control inflammation. Figure 8 presents a working model of the role of MG53 in renoprotection. During DAMP (TNFα) and PAMP (LPS) stimulation and ischemic stress, the NF-κB signalosome is activated in the kidney. MG53 protects the kidney by inhibiting the phosphorylation and activation of NF-κB signaling, stabilizing IκBα, and reducing p65 nuclear mobilization, blocking activation of pro-inflammatory programs. Although endogenous MG53 may mediate these effects, we suggest more robust protection may be afforded using MG53 therapeutically, which is feasible based on the ability of exogenous MG53 to reduce injury in UUO. Further investigation is warranted to understand how this myokine may be clinically exploited.
Figure 8. Schematic illustrating the proposed function of MG53 in the regulation of the NF-κB signalosome.
In response to tissue stress (UUO), DAMP (TNFα) or PAMP (LPS) stimuli, the canonical NF-κB pathway is induced via activation of the IKK complex, which leads to degradation of IκBα and the nuclear translocation of NF-κB. MG53 is proposed to modulate and suppress NF-κB transcriptional activity by directly binding to p65 and preventing its translocation to the nucleus. Additionally, MG53 might stabilize IκBa in the cytosol to prevent NF-κB activation.
Supplementary Material
Table S1. Primer sequences.
Supplementary Figure S1. mg53−/− mice display enhanced ECM protein deposition. Age-matched (10-months old) WT and mg53−/− kidney tissues were stained with DAPI (blue, left panels), α-SMA (green, 2nd panels), fibronectin (red, 3rd panels), and merged (right panels). Scale bar =25 μm. Quantification of renal fibrosis area (n=3 per group). Data are presented as means ± SD. Student’s t test. ***P <0.001.
Supplementary Figure S2. Distribution of skeletal muscle MG53 between nucleus and cytoplasm. Skeletal muscle derived from WT or mg53−/− mice was fractionated and immunoblotted with a custom-made anti-MG53 antibody (3 μg lysates) and cell compartment markers (20 μg lysates). Na-K-ATPase was used as a cell membrane marker, Histone-H3 as a nuclear marker and tubulin as a cytosol marker.
Supplementary Figure S3. HKC-8 renal proximal tubule cells specifically uptake Alexa Fluor 647 labeled rhMG53. $$PARABREAKHERE$$ rhMG53 (Trim-edicine) and BSA (ThermoFisher Scientific) were labelled with Alexa Flour (AF) Antibody Labeling kits (Life Technologies) according to manufacturer protocol. HKC-8 cells were cultured on Delta TPG glass-bottomed dish (Bioptechs Inc.) in DMEM with 10% FBS in the presence of labelled proteins, BSA-AF647 (left) and rhMG53-AF647 (right), for 24 h. Z-stack images were taken from LSM780 confocal microscope (Zeiss). Images show cells at the bottom of the dishes. Scale bar, 25 μm.
Supplementary Figure S4. Engineering RAW cells to mediate inducible MG53 secretion. (a) RAW cells were infected with Ad-tPA-MG53 for 24 hours and treated without (- Dox) or with Dox (+ Dox, 1 μg/ml) for an additional 24 hours to induce tPA-MG53 expression. Confocal images showed the induction of MG53 expression (custom-made anti-MG53 antibody, Red, lower panels) from macrophage-like RAW cells (F4/80 staining, Green, top panels). Scale bars, 25 μm. (b and c) Western blot analysis of cell lysates (in amount of 0.6, 3 and 6 μg) for induced expression of tPA-MG53 and MG53 from infected RAW cells (b) and the corresponding culture media concentrates (in volumes of 2, 5 and 10 μl) (c). Various quantities of rhMG53 (in amount of 0.2, 1, 0.05, 0.025 ng) were loaded as standards. Mw: molecular weight markers.
Translational Statement.
Chronic inflammation leads to fibrotic remodeling that may also underlie the transition from AKI to CKD. Activation of the pro-inflammatory transcription factor NF-κB is involved in the pathogenesis of kidney inflammation. Here, we provide evidence that MG53, a previously identified cell membrane repair protein, directly interacts with the NF-κB and reduces its transcriptional activity. Concomitantly, exogenously administered MG53 can decrease fibrotic remodeling of the inflamed kidney. These findings point to a protective interplay between MG53 and NF-κB to attenuate the development of inflammation-mediated kidney fibrosis. Pharmacologic administration of MG53 might be a promising approach for the treatment of progressive kidney fibrosis.
ACKNOWLEDGEMENTS
This work was supported by NIH R01-DK106394 (J. Ma, B.H. Rovin and P. Lin) and NIH R01-AG062896 (P. Lin and B.H. Rovin). This project was also partly supported by an intramural Lockwood fund from The Ohio State University (P. Lin) and an award from the National Center for Advancing Translational Sciences (P. Lin, Award Number UL1TR002733). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
DISCLOSURES
J. Ma has an equity interest in TRIM-edicine, Inc., which develops rhMG53 for treatment of human diseases. Patents on the use of MG53 are held by Rutgers University and The Ohio State University. All other authors declare no competing interests.
SUPPLEMENTARY MATERIAL
Supplementary information is available on Kidney International’s website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation 2019; 73: A7–a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation 2020; 75: A6–a7. [DOI] [PubMed] [Google Scholar]
- 3.Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 Inhibition for the Prevention and Treatment of Diabetic Kidney Disease: A Review. American journal of kidney diseases : the official journal of the National Kidney Foundation 2018; 72: 267–277. [DOI] [PubMed] [Google Scholar]
- 4.Hocher B, Tsuprykov O. Diabetic nephropathy: Renoprotective effects of GLP1R agonists and SGLT2 inhibitors. Nature reviews Nephrology 2017; 13: 728–730. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine 2010; 16: 535–543, 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canaud G, Brooks CR, Kishi S, et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Science translational medicine 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature medicine 2015; 21: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nature medicine 2015; 21: 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys BD. Mechanisms of Renal Fibrosis. Annual review of physiology 2018; 80: 309–326. [DOI] [PubMed] [Google Scholar]
- 10.Liu BC, Tang TT, Lv LL, et al. Renal tubule injury: a driving force toward chronic kidney disease. Kidney international 2018; 93: 568–579. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Sun SC. NF-kappaB in inflammation and renal diseases. Cell & bioscience 2015; 5: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song N, Thaiss F, Guo L. NFκB and Kidney Injury. Frontiers in immunology 2019; 10: 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade-Oliveira V, Foresto-Neto O, Watanabe IKM, et al. Inflammation in Renal Diseases: New and Old Players. Frontiers in pharmacology 2019; 10: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid S, Scholey J. Recent Approaches to Targeting Canonical NFκB Signalling in the Early Inflammatory Response to Renal IRI. Journal of the American Society of Nephrology : JASN 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann A, Levchenko A, Scott ML, et al. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science (New York, NY) 2002; 298: 1241–1245. [DOI] [PubMed] [Google Scholar]
- 16.Marko L, Vigolo E, Hinze C, et al. Tubular Epithelial NF-kappaB Activity Regulates Ischemic AKI. Journal of the American Society of Nephrology : JASN 2016; 27: 2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, Lai CF, Chang-Panesso M, et al. Proximal Tubule Translational Profiling during Kidney Fibrosis Reveals Proinflammatory and Long Noncoding RNA Expression Patterns with Sexual Dimorphism. Journal of the American Society of Nephrology : JASN 2020; 31: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singbartl K, Formeck CL, Kellum JA. Kidney-Immune System Crosstalk in AKI. Seminars in nephrology 2019; 39: 96–106. [DOI] [PubMed] [Google Scholar]
- 19.Hato T, Dagher PC. How the Innate Immune System Senses Trouble and Causes Trouble. Clinical journal of the American Society of Nephrology : CJASN 2015; 10: 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nature reviews Nephrology 2019. [DOI] [PubMed] [Google Scholar]
- 21.Peng H, Wang Q, Lou T, et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nature communications 2017; 8: 1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai C, Masumiya H, Weisleder N, et al. MG53 nucleates assembly of cell membrane repair machinery. Nature cell biology 2009; 11: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai C, Masumiya H, Weisleder N, et al. MG53 regulates membrane budding and exocytosis in muscle cells. The Journal of biological chemistry 2009; 284: 3314–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatakeyama S TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends in biochemical sciences 2017; 42: 297–311. [DOI] [PubMed] [Google Scholar]
- 25.Cai C, Weisleder N, Ko JK, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. The Journal of biological chemistry 2009; 284: 15894–15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duann P, Li H, Lin P, et al. MG53-mediated cell membrane repair protects against acute kidney injury. Science translational medicine 2015; 7: 279ra236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Zhu H, Zheng Y, et al. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. Journal of molecular and cellular cardiology 2015; 80: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia Y, Chen K, Lin P, et al. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nature communications 2014; 5: 4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan F, Huang T, Wang X, et al. The TRIM protein Mitsugumin 53 enhances survival and therapeutic efficacy of stem cells in murine traumatic brain injury. Stem cell research & therapy 2019; 10: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Y, Zhang B, Zhu H, et al. MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget 2016; 7: 22474–22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Duann P, Lin PH, et al. Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. The Journal of biological chemistry 2015; 290: 24592–24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian Z, Wang Q, Zhou X, et al. Sustained elevation of MG53 in the bloodstream increases tissue regenerative capacity without compromising metabolic function. Nature communications 2019; 10: 4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan F, Zhou X, Li P, et al. MG53 attenuates lipopolysaccharide-induced neurotoxicity and neuroinflammation via inhibiting TLR4/NF-kappaB pathway in vitro and in vivo. Progress in neuro-psychopharmacology & biological psychiatry 2019; 95: 109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Wang G, Zhang C, et al. MG53, A Novel Regulator of KChIP2 and Ito,f, Plays a Critical Role in Electrophysiological Remodeling in Cardiac Hypertrophy. Circulation 2019; 139: 2142–2156. [DOI] [PubMed] [Google Scholar]
- 35.Sermersheim M, Kenney AD, Lin PH, et al. MG53 suppresses interferon-β and inflammation via regulation of ryanodine receptor-mediated intracellular calcium signaling. Nature communications 2020; 11: 3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenney AD, Li Z, Bian Z, et al. Recombinant MG53 Protein Protects Mice from Lethal Influenza Virus Infection. American journal of respiratory and critical care medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Li X, Ong H, et al. MG53 suppresses NFκB activation to mitigate age-related heart failure. JCI insight 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney international 2009; 75: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 39.Guiteras R, Sola A, Flaquer M, et al. Macrophage Overexpressing NGAL Ameliorated Kidney Fibrosis in the UUO Mice Model. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2017; 42: 1945–1960. [DOI] [PubMed] [Google Scholar]
- 40.Higgins CE, Tang J, Higgins SP, et al. The Genomic Response to TGF-β1 Dictates Failed Repair and Progression of Fibrotic Disease in the Obstructed Kidney. Frontiers in cell and developmental biology 2021; 9: 678524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong P, Canaan A, Wang B, et al. The ubiquitin-like protein FAT10 mediates NF-kappaB activation. Journal of the American Society of Nephrology : JASN 2010; 21: 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisleder N, Takizawa N, Lin P, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science translational medicine 2012; 4: 139ra185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElhanon KE, Young N, Hampton J, et al. Autoantibodies targeting TRIM72 compromise membrane repair and contribute to inflammatory myopathy. The Journal of clinical investigation 2020; 130: 4440–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratliff BB, Abdulmahdi W, Pawar R, et al. Oxidant Mechanisms in Renal Injury and Disease. Antioxidants & redox signaling 2016; 25: 119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Wei Q, Liu J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney international 2017; 92: 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato Y, Yanagita M. Immunology of the ageing kidney. Nature reviews Nephrology 2019; 15: 625–640. [DOI] [PubMed] [Google Scholar]
- 47.O’Brown ZK, Van Nostrand EL, Higgins JP, et al. The Inflammatory Transcription Factors NFκB, STAT1 and STAT3 Drive Age-Associated Transcriptional Changes in the Human Kidney. PLoS genetics 2015; 11: e1005734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson DE, Ihekwaba AE, Elliott M, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science (New York, NY) 2004; 306: 704–708. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Song R, Feng Y, et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor α. Circulation 2015; 131: 795–804. [DOI] [PubMed] [Google Scholar]
- 50.Lee Y, Pei J, Baumhardt JM, et al. Structural prerequisites for CRM1-dependent nuclear export signaling peptides: accessibility, adapting conformation, and the stability at the binding site. Scientific reports 2019; 9: 6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bülow RD, Boor P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2019; 67: 643–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences.
Supplementary Figure S1. mg53−/− mice display enhanced ECM protein deposition. Age-matched (10-months old) WT and mg53−/− kidney tissues were stained with DAPI (blue, left panels), α-SMA (green, 2nd panels), fibronectin (red, 3rd panels), and merged (right panels). Scale bar =25 μm. Quantification of renal fibrosis area (n=3 per group). Data are presented as means ± SD. Student’s t test. ***P <0.001.
Supplementary Figure S2. Distribution of skeletal muscle MG53 between nucleus and cytoplasm. Skeletal muscle derived from WT or mg53−/− mice was fractionated and immunoblotted with a custom-made anti-MG53 antibody (3 μg lysates) and cell compartment markers (20 μg lysates). Na-K-ATPase was used as a cell membrane marker, Histone-H3 as a nuclear marker and tubulin as a cytosol marker.
Supplementary Figure S3. HKC-8 renal proximal tubule cells specifically uptake Alexa Fluor 647 labeled rhMG53. $$PARABREAKHERE$$ rhMG53 (Trim-edicine) and BSA (ThermoFisher Scientific) were labelled with Alexa Flour (AF) Antibody Labeling kits (Life Technologies) according to manufacturer protocol. HKC-8 cells were cultured on Delta TPG glass-bottomed dish (Bioptechs Inc.) in DMEM with 10% FBS in the presence of labelled proteins, BSA-AF647 (left) and rhMG53-AF647 (right), for 24 h. Z-stack images were taken from LSM780 confocal microscope (Zeiss). Images show cells at the bottom of the dishes. Scale bar, 25 μm.
Supplementary Figure S4. Engineering RAW cells to mediate inducible MG53 secretion. (a) RAW cells were infected with Ad-tPA-MG53 for 24 hours and treated without (- Dox) or with Dox (+ Dox, 1 μg/ml) for an additional 24 hours to induce tPA-MG53 expression. Confocal images showed the induction of MG53 expression (custom-made anti-MG53 antibody, Red, lower panels) from macrophage-like RAW cells (F4/80 staining, Green, top panels). Scale bars, 25 μm. (b and c) Western blot analysis of cell lysates (in amount of 0.6, 3 and 6 μg) for induced expression of tPA-MG53 and MG53 from infected RAW cells (b) and the corresponding culture media concentrates (in volumes of 2, 5 and 10 μl) (c). Various quantities of rhMG53 (in amount of 0.2, 1, 0.05, 0.025 ng) were loaded as standards. Mw: molecular weight markers.