Abstract
Rationale:
Cell dose and concentration play crucial roles in phenotypic responses to cell-based therapy for heart failure.
Objective:
To compare the safety and efficacy of two doses of allogeneic bone marrow-derived human mesenchymal stem cells (hMSC) identically delivered in patients with ischemic cardiomyopathy (ICM).
Methods and Results:
Thirty patients with ICM received in a blinded manner either 20 million (20M, n=15) or 100 million (100M, n=15) allogeneic hMSCs via transendocardial injection (10 0.5 cc injections/patient). Patients were followed for 12-months for safety and efficacy endpoints. There were no treatment-emergent serious adverse events (SAE) at 30 days or treatment related SAEs at 12 months. The Major Adverse Cardiac Event rate was 20.0% (95% CI, 6.9%, 50.0%) in 20M and 13.3% (95% CI, 3.5%, 43.6%) in 100M (p=0.58). Worsening heart failure re-hospitalization was 20.0% (95% CI, 6.9%, 50.0%) in 20M and 7.1% (95% CI, 1.0%, 40.9%) in 100M (p=0.27). Whereas scar size reduced to a similar degree in both groups: 20M by −6.4g (IQR, −13.5g, −3.4g, p=0.001) and 100M by −6.1g (IQR, −8.1g, −4.6g, p=0.0002), the ejection fraction (EF) improved only with 100M by 3.7 units (IQR, 1.1, 6.1, p=0.04). NYHA class improved at 12 months in 35.7% (95% CI, 12.7%, 64.9%) in 20M and 42.9% (95% CI, 17.7%, 71.1%) in 100M. Importantly, pro-BNP increased at 12 months in 20M by 0.32 log pg/mL (95% CI, 0.02, 0.62, p=0.039), but not in 100M (−0.07 log pg/mL; 95% CI, −0.36, 0.23, p=0.65; between group p=0.07).
Conclusion:
Although both cell doses reduced scar size, only the 100M dose increased EF. This study highlights the crucial role of cell dose in the responses to cell therapy. Determining optimal dose and delivery is essential to advance the field, decipher mechanism(s) of action, and enhance planning of pivotal Phase III trials.
Clinical Trial Registration:
NCTO2013674 [https://clinicaltrials.gov/ct2/show/NCT02013674]
Keywords: Stem cell, heart failure, mesenchymal stem cell, cell dose
Subject Terms: Cell Therapy, Clinical Studies, Stem Cells, Chronic Ischemic Heart Disease, Heart Failure
INTRODUCTION
As the field of cell-based therapy for cardiac regeneration continues to expand, clinical trials of cell-based therapy for heart disease continue to indicate that cell dose and concentration play crucial roles in clinical responses. Despite more than a decade of clinical research, investigations of these biological therapies for cardiac regeneration have mainly focused on the optimal cell type1–4, with only a handful of studies comparing different cell doses. Moreover, there is a great deal of inconsistency in the results from these few studies, with some demonstrating a direct and others an inverse dose relationship between the number of stem cells and regenerative capacity4–6. The POSEIDON trial5, the first randomized clinical trial to compare autologous vs. allogeneic human mesenchymal stem cells (hMSCs) at three different doses, demonstrated an inverse relationship between the total cells delivered and clinical outcomes in patients with chronic ischemic left ventricular dysfunction secondary to myocardial infarction (MI)5. Specifically, those who received the lowest dose of 20 million (M) cells demonstrated greater improvements in left-ventricular ejection fraction (EF) and end-systolic volume (ESV) compared to those who received the highest dose of 200M cells. In contrast, Quyyumi et al. reported a direct dose-dependent improvement of cardiac perfusion in a study utilizing autologous CD34+ stem cells for the treatment of ST elevated MI6. However, this same cell type produced an inverse dose-response relationship in a study of patients with refractory angina4, further calling into question the intuitive hypothesis that more cells yield better outcomes. Yet other sub-analyses of clinical trials that tested different cell doses failed to determine a linear pattern of dose response7, 8. These inconsistencies among the results from different trials examining optimal dosing reflect the complexity of dosing in cell-based therapies. As with pharmacologic agents, establishing an efficacious dose and cell concentration is critical to successful therapeutic outcomes. To address this issue, we randomized patients to receive one of two doses of allogeneic bone marrow-derived hMSCs and compared their safety and efficacy in patients with ICM.
METHODS
Study design and enrollment.
The TRansendocardial Stem Cell Injection Delivery Effects on Neomyogenesis STudy (The TRIDENT study) titled “A Phase II, Randomized, Blinded, Study of the Safety and Efficacy of Transendocardial Injection of Allogeneic Human Mesenchymal Stem Cells (20 Million or 100 Million Total MSCs) in Patients with Chronic Ischemic Left Ventricular Dysfunction Secondary to Myocardial Infarction” was conducted under FDA IND #13568. The primary objective was to demonstrate the safety of two doses of allogeneic hMSCs delivered by transendocardial stem cell injection (TESI) in patients with chronic LV dysfunction secondary to MI. The secondary objective was to evaluate the efficacy of the two hMSC doses. This trial was posted on clinicaltrials.gov (#NCT02013674). The institutional review board of the University of Miami Miller School of Medicine approved this study and all participants gave written informed consent.
Patients were enrolled at the University of Miami from April 29, 2014 to March 2, 2016 and protocol-specified follow up was completed on March 2017. Thirty patients were randomized in a 1:1 ratio to receive either 20M or 100M allogeneic hMSCs via TESI during cardiac catheterization. Randomization and data collection were performed using an electronic data entry system. Both the investigators and patients were masked to the hMSC dose, as were data analysts of endpoints. Unmasking was managed by a third party for statistical analysis. Only the unblinded study team at the third-party research organization and the Cell Therapy lab were knowledgeable about the unblinded treatment assignments. An independent data and safety monitoring board (DSMB) was responsible for safety oversight.
Patient population.
Patients eligible for inclusion were aged 21–90 with the diagnosis of chronic ischemic LV dysfunction secondary to MI as defined by previous MI documented by an imaging study demonstrating coronary artery disease with corresponding areas of akinesis, dyskinesis or severe hypokinesis on maximal appropriate medical therapy with a confirmed EF ≤ 50%. All patients were required to sign an informed consent form. A complete list of inclusion and exclusion criteria is described in the Table 1.
Table 1.
Major Inclusion and Exclusion Criteria
| Major Inclusion Criteria |
|
|
| • Diagnosis of chronic ischemic left ventricular dysfunction secondary to MI. |
| •Be a candidate for cardiac catheterization within 5 to 10 weeks of screening. |
| •Been treated with appropriate maximal medical therapy for heart failure or post-infarction left ventricular dysfunction. |
| •Ejection fraction less than or equal to 50%. |
| •Signing of informed consent form. |
|
|
| Major Exclusion Criteria |
|
|
| •Baseline glomerular filtration rate ≤35 mL/min/1.73m2. |
| •Presence of a mechanic aortic valve or heart constrictive device. |
| •Documented presence of aortic stenosis (aortic stenosis 1.5cm2 or less). |
| •Documented presence of moderate to severe aortic insufficiency (echocardiographic assessment of aortic insufficiency graded as ≥+2). |
| •Evidence of a life-threatening arrhythmia in the absence of a defibrillator (nonsustained ventricular tachycardia ≥ 20 consecutive beats or complete second or third degree heart block in the absence of a functioning pacemaker) or QTc interval > 550 ms on screening ECG. |
| •AICD firing in the past 60 days prior to study enrollment. |
| •Be eligible for or require coronary artery revascularization. |
| •Have a hematologic abnormality as evidenced by hematocrit < 25%, white blood cell < 2,500/μl or platelet values < 100,000/μl without another explanation. |
| •Have liver dysfunction, as evidenced by enzymes (ALT and AST) greater than three times the ULN. |
| •Have a coagulopathy condition = (INR > 1.3) not due to a reversible cause. |
| •Known, serious radiographic contrast allergy. |
| •Known allergies to penicillin or streptomycin. |
| •Organ transplant recipient. |
| •Have a history of organ or cell transplant rejection |
| •Clinical history of malignancy within 5 years (i.e., patients with prior malignancy must be disease free for 5 years), except curatively treated basal cell carcinoma, squamous cell carcinoma, or cervical carcinoma. |
| •Non-cardiac condition that limits lifespan to < 1 year. |
| •On chronic therapy with immunosuppressant medication. |
| •Serum positive for HIV, hepatitis BsAg, or viremic hepatitis C. |
| •Female patient who is pregnant, nursing, or of child-bearing potential and not using effective birth control. |
Study procedures and timeline.
Baseline studies were performed prior to TESI and included chemistry and hematology laboratory collection, echocardiography, ECG, computed tomography (CT) of the chest, abdomen and pelvis and cardiac imaging by CT. Patients were hospitalized for a minimum 2 days post-TESI and were followed at 2 weeks, 3– 6- and 12-months after cell treatment for further safety assessments, including interviews regarding adverse events (AEs) and measurements of other efficacy endpoints.
Allogeneic hMSCs for cell therapy.
Bone-marrow derived allogeneic hMSCs were isolated from 4 male donors with a median age of 24 years (range, 22–28 years) and manufactured at the University of Miami Interdisciplinary Stem Cell Institute and were delivered by TESI in passage 2, as previously described5, 9. All hMSCs met the following release criteria: cell viability >70%, positive for CD105+ and negative for CD45- by flow cytometry analysis, no growth of bacteria via Gram stain, negative for mycoplasma via polymerase chain reaction, and endotoxin level ≤5 EU/mL.
Transendocardial stem cell injection.
Cells were delivered by TESI to 10 LV during retrograde left heart catheterization using the Biocardia Helical Infusion Catheter (Biocardia)5, 10. Injections were targeted to the border zones of the chronically infracted myocardial territory determined by CT imaging and biplane left ventriculography.
Study endpoints.
The primary endpoint was the incidence of treatment-emergent serious adverse events (TE-SAEs) at one-month post-TESI as defined as a composite of death, nonfatal MI, stroke, hospitalization for worsening heart failure (WHF), cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias (>15 seconds or with hemodynamic compromise). Additional safety endpoints were assessed during the 6-month follow up period and the 12-month visit and included clinical assessment of AEs and serious adverse events (SAEs) along with surveillance testing including serial troponin and creatine kinase-MB, 24-hour ambulatory ECG, blood chemistry and hematology values, and ectopic tissue formation as measured by CT of the chest, abdomen and pelvis.
Secondary efficacy endpoints included cardiac CT and echocardiographic measures of regional and global LV function, shape, and infarct size. Other pre-specified endpoints were peak oxygen consumption (VO2), 6 minute walk test (6MWT), New York Heart Association (NYHA) functional class, Minnesota Living with Heart Failure Questionnaire (MLHFQ), incidence of Major Adverse Cardiac Events (MACE), serum tumor necrosis factor alpha (TNF-α), and pro-brain natriuretic peptide (pro-BNP) level, presented as log-transformed pg/mL values. MACE was assessed at 30, 180, and 365 days post-TESI and defined as the composite of death, hospitalization for WHF, or non-fatal recurrent MI.
Cardiac computed tomography imaging.
Contrast-enhanced CT was performed at baseline 128-slice and 12-month follow-up using a 128-slice CT scanning system (Siemens AS+, Siemens Medical Solutions)5, 11 to evaluate global LV function and volumes, sphericity index12, and infarct size, using (iNtuition software version 4.4.7.47; TeraRecon Inc., Foster City, Calif., USA). LV sphericity index was EDV/(πLVLd3/6)12. Scar size was evaluated using early enhancement defect (EED) as previously described5, 13.
Panel reactive antibodies and TNF-a.
Calculated panel reactive antibodies (cPRA) were measured at baseline and 6 months using Luminex 200. Serum samples were collected pre-TESI and 6 months post-TESI along with peripheral mononuclear cells isolated from heparinized blood by Ficoll sedimentation. Serum TNF-α was measured using human TNF-α enzyme-linked immunosorbent assay high sensitivity kit (eBiosciences).
Statistical analysis.
The sample size of 15 per treatment arm was chosen to be appropriate for a phase II study in this population. The anticipated 30-day proportion of TE-SAEs was 20%, which translates into 3 patients with events out of 15. When the true number of patients with TE-SAEs is 3, there is 65% power to rule out a number of patients experiencing the primary endpoint of 8 or higher. All patients who received study injection were included in the primary analysis. All statistical tests were performed at p=0.05 using two-sided tests. Baseline continuous variables were summarized by mean and standard deviation or median and interquartile range (IQR) as appropriate. Post-TESI continuous variables were estimated as the change from baseline and described using mean and 95% confidence interval (CI) or median and IQR. The frequency and percentages (based on the non-missing sample size) of observed levels were reported for all categorical measures. The proportion of patients in each group with a TE-SAE by 30 days, as defined above, was calculated. As no events were reported in either treatment group, statistical tests were not conducted. Outcomes which were collected at multiple follow-up visits were analyzed using a mixed model for repeated measures to compare treatment groups with treatment group considered an effect as well as a group-by-time interaction. Within group effects were described using model-estimated contrasts. Outcomes that were highly skewed were analyzed using ranked analysis of covariance adjusting for baseline, at each follow-up assessment. Within group effects were described using a Wilcoxon Signed Rank test. Categorical variables were compared between groups using Fisher’s exact tests. Analysis of time-to-event data was done using a two-sided Log-Rank test, censoring those who did not experience an event at their last known follow-up day. All data analyses and statistical computations were conducted with SAS, version 9.3 or higher (Cary, NC).
RESULTS
Patient population.
Fifteen patients were randomized to each of the 20M and 100M groups. The mean age of participants was 66.2±10.7 years, 10% were female, and 5 (16.7%) were Hispanic (Table 2). Fifty percent of patients had NYHA class II symptoms, markedly depressed median baseline EF (36.1%; IQR: 24.3%, 38.9%), and limited mean 6MWT (416.8±115.4m).
Table 2.
Baseline Characteristics
| Characteristics | Treatment Group | ||
|---|---|---|---|
| 20M | 100M | Total | |
| (n=15) | (n=15) | (n=30) | |
| Gender | |||
| Male | 12 (80.0) | 15 (100.0) | 27 (90.0) |
| Female | 3 (20.0) | 0 (0.0) | 3 (10.0) |
| Ethnicity | |||
| Hispanic or Latino | 2 (13.3) | 3 (20.0) | 5 (16.7) |
| Not Hispanic or Latino | 13 (86.7) | 12 (80.0) | 25 (83.3) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not Answered | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Race | |||
| American Indian/Alaskan | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian | 0 (0.0) | 1 (6.7) | 1 (3.3) |
| Hawaiian/Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Black or African American | 1 (6.7) | 0 (0.0) | 1 (3.3) |
| White | 14 (93.3) | 14 (93.3) | 28 (93.3) |
| More than One Race | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| History of Coronary Interventions | 15 (100.0) | 14 (93.3) | 29 (96.7) |
| Previously Referred for AICD Placement | 11 (73.3) | 12 (80.0) | 23 (76.7) |
| History of Atrial or Ventricular Arrhythmia | 5 (33.3) | 8 (53.3) | 13 (43.3) |
| History of Hypertension | 10 (66.7) | 7 (46.7) | 17 (56.7) |
| History of Congestive Heart Failure | 11 (73.3) | 12 (80.0) | 23 (76.7) |
| History of Valvular Heart Disease | 2 (13.3) | 2 (13.3) | 4 (13.3) |
| History of Smoking | 5 (33.3) | 12 (80.0) | 17 (56.7) |
| History of Diabetes | 3 (20.0) | 4 (26.7) | 7 (23.3) |
| Glomerular Filtration Rate (mL/min/1.73m2 ) | 70.0±24.4 | 79.2±20.4 | 74.6±22.6 |
| Age at injection (years) | 66.8±12.2 | 65.6±9.4 | 66.2±10.7 |
|
| |||
| Cardiac Function and Scar Size | |||
|
| |||
| Ejection Fraction (%) | 37.6 (24.3, 39.9) | 30.1 (22.3, 38.9) | 36.1 (24.3, 38.9) |
| End Systolic Volume (mL) | 140.3 (119.6, 262.5) | 177.3 (147.8, 249.4) | 168.8 (132.0,249.4) |
| End Diastolic Volume (mL) | 234.2 (191.8, 332.6) | 273.3 (234.1, 345.0) | 250.7 (214.6, 332.6) |
| Stroke Volume (mL) | 88.9±21.28 | 79.8±24.73 | 84.3±23.13 |
| End Diastolic Diameter (mm) | 58.3 (55.9, 72.5) | 64.3 (56.4, 67.3) | 60.9 (56.4, 67.3) |
| End Systolic Diameter (mm) | 52.4 (43.0, 58.8) | 53.7 (49.2, 57.5) | 52.8 (46.2, 57.5) |
| End-Diastolic Long Axis Diameter (mm) | 99.0±10.41 | 101.2±8.54 | 100.1±9.42 |
| Scar Size (g) | 17.9 (12.5, 23.1) | 16.2 (13.8, 24.3) | 17.1 (13.7, 23.9) |
| Scar Size / LV mass (%) | 9.9 (6.5, 16.6) | 9.7 (6.5, 12.3) | 9.8 (6.5, 14.4) |
|
| |||
| Functional Capacity and Quality of Life | |||
|
| |||
| NYHA Functional Class | |||
| I | 4 (26.7) | 6 (40.0) | 10 (33.3) |
| II | 8 (53.3) | 7 (46.7) | 15 (50.0) |
| III | 3 (20.0) | 1 (6.7) | 4 (13.3) |
| IV | 0 (0.0) | 1 (6.7) | 1 (3.3) |
| Peak VO2 (mL/kg/min) | 15.8±5.1 | 16.7±4.5 | 16.3±4.8 |
| Six Minute Walk Test (m) | 398.7±111.6 | 434.9±120.0 | 416.8±115.4 |
| MLHFQ score | 29.0 (21.0, 52.0) | 35.0 (6.0, 60.0) | 31.5 (9.0, 55.0) |
| FEV1 (%) | 72.7±15.1 | 76.9±15.5 | 74.8±15.2 |
| Pro-BNP (pg/mL) | 532.3 (373.5, 1530.0) | 377.7 (212.0,1246.0) | 443.9 (304.6, 1422.0) |
| Pro-BNP (log pg/mL) | 6.5±0.90 | 6.2±1.10 | 6.3±1.00 |
| TNFα (pg/mL) | 9.0 (5.7, 13.8) | 14.6 (11.7, 16.3) | 12.9 (7.6, 15.1) |
Values are mean±standard deviation, n (%), or median (interquartile range).
Safety.
TESI was technically successful in all 30 patients. Pre-specified criteria for stopping TESI procedure, which included hypotension or ischemia, did not occur. One patient in the 100M group received 9 injections only because there was not enough investigational product supplied on injection day. No patient had significant post-procedural pericardial effusion.
No TE-SAEs were experienced within 30-days at either dose level. Furthermore, the incidence of AEs by 30 days did not significantly differ by cell dose: 33.3% (95% CI, 15.4%, 62.5%) in 20M and 40% (95% CI, 20.3%, 68.2%) in 100M, (p=0.79) (Table 3). Only 1 SAE was experienced (Hematoma) in 20M by day 30. No other SAEs were experienced by day 30 in either dose group.
Table 3.
Safety Summary
| Treatment Group | ||||
|---|---|---|---|---|
|
| ||||
| 20M (n=15) | 100M (n=15) | |||
| 30 days post-TESI | ||||
| Incidence of AE | 5 | 33.3% (15.4, 62.5) | 6 | 40.0% (20.3, 68.2) |
| Incidence of SAE | 1 | 6.7% (1.0, 38.7) | 0 | 0% (0, 21.8) |
| Incidence of TE-SAE | 0 | 0% (0, 21.8) | 0 | 0% (0, 21.8) |
| Incidence of MACE | 0 | 0% (0, 21.8) | 0 | 0% (0, 21.8) |
| 6 months post-TESI | ||||
| Incidence of AE | 8 | 53.3% (31.3, 78.8) | 10 | 66.7% (43.6, 87.8) |
| Incidence of SAE | 4 | 26.7% (10.9, 56.4) | 2 | 13.3% (3.5, 43.6) |
| Incidence of MACE | 3 | 20.0% (6.9, 50) | 2 | 13.3% (3.5, 43.6) |
| Incidence of death | 0 | 0% (0, 21.8) | 1 | 6.7% (1.0, 38.7) |
| 1 year post-TESI | ||||
| Incidence of AE | 10 | 66.7% (43.6, 87.8) | 13 | 86.7% (65.4, 97.8) |
| Incidence of SAE | 7 | 46.7% (25.6, 73.7) | 5 | 33.3% (15.4, 62.5) |
| Incidence of MACE | 3 | 20.0% (6.9, 50) | 2 | 13.3% (3.5, 43.6) |
| Incidence of death | 0 | 0% (0, 21.8) | 1 | 6.7% (1.0, 38.7) |
Values are number of patients with events. Kaplan-Meier event rates and 95% CIs are presented; exact binomial CIs used for zero counts. AE = adverse event, CI = confidence interval, TE-SAE = treatment emergent serious adverse event, MACE = major adverse cardiac event(s).
Long term adverse events, rehospitalization, MACE and ectopic tissue formation.
The 6-month post-TESI SAE incidence was 26.7% (95% CI, 10.9%, 56.4%) in 20M and 13.3% (95% CI, 3.5%, 43.6%) in 100M, between groups (p=0.31). One death occurred post-TESI in 100M due to atherosclerotic cardiovascular disease on day 119 post-TESI. The event was considered unrelated to study treatment.
The 12-month re-hospitalization rate was 46.7% (95% CI, 25.6%, 73.7%) in 20M and 28.2% (95% CI, 11.6%, 58.9%) in 100M (p=0.21). The 12-month WHF re-hospitalization was 20.0% (95% CI, 6.9%, 50.0%) in 20M and 7.1% (95% CI, 1.0%, 40.9%) in 100M (p=0.27). MACE is defined as the composite of death, hospitalization for WHF, or non-fatal recurrent MI. Over 12 months, there were 3 MACE, event rate 20.0% (95% CI, 6.9%, 50%) in 20M and 2 events, event rate 13.3% (95% CI, 3.5%, 43.6%) in 100M (p=0.58). No ectopic tissue formation was identified over one year by CT in either group.
Arrhythmias.
During the study duration, no participants in either treatment group experienced sustained ventricular arrhythmia.
Left ventricular function and scar size.
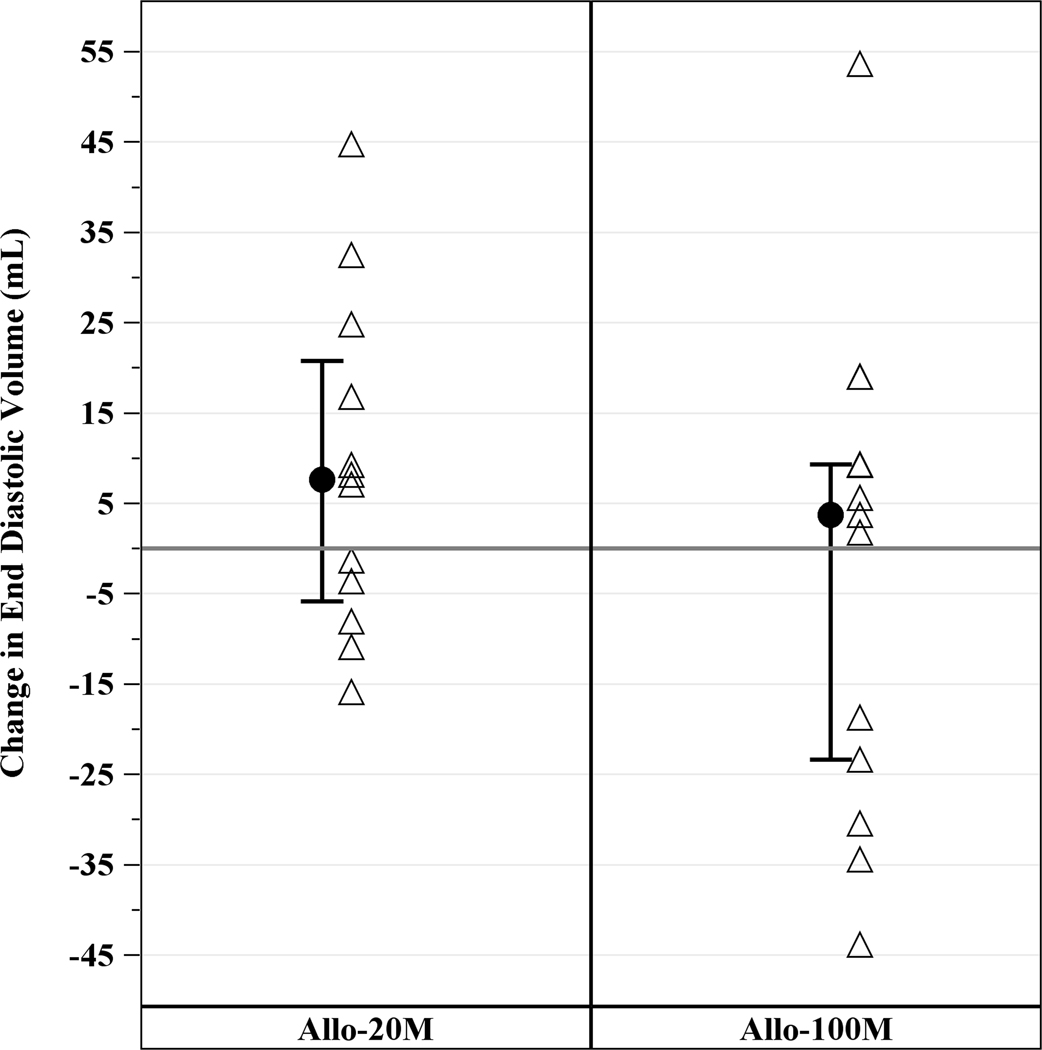
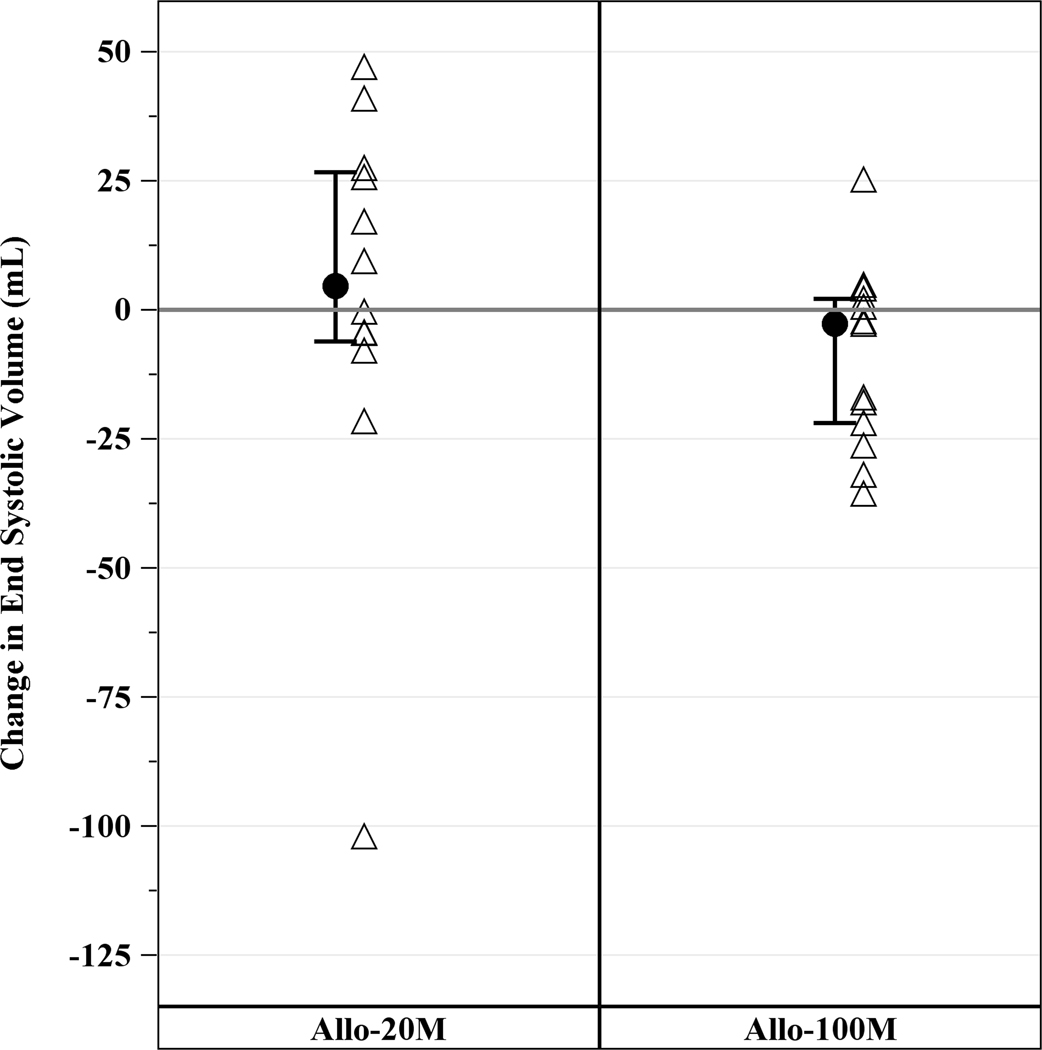
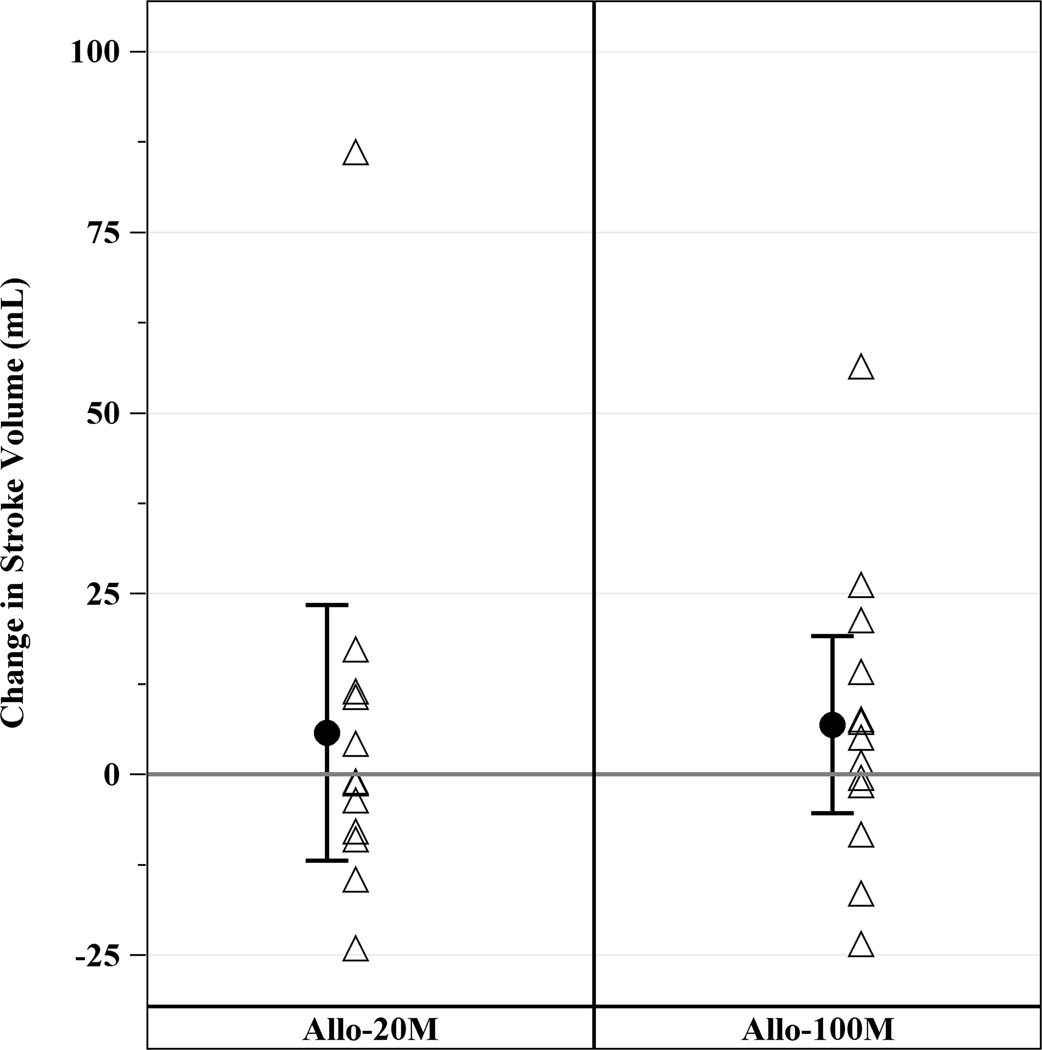
Cardiac CT revealed a median EF of 36.1% (IQR, 24.3%, 38.9%), LV end diastolic volume of 250.7mL (IQR, 214.6mL, 332.6mL), LV end systolic volume of 168.8mL (IQR, 132.0mL, 249.4mL), LV end diastolic diameter of 60.9mm (IQR, 56.4mm, 67.3mm), and median scar size of 17.1g (IQR, 13.7g, 23.9g). Allogeneic hMSCs therapy improved cardiac function in ICM patients as assessed by cardiac CT scan, with an increase in EF in the 100M and scar size reduction in both groups. EF increased in 100M by 3.73 units (IQR, 1.09, 6.10, p=0.04), but not in 20M (−0.27 units; IQR, −2.07, 3.35, p=0.85) at 12 months (between groups p=0.68; Figure 2A). End-diastolic long axis diameter decreased by −0.51mm (95% CI, −2.58mm, 1.55mm, p=0.61) from baseline to 12 months in 20M and −0.89mm (95% CI, −2.87mm, 1.09mm, p=0.36) in 100M, between groups (p=0.84). End diastolic volume (EDV; Figure 2B), end systolic volume (ESV; Figure 2C) and stroke volume (Figure 2D) did not significantly decrease from baseline. Sphericity index, end diastolic diameter and end systolic diameter did not change from baseline to 12 months in either group (data not shown).
Figure 2. Changes in LV Function from Baseline to 12 months post-TESI.
(A) Ejection fraction increased from baseline in 100 M but not in 20 M: 3.7 units (IQR, 1.09, 6.10, p=0.04), between groups (p=0.68). (B) End diastolic volume, (C) end systolic volume, and (D) stroke volume did not improve from baseline. *within-group p≤0.05.
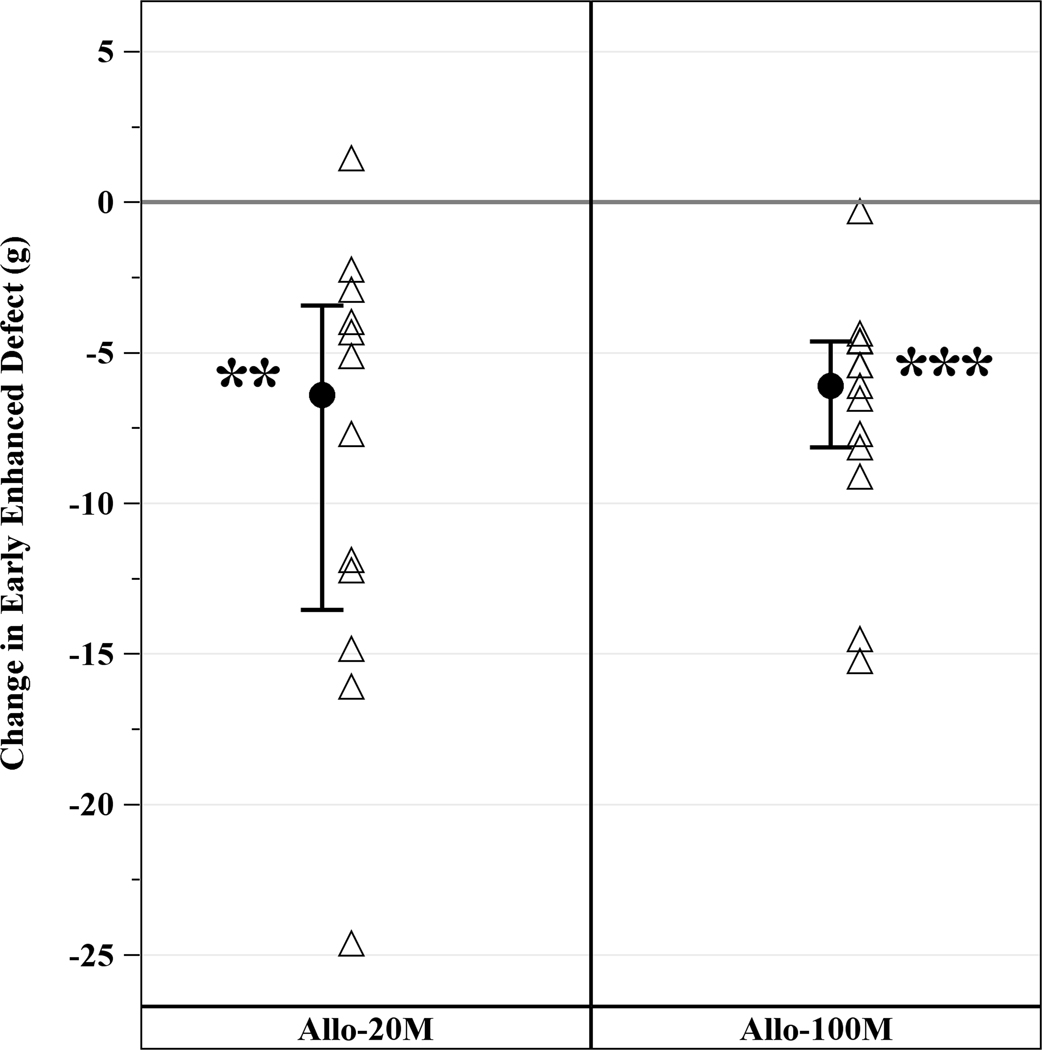
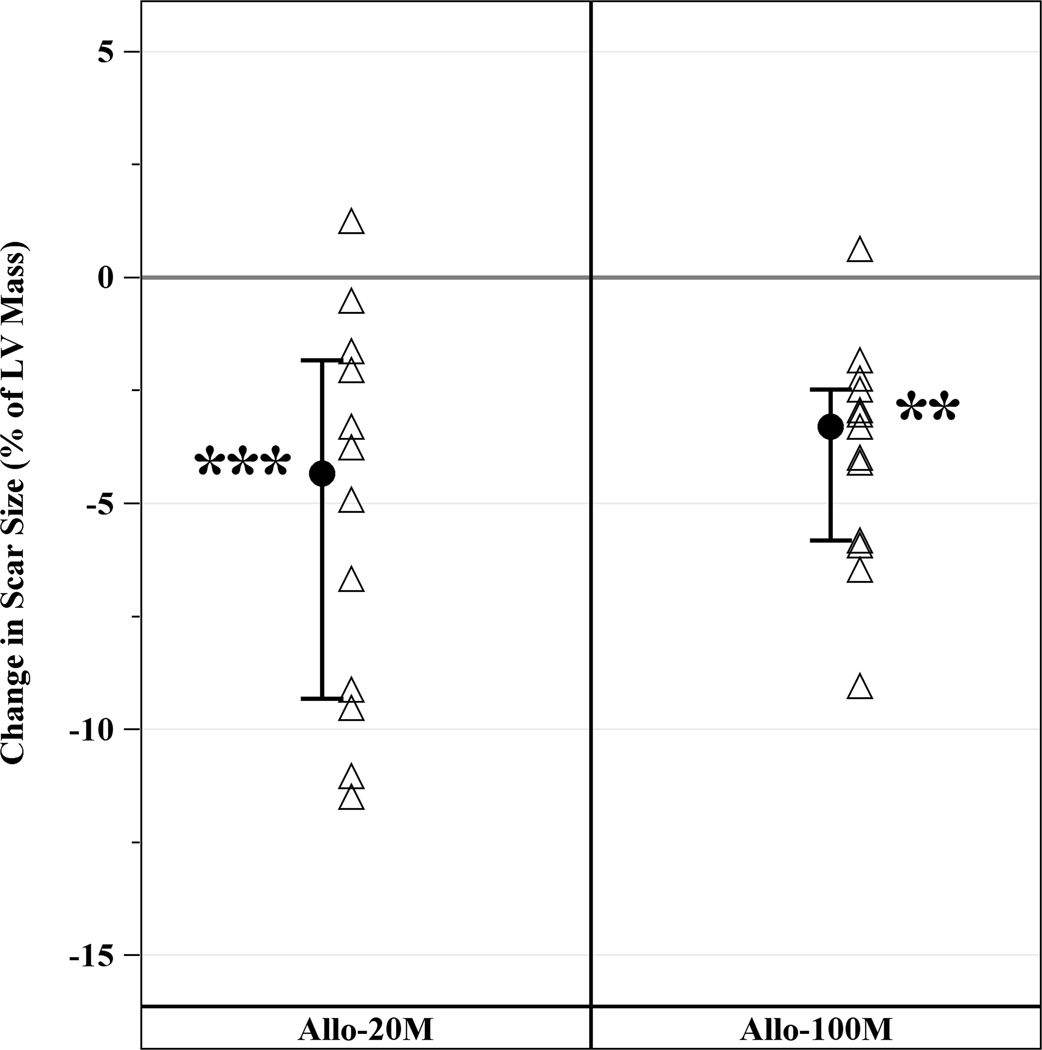
EED, a measure of infarct size, was 17.1g (IQR, 13.7g, 23.9g) at baseline and reduced to 10.0g (IQR, 8.6g, 17.5g) at 12 months. Both 20M and 100M reduced median EED by similar degree. In 20M, scar size decreased by −6.4g (IQR, −13.5g, −3.4g, p=0.001) and in 100M by −6.1g (IQR, −8.1g, −4.6g, p=0.0002) with no difference between groups (p=0.91) (Figure 3A). Infarct size was also expressed as percentage of LV mass (scar size/LV mass). Both groups reduced scar size as a percentage of the LV mass. Scar size as a percentage of LV mass decreased by 3.30% (−5.82%, −2.48%, p=0.0005) in 20M at 12 months post-TESI and by 4.34% (−9.32%, −1.84%, p=0.0015) in 100M, between group (p=0.64). These results support that 20M and 100M hMSCs decrease scar size to a similar degree (Figure 3B).
Figure 3. Changes in Scar Size from Baseline to 12 months post-TESI.
Both group reduced scar size and scar size as a fraction of LV mass by similar degree. (A) Scar size reduction in 20M by −6.4g (IQR, 13.5g, −3.4g, p=0.001) and 100M by −6.1g (IQR, −8.1g, −4.6g, p=0.0002), between groups (p=0.91). (B) Scar size as a percentage of LV mass decreased in 20M by 3.30% (−5.82%, −2.48%, p=0.0005) and 100M by 4.34% (−9.32%, −1.84%, p=0.0015), between groups (p=0.6). **Within-group p≤0.005, ***within-group p≤0.0005.
Functional status, quality of life, and pulmonary function.
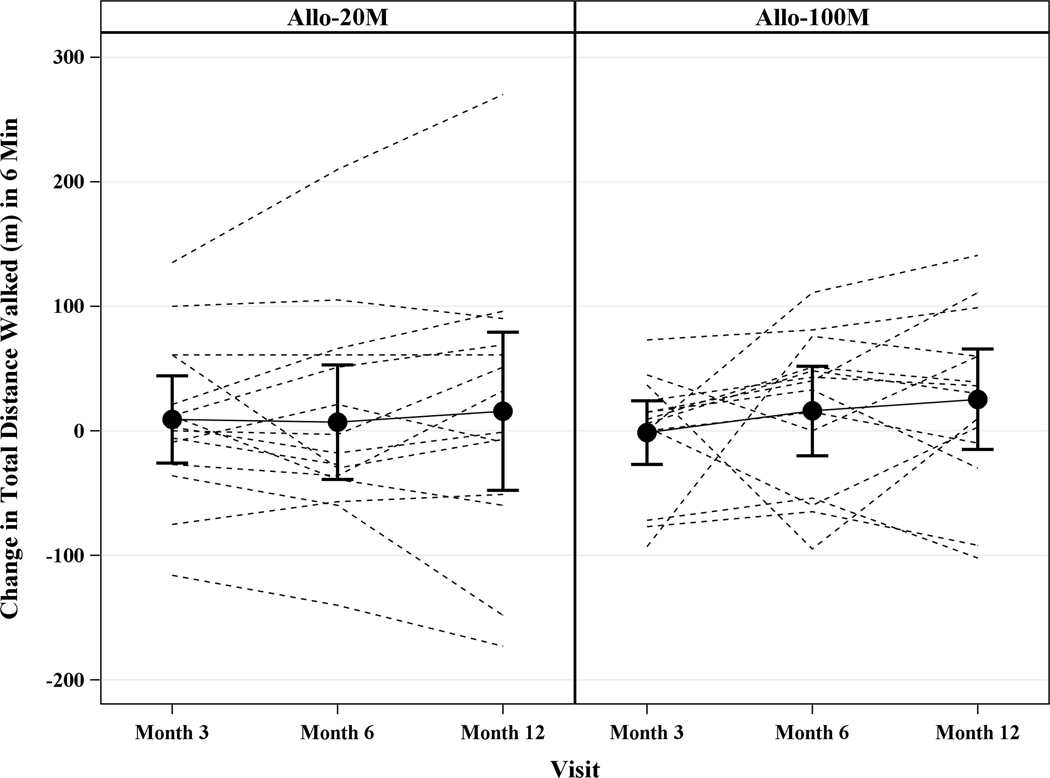
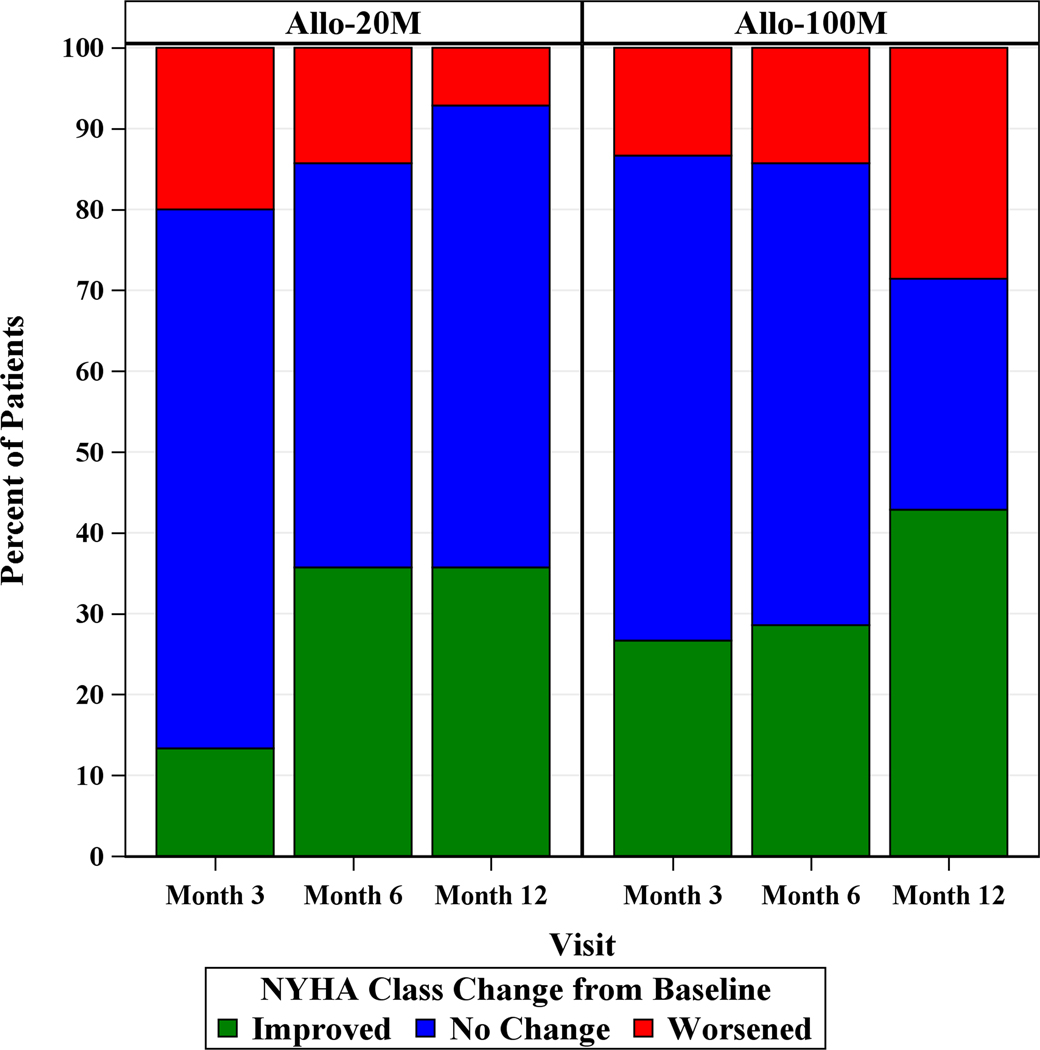
The 6MWT distance increased in 20M by 13.5m (95% CI, 334.5m, 484.9m, p=0.57) at 12 months and in 100M by 25.2m (95% CI, 380.4m, 545.6m, p=0.30). The between group difference was −12.72m (95% CI, −67.73m, 42.49m, p=0.64) and −14.24m (95% CI, −82.25m, 53.76m, p=0.67) from baseline to 6 or 12 months, respectively (Figure 4A). At 12 months 20M showed 35.7% (95% CI, 12.7%, 64.9%) improvements in NYHA functional class and 42.9% (95% CI, 17.7%, 71.1%) improved in 100M (between treatment group and change in NYHA class: p=0.22; Figure 4B). The median MLHFQ score at baseline was 29.0 (IQR, 21.0, 52.0) and at 12 months 25.0 (IQR, 4.0, 41.0) in 20M, and at baseline was 35.0 (IQR, 6.0, 60.0) and at 12 months 15.0 (IQR, 8.0, 64.0) in 100M (20M p=0.22, 100M p=0.23; Figure 4C). There were no differences in the peak VO2 and FEV1 at 6 or 12 months compared to baseline in either group.
Figure 4. Functional Capacity and Quality of Life in ICM Patients.
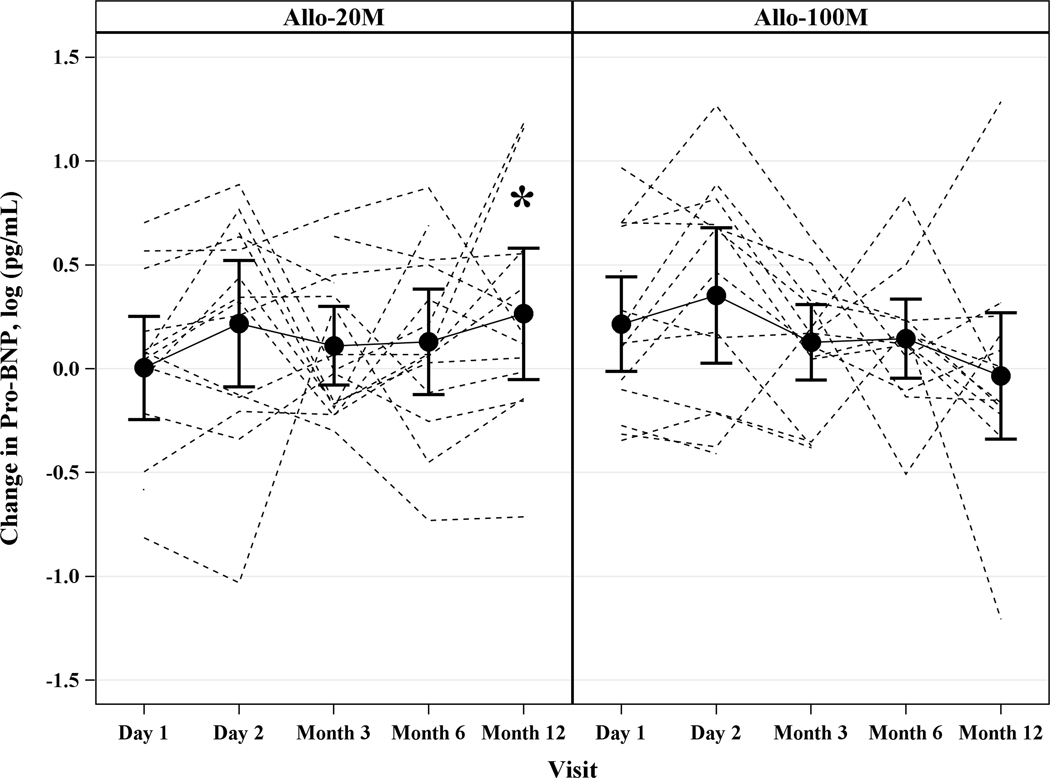
Depicted are changes in functional capacity, quality of life, and sexual quality of life. (A) 6MWT distance increased in 20M by 13.5m (95% CI, −34.48m, 61.46m, p=0.57) and in 100M by 25.2m (95% CI, −23.20m, 73.58m, p=0.30), between groups (p=0.64). (B) NYHA class improved to a similar degree in both dose groups: 35.7% in 20M and 42.9% in 100M after 1 year post-TESI. (C) MLHFQ score didn’t change over time (20M p=0.22, 100M p=0.23). (D) pro-BNP was maintained in 100M while it increased by 0.32 log pg/mL (p=0.038) in 20M. *Within-group p≤0.05.
Pro-BNP.
Pro-BNP increased from baseline to 12 months post-TESI: 0.32 log pg/mL (95% CI: 0.02, 0.6, p=0.039) in 20M, while 100M remained stable over time: −0.07 log pg/mL (95% CI: −0.36, 0.23, p=0.65). The between group difference at 12 months was −0.38 log pg/mL (95% CI: −0.8, 0.04, p=0.07) (Figure 4D).
Immune monitoring.
There were no rejection incidences as measured by the calculated panel reactive antibody assay (cPRA). Only 1 patient in 20M had no-to-low cPRA response. This cPRA response did not cause clinical immunologic rejection in the patient. These results confirm that allogeneic MSC therapy does not cause immunologic rejection (Table 4).
Table 4.
Recipient cPRA Change 6 Months Post-TESI
| Cell Type | ||
|---|---|---|
| cPRA Risk | 20M (n=15) | 100M (n=15) |
| No reaction to Low risk (0 – 20% cPRA) | 1 (6.7%) | 0 (0.0%) |
| Moderate risk (21 – 79% cPRA) | 0 (0.0%) | 0 (0.0%) |
| High risk (+80% cPRA) | 0 (0.0%) | 0 (0.0%) |
Values are n (%). cPRA = calculated panel reactive antibodies.
Both groups, 20M and 100M effectively reduced systemic inflammation as measured by serum TNF-α. Serum TNF-α decreased from baseline to 6 months in both groups (20M: −5.96pg/mL (IQR, −9.19pg/mL, −1.50pg/mL, p=0.01); 100M −9.37 pg/mL (IQR, −11.38pg/mL, −6.12pg/mL, p=0.0004); between groups (p=0.96) (Figure 5).
Figure 5. Effects of hMSCs on serum TNF-α in ICM Patients.
Cell treatment improved serum TNF-α at 6 months post-TESI by −5.96pg/mL in 20M (p=0.01) and −9.37pg/mL in 100M (p=0.0004), between groups (p=0.96). *Within-group p≤0.05, ***within-group p≤0.0005.
DISCUSSION
The TRIDENT study adds to a growing body of research on the safety of allogeneic hMSCs in ICM and addresses the issue of dosage in cell-based therapies, providing additional evidence of a direct relationship between cell dose and clinical efficacy. While several studies have demonstrated the safety of both autologous and allogeneic cell products3, 5, 14–18, the few preclinical19–22 and clinical studies4–6, 23–25 that examined dosing present conflicting and inconsistent results regarding the relationship between the quantity of cells delivered and clinical efficacy. Because they are biological products, the well-established principles of pharmacokinetics are not applicable to stem cells. This makes determination of the optimal dose difficult, especially when considering the complex mechanisms involved in the efficacy of these cells, including engraftment and paracrine signaling26, 27. Therefore, studies designed to specifically evaluate the appropriate quantity and concentration of transplanted cells are required to garner valuable information needed for larger phase II and III clinical trials.
The TRIDENT study was a randomized, double-blinded comparison of two doses of allogeneic hMSCs delivered by TESI to patients with ICM. The results indicate the safety and feasibility of TESI for both treatment groups and demonstrate the efficacy of these cells in reducing infarct scar size and improving cardiac function, with the 100M group imparting greater benefit. The primary objective of this study was to determine the safety of the two doses of allogeneic MSCs, and this trial supports that TESI of these cells is safe, as there were no TE-SAEs within the first 30 days or evidence of ectopic tissue formation at 12 months. A unique concern with allogeneic cell-based therapies is the potential for immune rejection. MSCs are well suited for use as allografts because they lack MHC class II molecules, lack T cell costimulatory molecules, and evade destruction by lymphocytes via paracrine signaling9, 26. In the present study, there was no clinical evidence of immune reactions. Only one patient had a detectable cPRA response, an incidence that is similar to previous studies of TESI of allogeneic hMSCs5, 9. These data further substantiate that hMSCs delivered as an allograft are safe and well tolerated in patients with ICM.
The TRIDENT study demonstrates that hMSCs are clinically efficacious in reducing scar size and improving cardiac function. Patients in both dose groups had reductions in infarct scar size, similar in magnitude to the allogeneic group in the POSEIDON study that compared allogeneic and autologous hMSCs in patients with ICM5. An intriguing finding of this study is that EF was improved only in the 100M, suggesting that the higher dose provides a greater benefit to cardiac function than the lower dose. Additionally, while pro-BNP increased over the study period in 20M, it remained stable in 100M, further supporting the superiority of the higher dose. Together, these findings support the clinical efficacy of allogeneic hMSCs in patients with ICM and the potential superiority of the 100M dose. Moreover, scar size reduction and functional restoration occurs at the sites of TESI vs non-TESI sites28.
We previously showed in the POSEIDON DCM trial9 that hMSC therapy produced favorable alterations in markers of chronic inflammation, known to be associated and predictive of disease progression in heart failure. Our present study yielded similar results, as both doses reduced serum levels of TNF-α, a proinflammatory cytokine and marker of heart disease progression29. Together, these results support the idea that MSCs exert their therapeutic benefits in part through anti-inflammatory effects.
To date, there have been few clinical studies examining multiple dosages of cell-based therapy. Quyyumi et al. observed a direct relationship between dose and efficacy in a study comparing intracoronary infusion of 5M, 10M and 15M autologous CD34+ cells in patients with acute ST-elevation MI6. They found that at 6 months, only patients receiving the higher doses (≥10M) had significant improvements in myocardial perfusion compared to placebo. Although this study demonstrated a direct dose response relationship, there are several factors that make it difficult to compare to our study. The acute injury process, route of administration, and timing of treatment could influence cell survival and efficacy. For example, the setting of acute MI is likely very hostile to transplanted cells and therefore higher doses may be required to overcome initial cell death secondary to hypoxia. Furthermore, intracoronary injection requires cells to extravasate and migrate to the areas of injury, which may result in lower engraftment rates than intramyocardial injection, thus requiring higher initial doses30. Another trial by Perin and colleagues compared three doses (25M, 75M and 150M) of allogeneic mesenchymal precursor cells delivered by TESI in patients with heart failure (either ischemic or non-ischemic)24. Similar to the TRIDENT findings, they found a direct dose response, with the 150M dose producing the greatest improvement in cardiac structure and function.
Conversely, other trials have suggested that there is an inverse relationship between dose and efficacy. Losordo et al. performed a study of autologous CD34+ cells delivered via TESI to patients with refractory angina in which 167 patients were randomized to receive either placebo or one of two doses (0.1M per kilogram or 0.5M per kilogram)4. Only the lowest dose group demonstrated significant improvement in frequency of angina, myocardial perfusion, and exercise tolerance. In a recent subanalysis of CHART-1, a phase III trial examining TESI of autologous lineage-directed cardiopoietic MSCs in patients with ICM31, Teerlink et al. found that patients who received <20 injections (and therefore fewer cells) had greater reverse remodeling than those receiving >20 injections23. However, the total number of injected cells was up to 600M, a dose that is much higher compared to our and other studies. A higher number of injections could potentially damage the myocardium leading to reduced efficacy, further complicating the relationship between cell quantity and clinical benefit.
Similarly, in our previous POSEIDON trial, which examined TESI of three doses (20M, 100M and 200M) of either autologous or allogeneic hMSCs in patients with ICM, patients who received the 20M dose had significantly greater improvements in EF and ESV compared to the 200M group5. These findings supported an inverse dose-response relationship, in contrast to our findings in TRIDENT. There were no significant differences between the 20M and 100M groups in the POSEIDON trial, but here we demonstrate the superiority of the 100M dose. This inconsistency may be because the sample size in POSEIDON was smaller for each dose and within each dose patients were further randomized either to autologous or allogeneic MSCs. Although a 200M dose was not included in this study, it may be that there is an upper limit or ceiling above which increases in the dose of allogeneic hMSCs do not improve efficacy. Additional disparities in the baseline characteristics of the POSEIDON trial compared to the TRIDENT trial in cardiac and functional parameters suggest that the severity of the disease, which was worse in the POSEIDON patients, can contribute to a different response in respect to the cell dose. In POSEIDON, patients with larger infarct sizes at baseline experienced greater infarct size reduction5. The discrepancies between the results of POSEIDON and TRIDENT further support the need for larger clinical trials examining a wider range of doses.
Given the paucity of trials designed to assess cell dose, and the discrepancies in the conclusions, great controversy still remains regarding optimal dosing in cell-based therapies for cardiac disease. The disparity in the results may be largely due to differences in the cell type, route of administration, and disease states examined. Given that transplanted cells must survive and interact with the surrounding environment, the disease state and mode of delivery must be consistent in order to compare dosing and efficacy. Regardless, the aforementioned studies did find significant differences between doses, which further emphasizes the importance of research into optimal dosage for cell-based therapies.
This phase II study is limited by the lack of a placebo group and small sample size. As such, further studies to examine efficacy should be performed with larger study populations and appropriate controls. In our study, some important parameters did not reach statistical significance, most likely due to the small number of patients per group. This study also only examines two doses, and higher or lower ranges may be more efficacious, but would require very large, multi-armed trials. Future larger studies are planned to investigate optimal dose for cardiac cell based therapy. The impact of baseline characteristics, including scar size and NYHA classification, also merits special attention in future studies, as efficacy may be impacted by severity of disease at baseline.
In conclusion, the TRIDENT study highlights the crucial role of cell dose and concentration in the responses to cell therapy for chronic ICM. Building upon previous data supporting the safety and efficacy of these cells, our findings support that hMSCs delivered by TESI are safe and feasible in patients with chronic ICM and that the higher 100M dose is superior to the 20M dose. These data support future investigation of hMSCs in phase III trials using the 100M dose, as it appears to be the most beneficial in this patient population, and further underscore the need for trials designed to evaluate optimal dosing for cell-based therapies.
Figure 1. Study Flow Chart.
Following screening, 30 patients were enrolled and randomized 1:1 to receive allogeneic hMSC treatment. Following safety evaluation at 30 day TE-SAEs and secondary endpoints.
Novelty and Significance.
What Is Known?
Human MSCs work through both paracrine signaling and heterocellular coupling mechanisms to improve cardiac function.
Defining the optimal cell dose and concentration would help enhance the therapeutic potential of MSCs.
What New Information Does This Article Contribute?
Allogeneic bone-marrow MSCs delivered by TESI to patients with ICM at cell doses of 20 million and 100 million are safe, as there were no treatment-emergent serious adverse events.
Allogeneic MSC therapy is associated with favorable effects on scar size reduction and cardiac function, with a greater benefit observed with the 100 million cell dose.
Human MSCs reduce serum TNF-α in patients with ICM.
Cell dosing plays an important role on clinical outcomes in patients with ischemic cardiomyopathy (ICM).
As the cell-based therapy field evolves, defining an optimal cell dose, cell concentration, and route of delivery is pivotal to enhance phenotypic responses to cell-based therapy. The TRIDENT trial compared two doses of allogeneic bone marrow-derived MSCs delivered by TESI in ICM patients. The results of this clinical trial indicate that both doses of allogeneic MSCs are well tolerated and associated with favorable effects on scar size and cardiac function, with a greater benefit observed with the 100 million cell dose. In addition, MSC therapy reduces serum TNF-α, a marker of chronic inflammation. This study highlights the crucial role of cell dose and concentration in cell therapy to advance the field and enhance standardization of future Phase III trials.
ACKNOWLEDGEMENTS
We thank the TRIDENT DSMB, the patients who participated in this trial, the bone marrow donors, and the staff of the cardiac catheterization laboratories at the University of Miami Hospital. We also thank Dr. Joel E. Fishman, Dr. Pradip M. Pattany, Erica C. Anderson, Jairo A. Tovar, Dr. Huw S. Kruger Gray, and the Sylvester Comprehensive Cancer Center Flow Cytometry Core Facility.
SOURCES OF FUNDING
This study was funded by the Starr and Soffer Family Foundations. Helical infusion catheters were provided by Biocardia INC.
DISCLOSURES
Dr. Hare discloses a relationship with Vestion Inc. that includes equity, board membership, and consulting, and Longeveron LLC that includes equity, board membership, and consulting. K. Valasaki discloses a relationship with Vestion Inc. that includes equity. Vestion Inc. did not participate in funding this work. A. Khan, and D. DiFede disclose a relationship with Longeveron LLC that includes consulting. Longeveron LLC did not participate in funding this work. D. DiFede discloses a relationship with BDS as a consultant. Drs. Hare and Heldman received research support from Biocardia. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms:
- 6MWT
6 minute walk test
- CI
confidence interval
- CT
computed tomography
- cPRA
calculated panel reactive antibodies
- EDV
end diastolic volume
- EED
early enhancement defect
- EF
ejection fraction
- ESV
end systolic volume
- ICM
ischemic cardiomyopathy
- IQR
interquartile range
- LV
left ventricular
- MACE
Major Adverse Cardiac Event
- M
million
- MI
myocardial infarction
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- NYHA
New York Heart Association
- SAE
serious adverse events
- TE-SAE
treatment-emergent serious adverse events
- TESI
transendocardial stem cell injection
- TNF-α
tumor necrosis factor alpha
- WHF
worsening heart failure
REFERENCES
- 1.de la Fuente LM, Stertzer SH, Argentieri J, Penaloza E, Miano J, Koziner B, Bilos C, Altman PA. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the tabmmi study). American heart journal. 2007;154:79 e71–77 [DOI] [PubMed] [Google Scholar]
- 2.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The focus-cctrn trial. JAMA : the journal of the American Medical Association. 2012;307:1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circulation research. 2011;108:792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA, Investigators AC. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circulation research. 2011;109:428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. Cd34(+) cell infusion after st elevation myocardial infarction is associated with improved perfusion and is dose dependent. American heart journal. 2011;161:98–105 [DOI] [PubMed] [Google Scholar]
- 7.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, Toma C, Davidson CJ, Barsness GW, Shavelle DM, Cohen M, Poole J, Moss T, Hyde P, Kanakaraj AM, Druker V, Chung A, Junge C, Preti RA, Smith RL, Mazzo DJ, Pecora A, Losordo DW. Preserve-ami: A randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous cd34+ cells in patients with left ventricular dysfunction post stemi. Circulation research. 2017;120:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA, Heldman AW, Miki R, Goldstein BJ, Mushtaq M, Levis-Dusseau S, Byrnes JJ, Lowery M, Natsumeda M, Delgado C, Saltzman R, Vidro-Casiano M, Pujol MV, Da Fonseca M, Oliva AA Jr., Green G, Premer C, Medina A, Valasaki K, Florea V, Anderson E, El-Khorazaty J, Mendizabal A, Goldschmidt-Clermont PJ, Hare JM. Allogeneic human mesenchymal stem cell infusions for aging frailty. The journals of gerontology. Series A, Biological sciences and medical sciences. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare JM, DiFede DL, Castellanos AM, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro-Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized comparison of allogeneic vs. Autologous mesenchymal stem cells for non-lschemic dilated cardiomyopathy: Poseidon-dcm trial. Journal of the American College of Cardiology. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (tac-hft) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. American heart journal. 2011;161:487–493 [DOI] [PubMed] [Google Scholar]
- 11.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, Halperin HR, Wu KC, Hare JM, Lima JA. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: Characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamas GA, Vaughan DE, Parisi AF, Pfeffer MA. Effects of left ventricular shape and captopril therapy on exercise capacity after anterior wall acute myocardial infarction. The American journal of cardiology. 1989;63:1167–1173 [DOI] [PubMed] [Google Scholar]
- 13.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA : the journal of the American Medical Association. 2014;311:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: The c-cure (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. Journal of the American College of Cardiology. 2013;61:2329–2338 [DOI] [PubMed] [Google Scholar]
- 17.Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sorensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (msc-hf trial). European heart journal. 2015;36:1744–1753 [DOI] [PubMed] [Google Scholar]
- 18.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic research in cardiology. 2008;103:525–536 [DOI] [PubMed] [Google Scholar]
- 20.Hamamoto H, Gorman JH 3rd, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: The effect of cell dosage. The Annals of thoracic surgery. 2009;87:794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. European heart journal. 2009;30:2722–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. European heart journal. 2008;29:251–259 [DOI] [PubMed] [Google Scholar]
- 23.Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G, Investigators C. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: Results from the congestive heart failure cardiopoietic regenerative therapy (chart-1) study. European journal of heart failure. 2017 [DOI] [PubMed] [Google Scholar]
- 24.Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A phase ii dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circulation research. 2015;117:576–584 [DOI] [PubMed] [Google Scholar]
- 25.Poglajen G, Sever M, Cukjati M, Cernelc P, Knezevic I, Zemljic G, Haddad F, Wu JC, Vrtovec B. Effects of transendocardial cd34+ cell transplantation in patients with ischemic cardiomyopathy. Circulation. Cardiovascular interventions. 2014;7:552–559 [DOI] [PubMed] [Google Scholar]
- 26.Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: Mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev. 2016;96:1127–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, Giacca M, Hare JM, Houser S, Lee RT, Marban E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte regeneration: A consensus statement. Circulation. 2017;136:680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, Nelson KH, Gerstenblith G, DiFede Velazquez DL, Breton E, Sitammagari K, Schulman IH, Taldone SN, Williams AR, Sanina C, Johnston PV, Brinker J, Altman P, Mushtaq M, Trachtenberg B, Mendizabal AM, Tracy M, Da Silva J, McNiece IK, Lardo AC, George RT, Hare JM, Heldman AW. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the percutaneous stem cell injection delivery effects on neomyogenesis (poseidon) randomized trial. Circulation research. 2014;114:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241 [DOI] [PubMed] [Google Scholar]
- 30.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. Journal of molecular and cellular cardiology. 2008;44:486–495 [DOI] [PubMed] [Google Scholar]
- 31.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz-Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guedes A, Heyse A, Moccetti T, Fernandez-Aviles F, Jimenez-Quevedo P, Bayes-Genis A, Hernandez-Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W, Program C. Cardiopoietic cell therapy for advanced ischaemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled chart-1 clinical trial. European heart journal. 2017;38:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]