Abstract
Light-emitting diodes (LEDs) and high-pressure sodium lamps (HPS) are among the most commonly used light sources for plant cultivation. The objective of this study was to evaluate the effect of two controlled-environment production systems differing in light sources on growth, photosynthetic activity, and secondary metabolism of common buckwheat. We hypothesized that LED light with the majority of red and blue waves would increase physiological and biochemical parameters compared to sunlight supplemented with HPS lamps. The experiment was performed in a phytotronic chamber (LEDs) and in a greenhouse (solar radiation supplemented with HPS lamps as a control). The effects were analyzed at the flowering phase with biometric measurements, leaf chlorophyll index, the kinetics of chlorophyll a fluorescence, content of soluble carbohydrates and phenolics in the leaves. Applied LED light decreased the biomass but stimulated the production of phenolics compared to control plants. In control plants, a positive correlation between flavonoid content and energy dissipation from photosystem II (DIo/CSm) was found, while in plants under LEDs total pool of phenolic content correlated with this parameter and the quantum yield of electron transport (φ Ro and ψ Ro) was lower than that of the control, probably affecting buckwheat biomass.
Subject terms: Plant physiology, Plant breeding
Introduction
Among primary environmental factors, light is probably the most important one affecting the growth and development of plants1. It is essential for germinating, seedling development, generative phase, photosynthesis productivity, and it is a significant signal to mediate substance metabolism for plant tolerance to environmental fluctuations2,3. Chlorophylls are the primary photosynthetic pigments that absorb primarily red and blue light, and so these wave ranges are thought to support plant development to the most significant degree. Blue light photoreceptors (cryptochromes and phototropins) and red/ far-red light receptors (phytochromes) monitor light spectra to control plant phenotype by regulating their growth and development. Another group of pigments, carotenoids, absorb violet and blue-green light. The examples of developmental responses include leaf expansion area, stem length, stomata opening, flowering, and phototropism1,4. Plants exposed to adverse fluctuations of environmental conditions exhibit photosynthesis disturbances. Analysis of chlorophyll a fluorescence (ChlF) is commonly used to study the photochemical efficiency of leaves and plant physiological conditions. It is a non-invasive and powerful tool in ecological and environmental studies of plant response to stress factors5. Light also affects the synthesis of some plant metabolites such as sugars, the primary photosynthetic assimilates, or phenolic compounds4,6, and regulates their secondary metabolism through induction of phenolic biosynthesis by affecting the activity of phenylalanine ammonia-lyase (PAL), which is the first enzyme in the phenylpropanoid pathway7,8. Phenylpropanoids originate from cinnamic acid, formed from phenylalanine in a reaction catalyzed by PAL, the offshoot point among primary (shikimic acid pathway) and secondary (phenylalanine pathway) metabolism9. Plants are potential sources of bioactive compounds such as phenolic compounds, demonstrating mainly antioxidant ability. Phenolics are recognized as pro-healthy components stored mainly in plant leaves. Secondary metabolites (such as phenolics) are accumulated often under stress conditions10. Sugars are significant regulators of metabolism; growth and their accumulation could be an indicator of photosynthetic efficiency11. The synthesis of phenolic compounds requires an extensive energy input, and therefore it depends on the accumulation of soluble sugars in the cells. To gain optimal photosynthetic productivity, each leaf within the plant canopy needs to adapt to fluctuations in light intensity and quality rapidly12. For example, Lin et al.13 proved that Red Blue White LED lights increased soluble sugar content and their accumulation in lettuce leaves. It positively affected their growth, development, and nutrition. It was also observed that high light intensity treatment supplemented with blue light reduced the quantum yield of photosystem II (PSII) and increased the accumulation of, i.e., pigment and phenolic content in L. sativa14. Phenolics affect various physiological processes related to plant growth and development, such as seed germination, cell division, flower development, and synthesis of photosynthetic pigments15,16. Various phenolic compounds have essential roles in human and animal functioning due to their practical, biological, and pharmacological effects, mainly because they protect the cell from oxidative stress. Gallic acid participates in cell apoptosis in different human diseases17; ferulic acid was involved in innovative mechanisms related to Alzheimer’s disease18. Chlorogenic acid demonstrates pharmacological properties, for example, antioxidant activity, antibacterial, antiviral and antimicrobial attributes19.
Controlled-environment plant production systems are widely used worldwide to produce plant materials or products of a quality that cannot be obtained in the natural environment. The primary environmental parameter controlled is temperature. However, environment control can contain other factors such as carbon dioxide levels, relative humidity, water, pest control, plant nutrients, and light. Therefore, manipulating light quality, spectrum, and light intensity to obtain better plant growth and quality has become a popular research object in recent years20. Artificial light can increase crop yield and nutritional value, especially during the late autumn and winter in greenhouse cultivation in northern latitudes2–4. In a greenhouse, the lighting conditions are difficult to control due to the change in light intensity depending on the season, time of day, and degree of cloudiness. On the other hand, daylight from dawn to dusk is the most natural factor for plant growth and development. The studies carried out in phytotronic chambers are, however, justified. Plant cultivation indoor allows to provide plants with more stable temperature, soil hydration, and light conditions specific to the species, and may also be important for urban agriculture. In the greenhouse or phytotronic conditions, more frequent crops can also be harvested, in some cases 2–3 times a year, and the growing conditions can be selected to obtain better plant quality in terms of the content of health-promoting compounds21. The most widespread light sources used for controlled environmental agriculture during the last decades are high-pressure sodium (HPS) lamps6,22. This kind of light provides high photon flux emission and long operational life but tends to use a large amount of electricity and radiates much heat, which leads to energy waste. Moreover, often their most intensive green and yellow bands of the spectrum are not optimal for photosynthesis4,23. Chlorophylls cannot absorb much light in the 450–550 nm spectrum, i.e., maximum sunlight intensity on Earth. However, this is the exact waveband where the carotenoids are the most efficient at light-absorbing24. light-emitting diodes (LEDs) proposed as an alternative light source have distinct advantages, such as low radiant heat output, reduced energy consumption, and composing their spectra to optimize photosynthesis and regulate plant growth and development3,6. However, LEDs should be compatible with the photosynthesis requirements of grown plants due to the specific role of each waveband of light. The study of monochromatic light creates a great opportunity to understand basic plant physiology processes. The current state of knowledge determines that blue and red light, are efficiently absorbed close to the surface while a green light contributes to deeper layers of the leaf and on the lower canopy level. It means that green light may drive photosynthesis in areas where other wavelengths are in a limited amount. The UV and far-red parts of the sunlight spectrum are important in defining photomorphogenesis21.
Buckwheat (Fagopyrum esculentum Moench) belongs to the Polygonaceae family, and it is considered a pseudo-cereal that constitutes a valuable food source in several regions of the world. Nowadays, it is becoming more popular because of its excellent nutritional qualities and gluten-free seeds. Its grains are rich in compounds such as polyphenols, lipids, dietary fiber, polysaccharides, and amino acids25,26. Therefore, this species is the subject of intensive research studies all over the world. Increasing yield productivity demands a better understanding of the environmental parameters that determine seed yield and seed composition. An increase in the assimilate synthesis is one of the major factors in plant productivity growth. Thus far, the intensity of buckwheat photosynthesis was studied, e.g., by Amelin et al.27. They concluded that the buckwheat cultivars with determined growth habit (det mutation) showed a higher photosynthesis rate at the grain filling stage than those with indeterminate growth habit. However, there are no data on the light quality impact on the growth and development of common buckwheat.
Our previous experiments found that common buckwheat plants sown and cultivated under sole HPS light at optimal temperature and humidity displayed abnormalities in morphology at the cotyledon stage and stopped growing. That disturbed ongoing experiments. However, the plants sown and grown to the cotyledon stage under solar light supplemented with HPS developed properly throughout further vegetative and generative stages28. The plants under both treatments grew at 25 + 2 °C/22 ± 2 °C day/night, with 55–60% humidity and under a 16 h photoperiod. It encouraged us to investigate this phenomenon in the current study. The hypothesis was that LED lamps emitting mainly blue and red waves, absorbed mainly by chlorophylls, can significantly influence common buckwheat's physiological and biochemical parameters. The objective of the present study was to evaluate the effect of two controlled-environment production systems differing in light sources on growth, photosynthetic activity, and bioactive compounds content in common buckwheat plants.
Results
Plant growth measurements
Common buckwheat plants grown under LED light were more compact (smaller leaf area and more internodes) and showed lower main stem height than the plants grown in the glasshouse with solar light supplemented with HPS lamps (Table 1). The number of internodes was not significantly different between both treatments. The plants grown under LED light showed lower fresh weight (FW) and dry weight (DW) of the aboveground parts than those grown in the glasshouse conditions. The leaf area was four times greater, and the FW of the leaf was three times higher for the plants grown under solar light supplemented with HPS lamps than those grown under LED light (Table 1; Fig. 1).
Table 1.
Main stem height, fresh (FW), and dry weight (DW) of the aboveground parts (stems with leaves) as well as leaf area of common buckwheat cv. ‘Panda’ grown under different controlled-environment production systems.
| Light | Main stem height [cm] | No. of the main stem internodes | Aboveground parts FW [g] | Aboveground parts DW [g] | The third leaf area [cm2] | The third leaf FW [g] |
|---|---|---|---|---|---|---|
| Control | 86.1 ± 1.7 | 9.7 ± 0.4 | 14.765 ± 1.2 | 1.875 ± 0.1 | 36.0 ± 2.5 | 0.6 ± 0.06 |
| LED | 72.5 ± 2.4** | 10.4 ± 0.4 | 11.014 ± 0.9* | 1.478 ± 0.2*** | 9.5 ± 0.8*** | 0.2 ± 0.02*** |
Control—solar light supplemented with HPS (High-Pressure Sodium) lamps; LED (Light-Emitting Diodes). Values represent means (n = 10) ± SE. Values marked with stars differ from the control significantly according to the Student’s t test: *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 1.

Differences in the leaf area in the plants grown in greenhouse conditions under solar light supplemented with HPS Agro Philips lamps (upper row) and the plants grown in phytotron chambers under LED light (bottom row). All presented leaves were collected at the same time from 8-week-old plants. The sample was the third, fully developed leaf in order from the top inflorescence.
ChlF (chlorophyll a fluorescence), chlorophyll index, total soluble carbohydrate, phenolic, and flavonoid content
The overall performance index of PSII (PI) was higher for plants grown under control conditions than in those grown in the phytotron chamber (Table 2). The values of energy used for electron transport (ETo/CSm), and the number of active reaction centers (RC/CSm) were not significantly different between plants grown under control light and LED light. Under LED light, the energy absorption (ABS/CSm), excitation energy trapped in PSII reaction centers (TRo/CSm), and energy dissipated from PSII (DIo/CSm) were higher than in control. The probability that a trapped exciton moves an electron into the electron transport chain beyond QA− (ψ Ro), as well as the quantum yield of electron transport from QA− to the PSI end electron acceptors (φ Ro) were higher in the plants grown under solar light supplemented with HPS lamps than under LED light. The values of δRo, denoting the efficiency with which an electron can move from the reduced intersystem of electron acceptors to the PSI end electron acceptors, did not differ between the treatments. Detailed, raw data can be found in the supplementary material (Supplementary Table S1).
Table 2.
Changes in the kinetics of chlorophyll a fluorescence in common buckwheat plants of cv. ‘Panda’ grown under different controlled-environment production systems.
| Light | PI | ABS/CSm | TRo/CSm | ETo/CSm | DIo/CSm | RC/CSm | ẟ Ro | φ Ro | ψ Ro |
|---|---|---|---|---|---|---|---|---|---|
| Control | 2.1 ± 0.2 | 1492 ± 10 | 1244 ± 10 | 594 ± 20 | 247 ± 3 | 633 ± 6.3 | 0.36 ± 0.01 | 0.14 ± 0.01 | 0.17 ± 0.01 |
| LED | 1.5 ± 0.2*** | 1591 ± 23*** | 1305 ± 25* | 545 ± 34 | 286 ± 11*** | 640 ± 21 | 0.36 ± 0.02 | 0.12 ± 0.01* | 0.14 ± 0.01*** |
Control—solar light supplemented with HPS (High-Pressure Sodium) lamps; LED (Light-Emitting Diodes). Values represent means (n = 20) ± SE. Values marked with stars differ from the control significantly according to the Student's t test: *p < 0.05; **p < 0.01; ***p < 0.001. ABS/CSm—energy absorption by antennas, DIo/CSm—energy dissipation from PSII, ETo/CSm—the energy used for electron transport, PI—performance index of PSII, RC/CSm—number of active reaction centers, TRo/CSm—excitation energy trapped in PSII, δ Ro—efficiency with which an electron can move from the reduced intersystem of electron acceptors to the PSI end electron acceptors, ψ Ro—probability, at time 0, that a trapped exciton moves an electron into the electron transport chain beyond QA −, φ Ro—quantum yield of electron transport from QA− to the PSI end electron acceptors.
Chlorophyll index was more significant in the leaves developed under solar light supported with HPS lamps than in the plants grown under LED light (Table 3). This effect is also shown in Fig. 1. Phenolic and flavonoid content was more significant in the leaves developed under LEDs light than under solar light supplemented with HPS (Table 3).
Table 3.
Chlorophyll index, the average content of total phenolicsa [µmol mg–1 DW] and total flavonoidsb [nmol mg–1 DW] in leaves of common buckwheat cv. 'Panda' under different controlled-environment production systems.
| Light | Chlorophyll index | Phenolic content [µmol mg–1 DW] | Total flavonoid content [nmol mg–1 DW] |
|---|---|---|---|
| Control | 18.9 ± 1.4 | 0.186 ± 0.015 | 52.4 ± 2.9 |
| LED | 14.5 ± 0.8** | 0.449 ± 0.020*** | 114.4 ± 5.8*** |
Control—solar light supplemented with HPS (High-Pressure Sodium) lamps; LED (Light-Emitting Diodes). Values represent means (n = 20 for chlorophyll index; n = 3 for phenolic and total flavonoid content) ± SE. Stars indicate significant difference between means; **p < 0.01, ***p < 0.001 (Student’s t test).
aµmol gallic acid equivalent (GAE)/mg–1 DW.
bµmol quercetin equivalent (QUE)/mg–1 DW.
Individual sugar content (SC)
Identification and quantification of sugar content in the leaves of common buckwheat showed significant differences between control and LED treatment (Fig. 2). The individuals studied sugars were significantly lower in the plants grown under LED, except for kestose and maltose. The highest differences were found for glucose (its amount was four times lower), fructose (five times lower), and sucrose (one and half times lower compared to that of the control).
Figure 2.
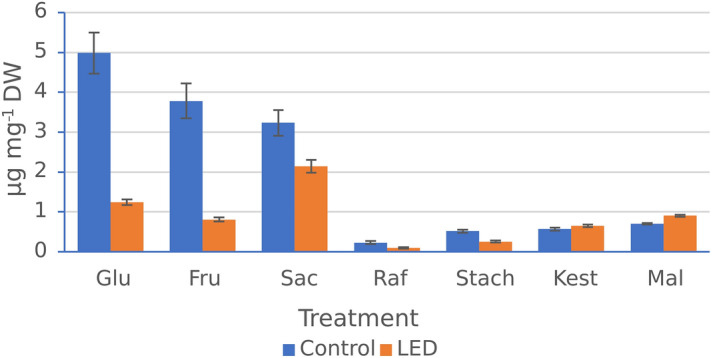
Individual sugar content in leaves of common buckwheat cv. ‘Panda’ under different controlled-environment production systems. Control—solar light supplemented with HPS (High-Pressure Sodium) lamps; LED (Light-Emitting Diodes), Raf—raffinose, Stach—stachyose, Kest—1-kestose, Mal—maltose, Glu—glucose, Fru—fructose, Suc—sucrose. Values represent means (n = 3) ± SE. Stars indicate significant difference between means; *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test).
Phenolic acid content
Plants grown under LED light produced significantly more amounts of the following phenolic acids: gallic, 3,4 dihydroxybenzoic, caffeic, vanillic, chlorogenic, homovanillic, syringic, benzoic, rosmarinic, and cinnamic than those grown under solar light supplemented with HPS lamps (Table 4). Buckwheat grown under solar light supplemented with HPS lamps contained higher p-hydroxybenzoic, ferulic, and salicylic acids than the plants treated with LEDs. The content of gentisic and p-coumaric acids remained at the same level under both light treatments.
Table 4.
The average amount of phenolic acids [µg mg–1 DW] in common buckwheat cv. 'Panda' grown under different controlled-environment production systems.
| Phenolic acid | Control [µg mg–1 DW] | LED [µg mg–1 DW] |
|---|---|---|
| Gallic acid (3,4,5-trihydroxybenzoic acid) | 0.044 ± 0.006 | 0.114 ± 0.012*** |
| 3,4-Dihydroxybenzoic acid | 0.008 ± 0.001 | 0.018 ± 0.002** |
| p-Hydroxybenzoic acid | 0.004 ± 0.001 | 0.002 ± 0.0002* |
| Gentisic acid (2,5-dihydroxybenzoic acid) | 0.005 ± 0.001 | 0.004 ± 0.001 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | 0.017 ± 0.002 | 0.050 ± 0.005*** |
| Vanillic acid (4-hydroxy-3-methoxybenzoic acid) | 0.007 ± 0.0007 | 0.014 ± 0.001** |
| Chlorogenic acid | 9.4 ± 1.4 | 25.6 ± 2.7*** |
| Homovanillic acid | 0.390 ± 0.051 | 1.080 ± 0.151*** |
| Syringic acid | 0.019 ± 0.003 | 0.061 ± 0.005*** |
| p-Coumaric acid | 0.018 ± 0.006 | 0.012 ± 0.003 |
| Ferulic acid | 0.015 ± 0.001 | 0.007 ± 0.0005** |
| Benzoic acid | 0.078 ± 0.017 | 0.440 ± 0.052*** |
| Sinapic acid | Nd | Nd |
| Salicylic acid | 0.002 ± 0.0002 | 0.001 ± 0.0001* |
| Rosmarinic acid | 0.009 ± 0.002 | 0.064 ± 0.008*** |
| Cinnamic acid | 0.005 ± 0.0005 | 0.010 ± 0.0007** |
Control—solar light supplemented with HPS (High-Pressure Sodium) lamps; LED (Light-Emitting Diodes). Values represent means (n = 3) ± SE. Values marked with stars differ from the control significantly according to the Student’s t test: *p < 0.05; **p < 0,01 ***p < 0.001. nd—not detected.
Correlation analyses
In control plants grown in a greenhouse under sunlight supplemented with HPS lamps, significant correlations were found between the performance index (PI) and the chlorophyll index in the leaves (r = 0.563; p < 0.05), with the fresh and dry weight of the aboveground parts (r = 0.537 and r = 0.617; p < 0.05, respectively), with the ChlF parameters such as φ Ro and ψ Ro (r = 0.845 and r = 0.823; p < 0.05, respectively) (Table 5). It is worth mentioning that the ChlF related to the quantum efficiency φ Ro and ψ Ro correlated with the fresh and dry mass of the aboveground parts. The energy dissipation from PSII (DIo/CSm) positively correlated with the content of flavonoids (r = 0.639; p < 0.05). In plants grown under LED light, PI correlated with the content of some individual sugars: glucose, sucrose, and maltose. In these plants, the DIo/CSm parameter correlated with the total phenolic content.
Table 5.
Pearson coefficients of linear correlation (p < 0.05) between selected studied parameters of common buckwheat cv. ‘Panda’ grown under different controlled-environment production systems.
| Variable | PI | Chl | FW | DW | DIo/CSm | φ Ro | ψ Ro | Flavonoids |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| PI | – | 0.563 | 0.537 | 0.617 | – | 0.845 | 0.823 | – |
| Chl | 0.563 | – | – | – | – | 0.705 | 0.723 | – |
| FW | 0.537 | – | – | 0.959 | – | 0.680 | 0.686 | – |
| DW | 0.617 | – | 0.959 | – | – | 0.639 | 0.643 | – |
| DIo/CSm | – | – | – | – | – | – | – | 0.639 |
| φ Ro | 0.845 | 0.705 | 0.680 | 0.639 | – | – | 0.998 | – |
| ψ Ro | 0.823 | 0.723 | 0.686 | 0.643 | – | 0.998 | – | – |
| Flavonoids | – | – | – | – | 0.639 | – | – | – |
| Variable | PI | Glu | Suc | Mal | DIo/CSm | Phenolics | ||
|---|---|---|---|---|---|---|---|---|
| LED | ||||||||
| PI | – | 0.565 | 0.456 | 0.744 | – | – | ||
| Glu | 0.565 | – | 0.953 | 0.793 | – | – | ||
| Suc | 0.456 | 0.953 | – | 0.692 | – | – | ||
| Mal | 0.744 | 0.793 | 0.692 | – | – | – | ||
| DIo/CSm | – | – | – | – | – | 0.653 | ||
| Phenolics | – | – | – | – | 0.653 | – | ||
Control—solar light supplemented with HPS (High-Pressure Sodium) lamps; LED (Light-Emitting Diodes); PI—performance index of PSII, Chl—chlorophyll index, FW—fresh weight, DW—dry weight, DIo/CSm—energy dissipation from PSII, ψ Ro—probability, at time 0, that a trapped exciton moves an electron into the electron transport chain beyond QA −, φ Ro—quantum yield of electron transport from QA− to the PSI end electron acceptors, Glu—glucose, Suc—sucrose, Mal—maltose.
Discussion
LED application is a proven and viable option of supplemental lighting in plant cultivation under controlled environmental conditions (e. g., Massa et al.29). The theoretical maximum efficiency occurs when the total input energy is transformed into the energy of photosynthetically active photons30,31. The blue LEDs are 93% efficient, white in 76%, and red in 81%. Therefore, horticultural LED lamps usually provide combinations of red (peak at 660 nm), blue (peak at 450 nm), and white or far-red (peak at 730 nm) emitting diodes. Other wavelengths are available, but they have lower photosynthetic efficiency30. Red and blue LED composition is commonly used for plants grown but still tested to intensify the horticultural production. Wojciechowska et al.32 reported that the value of performance index (PI) was the greatest in leaves of lamb lettuce (Valerianella locusta) under LED lamps compared to natural light. Our study analyzed the effect of sole LED light on various growth and photosynthetic parameters of common buckwheat, while plants grown under solar light supplemented with HPS lamps served as controls in greenhouse conditions. Our previous experiment conducted under solo HPS lamps indicated that the development of buckwheat plants stopped at the cotyledons stage due to inadequate composition of light spectra. A similar effect was observed in the case of winter rape (data not published). Interestingly, cereals grown under the same light spectrum in the same phytotron chamber developed properly (our observation provided in another study; unpublished). This effect suggests that dicotyledonous plants could have different light demands than monocotyledonous ones. The rapid climate changes taking place on Earth in recent years will probably force food producers to increasingly use the cultivation of plants under glasshouse conditions. Cultivation of plants outside the growing season requires artificial plant lighting, and for this purpose, the spectra emitted by different types of lamps should be adjusted to each plant species. In the case of common buckwheat, the use of solo HPS lamps, usually dedicated to greenhouse crops, was unsuccessful. Therefore, in our research, we used LED lamps with the spectrum most effectively in the photosynthesis process.
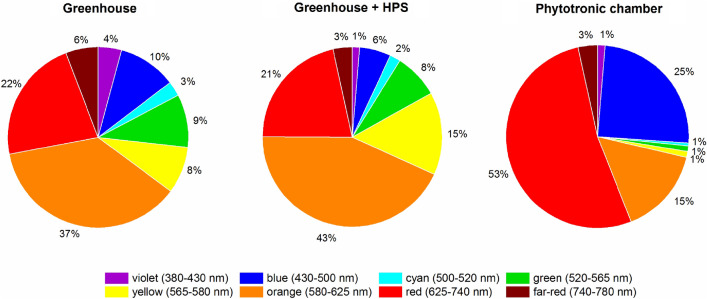
Lighting the plants in the greenhouse in the morning with HPS lamps slightly changed the spectrum of light reaching the plants. This spectrum was characterized by a lower proportion of blue light by 4%, violet and far-red light by 3%, and an increase in yellow light by 7% and orange light by 6%. It seems, however, that these relatively small changes could not have had a major impact on the studied parameters, taking into account that the HPS lamps were only used for 4 h early in a day when the solar radiation was weaker. The spectrum of sunlight and sunlight supplemented with HPS lamps was diametrically different from the spectrum of LED lamps. The LEDs in the phytotron chamber emitted 53% red light, 25% blue light, 15% orange light, 1% yellow and green light each. The percentage of far-red and violet light was the same as in the greenhouse. The mean intensity of daylight in the morning supplemented with HPS lamps was comparable to the light intensity emitted by LEDs in the phytotron chamber, but the intensity of sunlight at noon was very high and was 1300–1600 µmol m–2 s–1. It is worth noting that illumination with HPS lamps as well as solar radiation did not cause significant temperature fluctuations due to the air conditioning of the greenhouse chamber.
In our experiment, LEDs did not have the same effect on plant biomass as solar light supplemented with HPS lamps. The main effect of proper plant development and transition from the vegetative to the generative phase was achieved. In the case of plants grown under LEDs, practically all biometric parameters were significantly lower than that of the control. The most significant differences were observed in the leaf area: the leaves of the plants from the phytotronic chamber were almost four times smaller, which was obviously related to their three times less weight than the leaves of the control plants. The increase in biomass is influenced not only by its intensity but also by the spectrum of light21. Studies on the effect of monochromatic red light demonstrated that it inhibits the growth of biomass and leaf area while stimulating shoot elongation, number of leaves, and chlorophyll content33. It was also interesting that at the more significant amount of blue and red lights, most conducive to photosynthesis, the overall performance index of PSII (PI) was lower than in the plants grown in the greenhouse. In the plants grown in the phytotron chamber, the greater amount of energy dissipated from PSII (DIo/CSm) could be an effect of greater energy absorption by the reaction center (ABS/CSm) than in the plants grown in the greenhouse. The plants grown under LED and solar spectrum with HPS, demonstrated the same number of reaction centers (RC/CSm), although different chlorophyll amounts were detected. Hamdani et al.33 reported, that in rice leaves blue light compared to white light decreases the maximum efficiency of PSII (Fv/Fm), a lower rate of reduction of the excited electronic state of P700, and increases nonphotochemical quenching (NPQ). In turn, Su et al.34 observed an increase in photosynthesis rate in cucumber in monochromatic blue light which was associated with higher Rubisco biosynthesis. Hattori et al.35 showed that red light reduced stomatal density, stomatal conductance, the content of Rubisco, and decreased photosynthesis rate. In our experiment, we did not study the effect of monochromatic red light, but we should take into consideration the much higher participation of red light in radiation emitted by LEDs compared to sunlight. LEDs emitted twice more red light than blue light. It is possible that the performance index (PI) was significantly lower in plants grown under LEDs than in the glasshouse due to the high participation of red light and lower light intensity. Energy absorbed by reaction centers (RC) in PSII (ABS/CSm) was higher in plants grown in the phytotronic chamber than in the glasshouse. This fact could suggest an adaptation of the leaves to long vegetation in lower light intensity36. The higher values of TRo/CSm and DIo/CSm in these plants are the consequence of higher value ABS/CSm. In another study, barley leaves grown in sunlight demonstrated a higher CO2 assimilation rate and higher values of electron transport rate (ETR) than in the leaves grown in the shade37. At the same time, in the leaves growing in the shade a greater degree of energy dissipation was observed, which would confirm our results. Lazár38 suggested a decreased size of the pool of PSII and PSI electron carriers between QA− to ferredoxin in the leaves grown in the shadow.
As mentioned above, in plants under the LED lamps energy absorption (ABS/CSm) and energy dissipation from PSII (DIo/CSm) were higher than in plants grown in a greenhouse condition. This is most likely due to the greater amount of blue and red light absorbed mainly through chlorophyll. At the same time, the amount of absorbed energy did not reflect better quantum yield (ψ Ro and φ Ro), which were significantly lower than in plants grown in a greenhouse. A lower value of these parameters may also indicate the activation of cyclic photophosphorylation, in which mainly ATP is produced and NADPH not, which is needed for the reduction of 3-phosphoglyceric acid to 3-phosphoglyceraldehyde in the dark phase of photosynthesis. The lower value of these parameters may be related to lower plant weight and lower SC in plants growing under LED lamps. In control plants, high correlations between the values of both ψ Ro and φ Ro parameters with the fresh and dry weight of the plants were found. This effect suggests that the quantum efficiency in sunlight with the addition of HPS lamps was higher. In the case of the control plants, a high correlation between the flavonoid content and the energy dissipation (DIo/CSm) was also found. In plants growing under LED, dispersed energy correlated with the general pool of phenolic compounds. In studied controlled-environment production systems, these compounds probably acted as a photo-protective for the photosynthetic apparatus. In control plants, the parameters of PI, ψ Ro, and φ Ro strongly correlated with the content of chlorophyll index and with the fresh and dry weight of the aboveground parts of common buckwheat. This relationship was not found in plants growing under LED lamps. Under this treatment, PI, ψ Ro, and φ Ro were lower than the control, which could explain the lower FW and DW of aboveground parts of plants.
In our experiment, the plants grown in the greenhouse had a higher chlorophyll index. Chlorophyll biosynthesis requires light and its different qualities regulate the synthesis of photosynthetic pigments39,40. Blue light may promote the expression of enzymes that regulate the synthesis of chlorophyll, such as MgCH (magnesium chelatase), FeCH (ferrochelatase), and GluTR (glutamyl-tRNA reductase)40,41. However, red light reduces tetrapyrrole precursor 5-aminolevulinic acids, essential for chlorophyll biosynthesis40,42, which may explain the lower chlorophyll content in the plants exposed to LED light. In our study, red light seemed to be the more dominant factor regulating chlorophyll biosynthesis than lower light intensity. According to Tripathy and Brown43, red light at an intensity of 100 µmol m–2 s–1 stimulated chlorophyll synthesis, as opposed to a high intensity of 500 µmol m–2 s–1. In the case of buckwheat plants grown under LEDs with a 53% proportion of red light, i.e., 20% more than sunlight, we observed a significantly lower content of chlorophyll than in the plants grown in the daylight. According to Boardman44 and Lichtenthaler et al.45 leaves grown at low light intensity have more chlorophyll per unit weight, but lower content calculated per area of leaf surface compared to the leaves grown in the sunlight. In some plant species blue light may increase the value of the chlorophyll a/b ratio 21. In turn, Wang et al.46 stated that the combined Red and Blue LEDs increased chlorophyll content, photosynthesis rate, leaf number and area, and shoot mass. Muneer et al.47 showed that photosynthesis rate, stomatal conductance, and growth depended on Red light/Blue light ratio and these parameters increased with greater blue radiation. These authors stated that for photosynthesis rate and stomatal conductance in lettuce plants the most optimal is when the R/B ratio is 1. In the case of light in the phytotronic chamber, the R/B ratio was 2, in the greenhouse without HPS lamps (most of the day) it was also 2, and with HPS lamps R/B was 3.5. Therefore, the lower chlorophyll content in buckwheat leaves under LEDs may be the result of lower light intensity than a different light spectrum37. Although it cannot be excluded that a higher R/B ratio is more optimal for common buckwheat plants.
Landi et al.21 reported that typically monochromatic blue light does not have a significant effect on plant biomass compared to plants grown in multispectral light, but there are cases, where plants show an inhibitory effect of blue light on leaf growth, number, and size. However, we found significant differences between FW and DW of the aboveground parts with greater biomass for the plants grown under control conditions. Blue light is known to reduce elongation growth48–50, which depends on phytochrome activity affected by background light conditions51. It may explain why control plants were taller than those grown under LED spectra. Red light plays a significant role in shoot elongation and plant anatomy through phytochromes52,53. In common buckwheat growth, a dominant role of blue light was observed, which resulted in a smaller main shoot height in the plants grown under the LED spectrum. Also, the role of the green region of the spectrum, especially in photosynthesis in the deep layers of the mesophyll and the lower canopy levels, can be large enough. The green light is an influential wave band in supporting photosynthesis in higher plants, especially in leaves with higher chlorophyll index (Sun et al.54, Terashima55 and Ptushenko56), which occurred in our experiment in control plants.
Carbohydrates are products of photosynthesis that regulate the growth and development of leaves57. They participate in the synthesis of phenolic compounds that are activated in plant defense mechanisms during environmental stresses58. The quantitative and qualitative differences were found for individual soluble carbohydrates content. The monosaccharides (glucose and fructose) content in control plants was significantly higher than in the plants growing under LED. Under this treatment, performance index (PI) correlated with glucose, sucrose, and maltose. Monochromatic red light may induce the accumulation of carbohydrates in leaves due to the inhibition of the translocation of assimilates from source to sink tissue. On the second hand, red light induces the reduction of plant biomass and leaf area, provokes excessive stem elongation, and affects leaf number and chlorophyll content compared to plants grown in white light21. In turn, the photosynthetic rate in plants grown under blue LED light is similar to that in plants grown under white light and higher than in plants grown under red light21. In our opinion, the lower content of carbohydrates in common buckwheat plants under used LEDs was more related to lower photosynthetic activity than to the light spectrum. Biosynthesis of specified phenylpropanoids is activated during different stages of plant organogenesis in diverse tissues and responds to biotic and abiotic stress factors, e.g., UV-B irradiation9,59,60. Biosynthesis of phenylpropanoids requires an adequate flow of carbon via shikimate to the biosynthesis of aromatic amino acids: phenylalanine, tyrosine, and tryptophan. Aromatic amino acids serve as essential precursors to a wide range of secondary plant metabolites. The first step from primary into secondary metabolism is the phenylpropanoid pathway, an intermediate stage in specific branch pathways leading to targeted biosynthesis of, i.a., flavonoids9,59. Flavonoids may be included in colourful (flavonols, anthocyanins, and proanthocyanidins) and colourless compounds61. Colourless flavonoids, like simple phenolics, absorb mainly UV radiation in the range 335 nm for flavonoids and 280–315 nm for phenolic acids62. Some flavonoids absorb other wavelengths, for example, anthocyanins, which absorb the visible light of the solar spectrum63. Besides, some flavonoids show antioxidant properties, so they act as scavengers of reactive oxygen species64. Moreover, anthocyanins, by absorbing the fraction of yellow, green, and blue wavelengths, may significantly reduce the damage of PSII21. Phenolic compounds are known for their protective function against stress factors in harsh environmental conditions such as high temperature, salinity, heavy metal pollution, or ultraviolet radiation65. In the present experiment, total phenolic and flavonoid contents were significantly higher in the plants grown under light-emitting diodes than in control ones. The effect of monochromatic red and blue lights on secondary metabolites’ synthesis was studied in the case of Fagopyrum tataricum66,67. Blue light increased the content of anthocyanin, and the production of zeaxanthin, while reducing the content of total carotenoid compared to the treatment under white light. Lobiuc et al.68 reported that rosmarinic and gallic acids synthesis increased under LED blue light. Our results obtained for plants grown under LED light with 25% blue radiation may confirm it. Sytar et al.69 reported that direct sunlight with moderate temperature and high UV radiation increased the accumulation of total phenolics, flavonoids, and phenolic acids in lettuce leaves compared to the plants grown in the greenhouse with low UV radiation and high temperature. In our model of experiment, the temperature in the greenhouse was controlled with air-conditioning system and was comparable to the phytotronic chamber condition. We noticed the highest accumulation of total phenolics, flavonoids, and the majority of phenolic acids in buckwheat plants under LED light with the dominant role of red and blue radiation. Anthocyanin accumulation is induced by light and cytokinins21. The content of cytokinins in the shadow can be not sufficient to stimulate the accumulation of anthocyanins. In our experiment in phytotronic conditions with lower light intensity than in the greenhouse in the leaves of buckwheat, significantly higher phenolic accumulation was observed, and this was rather an effect of the light spectrum—mostly red and blue radiations, than light intensity. Blue and white light were observed to reinforce the production of phenolic acids7,70,71, which explains increased amounts of phenolic acids in the plants exposed to LEDs, with the more vigorous relative intensity of blue peak, than under control light conditions. Phenolic acids can be differentiated into two groups: derivatives of benzoic acid (hydroxybenzoic acids: benzoic, gallic, protocatechuic, p-hydroxybenzoic, vanillic, variations of dihydroxybenzoic acid) and derivatives of cinnamic acid (hydroxycinnamic acids: cinnamic, caffeic, p-coumaric, ferulic, sinapic)72. The amounts of the most studied benzoic acid derivatives increased in the plants grown under LEDs vs. control. Their accumulation, serving as a plant tolerance mechanism against abiotic stress, was stated in many plant species73. The genes encoding key enzymes, including HQT (hydroxycinnamoyl-CoA quinate hydroxycinnamoyl transferase) and PAL (phenylalanine ammonia-lyase) families, are upregulated under abiotic stresses, which boosts the biosynthesis of phenolic compounds, such as, e.g., chlorogenic acid. Finally, it may affect plant tolerance to the stress factor. Chlorogenic acid, an ester of trans-hydroxycinnamic acids and quinic acid, is involved in plant response to stress, e.g., pathogens and salinity74, and in our experiment, it was the most abundant acid with accumulation increasing more than two times in the leaves of plants grown under LED light vs. controls. The role of the specified light spectrum in photosynthetic efficiency and activity of biosynthetic pathways of valuable metabolites is essential for understanding the mechanisms of plant response to growth conditions, for example, in the greenhouse or phytotronic experiments.
Conclusions
We concluded that applied solo LED light in phytotronic conditions decreased the biomass growth of common buckwheat compared with solar light supplemented with HPS lamps. However, the used LEDs stimulated the production of phenolic compounds that are considered health-promoting due to their antioxidant properties.
Materials and methods
Plant material and growth conditions
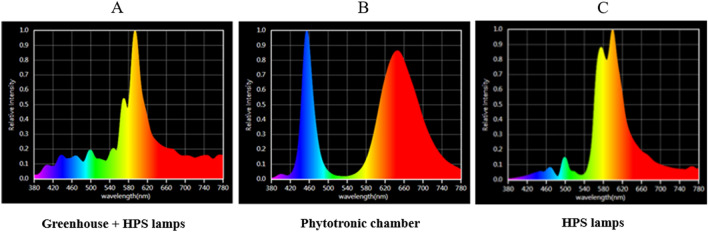
The seeds of F. esculentum Moench cv. 'Panda' were provided by breeders from Małopolska Plant Breeding in Polanowice, Poland. The experiments were carried out in plants grown under controlled conditions in a phytotron chamber and a greenhouse as a control. The plants were grown in pots (20 × 20 × 25 cm; five plants per pot; ten pots for each treatment) in commercial soil substrate (pH = 5.8) mixed 1:1 (v/v) with perlite. The plants were fertilized once a week with Hoagland medium75. The plants were cultivated for eight weeks in August and September 2020 at 25 + 2 °C/22 ± 2 °C day/night and 55–60% humidity. The greenhouse was located at latitude 50° 04′ 10.195″ N and longitude 19° 50′ 44.763″ E, the maximum sunlight intensity on a cloudless day was between 1300 and 1600 μmol (photons) m–2 s–1 of PPFD (photosynthetic photon flux density), the natural daylight lasted from 5:15 a.m. (sunrise) to 8:20 p.m. (sunset) at the beginning of cultivation and from 5.55 a.m. to 6.20 p.m. at the end of cultivation. In the greenhouse, plants were exposed to solar radiation supplemented with HPS lamps (Fig. 3). The HPS (AGRO Philips) lamps provided an additional 300 μmol (photons) m–2 s–1 of PPFD from 6:00 a.m. h to 10:00 p.m. under 16 h photoperiod. In the phytotronic chamber with LED light, plants were grown under a 16-h photoperiod with constant light intensity. In greenhouse conditions with daylight, in the period from August to the end of September, the daylight had to be supplemented with an additional light source to ensure the same photoperiod. HPS lamps were turned on in the morning so that the additional light source would not significantly increase the intensity of natural light. The greenhouse was air-conditioned preventing significant temperature fluctuations, which could occur on sunny days as well as under the influence of heat generated by HPS lamps. The used HPS lamps provided a golden yellow light and caused a color shift toward the yellow-orange end of the spectrum (Fig. 4C). The plants in the phytotron chamber were exposed to solo LED light (with blue, red, and addition of UV, green, yellow, orange, and far-red radiation, 200 W Full Spectrum LED Flood Light, Color Temp.: 380–840 nm) (Fig. 3 and 4B) and average 300 μmol (photons) m–2 s–1 of PPFD from 6:00 a.m. to 10:00 p.m. Composition of the solar light, and HPS lamps gave more green, yellow and orange waves than the LED spectrum (Fig. 3 and 4A). DLI (daily light integral) value was calculated to compare two studied sources of light: LED light in isolated chamber and greenhouse with daylight supplemented for 4 h with HPSs light. This parameter describes the number of photosynthetically active photons (photons in the PAR range) that are delivered to a specific area (1 m2) over a 24 h-period. DLI is calculated by measuring the photosynthetic photon flux density (PPFD) in μmol m−2 s−1 for a specific area as it changes throughout the day76–78. DLI = PPFD [μmol m−2 s−1] × (3600 × photoperiod)/1,000,000 [mol m−2 day−1]. The DLI value for the greenhouse with HPS was range from 10 mol m−2 day−1 during cloudy days to 38.5 mol m−2 day−1 in sunny days, while for the phytotronic chamber it was 23 mol m−2 day−1. The calculation suggests that in aproximately 9 h a day light under LEDs was lower than in the greenhouse.
Figure 3.
The percentage share of individual colours in the tested spectra. Greenhouse—solar radiation; Greenhouse + HPS—solar radiation supplemented with HPS lamps (High-Pressure Sodium); Phytotronic chamber—solo LED lamps (Light-Emitting diodes). Plants were grown in a greenhouse in the daylight (under 16-h photoperiod) supplemented with HPS lamps’ spectrum from 6.00 to 10.00 a.m., and in a phytotronic chamber with only LED spectrum. Greenhouse and Greenhouse + HPS were presented to demonstrate the influence of HPS spectrum on daylight.
Figure 4.
Light spectrum in a greenhouse under solar light supplemented with HPS Agro Phillips lamps (A; control), phytotron chamber with LED light (B), and the light spectrum emitted by solo HPS (C).
The light spectrum was recorded after germination at the top of 2-week-old seedlings by a spectrometer Lighting Passport Pro (Asensetek, Taiwan) with Spectrum Genius Cloud (Taiwan) software. The range of individual sub-regions of the visible light was selected according to Malacara79.
Plant growth measurements.
The measurements of the studied parameters were executed in the leaves (fully developed young leaves, third in the order from the top inflorescences) of 8-week-old plants at the stage of full flowering. Buckwheat blooming lasts throughout the entire vegetative phase. The measurements included shoot (whole aboveground parts), fresh weight (FW), and dry weight (DW), leaf area (LA), internode amount, and main stem height, and were done in ten replicates. Plant samples were transferred into a drying oven at 80 °C for 48 h to obtain dry weight. Every plant's LA (cm2) was measured with an LA meter (CI-202 Laser Area Meter, CID Bio-Science, WA, USA). The measurements were done for ten plants. The study was in compliance with relevant institutional, national, and international guidelines and legislation.
Chlorophyll a fluorescence (ChlF)
Before the measurements, the LED-light source of a fluorimeter was calibrated using an SQS light meter (Hansatech Ltd., King's Lynn, UK). Excitation irradiance intensity was 3000 [μmol m−2 s−1] (peak at 650 nm). Measurements were taken after 30 min of the leaf adaptation to darkness (clips with a 4 mm diameter hole). Changes in fluorescence were recorded during irradiation between 10 μs and 1 s. During the initial 2 ms, the data were collected every 10 μs with 12-bit resolution. After this period, the frequency of measurements was reduced automatically. The data were used to calculate the following parameters based on the theory of energy flow in PSII and the JIP test80,81: ABS/CSm—energy absorption by antennas, TRo/CSm—excitation energy trapped in PSII, ETo/CSm—the energy used for electron transport, DIo/CSm—energy dissipation from PSII, RC/CSm—number of active reaction centers, PI—performance index of PSII, ψ Ro—probability, at time 0, that a trapped exciton moves an electron into the electron transport chain beyond QA −, δ Ro—efficiency with which an electron can move from the reduced intersystem of electron acceptors to the PSI end electron acceptors, φ Ro—quantum yield of electron transport from QA− to the PSI end electron acceptors. The measurements included twenty plants per treatment.
Chlorophyll index
Leaf chlorophyll index was measured non-destructively with a hand-held chlorophyll meter (CL-01 Hansatech Instruments, King's Lynn, UK) in twenty repetitions for each treatment. We analyzed the third, fully developed leaf from the top inflorescence in eight-week-old plants. The measurements were done for twenty plants.
Biochemical analyses
Sample preparation for biochemical analyses
The samples involved fully developed young leaves, third in the order from the top inflorescences. The samples were collected, frozen in liquid nitrogen, and then lyophilized (LGA05, MLW, Leipzig, Germany, upgraded by JWE, Warsaw, Poland). Afterward, they were pulverized (MM400, Retsch, Haan, Germany), and the material was used for further analyses. Each sample for the biochemical analysis was 15 mg of DM.
Soluble sugar profile
Sugars were estimated using the method reported by Hura et al.82. The samples were extracted with ultra-pure water by shaking for 15 min at 30 Hz (MM 400, Retsch, Haan, Germany) and centrifuged for 5 min at 21,000×g (Universal 32R, Hettich, Germany). Then, the supernatant was collected, diluted with acetonitrile 1:1 (v/v), filtered (0.22 µm nylon membrane, Costar Spin-X, Corning, USA), and analyzed by high-performance liquid chromatography (HPLC) using an Agilent 1200 binary system (Agilent, Wolbrum, Germany) coupled with ESA Coulochem II electrochemical detector (ESA, Chelmsford, MA, USA). An RCX-10, 7 µm, 250 × 4.1 mm column (Hamilton, Reno, NV, USA) in a 75 mM NaOH solution gradient mode, and 500 mM sodium acetate in 75 mM NaOH solution at 1.5 ml/min, was used. Pulsed amperometric detection was employed on a gold electrode. Further technical details are given by Hura et al.82. The analysis was performed in three biological replicates.
Total phenolic content
The total soluble phenolic content was estimated according to the method reported by Singleton et al.83 with minor modifications. The extract was diluted in deionized water (0.5 cm3) with the addition of Folin–Ciocalteu reagent (0.2 cm3). After 10 min of incubation, saturated Na2CO3 (0.7 cm3) was added. Then, after a 2 h incubation, the samples were mixed and transferred into 96-well plates. The absorbance at 765 nm was read (Synergy II). Gallic acid was used as a standard. The analysis was carried out in three biological replicates.
Phenolic acid content
Phenolic acids were estimated according to Hura et al.82. The samples were extracted in an organic buffer (methanol/water/formic acid 15/4/1 /v/v/v). After evaporation under nitrogen stream (TurboVap LV), the residue was solubilized in 3% methanol in 1 M formic acid before clean-up on Discovery DPA-6S SPE cartridges (1 ml, 50 mg, Supelco). The eluate was evaporated under N2, reconstituted in 250 µl of methanol, and analyzed on Agilent Infinity 1260 UHPLC (Ultra-High-Performance Liquid Chromatography) with a fluorescence detector (FLD). The phenolic acids were separated on Zorbax Eclipse Plus Phenyl-Hexyl 3.5 µm 3.0 mm × 100 mm column (Agilent Technologies) under a linear gradient of 2% (v/v) formic acid aqueous solution versus methanol. Excitation and emission wavelengths were dynamically adjusted. Technical details are provided in Gołębiowska-Pikania et al.84. The analysis was done in three biological replicates.
Total flavonoid content
The analyses of total flavonoid content in the extracts were done according to Ramos et al.85, as reported by Klimek-Szczykutowicz et al.86. The samples were extracted with methanol, then 100 µl of the centrifuged extract were mixed with 40 µl of 10% AlCl3, filled with 5% acetic acid to a final volume of 1000 µl, and incubated for 20 min. Then the absorbance at 425 nm was read in 96-well plate format (Synergy II). Quercetin was used as a reference. The analysis was carried out in three biological replicates.
Statistical analysis
One-way analysis of variance (ANOVA) was performed using STATISTICA 13 package (Statsoft, Tulsa, OK, USA). Significance of differences between means obtained in the studied light conditions was marked with stars according to the Student's t test: *p < 0.05; **p < 0.01; ***p < 0.001. Values represent means ± SE (standard error). Pearson’s correlation coefficients were assumed as statistically significant at p < 0.05. The used parameters taken for correlation analysis referred to the same plants.
Supplementary Information
Author contributions
A.P. and M.H. conceived and designed the experiment; M.H., M.D., J. P., A.Sz., M.K. and M.Sz. performed the experiment and analyses; A.P., M.H., M.D. analyzed the data; M.H. and A.P. wrote the manuscript. All authors read the manuscript and accepted it.
Funding
This research was funded by the National Centre of Knowledge of Poland (No. 2017/25/B/NZ9/00148).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-04134-6.
References
- 1.Sullivan JA, Deng WX. From seed to seed: the role of photoreceptors in Arabidopsis development. Dev. Biol. 2003;260:289–297. doi: 10.1016/S0012-1606(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Chory J, Frankhauser C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004;38:87–117. doi: 10.1146/annual.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- 3.Bian Z, Jiang N, Grundy S, Lu C. Uncovering LED light effects on plant growth: New angles and perspectives—LED light for improving plant growth. Nutrition and energy-use efficiency. Acta Hortic. 2018;1227:491–498. doi: 10.17660/ActaHortic.2018.1227.62. [DOI] [Google Scholar]
- 4.Hytönen T, et al. Effects of LED light spectra on lettuce growth and nutritional composition. Light. Res. Technol. 2017 doi: 10.1177/1477153517701300. [DOI] [Google Scholar]
- 5.Kalaji HM, et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- 6.Wojciechowska R, Długosz-Grochowska O, Kołton A, Żupnik M. Effects of LED supplemental lightening on yield and some quality parameters of lab’s lettuce grown in two winter cycles. Sci. Hortic. 2015;187:80–86. doi: 10.1016/j.scienta.2015.03.006. [DOI] [Google Scholar]
- 7.Engelsma G. The mechanism of the changes in phenylalanine ammonia-lyase activity induced by ultraviolet and blue light in gherkin hypocotyls. Plant Physiol. 1974;54:702–705. doi: 10.1104/pp.54.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach A, Kapczyńska A, Dziurka K, Dziurka M. The importance of applied light quality on the process of shoot organogenesis and production of phenolics and carbohydrates in Lachenalia sp. cultures in vitro. S. Afr. J. Bot. 2018;114:14–19. doi: 10.1016/j.sajb.2017.10.015. [DOI] [Google Scholar]
- 9.Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghasemzadeh A, Ghasemzadeh N. Flavonoids, and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plant Res. 2011;5:6697–6703. doi: 10.5897/JMPR11.1404. [DOI] [Google Scholar]
- 11.Sami F, Yusuf M, Faizan M, Faraz A, Hayat S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016;109:54–61. doi: 10.1016/j.plaphy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R. Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 2018;11:1–10. doi: 10.1126/scisignal.aam9514. [DOI] [PubMed] [Google Scholar]
- 13.Lin, K. H. et al. The effects of red, blue and white light-emitting diodes (LEDs) on growth, development and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic.150, 86–91. 10.1016/j.scienta.2012.10.002 (2013).
- 14.Ouzounis, T., Parjilolaei, B. R., Frette, X., Rosenqvist, E. & Ottosen, C. O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front Plant Sci. 6, 19. 10.3389/fpls.2015.00019 (2015). [DOI] [PMC free article] [PubMed]
- 15.Ahmad SS, Tahir I. Regulatory role of phenols in flower development and senescence in the genus. Iris. Ind. J. Plant Physiol. 2016;22:135–140. doi: 10.1007/s40502-016-0278-4. [DOI] [Google Scholar]
- 16.Tanase, C., Bujor, O. C., Popa, V. I. Phenolic Natural Compounds and Their Influence on Physiological Processes in Plants, in Polyphenols in Plants. (2nd ed. Watson. R.R) 45–58. 10.1016/B978-0-12-813768-0.00003-7 (Cambridge, 2019).
- 17.Inoue M, Sakaguchi N, Isuzugawa K, Tani H, Ogihara Y. Role of reactive oxygen species in gallic acid-induced apoptosis. Biol. Pharm. Bull. 2000;23:1153–1157. doi: 10.1248/bpb.23.1153. [DOI] [PubMed] [Google Scholar]
- 18.Selka A, Ndongou Moutombi FJ, Cormier M, Touaibia M. Phenethyl esters and amide of ferulic acid, hydroferulic acid, homovanillic acid, and vanillic acid: Synthesis, free radicals scavenging activity, and molecular modeling as potential cholinesterases inhibitors. Molbank. 2020;2020:M1151. doi: 10.3390/M1151. [DOI] [Google Scholar]
- 19.Naveed M, et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 20.Ting, K. C., Mears, D. R. Overview of controlled environment plant production systems, in Proceedings of the International Symposium on Applied Technology of Greenhouse 117–114 (1991).
- 21.Landi M, Zivcak M, Sytar O, Brestic M, Allakhverdiev SI. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. BBA. 2020;1861:148131. doi: 10.1016/j.bbabio.2019.148131. [DOI] [PubMed] [Google Scholar]
- 22.Pinho P, et al. Evaluation of lettuce growth under multi-spectral-component supplemental solid state lighting in greenhouse environment. Int. Rev. Electr. Eng. 2007;2:854–860. [Google Scholar]
- 23.Randall WC, Lopez RG. Comparison of supplemental lighting from high-pressure sodium lamps and light emitting diodes during bedding plant seedling production. Hort. Sci. 2014;49:589–595. doi: 10.21273/HORTSCI.49.5.589. [DOI] [Google Scholar]
- 24.Hashimoto H, Uragami C, Cogdell RJ. Carotenoids and photosynthesis. Subcell. Biochem. 2016;79:111–139. doi: 10.1007/978-3-319-39126-7_4. [DOI] [PubMed] [Google Scholar]
- 25.Halbrecq, B., Rommedenne, P. & Ledent, J. F. Evolution of flowering. Ripening and seed set in buckwheat (Fagopyrum esculentum Moench.): Quantitative analysis. Europ. J. Agron. 23, 209–224. 10.1016/j.eja.2004.11.006 (2005).
- 26.Christa K, Soral-Śmietana M. Buckwheat grains and buckwheat products—nutritional and prophylactic value of their components—A review. Czech J. Food Sci. 2008;26:153–162. doi: 10.17221/1602-CJFS. [DOI] [Google Scholar]
- 27.Amelin AV, Fesenko AN, Chekalin EI, Fesenko IN, Zaikin VV. Higher yielding varieties of common buckwheat (Fagopyrum esculentum Moench) with determinate growth habit (single mutation det) manifest higher photosynthesis rate at stage of grain filling. Acta Agric. Slov. 2020;115:59–65. doi: 10.14720/aas.2020.115.1.1316. [DOI] [Google Scholar]
- 28.Hornyák M, et al. Photosynthetic activity of common buckwheat (Fagopyrum esculentum Moench) exposed to thermal stress. Photosynthetica. 2020;58:45–53. doi: 10.32615/ps.2019.140. [DOI] [Google Scholar]
- 29.Massa GD, Kim HH, Wheeler RM. Plant productivity in response to LED lighting. Hort. Sci. 2008;43:1951–1956. doi: 10.21273/HORTSCI.43.7.1951. [DOI] [Google Scholar]
- 30.Barber J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009;38:185–196. doi: 10.1039/B802262N. [DOI] [PubMed] [Google Scholar]
- 31.Kusuma P, Pattison PM, Bugbee B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020;7:56. doi: 10.1038/s41438-020-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojciechowska R, Kołton A, Długosz-Grochowska O, Żupnik M, Grzesiak M. The effect of LED lighting on photosynthetic parameters and weight of lamb's lettuce (Valerianella locusta) Fol. Horticult. 2013;25:41–47. doi: 10.2478/fhort-2013-0005. [DOI] [Google Scholar]
- 33.Hamdani S, Khan N, Perveen S, Qu M, Jiang J, Zhu XG. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019;139:107–121. doi: 10.1016/j.bbabio.2019.148131. [DOI] [PubMed] [Google Scholar]
- 34.Su N, Wu Q, Shen Z, Xia K, Cui J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 2014;73:227–235. doi: 10.1007/s10725-013-9883-7. [DOI] [Google Scholar]
- 35.Hattori T, Sonobe K, Inanaga S, An P, Tsuji W, Araki H, Eneji AE, Morita S. Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environ. Exp. Bot. 2007;60:177–182. doi: 10.1016/j.envexpbot.2006.10.004. [DOI] [Google Scholar]
- 36.Samoilova OP, Ptushenko VV, Kuvykin IV, Kiselev SA, Ptushenko OS, Tikhonov AN. Effects of light environment on the induction of chlorophyll fluorescence in leaves: A comparative study of Tradescantia species of different ecotypes. BioSystems. 2011;105(41–48):2011. doi: 10.1016/j.biosystems.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Zivcak M, Brestic M, Kalaji HM. Photosynthetic responses of sun-and shade-grown barley leaves to high light: is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014;119:339–354. doi: 10.1007/s11120-014-9969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazár D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct. Plant Biol. 2006;33:9–30. doi: 10.1071/FP05095. [DOI] [PubMed] [Google Scholar]
- 39.Jilani A, Kar S, Bose S, Tripathy BC. Regulation of the carotenoid content and chloroplast development by levulinic acid. Physiol. Plant. 1996;96:139–145. doi: 10.1111/j.1399-3054.1996.tb00194.x. [DOI] [Google Scholar]
- 40.Fan XX, et al. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013;153:50–55. doi: 10.1016/j.scienta.2013.01.017. [DOI] [Google Scholar]
- 41.Wang H, Gu M, Cui J, et al. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photoch. Photobio. B. 2009;96:30–37. doi: 10.1016/j.jphotobiol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Sood S, Gupta V, Tripathy BC. Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol. Biol. 2005;59:269–287. doi: 10.1007/s11103-005-8880-2. [DOI] [PubMed] [Google Scholar]
- 43.Tripathy BC, Brown CS. Root–shoot interaction in the greening of wheat seedlings grown under red light. Plant Physiol. 1995;107:407–411. doi: 10.1104/pp.107.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boardman NT. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977;28:355–377. doi: 10.1146/annurev.pp.28.060177.002035. [DOI] [Google Scholar]
- 45.Lichtenthaler HK, et al. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981;2:115–141. doi: 10.1007/BF00028752. [DOI] [PubMed] [Google Scholar]
- 46.Wang, J., Lu, W., Tong, Y., & Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci.7, 250.10.3389/fpls.2016.00250 (2016). [DOI] [PMC free article] [PubMed]
- 47.Muneer, S., Kim, E. J., Park, J. S., & Lee, J. H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci.15, 4657–4670. 10.3390/ijms15034657 (2014). [DOI] [PMC free article] [PubMed]
- 48.Hoenecke ME, Bula RJ, Tibbitts TW. Importance of ‘blue’ photon levels for lettuce seedlings grown under red-light-emitting diodes. Hort. Sci. 1992;27:427–430. doi: 10.21273/HORTSCI.27.5.427. [DOI] [PubMed] [Google Scholar]
- 49.Tripathy BC, Brown CS. Root-shoot interaction in the greening of wheat seedlings grown under red light. Plant Physiol. 1995;107:407–411. doi: 10.1104/pp.107.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernández R, Kubota C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016;121:66–74. doi: 10.1016/j.envexpbot.2015.04.001. [DOI] [Google Scholar]
- 51.Kong Y, Stasiak M, Dixon MA, Zheng Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018;155:345–359. doi: 10.1016/j.envexpbot.2018.07.021. [DOI] [Google Scholar]
- 52.Schuerger, A. C., Brown, C. S., Stryjewski, E. C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 79, 273–282. 10.1006/anbo.1996.0341 (1997). [DOI] [PubMed]
- 53.He J, Qin L, Chong ELC, Chong TW, Lee SK. Plant growth and photosynthetic characteristics of Mesembryantheum crystallinum grown aeroponically under different blue- and red-LEDs. Front. Plant. Sci. 2017;8:361. doi: 10.3389/fpls.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J, Nishio JN, Vogelmann TC. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998;39:1020–1026. doi: 10.1093/oxfordjournals.pcp.a029298. [DOI] [Google Scholar]
- 55.Terashima I, et al. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009;50:684–697. doi: 10.1093/pcp/pcp034. [DOI] [PubMed] [Google Scholar]
- 56.Ptushenko VV, et al. Possible reasons of a decline in growth of Chinese cabbage under a combined narrowband red and blue light in comparison with illumination by high-pressure sodium lamp. Sci. Hortic. 2015;194:267–277. doi: 10.1016/j.scienta.2015.08.021. [DOI] [Google Scholar]
- 57.Paul MJ, Pellny TK. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003;54:539–547. doi: 10.1093/jxb/erg052. [DOI] [PubMed] [Google Scholar]
- 58.Arnold TM, et al. Carbohydrate translocation determines the phenolic content of Populus foliage: A test of the sink-source model of plant defense. New. Phytol. 2004;164:157–164. doi: 10.1111/j.1469-8137.2004.01157.x. [DOI] [PubMed] [Google Scholar]
- 59.Douglas CJ. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996;1:171–178. doi: 10.1016/1360-1385(96)10019-4. [DOI] [Google Scholar]
- 60.Wang J, Yuan B, Huang B. Differential heat-induced changes in phenolic acids associated with genotypic variations in heat tolerance for hard fescue. Crop Sci. 2019;59:667–674. doi: 10.2135/cropsci2018.01.0063. [DOI] [Google Scholar]
- 61.Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 62.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 63.Landi M, Tattini M, Gould KS. Multiple functional roles of anthocyanins in plant–environment interactions. Environ. Exp. Bot. 2015;119:4–17. doi: 10.1016/j.envexpbot.2015.05.012. [DOI] [Google Scholar]
- 64.Ferreyra, M. L. F., Serra, P., & Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant.173, 736–749. 10.1111/ppl.13543 (2021) [DOI] [PubMed]
- 65.Sharma A, et al. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24:2452. doi: 10.3390/molecules24132452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuan, P. A. et al. Effects of white, blue, and red light-emitting diodes on carotenoid biosynthetic gene expression levels and carotenoid accumulation in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). J. Agric. Food Chem. 61, 12356–12361. 10.1021/jf4039937 (2013). [DOI] [PubMed]
- 67.Thwe AA, et al. Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in Fagopyrum tataricum sprouts. J. Agric. Food Chem. 2014;62:4839–4845. doi: 10.1021/jf501335q. [DOI] [PubMed] [Google Scholar]
- 68.Lobiuc, A. et al. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules22, 2111. 10.3390/molecules22122111 (2017). [DOI] [PMC free article] [PubMed]
- 69.Sytar O, et al. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018;239:193–204. doi: 10.1016/j.scienta.2018.05.020. [DOI] [Google Scholar]
- 70.Szopa, A., Ekiert, H., Szewczyk, A. & Fugas, E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Rutagra veolens ssp. divaricata (Tenore) Gams, under different light conditions. Plant Cell Tissue Organ Cult.110, 329–336. 10.1007/s11240-012-0154-5 (2012).
- 71.Długosz-Grochowska O, Kołton A, Wojciechowska R. Modifying folate and polyphenol concentrations in Lamb's lettuce by the use of LED supplemental lighting during cultivation in greenhouses. J. Funct. Foods. 2016;26:228–237. doi: 10.1016/j.jff.2016.07.020. [DOI] [Google Scholar]
- 72.Goleniowski, M. E., Bonfill, M., Cusido, R. & Palazon, J. Phenolic acids, in Natural Products. (ed. Ramawat, K.; Mérillon, J.M.) 1951–1973. 10.1007/978-3-642-22144-6_64 (Springer, 2013).
- 73.Mahdavi V, Farimani MM, Fathi F, Ghassempour A. A targeted metabolomics approach toward understanding metabolic variations in rice under pesticide stress. Anal. Biochem. 2015;478:65–72. doi: 10.1016/j.ab.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 74.Yan, K., Cui, M., Zhao, S., Chen, X. & Tang, X. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonicaThunb.). Front Plant Sci.18, 1563. 10.3389/fpls.2016.01563 (2016). [DOI] [PMC free article] [PubMed]
- 75.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif. Agr. Exp. Sta. Cir. 1950;347:32. [Google Scholar]
- 76.Bula RJ, et al. Light-emitting diodes as a radiation source for plants. Hort Sci. 1991;26:203–205. doi: 10.21273/HORTSCI.26.2.203. [DOI] [PubMed] [Google Scholar]
- 77.Korczynski PC, Logan J, Faust JE. Mapping monthly distribution of daily light integrals across the contiguous United States. HortTechnology. 2002;12:12–16. doi: 10.21273/HORTTECH.12.1.12. [DOI] [Google Scholar]
- 78.Faust JE, Holcombe V, Rajapakse NC, Layne DR. The effect of daily light integral on bedding plant growth and flowering. HortScience. 2005;40:645–649. doi: 10.21273/HORTSCI.40.3.645. [DOI] [Google Scholar]
- 79.Malacara, D. Color Vision and Calorimetry: Theory and Applications, 2nd ed. 10.1117/3.881172 (SPIE Press, 2011).
- 80.Lazár D. Chlorophyll a fluorescence induction. BBA. 1999;1412:1–28. doi: 10.1016/s0005-2728(99)00047-x. [DOI] [PubMed] [Google Scholar]
- 81.Strasser, R. J., Srivatava, A., Tsimilli-Michael, M. The fluorescence as tool to characterize and screen photosynthetics samples. (ed. Yunus M., Pathre U., Mohanty P.) 45–483 (Bristol, 2000).
- 82.Hura T, Dziurka M, Hura K, Ostrowska A, Dziurka K. Different allocation of carbohydrates and phenolics in dehydrated leaves of triticale. J. Plant Physiol. 2016;202:1–9. doi: 10.1016/j.jplph.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 83.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 84.Gołębiowska-Pikania G, et al. Changes in phenolic acid abundance involved in low temperature and Microdochium nivale (Samuels and Hallett) cross tolerance in winter triticale (×Triticosecale Wittmack) Acta Physiol. Plant. 2019;41:38. doi: 10.1007/s11738-019-2823-z. [DOI] [Google Scholar]
- 85.Ramos RTM, Bezerra ICF, Ferreira MRA, Soares LAL. Spectrophotometric quantification of flavonoids in herbal material, crude extract, and fractions from leaves of Eugenia uniflora Linn. Pharmacognosy Res. 2017;9:253–260. doi: 10.4103/pr.pr_143_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klimek-Szczykutowicz M, et al. Phytochemical and biological activity studies on Nasturtium officinale (watercress) microshoot cultures grown in RITA® temporary immersion systems. Molecules. 2020;25:5257. doi: 10.3390/molecules25225257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.