Abstract
This multi-center, retrospective study aimed to clarify the factors affecting drug retention of the Janus kinase inhibitors (JAKi) including baricitinib (BAR) and tofacitinib (TOF) in patients with RA. Patients were as follows; females, 80.6%; age, 60.5 years; DAS28-ESR, 4.3; treated with either BAR (n = 166) or TOF (n = 185); bDMARDs- or JAKi-switched cases (76.6%). The reasons for drug discontinuation were classified into four major categories. The drug retention was evaluated at 24 months using the Kaplan–Meier method and multivariate Cox proportional hazards modelling adjusted by confounders. Discontinuation rates for the corresponding reasons were as follows; ineffectiveness (22.3%), toxic adverse events (13.3%), non-toxic reasons (7.2%) and remission (0.0%). Prior history of anti-interleukin-6 receptor antibody (aIL-6R) ineffectiveness significantly increased the risk of treatment discontinuation due to ineffectiveness (p = 0.020). Aging (≥ 75 years) (p = 0.028), usage of PSL ≥ 5 mg/day (p = 0.017) and female sex (p = 0.041) significantly increased the risk of treatment discontinuation due to toxic adverse events. Factors not associated with treatment discontinuation were: number of prior bDMARDs or JAKi, concomitant MTX usage, difference of JAKi, and prior use of TNF inhibitor, CTLA4-Ig or other JAKi.
Subject terms: Rheumatic diseases, Outcomes research
Introduction
The recommendations of the 2019 European League Against Rheumatism (EULAR) stated that the efficacies of anti-interleukin (IL)-6 receptor antibody (aIL-6R; tocilizumab and sarilumab), cytotoxic T lymphocyte-associated antigen-4-Ig (CTLA4-Ig; abatacept) and Janus kinase inhibitors (JAKi) such as baricitinib (BAR; a JAK1 and JAK2 inhibitor) and tofacitinib (TOF; a JAK1 and JAK3 inhibitor) are considered equivalent to those of tumor necrosis factor inhibitors (TNFi) in both Phase II and Phase III treatments of rheumatoid arthritis (RA)1. The authors reported no significant differences in outcomes between biological disease-modifying antirheumatic drugs (bDMARDs) and JAKi therapy, irrespective of their targets.
JAKi inhibits the JAK-signal transducer and activator of transcription pathways system, which leads to the inhibition of IL-6 and various other cytokines2. Five JAKi, including TOF (2013), BAR (2017), peficitinib (2019), upadacitinib (2020) and filgotinib (2020), have been approved for use in Japan—the only country to have approved five JAKi.
In real-world settings, JAKi tends to be introduced in patients with intolerance to methotrexate (MTX) due to comorbidities or with multiple bDMARDs failures—quite different from those recruited in randomised controlled trials. Therefore, it is of great interest to investigate factors affecting the effectiveness and safety of JAKi in ‘difficult-to-treat’ RA patients, especially those who were previously treated with TNFi, aIL-6R, CTLA4-Ig or another JAKi.
The performance of bDMARDs has increasingly been investigated through recent cohort-based observational studies3,4 in which drug retention is considered a major index of both treatment safety and effectiveness5,6. We have recently reported the drug retention rates of bDMARDs7–12, factors affecting the efficacy of bDMARDs13,14 and factors affecting the achievement of bDMARDs-free remission15 on the basis of findings from our cohort. The aim of the present multicenter, retrospective study is to clarify the factors affecting drug retention of a JAKi (BAR or TOF) in real-world settings.
Methods
Study design and patients
The Kansai Consortium for Well-being of Rheumatic Disease Patients (ANSWER) cohort is an observational, multicenter registry of patients with RA in the Kansai district of Japan7–12. Data were retrospectively collected from patients who were examined at seven major university-related hospitals (Kyoto University, Osaka University, Osaka Medical College, Kansai Medical University, Kobe University, Nara Medial University and Osaka Red Cross Hospital). RA was diagnosed on the basis of the 1987 RA classification criteria of the American College of Rheumatology (ACR)16 or the 2010 ACR/EULAR RA classification criteria17.
Patients who were treated with either BAR or TOF between 2013 and 2020, with complete data on the start and discontinuation dates and the reasons for discontinuation, were included in this study. Additional data were collected, including baseline demographic data (age, sex); disease duration; disease activity (disease activity score in 28 joints using erythrocyte sedimentation rate [DAS28-ESR]); Clinical Disease Activity Index (CDAI) score; concomitant doses (calculated as a blank when not combined) and ratios of MTX and glucocorticoid (GC) (prednisolone [PSL] equivalent); concomitant ratios of other conventional disease-modifying antirheumatic drugs (csDMARDs), such as salazosulfapyridine, bucillamine, iguratimod, tacrolimus and leflunomide; rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody positivity; and Health Assessment Questionnaire Disability Index score7–9. Patients were categorized by age: young, < 65 years; old, 65–74 years; and very old, ≥ 75 years18 and by concomitant dose of PSL (< 5 or ≥ 5 mg/day)19 because previous reports had demonstrated that these categories are associated with drug retention of bDMARDs and JAKi.
In Japan, public national health insurance covers 70%–90% of medical expense, and bDMARDs or JAKi can be administered at the discretion of attending rheumatologists, in accordance with the Japan College of Rheumatology guidelines20–22. The dose of each agent is determined in accordance with the manufacturer’s recommendation. Drug retention was retrospectively evaluated as the duration until definitive treatment interruption. The reasons for discontinuation were classified into four major categories as follows: (1) lack of effectiveness (including primary and secondary); (2) toxic adverse events (infection, skin or systemic reaction and other toxic events, including haematologic, pulmonary, renal, cardiovascular complications and malignancies); (3) non-toxic reasons (patient preference, change in hospital, desire for pregnancy, etc.); and (4) disease remission7–9,11,12. Physicians were allowed to cite only one reason for discontinuation.
Statistical analyses
Differences in baseline clinical characteristics between the groups were assessed using the Mann–Whitney U test (for continuous variables) and the chi-squared test (for categorical variables). The Kaplan–Meier method adjusted by potential confounders was used to examine the survival curves for the agents, as determined by the specified causes. Hazard ratios (HR) and Cox p-value for each discontinuation reason of treatment at 24 months were analyzed using multivariate Cox proportional hazards modelling, by including all of the potential confounders and excluding other non-relevant discontinuation reasons3,7–9. The analysis was adjusted for potential confounders that could influence drug retention, as previously described (age; sex; disease duration; concomitant PSL and MTX use; difference of JAKi; number of switched bDMARDs or JAKi; prior use of TNFi, aIL-6R, CTLA4-Ig or other JAKi)3,23–26. Some minor missing baseline data such as disease activities were extracted by last observation carried forward, which were excluded from the adjustment confounders. In evaluating the effects of prior treatment on drug retention, patients with at least one history of discontinuation due to ineffectiveness in the same drug categories (TNFi, aIL-6R, CTLA4-Ig or JAKi) were categorized as “drug ineffectiveness”. Other 3 reasons for discontinuation excluding ineffectiveness was categorized as “drug intolerance”. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R software (R Foundation for Statistical Computing, Vienna, Austria)27. A two-sided p value < 0.05 was considered statistically significant.
Ethical statement
The representative facility of this registry was Kyoto University, and this observational study was conducted in accordance with the Declaration of Helsinki, with the approval of the ethics committees of the following seven institutes: Kyoto University (2016-03-24/approval No. R053), Osaka University (2015-11-04/approval No. 15300), Osaka Medical College (2014-07-14/approval No. 1529), Kansai Medical University (2017-11-21/approval No. 2014625), Kobe University (2015-03-20/approval No. 1738), Nara Medial University (2018-01-23/approval No. 1692), and Osaka Red Cross Hospital (2015-09-01/approval No. 644). The board of the Osaka University Hospital Ethics Committee waived the requirement for patient informed consent because of the anonymous nature of the data. Written informed consent was obtained from the participants in other institutes.
Results
Clinical characteristics
Table 1 presents the baseline clinical characteristics of the patients at initiation of treatment with each agent. Most of the patients who experienced prior JAKi were switched from TOF to BAR (n = 30) or from BAR to TOF (n = 8). Overall, patients were treated by a low dose and ratio of MTX, and had mostly switched from other bDMARDs or JAKi, suggesting ‘difficult-to-treat’ backgrounds.
Table 1.
Patients’ clinical characteristics at initiation of treatment with each agent.
| Variable | BAR (n = 166) | TOF (n = 185) | p value |
|---|---|---|---|
| Age (years) | 60.2 ± 13.5 | 60.7 ± 13.1 | 0.87 |
| Female sex (%) | 86.7 | 75.1 | 0.009 |
| Disease duration (years) | 12.6 ± 10.6 | 9.7 ± 8.3 | 0.016 |
| RF positivity (%) | 86.1 | 81.6 | 0.50 |
| ACPA positivity (%) | 82.0 | 83.1 | 0.93 |
| DAS28-ESR | 4.3 ± 1.3 | 4.3 ± 1.3 | 0.98 |
| CDAI | 17.2 ± 11.0 | 18.8 ± 11.1 | 0.16 |
| HAQ-DI | 0.9 ± 0.7 | 0.9 ± 0.8 | 0.81 |
| PSL use (%) | 42.8 | 50.3 | 0.19 |
| PSL dose (mg/day) | 4.7 ± 3.2 | 5.7 ± 3.3 | 0.022 |
| MTX use (%) | 64.5 | 57.3 | 0.048 |
| MTX dose (mg/week) | 8.7 ± 3.1 | 9.2 ± 3.3 | 0.35 |
| SASP use (%) | 11.4 | 23.8 | 0.004 |
| BUC use (%) | 7.8 | 8.6 | 0.93 |
| IGU use (%) | 13.3 | 17.8 | 0.30 |
| TAC use (%) | 15.7 | 9.7 | 0.13 |
| LEF use (%) | 0.0 | 0.0 | N.A |
| bDMARDs or JAKi naive (%) | 22.3 | 24.3 | 0.75 |
| 2nd bDMARDs or JAKi (%) | 23.5 | 24.3 | 0.86 |
| 3rd bDMARDs or JAKi (%) | 26.5 | 16.2 | 0.018 |
| ≥ 4th bDMARDs or JAKi (%) | 27.7 | 35.1 | 0.14 |
| Prior TNFi use (%) | 57.8 | 65.9 | 0.15 |
| Prior aIL-6R use (%) | 36.1 | 40.5 | 0.46 |
| Prior CTLA4-Ig use (%) | 31.9 | 25.4 | 0.22 |
| Prior JAKi use (%) | 20.5 | 6.5 | < 0.001 |
| Prior JAKi | TOF (n = 30), BAR (n = 1), PEF (n = 3) | TOF (n = 4), BAR (n = 8) | N.A |
Values are presented as mean ± standard deviation or percentage. Differences between the groups were assessed by the Mann–Whitney U test or the chi-squared test.
N.A. not applicable, BAR baricitinib, TOF tofacitinib, RF rheumatoid factor, ACPA anticyclic citrullinated peptide antibody, DAS28-ESR Disease Activity Score in 28 joints using erythrocyte sedimentation rate, CDAI clinical disease activity index, HAQ-DI Health Assessment Questionnaire disability index, PSL prednisolone, MTX methotrexate, SASP salazosulfapyridine, BUC bucillamine, IGU iguratimod, TAC tacrolimus, LEF leflunomide, bDMARDs biological disease-modifying antirheumatic drugs, JAKi Janus kinase inhibitor, TNFi tumour necrosis factor inhibitors, aIL-6R anti-interleukin-6 receptor, CTLA4-Ig cytotoxic T lymphocyte-associated antigen-4-Ig, PEF peficitinib.
Reasons and rates of drug discontinuation
In total, the adjusted discontinuation rates for the corresponding reasons at 24 months were as follows: lack of effectiveness (22.3%), toxic adverse events (13.3%), non-toxic reasons (7.2%) and remission (0.0%). We further investigated the factors affecting discontinuation due to lack of effectiveness and toxic adverse events, using multivariate Cox proportional hazards modelling. Prior use of aIL-6R significantly increased the risk of treatment discontinuation due to lack of effectiveness (HR, 2.07; p = 0.021), and prior use of TNFi tended to increase the risk of treatment discontinuation due to lack of effectiveness (HR, 1.90; p = 0.075) (Table 2).
Table 2.
Cox proportional hazard analysis for the risk factors of treatment discontinuation due to lack of effectiveness.
| Variable | HR (95% CI) | p value |
|---|---|---|
| Prior aIL-6R use (%) | 2.07 (1.12–3.83) | 0.021 |
| Prior TNFi use (%) | 1.90 (0.94–3.84) | 0.075 |
| Age (years) | 0.99 (0.97–1.01) | 0.14 |
| Sex (male) | 0.65 (0.34–1.21) | 0.17 |
| Disease duration (years) | 0.98 (0.95–1.01) | 0.20 |
| MTX use (%) | 1.21 (0.71–2.04) | 0.49 |
| PSL use (≥ 5 mg/day) | 0.86 (0.50–1.47) | 0.57 |
| Switched number of bDMARDs or JAKi | 0.93 (0.71–1.22) | 0.58 |
| Difference of JAKi (TOF use) | 1.11 (0.64–1.94) | 0.70 |
| Prior JAKi use (%) | 1.13 (0.48–2.69) | 0.78 |
| Prior CTLA4-Ig use (%) | 1.02 (0.53–1.96) | 0.97 |
HR hazard ratio, CI confidence interval, aIL-6R anti-interleukin-6 receptor, TNFi tumour necrosis factor inhibitors, MTX methotrexate, PSL prednisolone, bDMARDs biological disease-modifying antirheumatic drugs, JAKi Janus kinase inhibito, TOF tofacitinib, CTLA4-Ig cytotoxic T lymphocyte-associated antigen-4-Ig.
As for discontinuation due to toxic adverse events, significant confounders were aging (HR, 1.04; p = 0.015), PSL usage ≥ 5 mg/day (HR, 2.21; p = 0.017) and male sex (HR, 0.33; p = 0.041) (Table 3).
Table 3.
Cox proportional hazard analysis for the risk factors of treatment discontinuation due to toxic adverse events.
| Variable | HR (95% CI) | p value |
|---|---|---|
| Age (years) | 1.04 (1.01–1.06) | 0.015 |
| PSL use (≥ 5 mg/day) | 2.21 (1.15–4.23) | 0.017 |
| Sex (male) | 0.33 (0.11–0.95) | 0.041 |
| Disease duration (years) | 1.01 (0.98–1.04) | 0.64 |
| Prior aIL-6R use (%) | 0.83 (0.35–1.97) | 0.67 |
| MTX use (%) | 1.14 (0.58–2.27) | 0.70 |
| Prior JAKi use (%) | 1.22 (0.41–3.61) | 0.72 |
| Difference of JAKi (TOF use) | 0.91 (0.45–1.85) | 0.79 |
| Prior CTLA4-Ig use (%) | 0.96 (0.42–2.19) | 0.93 |
| Switched number of bDMARDs or JAKi | 1.01 (0.71–1.46) | 0.94 |
| Prior TNFi use (%) | 1.03 (0.41–2.60) | 0.96 |
HR hazard ratio, CI confidence interval, PSL prednisolone, aIL-6R anti-interleukin-6 receptor, MTX methotrexate, JAKi Janus kinase inhibitor, TOF tofacitinib, CTLA4-Ig cytotoxic T lymphocyte-associated antigen-4-Ig, bDMARDs biological disease-modifying antirheumatic drugs, TNFi tumour necrosis factor inhibitors.
The adjusted drug retention rates for the corresponding reasons and the statistical differences between the groups were as follows.
Between BAR and TOF, there were no significant differences in the retention rates due to lack of effectiveness (BAR, 84.6% vs. TOF, 75.9%; p = 0.70) or toxic adverse events (BAR, 82.7% vs. TOF, 87.5%; p = 0.79; data not shown).
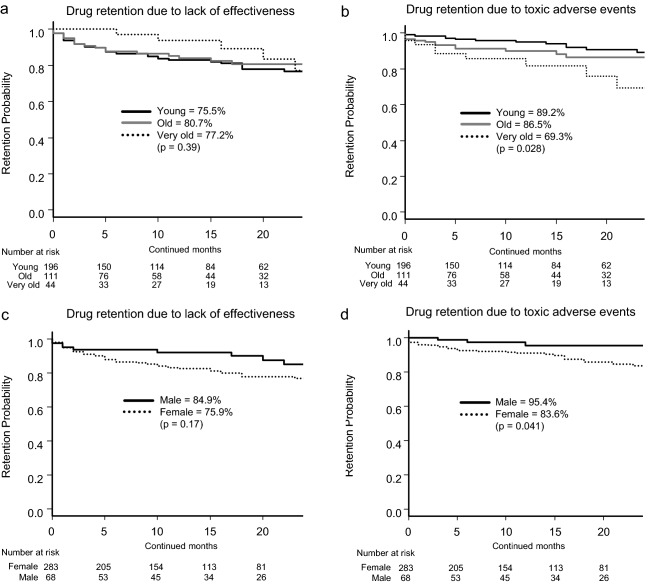
With regard to age, retention rates due to lack of effectiveness were: young, 75.5% vs. old, 80.7% vs. very old, 77.2% (p = 0.39; Fig. 1a) and those of toxic adverse events were: young, 89.2% vs. old, 86.5% vs. very old, 69.3% (p = 0.028; Fig. 1b). With regard to sex, retention rates due to lack of effectiveness were: male, 84.9% vs. female, 75.9% (p = 0.17; Fig. 1c) and those of toxic adverse events were: male, 95.4% vs. female, 83.6% (p = 0.041; Fig. 1d). Taken together, very old patients (≥ 75 years) and female patients showed higher risk of treatment discontinuation due to toxic adverse events.
Figure 1.
Adjusted drug retention by age and sex. Adjusted drug retention between young (< 65 years), aged (65–74 years), and very old (≥ 75 years) groups, due to (a) lack of effectiveness and (b) toxic adverse events, and adjusted drug retention between male and female, due to (c) lack of effectiveness and (d) toxic adverse events.
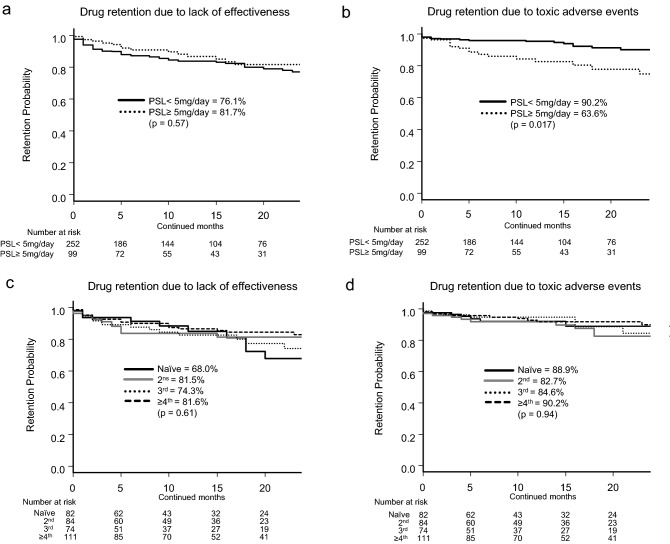
On the other hand, regarding concomitant GC dose, retention rates for discontinuation due to lack of effectiveness were PSL < 5 mg/day, 76.1% vs. PSL ≥ 5 mg/day, 81.7% (p = 0.57; Fig. 2a) and those for toxic adverse events were PSL < 5 mg/day, 90.2% vs. PSL ≥ 5 mg/day, 63.6% (p = 0.017; Fig. 2b). As for the influence of switched number of bDMARDs or JAKi, retention rates due to lack of effectiveness were: naïve, 68.0% vs. 2nd, 81.5% vs. 3rd, 74.3% vs. 4th or more, 81.6% (p = 0.61; Fig. 2c), and those of toxic adverse events were: naïve, 88.9% vs. 2nd, 82.7% vs. 3rd, 84.6% vs. 4th or more, 90.2% (p = 0.94; Fig. 2d). Concomitant PSL ≥ 5 mg/day showed higher risk of treatment discontinuation due to toxic adverse events.
Figure 2.
Adjusted drug retention by PSL dose and bDMARD/JAKi therapy. Adjusted drug retention between concomitant PSL < 5 mg/day and PSL ≥ 5 mg/day, due to (a) lack of effectiveness and (b) toxic adverse events, and adjusted drug retention between switched bDMARD/JAKi groups due to (c) lack of effectiveness and (d) toxic adverse events. PSL prednisolone, bDMARD biological disease-modifying antirheumatic drug, JAKi Janus kinase inhibitor.
Regarding prior TNFi treatment, the retention rates were: without prior TNFi, 80.4% vs. prior TNFi intolerance, 88.3% vs. prior TNFi ineffectiveness, 71.4% (p = 0.17; Fig. 3a). As for prior aIL-6R treatment, the adjusted retention rates were: without prior aIL-6R, 81.8% vs. prior aIL-6R intolerance, 83.5% vs. prior aIL-6R ineffectiveness, 63.3% (p = 0.020; Fig. 3b). Regarding prior CTLA4-Ig treatment, the adjusted retention rates were: without prior CTLA4-Ig, 73.4% vs. prior CTLA4-Ig intolerance, 95.9% vs. prior CTLA4-Ig ineffectiveness, 81.8% (p = 0.39; Fig. 3c). As for prior JAKi treatment, the adjusted retention rates were: without prior JAKi, 76.1% vs. prior JAKi intolerance, 84.9% vs. prior JAKi ineffectiveness, 85.2% (p = 0.80; Fig. 3d). Taken together, history of prior aIL-6R ineffectiveness showed higher risk of treatment discontinuation due to lack of effectiveness. History of prior TNFi, CTLA4-Ig or other JAKi ineffectiveness did not significantly affect following JAKi treatment retention.
Figure 3.
Adjusted drug retention by TNFi, aIL-6R, CTLA4-Ig and JAKi experience. Adjusted drug retention due to lack of effectiveness between (a) non-TNFi-experienced, prior TNFi intolerance, and prior TNFi ineffectiveness groups, (b) non-aIL-6R-experienced, prior aIL-6R intolerance, and prior aIL-6R ineffectiveness groups, (c) non-CTLA4-Ig-experienced, prior CTLA4-Ig intolerance, and prior CTLA4-Ig ineffectiveness groups, (d) and non-JAKi-experienced, prior JAKi intolerance, and prior JAKi ineffectiveness groups. TNFi tumour necrosis factor inhibitor, aIL-6R anti-interleukin-6 receptor, CTLA4-Ig cytotoxic T lymphocyte-associated antigen-4-Ig, JAKi Janus kinase inhibitor.
Discussion
Regarding the difference between BAR and TOF, a previous meta-analysis revealed that, in patients with inadequate response to csDMARDs or bDMARDs, BAR and TOF were similarly efficacious28, which accords with the results of the present study.
Concerning the effect of aging, the RA-BUILD and RA-BEAM studies of BAR showed similar clinical efficacy between young (< 50 years) and old (≥ 65 years) patients29, similar to the phase III and long-term extension studies of TOF30. However, elderly patients (≥ 65 years) tended to show a higher rate of discontinuation of BAR treatment due to adverse events (8.8%) than younger patients (< 50 years, 2.3%)29, similar to results for TOF30. Greater age was associated with increased risk of herpes zoster (HZ)31, major adverse cardiovascular events32 and gastrointestinal perforation in TOF treatment33. Thus, in JAKi treatment, aging may not affect efficacy but may attenuate safety.
With regard to sex, females had higher risk of HZ compared with males in TOF treatment30. HZ is one of the most frequent adverse events in JAKi treatment, and has higher incidence in Japan, compared with western countries34. This may lead to the higher rate of discontinuation due to toxic adverse events in our present study. RA disease activity tend to be higher in female, whereas clinical response to csDMARDs and bDMARDs appears to be better in male35. In our present study, although the baseline disease activities (DAS28-ESR and CDAI) were similar (data not shown), drug retention of JAKi due to lack of effectiveness tended to be higher in male compared to female. This tendency may be similar among these anti-rheumatic agents.
Concomitant use of GC with TOF did not affect clinical or radiographic efficacy36. However, the occurrence of HZ doubled in oral GC use30; oral GC (> 7.5 mg/day of PSL) was a risk factor for serious infections (including HZ) in TOF treatment31. In the present study, patients with PSL ≥ 5 mg/day were at higher risk of toxic adverse events, similar to the results for bDMARDs in Japanese RA patients19,37.
Another concern is whether the number or mode of action of prior bDMARDs or JAKi may affect the drug retention of JAKi. Although improvement in disease activity was greatest in the bDMARDs-naïve group, both BAR and TOF were effective in patients refractory to multiple bDMARDs38. In addition, prior use of bDMARDs did not affect the clinical efficacy of BAR39; the clinical efficacy of BAR was similar regardless of previous multiple bDMARD use40. Concerning the mode of action of prior bDMARDs or JAKi, prior use of non-TNFi (n = 31, including aIL-6R and CTLA4-Ig) or JAKi was associated with diminished improvement of DAS28-C-reactive protein (CRP) in BAR treatment40. However, non-TNFi (such as aIL-6R and JAKi) may overly downregulate CRP levels by inhibiting IL-6 signalling regardless of its actual disease activity. Therefore, using DAS28-CRP to evaluate disease activity in aIL-6R or JAKi treatment may overestimate their clinical response, and also underestimate following treatment response. Considering the underlying mechanisms, BAR inhibits JAK1 and JAK2 signalling, while TOF inhibits JAK1 and JAK3 signalling, which are mainly involved in IL-6 production2. Thus, patients who showed ineffectiveness to aIL-6R may not be fully rescued by BAR or TOF. However, JAK2 is also involved in Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF), which initiates arthritis and pain41, and interferon-γ production, which activates macrophages. JAK3 is also involved in IL-2 and IL-21 production, which promote T-cell activation and differentiation, and play important roles in the pathology of RA42. Thus, some patients who are dominated by cytokines other than IL-6 may be rescued by switching between BAR and TOF, although further detailed examinations are required.
MTX inhibits not only IL-6 but also inhibits IL-1, matrix metalloproteinases and RF, which play important roles in joint destruction43. Indeed, BAR monotherapy was inferior to BAR + MTX in radiographic progression44. However, drug retention of BAR due to ineffectiveness45 and also drug retention of BAR and TOF in our present study were not significantly affected by concomitant MTX. Taken together, the effectiveness of JAKi in inhibiting joint destruction may be superior in combination with MTX, although drug retention based on clinical settings may be similar compared with monotherapy. The effectiveness of low-dose MTX in Japanese populations should be considered. Intra-erythrocyte MTX-polyglutamate concentration, which is considered a useful biomarker of MTX efficacy, was 65 nmol/L with MTX of 13.4 mg/week in patients from the United States, and reached 94 nmol/L with MTX of 10.3 mg/week in Japanese patients46.
The limitations of the present study are as follows. First, although patients were followed by experienced senior rheumatologist of university-related hospitals, the reasons for discontinuation depended on the decisions of different physicians without standardized criteria. Second, according to the Japanese guidelines, TNFi, aIL-6R, or CTLA4-Ig are equally recommended in patients who showed inadequate response to csDMARDs, and JAKi are mainly recommended in patients who showed inadequate response to bDMARDs, which may differ from that of western countries and also affected the results. Third, as the initial dose of each agent was determined according to the manufacturer’s recommendations, minor changes of dose of each agent during the period couldn’t be monitored. Fourth, comorbidities, which can potentially affect drug retention, were not evaluated. Fifth, a relatively small number of prior JAKi-experienced patients may have affected the results. Sixths, the U.S. Food and Drug Administration recently alerted that increased risk of serious cardiovascular events and malignancy of JAKi compared to TNFi, which may affect the results of long-term treatment. However, the strength of this study is that it is the first to evaluate factors affecting plural JAKi retention, by adjusting clinical backgrounds according to prior history of TNFi, aIL-6R, CTLA4-Ig and JAKi, especially in “difficult-to-treat” RA patients who may not be included in randomized controlled trials.
In conclusion, concerning BAR or TOF treatment, prior history of aIL-6R discontinuation due to ineffectiveness may increase the risk of treatment discontinuation due to ineffectiveness. On the other hand, aging (≥ 75 years), concomitant PSL ≥ 5 mg/day, and female sex may increase the risk of treatment discontinuation due to toxic adverse events. These novel findings may provide new insight for the management of JAKi in clinical practice.
Acknowledgements
We thank all the medical staff at all the institutions who participated in the ANSWER cohort for providing the data.
Author contributions
K.E. was responsible for conception and design. K.E., T.H., Y.M., Y.O., M.Ha., K.M., A.O., S.J., R.H., T.T., A.Y., Y.S., H.A., M.K., and E.Y. contributed to data extraction and interpretation. K.E., W.Y., and K.Y. contributed to the design and conduction of statistical analysis. K.E. and M.Hi. prepared the manuscript. A.K., M.Hi., S.O., and K.N. supervised the manuscript. All the authors read and approved the final manuscript.
Funding
The study reported in this publication uses the ANSWER Cohort, was supported by grants from 11 pharmaceutical companies (AbbVie GK, Asahi-Kasei, Ayumi, Chugai, Eisai, Eli Lilly, Janssen K.K, Ono, Sanofi K.K, Teijin Healthcare, and UCB Japan) and an information technology service company (CAC). This study was conducted as an investigator-initiated study. These companies had no roles in the study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Competing interests
KE is affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University, Graduate School of Medicine, which is supported by Taisho. KE has received research grants from AbbVie, Amgen, Asahi-Kasei, Astellas, Chugai, Eisai, Mitsubishi-Tanabe, Ono Pharmaceutical, Teijin Pharma, and UCB Japan. KE has received payments for lectures from AbbVie, Amgen, Asahi-Kasei, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Janssen, Mitsubishi-Tanabe, Ono Pharmaceutical, Pfizer, Sanofi, and UCB Japan. TH received a research grant and/or speaker fee from Astellas, Chugai, GlaxoSmithKline, Nippon Shinyaku, and Eisai. YM received a research grant and/or speaker fee from Eli Lilly, Chugai, Pfizer, Bristol-Myers Squibb, and Mitsubishi-Tanabe. MHashimoto received a research grant and/or speaker fee from Mitsubishi-Tanabe, Eisai, Eli Lilly, Bristol-Myers Squibb, and Novartis Pharma. KM is affiliated with a department that is financially supported by four pharmaceutical companies (Asahi-Kasei, Mitsubishi-Tanabe, Chugai, Ayumi, and UCB Japan) and the city government (Nagahama City). KM received a speaker fee from Eisai. TT is affiliated with a department that is financially supported by six pharmaceutical companies (Mitsubishi-Tanabe, Chugai, Ayumi, Astellas, Eisai, and Takeda). TT received a research grant from Chugai, cover letter and a speaker fee from Astellas, Chugai, Eisai, Mitsubishi-Tanabe, AbbVie, Bristol-Myers Squibb, Ayumi, Daiichi Sankyo, Eisai, Takeda, and Asahi-Kasei. AO received a speaker fee from Chugai, Ono Pharmaceutical, Eli Lilly, Mitsubishi-Tanabe, Asahi-Kasei, and Takeda. SJ has received speaking fees from AbbVie, Asahi-Kasei, Bristol-Myers Squib, Chugai, Eisai, Eli Lilly, Janssen Pharmaceutical, Mitsubishi-Tanabe, and Ono Pharmaceutical. RH received a speaker fee from AbbVie. MHirao received a speaker fee from Astellas, Ono Pharmaceutical, Eli Lilly, Mitsubishi-Tanabe, Pfizer, Ayumi, and Takeda. AK received a research grant and/or speaker fee from Mitsubishi-Tanabe, Chugai, Eisai, Asahi-Kasei, Astellas, AbbVie, Bristol-Myers Squibb, Ono Pharmaceutical, and Pfizer. KN has received a research grant from Astellas, and supervises the Department of Musculoskeletal Regenerative Medicine, Osaka University, Graduate School of Medicine, which is supported by Taisho. WY, YS, HA, AY, MK, KY, YO, YE, and SO have no financial conflicts of interest to disclose concerning this manuscript. These companies had no role in the study design, data collection, data analysis, data interpretation, and preparation of the manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smolen JS, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 2.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017;13:234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- 3.Du Pan SM, et al. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009;61:560–568. doi: 10.1002/art.24463. [DOI] [PubMed] [Google Scholar]
- 4.Favalli EG, et al. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: Real-life data from a local registry. Arthritis Care Res. (Hoboken) 2016;68:432–439. doi: 10.1002/acr.22788. [DOI] [PubMed] [Google Scholar]
- 5.Hyrich KL, Watson KD, Lunt M, Symmons DP. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology (Oxford) 2011;50:117–123. doi: 10.1093/rheumatology/keq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neovius M, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann. Rheum. Dis. 2015;74:354–360. doi: 10.1136/annrheumdis-2013-204128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebina K, et al. Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis: The ANSWER cohort study. PLoS ONE. 2019;14:e0216624. doi: 10.1371/journal.pone.0216624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebina K, et al. Drug tolerability and reasons for discontinuation of seven biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res. Ther. 2019;21:91. doi: 10.1186/s13075-019-1880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebina K, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis: The ANSWER cohort study. PLoS ONE. 2018;13:e0194130. doi: 10.1371/journal.pone.0194130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebina K, et al. Drug retention of sarilumab, baricitinib, and tofacitinib in patients with rheumatoid arthritis: The ANSWER cohort study. Clin. Rheumatol. 2021 doi: 10.1007/s10067-021-05609-7. [DOI] [PubMed] [Google Scholar]
- 11.Ebina K, et al. Drug retention of secondary biologics or JAK inhibitors after tocilizumab or abatacept failure as first biologics in patients with rheumatoid arthritis -the ANSWER cohort study. Clin. Rheumatol. 2020;39:2563–2572. doi: 10.1007/s10067-020-05015-5. [DOI] [PubMed] [Google Scholar]
- 12.Ebina K, et al. Drug retention of 7 biologics and tofacitinib in biologics-naive and biologics-switched patients with rheumatoid arthritis: The ANSWER cohort study. Arthritis Res. Ther. 2020;22:142. doi: 10.1186/s13075-020-02232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jinno S, et al. Comparison of the drug retention and reasons for discontinuation of tumor necrosis factor inhibitors and interleukin-6 inhibitors in Japanese patients with elderly-onset rheumatoid arthritis-the ANSWER cohort study. Arthritis Res. Ther. 2021;23:116. doi: 10.1186/s13075-021-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda Y, et al. Comparison of efficacy between anti-IL-6 receptor antibody and other biological disease-modifying antirheumatic drugs in the patients with rheumatoid arthritis who have knee joint involvement: The ANSWER cohort, retrospective study. Rheumatol. Int. 2021 doi: 10.1007/s00296-021-04862-y. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, et al. Factors associated with the achievement of biological disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: The ANSWER cohort study. Arthritis Res. Ther. 2018;20:165. doi: 10.1186/s13075-018-1673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Aletaha D, et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 18.Lahaye C, et al. Effectiveness and safety of abatacept in elderly patients with rheumatoid arthritis enrolled in the French Society of Rheumatology's ORA registry. Rheumatology (Oxford) 2016;55:874–882. doi: 10.1093/rheumatology/kev437. [DOI] [PubMed] [Google Scholar]
- 19.Koike T, et al. Effectiveness and safety of tocilizumab: Postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J. Rheumatol. 2014;41:15–23. doi: 10.3899/jrheum.130466. [DOI] [PubMed] [Google Scholar]
- 20.Kawahito Y. Guidelines for the management of rheumatoid arthritis. Nihon Rinsho. 2016;74:939–943. [PubMed] [Google Scholar]
- 21.Koike R, et al. Japan College of Rheumatology 2009 guidelines for the use of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, in rheumatoid arthritis. Mod. Rheumatol. 2009;19:351–357. doi: 10.1007/s10165-009-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike R, Takeuchi T, Eguchi K, Miyasaka N. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod. Rheumatol. 2007;17:451–458. doi: 10.1007/s10165-007-0626-3. [DOI] [PubMed] [Google Scholar]
- 23.Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: A cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664–1668. doi: 10.1093/rheumatology/keu158. [DOI] [PubMed] [Google Scholar]
- 24.Gabay C, Riek M, Scherer A, Finckh A. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54:1664–1672. doi: 10.1093/rheumatology/kev019. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg JD, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: Results from the US CORRONA registry. Ann. Rheum. Dis. 2012;71:1134–1142. doi: 10.1136/annrheumdis-2011-150573. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen TS, et al. Effectiveness and drug adherence of biologic monotherapy in routine care of patients with rheumatoid arthritis: A cohort study of patients registered in the Danish biologics registry. Rheumatology. 2015;54:2156–2165. doi: 10.1093/rheumatology/kev216. [DOI] [PubMed] [Google Scholar]
- 27.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae SC, Lee YH. Comparison of the efficacy and safety of tofacitinib and baricitinib in patients with active rheumatoid arthritis: A Bayesian network meta-analysis of randomized controlled trials. Z. Rheumatol. 2019;78:559–567. doi: 10.1007/s00393-018-0531-5. [DOI] [PubMed] [Google Scholar]
- 29.Fleischmann R, et al. Safety and efficacy of baricitinib in elderly patients with rheumatoid arthritis. RMD Open. 2017;3:e000546. doi: 10.1136/rmdopen-2017-000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis JR, et al. Risk for herpes zoster in tofacitinib-treated rheumatoid arthritis patients with and without concomitant methotrexate and glucocorticoids. Arthritis Care Res. (Hoboken) 2019;71:1249–1254. doi: 10.1002/acr.23769. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2924–2937. doi: 10.1002/art.38779. [DOI] [PubMed] [Google Scholar]
- 32.Charles-Schoeman C, et al. Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1450–1459. doi: 10.1002/art.40911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: Risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68:2612–2617. doi: 10.1002/art.39761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harigai M, et al. Safety profile of baricitinib in Japanese patients with active rheumatoid arthritis with over 1.6 years median time in treatment: An integrated analysis of Phases 2 and 3 trials. Mod. Rheumatol. 2020;30:36–43. doi: 10.1080/14397595.2019.1583711. [DOI] [PubMed] [Google Scholar]
- 35.Favalli EG, et al. Sex and management of rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2019;56:333–345. doi: 10.1007/s12016-018-8672-5. [DOI] [PubMed] [Google Scholar]
- 36.Charles-Schoeman C, et al. Effect of glucocorticoids on the clinical and radiographic efficacy of tofacitinib in patients with rheumatoid arthritis: A posthoc analysis of data from 6 phase III studies. J. Rheumatol. 2018;45:177–187. doi: 10.3899/jrheum.170486. [DOI] [PubMed] [Google Scholar]
- 37.Mori S, et al. Comparative risk of hospitalized infection between biological agents in rheumatoid arthritis patients: A multicenter retrospective cohort study in Japan. PLoS ONE. 2017;12:e0179179. doi: 10.1371/journal.pone.0179179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitton J, Melville AR, Emery P, Nam JL, Buch MH. Real-world single centre use of JAK inhibitors across the rheumatoid arthritis pathway. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa858. [DOI] [PubMed] [Google Scholar]
- 39.Spinelli FR, et al. Effectiveness and safety of baricitinib in rheumatoid arthritis: A monocentric, longitudinal, real-life experience. Clin. Exp. Rheumatol. 2020;39:525–531. [PubMed] [Google Scholar]
- 40.Takahashi N, et al. Predictors for clinical effectiveness of baricitinib in rheumatoid arthritis patients in routine clinical practice: Data from a Japanese multicenter registry. Sci. Rep. 2020;10:21907. doi: 10.1038/s41598-020-78925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirota K, et al. Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity. 2018;48:1220–1232. doi: 10.1016/j.immuni.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: A review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 43.Swierkot J, Szechinski J. Methotrexate in rheumatoid arthritis. Pharmacol. Rep. 2006;58:473–492. [PubMed] [Google Scholar]
- 44.Fleischmann R, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69:506–517. doi: 10.1002/art.39953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guidelli GM, et al. Efficacy and safety of baricitinib in 446 patients with rheumatoid arthritis: A real-life multicentre study. Clin. Exp. Rheumatol. 2020;39:868–783. [PubMed] [Google Scholar]
- 46.Takahashi C, et al. Association of erythrocyte methotrexate-polyglutamate levels with the efficacy and hepatotoxicity of methotrexate in patients with rheumatoid arthritis: A 76-week prospective study. RMD Open. 2017;3:e000363. doi: 10.1136/rmdopen-2016-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.