Abstract
Using high-depth whole genome sequencing of F0 mating pairs and multiple individual F1 offspring, we estimated the nuclear mutation rate per generation in the malaria vectors Anopheles coluzzii and Anopheles stephensi by detecting de novo genetic mutations. A purpose-built computer program was employed to filter actual mutations from a deep background of superficially similar artifacts resulting from read misalignment. Performance of filtering parameters was determined using software-simulated mutations, and the resulting estimate of false negative rate was used to correct final mutation rate estimates. Spontaneous mutation rates by base substitution were estimated at 1.00 × 10−9 (95% confidence interval, 2.06 × 10−10—2.91 × 10−9) and 1.36 × 10−9 (95% confidence interval, 4.42 × 10−10—3.18 × 10−9) per site per generation in A. coluzzii and A. stephensi respectively. Although similar studies have been performed on other insect species including dipterans, this is the first study to empirically measure mutation rates in the important genus Anopheles, and thus provides an estimate of µ that will be of utility for comparative evolutionary genomics, as well as for population genetic analysis of malaria vector mosquito species.
Subject terms: Evolutionary genetics, Evolutionary biology, Mutation, Sequencing, Entomology
Introduction
The process of evolution depends on the occurrence of new mutations which provide genetic variation and influence phenotypic traits1. The rate of de novo mutations is a key determinant of the rate of evolution of an organism under a molecular clock model2. DNA repair mechanisms normally ensure that genetic material is copied with fidelity during meiosis and transferred from one generation to the next3. However, at some small frequency, the transmission of damaged and improperly repaired DNA, or of DNA with replication errors, to the next generation causes a germline mutation in the offspring, which may then be subject to natural selection4. The precise estimation by empirical methods of the de novo germline mutation rate in multicellular organisms with large genome sizes has remained a great challenge even with the advent of next-generation DNA sequencing technologies because of inherent limitations, biases, and errors5.
Previous studies have been conducted to estimate the mutation rate per generation in organisms ranging from prokaryotes to eukaryotes including invertebrates and vertebrates6. The rate of the mutation per site per generation in vertebrates was estimated as 4.6 × 10–9 in a bird species (the flycatcher Ficedula albicollis)7, 4.5 × 10–9 in wolves (Canis lupus)8, and 5.4 × 10−9 in mouse (Mus musculus)9. The spontaneous mutation per base pair per generation for African green monkeys (Chlorocebus sabaeus)10 was estimated at a rate of 0.94 × 10–8 which is slightly lower than the rate of 1.2 × 10–8 per base pair per generation that was reported in chimpanzees (Pan troglodytes)11. The mutation rate per base per generation estimated in humans was 1.2 × 10−8 in two separate studies12,13 and was associated with a higher mutation rate in exons compared to introns14. Recent studies reveal that many factors may affect mutations rates, including genomic heterogeneity, population differences, and both cis- and trans-acting factors that influence mutagenic processes15–17.
In invertebrate species, Keightley et al. estimated a similar mutation rate per generation between two insect species in consecutive studies: Drosophila melanogaster18 at 2.8 × 10−9 and Heliconius melpomene19 at 2.9 × 10−9. Likewise, estimated mutation rates in two bee species were very close. Honeybee (Apis mellifera)20 was estimated at 3.4 × 10−9 and bumblebee (Bombus terrestris)21 at 3.6 × 10−9 per haploid genome, which is approximately 20–24% higher than H. melpomene and D. melanogaster. The rates for the two bee species were nearly identical despite a ~ 4-fold difference in recombination rate. Oppold and Pfenninger22 presented a mutation rate per generation for a non-biting midge, Chrironomus riparius, at the lower range of other insect rates (2.1 × 10−9). These reports in insects laid the groundwork for our study of mosquitoes reported here.
Female mosquitoes of the genus Anopheles are well known as malaria vectors, but not all species within the genus are efficient transmitters of human malaria. Anopheles coluzzii and Anopheles stephensi are two of the most intensively studied mosquito species. This focus is because they are the main human malaria vectors, the former in Africa and the latter in Asia, mainly in the Indian subcontinent region and Middle East23,24. The infected female Anopheles mosquito is responsible for transmitting the malaria parasites (Plasmodium species) to people through their bites25. Characteristically, Anopheles mosquitoes mate in flight within a swarm where groups of male mosquitoes gather and attract virgin females at dusk. Typically, a single male mosquito copulates with each female26,27. The karyotype of anopheline mosquitos is comprised of one pair of sex chromosomes (XX for female and XY for male) and two pairs of submetacentric autosomes. The longer arm is designated the right arm and the autosomal compliment is typically designated R and L thus yielding 2R, 2L and 3R, 3L28.
This study obtained mutation rate estimations for A. coluzzii, an Afrotropical malaria mosquito belonging to the Anopheles gambiae species complex29, and A. stephensi, an urban malaria vector widespread in Asia24 and recently emerging as a major malaria vector, with life-threatening potential, in east Africa30,31. The A. gambiae complex is comprised of at least nine closely related, homomorphic sibling species, varying in their geographical distributions32,33. Some act as dominant vectors of malaria, some as secondary vectors34, and others are non-vector species23. Like other sibling species of this group, A. coluzzii, plays a crucial role in human malaria transmission in west and central Africa35,36 and its genome has been intensively studied37.
Several approaches have been applied for the estimation of mutation rates in eukaryotes, including indirect methods that utilize established principles of population genetic theory to infer and extrapolate from polymorphisms detected in sequenced genomic subregions38–40. The advent of next-generation sequencing (NGS) established a new modality for the identification of de novo mutations by applying whole-genome sequencing to a pedigree, followed by a site-by-site genomic comparison of parents and offspring8,20,21. The observation of de novo mutations from high depth genome sequencing of parents and offspring is a conceptually simple and effective approach, although it is complicated by errors intrinsic to the nature of the NGS data and its analysis41,42. Here, we adopted an insect-favorable method for direct detection of mutations and estimation of the base substitution rate per generation using parent-offspring, multi-fold genome sequencing as initially proposed by Keightley et al.18. This is an effective approach of direct estimation by categorizing a large set of offspring into two classes, focal and bait, to minimize false positive mutations from the variant call set19.
In the current study, we sequenced the genomes of parents and numerous offspring of the malaria mosquitos A. coluzzii and A. stephensi. Variant calls generated from the sequence dataset for each species were then examined for evidence of point mutations by applying a series of filtering criteria to exclude false positives. Estimation of de novo mutation rate per generation in two Anopheles species can illustrate variation in mutation rates across species in this important group of mosquitos and can offer an open path for the interpretation of the molecular clock and demographic history.
Results
Successful mating and offspring generation was obtained with a female:male ratio of 1:4 for Anopheles coluzzii, and 1:2 for A. stephensi. Fatherhood was determined using whole genome sequencing information obtained for each potential male parent. Parental and focal offspring genomes were sequenced at a high coverage (≥ 20), whereas “bait offspring” were sequenced at lower coverage (≤ 20) (Supplementary Tables 1 and 2). One A. coluzzii offspring sample (Ac-F1-F13) was removed from analysis due to low yield and poor mappability (< 2%). We obtained 10 focal and 19 bait offspring for A. coluzzii and 13 focal and 17 bait offspring for A. stephensi (Table 1). Mean fold genome coverage for focal offspring ranged from 22.1 to 33.1 for A. coluzzii and from 30.2 to 46.1 for A. stephensi. Mean fold genome coverage ranges for the bait offspring were from 8.9 to 18.0 for A. coluzzii and from 15.1 to 20.1 for A. stephensi. Parents of both Anopheles species achieved mean genome coverage of ~ 27 times or higher, except the female parent of A. coluzzii which had a comparatively lower coverage mean of 20.6 (Table 1; Supplementary Tables 1 and 2).
Table 1.
Whole genome sequencing coverage results for each sample categorization.
| Species | Sample type | N | Coverage | |||
|---|---|---|---|---|---|---|
| 25th | Median | 75th | Mean | |||
| Anopheles coluzzii | Female parent | 1 | 16 | 21 | 25 | 20.4 |
| Putative male parent | 4 | 24 | 31 | 40 | 32.4 | |
| Focal offspring | 10 | 22.1 | 26 | 32 | 25.7 | |
| Bait offspring | 19 | 8.9 | 13 | 17 | 13.1 | |
| Anopheles stephensi | Female parent | 1 | 30 | 36 | 41 | 35.7 |
| Putative male parent | 2 | 22 | 28 | 34 | 28.2 | |
| Focal offspring | 13 | 27 | 32 | 38 | 32.2 | |
| Bait offspring | 17 | 11 | 14 | 17 | 14.4 | |
Number of samples (N) and whole genome coverage results for parents and offspring types (focal and bait) for A. coluzzii and A. stephensi.
25th 25th percentile, 75th 75th percentile.
Male parent identification
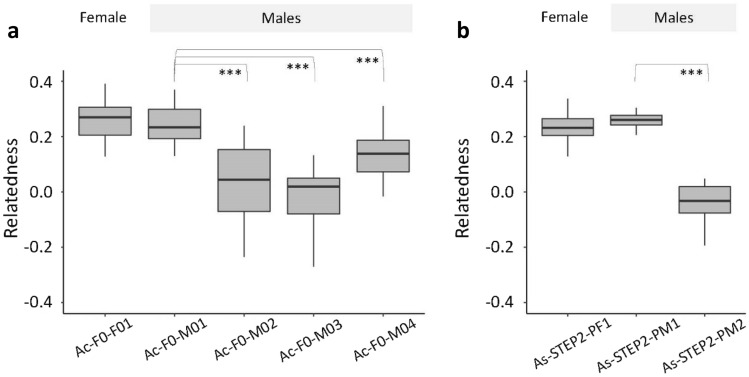
After joint variant calling for each species, true fathers were identified by relatedness43 and Mendelian inheritance-based analysis, both using only autosomal, biallelic SNPs from all offspring. For A. coluzzii, male Ac-F0-M01 presented a significantly higher relatedness with offspring specimens than any other male (p-value < 0.0001; Fig. 1a). The same male sample showed the highest proportion of observed heterozygous genotypes among all offspring based on expected heterozygosity, i.e., when both parents are homozygous for different alleles (Table 2). For A. stephensi, male As19-STE2-PM1 was closely related to the offspring by both methods: relatedness test (p-value < 0.0001; Fig. 1b) and Mendelian analysis (Table 2).
Figure 1.
Male parent identification of A. coluzzii and A. stephensi offspring. Boxplots of relatedness values between each potential male parent and all offspring. Both species had a unique female parent and 4 potential male parents for A. coluzzii and 2 for A. stephensi. ***p-value < 0.0001; Wilcoxon rank-sum test.
Table 2.
Mendelian inheritance for male parent identification.
| Species | Parent pair (female, male) | Homozygous for different allele | Number of sites | Heterozygous genotypes in all offspring (percentage) |
|---|---|---|---|---|
| Anopheles coluzzii |
Ac-F0-F01 Ac-F0-M01 |
Ref × Alt Alt × Ref |
9038 8227 |
6962 (77.03%) 6584 (80.03%) |
|
Ac-F0-F01 Ac-F0-M02 |
Ref × Alt Alt × Ref |
40,095 39,555 |
8 (0.02%) 2 (0.01%) |
|
|
Ac-F0-F01 Ac-F0-M03 |
Ref × Alt Alt × Ref |
33,316 28,923 |
3 (0.01%) 15 (0.05%) |
|
|
Ac-F0-F01 Ac-F0-M04 |
Ref × Alt Alt × Ref |
18,591 14,986 |
5 (0.03%) 3 (0.02%) |
|
| Anopheles stephensi |
As19-STE2-PF1 As19-STE2-PM1 |
Ref × Alt Alt × Ref |
19,740 29,616 |
16,523 (83.70%) 25,100 (84.75%) |
|
As19-STE2-PF1 As19-STE2-PM2 |
Ref × Alt Alt × Ref |
30,949 43,070 |
44 (0.14%) 42 (0.10%) |
The Mendelian inheritance-based approach for paternity analysis reveals the number of sites at which all offspring are heterozygous for different pairs of parents where parents are homozygous for different alleles.
Identification of candidate de novo mutations
The whole-genome variant calling algorithm returned a variant call format file (VCF) for each species. 6,150,332 variants were called for A. coluzzii, and 2,556,842 for A. stephensi. After filtering for biallelic SNPs, a total of 5,141,452 variant sites were found in A. coluzzii and 2,189,630 in A. stephensi (Table 3). These were the set of biallelic variant sites used for identification of candidate de novo mutations using a program in Perl that filters each criteria step. The first criterion applied was calling both parents as homozygous for the reference allele with a minimum read depth of 10, which yielded a total of 298,527 and 45,338 variant sites in A. coluzzii and A. stephensi, respectively (Table 3). Next, variant sites with alternative alleles that were either in the bait offspring or not called in every focal offspring were filtered out, resulting in 256,261 sites for A. coluzzii, and 33,203 for A. stephensi. From those results, homozygous sites were filtered out leaving 170 heterozygous sites in one or two focal offspring in A. coluzzii, and 46 heterozygous sites in A. stephensi. Finally, in A. coluzzii, 8 autosomal sites were identified as candidate mutations based on their proportion of reference and alternative allele reads, with an average read depth of 33.8 for the parents and 28.3 for all focal offspring (Supplementary Table 3). In A. stephensi, 4 candidate mutations were detected on autosomes and two on the X chromosome with an overall average read depth of 30.1 for the parents and 24.7 for all focal offspring (Supplementary Table 4).
Table 3.
Candidate mutation detection summary.
| Filtering site steps | Anopheles coluzzii | Anopheles stephensi |
|---|---|---|
| Total variants | 6,150,332 | 2,556,842 |
| Biallelic SNPs | 5,141,452 (83.6%) | 2,189,630 (85.6%) |
| Reference homozygous sites DP ≥ 10 | 298,527 (4.9%) | 45,338 (1.8%) |
| Sites without alternate allele in bait offspring | 277,677 (4.5%) | 35,959 (1.4%) |
| Sites called in every focal offspring | 256,261 (4.2%) | 33,203 (1.3%) |
| One or two heterozygous allele in focal | 170 | 49 |
| 27% < AAF < 73% on a heterozygous site | 8 | 6 |
The number of sites filtered out at each step of the candidate mutation detection protocol.
DP depth, AAF alternate allele frequency.
Manual curation and validation by Sanger sequencing
After examination on IGV, 9 out of 14 detected candidate mutations were forwarded for Sanger sequencing confirmation: 4 for A. coluzzii and 5 for A. stephensi (Table 4). Acceptable candidate mutations were found in the heterozygous condition in a single focal offspring and were entirely absent in reads from the parents. Those candidates that were rejected displayed clear evidence of being artifacts of read mis-mapping. Mis-mapping was typically in the form of nearby variants on the same reads that segregated identically with the candidate SNP (Supplementary Figs. 1 and 2). Sanger sequencing for the parent–offspring trio confirmed 3 candidates for A. coluzzii and all 5 in A. stephensi. Confirmed mutations were all found in non-coding regions. Half of them were transitions and the other half transversions.
Table 4.
Candidate de novo mutations detected in A. coluzzii and A. stephensi by whole genome sequencing and validated by Sanger re-sequencing.
| Chromosome, position | Ref | Alt | Focal offspring | Allele depth | Mean depth | Confirmed | Location | ||
|---|---|---|---|---|---|---|---|---|---|
| Ref | Alt | Parents | Focal | ||||||
| Anopheles coluzzii | |||||||||
| 2L, 21501855 | A | G | Ac-F1-M01 | 16 | 6 | 35 | 28.1 | No | – |
| 3L, 10978191 | G | A | Ac-F1-M04 | 8 | 9 | 31.5 | 26.9 | Yes | Intron |
| 3L, 30182152 | A | C | Ac-F1-M05 | 15 | 8 | 31.5 | 28.2 | Yes | Intergenic |
| 3L, 32059779 | C | T | Ac-F1-F09 | 11 | 7 | 26.5 | 17.8 | Yes | Intergenic |
| Anopheles stephensi | |||||||||
| X, 1692026 | C | T | As19-STE2-F23 | 18 | 8 | 22 | 18.6 | Yes | Intergenic |
| X, 14920753 | C | T | As19-STE2-M15 | 0 | 12 | 27 | 17.8 | Yes | Intron |
| 2, 29186821 | T | A | As19-STE2-M09 | 15 | 19 | 39.5 | 30.8 | Yes | Intergenic |
| 3, 6181251 | G | C | As19-STE2-M02 | 13 | 14 | 26.5 | 26.3 | Yes | Intron |
| 3, 56020205 | G | T | As19-STE2-F14 | 10 | 9 | 35.5 | 27.2 | Yes | Intergenic |
Genomic position, reference (Ref.) and alternate (Alt.) alleles in focal offspring and allele depth. Read depth mean for parents and focal offspring. All candidates were re-sequenced by Sanger sequencing for validation.
Synthetic mutations analysis
We generated synthetic mutations to estimate the false negative rate (FNR) of our mutation detection protocol for each species (see “Methods”). In total, 1000 synthetic mutations were inserted in each of the focal offspring (A. coluzzii—10 individuals; A. stephensi—13 individuals) across randomly selected autosomal sites where both parents achieved a read depth of 10 or higher. For A. coluzzii, of the 10,000 synthetic mutations introduced, 9914 had a read depth of 10 or higher for focal offspring, and therefore these were considered for FNR calculation. Ultimately, our de novo mutation detection program detected 7,658 synthetic mutations that passed all filters. This implies a FNR of 22.8% for the A. coluzzii analysis (2,256 synthetic mutations were not detected out of 9,914 inserted in callable sites; Supplementary Table 6). In A. stephensi, 12,724 out of 13,000 synthetic mutations were callable, of which 9563 were detected. Therefore, this species presented a FNR of 24.8% (Supplementary Table 6).
Mutation rate calculation
Our direct sequencing of the offspring of a single mating confined the measurement to a single generation and the calculation was resolved to a simple ratio of mutations per diploid genome corrected for false negatives. We confirmed three de novo mutations in 3.89 × 109 callable sites for A. coluzzii and five in 4.88 × 109 sites for A. stephensi. Estimated mutation rates for each species were 1.00 × 10−9 per site per generation (95% confidential interval, 2.06 × 10−10–2.91 × 10−9) for A. coluzzii and 1.36 × 10−9 per site per generation (95% confidential interval, 4.42 × 10−10—3.18 × 10−9) for A. stephensi (Supplementary Table 6).
Discussion
All eight mutations detected occurred in non-coding regions with equal numbers of transitions and transversions. Five out of the eight de novo mutations detected occurred in male offspring. Male mutation bias in eukaryotes is well known14,44,45. Sanger sequencing validation identified only a single false positive mutation in A. coluzzii among all the de novo mutations detected by our bioinformatics pipeline. The minimal false-positive rates supported previous studies which showed that higher sequencing depth in multiple samples reduced the false positive rate. These previous studies reveal that 30× and higher read depth data in multiple samples gives 99% genotype accuracy, but lowering read depth increases false-positive genotypes due to mis-mapping and paralogous reads18,46,47.
Like reports in bees and insects18–21, estimated mutation rates in A. coluzzii and A. stephensi were similar to each other. Overall, data for mutation rates in bees, flies, and mosquitos confirm that mutation rates are very close among species within the class Insecta, whereas values among mammal species differ by nearly an order of magnitude (Table 5). Interestingly, genome sizes among these eukaryotic species are not similar (Table 5), supporting the suggestion that genome size does not significantly influence mutation events in eukaryotes7,19,48. Conversely, there is a negative correlation (R2 = 0.5953) between mutation rate and generation time (Fig. 2). Species with long generation times (i.e., few generations per year) tend to have high mutation rates and vice versa, a trend that is apparent across distant taxa from insects to mammals. This might be a mechanism to balance mutation load over evolutionary timescales49. The mutation rate of a species is a vital parameter applied in evolutionary and population genetics, but estimating mutation rates is a formidable challenge48.
Table 5.
Available direct estimates of mutation rate in vertebrates and invertebrates.
| Species | Mutation rate | Genome size (Mb) | References |
|---|---|---|---|
| Vertebrates | |||
| Homo sapiens | 1.1 × 10–8 | 3232 | 41 |
| Pan troglodytes | 1.2 × 10–8 | 3309 | 11 |
| Chlorocebus aethiops | 0.94 × 10–8 | 2797 | 10 |
| Gorilla gorilla | 1.22 × 10–8 | 3084 | 76 |
| Canis lupus | 4.5 × 10–9 | 2350 | 8 |
| Mus musculus | 5.4 × 10–9 | 2671 | 9 |
| Ficedula albicollis | 4.6 × 10–9 | 1118 | 7 |
| Invertebrates | |||
| Apis mellifera | 3.4 × 10–9 | 247 | 20 |
| Bombus terrestris | 3.6 × 10–9 | 433 | 21 |
| Drosophila melanogaster | 2.8 × 10–9 | 148 | 18 |
| Heliconius melpomene | 2.9 × 10–9 | 269 | 19 |
| Chironomus riparius | 2.1 × 10–9 | 210 | 22 |
| Anopheles coluzzii | 1.0 × 10–9 | 280 | This study |
| Anopheles stephensi | 1.4 × 10–9 | 240 | This study |
Mutation rate and genome size of different species of vertebrates and invertebrates.
Figure 2.
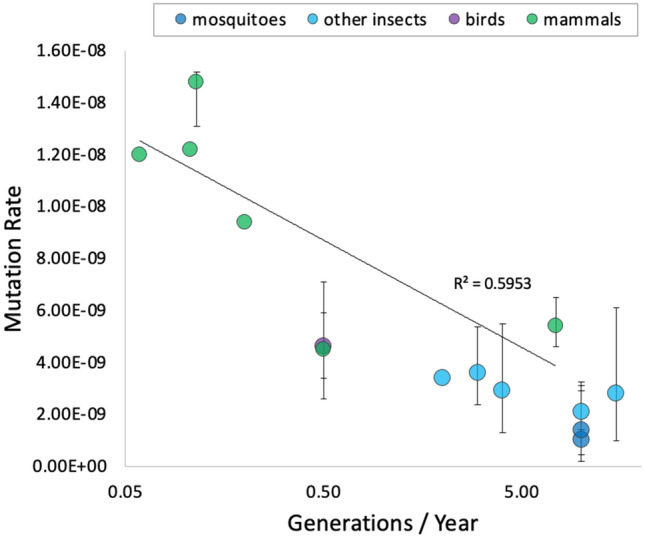
Mutation rate estimation and number of generations per year. Plot of number of generations per year (x-axis) against mutation rate estimations (y-axis) for six species of mammals, green circles (Canis lupus, Mus musculus, Chlorocebus sabaeus, Gorilla gorilla, Pan troglodytes and Homo sapiens), a bird, violet circle (Ficedula albicollis), the two species of mosquitoes estimated in this study, blue circles and five non-mosquito insects (Chironomus riparius, Drosophila melanogaster, Apis mellifera, Bombus terrestris, Heliconius melpomene) cyan circles. Error bars correspond to 95% CI values when provided.
Point mutations occur in both non-coding and coding regions and are typically characterized as neutral50, beneficial51, or deleterious52 based on their effect on fitness. As the names imply, neutral mutations show negligible effects on fitness, beneficial mutations increase the fitness of carriers and their frequencies tend to increase because of positive selection, and deleterious mutations decrease the fitness of carriers and tend to be removed from a population by purifying selection. Mutations in non-coding sequence are not necessarily neutral because they may alter protein binding sites that have direct consequences on gene regulation53,54. All mutations in this study were identified in non-coding regions.
The mutation rate per generation in a eukaryotic species is extremely low, meaning that only a few mutations evolve within an individual’s entire genome. Detecting newly risen mutations efficiently is a tedious quest, especially in large genomes. We have found the approach presented herein to be very effective and it is our hope that it will prove useful if applied to other mosquito and insect species.
Methods
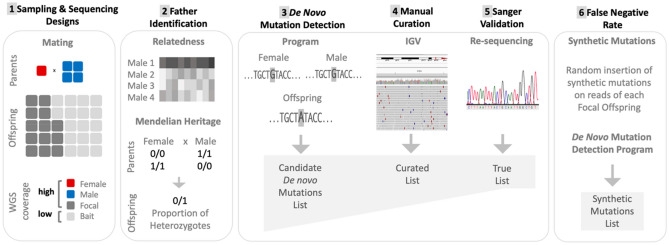
The workflow for this study, depicted schematically in Fig. 3, consisted of sample collection, high-throughput genome sequencing, variant calling, confirmation of paternity, detection of putative de novo mutations in offspring, verification of mutations, and estimation of the false negative rate by use of simulated mutations.
Figure 3.
Experimental and analytical designs. Schematics of experimental and analytical procedures for direct mutation rate estimation which includes a series of parameters to exclude false positive mutations and account for false negatives. (1) Sampling & sequencing designs: experimental female and male mating, and whole genome sequencing of parents and offspring at high and low coverage levels. (2) Father identification: two methods are represented (relatedness and Mendelian heritage). (3) De novo mutation detection: candidate de novo mutation is absent from both parents. (4) Manual curation: Integrated Genome Viewer (IGV) for mapping visualization of reads of candidate mutation list. (5) Sanger validation: PCR amplification and re-sequencing of candidate mutation. (6) False Negative Rate (FNR): 1000 synthetic mutations were inserted in each focal offspring; reads are processed and the de novo mutation detection program performed as in (3).
Mosquitoes
Anopheles coluzzii (MOPTI strain; MRA-763) and A. stephensi (STE2 strain; MRA-128) mosquito eggs were obtained from the Malaria Research and Reference Reagent Resource Center (BEI Resources, VA, USA) and reared at 26 °C and 80% relative humidity for multiple generations prior to experimental setup. Pupae were separated by sex and distributed in replicates with varying ratios of multiple males to single females to achieve mating. Adults were allowed to mate for four days before blood-feeding on heparin-treated bovine blood using a Hemotek artificial blood feeder (Hemotek Ltd, UK). Egg cups were placed in each mating cage after blood-feeding and hatched larvae were counted and transferred to a larger container for subsequent rearing. Replicates chosen for further analysis (one for each species) were those with the fewest F0 males but producing more than 30 F1 individuals, which were reared to adulthood, sorted by sex, and stored in 80% ethanol at −20 °C until DNA extraction.
Whole genome sequencing
For each species, the genomes of the female, potential male parents, and a subset of the offspring (designated ‘focal’) were sequenced at a target depth of 30×, while the remaining offspring (designated ‘bait’) were sequenced at a lower target depth of 15×. This reflects a strategy to minimize false positive mutations by dividing the offspring into two arbitrary classes: focal and bait. Only sites in focal offspring were checked for candidate mutations, and the sites checked included only those without variants in the bait offspring. This resulted in an efficient filtering method to detect sites in the genome that are prone to erroneous read alignment. Since mis-mapping errors are expected to be frequent at the sites where they occur, they should be detected even at the lower depth of coverage employed for bait samples, thus economizing on sequencing resources.
Prior to DNA extraction, the lower abdominal segment of females (containing the spermathecae) were removed to prevent sperm DNA contamination in downstream sequencing analysis. DNA extraction was carried out using a Qiagen Biosprint 96 extraction robot (Qiagen, Hilden, Germany) following a protocol established in our lab for increased DNA yield55. Samples were quantified using a Qubit 2.0 Fluorometer (Life Technologies, CA, USA) and prepared for Illumina sequencing using 10 ng DNA input following the KAPA HyperPlus Prep kit manufacturer’s protocol (KAPA Biosystems, MA, USA) with minor modifications56. Resulting DNA libraries were pooled and submitted for sequencing as 150 bp paired-end reads on the Illumina HiSeq 4000 platform at the UC Davis Genome Center.
Pre-processing of sequence data, mapping, variant calling
Low-quality and adapter sequences were removed from reads using Trimmomatic v0.3957 with settings illuminaclip:2:30:10, leading:3, trailing:3, slidingwindow:4:15, minlen:36. Reads were marked for PCR duplicates using Sambamba v0.6.758. The trimmed paired reads of both species were mapped onto their respective reference genomes using BWA MEM v0.7.1759 with default settings. Anopheles gambiae reference genome ‘PEST AgamP460,61 was the mapping target for A. coluzzii samples. The PEST genome originates from an A. gambiae-A. coluzzii hybrid laboratory strain and is commonly regarded as suitable for genomic analysis of both species62,63. The sequence reads of A. stephensi were mapped onto the recently assembled genome of A. stephensi ‘Indian’ strain64. Mapping quality control statistics were generated with Qualimap v2.2.165. Joint variant calling was performed using Freebayes v1.1.166 and biallelic SNPs were extracted from the VCF file using BCFtools v1.967.
Male parent identification
Various inheritance-based approaches are available for kinship analysis based on identification of common alleles shared by descent68–70. Here, we adopted similar approaches, using autosomal biallelic variant sites to identify the male parent from among potential fathers in each grouping by applying two strategies: (i) relatedness levels between parents and offspring were estimated by the KING inference method43 as implemented in –relatedness2 from VCFtools71; and (ii) concordance with expectations of Mendelian inheritance were assessed at variant sites where the genotypes of the parents are homozygous for different alleles, and therefore the offspring are expected to present only heterozygous genotypes. For the second method, separate analyses were performed for each potential male parent in combination with the female parent using a custom Perl script. The pair with the highest number of sites that were heterozygous in all offspring was identified as the true mating pair. After identification of the true fathers, the irrelevant F0 male samples were removed from the dataset.
Calling candidate mutations
Starting with the biallelic SNPs for each species in VCF format we applied a defined program in Perl to filter each variant site according to a set of criteria which define a candidate mutation as follows:
(i) Both parents are homozygous for the reference allele with read depth (DP) ≥ 10, and no alternate allele reads present (ADALT = 0).
(ii) All bait offspring with called genotypes are homozygous for the reference allele with no alternate allele reads present (ADALT = 0).
(iii) All focal offspring have a called genotype and either 1 or 2 focal offspring are called as heterozygotes (or are hemizygous for the alternate allele in the case of male offspring at sites on the X chromosome) and DP ≥ 10, while the remaining focal offspring are homozygous for the reference allele with no alternate allele reads present (ADALT = 0).
(iv) For the heterozygous focal offspring in (iii): at sites on the autosomes, and for female offspring at sites on the X chromosome the number of alternate allele reads present (ADALT) must be related to the total read depth (DP): 0.27 × DP ≤ ADALT ≤ 0.73 DP.
Manual curation of candidate mutations
All candidate mutations detected by our program were examined using Integrated Genome Viewer (IGV)72 which facilitates visualization of aligned reads from the original BAM files. In order to rule out obvious mis-mapping artifacts candidate mutations were considered within the context of all overlapping reads and adjacent reads and their variant sites.
Sanger sequencing
After manual curation with IGV, we evaluated all remaining candidates by Sanger sequencing to identify true mutations. For each candidate site, two or more sets of primers were designed to amplify the locus of interest from the DNA of parent–offspring trios (the mutated individual and both parents). Primers were designed based on the reference genome sequence of each species using Primer-BLAST73. Polymerase Chain Reaction (PCR) performed in an ABI Veriti thermal cycler (Applied Biosystems, USA) in 25 µl reaction volume containing: 12.5 µl 2 × GoTaq Green Master Mix (Promega), 1 µl of 10 pmol/µl of each forward and reverse primer, 9 µl of nuclease free water, and 1.5 µl of template DNA. Thermocycle conditions for the reaction were as follows: initial denaturation of 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, with 30 s of extension, and a final extension of 72 °C for 5 min. Amplicons were cleaned using 1.8 × SPRI magnetic beads and sequenced on both strands at the UCDNA Sequencing Facility (Davis, CA, USA) using BigDye Terminator v. 3.1 chemistry on an ABI Prism 3730 Genetic Analyzer (Applied Biosystems, USA). Chromatograms were analyzed for each member of a trio to verify the presence of the mutation in the offspring but not in either parent. Genomic annotations for each mutation site were obtained from the published general feature format (GFF) files of A. coluzzii74 and A. stephensi64.
Callable sites
The great majority of genomic sites are invariant within the pedigrees sequenced, and thus are not captured in the output of joint variant calling. On the other hand, not all sites absent from the variant calling results can confidently be assumed to be invariant. At sites with very low or no read coverage, a mutation will not be detected even when present. We reasoned that a read coverage of 10× will allow variant detection. Thus, to obtain an estimate of callable sites we defined the sets of all genomic coordinates with aligned read depth greater than 10× in each parent and each focal offspring, then intersected these three sets for each parent–offspring trio. A minority of sites reported by the joint variant caller were removed from the result for each trio since these sites (all except the handful of final candidate mutations) by failing our filtering regime had all proven to be un-callable by our methods. The remaining callable sites for each trio were summed to give the total number of callable genomic coordinates across all focal offspring. The final number of callable sites was doubled to account for the presence of diploid chromosomes.
Synthetic mutations
To estimate the false negative rate (type 2 error), we added 1000 faux (= synthetic) mutations to each focal offspring sequence data and measured the ability of our analytical pipeline to detect them19. Synthetic mutations were randomly inserted in autosomal sites using BAMSurgeon software75. The introduction of a mutation was performed by changing a fraction of reads overlapping the site and replacing the existing base with an alternate base randomly selected for the site. The number of reads altered for each synthetic mutation was determined based on an empirical distribution of alternate allele read depths at all heterozygous autosomal sites in the full dataset for all offspring for each species. These empirical distributions were calculated for each overall read depth between 10 and 100. When a synthetic mutation was created at a site with a given overall read depth, the quantity of alleles altered was drawn from the appropriate, corresponding distribution for that depth. To calculate empirical distributions, determination of heterozygosity relied on the principles of Mendelian inheritance: when each parent was homozygous for a different allele, all offspring were assumed to be heterozygous at that site. For example, for A. coluzzii there were 82,616 such heterozygous sites where overall read depth was 12 (Supplementary Table 5). The empirical distributions for alternate allele depth at these sites, as well as the corresponding binomial distribution calculated from the data mean and variance, are shown in Supplementary Fig. 3. For a synthetic mutation site with depth 12, a random integer N would be chosen from the empirical frequency distribution and N reads would be changed to the randomly chosen alternate base in the focal individual’s BAM file. After introduction of synthetic mutations, all BAM files were converted into FASTQ format and then processed with the mapping, variant calling, and filtering methods described above. For each species, dividing the number of synthetic mutations detected by the number of synthetic mutations inserted into callable sites provided an estimate of the false negative rate (FNR) which was used as a correction factor in the final mutation rate calculation.
Estimation of mutation rate
For the calculation of the mutation rate (µ), we desire the number of mutations per genome per generation. The enumerated total of mutations we detected in each species serves as the numerator. For the denominator we require an estimate of the number of callable sites, that is sites where we can confidently determine the presence or absence of a mutation, should one be present. For each species the corrected mutation rate was calculated as follow:
Supplementary Information
Acknowledgements
This work was supported by grants from the University of California Irvine Malaria Initiative and Open Philanthropy Project (A20-3521). This work was also supported in part by the Tata Institute for Genetics and Society (TIGS), India. We appreciate the efforts of Suresh Subramani, Ethan Bier and Sarah Werner TIGS-UCSD and acknowledge their work under TIGS. We are grateful to the UC Davis Genome Center for providing the necessary facilities to carry out the work.
Author contributions
I.R. data analysis, wrote paper. Me.C. data analysis, wrote paper. T.C. data analysis. Ma.C. lab experiments, data analysis, wrote paper. A.W. lab experiments. H.G. lab experiments. Y.L. conceived study, data analysis. H.S. conceived study, data analysis. G.C.L. conceived study, wrote paper.
Data availability
Whole genome sequence data included in this study are deposited in NCBI GenBank under BioProject PRJNA732889. Anopheles stephensi from accession number SAMN19349627–SAMN19349659 and A. coluzzii SAMN19355128–SAMN19355162.
Code availability
Custom codes used for the analysis are available on GitHub page: https://github.com/vectorgenetics-lab/mutation-rate.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Iliyas Rashid and Melina Campos.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03943-z.
References
- 1.Kong A, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altenberg L. An evolutionary reduction principle for mutation rates at multiple loci. Bull. Math. Biol. 2011;73:1227–1270. doi: 10.1007/s11538-010-9557-9. [DOI] [PubMed] [Google Scholar]
- 3.Yao NY, O'Donnell ME. Evolution of replication machines. Crit. Rev. Biochem. Mol. Biol. 2016;51:135–149. doi: 10.3109/10409238.2015.1125845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017;58:235–263. doi: 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake JW. The distribution of rates of spontaneous mutation over viruses, prokaryotes, and eukaryotes. Ann. N. Y. Acad. Sci. 1999;870:100–107. doi: 10.1111/j.1749-6632.1999.tb08870.x. [DOI] [PubMed] [Google Scholar]
- 7.Smeds L, Qvarnström A, Ellegren H. Direct estimate of the rate of germline mutation in a bird. Genome Res. 2016;26:1211–1218. doi: 10.1101/gr.204669.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch E, et al. De novo mutation rate estimation in wolves of known pedigree. Mol. Biol. Evol. 2019;36:2536–2547. doi: 10.1093/molbev/msz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchimura A, et al. Germline mutation rates and the long-term phenotypic effects of mutation accumulation in wild-type laboratory mice and mutator mice. Genome Res. 2015;25:1125–1134. doi: 10.1101/gr.186148.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeifer SP. Direct estimate of the spontaneous germ line mutation rate in African green monkeys. Evolution. 2017;71:2858–2870. doi: 10.1111/evo.13383. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumoto S, et al. Direct estimation of de novo mutation rates in a chimpanzee parent-offspring trio by ultra-deep whole genome sequencing. Sci. Rep. 2017;7:13561. doi: 10.1038/s41598-017-13919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad DF, et al. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 2011;43:712–714. doi: 10.1038/ng.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milholland B, et al. Differences between germline and somatic mutation rates in humans and mice. Nat. Commun. 2017;8:15183. doi: 10.1038/ncomms15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Galindo M, Casillas S, Weghorn D, Barbadilla A. Germline de novo mutation rates on exons versus introns in humans. Nat. Commun. 2020;11:3304. doi: 10.1038/s41467-020-17162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson J, et al. Extremely rare variants reveal patterns of germline mutation rate heterogeneity in humans. Nat. Commun. 2018;9:3753. doi: 10.1038/s41467-018-05936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan C, et al. Reduced intrinsic DNA curvature leads to increased mutation rate. Genome Biol. 2018;19:132. doi: 10.1186/s13059-018-1525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narasimhan VM, et al. Estimating the human mutation rate from autozygous segments reveals population differences in human mutational processes. Nat. Commun. 2017;8:303. doi: 10.1038/s41467-017-00323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keightley PD, Ness RW, Halligan DL, Haddrill PR. Estimation of the spontaneous mutation rate per nucleotide site in a Drosophila melanogaster full-sib family. Genetics. 2014;196:313–320. doi: 10.1534/genetics.113.158758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keightley PD, et al. Estimation of the spontaneous mutation rate in Heliconius melpomene. Mol. Biol. Evol. 2015;32:239–243. doi: 10.1093/molbev/msu302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, et al. Parent-progeny sequencing indicates higher mutation rates in heterozygotes. Nature. 2015;523:463–467. doi: 10.1038/nature14649. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, et al. Direct determination of the mutation rate in the bumblebee reveals evidence for weak recombination-associated mutation and an approximate rate constancy in insects. Mol. Biol. Evol. 2017;34:119–130. doi: 10.1093/molbev/msw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppold AM, Pfenninger M. Direct estimation of the spontaneous mutation rate by short-term mutation accumulation lines in Chironomus riparius. Evolut. Lett. 2017;1:86–92. doi: 10.1002/evl3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinka ME, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinka ME, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: What makes a malaria vector? Trends Parasitol. 2010;26:130–136. doi: 10.1016/j.pt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Poda SB, et al. Sex aggregation and species segregation cues in swarming mosquitoes: Role of ground visual markers. Parasit. Vectors. 2019;12:589. doi: 10.1186/s13071-019-3845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabaté A, et al. Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evol. Biol. 2011;11:184. doi: 10.1186/1471-2148-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artemov GN, Stegniy VN, Sharakhova MV, Sharakhov IV. The development of cytogenetic maps for malaria mosquitoes. Insects. 2018;9:121. doi: 10.3390/insects9030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coluzzi, M., Sabatini, A., della Torre, A., Di Deco, M. A. & Petrarca, V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science298, 1415–1418. 10.1126/science.1077769 (2002). [DOI] [PubMed]
- 30.Seyfarth M, Khaireh BA, Abdi AA, Bouh SM, Faulde MK. Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: Populations established-malaria emerging. Parasitol. Res. 2019;118:725–732. doi: 10.1007/s00436-019-06213-0. [DOI] [PubMed] [Google Scholar]
- 31.Sinka ME, et al. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. U S A. 2020;117:24900–24908. doi: 10.1073/pnas.2003976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontaine, M. C. et al. Mosquito genomics. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science347, 1258524. 10.1126/science.1258524 (2015). [DOI] [PMC free article] [PubMed]
- 33.Wiebe A, et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar. J. 2017;16:85. doi: 10.1186/s12936-017-1734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogola EO, et al. Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasites Vectors. 2018;11:577. doi: 10.1186/s13071-018-3171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong D, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci. Rep. 2020;10:16139. doi: 10.1038/s41598-020-73073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coetzee M, et al. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 37.Miles A, et al. Genetic diversity of the African malaria vector Anopheles gambiae. Nature. 2017;552:96–100. doi: 10.1038/nature24995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura M. Evolutionary rate at the molecular level. Nature. 1968;217:624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- 39.Foster KR, Wenseleers T, Ratnieks FL. Kin selection is the key to altruism. Trends Ecol. Evol. 2006;21:57–60. doi: 10.1016/j.tree.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genetics. 2000;156:297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roach JC, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science. 2010;328:636–639. doi: 10.1126/science.1186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Fan HC, Behr B, Quake SR. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell. 2012;150:402–412. doi: 10.1016/j.cell.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manichaikul A, et al. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell CD, Eichler EE. Properties and rates of germline mutations in humans. Trends Genet. 2013;29:575–584. doi: 10.1016/j.tig.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson Sayres, M. A. & Makova, K. D. Genome analyses substantiate male mutation bias in many species. Bioessays33, 938–945. 10.1002/bies.201100091 (2011). [DOI] [PMC free article] [PubMed]
- 46.Bentley DR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Chen W, Liu EY, Zhou YH. Single nucleotide polymorphism (SNP) detection and genotype calling from massively parallel sequencing (MPS) data. Stat. Biosci. 2013;5:3–25. doi: 10.1007/s12561-012-9067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch M. Evolution of the mutation rate. Trends Genet. 2010;26:345–352. doi: 10.1016/j.tig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin AP, Palumbi SR. Body size, metabolic rate, generation time, and the molecular clock. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4087–4091. doi: 10.1073/pnas.90.9.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochman H. Neutral mutations and neutral substitutions in bacterial genomes. Mol. Biol. Evol. 2003;20:2091–2096. doi: 10.1093/molbev/msg229. [DOI] [PubMed] [Google Scholar]
- 51.Marad DA, Buskirk SW, Lang GI. Altered access to beneficial mutations slows adaptation and biases fixed mutations in diploids. Nat Ecol. Evolut. 2018;2:882–889. doi: 10.1038/s41559-018-0503-9. [DOI] [PubMed] [Google Scholar]
- 52.Eyre-Walker A, Keightley PD. The distribution of fitness effects of new mutations. Nat. Rev. Genet. 2007;8:610–618. doi: 10.1038/nrg2146. [DOI] [PubMed] [Google Scholar]
- 53.Keightley PD, Gaffney DJ. Functional constraints and frequency of deleterious mutations in noncoding DNA of rodents. Proc. Natl. Acad. Sci. U S A. 2003;100:13402–13406. doi: 10.1073/pnas.2233252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loehlin DW, Ames JR, Vaccaro K, Carroll SB. A major role for noncoding regulatory mutations in the evolution of enzyme activity. Proc. Natl. Acad. Sci. U S A. 2019;116:12383–12389. doi: 10.1073/pnas.1904071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieman, C. C., Yamasaki, Y., Collier, T. C. & Lee, Y. A DNA extraction protocol for improved DNA yield from individual mosquitoes. F1000Res4, 1314. 10.12688/f1000research.7413.1 (2015). [DOI] [PMC free article] [PubMed]
- 56.Yamasaki, Y. K. et al. Improved tools for genomic DNA library construction of small insects (version 1; not peer reviewed). F1000Research5, 211 (2016).
- 57.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: Fast processing of NGS alignment formats. Bioinformatics. 2015;31:2032–2034. doi: 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 1303 (2013).
- 60.Holt RA, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 61.Sharakhova MV, et al. Update of the Anopheles gambiae PEST genome assembly. Genome Biol. 2007;8:R5. doi: 10.1186/gb-2007-8-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Love RR, et al. Chromosomal inversions and ecotypic differentiation in Anopheles gambiae: The perspective from whole-genome sequencing. Mol. Ecol. 2016;25:5889–5906. doi: 10.1111/mec.13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anopheles gambiae 1000 Genomes Consortium. Genome variation and population structure among 1142 mosquitoes of the African malaria vector species Anopheles gambiae and Anopheles coluzzii. Genome Res.30, 1–14. 10.1101/gr.262790.120 (2020). [DOI] [PMC free article] [PubMed]
- 64.Chakraborty M, et al. Hidden genomic features of an invasive malaria vector, Anopheles stephensi, revealed by a chromosome-level genome assembly. BMC Biol. 2021;19:28. doi: 10.1186/s12915-021-00963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okonechnikov K, Conesa A, García-Alcalde F. Qualimap 2: Advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32:292–294. doi: 10.1093/bioinformatics/btv566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garrison, E. & Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 1207 (2012).
- 67.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill WG, Weir BS. Variation in actual relationship as a consequence of Mendelian sampling and linkage. Genet. Res. 2011;93:47–64. doi: 10.1017/s0016672310000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Städele V, Vigilant L. Strategies for determining kinship in wild populations using genetic data. Ecol. Evol. 2016;6:6107–6120. doi: 10.1002/ece3.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Odero JO, Fillinger U, Rippon EJ, Masiga DK, Weetman D. Using sibship reconstructions to understand the relationship between larval habitat productivity and oviposition behaviour in Kenyan Anopheles arabiensis. Malar. J. 2019;18:286. doi: 10.1186/s12936-019-2917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye J, et al. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giraldo-Calderón GI, et al. VectorBase: An updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015;43:D707–713. doi: 10.1093/nar/gku1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ewing AD, et al. Combining tumor genome simulation with crowdsourcing to benchmark somatic single-nucleotide-variant detection. Nat. Methods. 2015;12:623–630. doi: 10.1038/nmeth.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Besenbacher S, Hvilsom C, Marques-Bonet T, Mailund T, Schierup MH. Direct estimation of mutations in great apes reconciles phylogenetic dating. Nat. Ecol. Evolut. 2019;3:286–292. doi: 10.1038/s41559-018-0778-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Whole genome sequence data included in this study are deposited in NCBI GenBank under BioProject PRJNA732889. Anopheles stephensi from accession number SAMN19349627–SAMN19349659 and A. coluzzii SAMN19355128–SAMN19355162.
Custom codes used for the analysis are available on GitHub page: https://github.com/vectorgenetics-lab/mutation-rate.