Abstract
Background:
In Helicobacter pylori-driven gastric cancer, mucosal colonization induces chronic inflammation that may variably progress to cancer. Prospective studies of circulating inflammation-related proteins have suggested weak associations with gastric cancer risk. To assess potential utility as a screening tool in clinical settings, we examined circulating levels of a wide range of key inflammation molecules for associations with early-stage gastric cancer.
Methods:
We used pre-treatment EDTA plasma from 239 individuals with early-stage noncardia gastric cancer (203 stage I and 36 stage II) and 256 age-frequency-matched H. pylori-seropositive cancer-free controls within the Hospital-based Epidemiologic Research Program at Aichi Cancer Center. Levels of 92 biomarkers were measured by proximity extension assays using Olink’s Proseek Immuno-oncology Panel. Odds ratios (ORs) for association with gastric cancer risk were calculated for quantiles (two to four categories) of each biomarker from unconditional logistic regression models, adjusted for age, sex, smoking and alcohol consumption. Two-sided p-values <0.05 were considered as significant. The false discovery rate (FDR) was used to correct for multiple comparisons.
Results:
Of 83 evaluable biomarkers, lower levels of TNFRSF12A (per quartile OR, 0.82; nominal p-trend=0.02) and ADGRG1 (per quartile OR, 0.84; nominal p-trend=0.03) were associated with early-stage gastric cancer but were not statistically significant after FDR correction.
Conclusion:
Our study did not identify any inflammation-related biomarkers that may be useful for early disease detection. To date, this is the first assessment of circulating inflammation-related proteins in early-stage gastric cancer. Given the complex inflammation processes preceding malignant transformation, further investigation of other biomarkers is warranted.
Keywords: Gastric cancer, Inflammation, HERPACC
INTRODUCTION
Gastric cancer (GC) is the fourth leading cause of cancer deaths worldwide (Sung et al, 2021). In its early clinical stages, this neoplasia is treatable. In Japan, ~70% of patients with GC are diagnosed at early stages (I-II) often detected through the national endoscopy screening program (Katai et al., 2018). The estimated 5-year overall survival rates of GC patients with stages IA, IB and II disease are 92%, 84% and 71%, respectively (Katai et al., 2018).
In Helicobacter pylori-driven GC, mucosal colonization induces chronic inflammation with variable progression to cancer. Gastric mucosal regeneration after injury involves a complex interaction of various signaling pathways and regulators. If locally produced molecules from the gastric tissue microenvironment were to recirculate via the bloodstream, blood levels could be informative either as markers of local activity or reflecting systemic effects. Prospective studies of circulating inflammation-related proteins have suggested weak associations with GC risk (Epplein et al., 2013,Camargo et al., 2019). To assess potential utility as predictive biomarkers for clinical screening, we examined levels of key immune and inflammation molecules for associations with early-stage noncardia GC.
MATERIALS AND METHODS
Study Population
All participants in the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) study were first-visit outpatients at the cancer center who provided lifestyle data and pretreatment blood samples (Tajima et al., 2000). Among the participants recruited between 2001–2005 (HERPACC-II), 239 patients with early-stage (153 IA, 50 IB and 36 II) noncardia GC (ICD-O-3 codes C16.1-C16.9) were age-frequency matched to 256 cancer-free H. pylori seropositive controls for this study.
Informed consent was obtained from all individuals. The HERPACC study was approved by the Aichi Cancer Center and Nagoya University.
Laboratory Methods
EDTA plasma samples from cases, controls and replicates (n=34) were tested for 92 proteins by multiplex proximity extension assays (Proseek Multiplex Immuno-oncology Panel, Olink Bioscience, Uppsala, Sweden). Blinded testing was performed on a Fluidigm Biomark reader at the facility of the kit manufacturer. The assay panel includes proteins related to apoptosis, chemotaxis, autophagy, modulation of tumor immunity, vascularization and tissue remodeling. Relative levels were calculated from quantitative PCR cycle threshold values with corrections for assay variation and expressed as normalized values. Seven biomarkers (IFN-beta, IFN-gamma, IL1-alpha, IL35, IL13, IL2 and IL33) were undetectable in >90% of samples and were excluded from the analysis. IL5 and VEGFC were excluded because of high (>40%) coefficients of variation (CVs). Of the remaining 83 biomarkers, 68 had CVs <15%, 11 between 15% and 30%, and 4 between 31% and 40%.
Statistical Analysis
Biomarker levels were analyzed as ordinal variables (two to four categories depending upon the proportion of measurements below the lower limit of detection) based on distributions among controls. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association of each biomarker with GC were estimated from unconditional logistic regression models adjusted for age, sex, smoking status and alcohol drinking status. False discovery rate (FDR) corrected Ptrend values were also calculated to account for multiple testing. Analyses were stratified by histology (74 diffuse-type tumors, 82 intestinal-type and 83 mixed-type by Lauren classification), with a test for heterogeneity among strata by Wald test. We also restricted to non-atrophy controls (n=164) as determined by normal pepsinogen (PG) levels (PG I >70 ng/ml or PG I/PG II > 3 ng/ml). Analyses were conducted using SAS version 9.4 software (SAS Inc, Cary, NC).
RESULTS
Characteristics of GC cases and controls are presented in Table 1. Compared with the controls, cases were less likely to be male.
Table 1.
Baseline characteristics of HERPACC Study early-stage gastric cancer cases and controls
|
H. pylori-seropositive
controls n=256 |
Early-stage gastric cancer cases
n=239 |
p-value | |
|---|---|---|---|
| Age in years, mean (SD) | 60 (10) | 59 (10) | 0.21 |
| Male, n (%) | 209 (81.6) | 179 (71.3) | 0.01 |
| Cigarette smoking status, n (%) | |||
| Never | 87 (34.0) | 82 (34.3) | 0.28* |
| Former | 88 (34.4) | 61 (25.5) | |
| Current | 81 (31.6) | 96 (40.2) | |
| Alcohol drinking status, n (%) | |||
| Never | 78 (30.5) | 87 (36.4) | 0.18* |
| Former | 7 (2.7) | 5 (2.1) | |
| Current | 171 (66.8) | 147 (61.5) |
Abbreviations: SD, standard deviation
p-value calculated by Mantel-Haenszel chi-square
Figure 1 shows the 83 evaluable biomarkers and their adjusted per quantile increase ORs for associations with early-stage GC risk. Levels of TNFRSF12A (OR per quartile, 0.82; nominal p-trend=0.02) and ADGRG1 (OR, 0.84; p-trend=0.03) were inversely associated with GC. These associations varied by histology (p-heterogeneity <0.05), with stronger TNFRSF12A results for intestinal-type tumors (OR, 0.67; 95%CI, 0.53–0.86) and stronger ADGRG1 results for diffuse-type tumors (OR, 0.73; 95%CI 0.56–0.94). Restricting to non-atrophy controls, levels of ADGRG1 (OR per quartile, 0.80; nominal p-trend=0.02) and MIC-A/B (OR, 1.23; p-trend=0.03) were associated with GC. However, no association met statistical significance after FDR correction.
Figure 1.
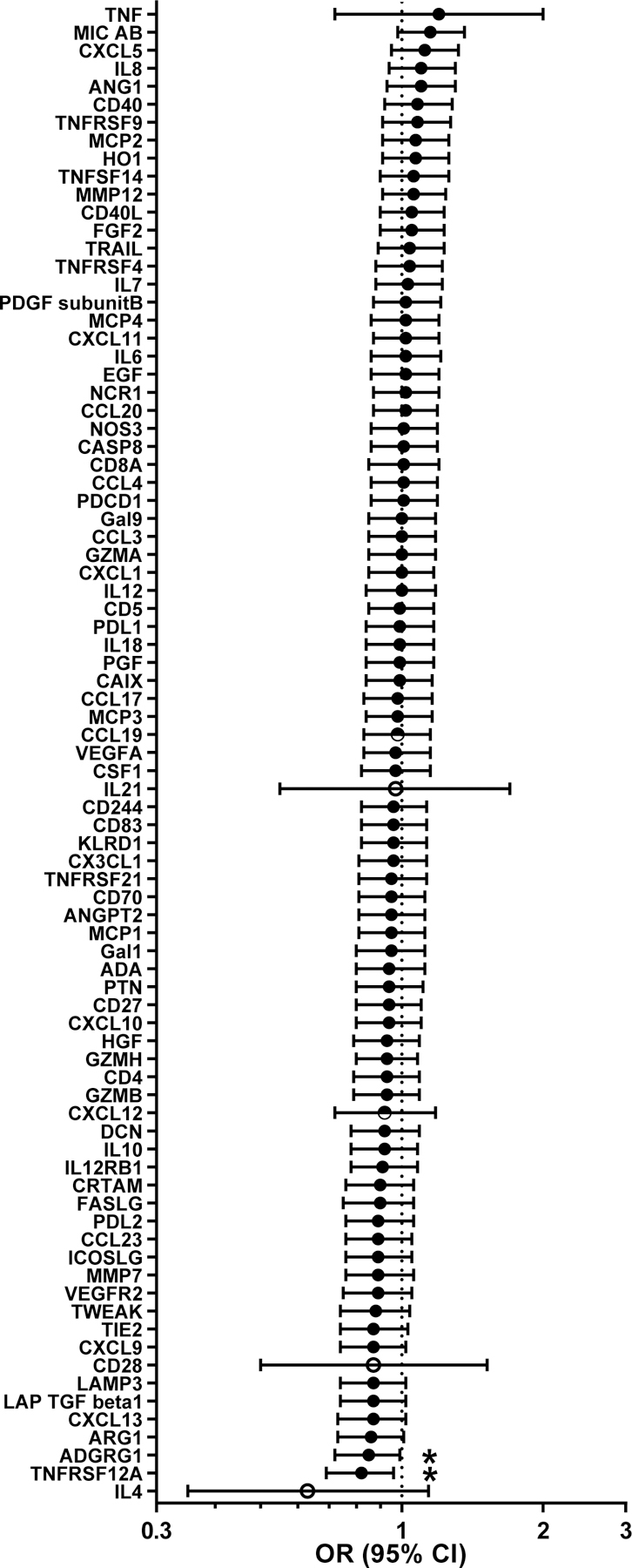
Estimated odds ratios (ORs) and 95% confidence intervals (CIs) of early-stage noncardia gastric cancer risk per quantile increase of biomarker levels. Unconditional logistic regression models were adjusted for age (continuous), sex, cigarette smoking status (never, former, current) and alcohol drinking status (never, former, current). Biomarker levels were analyzed by quartiles (filled circles), three categories (undetectable, < median vs. ≥ median; half-filled circle) and two categories (undetectable vs. detectable levels; open circles). * indicates nominal Ptrend<0.05
DISCUSSION
Circulating inflammation-related proteins may be important mediators or biomarkers of gastric carcinogenic mechanisms. However, in this first assessment of early-stage noncardia GC, we found no significant associations with key immune- and inflammation-related molecules. Strengths of our study include using well-characterized pre-treatment biospecimens, adjustment for H. pylori infection and evaluation of a broad range of plausible carcinogenic pathways.
Our findings add to the understanding of the complex role of inflammation in carcinogenesis. Evaluating a wide-range of pro- and anti-inflammatory cytokines, growth factors, soluble receptors and angiogenesis factors, we and others have shown that prediagnostic circulating levels of multiple proteins have null-to-weak associations with GC risk (Sasazuki et al., 2010,Epplein et al., 2013,Camargo et al., 2019). Similar findings have been reported for colorectal (Song et al., 2018) and esophageal cancers (Aversa et al., 2020). Speculations for these weak associations are a well-controlled tissue permeability and increased state of immunological tolerance in the presence of confined mucosal lesions.
In conclusion, our study did not identify any inflammation-related biomarkers that may be useful for the early detection of GC. Further investigation of other biomarkers is warranted.
ACKNOWLEDGMENTS
The authors thank the physicians, nurses, technical and administrative staff at Aichi Cancer Center Hospital for their invaluable assistance conducting the HERPACC study.
The HERPACC study was supported by the Ministry of Education, Science, Sports, Culture and Technology of Japan Grant-in-Aid for Scientific Research; Ministry of Health, Labour and Welfare of Japan Grant-in-Aid for Cancer Research and Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control; and Uehara Memorial Foundation research grant. This analysis was supported by the Intramural Research Program of the U.S. National Cancer Institute.
Footnotes
Compliance with ethical standards
Conflict of interest: The authors declare no conflicts of interest
Ethical approval: Informed consent was obtained from all individuals. The HERPACC study was approved by the Aichi Cancer Center and Nagoya University.
REFERENCES
- Aversa J, Song M, Shimazu T, Inoue M, Charvat H, Yamaji T et al. (2020). Prediagnostic circulating inflammation biomarkers and esophageal squamous cell carcinoma: A case-cohort study in Japan. International journal of cancer Journal international du cancer 147(3):686–691.. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:caac.21660. [DOI] [PubMed] [Google Scholar]
- Camargo MC, Song M, Shimazu T, Charvat H, Yamaji T, Sawada N et al. (2019). Circulating Inflammation Markers and Risk of Gastric and Esophageal Cancers: A Case-Cohort Study Within the Japan Public Health Center-Based Prospective Study. Cancer Epidemiol Biomarkers Prev 28(4):829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epplein M, Xiang YB, Cai Q, Peek RM Jr., Li H, Correa P et al. (2013). Circulating cytokines and gastric cancer risk. Cancer Causes Control 24(12):2245–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I et al. (2018). Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 21(1):144–154. [DOI] [PubMed] [Google Scholar]
- Sasazuki S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T et al. (2010). Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan Public Health Center-based prospective study. Carcinogenesis 31(4):712–718. [DOI] [PubMed] [Google Scholar]
- Song M, Sasazuki S, Camargo MC, Shimazu T, Charvat H, Yamaji T et al. (2018). Circulating Inflammatory Markers and Colorectal Cancer Risk: A Prospective Case-cohort Study in Japan. International journal of cancer Journal international du cancer [DOI] [PMC free article] [PubMed]
- Tajima K, Hirose K, Inoue M, Takezaki T, Hamajima N, Kuroishi T (2000). A Model of Practical Cancer Prevention for Out-patients Visiting a Hospital: the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Asian Pac J Cancer Prev 1(1):35–47. [PubMed] [Google Scholar]


