Abstract
OBJECTIVE
To explore the correlates of diabetes-related distress (DD) with psychometrically valid assessments of emotional regulation in individuals with type 1 and type 2 diabetes.
RESEARCH DESIGN AND METHODS
Adults with diabetes (n = 298) were assessed for psychological issues possibly associated with diabetes and were further evaluated with measures of negative emotional experience (ER-Exp) and skill at regulating such experiences (ER-Skill) and measures of DD, perceived psychosocial stress, diabetes literacy, and diabetes self-care.
RESULTS
ER-Exp was directly related to DD, while ER-Skill was inversely related to DD. Together, these ER variables displayed a medium-size relationship (β = 0.45) with DD. Inclusion of variables related to diabetes self-care and perceived psychosocial stress was associated with only an 18% reduction (i.e., β = 0.45 to β = 0.38) in the strength of this relationship, while the magnitude of relationships between DD and perceived psychosocial stress (β = 0.15) and diabetes self-care (β = −0.09) was relatively small.
CONCLUSIONS
These data suggest that DD is meaningfully linked with negative emotionality, and skill at regulating such emotions, in adults with diabetes. This relationship appears to be stronger than that between DD and perceived psychological stress or diabetes self-care. If so, DD (and possibly A1C) may be improved in those with diabetes and difficulties with negative emotionality.
Introduction
Currently, 34 million Americans are living with diabetes (1), a chronic illness requiring multiple self-care behaviors to slow the onset of complications affecting all major organ systems in the body. Despite an increase in behavioral modifications, medications, and device technologies since the discovery of insulin, <50% of people with diabetes achieve a glycemic target of A1C <7.0% (<53 mmol/mol), indicating that reaching glycemic targets is beyond the reach of many with diabetes (2,3).
While achievement of glycemic management targets is desirable for individuals and their providers, multiple factors, both internal and external to the individual, pose substantial barriers to achieving this goal. Diabetes-related distress (DD), the emotional distress specific to the regimen, burden, and interpersonal factors involved in daily care of diabetes, is common. Of adults with diabetes, (38–45% [4]) report at least moderate levels of DD (4,5). DD is inversely related (and independent of depression) with quality of life measures (6). In addition, DD is associated with reduced diabetes self-care behaviors (7) and demonstrates an independent association with A1C, with fluctuations of DD going hand in hand with changes over time in A1C levels (4,8). Recent studies show that DD is more prevalent than depressive disorders (9) and that DD, itself, should be targeted for treatment in people with diabetes (10).
Therapeutic approaches to treating DD, ranging from multidisciplinary diabetes education to psychological interventions (7), have resulted in only limited improvements in A1C. This may be because DD is better related to the patient’s facility in emotional regulation, specifically, to the nature of emotional experience and to skill in managing emotional responses, which have not yet been targeted in intervention studies of DD in people with diabetes. To this point, Hughes, Berg, and Wiebe (11) have reported that low emotional processing, along with low self-control, was related to the poorest A1C levels in adolescents with type 1 diabetes (T1D). Further, they noted that emotion processing predicted A1C levels better than other variables (e.g., self-efficacy, adherence to medical regimens, etc.).
The experience, processing, and understanding of and coping with emotions are broadly referred to as emotional regulation (12). Issues in “emotionality” are manifest when individuals feel an excess of negative emotion, either in general or in response to daily stressors, and/or in shifting between different negative emotional states, and are labeled as “emotional experience” (ER-Exp). Difficulty in identifying, evaluating, and controlling the expression of emotion in an appropriate manner is referred to skill in emotion management (ER-Skill) (13). ER-Exp and ER-Skill are inversely related, and the presence of poor ER-Skill is, not surprisingly, associated with increases in ER-Exp (negative emotionality). Given that living with diabetes involves considerable time and energy from those with diabetes and, even if optimally performed, self-care behaviors do not often result in desired blood glucose levels, feelings of frustration, helplessness, and hopelessness (DD, ER-Exp) occur on a regular basis. Thus, we posit that a critical barrier to treatment in these individuals is broad difficulty with negative ER-Exp and, likely, less ER-Skill (5,14).
Support for this idea come from post hoc analyses (15) from a large treatment study of DD in those with T1D comparing a low-intensity emotion-management intervention (i.e., 1-day workshop with four online video sessions over 3 months) with a psycho-educational intervention (16). With combination of all available data, a construct of “emotion management” was associated with reducing DD, increasing self-care, and reducing A1C levels. Examination of these relationships suggested that adverse judgment of emotions, emotional reactivity, and, to a lesser degree, poor emotion processing were related to DD (15), setting the stage for future DD treatment studies to focus more on emotion management.
In this study, we examined data from our Psychosocial Evaluation and Treatment Program in adult patients with T1D and type 2 diabetes (T2D). We were specifically interested in examining the relationships between DD and measures of negative ER-Exp and ER-Skill. We hypothesized that DD would be associated with variables related to both constructs even in the context of other relevant variables such as diabetes literacy, diabetes self-care, and perceived stress.
Research Design and Methods
Study Participants
Participants were recruited among individuals with T1D and T2D receiving care at the Kovler Diabetes Center program at the University of Chicago Medical Center between 2012 and 2016. Each study participant was followed by a board-certified endocrinologist specializing in the treatment of diabetes. Study participants were referred to the Director (T.D.) of our Psychosocial Evaluation and Treatment Program when Kovler Center physicians judged the presence of psychosocial issues that might affect the study participant’s care. After giving informed consent agreeing that their data would be used for research purposes without identifying them, study participants were evaluated by clinical psychological externs and interns with a structured clinical assessment. As part of that assessment all study participants completed a screening survey and, at a second visit, a series of other assessments relevant to diabetes (see below). The study was approved by the UChicago Medicine Institutional Review Board.
Assessments
Screening
After referral, our staff completed a screening survey to collect data related to demographics (e.g., age, sex, ethnicity, annual income), cognitive function, diabetes treatment history, and personal/family psychiatric history and DD. At a second visit, study participants also completed a set of clinically relevant questionnaires including Literacy Assessment for Diabetes (LAD) (17), Diabetes Self-Care Inventory-revised (SCI-R) (18), Perceived Stress Scale (PSS) (19), Affect Intensity Measure (AIM) (20), Affect Lability Scale (ALS) (21), and Trait Meta-Mood (TMM) Scale (22).
Assessment of DD
As part of the screening process, six questions were included to gauge levels of DD. Three items were from Problem Areas in Diabetes (PAID) (23) (e.g., “feeling alone with diabetes”) and three from the Diabetes Distress Scale (DDS) (24) (e.g., “feeling that diabetes is taking up too much of your mental and physical energy every day”). Studied, together, both scales are valid and reliable assessments of DD (25). The DD screen demonstrated very good internal consistency (α = 0.89) and correlated significantly with a measure of quality of life (26) (r = −0.41, P < 0.001), as does the full DDS (6). Examination of a subset of 20 individuals with diabetes, who also completed the full DDS (24), revealed a large-sized correlation between the DD screen and the full DDS (r = 0.63, P < 0.01). DD screen scores also correlated significantly with three of the four DDS subscores (Emotional Burden, r = 0.77, P < 0.001; Interpersonal Distress, r = 0.59, P < 0.01; and Regimen-Related Distress, r = 0.59, P < 0.01) but not Physician-Related Distress (r = −0.09, P = 0.700).
Assessments Related to DD
In addition to screening measures, study participants also completed two questionnaires related to diabetes literacy and diabetes self-care and four questionnaires related to psychological stress, negative emotionality, and skill at regulating emotion.
LAD.
The LAD is a word recognition test composed of third grade reading level word lists in ascending difficulty. As such, it measures the study participant’s ability to pronounce terms they would encounter during clinic visits and in reading menu and self-care instructions. Reliability and validity have been established (17).
SCI-R.
The SCI-R is a 15-item questionnaire, scored on a 0–4 Likert scale (ranging from “never” to “always”), assessing self-care behaviors related to diabetes in the past 1–2 months (e.g., “checked your blood glucose with monitor”). The SCI-R has no subscales, and its items have good internal consistency (α = 0.84).
PSS.
The PSS (19) is a 10-item questionnaire, with no subscales, scored on a 0–4 Likert scale (ranging from “never” to “very often”), assessing perceived psychological stress in the past month (e.g., “in the last month, how often have you felt difficulties were piling up so high that you could not overcome them”; α = 0.87).
AIM.
The AIM (20) is a 40-item questionnaire, scored on a 0–5 Likert scale (i.e., from “never” to “always”), assessing intensity of affect. The AIM includes three affect-related subscales: Negative Intensity (e.g., “my friends would probably say I’m a tense or ‘high-strung’ person”; α = 0.70), Negative Reactivity (e.g., “sad movies deeply touch me”; α = 0.70), and Positive Reactivity (e.g., “my happy moods are so strong that I feel like I’m in heaven”; α = 0.85). For this study, focusing on negative affect, we used the former two subscales (α = 0.81 for the set of items).
ALS.
The ALS (21) is a 54 item questionnaire, scored on a 0–3 Likert scale (i.e., from “definitely not characteristic of me” to “very characteristic of me”), regarding shifts of moods over the course of minutes to hours (e.g., “there are times when all I can think about is how worthless I am and then very soon afterwards all I can think about are the things that I am worried about”). The ALS has six subscales for Depression, Anxiety, Hypomania, Biphasic, Anger, and Anxiety/Depression. Internal consistency for theses subscales ranged from α = 0.81 to α = 0.90.
TMM.
The TMM (22) is a 30-item questionnaire, scored on a 0–3 Likert scale (from “strongly disagree” to “strongly agree”), with three subscales assessing how an individual relates with his/her emotions. The TMM has three subscales, Attention to Emotion (e.g., “I often think about my feelings”; α = 0.77); Clarity of Emotion (e.g., “I usually know my feelings about a matter”; α = 0.79), and Repair of Emotion (e.g., “when I become upset I remind myself of all the pleasures in life”; α = 0.64). For this study, focusing on the skill of regulating negative emotion, we used the latter two subscales (α = 0.81 for the set of items).
Statistical Analysis
Statistical analysis of these data involved χ2, t test, ANCOVA, Pearson correlation, multiple regression, and ANCOVA, all at a two-tailed α level of 0.05. Data regarding the internal consistency (Cronbach α) of the measures were calculated from the participants in this sample rather than the literature. In addition to analysis of scores from individual measures, summary scores were also calculated to produce variables to more parsimoniously reflect the relationship between DD and emotion-related variables of interest. First, we examined the relationships between DD and overall scores for the AIM, ALS, and TMM variables. Second, we examined the relationships between DD and subscales of these emotion-related variables. Third, we created summary variables to reflect emotion regulation variables related to the experience of negative emotion (ER-Exp) and skill at regulation of negative emotions (ER-Skill). Finally, we created a composite ER-Exp/ER-Skill variable to reflect global emotion regulation.
Results
Patient Characteristics: Overall and T1D Versus T2D
Demographics
A total of 298 adult study participants took part in this project. The characteristics of the full sample are listed in the Table 1. The sample was split between those with T1D (n = 125 [42%]) and T2D (n = 173 [58%]). Participants with T1D differed from those with T2D in age, ethnicity, and distribution of annual income but not in distribution of sex.
Table 1.
Characteristics of subjects
| Total sample (N = 298) | T1D (N = 125) | T2D (N = 173) | |
|---|---|---|---|
| Demographic variables | |||
| Age | 49.5 ± 17.6 | 36.8 ± 16.0 | 58.6 ± 12.3a |
| Sex (% female) | 57.4 | 54.4 | 59.5 |
| Race (% non-White) | 51.3 | 25.6 | 69.9a |
| Income (% <$20,000, $20,000–60,000, >$60,000) | 38, 26, 36 | 29, 22, 49 | 45, 29, 26a |
| Diabetes-related variables | |||
| Years with diabetes | 34.1 ± 18.7 | 18.8 ± 18.4 | 45.2 ± 13.5a |
| Insulin dependent (%) | 77.9 | 100 | 61.8a |
| DD screen | 6.9 ± 6.2 | 7.1 ± 5.8 | 6.7 ± 6.4 |
| DL (LAD) | 55.7 ± 4.1 | 57.6 ± 2.6 | 54.2 ± 4.4a |
| DSC (SCI-R) | 49.2 ± 9.5 | 51.5 ± 9.9 | 47.6 ± 8.8a |
| Psychometric variables | |||
| Global Negative Emotionality (AIM) | 32.8 ± 9.2 | 32.6 ± 9.7 | 32.9 ± 8.7 |
| Global Affect Lability (ALS) | 105.2 ± 30.0 | 102.6 ± 30.1 | 107.1 ± 29.8 |
| Global Emotional Skills (TMM) | 46.2 ± 8.4 | 46.3 ± 8.7 | 46.2 ± 8.1 |
| Perceived Stress (PSS) | 20.3 ± 4.8 | 20.5 ± 4.7 | 20.2 ± 4.9 |
Data are means ± SD or %. DL, diabetes literacy; DSC, diabetes self-care.
P ≤ 0.001.
Years of Diabetes, Diabetes Literacy, and Diabetes Self-care
Compared with patients with T2D, patients with T1D had fewer years of diabetes duration and higher LAD and SCI-R scores.
Behavioral Variables and History of Mental Health Treatment
Participants with T1D did not differ from those with T2D in DD screen, negative emotionality, affect lability, emotional skill, or psychosocial stress scores. Nearly two-fifths of all participants (119 of 298 [39.1%]) had a history of mental health treatment, primarily for “depression/anxiety” (56 of 298 [18.8%]) or for “life issues” (50 of 298 [16.8%]); participants with T1D did not differ from those with T2D in history of mental health treatment.
DD
For all participants, mean ± SD total and item DD screen scores were 6.9 ± 6.2 and 1.1 ± 1.0, respectively. While both scores represent only a “minor” level of DD, 64.4% of participants endorsed at least one DD screen item at a “moderate” level. These participants had substantially higher DD screen scores than those who endorsed no item at higher than a “minor” level (total score, 9.7 ± 5.7 vs. 1.3 ± 1.2, F[7, 282] = 205.64, P < 0.001; item score, 1.6 ± 0.9 vs. 0.2 ± 0.2, F[7, 282] = 205.64, P < 0.001). Participants with T1D did not differ from those with T2D in mean score, mean item score, or the proportion of participants endorsing at least one DD item at the “moderate” level.
DD and Emotion-Related Variables
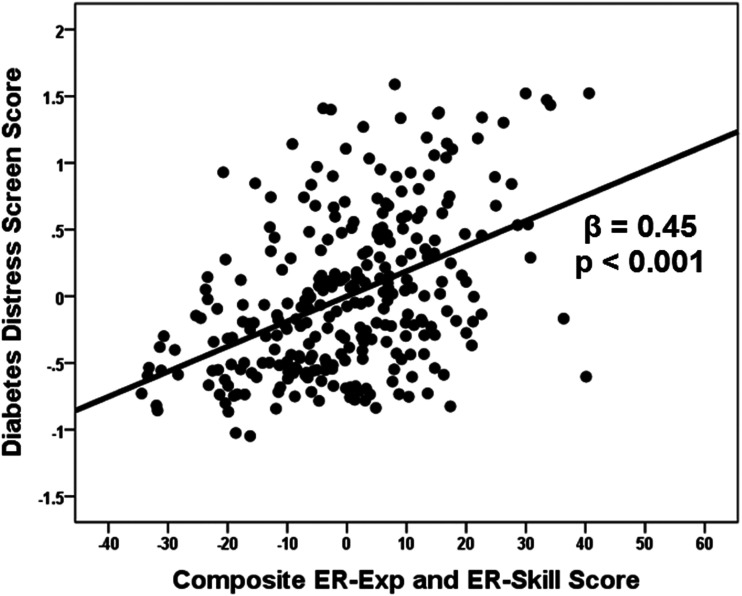
Separate multiple regression analysis (with age, sex, ethnicity, income, type of diabetes, and insulin dependence on step 1) revealed that DD correlated significantly with scores for AIM Negativity (β = 0.22, P < 0.001), ALS Global (β = 0.39, P < 0.001), and TMM Emotion Skill (P = −0.36, P < 0.001). Subsequent multiple regression analysis of the AIM Negativity subscales revealed a unique relationship between DD and Negative Intensity (β = 0.23, P = 0.002) but not Negative Reactivity (β = 0.02, P = 0.807). Similar analysis of the six ALS subscales revealed a unique relationship between DD and ALS Anxiety/Depression (β = 0.29, P = 0.012), while inverse, and unique, relationships were observed between DD and the two Emotion Skill subscales TMM Clarity (β = −0.23, P < 0.001) and TMM Repair (β = −0.21, P = 0.001). Accordingly, DD correlated uniquely with two variables reflecting ER-Exp and, uniquely, with two variables reflecting ER-Skill. While the source variables for ER-Exp (β = 0.47, P < 0.001) and those for ER-Skill (β = −0.39, P < 0.001) were also correlated, overlap in each case was modest, indicating that the source variables could be combined into composite ER-Exp and composite ER-Skill variables for further analysis. Placed in the same model, DD correlated, uniquely, with both ER-Exp (β = 0.28, P < 0.001) and ER-Skill (β = −0.25, P < 0.001). Substituting a global composite variable, representing both ER-Exp and ER-Skill (while taking account of their opposing directionality with DD), yielded a larger relationship with DD (β = 0.45, P < 0.001) (Fig. 1).
Figure 1.
Partial plot for the relationship between DD screen score and composite ER-Exp/ER-Skill in adult patients with T1D and T2D. Independent variables included age, sex, ethnicity, annual income, type of diabetes, insulin dependence, and composite ER-Exp/ER-Skill score (F[7, 290] = 15.40, P < 0.001).
DD and Other Relevant Variables
Separate multiple regression analysis (with age, sex, ethnicity, income, type of diabetes, and insulin dependence on step 1) revealed significant relationships between DD and PSS (β = 0.28, P < 0.001) and SCI-R (β = −0.17, P < 0.005), but not LAD (β = −0.09, P = 0.192). Placing these variables in the same model revealed unique relationships between DD and PSS (β = 0.27, P < 0.001) and between DD and SCI-R (β = −0.14, P < 0.02). Placing ER-Exp and ER-Skill variables in the same model with PSS and SCI-R revealed significant relationships between DD and ER-Exp (β = 0.23, P < 0.001), ER-Skill (β = −0.23, P < 0.001), and PSS (β = 0.15, P < 0.01) but only a trend toward statistical significance for SCI-R (β = −0.09, P = 0.082). Substituting the composite ER-Exp/ER-Skill variable in the model resulted in a medium-sized relationship with DD (β = 0.38, P < 0.001) without any change in the β-coefficients for PSS or SCI-R.
Conclusions
These data suggest that DD is meaningfully linked with two related, but modestly overlapping, aspects of negative emotionality and regulation of negative emotion. ER-Exp is directly related to DD, while ER-Skill is inversely related to DD. While the β-coefficients for ER-Exp and ER-Skill both are similar and in the modest range, the combination of the two demonstrates a stronger relationship of medium size (β = 0.45). Inclusion of variables related to diabetes self-care (SCI-R) and perceived psychological stress (PSS) was associated with only an 18% reduction (i.e., β = 0.45 to β = 0.38) in the strength of this relationship. At the same time, while statistically significant, the magnitude of the relationships between DD and PSS and SCI-R were modest (β = 0.15) or small (β = −0.09), respectively. These data highlight the likelihood that constructs of negative emotionality and regulation of such emotions are quite relevant to the construct of DD.
DD, the distress associated with coping with diabetes, is now recognized as a critical characteristic of suboptimal glycemic management among those with diabetes (7,10). It is common (4,5) in adults with T1D and T2D, inversely related with quality of life (6), more prevalent than depressive disorders (9), associated with reduced diabetes self-care behaviors (7), and independently associated with A1C levels (4,8).
In this study, we found that both ER-Exp and suboptimal ER-Skill at regulating negative emotion were related to DD. This is not surprising, given that the nature of DD is characterized by negative emotionality related to having diabetes and that skill at reducing such emotions would be relatively low. Our findings are also consistent with study results suggesting a role for reduced effective emotion management being associated with increased DD, in turn associated with reduced self-care and higher A1C levels. The magnitude of the relationships between DD and our emotional regulation variable was comparable with that in another study (β = 0.38 vs. β = 0.36), though the magnitude of the relationship between DD and self-care was comparatively less (β = 0.09 vs. β = 0.19). Our study, also, included a variable related to perceived psychological stress (not related to having diabetes), and it was significantly related to our variable of DD (β = 0.15), suggesting that this variable may also be important to consider in future treatment studies of DD, given that psychological stress also affects glucose levels in those with diabetes (27,28).
This study was cross-sectional and cannot speak to directionality. However, two small studies suggest that targeted emotional skill (29,30) or both negative emotionality and emotional skill (31) in the context of an in-person, multiweek, cognitive behavioral intervention can reduce DD (31) and A1C levels (29,31) to a substantial degree. Considering all factors, it is also likely that treating suboptimal emotional regulation, in itself, can reduce blood glucose levels. First, in a previous study in those with T2D (32), we found that while emotional regulation was related to baseline levels of A1C, it continued to correlate with A1C levels even when DD and self-care variables were included in the statistical model. A similar finding has also been reported in patients with T1D (33). Second, studies in healthy adults show that enhancing negative emotion increases, while enhancing positive emotion reduces, blood glucose in those characterized with poor emotion regulation (34), which is how we could characterize people with diabetes and elevated DD.
Like all studies, this study has strengths and limitations. Strengths include a reasonably large sample in those with T1D and T2D and the use of well-validated measures of emotionality and skill at emotional regulation.
One limitation includes the fact that our study participants were drawn from an endocrine clinic specializing in the treatment of diabetes (Kovler Diabetes Center). Thus, our patients may not reflect a broader group of individuals with diabetes treated in a primary care clinic or in the community. In addition, we did not evaluate all individuals being treated in this clinic; we only evaluated those referred to us for psychosocial evaluation/treatment, which was ∼25–30% of the clinic population. As such, our sample may overrepresent those with diabetes and serious psychological issues. That said, only ∼40% of our sample had a psychiatric or psychologic treatment history, a rate comparable with that seen in people with diabetes in the community (35,36). Another limitation is that our measure of DD was a brief screening tool drawn from the DDS and PAID (precursor of the DDS). For the purposes of our Psychosocial Evaluation and Treatment Program in adults with diabetes, we chose to use this DD screening tool to limit patient burden during initial evaluation. That said, the DD screening tool that we used displayed excellent internal consistency, was inversely associated with a quality of life measure, and was highly correlated with the score on the full DDS (in patients who completed both). This screening tool also correlated with each of the four DDS subscales, except for physician-related distress. While the screening tool did not include physician-related distress items, most patients (75%) completing the full DDS endorsed no physician-related distress items. It is likely that physician-related distress is uncommon in those treated in our clinic because the Kovler Clinic physicians are both highly knowledgeable about diabetes and attentive to needs of these patients.
Conclusion
These data suggest that DD is meaningfully linked with negative emotionality and skill at emotional regulation in adults with diabetes and that this relationship is considerably stronger than that with perceived psychological stress or diabetes self-care. Given other studies examining the construct of emotionality (15,37), and preliminary results from small treatment studies targeting emotionality/emotional regulation (29–31), these data suggest that DD and A1C may be improved especially in those with diabetes and difficulties with emotionality. Finally, data of this kind could be used to develop a metric to identify those with diabetes who have difficulties with emotional regulation, who have elevated DD and have less than optimal glycemic regulation.
Article Information
Acknowledgments. The authors acknowledge Joselyn Gomez (Chicago, IL) for her coordination of the data collection in this study.
Funding. This work was supported by the Kovler Diabetes Center at the University of Chicago.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.F.C. researched the data and wrote the manuscript. S.L., J.J., K.W., T.D., L.P., and M.d.G. contributed to discussion and reviewed and edited the manuscript. E.F.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA, Centers for Disease Control and Prevention, 2020 [Google Scholar]
- 2.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract 2011;65:314–322 [DOI] [PubMed] [Google Scholar]
- 3.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 5.Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: emerging findings. Psychosomatics 2011;52:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carper MM, Traeger L, Gonzalez JS, Wexler DJ, Psaros C, Safren SA. The differential associations of depression and diabetes distress with quality of life domains in type 2 diabetes. J Behav Med 2014;37:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturt J, Dennick K, Hessler D, Hunter BN, Oliver J, Fisher L. Effective interventions for reducing diabetes distress: systematic review and meta-analysis. Int Diab Nurs 2015;12:40–55 [Google Scholar]
- 8.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med 2008;25:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hessler D, Fisher L, Polonsky W, et al. There is value in treating elevated levels of diabetes distress: the clinical impact of targeted interventions in adults with type 1 diabetes. Diabet Med 2020;37:71–74 [DOI] [PubMed] [Google Scholar]
- 11.Hughes AE, Berg CA, Wiebe DJ. Emotional processing and self-control in adolescents with type 1 diabetes. J Pediatr Psychol 2012;37:925–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In Handbook of Emotion Regulation. Gross JJ, Ed. New York, NY, Guilford Press, 2007, pp. 3–24 [Google Scholar]
- 13.Salovey P, Grewal D. The science of emotional intelligence. Curr Dir Psychol Sci 2005;14:281–285 [Google Scholar]
- 14.Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;333:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher L, Hessler D, Polonsky W, et al. Emotion regulation contributes to the development of diabetes distress among adults with type 1 diabetes. Patient Educ Couns 2018;101:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher L, Hessler D, Polonsky WH, et al. T1-REDEEM: a randomized controlled trial to reduce diabetes distress among adults with type 1 diabetes. Diabetes Care 2018;41:1862–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath CR, Sylvester ST, Yasek V, Gunel E. Development and validation of a literacy assessment tool for persons with diabetes. Diabetes Educ 2001;27:857–864 [DOI] [PubMed] [Google Scholar]
- 18.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-revised with adults. Diabetes Care 2005;28:1346–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396 [PubMed] [Google Scholar]
- 20.Larsen RJ, Diener E. Affect intensity as an individual difference characteristic: a review. J Res Pers 1987;21:1–39 [Google Scholar]
- 21.Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. J Clin Psychol 1989;45:786–793 [DOI] [PubMed] [Google Scholar]
- 22.Salovey P, Mayer H, Goldman S, Turvey C, Palfai T. Emotional attention, clarity and repair: exploring emotional intelligence using the Trait Meta-Mood Scale. In Emotion, Disclosure and Health. Pennebaker JW, Ed. Washington, DC, American Psychological Association, 1995 [Google Scholar]
- 23.McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia 2010;53:66–69 [DOI] [PubMed] [Google Scholar]
- 24.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 25.Schmitt A, Reimer A, Kulzer B, Haak T, Ehrmann D, Hermanns N. How to assess diabetes distress: comparison of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Diabet Med 2016;33:835–843 [DOI] [PubMed] [Google Scholar]
- 26.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993;29:321–326 [PubMed] [Google Scholar]
- 27.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol 2017;13:547–560 [DOI] [PubMed] [Google Scholar]
- 28.Hilliard ME, Yi-Frazier JP, Hessler D, Butler AM, Anderson BJ, Jaser S. Stress and A1c among people with diabetes across the lifespan. Curr Diab Rep 2016;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karahan TF, Yalcin BM. The effects of an emotional intelligence skills training program on anxiety, burnout and glycemic control in type 2 diabetes mellitus patients. Turkiye Klinikleri J Med Science 2009;29:16–24 [Google Scholar]
- 30.Yalcin BM, Karahan TF, Ozcelik M, Igde FA. The effects of an emotional intelligence program on the quality of life and well-being of patients with type 2 diabetes mellitus. Diabetes Educ 2008;34:1013–1024 [DOI] [PubMed] [Google Scholar]
- 31.Coccaro EF, Busby AK, Potts TM, Philipson L, Drossos T. 1120-P: Emotion-focused CBT reduces diabetes distress and HbA1c levels in patients with T2D: a pilot study. Diabetes 2019;68(Suppl. 1):1120-P [Google Scholar]
- 32.Coccaro EF, Drossos T, Phillipson L. HbA1c levels as a function of emotional regulation and emotional intelligence in patients with type 2 diabetes. Prim Care Diabetes 2016;10:334–341 [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Aranda D, Zysberg L, García-Linares E, Castellano-Guerrero AM, Martínez-Brocca MA, Gutiérrez-Colosía MR. Emotional abilities and HbA1c levels in patients with type 1 diabetes. Psychoneuroendocrinology 2018;93:118–123 [DOI] [PubMed] [Google Scholar]
- 34.Niven K, Totterdell P, Miles E, Webb TL, Sheeran P. Achieving the same for less: improving mood depletes blood glucose for people with poor (but not good) emotion control. Cogn Emotion 2013;27:133–140 [DOI] [PubMed] [Google Scholar]
- 35.Lin EH, Von Korff M, Alonso J, et al. Mental disorders among persons with diabetes--results from the World Mental Health Surveys. J Psychosom Res 2008;65:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maia AC, Braga AdeA, Brouwers A, Nardi AE, Oliveira e Silva AC. Prevalence of psychiatric disorders in patients with diabetes types 1 and 2. Compr Psychiatry 2012;53:1169–1173 [DOI] [PubMed] [Google Scholar]
- 37.Fisher L, Hessler D, Polonsky W, Strycker L, Bowyer V, Masharani U. Toward effective interventions to reduce diabetes distress among adults with type 1 diabetes: enhancing emotion regulation and cognitive skills. Patient Educ Couns 2019;102:1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]