Abstract
Significance: Cutaneous scarring affects millions of patients worldwide and results in significant financial and psychosocial burdens. Given the immune system's intricate involvement in the initiation and progression of wound healing, it is no surprise that the scarring outcome can be affected by the actions of various immune cells and the cytokines and growth factors they produce. Understanding the role of T cells in regulating immune responses and directing the action of wound mesenchymal cells is essential to developing antifibrotic therapies to reduce the burden of scarring.
Recent Advances: As the immune system is intimately involved in wound healing, much work has examined the impact of T cells and their cytokines on the final wound outcome. New innovative tools for studying T cells have resulted in more sophisticated immunophenotyping capabilities and the ability to examine effects of individual cytokines in the wound environment.
Critical Issues: Despite continued advances in the study of specific immune cells and their effects on dermal fibrosis, minimal progress has been made to modulate immune responses to result in improved wound cosmesis.
Future Directions: The actions of T cells represent potential pharmacologic targets that could lead to novel bioengineered or immunoengineered therapies to improve the lives of people with cutaneous scarring.
Keywords: lymphocyte, fibrosis, scarring, immune, inflammation

Sundeep G. Keswani, MD, FACS, FAAP
Scope and Significance
Normal mammalian cutaneous wound healing inevitably results in some degree of dermal scarring. While this aesthetically displeasing phenotype is likely the result of evolutionary pressure for rapid healing of contaminated wounds, it results in a healed area that will never completely recover the tensile strength of unwounded skin.1 Wound healing involves a dynamic interplay between skin-resident cells and infiltrating cells of both the innate and adaptive immune systems. These immune cells not only perform an essential antimicrobial function but also govern the transition from an acute inflammatory phase to the reparative phases of healing, guided in part by T cells. Understanding the role of T cells in cutaneous fibrosis is essential to develop therapeutics that may prevent or even reverse scarring, thus combating the problematic psychosocial and economic burden that scarring has on modern society.2
Translational Relevance
Despite numerous studies of lymphocyte impact on fibrogenesis in various organ systems, little primary research has focused on their role in cutaneous scarring, particularly the contribution of various T cell subsets to this process. As the immune system's involvement in wound healing has come to the forefront of basic wound healing research, this review serves to summarize recent seminal discoveries of the involvement of T cells in cutaneous scarring and stimulate further research into this incredibly complex and important subject matter.
Clinical Relevance
Millions of patients suffer from surgical scarring and burn contracture.1 Despite decades of research, the magic bullet of regenerative healing has remained elusive. The immune system is deeply intertwined in the wound healing response and thus represents a potential target for therapeutics. Immunomodulation and cell-based therapies are currently being developed to ameliorate autoimmune conditions and graft-versus-host disease, and better understanding of how the immune system contributes to scarring can aid in applying these types of therapies to improve the lives of patients affected by scarring.
The Intricate Inflammatory Response in Wound Healing
The process of cutaneous wound healing is traditionally divided into four mutually inclusive stages: hemostasis, inflammation, proliferation, and remodeling. While scar formation occurs primarily in the remodeling phase, the preceding healing steps, particularly inflammation, significantly impact the final wound healing outcome.
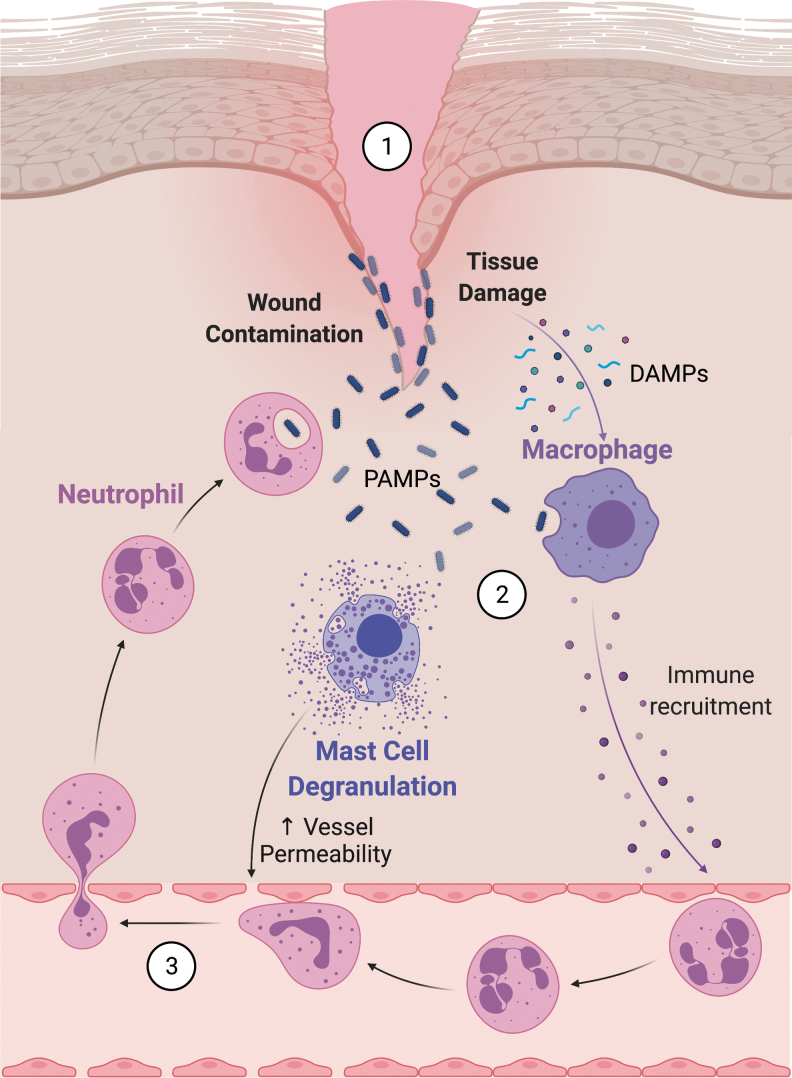
Lasting around 6 days, the inflammatory response originates with tissue injury and involves influx and activation of various waves of immune cells (Fig. 1). It is initiated by molecular signals from injured keratinocytes and fibroblasts in the form of DNA, RNA, uric acid, and extracellular matrix (ECM) components, together classified as damage-associated molecular patterns (DAMPs).3 Further inflammatory cell recruitment to a wound can be driven by bacterial pathogens present in the wound, or pathogen-associated molecular patterns (PAMPs), which along with DAMPs are recognized by skin-resident immune cells such as dendritic cells, innate lymphoid cells, and macrophages, leading to cytokine and chemokine production.4 PAMPs and local tissue injury signals also activate resident mast cells to degranulate, releasing cytokines and chemokines that serve to attract circulating immune responders.5 Neutrophils are the first innate immune cells to be attracted by these chemokines, specifically by interleukin-8 (IL-8) produced by skin-resident cells. Skin-resident macrophages, activated by DAMPs, initially contribute to the acute inflammatory response and participate in phagocytosis of foreign material and cellular debris. Circulating monocytes—macrophage precursors—are rapidly drawn to the wound by IL-6 and monocyte chemoattractant protein-1 (MCP-1).6 As the inflammatory response proceeds, ingestion of apoptotic neutrophils triggers macrophages to signal an end to this response by secreting transforming growth factor-beta (TGF-β) and prostaglandins.7 Dysregulated macrophage responses can cause prolongation of the inflammatory phase, resulting in chronic nonhealing wounds.8
Figure 1.
Initiating the inflammatory response. (1) Tissue injury and cell death release DAMPs that stimulate macrophages (2) to release proinflammatory cytokines. Simultaneously, bacterial contamination signals both macrophages and mast cells through PAMPs, leading to further chemokine release and mast cell degranulation. Mast cells release histamine that facilitates immune cell migration into tissues by increasing blood vessel permeability. (3) The end result is increased immune cell infiltration into the wound to participate in phagocytosis of pathogens and necrotic debris. Cells are not drawn to scale. Image created using BioRender.com. DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern. Color images are available online.
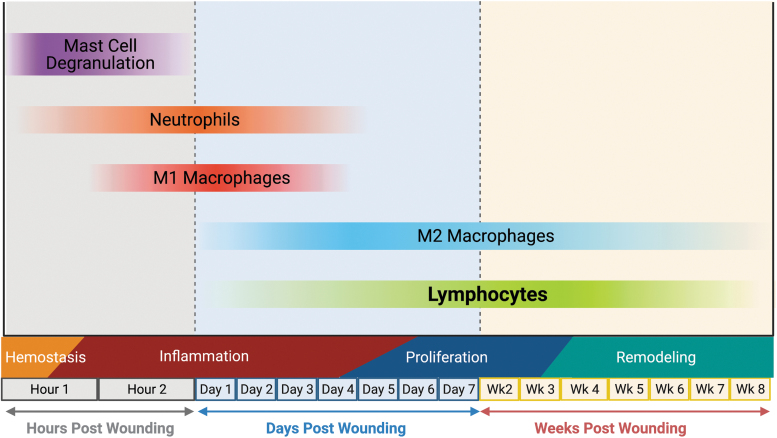
Lymphocytes, drawn by chemokines produced in the wound, such as CCL3, CCL4, and CCL5, are the final immune cells to infiltrate into the wound.6,9 While historically T cells were seen as arriving late in the inflammatory process, Wang et al. recently demonstrated that T cells are present in murine wounds within 24 h of wounding and remain present for at least 30 days, with CD4+ T cells representing the most abundant T cell subset present in the wound healing process.10 Findings such as this indicate that T cells likely not only play an important role in regulating the inflammatory response but may also continue to modulate cells in the wound during the proliferative and remodeling phases, although confirmatory functional studies were not conducted in that work. The time course of immune cell activity in wound healing is demonstrated in Fig. 2.
Figure 2.
Timing of immune cell activity following injury. Tissue injury rapidly results in hemostasis, which is followed shortly by mast cell degranulation and recruitment of neutrophils and macrophages to the wound to perform antimicrobial functions. As the inflammatory phase winds down, macrophages shift their phenotype to the more reparative M2s. Lymphocytes are recruited to the wound early in inflammation and persist in the wound at low levels for weeks following injury. Image created using BioRender.com. M2s, alternatively activated macrophages. Color images are available online.
While T cells have been an area of focus in many categories of diseases, our understanding of lymphocyte function in wound healing is limited. In this study, we illustrate the roles of T cells in wound healing by summarizing current literature with an emphasis on the common outcome of wound healing—fibrosis.
Lymphocyte Roles in Wound Healing
Lymphocytes arise from lymphoid progenitor cells and comprise the adaptive immune system, meaning they react to specific foreign antigens, unlike the innate immune system. In the broadest sense, lymphocytes can be divided into two major classes: T, or thymic-derived, and B, or bone marrow-derived, lymphocytes. Activated B cells mature into plasma cells that produce antibodies, while activated T cells differentiate into unique phenotypic subtypes that are distinguished by surface markers, transcription factors, and cytokine production. This review will not delve into the complexities of comprehensive T cell immunophenotyping, but instead will focus on the pertinent classes that are most established in the wound healing literature. T cells are of particular interest because they serve as assistants and regulators of immune response. CD3+ T cells can be subdivided into CD4+ cells, which recognize antigen presentation by major histocompatibility complex (MHC) class II, and CD8+, which rely on MHC class I signaling. While CD8+ T cells do produce some inflammatory cytokines, their primary active function is cytotoxicity, which distinguishes them from CD4+ T cells whose principal effector role is production of cytokines that modulate both innate and adaptive immune responses. Therefore, T cells are significant in wound healing research as they have the potential to act as conductors of the symphony of the cellular response to injury and can nudge the overall balance of inflammatory and fibrotic factors in a wound toward either a more regenerative or fibrotic outcome.
Exactly how lymphocytes tip the scales with regard to scarless versus profibrotic wound healing is rather opaque. An early study demonstrated that wounds in athymic nude mice, which lack typical T cell maturation, healed with greater tensile strength likely due to greater collagen content.11 They also revealed that transfer of T cells into these mice decreased collagen in the wounds. By contrast, another study in athymic mice, which focused on regenerative wound healing, demonstrated that these mice healed in a way that resembles scarless fetal wound healing, including regrowth of musculature.12 The authors further demonstrated that in this particular athymic strain, Athymic Nude-nu (B6.Cg-Foxn1nu), healing occurs with elevated hyaluronan (HA), low collagen content, and decreased arbiters of fibrosis, TGF-β1 and PDGF- B.13 From these results, one could draw the conclusion that T cells have a negative impact on cutaneous scarring. Contrary to that conclusion, our group has demonstrated that SCID mice (B6.CB17-Prkdcscid/SzJ), lacking functional T and B cells, have exaggerated scarring compared with wild-type (WT) controls, with reconstitution of total lymphocytes or CD4+ T cells alone leading to a reduction in fibrosis and inflammation.10 While the purpose of the utilization of SCID and athymic animals is to provide a model deficient in lymphocytes, there are other alterations to the immune environment and responses in immunodeficient models that may alter the wound healing phenotype such as upregulated macrophages, neutrophils, and complement activation.14–17 Although these conflicting findings may be due to genetic variation in the models, they do highlight that T cells play a greater role in the wound outcome than previously recognized, and investigations into specific lymphocyte phenotypic subsets are warranted.
Involvement of T cells in wound healing and scarring
As mentioned previously, T cells differ from B cells, in that they develop in the thymus, as opposed to the bone marrow, and constitutively express the surface molecule, CD3. CD3 acts as a coreceptor for the T cell receptor alpha–beta chains, allowing specific antigen binding imperative for T cell activation. T cell activation, in the context of the cytokine milieu such as interleukins, triggers transcription factors that press naïve T cells to differentiate into one of several effector or memory cells. A summary of the most well-established T cells and their respective surface markers, transcription factors, and cytokine profiles is listed in Table 1.
Table 1.
CD4+ Subsets and Their Defining Characteristics
| CD4+ Subset | Polarization Signal | Primary Transcription Factor | Surface Marker | Cytokine Profile | References |
|---|---|---|---|---|---|
| Th1 | IL-12 | T-bet | Nonspecific | IFN-γ | 78–80 |
| Th2 | IL-4 | GATA-3 | Nonspecific | IL-4, IL-5, IL-10, and IL-13 | 79,80 |
| Th17 | TGF-β and IL-6 | RORγt | Nonspecific | IL-17 and IL-22 | 81,82 |
| Treg | TGF-β | Foxp3 | CD25 (Mouse) | IL-10 and TGF-β | 83,84 |
| Tr1 | IL-10 and IL-27 | IRF4, c-Maf, and AhR | CD49b, LAG-3, CD226, CCR5, and PD-1 | IL-10 and TGF-β | 45,85 |
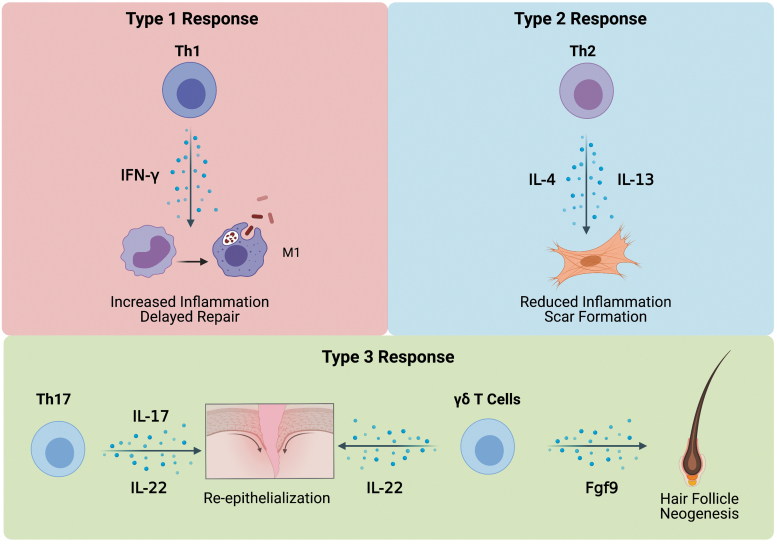
Immunologists have generated a classification system that divides immune responses into three types (Fig. 3), all of which play a role in physiologic wound healing. The initial cytokine milieu is determined, in part, by responses of innate lymphoid cells, of which there are three types that correspond to their associated immune response. Bacterial contamination principally stimulates type 1 and type 3 innate lymphoid cells to produce cytokine profiles that activate macrophages and preferentially polarize helper T cells toward type 1 helper T cells (Th1s) and type 17 helper T cells (Th17s).4 Type 1 immune responses are driven by Th1s and strongly depend on the proinflammatory cytokine they produce, interferon gamma (IFN-γ). Type 2 immune responses rely on IL-4 and IL-13 production from type 2 helper T cells (Th2s), and type 3 responses are associated with IL-17 and IL-22 from Th17s.18 Immune responses are dynamic and fluid in wound healing, beginning with a type 1 response in the acute inflammatory phase, which transitions to a type 2 reparative response through macrophage signaling.18 The type 3 immune response overlaps with the first two types during wound healing, where it stimulates inflammation through neutrophil recruitment and promotes reepithelialization.18 The following subsections will briefly discuss the fibrogenic role of CD8+ cells, followed by the roles of Th1, Th2, Th17, Foxp3+ regulatory T cells (Treg), and type 1 regulatory T cell (Tr1) subsets and their respective effector cytokines in cutaneous scarring.
Figure 3.
The types of immune responses and their involvement in wound healing. The type 1 immune response is primarily driven by interferon-gamma, which is released by Th1s. IFN-γ is proinflammatory and aids in polarizing macrophages to the M1 phenotype. As the inflammatory phase progresses, it is shifted into a type 2 response as T cells are polarized toward Th2s. Th2s produce cytokines IL-4 and IL-13, which, although anti-inflammatory, also have significant profibrotic effects. Overlapping with the type 1 and 2 immune responses, the type 3 responses driven by Th17s and γδ T cells result in reepithelialization of the wound as well as hair follicle neogenesis. Cells are not drawn to scale. Image created using BioRender.com. Th1s, type 1 helper T cells; Th17s, type 17 helper T cells; Th2s, type 2 helper T cells; M1, classically activated macrophage. Color images are available online.
CD8+ T cells
Cytotoxic or CD8+ T cells are essential for destruction of virally infected cells. CD8+ T cells produce IL-13, a profibrotic cytokine, and migrate to the skin in patients with systemic sclerosis and have been shown in vitro to increase Col1 production by dermal fibroblasts.19,20 Beyond systemic sclerosis, CD8+ T cells play a profibrotic role in thyroid, lung, and renal fibrosis.21 In CD8+-deficient mice, wounds displayed decreased infiltration of inflammatory cells such as CD4+ cells, macrophages, and neutrophils. This culminated in no difference in the wound closure rate and collagen content in wounds between WT and CD8+-deficient mice, and scarring was not specifically evaluated.22 Unfortunately, CD8+ involvement in cutaneous scarring is underexplored, thus we will focus on the impact of CD4+ T cells.
Type 1 helper T cells
Th1s are major players in the propagation of cell-mediated inflammation; however, they have not been extensively studied in cutaneous fibrosis. They characteristically produce IL-2 and IFN-γ.23 While IL-2 serves to activate and act as a survival signal for Tregs, helper T cells, and cytotoxic T cells, IFN-γ is widely accepted to be a proinflammatory cytokine, although with potential antifibrotic effects.24 In one study, IFN-γ knockout mice healed with reduced inflammation, but greater fibrosis, highlighting the concept that inflammation and fibrogenesis do not necessarily go hand in hand.25 When recombinant IFN-γ was injected into palatal wounds in rats, the wounds healed with fewer myofibroblasts, resulting in decreased collagen deposition.26 While in vitro studies have demonstrated that IFN-γ reduces alpha smooth muscle actin (αSMA) and ECM production in normal human skin fibroblasts, these effects are not seen in keloid-derived fibroblasts in patients.27 These results indicate intrinsic differences in normal and keloid fibroblasts, highlighting limitations in the antiscarring therapeutic utility of IFN-γ. Nevertheless, the evidence for antifibrotic effects of IFN-γ must be viewed in the context of the Th1 response.
One major way that Th1s can influence the healing process is by influencing macrophage polarization to the M1 phenotype. Known as classically activated macrophages, M1s are distinguished from alternatively activated macrophages (M2s) by low IL-10 secretion coupled with elevated proinflammatory cytokine production and greater phagocytotic propensity. This destruction often lacks specificity, however, leading to inadvertent damage of healthy or host tissues during the acute inflammatory phase of wound healing.28 M1s stimulate Th1 aggregation to injury sites, generating a feed-forward loop in which Th1s continue to drive M1 polarization through IFN-γ secretion, and vice versa, which when prolonged leads to more scarring.28–30 In conclusion, while IFN-γ may have potential to be an antifibrotic effector, the association of a Th1-mediated response and the resultant inflammatory tissue damage prevents IFN-γ from being a mediator of reduced scarring in physiologic healing.
Type 2 helper T cells
Th2 polarization from naïve T cells is driven by the transcription factor, GATA3, resulting in cells that produce a characteristic cytokine milieu consisting of IL-4, IL-5, IL-13, and the known anti-inflammatory mediator, IL-10. IL-10, in particular, not only has immune dampening effects but has also been repeatedly demonstrated to be antifibrotic and promote regenerative healing in multiple organ systems, including the skin.31 Despite production of IL-10, Th2 responses are associated with tissue fibrosis, with the balance of fibrotic outcome shifted in favor of ECM deposition by the strong profibrotic effects of IL-13 and, to a lesser extent, IL-4.
IL-13 and IL-4 are major stimulators of collagen synthesis and the TGF-β1 fibrotic pathway and lead to proliferation of fibroblasts and ECM deposition.32,33 IL-4 and IL-13 binding results in activation of the JAK intracellular pathway, leading to phosphorylation of STAT6.32,34 STAT6 then activates genes promoting collagen synthesis and TGF-β1 expression.34 An exaggerated Th2 response is responsible for fibrosis in hepatic and schistosomal infection models, primarily due to overproduction of IL-13.35,36 IL-33, a cytokine released by dying fibroblasts, epithelial cells, and endothelial cells, stimulates an increase in the Th2 cytokines, IL-4, IL-5, and IL-13, when injected intradermally in mice, culminating in dermal fibrosis.37 Interestingly, this fibrotic response was dependent on functional eosinophils that are known to attract Th2s as well as stimulate Th2 differentiation from naïve T cells.38 Although IL-4 and IL-13 are produced in great quantities by Th2s, they are also produced by other innate immune cells that are all drawn to wounds in large numbers by IL-33 and other alarmins.18 While the involvement of type 2 cytokines is strongly implicated in scarring following injury, the specific role of Th2s has not been adequately demonstrated in skin fibrosis, and further study is warranted.
Further complicating things, the Th2 cytokines, IL-4 and IL-13, also serve to instigate polarization of macrophages to the reparative M2 phenotype. Deletion of the IL-4Rα receptor in M2s results in delayed wound closure and anomalous collagen fibril assembly, signifying the importance of Th2 cytokines in the M2-mediated wound healing process.39
Together, the studies on Th1s and Th2s demonstrate that the shift from the inflammatory type 1 response to the reparative type 2 process is critical for healing of wounds as some fibrosis is intrinsically necessary for the successful resolution of physiological wound healing. It is possible that regenerative wound healing does not require absolute containment of Th1 or Th2 responses, but instead needs a mediator that can delicately shift the balance from fibrosis to regeneration.
Foxp3+ regulatory T cells
Tregs, defined by expression of the lineage-determining transcription factor, Foxp3, make up ∼20% of CD4+ cells residing in adult human skin and play a crucial role in attenuating inflammation during normal wound healing. In human skin, Tregs tend to display a memory phenotype and are localized in areas of the epidermis and dermis that surround hair follicles.40 While Tregs do secrete the regulatory and antifibrotic cytokine, IL-10, they also secrete TGF-β1, which dually acts as an anti-inflammatory factor and an intensely profibrotic growth factor.
Treg levels, similar to overall lymphocytes, peak around day 7 of wounding, although it is unknown if this activated population arises from skin-resident or circulating Tregs.41 Ablation of Tregs in mouse excisional wound models leads to increased IFN-γ levels and proinflammatory macrophages, resulting in a prolonged type 1 inflammatory response and delaying wound closure.41,42 In a mouse model of bleomycin-induced dermal fibrosis, knockdown of Tregs resulted in increased myofibroblast activation with subsequent skin fibrosis.43 Tregs are also implicated in the pathologic fibrosis of keloids, as they have been shown in vitro to increase collagen expression by fibrocytes, profibrotic cells of myeloid origin.44 This increase in collagen expression was demonstrated to be mediated by Treg production of TGF-β. Our own work with Tregs demonstrated that adoptively transferring Tregs into SCID mice results in decreased αSMA, but was not sufficient to reduce collagen content in our model, indicating that Tregs may require effector T cells that SCID mice lack to have antifibrotic effects.10 Interestingly, a large proportion of Tregs residing in skin express GATA3, the primary transcription factor that determines Th2 polarization. In fact, simply depleting Tregs in unwounded skin results in significant increases in Th2 cytokine content, leading to pathologic dermal fibrosis.42,43 In murine wounding experiments, depletion of Tregs similarly results in an enriched proportion of T-bet and GATA3 expression in T cells in the wound.42 This finding indicates that skin Tregs moderate a Th1/Th2 response that is key in preventing dermal fibroblast activation and fibrosis. Results from these experiments not only underscore the importance of Tregs in preventing excessive fibrosis but also indicate that the actions of Tregs alone are not enough to promote regenerative healing.
Type 1 regulatory T cells
Tr1s differ from Tregs, in that they are peripherally induced and exert their immunosuppressive effects through IL-10 and TGF-β signaling independent of Foxp3.45 Identification of Tr1s can be somewhat challenging; however, consensus in the literature suggests that Tr1s in humans and mice specifically coexpress CD49b and LAG-3, along with nonspecific markers such as ICOS, CCR5, PD-1, CTLA-4, TIM-3, TIGIT, and CD226.45,46 The cytokine profile of Tr1s can vary based upon the local cytokine milieu, but all methods of stimulation result in an abundance of IL-10 production and relatively low IL-4 production, which distinguish Tr1s from Th2s. Tr1s, in fact, act to inhibit the Th2 response in allergen models of type 2 immune responses in mice.47 Most research on the role of Tr1s has focused on their mediation of allergic responses and autoimmune disorders, rather than their role in wound healing. Their ability to produce IL-10 in great quantities, while producing less profibrotic TGF-β than Foxp3+ Tregs, may make them ideal targets for reduction of scarring.48
Lymphocytes and interleukins of the type 3 immune response
The type 3 immune response is driven by IL-17 and IL-22 production from Th17s, IL-17+ CD8 T cells, and γδ T cells, the latter of which have gamma and delta subunits of the T cell receptor rather than alpha and beta subunits such as Th1s, Th2s, and Tregs.18
Th17s are defined by their production of IL-17, which contributes to skin and lung fibrosis in bleomycin mouse models.49,50 In humans, IL-17 enhances fibroblast proliferation and their secretion of inflammatory cytokines, MCP-1, IL-6, and IL-8, while also increasing levels of matrix metalloproteinase-1 and -3.50–52 Conversely, IL-17 has been shown to downregulate connective tissue growth factor in human fibroblasts, a promoter of fibrosis upstream of TGF-β1, suggesting a possible role in scar reduction.53 Th17s have been shown to inhibit collagen production by human fibroblasts and were inversely correlated with the level of skin fibrosis in patients with systemic sclerosis.54,55 However, these results were disputed by another study that demonstrated that IFN-γ−expressing Th17s increased expression of αSMA and COL1A1, the effects of which were reversed when IL-21, another cytokine produced by Th17s, was neutralized.56
Th17s are also known to yield IL-22, a cytokine similarly produced by γδ T cells, which is structurally similar to IL-10, although it acts through different mechanisms.57 IL-22 is known to increase keratinocyte proliferation and migration, stimulate production of collagen by dermal fibroblasts, and promote myofibroblast differentiation following wounding.57,58 γδ T cells in mice reside in the epidermal and dermal layers of the skin and function in wound healing to support reepithelialization through production of keratinocyte growth factors.59 Notably, loss of γδ T cell function results in prolonged wound healing, particularly in diabetic mice.59,60 These cells also appear to be mediators of fibrosis given their involvement in the pathogenesis of systemic sclerosis. When dermal fibroblasts are cultured in γδ T cell-conditioned medium, they increase synthesis of collagen in a TGF-β–dependent manner.61 Despite this profibrotic activity of γδ T cells, they demonstrate a beneficial role in wound-induced hair follicle neogenesis, increasing proliferation of hair follicle stem cells through production of fibroblast growth factor 9 (FGF9).62,63
While the production of type 3 immune cytokines, IL-17 and IL-22, has been studied in wound healing, actions of Th17s and γδ T cells themselves still require further study to determine their effects on fibrosis in the context of wound healing and if their role in promulgating fibrosis is offset by aiding regeneration of hair follicles.
The Fetal Cutaneous Immune System: Clues to Regenerative Healing
The mid-gestation fetus has the ability to heal incisions and small wounds regeneratively, with no scars and restoration of dermal architecture, musculature, and hair follicles.64–66 IL-10 is present in greater quantities in fetal skin compared with adult skin, and the fetal regenerative phenotype is dependent on IL-10 signaling.67,68 As immune cells are the greatest sources of IL-10 production, the fetal cutaneous immune response to injury is of particular interest to those seeking to recapitulate that healing phenotype. αβ-T cells begin to migrate to and reside in human fetal skin by the second trimester and, as such, there are fewer CD3+ T cells in fetal skin at this time point relative to adult skin.69,70 Overall, fetal wounds exhibit less inflammatory cell infiltration than postgestational wounds.71 Upon stimulation, fetal T cells have recently been shown to have greater capacity for IFN-γ production relative to IL-13, and while the effects of this have not been investigated in terms of dermal wound healing, babies with high levels of IFN-γ in cord blood are less likely to develop atopic dermatitis, a condition that involves inflammation of the skin.69,72 Tregs make up about 10–20% of T cells in human fetal skin, a similar percentage is seen in adults; however, it is unknown if fetal cutaneous Tregs have a greater regulatory effect when compared with adult Tregs.40,73 While fetal Tregs have been shown to have greater expression of Foxp3 and PD-1, a marker of Treg suppressive ability, the functional effects of these genes in fetal wound healing have not been elucidated.69 With recent advances in immune cell phenotyping and imaging, one should expect more clues to the function of fetal T cells in the coming years.
Potential Immunomodulatory Therapeutics
Despite billions of dollars spent annually in the United States on wound care, an effective therapeutic that reliably prevents or reverses scarring does not exist.74 Given the integral involvement of lymphocyte cytokines in the pathogenesis of fibrosis, immunomodulatory medications may be the future of scar-modulating treatment. For example, it would be logical to target the Th2 cytokines, IL-4 and IL-13, as they have been shown to contribute significantly to scarring.
In 2017, a phase 2b trial (NCT02345070) was completed, which investigated the efficacy of SAR15697 (an antibody that binds and neutralizes both IL-4 and IL-13) in the treatment of idiopathic pulmonary fibrosis, although it did not show a significant difference in the primary end point compared with placebo.75 Another clinical trial involving SAR15697 is currently in phase 2 to investigate the antibody's utility in the treatment of diffuse systemic sclerosis (NCT02921971).
A possible therapeutic strategy also involves using enriched populations of lymphocytes themselves to treat wounds. A preclinical study treated wounds of diabetic mice topically with B cells, resulting in more rapid wound closure.76 Similarly, topically applying mixed-cell sheets containing fibroblasts and peripheral blood mononuclear cells, ∼65% of which are lymphocytes, resulted in increased angiogenesis and more expedited wound healing.77
While none of these studies have explicitly focused on scarring in cutaneous wounds, the demonstrated safety in human trials and validated biological effects of topical immune cells in animal models are encouraging results that may bring immunomodulatory therapies to the forefront in wound healing research.
Summary
In summary, the actions of a variety of T cells are an integral part of wound healing, but the full antifibrotic potential of these cells has yet to be unlocked. Successful wound healing requires a complex interplay of immune cells, fibroblasts, epithelial cells, and endothelial cells. When one type of immune response is dysregulated, maladaptive effects tend to occur, such as delayed wound closure or excessive fibrosis. Tregs appear to have a role in maintaining the balance of these immune responses to prevent excessive fibrosis, but are not enough on their own to reverse scarring. Further understanding of the complexities of T cells in wound healing may allow for development of therapeutics that find an optimal balance between the inflammatory response and tissue repair to bring about antiscarring effects.
Take-Home Messages
Scarring is a global economic and psychosocial burden for patients.
T cells influence the wound healing phases through secretion of cytokines.
Three types of immune responses play different roles in wound healing: type 1 leads to inflammation and destruction of microbes, type 2 transitions the wound to the proliferative stage of healing, and type 3 is involved in reepithelialization and hair follicle neogenesis.
Macrophage polarization mirrors the phase of wound healing, with polarization toward M2s aiding the resolution of the inflammatory phase.
Tregs and Tr1s secrete the anti-inflammatory cytokine, IL-10, which is known to have pleiotropic antifibrotic properties across several organ systems, including skin.
Further research on the role of T cells in fibrotic and fetal regenerative wound healing could lead to novel antiscarring therapeutics.
Abbreviations and Acronyms
- αSMA
alpha smooth muscle actin
- CCL
chemokine ligand
- DAMPs
damage-associated molecular patterns
- ECM
extracellular matrix
- FGF9
fibroblast growth factor 9
- Foxp3
forkhead box protein P3
- HA
hyaluronan
- IFN-γ
interferon gamma
- IL
interleukin
- M1s
classically activated macrophages
- M2s
alternatively activated macrophages
- MCP-1
monocyte chemoattractant protein-1
- MHC
major histocompatibility complex
- PAMPs
pathogen-associated molecular patterns
- PDGF-B
platelet-derived growth factor-B
- SCID
severe combined immunodeficiency
- TCR
T cell receptor
- TGF-β
transforming growth factor-beta
- Th1s
type 1 helper T cells
- Th17s
type 17 helper T cells
- Th2s
type 2 helper T cells
- Tr1s
type 1 regulatory T cells
- Tregs
Foxp3+ regulatory T cells
Acknowledgments and Funding Sources
S.G.K is supported by a National Institutes of Health grant (GM111808). The authors acknowledge the editorial support of Monica Fahrenholtz, PhD, from the Office of Surgical Research Administration at Texas Children's Hospital.
Author Disclosure and Ghostwriting
No competing financial interests exist for any of the authors of this article.
About the Authors
Walker D. Short, MD, is a general surgery resident at Baylor College of Medicine, completing his research fellowship in the Laboratory for Regenerative Tissue Repair (LRTR). Xinyi Wang, PhD, is an Assistant Professor in the LRTR. Sundeep G. Keswani, MD, FACS, FAAP, is the Surgical Director of Basic Science Research; Professor of Surgery, Pediatrics, and Obstetrics and Gynecology; Chief of Pediatric Surgery at Baylor College of Medicine/Texas Children's Hospital; and the Principal Investigator of the LRTR.
References
- 1. Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ 2003;326:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 2008;61:1049–1058. [DOI] [PubMed] [Google Scholar]
- 3. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008;8:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vivier E, Artis D, Colonna M, et al. Innate lymphoid cells: 10 Years On. Cell 2018;174:1054–1066. [DOI] [PubMed] [Google Scholar]
- 5. Wilgus TA, Wulff BC. The importance of mast cells in dermal scarring. Adv Wound Care (New Rochelle) 2014;3:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balaji S, Watson CL, Ranjan R, King A, Bollyky PL, Keswani SG. Chemokine involvement in fetal and adult wound healing. Adv Wound Care (New Rochelle) 2015;4:660–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep 2018;7:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am 1997;77:509–528. [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Balaji S, Steen EH, et al. T lymphocytes attenuate dermal scarring by regulating inflammation, neovascularization, and extracellular matrix remodeling. Adv Wound Care (New Rochelle) 2019;8:527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbul A, Shawe T, Rotter SM, Efron JE, Wasserkrug HL, Badawy SB. Wound healing in nude mice: a study on the regulatory role of lymphocytes in fibroplasia. Surgery 1989;105:764–769. [PubMed] [Google Scholar]
- 12. Gawronska-Kozak B. Regeneration in the ears of immunodeficient mice: identification and lineage analysis of mesenchymal stem cells. Tissue Eng 2004;10:1251–1265. [DOI] [PubMed] [Google Scholar]
- 13. Gawronska-Kozak B, Bogacki M, Rim JS, Monroe WT, Manuel JA. Scarless skin repair in immunodeficient mice. Wound Repair Regen 2006;14:265–276. [DOI] [PubMed] [Google Scholar]
- 14. Guhad FA, Jensen HE, Hau J. Complement activation in SCID and nude mice is related to severity of tissue inflammation in the Candida mastitis model. FEMS Microbiol Lett 2000;192:27–31. [DOI] [PubMed] [Google Scholar]
- 15. Laufer J, Katz Y, Passwell JH. Extrahepatic synthesis of complement proteins in inflammation. Mol Immunol 2001;38:221–229. [DOI] [PubMed] [Google Scholar]
- 16. Santini SM, Rizza P, Logozzi MA, et al. The SCID mouse reaction to human peripheral blood mononuclear leukocyte engraftment. Neutrophil recruitment induced expression of a wide spectrum of murine cytokines and mouse leukopoiesis, including thymic differentiation. Transplantation 1995;60:1306–1314. [PubMed] [Google Scholar]
- 17. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166–6173. [DOI] [PubMed] [Google Scholar]
- 18. Boothby IC, Cohen JN, Rosenblum MD. Regulatory T cells in skin injury: at the crossroads of tolerance and tissue repair. Sci Immunol 2020;5:eaaz9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furue M, Mitoma C, Mitoma H, et al. Pathogenesis of systemic sclerosis-current concept and emerging treatments. Immunol Res 2017;65:790–797. [DOI] [PubMed] [Google Scholar]
- 20. Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA Jr. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 2013;65:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang M, Zhang S. T cells in fibrosis and fibrotic diseases. Front Immunol 2020;11:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L, Mehta ND, Zhao Y, DiPietro LA. Absence of CD4 or CD8 lymphocytes changes infiltration of inflammatory cells and profiles of cytokine expression in skin wounds, but does not impair healing. Exp Dermatol 2014;23:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015;74:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, Design and application. Trends Immunol 2015;36:763–777. [DOI] [PubMed] [Google Scholar]
- 25. Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol 2004;172:1848–1855. [DOI] [PubMed] [Google Scholar]
- 26. Cornelissen AM, Maltha JC, Von den Hoff JW, Kuijpers-Jagtman AM. Local injection of IFN-gamma reduces the number of myofibroblasts and the collagen content in palatal wounds. J Dent Res 2000;79:1782–1788. [DOI] [PubMed] [Google Scholar]
- 27. Hasegawa T, Nakao A, Sumiyoshi K, Tsuboi R, Ogawa H. IFN-gamma fails to antagonize fibrotic effect of TGF-beta on keloid-derived dermal fibroblasts. J Dermatol Sci 2003;32:19–24. [DOI] [PubMed] [Google Scholar]
- 28. Ley K. M1 Means Kill; M2 Means Heal. J Immunol 2017;199:2191–2193. [DOI] [PubMed] [Google Scholar]
- 29. Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 1983;158:670–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci 2017;18:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, Keswani SG. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care (New Rochelle) 2020;9:184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen JK, Austin E, Huang A, Mamalis A, Jagdeo J. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res 2020;312:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015;75:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 1999;104:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 2000;164:2585–2591. [DOI] [PubMed] [Google Scholar]
- 37. Rankin AL, Mumm JB, Murphy E, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol 2010;184:1526–1535. [DOI] [PubMed] [Google Scholar]
- 38. Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol 2010;88:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knipper JA, Willenborg S, Brinckmann J, et al. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 2015;43:803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, et al. Memory regulatory T cells reside in human skin. J Clin Invest 2014;124:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nosbaum A, Prevel N, Truong HA, et al. Cutting Edge: regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol 2016;196:2010–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haertel E, Joshi N, Hiebert P, Kopf M, Werner S. Regulatory T cells are required for normal and activin-promoted wound repair in mice. Eur J Immunol 2018;48:1001–1013. [DOI] [PubMed] [Google Scholar]
- 43. Kalekar LA, Cohen JN, Prevel N, et al. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Sci Immunol 2019;4:eaaw2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Jin Q, Fu X, Qiao J, Niu F. Connection between T regulatory cell enrichment and collagen deposition in keloid. Exp Cell Res 2019;383:111549. [DOI] [PubMed] [Google Scholar]
- 45. Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The Biology of T Regulatory Type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 2018;49:1004–1019. [DOI] [PubMed] [Google Scholar]
- 46. Huang W, Solouki S, Carter C, Zheng SG, August A. Beyond Type 1 Regulatory T Cells: co-expression of LAG3 and CD49b in IL-10-Producing T Cell Lineages. Front Immunol 2018;9:2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol 2000;165:4848–4853. [DOI] [PubMed] [Google Scholar]
- 48. Lim JY, Im KI, Lee ES, et al. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep 2016;6:26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson MS, Madala SK, Ramalingam TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 2010;207:535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol 2008;35:515–519. [PubMed] [Google Scholar]
- 51. Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 1996;183:2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kurasawa K, Hirose K, Sano H, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum 2000;43:2455–2463. [DOI] [PubMed] [Google Scholar]
- 53. Nakashima T, Jinnin M, Yamane K, et al. Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J Immunol 2012;188:3573–3583. [DOI] [PubMed] [Google Scholar]
- 54. Brembilla NC, Montanari E, Truchetet ME, Raschi E, Meroni P, Chizzolini C. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res Ther 2013;15:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Truchetet ME, Brembilla NC, Montanari E, et al. Interleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis Rheum 2013;65:1347–1356. [DOI] [PubMed] [Google Scholar]
- 56. Xing X, Li A, Tan H, Zhou Y. IFN-gamma(+) IL-17(+) Th17 cells regulate fibrosis through secreting IL-21 in systemic scleroderma. J Cell Mol Med 2020;24:13600–13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174:3695–3702. [DOI] [PubMed] [Google Scholar]
- 58. McGee HM, Schmidt BA, Booth CJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol 2013;133:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Munoz LD, Sweeney MJ, Jameson JM. Skin Resident gammadelta T cell function and regulation in wound repair. Int J Mol Sci 2020;21:9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Z, Xu Y, Zhang X, et al. Defects in dermal Vgamma4 gamma delta T cells result in delayed wound healing in diabetic mice. Am J Transl Res 2016;8:2667–2680. [PMC free article] [PubMed] [Google Scholar]
- 61. Ohtsuka T. Effect of gammadelta T cell supernatant on human skin fibroblast proliferation and collagen production—possible role of transforming growth factor-beta and basic fibroblast growth factor. Int J Dermatol 2008;47:1135–1140. [DOI] [PubMed] [Google Scholar]
- 62. Lee P, Gund R, Dutta A, et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of gammadeltaT-cells. Elife 2017;6:e28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gay D, Kwon O, Zhang Z, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med 2013;19:916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 1979;381:353–361. [DOI] [PubMed] [Google Scholar]
- 65. Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg 1990;25:63–68; discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 66. Adzick NS, Harrison MR, Glick PL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg 1985;20:315–319. [DOI] [PubMed] [Google Scholar]
- 67. Gordon A, Kozin ED, Keswani SG, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen 2008;16:70–79. [DOI] [PubMed] [Google Scholar]
- 68. Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg 2000;35:866–872; discussion 872–873. [DOI] [PubMed] [Google Scholar]
- 69. Dhariwala MO, Karthikeyan D, Vasquez KS, et al. Developing human skin contains lymphocytes demonstrating a memory signature. Cell Rep Med 2020;1:100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Walraven M, Talhout W, Beelen RH, van Egmond M, Ulrich MM. Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen 2016;24:533–541. [DOI] [PubMed] [Google Scholar]
- 71. Moore AL, Marshall CD, Barnes LA, Murphy MP, Ransom RC, Longaker MT. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip Rev Dev Biol 2018;7:10..1002/wdev.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet 1994;344:983–985. [DOI] [PubMed] [Google Scholar]
- 73. Schuster C, Vaculik C, Prior M, et al. Phenotypic characterization of leukocytes in prenatal human dermis. J Invest Dermatol 2012;132:2581–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle) 2019;8:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raghu G, Richeldi L, Crestani B, et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772). Eur Respir J 2018;52:1801130. [DOI] [PubMed] [Google Scholar]
- 76. Sirbulescu RF, Boehm CK, Soon E, et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen 2017;25:774–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mizoguchi T, Ueno K, Takeuchi Y, et al. Treatment of Cutaneous Ulcers with Multilayered Mixed Sheets of Autologous Fibroblasts and Peripheral Blood Mononuclear Cells. Cell Physiol Biochem 2018;47:201–211. [DOI] [PubMed] [Google Scholar]
- 78. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986;136:2348–2357. [PubMed] [Google Scholar]
- 79. Assenmacher M, Lohning M, Scheffold A, et al. Commitment of individual Th1-like lymphocytes to expression of IFN-gamma versus IL-4 and IL-10: selective induction of IL-10 by sequential stimulation of naive Th cells with IL-12 and IL-4. J Immunol 1998;161:2825–2832. [PubMed] [Google Scholar]
- 80. Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol 2014;841:15–44. [DOI] [PubMed] [Google Scholar]
- 81. Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204:1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008;159:1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–1061. [PubMed] [Google Scholar]
- 84. Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 2007;110:2983–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013;19:739–746. [DOI] [PubMed] [Google Scholar]