Abstract
Drosophila’s circadian clock can be perturbed by magnetic fields, as well as by lithium administration. Cryptochromes are critical for the circadian clock. Further, the radical pairs in cryptochrome also can explain magnetoreception in animals. Based on a simple radical pair mechanism model of the animal magnetic compass, we show that both magnetic fields and lithium can influence the spin dynamics of the naturally occurring radical pairs and hence modulate the circadian clock’s rhythms. Using a simple chemical oscillator model for the circadian clock, we show that the spin dynamics influence a rate in the chemical oscillator model, which translates into a change in the circadian period. Our model can reproduce the results of two independent experiments, magnetic field and lithium effects on the circadian clock. Our model predicts that stronger magnetic fields would shorten the clock’s period. We also predict that lithium influences the clock in an isotope-dependent manner. Furthermore, our model also predicts that magnetic fields and hyperfine interactions modulate oxidative stress. The findings of this work suggest that the quantum nature of radical pairs might play roles in the brain, as another piece of evidence in addition to recent results on xenon anesthesia and lithium effects on hyperactivity.
Subject terms: Computational biophysics, Biological physics, Quantum physics, Biophysical chemistry, Neuroscience, Molecular medicine
Introduction
All organisms, including microbes, plants, and animals, use an endogenous timekeeping system, namely the circadian clock (CC), which helps organisms to adapt to the 24-h cycle of the earth to control their daily physiology and behavior rhythms. Molecular pacemakers inside organisms drive the CC. In mammals, the coordination of essential behavioral, hormonal, and other physiological rhythms throughout the body relies on the CC1. It is also known that the circadian clock modulates cognitive activities2–5 and is linked to mood disorders6. In Drosophila, the CC controls the timing of eclosion and courtship, the period of rest and activity, and the timing of feeding; it also influences temperature preference7,8. Despite the differences in molecular components of the CCs, their features, organization, and the molecular mechanism that generate rhythmicity are very alike across organisms9.
Environmental cues such as light, food, and temperature can modulate the rhythmicity of the CC10. It is also known that the CC is susceptible to external magnetic fields (MFs). In the 1960s, Brown et al.11 found that small changes in the intensity of Earth’s MF synchronize the CCs of fiddler crabs and other organisms. Since then, the effects of external MF on the CC have been observed in multiple studies12–24. Similarly, Yoshii et al.25 have shown the effects of static MFs on the CC of Drosophila and found that exposure to these fields exhibited enhanced slowing of clock rhythms in the presence of blue light, with a maximal alteration at , and reduced effects at both lower and slightly higher field strengths. However, the exact mechanism behind this phenomenon is still mostly unknown.
Additionally, a growing body of evidence points to the circadian cycles as a target for bipolar disorder treatments26,27. Bipolar disorder is correlated with disruptions in circadian rhythms26,27 and abnormalities in oxidative stress28–35. Lithium is the first-line treatment for bipolar disorders36,37, yet the exact mechanisms and pathways underlying this treatment are under debate. It has been shown that lithium treatment for hyperactivity in rats is isotope dependent38. Lithium has two stable isotopes, and , which have different nuclear spin angular momentum, and , respectively. In a recent study, it has also been proposed that lithium affects hyperactivity via the clock center channel in the brain39. This study further predicted that the magnetic field would influence the potency of lithium treatment. Furthermore, Dokucu et al.40 showed that in Drosophila lithium lengthens the period of the CC. Here, based on these findings, we propose a mechanism that can explain both the MF effects and lithium effects on Drosophila’s CC.
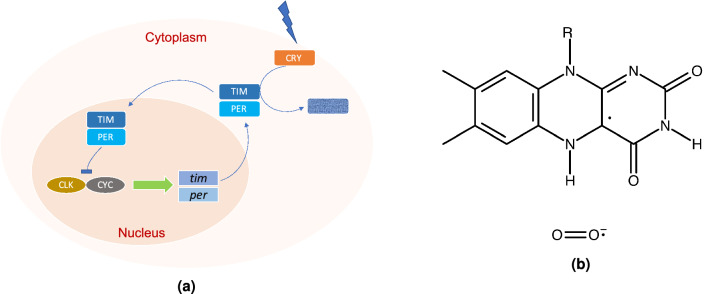
In the CC of Drosophila, the CLOCK (CLK) and CYCLE (CYC) transcription factors form a heterodimeric complex and promote the period (per) and timeless (tim) transcription mRNAs, which result in the assembly of the PERIOD (PER) and TIMELESS (TIM) proteins in the cytoplasm41, shown in Fig. 1a. During the night, PER and TIM accumulate and form a heterodimer. The TIM/PER complex enters the nucleus and then promotes the phosphorylation of CLK/CYC, which inhibits the promotion of the per and tim mRNAs. During the day, TIM and PER are gradually degraded, and consequently, CLK/CYC are released from repression to start a new cycle.
Figure 1.
(a) Simplified models of the circadian clock feedback loop in Drosophila. CLOCK (CLK) and CYCLE (CYC) promote the tim and per genes. PER and TIM first accumulate in the cytoplasm and then enter into the nucleus to block their gene transcription. Upon light, absorption CRY binds to TIM, and this results in the degradation of TIM67,68. (b) Flavinsemiquinone, , and superoxide radical pair in CRY, considered in the RPM model in the present work. The radical pair undergoes interconversion between singlet and triplet states.
In this process, light activation of cryptochrome (CRY) protein is critical for the rhythmicity of the CC. CRYs regulate growth and development in plants; they also act as photo-receptors in some animal’s CC, where they are necessary components of the circadian clock42,43. In Drosophila’s CC, upon light absorption, CRY undergoes a conformational change that allows it to bind TIM7,10,44 which results in the degradation of TIM and hence resetting the clock, see Fig. 1a. In Drosophila, CRYs contain the flavin adenine dinucleotide (FAD) cofactor, which is the photoactive cofactor. Upon blue-light absorption, FAD can go through various redox states. In insects, this process produces the anionic semiquinone FAD, , and reactive oxygen species (ROS), which are thought to be the key signaling states for initiating TIM degradation45,46. In mammals, CRY’s do not bind FAD. However, CRY’s are essential for the development of intercellular networks in the suprachiasmatic nucleus (SCN), a circadian pacemaker in the brain, that subserve coherent rhythm expression; the network synchronizes cellular oscillators and corrects errors47.
It has been known for many years that migratory birds use Earth’s magnetic field for finding their way during migrations. Later, it was proposed that the radical pair mechanism (RPM) could be the key for birds’ magnetoreception48. Ritz et al.49 proposed that the candidate protein for such a mechanism could be the CRY in the retina of birds. Ever since, there have been extensive studies on that hypothesis, and to date, it is the most promising model for avian magnetoreception50–52 in birds, sharks, sea turtles, monarch butterflies, fruit flies, etc. These models are based on the study of the dynamics of the created pair of radicals which can be in a superposition of singlet (S) and triplet (T) states53, depending on the parent molecule’s spin configuration54. The key elements in such reactions are radical molecules—transient molecules with an odd number of electrons in their outer molecular shell. Protons, neutrons, and electrons possess spin angular momentum, an inherently quantum characteristic. In a simple picture, quantum spins are like tiny magnets; any other spins or magnetic field in the vicinity could alter their states. In the framework of RPM for avian magnetoreception, it is thought that in CRY RPs can be in the form of anionic semiquinone FAD radical () and terminal tryptophan radical ()48,55–57. It is also well-known that the superoxide radical, , can be an alternative partner for the flavin radical58–61. It has also been proposed that RPs can play important roles in the magnetosensitivity of Drosophila’s CC21,25,62.
Applied magnetic fields can also influence oxidative stress in the presence of CRY63. Moreover, the CC rhythmicity is associated with an endogenous rhythm in the generation of ROS64. Thus it seems pertinent to explore the connection between magnetic field effects and ROS role in the CC. It has recently been proposed that radical pairs (RPs) could play roles in other brain functions. Dufor et al.65 propose that weak MFs activate cellular signaling cascade in neural circuits via acting through CRY, most likely by modulating the state of RPs. The authors concluded that the presence of CRY is critical in axon outgrowth under low-intensity repetitive transcranial magnetic stimulation (rTMS). It has also been suggested that RP may help explain xenon-induced anesthesia66 and the lithium effects on mania39. It, therefore, seems RPs could play critical roles in the functionalities of the brain in general and the CC in particular.
The circadian oscillations in Drosophila can be modeled by incorporating the formation of a complex between the PER and TIM proteins and introducing negative feedback loops69, which are the key to the rhythmicity of PER and TIM and their mRNA transcription. The models can be described by a set of a few kinetic equations70. However, modeling Drosophila CC70 can be further simplified into two nonlinear equations67. Furthermore, Player et al.71 show that quantum effects such as magnetic field effects and hyperfine interaction of radical pairs can be introduced to the chemical oscillator by considering the quantum effects on the corresponding reaction rates.
Here, we propose that the RPM could be the underlying mechanism behind the lithium treatment effects and MF effects on Drosophila’s CC. MF via the Zeeman interaction and lithium nucleus via HFIs modulate the recombination dynamics of singlet-triplet interconversion in the naturally occurring RPs in the [ ... ] complex, shown in Fig. 1b, and hence influence the period of the CC.
In the following, we review the quantitative experimental results for the effects of applied magnetic field25 and lithium40 on the period of Drosophila’s CC. Next, we briefly describe the quantum spin dynamics for the radical pair model where the magnetic field effects and the HFIs are relevant. Moving on, we present our singlet yield calculation for the RP system, inspired by the CRY-based model of birds’ avian magnetoreception72. Later we use a simple model for the mathematical presentation of Drosophila’s CC, following the work on Tyson et al.67. Then we introduce the quantum effect to the period of the CC model, and we show the consistency of our model’s predictions and the experimental findings on the magnetic field and lithium treatment effects. Finally, we discuss new predictions for experiments.
Results
Magnetic field and lithium treatment effects on circadian clock and RPM
Results from prior experiments
Here, we focus on the effects of static MF on Drosophila’s CC observed by Yoshii et al.25. The authors conducted experiments to observe the effects of static magnetic fields with different intensities, [0, 150, 300, 500] , on changes in the period of Drosophila’s CC under blue light illumination, shown in Table 1. These magnetic fields are, excepting the control of , approximately 3, 6, and 10 times stronger than natural magnetic fields, respectively. That observation revealed that the period alterations significantly depended on the strength of the magnetic field such that the period change reached a maximum of at . In this experiment, the geomagnetic field was shielded, and the arrhythmic flies were excluded from the analysis. We also consider the results of the experiment conducted by Dokucu et al.40 observing the effects of chronic lithium administration on Drosophila’s CC for a range of doses [0, 300] mM. It was shown that lithium treatment lengthens the CC with a maximum prolongation of h at 30 mM of lithium compared to zero lithium intake, see Table 2. In that work, the lethality of lithium up to 30 mM was relatively low until the end of the experiments. Here, we consider that 30 mM is the optimal concentration of lithium where all RPs interact with lithium atoms. We assume that the lithium administered in that work was in its natural abundance, 92.5% and 7.5% of and , respectively. Here we will refer to the natural lithium as Li. In our model here, of MF and 0 mM of lithium are our control sets for MF and lithium effects on the CC.
Table 1.
Period Changes in the free-running rhythm of Drosophila after application of magnetic fields (MFs) under blue light illumination and lithium administration, taken from the work of Yoshii et al.25.
| Applied MF | Period Change (h) | Number of flies |
|---|---|---|
| 0 | 27 | |
| 150 | 26 | |
| 300 | 23 | |
| 500 | 25 | |
| Relative period change (h) 300-0 |
Table 2.
Period in the free-running rhythm of Drosophila for zero and 30 mM intake of lithium, taken from the work of Dokucu et al.40.
| Lithium dose (mM) | Period (h) | Number of flies |
|---|---|---|
| 0 | 311 | |
| 30 | 44 | |
| Relative period change (h) 30-0 |
RPM model
We develop an RP model to reproduce static MFs and lithium administration effects on the rhythmicity of Drosophila’s CC observed in Ref.25 and Ref.40, respectively. Taking into account the facts that the CC is associated with oxidative stress levels under light exposure45,46,64,73,74 and applied MF75,76, and the CC is affected by lithium intake, we propose that the applied magnetic field interacts with the spins of RPs on FADH and superoxide, and the nuclear spin of lithium modulates the spin state of the radical on superoxide. The correlated spins of RP are assumed to be in the [ ... ] form, where the unpaired electron on each molecule couples to the nuclear spins in the corresponding molecule, see Fig. 1b. In [ ... ].
We consider a simplified system in which the unpaired electron is coupled to the flavin’s nitrogen nucleus with an isotropic HF coupling constant (HFCCs) of 77. In this model, for simplicity, we consider only Zeeman and HF interactions48,78. Following the work of Hore72, the anisotropic components of the hyperfine interactions are excluded, which are only relevant when the radicals are aligned and immobilized79. The RPs are assumed to have the g-values of a free electron. The Hamiltonian for the RP system reads as follows:
| 1 |
where and are the spin operators of radical electron A and B, respectively, is the nuclear spin operator of the isoalloxazine nitrogen of , similar to Refs.39,72, is the nuclear spin operator of the Li nucleus, and are HFCCs, taken from39,77, and is the Larmor precession frequency of the electrons due to the Zeeman effect. Ref72 uses two nitrogen atoms, while for the purpose of the computational cost we use only the largest one without loss of generality. Of note, oxygen has a zero nuclear spin and thus its HFCC equals zero, (), however in the model for lithium effects corresponds to the nuclear spin of lithium. We assumed that the RPs start off from singlet states (see the Discussion section).
Singlet yield calculation
The singlet yield resulting from the radical pair mechanism can be obtained by solving the Liouville-von Neumann equation for the spin state of the radical pair throughout the reaction. Using the eigenvalues and eigenvectors of the Hamiltonian, the ultimate singlet yield, , for periods much greater than the RP lifetime72 has the following form:
| 2 |
where , , is the nuclear spin multiplicity, is the singlet projection operator, and are eigenstates of with corresponding eigenenergies of and , respectively, k is the RP reaction rate, and r is the RP spin-coherence lifetime rate (relaxation rate).
Here we look at the sensitivity of the singlet yield to changes in the strength of the external magnetic field for the [ ... ] radical complex. Figure 2 illustrates the dependence of the singlet yield of the [ ... ] complex on external magnetic field B with a maximum yield in [280–360] T for and with . In our model, the magnetic dependence of singlet yield is the foundation of the magnetic sensitivity of the circadian clock. Using the singlet yield, we can reproduce the experimental finding on the effects of applied MF25 and lithium administration40 on the period of the circadian clock of Drosophila, as we discuss below. It is worth mentioning that the singlet-product of the RP system in [ ... ] is 80, which is the major ROS in redox regulation of biological activities and signaling81.
Figure 2.
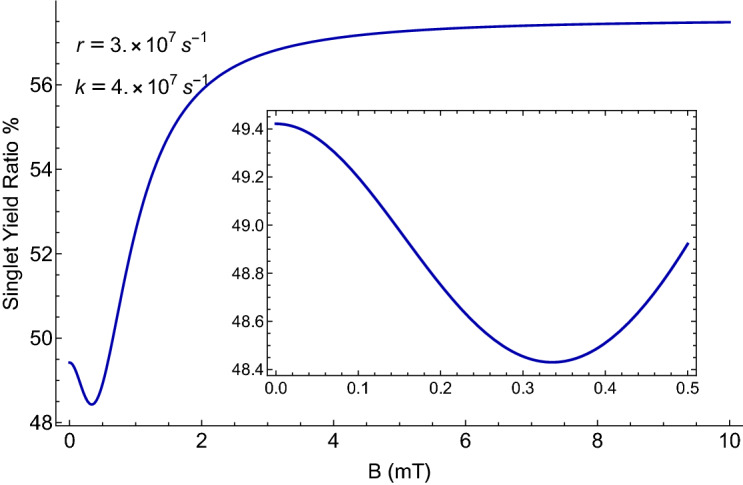
The dependence of the singlet yield of the [ ... ] complex on external magnetic field B for , reaction rate k, and relaxation rate r. The singlet yield reaches a minimum value of 48.45% in [280–360] (see the inset).
Circadian clock model
We use a simple mathematical model for the circadian clock of Drosophila, following the work of Tyson et al.67. Despite its simplicity, the model is very well known69,82–84 and captures the most important part of the clock’s function. In this model, PER monomers are rapidly phosphorylated and degraded, whereas PER/TIM dimers are less susceptible to proteolysis, shown in Fig. 1a. In this context, it is also assumed that the cytoplasmic and nuclear pools of dimeric protein are in rapid equilibrium. With these considerations, it is possible to write the mathematical model in two coupled equations as follows:
| 3 |
| 4 |
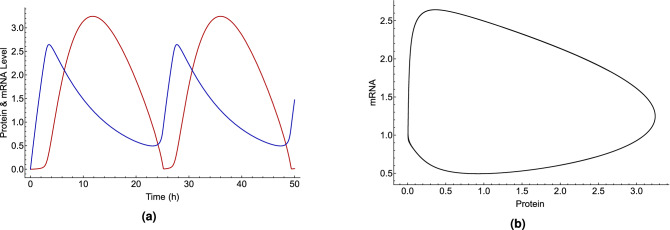
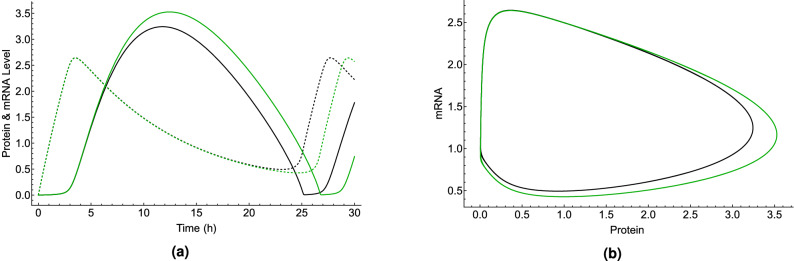
where , and M(t) are the total protein and the mRNA concentrations, respectively. For the descriptions and values of the parameters, see Table 3. In this simple model, represents the role of CRY’s light activation and hence proteolysis of protein. By solving Eqs. 3 and 4, we obtain the oscillation of protein and mRNA concentrations. Figure 3 shows the explicit time-dependence of protein and mRNA concentrations and the parametric representation of the chemical oscillator limit cycle for Drosophila’s CC. To obtain the period of the clock, we take the average differences between successive peaks and likewise troughs of either or M(t) by keeping track of when the derivative is zero.
Table 3.
Parameter values for the circadian clock of Drosophila, taken from the work of Tyson et al.67. and are characteristic concentrations for mRNA and protein, respectively.
| Name | Value | Units | Description |
|---|---|---|---|
| 1.0 | Maximum rate of mRNA synthesis | ||
| 0.1 | mRNA degradation rate constant | ||
| 0.5 | mRNA rate constant | ||
| 10 | of monomer phosphorylation | ||
| 0.03 | of dimer phosphorylation | ||
| 0.1 | Proteolysis rate constant caused by CRY activation | ||
| 200 | Dimerization equilibrium constant | ||
| 0.1 | Dimer concentration at the half-maximum transcription rate | ||
| 0.05 | Michaelis constant for protein kinase (DBT) |
Figure 3.
(a) Explicit time-dependence of the concentrations of protein [red] and mRNA [blue] and (b) Parametric representations of oscillations in the concentrations of protein and mRNA, shown as a limit cycle in Drosophila’s circadian clock model using Eqs. (3) and (4), and the parameters from Table 3.
Effects of singlet yield change on circadian clock
The effects of applied magnetic fields and hyperfine interactions can be introduced to the chemical oscillator of the circadian clock by modifying the rate 71, following the work of Player et al., see Methods. In the CC Eqs. (3) and (4) the corresponding rate is , which represents the role of CRY’s light activation and hence proteolysis of protein and is 0.1 for the natural cycle of the clock. Hence for the occasions with no singlet yields effects, this value must be retained. The singlet yield effects on can be written as follows:
| 5 |
where , , and are the modified rate constant , the singlet yield with no quantum effects, and the singlet yield resulted from quantum effects due to the Zeeman and/or hyperfine interactions, respectively.
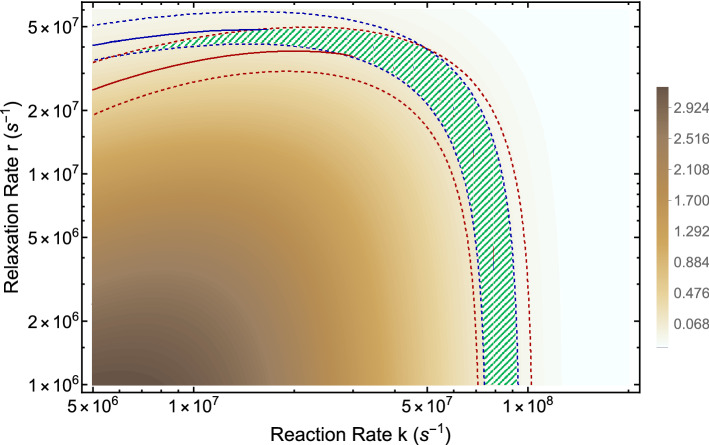
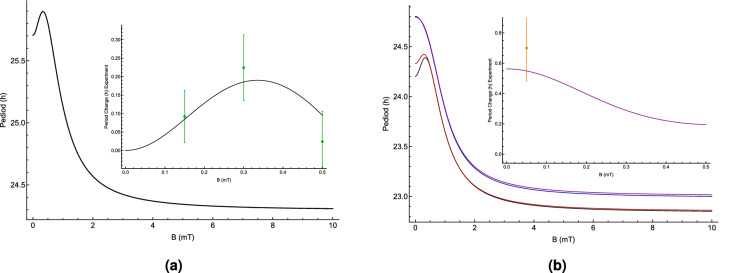
Based on the above considerations, here, we calculate the explicit effects of an applied magnetic field and the hyperfine interactions on the period of the CC. Using Eqs. (3), (4), and (5), we explored the parameter space of relaxation rate r and recombination rate k in order to find allowed regions for which our model can reproduce both experimental findings of static MF of 25 and 30 mM of lithium40 effects on Drosophila’s CC, which respectively lengthen the clock’s period by and . The results are shown in Fig. 4. We find an allowed region where the model reproduces both experiments, see Fig. 4. The parameters for calculating the period of the circadian clock are taken from Table 3. As discussed above, corresponds to the degradation of TIM due to blue light exposure. For the MF effects under blue light illumination, we set to obtain the control period of the circadian clock under blue light illumination observed in Ref.25. Figure 5 shows the effects of lithium on the rhythmicity of CC, such that lengthens the period of the clock longer than . For the effects of lithium on the circadian clock, the geomagnetic field of is taken into account. Figure 6 shows the effects of MF on the CC.
Figure 4.
The RPM model can reproduce both magnetic field and lithium effects. The comparison between period changes due to applied magnetic fields measured in the experiment25, , and obtained by the RPM model, , where is the difference between period changes at 300 T and 0 T, . The solid blue line indicates and the dashed blue line indicates the region where . The difference between period changes due to the lithium administration measured in the experiment40, , and obtained by the RPM model, is presented by red lines. The solid red line indicates h and the dashed red line indicates the region where , . The green shaded color indicates the regions where the RPM model can reproduce both magnetic field25 and lithium40 effects on Drosophila’s CC. The parameters for calculating the period of the circadian clock are taken from Table 3, except that for the MF effects under blue light illumination .
Figure 5.
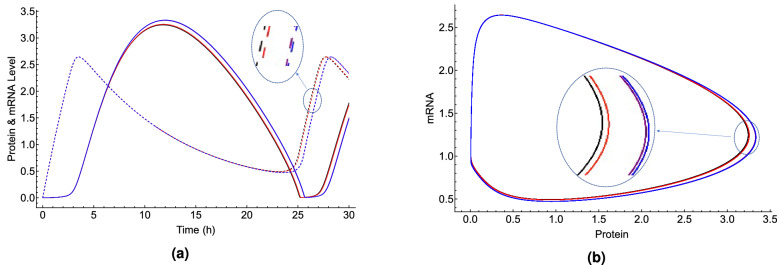
Lithium effects on the circadian clock are reproduced by the RPM model. (a) Explicit time-dependence of the concentrations of protein [the solid lines] and mRNA [the dashed lines] and (b) Parametric representations of oscillations in the concentrations of protein and mRNA, in Drosophila’s circadian clock model using the parameters from Table 3. The black, red, blue and purple colors indicate zero-lithium, , , and Li, respectively. Lithium administration prolongs the period of the clock, such that has more potency than .
Figure 6.
Magnetic field effects on the circadian clock are reproduced by the RPM model. (a) Explicit time-dependence of the concentrations of protein [the solid lines] and mRNA [the dashed lines] and (b) Parametric representations of oscillations in the concentrations of protein and mRNA, in Drosophila’s circadian clock model using the parameters from Table 3, except . The black and green colors indicate zero-MF and MF effects, respectively.
The model here reproduces the dependence of the CC’s period on the applied MF’s strength and Li administration, shown in Fig. 7. The model predicts that further increases in the intensity of the MF would shorten the period of the clock significantly. For the cases considering MF effects solely, for both the experimental data and the RPM model, the period reaches a maximum between and and exhibits reduced effects at both lower and slightly higher field strengths, shown in Fig. 7a. For the cases of or without lithium intake, the largest prolongation of the period occurs in the same range of magnetic field as well, shown in Fig. 7b. Another prediction of the model is that prolongs the clock’s period stronger than , which has a smaller spin compared to , see Fig. 7b. In this model, in the cases where lithium effects are considered, the geomagnetic effects of are also considered. For the comparison between our model and the experimental data on the lithium effects, we assume that natural lithium was administered in the experiment40. Following Ref.39, we considered . Note that moderate changes to the HFCC of lithium do not impact our results. Figure 7 shows that the dependence of the period on applied MFs and lithium effects calculated by the RPM model used in the present work is consistent with the experimental observations. We compare the maximum lengthening of the period in both the RPM model and experimental data25, and , respectively, where . The results from the RPM fall into the uncertainty of the experimental data, .
Figure 7.
The dependence of the period of Drosophila’s circadian clock calculated by the RPM model on the static magnetic field strength B without (a) and with (b) lithium effects for T, , relaxation rate , and reaction rate . Higher magnetic field intensities shorten the period of the circadian clock. For the case without lithium effects (a), the applied magnetic field lengthens the period of the clock to a maximum in [280–360] T and reduces effects at both lower and higher field strengths. The comparison between the dependence of the period on applied magnetic field calculated by the RPM model [black line in the inset of plot (a)] and the experimental findings [green dots with error-bars] of Ref.25. (b) The effects of Li [purple], [red], [blue], and zero Li [black]. The inset indicates the comparison between the effects of Li on the period of the clock calculated by the RPM model [purple line] and the experimental findings [orange dots with error-bars] of Ref.40. The results from the RPM fits into the uncertainty of the experimental data, such that .
Discussion
In this project, we aimed to probe whether a RP model can explain the experimental findings for both the effects of static magnetic field25 and lithium40 on the circadian clock in Drosophila. We showed how the quantum effects affect the rates, which then yields a change in the period of the clock. This is a significant step forward compared to the previous studies on xenon anesthesia66 and the lithium effects on hyperactivity39, where the quantum effects were correlated to experimental findings without explicitly modeling the related chemical reaction networks. With a set of reasonable parameters, our model reproduces the experimental findings, as shown in Figs. 4, and 7. In addition, this strengthens the previously proposed explanation for the effects of lithium on hyperactivity39 via the circadian clock.
We proposed that applied magnetic fields and nuclear spins of lithium influence the spin state of naturally occurring [ ... ] radical pairs in the circadian clock. This is inspired by the observations that the Drosophila circadian clock is altered by external magnetic fields24,25, which is accompanied by modulations in the ROS level64,75, and by lithium administration40. Let us note that it has also been suggested that lithium exerts its effects by inhibiting Glycogen synthase kinase-3 (GSK-3)85,86 . However, while the presence of RPs is a natural explanation for magnetic field effects, their existence in GSK-3 requires experimental support.
The suggested [...] radical pairs depend on a form of FAD in the Drosophila CRY photocycle that is thought to be unusual. However, Baik et al. showed the possibility that light-activated CRY in Drosophila neurons express a neutral semiquinone state87. They conclude that further investigation would shed more light on whether the red light responses are due to Drosophila CRY signaling or opsin signaling or a combination of both. On the other hand, Chandrasekaran et al. reported that Drosophila CRY mutants that can form are incapable of light-activated conformational changes88. However, more recent studies show that alternative signaling pathways may be involved in the magnetosensitivity of the Drosophila circadian clock89. Thus, other forms of radical pairs, e.g. [ ... ], could also be considered instead of [...] to explain the magnetosensitivity of the Drosophila circadian clock.
Of note, there is a large body of evidence that ROS are involved in the context of magnetosensing and the circadian clock modulations63,64,73,74,76,90. it has been shown that oscillating magnetic fields at Zeeman resonance can influence the biological production of ROS in vivo, indicating coherent S-T mixing in the ROS formation80. Additionally, it has been observed that extremely low frequency pulsed electromagnetic fields cause defense mechanisms in human osteoblasts via induction of and 75. Sherrard et al.63 observed that weak pulsed electromagnetic fields (EMFs) stimulate the rapid accumulation of ROS, where the presence of CRY was required63. The authors of that work concluded that modulation of intracellular ROS via CR represents a general response to weak EMFs. Further, Sheppard et al.90 demonstrated that MFs of a few millitesla can indeed influence transfer reactions in Drosophila CRY. It has also been shown that illumination of Drosophila CRY results in the enzymatic conversion of molecular oxygen to transient formation of superoxide and accumulation of hydrogen peroxide in the nucleus of insect cell cultures73. These findings indicate the light-driven electron transfer to the flavin in CRY signaling74.
The feasibility for the radical to be involved in the RPM is a matter of debate in this scenario due to its likely fast spin relaxation rate r. Because of fast molecular rotation, the spin relaxation lifetime of is thought to be on the orders of 1 ns91,92. Nonetheless, it has also been pointed out that this fast spin relaxation can be decreased on account of its biological environment. Additionally, Kattnig et al.93,94 proposed that scavenger species around can also reduce its fast spin relaxation. Moreover, in such a model, the effects of exchange and dipolar interactions can also be minimized.
It is often assumed that in the RP complexes involving superoxide are formed in triplet states, as opposed to the case considered here. This is because the ground state of the oxygen molecule is a triplet state. The initial state for RP formation could also be its excited singlet state, which is a biologically relevant ROS95–97. Further, the transition of the initial RP state from triplet to singlet could also take place due to spin-orbit coupling98,99.
Our model predicts that increasing the intensity of the applied magnetic field will shorten the period of the clock. This is a significant new prediction of our model that would be very interesting to check. The isotopic-dependence of the period is another prediction of our present model, such that lengthens the period of the clock longer than .
The circadian clock not only controls the rhythms of the biological processes, but it also has intimate connections to other vital processes in the body100 and particularly in the brain101. It has been suggested that environmental perturbations in the circadian period could increase the risk of selected cancers and hence the circadian clock could be a therapeutic target for cancer risks102. It also appears that the way drugs function depends on the circadian clock103,104. Notably, it has been shown that the circadian clock is vital for maintaining the anti-oxidative defense105. Moreover, it has been suggested that the circadian clock could be a new potential target for anti-aging106,107 and neurodegenerative disorders therapeutics108. Thus this project also paves a potential path to study other functionalities of the body and the brain connected to the circadian clock in the light of the RPM.
To sum up, our results suggest that quantum effects may underlie the magnetic field and lithium effects on the circadian clock. A similar mechanism is likely to be at the heart of magnetoreception in animals109, xenon-induced anesthesia66, and lithium treatment for mania39. Our work is thus another piece of evidence that quantum effects may play essential roles in the brain’s functionalities110–121.
Methods
Quantum effects and chemical oscillator
The effects of applied magnetic fields and hyperfine interactions can be introduced, following the work of Player et al.71, by assuming that the FAD signaling of CRY to TIM and hence the TIM degradation in the CC process proceed by RPs, shown in Eq. 6:
| 6 |
where rate constants and are assumed to conserve electron spin. External magnetic fields and the hyperfine interactions can influence the overall rate of production of by altering the extent and timing of coherent singlet/triplet interconversion in RP and so changing the probability that it reacts to give rather than returning to . Based on a spin dynamics calculation, one can describe the effect of applied magnetic fields and the HFIs on the kinetics of the CC simply by modifying the rate constant 122–125 which corresponds to the degradation of TIM in Eqs. (3) and (4). The spin dynamics of the RP in Eq. (6) can be written as follows:
| 7 |
where is the Liouvillian, [..., ...] and are the commutator and anti-commutator operators, is the spin density operator of the RP system; its trace, , equals the concentration of RPs divided by the fixed concentration of in Eq. 6. As RPs are short-lived intermediates, their concentrations are very low, and hence one can obtain the steady-state solutions as follows (see Ref.71):
| 8 |
where is the singlet yield of the RPM. It is, therefore, possible to introduce the singlet yield of the RPM to the chemical reaction by modifying the rate . In the CC Eqs. (3) and (4) the corresponding rate is , which is for the natural cycle of the clock without blue light illumination and for blue light illumination. The singlet yield effects on can be written as follows:
| 9 |
where , , and are the modified rate constant , the singlet yield with no quantum effects, and the singlet yield resulted from quantum effects due to the Zeeman and/or hyperfine interactions, respectively.
Acknowledgements
The authors would like to thank Rishabh, Dennis Salahub, Wilten Nicola, Gabriel Bertolesi, Sarah McFarlane, and Nilakshi Debnath for their valuable input. The authors also acknowledge Compute Canada for its computing resources. This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Author contributions
H.ZH. and C.S. conceived the project; H.ZH. performed the calculations; H.ZH. and C.S. wrote the paper; C.S. supervised the project.
Data availability
The generated datasets and computational analysis are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hadi Zadeh-Haghighi, Email: hadi.zadehhaghighi@ucalgary.ca.
Christoph Simon, Email: csimo@ucalgary.ca.
References
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyriacou CP, Hastings MH. Circadian clocks: Genes, sleep, and cognition. Trends Cognit. Sci. 2010;14:259–267. doi: 10.1016/j.tics.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Aten S, et al. mir-132 couples the circadian clock to daily rhythms of neuronal plasticity and cognition. Learn. Mem. 2018;25:214–229. doi: 10.1101/lm.047191.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teodori L, Albertini MC. Shedding light into memories under circadian rhythm system control. Proc. Natl. Acad. Sci. 2019;116:8099–8101. doi: 10.1073/pnas.1903413116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver R, Kriegsfeld LJ. Circadian rhythms have broad implications for understanding brain and behavior. Eur. J. Neurosci. 2014;39:1866–1880. doi: 10.1111/ejn.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Therapeut. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allada R, Chung BY. Circadian organization of behavior and physiology in drosophila. Ann. Rev. Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, Y. & Emery, P. Molecular and neural control of insect circadian rhythms. In Insect molecular biology and biochemistry, 513–551 (Elsevier, 2012).
- 9.Tataroglu O, Emery P. Studying circadian rhythms in drosophila melanogaster. Methods. 2014;68:140–150. doi: 10.1016/j.ymeth.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2. [DOI] [PubMed] [Google Scholar]
- 11.Brown, F. A. Response to pervasive geophysical factors and the biological clock problem. In Cold Spring Harbor Symposia on Quantitative Biology, vol. 25, 57–71 (Cold Spring Harbor Laboratory Press, 1960).
- 12.Bliss VL, Heppner FH. Circadian activity rhythm influenced by near zero magnetic field. Nature. 1976;261:411–412. doi: 10.1038/261411a0. [DOI] [PubMed] [Google Scholar]
- 13.Contalbrigo L, et al. Effects of different electromagnetic fields on circadian rhythms of some haematochemical parameters in rats. Biomed. Environ. Sci. 2009;22:348–353. doi: 10.1016/S0895-3988(09)60067-2. [DOI] [PubMed] [Google Scholar]
- 14.Marley R, Giachello CN, Scrutton NS, Baines RA, Jones AR. Cryptochrome-dependent magnetic field effect on seizure response in drosophila larvae. Sci. Rep. 2014;4:1–4. doi: 10.1038/srep05799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Close JP. The compass within the clock-part 1: The hypothesis of magnetic fields as secondary zeitgebers to the circadian system–logical and scientific objections. Hypothesis. 2014;12:e1. [Google Scholar]
- 16.Close JP. The compass within the clock-part 2: Does cryptochrome radical-pair based signalling contribute to the temperature-robustness of circadian systems. Hypothesis. 2014;12:e3. [Google Scholar]
- 17.Fedele G, et al. Genetic analysis of circadian responses to low frequency electromagnetic fields in drosophila melanogaster. PLoS Genet. 2014;10:e1004804. doi: 10.1371/journal.pgen.1004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewczuk, B. et al. Influence of electric, magnetic, and electromagnetic fields on the circadian system: current stage of knowledge. BioMed research international2014 (2014). [DOI] [PMC free article] [PubMed]
- 19.Vanderstraeten J, Burda H, Verschaeve L, De Brouwer C. Could magnetic fields affect the circadian clock function of cryptochromes? Testing the basic premise of the cryptochrome hypothesis (elf magnetic fields) Health Phys. 2015;109:84–89. doi: 10.1097/HP.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 20.Manzella N, et al. Circadian gene expression and extremely low-frequency magnetic fields: An in vitro study. Bioelectromagnetics. 2015;36:294–301. doi: 10.1002/bem.21915. [DOI] [PubMed] [Google Scholar]
- 21.Bartos P, et al. Weak radiofrequency fields affect the insect circadian clock. J. Royal Soc. Interface. 2019;16:20190285. doi: 10.1098/rsif.2019.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai, H. Genetic effects of non-ionizing electromagnetic fields. Electromagn. Biol. Med. 1–10 (2021). [DOI] [PubMed]
- 23.Thöni, V., Oliva, R., Mauracher, D. & Egg, M. Therapeutic nuclear magnetic resonance affects the core clock mechanism and associated hypoxia-inducible factor-1. Chronobiol. Int. 1–15 (2021). [DOI] [PubMed]
- 24.Xue X, et al. Biological effects of space hypomagnetic environment on circadian rhythm. Front. Physiol. 2021;12:254. doi: 10.3389/fphys.2021.643943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshii T, Ahmad M, Helfrich-Förster C. Cryptochrome mediates light-dependent magnetosensitivity of drosophila’s circadian clock. PLoS Biol. 2009;7:e1000086. doi: 10.1371/journal.pbio.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu T, Bragança M. The bipolarity of light and dark: A review on bipolar disorder and circadian cycles. J. Affect. Disord. 2015;185:219–229. doi: 10.1016/j.jad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi JS, Hong H-K, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yumru M, et al. Oxidative imbalance in bipolar disorder subtypes: A comparative study. Prog. Neuro-Psychopharmacol. Biol. Psych. 2009;33:1070–1074. doi: 10.1016/j.pnpbp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Salim S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014;12:140–147. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado-Vieira R, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neurosci. Lett. 2007;421:33–36. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 32.Andreazza AC, et al. Oxidative stress markers in bipolar disorder: A meta-analysis. J. Affect. Disord. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Lee S-Y, et al. Oxidative/nitrosative stress and antidepressants: Targets for novel antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psych. 2013;46:224–235. doi: 10.1016/j.pnpbp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psych. Res. 2014;218:61–68. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Berk M, et al. Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor rev-erb is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Lu W-Q, Beesley S, Loudon AS, Meng Q-J. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PloS One. 2012;7:e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ettenberg A, et al. Differential effects of lithium isotopes in a ketamine-induced hyperactivity model of mania. Pharmacol. Biochem. Behav. 2020;190:172875. doi: 10.1016/j.pbb.2020.172875. [DOI] [PubMed] [Google Scholar]
- 39.Zadeh-Haghighi H, Simon C. Entangled radicals may explain lithium effects on hyperactivity. Sci. Rep. 2021;11:12121. doi: 10.1038/s41598-021-91388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dokucu ME, Yu L, Taghert PH. Lithium-and valproate-induced alterations in circadian locomotor behavior in drosophila. Neuropsychopharmacology. 2005;30:2216–2224. doi: 10.1038/sj.npp.1300764. [DOI] [PubMed] [Google Scholar]
- 41.Tataroglu O, Emery P. The molecular ticks of the drosophila circadian clock. Curr. Opin. Insect Sci. 2015;7:51–57. doi: 10.1016/j.cois.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emery P, et al. Drosophila cry is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 43.Chaves I, et al. The cryptochromes: Blue light photoreceptors in plants and animals. Ann. Rev. Plant Biol. 2011;62:335–364. doi: 10.1146/annurev-arplant-042110-103759. [DOI] [PubMed] [Google Scholar]
- 44.Ceriani MF, et al. Light-dependent sequestration of timeless by cryptochrome. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 45.Ozturk N, Selby CP, Annayev Y, Zhong D, Sancar A. Reaction mechanism of drosophila cryptochrome. Proc. Natl. Acad. Sci. 2011;108:516–521. doi: 10.1073/pnas.1017093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaidya AT, et al. Flavin reduction activates drosophila cryptochrome. Proc. Natl. Acad. Sci. 2013;110:20455–20460. doi: 10.1073/pnas.1313336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Ann. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hore PJ, Mouritsen H. The radical-pair mechanism of magnetoreception. Ann. Rev. Biophys. 2016;45:299–344. doi: 10.1146/annurev-biophys-032116-094545. [DOI] [PubMed] [Google Scholar]
- 49.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J, et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature. 2021;594:535–540. doi: 10.1038/s41586-021-03618-9. [DOI] [PubMed] [Google Scholar]
- 51.Wan G, Hayden AN, Iiams SE, Merlin C. Cryptochrome 1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat. Commun. 2021;12:1–9. doi: 10.1038/s41467-021-21002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones AR. Magnetic field effects in proteins. Molecul. Phys. 2016;114:1691–1702. [Google Scholar]
- 53.Steiner UE, Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 1989;89:51–147. [Google Scholar]
- 54.Timmel CR, Till U, Brocklehurst B, Mclauchlan KA, Hore PJ. Effects of weak magnetic fields on free radical recombination reactions. Molecul. Phys. 1998;95:71–89. doi: 10.1080/09553000050176270. [DOI] [PubMed] [Google Scholar]
- 55.Giovani B, Byrdin M, Ahmad M, Brettel K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Molecul. Biol. 2003;10:489–490. doi: 10.1038/nsb933. [DOI] [PubMed] [Google Scholar]
- 56.Hong G, Pachter R, Essen L-O, Ritz T. Electron transfer and spin dynamics of the radical-pair in the cryptochrome from chlamydomonas reinhardtii by computational analysis. The J. Chem. Phys. 2020;152:065101. doi: 10.1063/1.5133019. [DOI] [PubMed] [Google Scholar]
- 57.Hochstoeger T, et al. The biophysical, molecular, and anatomical landscape of pigeon cry4: A candidate light-based quantal magnetosensor. Sci. Adv. 2020;6:eabb9110. doi: 10.1126/sciadv.abb9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller P, Ahmad M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin-superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011;286:21033–21040. doi: 10.1074/jbc.M111.228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero E, Gómez Castellanos JR, Gadda G, Fraaije MW, Mattevi A. Same substrate, many reactions: Oxygen activation in flavoenzymes. Chem. Rev. 2018;118:1742–1769. doi: 10.1021/acs.chemrev.7b00650. [DOI] [PubMed] [Google Scholar]
- 60.Chaiyen P, Fraaije MW, Mattevi A. The enigmatic reaction of flavins with oxygen. Trends Biochem. Sci. 2012;37:373–380. doi: 10.1016/j.tibs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Mondal P, Huix-Rotllant M. Theoretical insights into the formation and stability of radical oxygen species in cryptochromes. Phys. Chem. Chem. Phys. 2019;21:8874–8882. doi: 10.1039/c9cp00782b. [DOI] [PubMed] [Google Scholar]
- 62.Gegear RJ, Foley LE, Casselman A, Reppert SM. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature. 2010;463:804–807. doi: 10.1038/nature08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sherrard RM, et al. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 2018;16:e2006229. doi: 10.1371/journal.pbio.2006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edgar RS, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dufor T, et al. Neural circuit repair by low-intensity magnetic stimulation requires cellular magnetoreceptors and specific stimulation patterns. Sci. Adv. 2019;5:eaav9847. doi: 10.1126/sciadv.aav9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith J, Zadeh-Haghighi H, Salahub D, Simon C. Radical pairs may play a role in xenon-induced general anesthesia. Sci. Rep. 2021;11:6287. doi: 10.1038/s41598-021-85673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyson JJ, Hong CI, Thron CD, Novak B. A simple model of circadian rhythms based on dimerization and proteolysis of per and tim. Biophys. J. 1999;77:2411–2417. doi: 10.1016/S0006-3495(99)77078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leloup J-C, Gonze D, Goldbeter A. Limit cycle models for circadian rhythms based on transcriptional regulation in drosophila and neurospora. J. Biol. Rhythms. 1999;14:433–448. doi: 10.1177/074873099129000948. [DOI] [PubMed] [Google Scholar]
- 69.Goldbeter A. Computational approaches to cellular rhythms. Nature. 2002;420:238–245. doi: 10.1038/nature01259. [DOI] [PubMed] [Google Scholar]
- 70.Leloup J-C, Goldbeter A. A model for circadian rhythms in drosophila incorporating the formation of a complex between the per and tim proteins. J. Biol. Rhythms. 1998;13:70–87. doi: 10.1177/074873098128999934. [DOI] [PubMed] [Google Scholar]
- 71.Player TC, Baxter ED, Allatt S, Hore P. Amplification of weak magnetic field effects on oscillating reactions. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-88871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hore PJ. Upper bound on the biological effects of 50/60 hz magnetic fields mediated by radical pairs. Elife. 2019;8:e44179. doi: 10.7554/eLife.44179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. Cry, a drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 74.Arthaut L-D, et al. Blue-light induced accumulation of reactive oxygen species is a consequence of the drosophila cryptochrome photocycle. PloS One. 2017;12:e0171836. doi: 10.1371/journal.pone.0171836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ehnert S, et al. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of and . Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-14983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pei J-F, et al. Diurnal oscillations of endogenous sustained by p66 shc regulate circadian clocks. Nat. Cell Biol. 2019;21:1553–1564. doi: 10.1038/s41556-019-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee AA, et al. Alternative radical pairs for cryptochrome-based magnetoreception. J. Royal Soc. Interface. 2014;11:20131063. doi: 10.1098/rsif.2013.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Efimova O, Hore P. Role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophys. J. 2008;94:1565–1574. doi: 10.1529/biophysj.107.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulten K, Staerk H, Weller A, Werner H-J, Nickel B. Magnetic field dependence of the geminate recombination of radical ion pairs in polar solvents. Z Phys. Chem. 1976;101:371–390. [Google Scholar]
- 80.Usselman RJ, et al. The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Sci. Rep. 2016;6:1–6. doi: 10.1038/srep38543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sies H, Jones DP. Reactive oxygen species (ros) as pleiotropic physiological signalling agents. Nat. Rev. Molecul. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 82.Novák B, Tyson JJ. Design principles of biochemical oscillators. Nat. Rev. Molecul. Cell Biol. 2008;9:981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasty J, McMillen D, Collins JJ. Engineered gene circuits. Nature. 2002;420:224–230. doi: 10.1038/nature01257. [DOI] [PubMed] [Google Scholar]
- 84.Lowrey PL, Takahashi JS. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Ann. Rev. Genet. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- 85.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 86.Padiath QS, Paranjpe D, Jain S, Sharma VK. Glycogen synthase kinase 3 as a likely target for the action of lithium on circadian clocks. Chronobiol. Int. 2004;21:43–55. doi: 10.1081/cbi-120027981. [DOI] [PubMed] [Google Scholar]
- 87.Baik LS, et al. Distinct mechanisms of drosophila cryptochrome-mediated light-evoked membrane depolarization and in vivo clock resetting. Proc. Natl. Acad. Sci. 2019;116:23339–23344. doi: 10.1073/pnas.1905023116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandrasekaran S, et al. Tuning flavin environment to detect and control light-induced conformational switching in drosophila cryptochrome. Commun. Biol. 2021;4:1–12. doi: 10.1038/s42003-021-01766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bradlaugh, A. A. et al. Essential elements of radical pair magnetosensitivity in drosophila. bioRxiv (2021). [DOI] [PMC free article] [PubMed]
- 90.Sheppard DM, et al. Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci. Rep. 2017;7:1–7. doi: 10.1038/srep42228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Player TC, Hore P. Viability of superoxide-containing radical pairs as magnetoreceptors. The J. Chem. Phys. 2019;151:225101. doi: 10.1063/1.5129608. [DOI] [PubMed] [Google Scholar]
- 92.Hogben HJ, Efimova O, Wagner-Rundell N, Timmel CR, Hore P. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: Origin of zeeman resonances observed by in vivo epr spectroscopy. Chem. Phys. Lett. 2009;480:118–122. [Google Scholar]
- 93.Kattnig DR. Radical-pair-based magnetoreception amplified by radical scavenging: Resilience to spin relaxation. The J. Phys. Chem. B. 2017;121:10215–10227. doi: 10.1021/acs.jpcb.7b07672. [DOI] [PubMed] [Google Scholar]
- 94.Kattnig DR, Hore P. The sensitivity of a radical pair compass magnetoreceptor can be significantly amplified by radical scavengers. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-09914-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerver ED, et al. In situ detection of spontaneous superoxide anion and singlet oxygen production by mitochondria in rat liver and small intestine. The Histochem. J. 1997;29:229–237. doi: 10.1023/a:1026453926517. [DOI] [PubMed] [Google Scholar]
- 96.Miyamoto S, Martinez GR, Medeiros MH, Di Mascio P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B: Biol. 2014;139:24–33. doi: 10.1016/j.jphotobiol.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 97.Kanofsky JR. Singlet oxygen production by biological systems. Chemico-Biol. Interact. 1989;70:1–28. doi: 10.1016/0009-2797(89)90059-8. [DOI] [PubMed] [Google Scholar]
- 98.Goushi K, Yoshida K, Sato K, Adachi C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photon. 2012;6:253–258. [Google Scholar]
- 99.Fay TP, Manolopoulos DE. Radical pair intersystem crossing: Quantum dynamics or incoherent kinetics? The J. Chem. Phys. 2019;150:151102. doi: 10.1063/1.5095204. [DOI] [PubMed] [Google Scholar]
- 100.Ruan W, Yuan X, Eltzschig HK. Circadian rhythm as a therapeutic target. Nat. Revi. Drug Discov. 2021;20:287–307. doi: 10.1038/s41573-020-00109-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: Circadian rhythms in human cognition. Cognit. Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]
- 102.Kelleher FC, Rao A, Maguire A. Circadian molecular clocks and cancer. Cancer Lett. 2014;342:9–18. doi: 10.1016/j.canlet.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 103.Nahmias, Y. & Androulakis, I. P. Circadian effects of drug responses. Ann. Rev. Biomed. Eng.23 (2021). [DOI] [PMC free article] [PubMed]
- 104.Raz A. Perspectives on the efficacy of antidepressants for child and adolescent depression. PLoS Med. 2005;3:e9. doi: 10.1371/journal.pmed.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krishnan N, Davis AJ, Giebultowicz JM. Circadian regulation of response to oxidative stress in drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008;374:299–303. doi: 10.1016/j.bbrc.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulgherait M, et al. Circadian regulation of mitochondrial uncoupling and lifespan. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-15617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Acosta-Rodríguez VA, Rijo-Ferreira F, Green CB, Takahashi JS. Importance of circadian timing for aging and longevity. Nat. Commun. 2021;12:1–12. doi: 10.1038/s41467-021-22922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mattis J, Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends in Endocrinol. Metab. 2016;27:192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mouritsen H. Long-distance navigation and magnetoreception in migratory animals. Nature. 2018;558:50–59. doi: 10.1038/s41586-018-0176-1. [DOI] [PubMed] [Google Scholar]
- 110.Hameroff SR, Craddock TJ, Tuszynski JA. Quantum effects in the understanding of consciousness. J. Integr. Neurosci. 2014;13:229–252. doi: 10.1142/S0219635214400093. [DOI] [PubMed] [Google Scholar]
- 111.Fisher MP. Quantum cognition: The possibility of processing with nuclear spins in the brain. Ann. Phys. 2015;362:593–602. [Google Scholar]
- 112.Simon C. Can quantum physics help solve the hard problem of consciousness? J. Conscious. Stud. 2019;26:204–218. [Google Scholar]
- 113.Adams B, Petruccione F. Quantum effects in the brain: A review. AVS Quant. Sci. 2020;2:022901. [Google Scholar]
- 114.Kumar S, Boone K, Tuszyński J, Barclay P, Simon C. Possible existence of optical communication channels in the brain. Sci. Rep. 2016;6:36508. doi: 10.1038/srep36508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gauger EM, Rieper E, Morton JJ, Benjamin SC, Vedral V. Sustained quantum coherence and entanglement in the avian compass. Phys. Rev. Lett. 2011;106:040503. doi: 10.1103/PhysRevLett.106.040503. [DOI] [PubMed] [Google Scholar]
- 116.Bandyopadhyay JN, Paterek T, Kaszlikowski D. Quantum coherence and sensitivity of avian magnetoreception. Phys. Rev. Lett. 2012;109:110502. doi: 10.1103/PhysRevLett.109.110502. [DOI] [PubMed] [Google Scholar]
- 117.Cai J, Guerreschi GG, Briegel HJ. Quantum control and entanglement in a chemical compass. Phys. Rev. Lett. 2010;104:220502. doi: 10.1103/PhysRevLett.104.220502. [DOI] [PubMed] [Google Scholar]
- 118.Kominis I. Magnetic sensitivity and entanglement dynamics of the chemical compass. Chem. Phys. Lett. 2012;542:143–146. [Google Scholar]
- 119.Pauls JA, Zhang Y, Berman GP, Kais S. Quantum coherence and entanglement in the avian compass. Phys. Rev. E. 2013;87:062704. doi: 10.1103/PhysRevE.87.062704. [DOI] [PubMed] [Google Scholar]
- 120.Tiersch M, Guerreschi G, Clausen J, Briegel H. Approaches to measuring entanglement in chemical magnetometers. The J. Phys. Chem. A. 2014;118:13–20. doi: 10.1021/jp408569d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y, Berman GP, Kais S. Sensitivity and entanglement in the avian chemical compass. Phys. Rev. E. 2014;90:042707. doi: 10.1103/PhysRevE.90.042707. [DOI] [PubMed] [Google Scholar]
- 122.Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 123.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the drosophila clock by photic regulation of per and a per-tim complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- 124.Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of timeless and entrainment of the drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 125.Zeng H, Qian Z, Myers MP, Rosbash M. A light-entrainment mechanism for the drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The generated datasets and computational analysis are available from the corresponding author on reasonable request.