Abstract
Background:
Acute kidney injury is a common complication following the Norwood operation. Most neonatal studies report acute kidney injury peaking within the first 48 hours after cardiac surgery. The aim of this study was to evaluate if persistent acute kidney injury (>48 postoperative hours) after the Norwood operation was associated with clinically relevant outcomes.
Methods:
Two-centre retrospective study among neonates undergoing the Norwood operation. Acute kidney injury was initially identified as developing within the first 48 hours after cardiac surgery and stratified into transient (≤48 hours) and persistent (>48 hours) using the neonatal modification of the Kidney Disease: Improving Global Outcomes serum creatinine criteria. Severe was defined as stage ≥2. Primary and secondary outcomes were mortality and duration of ventilation and hospital length of stay.
Results:
One hundred sixty-eight patients were included. Transient and persistent acute kidney injuries occurred in 24 and 17%, respectively. Cardiopulmonary bypass and aortic cross clamp duration, and incidence of cardiac arrest were greater among those with persistent kidney injury. Mortality was four times higher (41 versus 12%, p < 0.001) and mechanical ventilation duration 50 hours longer in persistent acute kidney injury patients (158 versus 107 hours; p < 0.001). In multivariable analysis, persistent acute kidney injury was not associated with mortality, duration of ventilation or length of stay. Severe persistent acute kidney injury was associated with a 59% increase in expected ventilation duration (aIRR:1.59, 95% CI:1.16, 2.18; p = 0.004).
Conclusions:
Future large studies are needed to determine if risk factors and outcomes change by delineating acute kidney injury into discrete timing phenotypes.
Keywords: Acute kidney injury, Norwood operation, outcomes, neonatal, transient, persistent
Acute kidney injury is a well-known complication following paediatric cardiac surgery with rates ranging from 10 to 60% depending on the definition applied.1–7 Irrespective of the definition, acute kidney injury has been consistently associated with increased morbidity and mortality across heterogeneous cohorts of children undergoing congenital cardiac surgery.2,5,6,8,9
Studies among critically ill children and neonates and have not explored the trajectory and variations in acute kidney injury time course but have rather focused on peak injury,1,2,5,6 with most episodes peaking within the first 24–48 postoperative hours.3 It is possible that the phenotype of transient acute kidney injury that develops early in the postoperative course and resolves quickly may be different from acute kidney injury that persists. A recent consensus statement suggests that the meaningful time period for which acute kidney injury diagnosis may be most associated with outcomes is after 48 hours.10
The Norwood procedure is one of the most complex neonatal cardiac surgical procedures performed and is associated with high rates of acute kidney injury.6,11,12 The single ventricle reconstruction trial reported an association between lower surgeon Norwood volume and acute kidney injury13 but the timing of acute kidney injury was not described.
The purpose of this study was to determine if unique acute kidney injury temporal phenotypes were associated with unique patient outcomes following the Norwood procedure.
Materials and methods
We performed a 2-center retrospective chart review of all neonates with single right ventricle congenital heart disease undergoing the Norwood operation (stage 1 palliation) from January 2009 to December 2015 (Center 1 – Children’s Hospital Colorado) and December 2008 to February 2017 (Center 2 – Children’s of Alabama). The varying time frames between centres were related to years of inclusion in an existing surgical/research database at both centres, and a change in surgeon at centre 1. Institutional Review Board approval was obtained with a waiver of informed consent.
Cases were identified from the cardiac surgery database at both institutions. Neonates with a diagnosis of hypoplastic left heart syndrome (HLHS) and other single right ventricle variants such as double outlet right ventricle with mitral atresia and right dominant atrioventricular septal defect were included. Patients who required extracorporeal membrane oxygenation (ECMO) or dialysis preoperatively, and those with known kidney abnormalities were excluded from the analysis. The reporting of this study conforms to The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.14 Demographic and clinical data were abstracted from the electronic health record at each site.
The Norwood procedure was performed using a standard technique. Provision of pulmonary blood flow included placement of a right ventricle to pulmonary artery conduit using a Gore-Tex tube graft or a modified Blalock Thomas-Taussig Shunt. Determination of shunt type was at the discretion of the performing surgeon. Continuous low-flow cerebral perfusion was used for all cases during arch reconstruction. Pre-operative methylprednisolone, but not intraoperative methylprednisolone, was administered in all cases. All patients received zero-balance ultrafiltration during cardiopulmonary bypass and/or single-pass ultrafiltration after cardiopulmonary bypass. Delayed sternal closure was utilized per surgeon discretion and the duration recorded. Peritoneal drains were placed at Site 1 based on surgeon discretion and were only used for passive drainage. At site 2, starting in January, 2011, all patients received prophylactic peritoneal dialysis. Dialysis was considered prophylactic if started by 6 a.m. on the first post-operative morning.15 Passive drainage or treatment dialysis was utilized as needed.
Definitions
The neonatal modification of the Kidney Disease: Improving Global Outcomes serum creatinine criteria was used to define acute kidney injury.16–18 Transient acute kidney injury was defined as any stage on postoperative days 0, 1 and 2 with return to baseline on postoperative day 3. Persistent acute kidney injury was defined as any stage on postoperative days 0–2 with persistence on post-operative day 3. Severe acute kidney injury was defined as either stage 2 or 3 (at least a doubling of serum creatinine from baseline). Patients who received a peritoneal dialysis catheter for passive drainage or prophylactic dialysis were classified as having acute kidney injury based on the definition described above, and not automatically given a stage 3 diagnosis. Urine output criteria were not used for acute kidney injury diagnosis due to the ubiquitous use of either prophylactic peritoneal dialysis or passive peritoneal drainage at both centres, which is known to reduce urine volume.19 Daily percent fluid overload was calculated as previously described20 and is reported as cumulative daily percent fluid overload.
The primary outcome was in-hospital mortality. Secondary outcomes included duration of mechanical ventilation and hospital length of stay among survivors. Duration of mechanical ventilation was defined as the time in hours from post-surgical admission until the first successful extubation (success was defined as being extubated for ≥ 48hours). Days in the intensive care unit (ICU) was defined as time from post-surgical admission until discharge from the ICU. Hospital length of stay was defined as the time from post-surgical admission until discharge.
Statistical analysis
Descriptive analyses [means, standard deviations, medians, interquartile ranges, frequency distributions (%)] were used to describe patient demographics, clinical characteristics and outcomes. Chi-square, Fisher exact, Mann–Whitney U-test and Student’s t-test were used to compare categorical and continuous variables as appropriate. Multivariate generalized linear models with log-link and negative binomial distributions were used to assess the variables associated with the outcomes of mechanical ventilation in hours and hospital length of stay. Estimated relative risk-type estimates with 95% confidence intervals were determined for the outcomes of mechanical ventilation duration and hospital length of stay. Multivariate logistic regression models were used to assess the factors associated with mortality. Wald tests assessed group difference. Variable selection used a stepwise method informed by clinical insight and guided by Akaike Information Criterion (AIC). Covariates identified as clinically correlated with acute kidney injury were considered in the final models (p-value < 0.15). When covariates were highly correlated or collinear, investigators identified the covariate for inclusion in the model. The final multivariate models included cardiac arrest, aortic cross clamp, centre and cardiopulmonary bypass duration. Incidence Rate Ratios (IRRs) and odds ratio (OR) with 95% CI for the most parsimonious model fit are reported. All hypothesis tests were two-tailed. A P-value of < 0.05 indicated statistical significance. All analyses were performed in SAS (version 9.4, Cary, NC).
Results
One hundred sixty-eight patients were included. There was a slight preponderance of males (66%). Provision of pulmonary blood flow was via a right ventricle to pulmonary artery conduit in 82% of cases. In-hospital mortality was 17% (n = 28). Median duration of ventilation among survivors was 118 (IQR:84, 166) hours. Median ICU and hospital length of stay among survivors was 17 (IQR:12, 26) and 28 (IQR: 18, 40) days, respectively. Data are summarized in Table 1. Center differences were found in patient demographics, clinical characteristics and outcomes, including the incidence of transient and persistent acute kidney injury (Supplemental Table 1).
Table 1.
Demographics and peri- and post-operative characteristics and outcomes of all patients included the study cohort
| Variable | (n =168) |
|---|---|
| Demographics and Pre-operative Characteristics | |
| Age at surgery, days | 5.6 ± 2.5 |
| Gender: Male, n (%) | 111 (66) |
| Gestational age, weeks | 38.4 ± 1.5 |
| Weight, kilograms | 3.2 ± 0.5 |
| Genetic abnormalities: Yes, n (%) | 3 (2) |
| Pre-operative MV: Yes, n (%) | 32 (19) |
| Operative Characteristics | |
| CPB duration, minutes | 174 ± 52 |
| ACC, minutes | 72 ± 21 |
| Circulatory arrest, minutes | 11 ± 17 |
| Shunt type, n (%) | |
| Sano | 138 (82) |
| mBTTS | 30 (18) |
| ECMO in operating room, n (%) | 4 (2) |
| Open Sternum: Yes, n (%) | 114 (68) |
| Post-operative characteristics | |
| Post-operative ECMO, n (%) | 20 (12) |
| Post-operative cardiac arrest, n (%) | 28 (17) |
| VIS | |
| Admit | 15 ± 6 |
| POD-1 | 21 ± 12 |
| POD-2 | 17 ± 9 |
| POD-3 | 13 ± 7 |
| Percentage of Cumulative daily fluid overload, (L/kg)*100% | |
| 0 | 2.6 ± 6.6 |
| 1 | 2.0 ± 9.3 |
| 2 | −2.2 ± 10.7 |
| 3 | −6.2 ± 11.6 |
| 4 | −7.8 ± 13.3 |
| Peritoneal drain (yes), n (%) | 118 (70) |
| Haemodialysis, n (%) | 7 (4) |
| AKI | |
| None | 99 (59) |
| Transient | 40 (24) |
| Persistent | 29 (17) |
| Outcomes | |
| Reintubated within 48 hours, n (%) | 27 (16) |
| Duration of MV, hours | 118 (84,166) |
| ICU length of stay, days | 17 (12, 26) |
| Hospital length of stay, days | 28 (18, 40) |
| Mortality, n (%) | 28 (17) |
Continuous data presented as mean ± standard deviation or median (IQR). ACC = aortic cross clamp; AKI = acute kidney injury; CPB = cardiopulmonary bypass; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; Mbtts = modified Blalock-Thomas-Taussig shunt; MV = mechanical ventilation; MV = mechanical ventilation; POD = post-operative day; UOP = urine output; VIS = vasoactive inotrope score.
Acute kidney injury in the first 48 hours post-operative occurred in 69 (41%) of which transient acute kidney injury occurred in 23.8% (n = 40) and persistent acute kidney injury occurred in 17.3% (n = 29). Comparisons across all three groups (none, transient and persistent acute kidney injuries) are summarized in Supplemental Table 2. Comparisons of patients with no/transient acute kidney injury versus persistent acute kidney injury are summarized in Table 2. Severe persistent acute kidney injury (stage 2/3) occurred in 62% (n = 18). Cardiopulmonary bypass duration and aortic cross clamp time were significantly longer among those with persistent acute kidney injury compared with those with no/transient acute kidney injury (both p = 0.02). A greater proportion of patients with persistent acute kidney injury experienced cardiac arrest (41.4% versus 11.5%; p < 0.001) and received post-operative extracorporeal membrane oxygenation (27.6% versus 8.6%; p = 0.01). Peritoneal dialysis catheter use was not different between those with no/transient and persistent acute kidney injury (p = 0.27). Cumulative daily percent fluid overload was significantly higher on postoperative days 0 and 1 in patients with persistent acute kidney injury as compared with those with no/transient acute kidney injury (p = 0.04 and p = 0.005, respectively).
Table 2.
Demographics and peri- and post-operative characteristics and outcomes among patients with none/transient and persistent acute kidney injuries
| Variable | None/Transient) AKI (n = 139) | Any (Persistent) AKI (n = 29) | P Value |
|---|---|---|---|
| Demographics and pre-operative characteristics | |||
| Age at surgery, days | 5 ± 2 | 6 ± 2 | 0.22 |
| Gender: Male, n (%) | 98 (71) | 13 (45) | 0.02 |
| Gestational age, weeks | 39 ± 2 | 38 ± 2 | 0.08 |
| Weight, kilograms | 3.2 ± 0.5 | 3.1 ± 0.5 | 0.19 |
| Pre-operative MV: Yes, n (%) | 27 (19) | 5 (17) | 0.99 |
| Operative Characteristics | |||
| CPB, minutes | 170 ± 50 | 194 ± 59 | 0.02 |
| ACC, minutes | 70 ± 21 | 80 ± 20 | 0.02 |
| Circulatory arrest, minutes | 10 ± 16 | 16 ± 21 | 0.09 |
| Shunt type, n (%) | 0.86 | ||
| Sano | 115 (83) | 23 (79) | |
| mBTTS | 24 (17) | 6 (21) | |
| ECMO in operating room, n (%) | 2 (1) | 2 (7) | 0.28 |
| Open Sternum: Yes, n (%) | 94 (67.6) | 20 (69.0) | 0.99 |
| Post-operative Characteristics | |||
| Cardiac arrest | 16 (12) | 12 (41) | 0.0004 |
| VIS | |||
| Admit | 15 ± 6 | 15 ± 6 | 0.69 |
| POD-1 | 20 ± 8 | 30 ± 22 | <0.001 |
| POD-2 | 16 ± 9 | 21 ± 10 | 0.006 |
| POD-3 | 12 ± 7 | 16 ± 7 | 0.01 |
| Post-operative ECMO, n (%) | 12 (9) | 8 (28) | 0.01 |
| Post-operative cardiac arrest, n (%) | 16 (12) | 12 (41) | <0.001 |
| Peritoneal drain, n (%) | 95 (68) | 23 (79) | 0.27 |
| Cumulative percent fluid overload (daily) | |||
| 0 | 2.11 ± 6.1 | 4.9 ± 8.3 | 0.04 |
| 1 | 1.1 ± 8.8 | 6.3 ± 10.4 | 0.005 |
| 2 | −2.9 ± 9.8 | 1.2 ± 13.7 | 0.06 |
| 3 | −6.4 ± 10.8 | −5.1 ± 14.9 | 0.57 |
| 4 | −7.1 ± 12.7 | −11.2 ± 15.8 | 0.13 |
| Outcomes | |||
| Mortality, n (%) | 16 (12) | 12 (41) | <0.001 |
| Duration of MV, hours, median [IQR] | 107 (72, 150) | 158 (142, 308) | <0.001 |
| ICU length of stay, days (n = 148) | 18 (12, 28) | 16 (13, 22) | 0.65 |
| Hospital length of stay, days | 29 (20, 40) | 22 (14, 41) | 0.17 |
Continuous data presented as mean ± standard deviation or median (IQR). ACC = aortic cross clamp; AKI = acute kidney injury; CPB = cardiopulmonary bypass; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; mBTTS = modified Blalock-Thomas-Taussig shunt; MV = mechanical ventilation; MV = mechanical ventilation; POD = post-operative day; UOP = urine output; VIS = vasoactive inotrope score.
Mortality among those with persistent acute kidney injury (41.4%, n = 12) was significantly higher compared with those with no/transient acute kidney injury (11.5%, n = 16) (p < 0.001). Duration of ventilation in survivors was also significantly longer among those with persistent acute kidney injury (158, IQR: 142, 308 hours) compared to those with no/transient acute kidney injury (107, IQR: 72, 150 hours)(p < 0.001). There was no difference in ICU and hospital length of stay between groups.
Multivariable logistic regression demonstrated no association of persistent acute kidney injury with mortality (reference: none/transient) (aOR: 2.1, 95% CI: 0.6–7.1, p = 0.26). The adjusted odds of mortality increased 5.2-fold in those with cardiac arrest (aOR: 5.2, 95% CI: 2.8–9.5; p < 0.001) (Table 3). Similarly, for every 30-minute increase in cardiopulmonary bypass duration, the adjusted odds of mortality increased 50% (aOR: 1.5, 95% CI: 1.1–2.1; p = 0.02). When only considering those with severe persistent acute kidney injury, there was no association with mortality (Table 4).
Table 3.
Association of persistent acute kidney injury with the outcomes of mortality, ventilation duration, hospital length of stay and ICU length of stay
| Mortality | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Persistent AKI (ref: transient/no AKI) | 2.2 (0.60, 7.20) | 0.21 |
| CBP (every 30 min increase) | 1.40 (1.10, 2.00) | 0.02 |
| Cardiac Arrest | 4.80 (2.70, 8.54) | <0.0001 |
| Center (Ref: Center-2) | 1.03 (0.59, 1.79) | 0.92 |
| Ventilation Duration | Incidence Rate Ratio (95% CI) | P-value |
| Persistent AKI (ref: transient/no AKI) | 1.25 (0.97, 1.62) | 0.09 |
| CBP (every 30 min increase) | 1.03 (0.97, 1.09) | 0.40 |
| Cardiac Arrest | 1.39 (1.17, 1.64) | 0.0001 |
| Center (Ref: Center-2) | 1.13 (1.03, 1.24) | 0.007 |
| ICU Length of Stay | Incidence Rate Ratio (95% CI) | P-value |
| Persistent AKI (ref: transient/no AKI) | 0.95 (0.73, 1.24) | 0.71 |
| CBP (every 30 min increase) | 0.99 (0.93 1.06) | 0.82 |
| Cardiac Arrest | 1.27 (1.08 1.50) | 0.004 |
| Center (Ref: Center-2) | 1.08 (0.99 1.19) | 0.10 |
| Hospital Length of Stay | Incidence Rate Ratio (95% CI) | P-value |
| Persistent AKI (ref: transient/no AKI) | 1.24 (0.97, 1.58) | 0.08 |
| CBP (every 30 min increase) | 1.05 (0.99, 1.12) | 0.18 |
| Cardiac Arrest | 1.18 (1.02, 1.38) | 0.03 |
| Center (Ref: Center-2) | 0.95 (0.87, 1.03) | 0.23 |
Abbreviations: AKI = acute kidney injury; CI = confidence interval; CPB = cardiopulmonary bypass.
Table 4.
Association of severe persistent acute kidney injury with the outcomes of mortality and hospital length of stay.
| Mortality | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Severe Persistent AKI (ref: transient/no AKI) | 3.00 (0.74, 12.17) | 0.12 |
| CBP (every 30 min increase) | 1.47 (1.05, 2.05) | 0.02 |
| Cardiac Arrest | 5.03 (2.85, 8.90) | <0.0001 |
| Center (Ref: Center-2) | 0.96 (0.55, 1.69) | 0.89 |
| ICU Length of Stay | ||
| Persistent AKI (ref: transient/no AKI) | 1.05 (0.75, 1.47) | 0.77 |
| CBP (every 30 min increase) | 0.99 (0.93 1.06) | 0.84 |
| Cardiac Arrest | 1.27 (1.08 1.50) | 0.005 |
| Center (Ref: Center-2) | 1.08 (0.99 1.19) | 0.09 |
| Hospital Length of Stay | Incidence Rate Ratio (95% CI) | P-value |
| Severe Persistent AKI (ref: transient/no AKI) | 1.11 (0.81, 1.50) | 0.52 |
| CBP (every 30 min increase) | 1.05 (0.99, 1.12) | 0.10 |
| Arrest | 1.20 (1.03, 1.39) | 0.02 |
| Center (Ref: Center-2) | 0.95 (0.87, 1.03) | 0.23 |
Abbreviations: AKI = acute kidney injury; CI = confidence interval, CPB = cardiopulmonary bypass.
There was no association between persistent acute kidney injury and an expected increase in ventilation duration after adjusting for covariates (aIRR: 1.3, 95% CI: 1.0–1.6, p = 0.08). Both cardiac arrest (IRR: 1.4 95% CI: 1.2–1.6; p < 0.001) and open sternum (aIRR: 1.3, 95% CI: 1.0–1.6; p = 0.04) were associated with increases in expected ventilation duration after adjusting for confounding variables (Table 3). When only severe persistent acute kidney injury was considered, there was a 60% increase in the expected duration of ventilation after adjusting for confounders (aIRR: 1.6, 95% CI: 1.2, 2.2; p = 0.004). Both cardiac arrest and open sternum were also associated with an expected increase in the duration of ventilation after adjusting for confounding factors (Fig 1).
Figure 1.
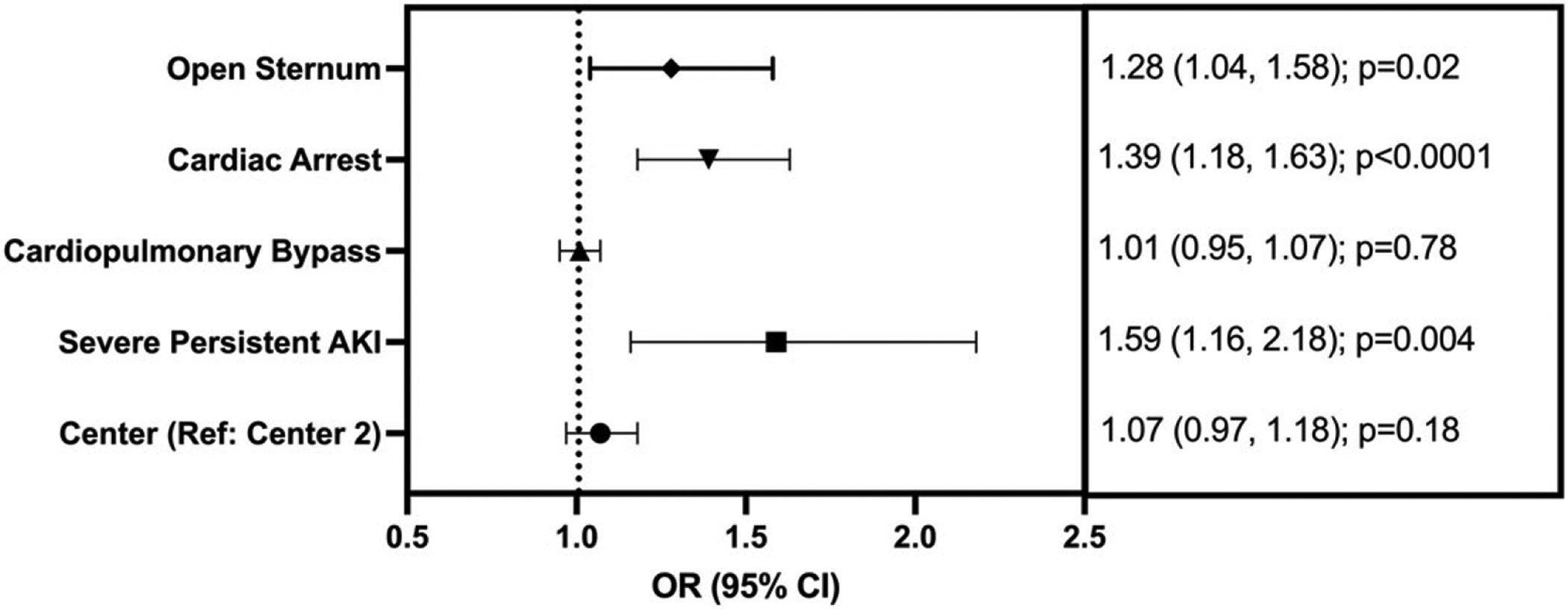
Forest plot demonstrating associations of clinical factors and expected increase (incidence rate ratio with 95% confidence intervals) with mechanical ventilation. Severe persistent acute kidney injury was defined as >48 hours and stage 2 or 3. Cardiopulmonary bypass is notated for every 30-minute increase.
There was no association of persistent acute kidney injury and severe persistent acute kidney injury with an adjusted expected increase in ICU length of stay (Tables 3 and 4) Cardiac arrest and open sternum were both associated with an expected increase in the expected ICU length of stay. Persistent acute kidney injury was not associated with an expected increase in hospital length of stay after adjusting for confounding variables (aIRR: 1.2, 95% CI: 1.0–1.6, p = 0.09). Cardiac arrest was associated with an expected increase in hospital length of stay after adjusting for confounders (aIRR: 1.2, 95% CI: 1.0–1.4, p = 0.03) (Table 3). Assessment of severe persistent acute kidney injury as the predictor, there was no association with an expected increase in the ICU or hospital length of stay (Table 4).
Discussion
This study evaluates the impact of duration of acute kidney injury in neonates who have undergone the Norwood operation. The incidence of acute kidney injury in this cohort was just over 40%, but the majority of cases were transient with no obvious impact on postoperative outcomes. Only 17% of patients had persistent acute kidney injury; almost two thirds of which had severe (stage 2/3) disease. Persistent acute kidney injury was associated with more early fluid overload and measures of haemodynamic instability (higher vasoactive inotrope score, cardiac arrest and extracorporeal membrane oxygenation) when compared with none/transient acute kidney injury, but it was not associated with clinically relevant outcomes (mortality, ventilation, length of stay). However, severe persistent acute kidney injury was independently associated with an increase in the expected duration of ventilation. Our data suggest that there are different phenotypes of acute kidney injury with respect to duration in this cohort, with a differential impact on outcomes. Moreover, while we did not identify an association between severe persistent acute kidney injury with mortality and hospital length of stay, the directionality of the odds and incidence rate ratios suggest that with increased power, we would identify important associations.
The 16th Acute Dialysis Quality Initiative (ADQI) recommends inclusion of the temporal characteristics of acute kidney injury, and suggests acute kidney injury diagnosis after 48 hours may be most associated with outcomes.10 However, most studies define early acute kidney injury as that present anytime, from immediately postoperative through the first post-operative week.2,5–7 A recent systematic review and meta-analysis demonstrated increased risk of death, cardiovascular events and chronic kidney disease with increased duration (persistence) of acute kidney injury.21 We defined both transient and persistent acute kidney injury phenotypes similar to this meta-analysis, with the hypothesis that a transient rise of serum creatinine in the immediate postoperative period that normalized by postoperative day 3 (~48 hours) would be of little clinical significance and may not reflect true renal injury. The limitations of serum creatinine for detection of acute kidney injury, especially in paediatric cardiac surgery are well described,22,23 including the possibility of delayed decrement due to altered single ventricle physiology, even in the absence of impaired tissue oxygen delivery. Indeed, we saw transient and persistent acute kidney injury had differential impact on outcomes as persistent acute kidney injury was more frequent in patients with increased cardiac surgery support times and was associated with worse outcomes with respect to fluid overload and haemodynamic instability. Furthermore, only severe persistent acute kidney injury was associated with prolonged ventilation, which has been reported in other studies.1,2,5
We did not find association of persistent acute kidney with ventilation duration, LOS, or mortality. Our study is different from other neonatal cardiac surgery studies that report acute kidney injury-related outcome differences with the major difference being the inclusion of heterogeneous cohorts with respect to risk and complexity.2,4 Our study represents a homogeneous cohort of postoperative neonates that is recognized as the highest risk surgical cohort with the most frequent complications such as cardiac arrest, extracorporeal membrane oxygenation utilization, infection, etc., which are often collinear with acute kidney injury, and have more significant impact on morbidity and mortality.24 Patients that had cardiac arrest represent nearly 50% of the persistent acute kidney injury cases in this study. Other non-patient condition factors, including proximity of the patient to a major paediatric centre, and time allotted to work singularly on oral feeds may impact hospital length of stay for postoperative Norwood patients thereby negating the utility of length of stay metric in this population. Our findings are not intended to marginalize the impact of acute kidney injury on outcomes after the Norwood operation. Rather we point out unique challenges to determination of outcomes impact in this cohort and highlight that not all acute kidney injury is created equally, especially as it relates to the different phenotypes, duration and timing of diagnosis.
Given the limitations of creatinine, we propose that utilization of a combination biomarker-based approach may be able to delineate unique patient classifications that ultimately predict distinct persistent acute kidney injury severity in this cohort. Basu and colleagues25 combined a functional damage biomarker with a tubular damage biomarker to form a composite biomarker for prediction of discrete acute kidney injury phenotypes in children undergoing cardiac surgery. They found that the likelihood of persistent acute kidney injury was 15 times greater in those with both functional and tubular biomarker positivities. This approach has also been reported among critically ill adults, where the composite improved prediction of severe acute kidney injury, need for renal replacement therapy or death.26
The strength of this study is its 2-center approach encompassing a very homogeneous cohort of patients. This strength is also a limitation, in that there are inherent practice differences across centres that may impact outcomes which were identified in the multivariable analysis. Other limitations include the retrospective design through which only associations rather than causation can be established. The number of patients with persistent acute kidney injury was relatively small leading to the potential for insufficient power to identify differences between no/transient and persistent acute kidney injuries. We only assessed for acute kidney injury most proximate to surgery and did not account for additional episodes from reoperations and reinterventions after the third postoperative day which could have impacted the associations with outcomes. Use of diuretics, including the timing of initiation was not captured. We suspect there are likely centre differences regarding diuretic use, peritoneal drains were more ubiquitously used at Center 2. Finally, current neonatal acute kidney injury definitions may be limited in neonatal cardiac surgery resulting in misclassification.
In conclusion, phenotypic classification of transient and persistent acute kidney injuries may further delineate associations with outcomes. Future large multi-centre studies that evaluate acute kidney injury phenotypes among homogeneous cohorts of children following cardiac surgery, coupled with clinically available biomarkers should be explored.
Supplementary Material
Financial support.
JTB 0.2 FTE was supported by the Department of Pediatrics and Research Institute at the University of Colorado/Children’s Hospital Colorado for KMG and DES.
DES was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) career development award program (K08DK109226-01A1).
Footnotes
Conflicts of interest. Dr. Borasino’s institution received funding from Octopharma (post marketing safety study for kids) and Asklepion (RCT of citrulline for ALI after CPB), and he received funding from Banner Health (consulting). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on research in children and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Colorado Multiple Institutional Review Board and the University of Alabama Institutional Review Board.
Supplementary material. To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121002560
References
- 1.Blinder JJ, Asaro LA, Wypij D, et al. Acute kidney injury after pediatric cardiac surgery: a secondary analysis of the safe pediatric euglycemia after cardiac surgery trial. Pediatr Crit Care Med 2017; 18: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg 2012; 143: 368–374. [DOI] [PubMed] [Google Scholar]
- 3.Gist KM, Blinder JJ, Bailly D, et al. Neonatal and paediatric heart and renal outcomes network: design of a multi-centre retrospective cohort study. Cardiol Young 2019; 29: 511–518. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham TW, Tan Y, Krawczeski CD, et al. Incidence and impact of acute kidney injury in patients with hypoplastic left heart syndrome following the hybrid stage 1 palliation. Cardiol Young 2021; 31: 414–420. doi: 10.1017/S1047951120004199 [DOI] [PubMed] [Google Scholar]
- 5.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med 2011; 39: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SooHoo MM, Patel SS, Jaggers J, Faubel S, Gist KM. Acute kidney injury defined by fluid corrected creatinine in neonates after the Norwood procedure. World J Pediatr Congenit Heart Surg. 2018; 9: 513–521. [DOI] [PubMed] [Google Scholar]
- 7.Morgan CJ, Zappitelli M, Robertson CM, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr 2013; 162: 120–127 e121. [DOI] [PubMed] [Google Scholar]
- 8.Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 2017; 1: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of acute kidney injury in critically Ill children and young adults. N Engl J Med 2017; 376: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol 2017; 13: 241–257. [DOI] [PubMed] [Google Scholar]
- 11.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 2010; 362: 1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia RU, Natarajan G, Walters HL, Delius RE, Aggarwal S. Acute kidney injury following first-stage palliation in hypoplastic left heart syndrome: hybrid versus Norwood palliation. Cardiol Young 2018; 28: 261–268. [DOI] [PubMed] [Google Scholar]
- 13.Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the pediatric heart network single ventricle reconstruction trial. J Thorac Cardiovasc Surg 2012; 144: 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 15.Sasser WC, Dabal RJ, Askenazi DJ, et al. Prophylactic peritoneal dialysis following cardiopulmonary bypass in children is associated with decreased inflammation and improved clinical outcomes. Congenit Heart Dis 2014; 9: 106–115. [DOI] [PubMed] [Google Scholar]
- 16.KDIGO AKI. Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138. [Google Scholar]
- 17.Zappitelli M, Ambalavanan N, Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res 2017; 82: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney injury. Pediatrics 2015; 136: e463–e473. [DOI] [PubMed] [Google Scholar]
- 19.Gist KM, Henry BM, Borasino S, et al. Prophylactic peritoneal dialysis after the arterial switch operation: a retrospective cohort study. Ann Thorac Surg 2021; 111: 655–661. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001; 107: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 21.Mehta S, Chauhan K, Patel A, et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol 2018; 19: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 2011; 58: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bojan M, Lopez-Lopez V, Pouard P, Falissard B, Journois D. Limitations of early serum creatinine variations for the assessment of kidney injury in neonates and infants with cardiac surgery. PLoS One 2013; 8: e79308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabbutt S, Schuette J, Zhang W, et al. A novel model demonstrates variation in risk-adjusted mortality across pediatric cardiac ICUs after surgery. Pediatr Crit Care Med 2019; 20: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014; 64: 2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon BA, Galligan M, Redahan L, et al. Biomarker predictors of adverse acute kidney injury outcomes in critically ill patients: the Dublin acute biomarker group evaluation study. Am J Nephrol 2019; 50: 19–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


