Abstract
Significance: Skin scarring is a permanent, irreversible end point of cutaneous injury. However, not everyone will acquire the same exact scar type. Skin scarring is generally recognized as complex with significant variability in individuals' scar type and response to treatment. Despite these tangible differences in treatment response, to date there has been no simplified approach in defining spectrum of skin scarring in relation to prediction and outcome post-treatment. Thus, in this study we propose that skin scarring consists of distinct endotypes, which is characterized by their specific pathology. Four distinct scar endotypes can be observed: (1) Stretched (flat), (2) Contracted, (3) Atrophic (depressed), and (4) Raised scarring, which can be abbreviated to S.C.A.R. endotypes. Each of these endotypes can certainly include subphenotypes and each phenotype can be present in more than one endotype. To define these endotypes, we also present a structured approach in assessment of all relevant parameters in skin scar evaluation including clinical (scar symptoms and signs) and nonclinical parameters (device measurements of structural, mechanical, and physiological properties of scars as well as gene and protein laboratory studies).
Recent Advances: Scars can be phenotypically characterized based on a multitude of parameters assessed; however, not all scar types will share all the same characteristics. This leads to the question of whether skin scarring is a single disease entity with varying phenotypic characteristics or should be classed as several disease entities that have certain similar parameters. We suggest the latter and propose distinct scarring phenotypes arise mainly owing to genetic and environmental susceptibilities associated with the development of each specific scar endotype. Characteristic features of skin scarring, however, can be objectively and quantitively evaluated and used as an aid in the theranostic goal-directed management of scarring.
Critical Issues: The concept of identifying different endotypes is key in formulating personalized treatments with improved outcomes beyond what is achieved with current nonspecific approaches in scar management. This approach has gained interest and significant traction in several other medical conditions including asthma, rheumatoid arthritis, and atopic dermatitis.
Future Directions: To begin identifying distinct endotypic features in skin scarring, it is important to have a better understanding of underlying pathological mechanisms leading to further insight into the heterogeneous nature of skin scarring endotypes. This approach may lead to improved theranostic outcomes and further understanding of the pathophysiology of the complex nature of human skin scarring.
Keywords: dermal fibrosis, raised dermal scarring, atrophic scarring, contracted scarring, skin scar endotypes, stretched (flat) scarring

Ardeshir Bayat, MBBS, PhD
Scope and Significance
Skin scarring is a permanent, irreversible end point of cutaneous injury and this occurs in ∼100 million people in the developed world alone per year.1,2 However, not everyone will acquire the same exact scar type. Skin scarring is generally recognized as complex with significant variability in individuals' scar type and response to treatment.
Despite these tangible differences in treatment response, to date there has been no simplified approach in defining spectrum of skin scarring in relation to prediction and outcome post-treatment. The concept of identifying different endotypes has gained great interest in a number of other medical conditions and this has led to the idea that we can begin to identify distinct endotypes in skin scarring.3–11 Thus, we propose that skin scarring consists of distinct endotypes that is characterized by their specific pathology.
Translational Relevance
Scars can be phenotypically characterized based on a multitude of parameters; however, not all scar types will share all the same characteristics. This leads to the question of whether skin scarring is a single disease entity with varying phenotypic characteristics or should be classed as several disease entities that have certain similar parameters. We suggest the latter and propose distinct scarring phenotypes arise mainly because of genetic and environmental susceptibilities associated with the development of each specific scar endotype.
Characteristic features of skin scarring, however, can be objectively and quantitively evaluated and used as an aid in the theranostic goal-directed management of scarring.12,13 Endotype definition would enable identification of novel therapeutic targets and biomarkers, standardize improved scar management approaches, and predict response to therapies more effectively.
Clinical Relevance
Effective therapies are lacking to manage skin scarring and thus, treating them effectively has remained a challenge. Defining skin scarring endotypes would allow clinicians to treat these scars using the same standardized process and from a disease entity where they base their treatment options on the baseline parameters identified. In this study, we identify four distinct scar endotypes: (1) Stretched (flat), (2) Contracted, (3) Atrophic (depressed), and (4) Raised scarring, which can be abbreviated to S.C.A.R. endotypes. Each of these endotypes can certainly include subphenotypes, which are a subset of a phenotype that is characteristic of a population, and each phenotype can be present in more than one endotype.
The term phenotype is defined as the observable physical properties and in skin scarring we have described a number of parameters categorized by anatomical, mechanical, and physiological scar characteristics, whereas an endotype can be defined as a distinct functional or pathophysiological mechanism underlying the disease's visible features/phenotype. To define these endotypes, we also present a structured approach in assessment of all relevant parameters in skin scar evaluation including, clinical (scar symptoms and signs) and nonclinical parameters (device measurements of structural, mechanical, and physiological properties of scars as well as gene and protein laboratory studies).
Background
Skin scarring is a permanent, irreversible end point of cutaneous wound healing and this occurs in ∼100 million people in the developed world alone per year.1 Scar tissue can be more rigid than normal skin, can be weaker and inhibit normal skin function that can lead to problems including restriction of movement and thermoregulation, and so on. Furthermore, visible scars can be esthetically displeasing and lead to psychological distress.1,14 However, not everyone will acquire the exact same scar type.
Endogenous factors including mechanical stretching can lead to the development of raised skin scarring demonstrating unique phenotypic characteristics such as the butterfly-shaped keloid scars postmedian sternotomy.15 In certain individuals and anatomic sites, excessive fibrosis may lead to hypertrophic scarring or keloid disease formation.14,15 Skin scarring is generally recognized as complex with significant variability in individuals' scar type and response to treatment.
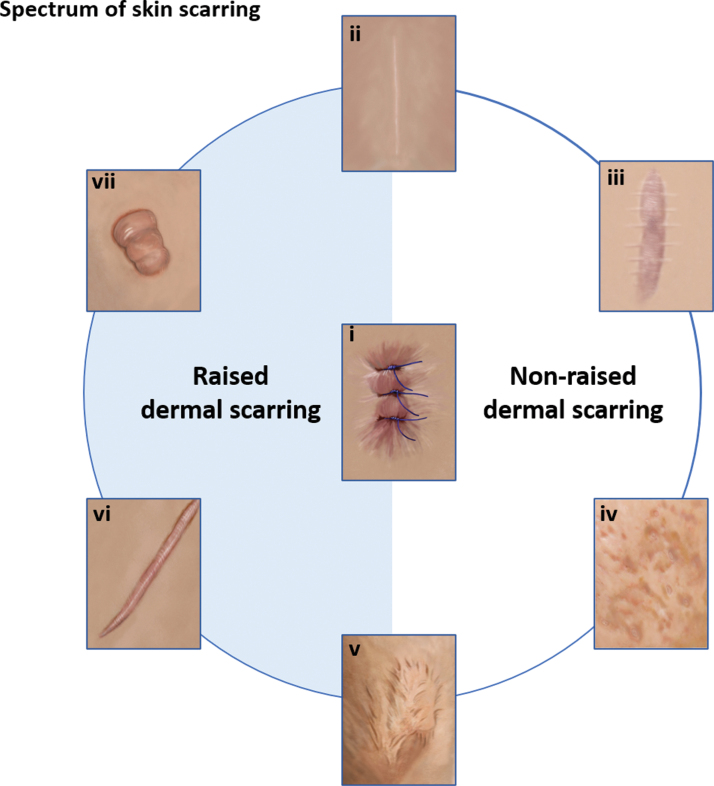
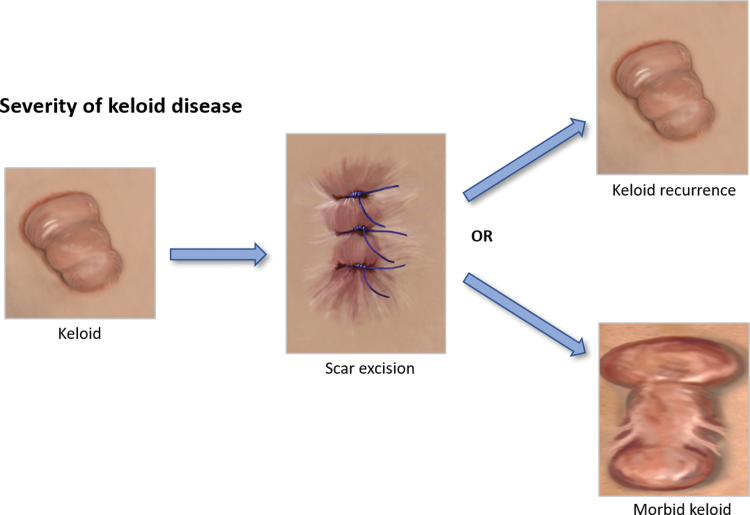
There is a broad spectrum of scar types ranging from normal flat fine line, which is generally considered to be the optimal scar, as opposed to stretched or widespread such as stretch marks, atrophic such as acne scarring, contracted and raised scars including hypertrophic and keloid (Fig. 1).2 Keloids can also become morbid keloids, for example, when a keloid has been removed, it can recur and become much larger and spread beyond the confines of the original wound site (Fig. 2).2
Figure 1.
Spectrum of skin scarring. This diagram demonstrates the range of scar types that occur in human skin including either raised or nonraised dermal scarring tendencies from (i) wounding, a scar can be a (ii) fine-line normal scar, (iii) a stretched or widespread scar such as stretch marks, (iv) an atrophic scar such as acnes scarring, (v) a contracted scar following burns, or can lead to raised dermal scarring including (vi) hypertrophic scarring or (vii) keloid scarring. Color images are available online.
Figure 2.
Severity of keloid disease. This diagram highlights that a keloid scar can be excised and then can either recur over time or grow back to become a much larger, morbid keloid scar. Color images are available online.
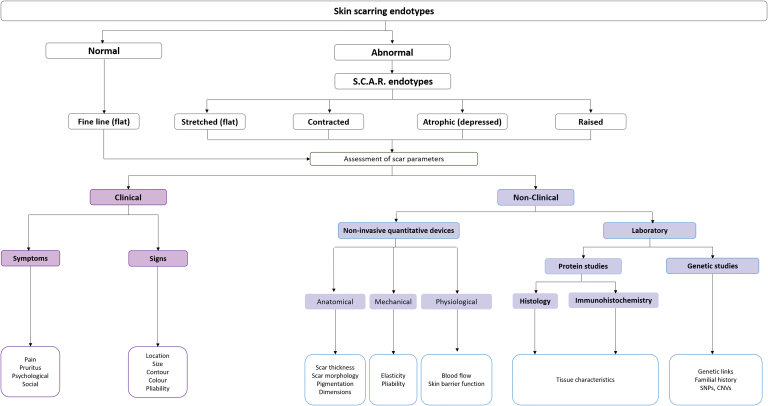
Scars can be phenotypically characterized based on a multitude of parameters, however, not all scar types, share all the same characteristics (Fig. 3). For example, a hypertrophic or keloid scar will display greater scar thickness compared with a flat, fine-line scar.3 An imbalanced or persistent inflammatory response is believed to contribute to scar formation and fibrosis; however, a fine-line scar may display greater inflammation or blood flow compared with normal skin but a keloid scar will have a much higher inflammatory response compared with the fine-line scar.7
Figure 3.
Proposed structured assessment. A flowchart to demonstrate a proposed structured assessment and the range of scar endotypes that they can be subdivided under normal and abnormal with abnormal including stretched, contracted, atrophic, and raised endotypes. Assessment of scar parameters including clinical and nonclinical methods. Clinical including assessment of scar signs and symptoms. Nonclinical including the use of noninvasive quantitative devices evaluating the anatomical, mechanical, and physiological parameters that can be measured for all scar types in humans. Examples of anatomical parameters (scar thickness, morphology, pigmentation, and dimensions) are presented, mechanical parameters (pliability and elasticity), and physiological parameters (skin barrier function, blood flow, and inflammation). All parameters that are measured are then validated by laboratory methods including gene and protein studies. CNVs, copy number variations; SNPs, single nucleotide polymorphisms. Color images are available online.
This likely leads to the question of whether skin scarring is a single disease entity with varying phenotypic characteristics or should be classed as several disease entities, which have certain similar parameters. We suggest the latter and propose distinct scarring phenotypes arise mainly owing to genetic and environmental susceptibilities associated with the development of each specific scar endotype.
The term phenotype is defined as the observable physical properties and in skin scarring we have described a number of parameters categorized by anatomical, mechanical, and physiological characteristics and the response to different treatments. To date, the phenotypic characterization of skin scarring has predominantly relied on visual inspection and rating using established analog scales.12,13 One of the most commonly used scales is the Vancouver Scar Scale (VSS), which is used to assess the scar with four specific parameters including pigmentation, pliability, vascularity, and height (i.e., thickness). However, VSS has been considered subjective and with poor sensitivity and inter-rater reliability, and the scores are not sensitive to minor changes of colors.12
Therefore, there has been a need to develop standardized, objective, and quantitative methods to accurately evaluate healing and scaring, which could become a standard tool in the evaluation of surgical outcomes. Our group has used a number of objective quantitative tools to objectively define these criteria and outcomes by measuring the change in the scar parameters from baseline (e.g., blood flow and pigmentation) throughout treatment and this can direct the response to treatment while also objectively defining normal parameters of skin scarring. These phenotypes tend to provide clinically relevant information with regard to the presentation of the scarring and treatment response.
The concept of identifying different endotypes is key in formulating personalized treatments with improved outcomes beyond what is achieved with current nonspecific approaches in scar management. This approach has gained interest and significant traction in several other medical conditions including asthma, rheumatoid arthritis (RA), and atopic dermatitis.3–11 Endotype can be defined as a distinct functional or pathophysiological mechanism underlying the disease's visible features/phenotype.16 Therefore, together with genetic and/or environmental influences, the endotype can explain the clinical presentation, epidemiology, and response to different treatments.
Despite these tangible differences in treatment response in skin scarring, to date there has been no simplified approach in defining spectrum of skin scarring in relation to prediction and outcome post-treatment. Thus, we propose that skin scarring consists of distinct endotypes, which is characterized by their specific pathology. To define these endotypes, we also present a structured approach in assessment of all relevant parameters in skin scar evaluation.
Discussion
Defining scar endotypes
The concept of identifying different endotypes has gained great interest in a number of other medical conditions including asthma, RA, and atopic dermatitis.3–11 Asthma is now considered an umbrella diagnosis for several diseases with distinct mechanistic pathways (endotypes) and variable clinical presentations (phenotypes).3–5 The definition of these endotypes is central to asthma management owing to inherent therapeutic and prognostic implications.5
This is also true for RA, which is thought to comprise multiple clinical and molecular endotypes,9 being defined as subtypes of a disease with distinctive underlying pathological mechanisms.10,11 An RA patient's clinical presentation, response to treatment, and rates of disease progression may be determined by their endotype.9 Therefore, well-characterized endotypes may provide patients and physicians with a means for targeted treatment against a patient's individual molecular disease pathogenesis. Different endotypes of atopic dermatitis have also been proposed based on age, disease chronicity, ethnicity, filaggrin and IgE status, and underlying molecular mechanisms.6–8
The nature of injury, depth, location, tensional stress, infection, and severity can determine the level of skin scarring observed. Mechanical tension has been known to promote phenotypic alteration in fibroblasts throughout wound healing and it has been suggested that mechanical forces can influence the development of keloid scars in certain anatomical sites, which are under high tension.15 In addition, the age of the scar can have an effect on the treatment response. For example, immature scars, which are those that are still in the process of maturing and remodeling, can sometimes develop into hypertrophic scars if symptoms persist after 1 month. Therefore, a preventative strategy is recommended such as laser therapy.1,14
Current scar management advocates the prevention of scarring as it is better to prevent formation than to rely on management once a scar has formed. Examples of prevention techniques are the use of certain surgical techniques such as closing skin edges with minimal tension.15 Furthermore, identifying patients at high risk of developing scars is essential. First-line treatments are silicon-based products, which are applied after epithelialization. Intralesional corticosteroid injections are considered second-line approaches, whereas laser therapies are third line.14 There are a number of different therapies that have been suggested for the management of different types of skin scarring; however, the heterogeneity of skin scarring advocates against the therapeutic approaches still currently used, which can often be one standard approach.
More importantly, genetic predisposition has been demonstrated to affect the level and quality of scar tissue formation in the wound. We propose that distinct scarring phenotypes arise mainly because of genetic and environmental susceptibilities associated with the development of each specific scar endotype. Each of these endotypes can certainly include subphenotypes and each phenotype can be present in more than one endotype.
To define these endotypes, we also present a structured approach in assessment of all relevant parameters in skin scar evaluation including, clinical (scar symptoms and signs) and nonclinical parameters (device measurements of structural, mechanical, and physiological properties of scars as well as gene and protein laboratory studies). There have been a number of clinical human studies that have identified skin scar phenotypes, which include their visible features and behavior of the scars.14,15,17–19 Characteristic features of skin scarring can be objectively and quantitatively evaluated and used as an aid in the theranostic goal-directed management of scarring.
We have applied a number of noninvasive objective assessment modalities to monitor the progression of healing based on a number of specific parameters.20–24 These measurements can be applied to any of the scar endotypes. The scar phenotypes outlined above are predominantly based on visual assessment including, scar color, thickness, pliability, and volume. Our group work has been focused on objectively defining a number of these criteria and outcomes.25–33 We have used various technologies, which provide an objective assessment including the evaluation of the structural and physical parameters of a wound or scar.
It is useful to consider the anatomical, mechanical, and physiological characteristics of wound healing.20 Anatomical parameters include skin thickness, pigmentation, wound dimensions, texture, and tissue morphology; mechanical parameters include elasticity and pliability of the skin; and physiological characteristics include blood flow, skin barrier function, and sebaceous glands. These devices enabled the objective and reproducible evaluation of these parameters before, during, and after the treatment.20
The color of the skin is an important component that is largely determined by the distribution of blood vessels and pigmentation.34 We used device analysis of skin color using spectrophotometric intracutaneous analysis (SIAscopy) and a color probe in a number of studies.27–33 This enabled us to generate baseline values of scar pigmentation. In addition, the devices that measure the blood flow in the scar microcirculation were used such as full-field laser perfusion imaging and dynamic optical coherence tomography producing images and quantitative data.25,26 These devices were utilized in our randomized human clinical trials and provided essential information over the course of healing, and all devices and parameters measured can be used to assess any of the scar endotypes identified.25,26
Objective noninvasive measuring devices, such as the ones discussed in this study, are essential in both clinical practice and research, especially when monitoring the response to different therapies. The use of these technologies could be useful in future as a standard for investigation and could aid as a marker for healing. Challenges regarding these tools are that there is no single device that can measure all the scar parameters simultaneously; therefore, it is vital to choose the correct device. Furthermore, some instruments have not yet been evaluated in all wound or scar types; therefore, it would be useful to investigate this further in all devices.
A number of other parameters were not included for identification of scar endotypes. For example, comorbidities were considered unsuitable because of the fact they can interact with different pathophysiological processes and could alter the phenotype but not the endotype. Patient compliance was also not used as this can influence the way the scar heals owing to smoking, adherence to treatments, or the severity of the scarring and can prove difficult to link with a specific endotype.
Description of scar endotypes
Four distinct scar endotypes can be observed based on their genetic and environmental influences: (1) Stretched (flat), (2) Contracted, (3) Atrophic (depressed), and (4) Raised scarring, which can be abbreviated to S.C.A.R. endotypes. Here, we suggest that the identification of these endotypes may lead to improved therapeutic outcomes and further understanding of the pathophysiology of the complex nature of human skin scarring.
Stretched (flat) scar endotype
The stretched (flat) scar endotype is considered closest to the normal fine-line scar type and often the least symptomatic endotype. When the skin heals, a scar will flatten and change color and in time, many flat scars become similar in color and pigmentation to that of the surrounding skin, although some may remain slightly paler or darker.1 Normal fine-line scars tend to be paler in color, remain flush with the skin surface, and are symptomless, whereas stretched scars are also flat, pale, pliable, and symptomless, but by definition are widespread and can be cosmetically regarded as unacceptable.1,29 With regard to response to treatment, flat scar endotypes tend to respond well to surgical treatment if required; exception are those not in parallel to Langer's lines.35
Environmental factors influencing the formation of stretched scars include tension or stretch to the wound site or improper (loose) suturing and in situ foreign body while the wound is healing.15 The genetic aspect of acquiring a flat or stretched scar endotype is unknown but there seems to be no genetic susceptibility. Although they are not the same as a stretched scar, striae distensae are an example of a stretched flat nontraumatic scar. They tend to lose their tensile strength rather than being a new scar that stretches. Nevertheless, striae have been shown to occur in people with dark skin, more specifically, resulting in striae nigrae and striae caerulea, owing to increased melanization.17 Despite striae being very common in Caucasian people, striae severity has been noted to be more severe in darker skinned women compared with Caucasian people within the same geographic region.36
Contracted scar endotype
Contracture scars are most common after burn injuries and can be difficult to resolve with high incidence of recurrence after surgical treatment.19 Contracted scars tend to disrupt function and movement if located adjacent to a joint surface and develop a shortening in the skin and they can become hypertrophic and dysfunctional.19,37
These scars can be exacerbated or influenced by environmental factors such as infection, slow wound closure, depth of wound site, or size of wound.38 Contracted scars can be included in the raised scar endotype category; however, this scar type requires a different type of management. Many first-line treatments, including silicon gel therapy, do not tend to be effective in improving this endotype, with the most common therapy advocated as surgical release.37 There may be a genetic influence to the formation of this abnormal type of scarring including sex and age but no specific gene or group of genes has yet been identified to cause contractile scarring.39
Atrophic (depressed) scar endotype
Atrophic or depressed scar endotypes can be described as asymptomatic, depressed below the surface of the surrounding skin, and are classified according to depth and size.18 They tend to be pliable and soft and histopathologically show a destruction of collagen in the dermis. Atrophy and fibrous tissue formation following improvement of acne is the etiology of depression in acne scars.40 Their genetic susceptibility is unknown but they respond well to treatments, although reports of changes in skin texture and color have been reported specifically with acne treatments including chemical peels, surgical subcision, and laser therapies.
Raised scar endotype
The raised scar endotype are defined as scars that are raised above the surface of the surrounding skin and include hypertrophic and keloid scars. This endotype is often considered on the most severe end of the spectrum of skin scarring. The histologic composition of these scars can vary depending on the age, sex, and race of the individual, as well as the duration, location, and mechanism of injury.41 For example, keloid scars can result from a variety of causes involving deep dermal injury, with the most common being ear piercings, postelective surgery, acne, trauma, or burns.41 Environmental factors can influence the raised scar endotype. Furthermore, increasing tension placed upon the lesion in anatomically high-risk sites and orientations, and the presence of infection and foreign bodies, may augment the risk of keloid disease formation.42–44 The raised scar endotype can be further subdivided into hypertrophic and keloid scarring endotypes.
Hypertrophic scar endotype
Hypertrophic scars remain within the boundaries of the original wound, are raised, hyperpigmented, regress spontaneously, and can be pruritic and painful.14 These scars tend to arise in locations such as joints.1 Histologically they show prolonged inflammation, increased collagen, and show a swirling nodular pattern of collagen fibers. They also display excessive extracellular matrix accumulation.2 Genetically, there has been tendency to show more prevalence in darker skinned individuals but there has been no strong link identified.45 With regard to treatment response, they do not always respond well but they tend to regress and flatten over time. Treatments for hypertrophic scar endotype can include silicon sheeting, surgical excision, pressure therapy, steroids, or laser therapy,14,45 which can be similar to keloid scar endotype treatment but they tend to respond better to these therapies.
Keloid scar endotype
Keloid scars that are also raised, painful, pruritic, and hyperpigmented but spread beyond the margins of the original injury site, invade the surrounding tissue, do not regress spontaneously, continue to grow over time, and can recur following treatment.15 Keloid scars tend to arise in locations that have high tension such as the sternum and display reduced pliability, feel more rigid, firm, and have less elasticity compared with surrounding skin.14,46 Histologically, they display a swirling nodular pattern of collagen fibers with increased collagen and fibronectin.2 They show aggressive, invasive, expansile behavior with prolonged inflammation and excessive extracellular matrix accumulation.14,47
Genetic factors that influence the keloid scar endotype include the influence of ethnicity and age. Keloid scars are considered to be an important clinical problem in certain ethnic populations, as there is a higher prevalence in individuals with pigmented skin.42,43 Keloids, in particular, are reported to be especially high in individuals of African, Asian, and Hispanic descent.42 With regard to treatment, certain laser therapies are not advocated in darker skin types.43 Bayat et al. studied the keloid in Afro-Caribbean patients and found that young female patients and those with a positive family history of keloid were associated with the development of keloid in multiple anatomic sites.44 Therefore, this is relevant in predicting keloid response to treatment and prognosis and in identifying those at higher risk of disease recurrence. In addition, keloids can develop at any age but tend to be most prevalent in individuals between 10 and 30 years of age.44 Thus, it is crucial to define this endotype to tailor adequate treatments. Treatments such as electrical stimulation and photodynamic therapy have shown promise in the treatment of keloid scar endotypes, whereas these treatments are not used for other endotypes including atrophic or contracted.28,46
Proposed structured assessment of scar endotypes
Our research has focused on objectively defining scar criteria and outcomes. We have been able to measure the baseline values for a number of specific parameters including blood flow, pigmentation, collagen, elastin/pliability, and scar thickness. These measurements have also been used throughout trials for monitoring response to treatment, which enabled us to measure response at the same time as objectively defining normal parameters of healing. We have conducted a number of randomized placebo-controlled trials assessing the efficacy of a topical formulation in the treatment of flat skin scars.25,30 These studies have enabled the use of a range of noninvasive objective modalities to guide laboratory work to assess if these topicals had a positive effect on baseline scar characteristics over time.
Based on our research and clinical experience we offer a proposed structured assessment of all relevant parameters in skin scar evaluation (Fig. 4). Assessment should include a detailed scar history, full medical history, a psychosocial assessment, and any family history of previous scarring (determine family scar endotypes). In addition, a through clinical assessment of the scars themselves should be performed, including signs and symptoms including; location, size, contour, color, pliability, pain, and pruritus (always useful to make a record of vaccination scar type and body piercing/tattooing outcome). Subsequently, additional assessments if possible should include the use of noninvasive quantitative device measurements including that can objectively measure anatomical, mechanical, and physiological parameters of skin scarring. Following these baseline guidance measurements, laboratory assessments can be useful when indicated, particularly relevant in keloid scar endotypes. These laboratory measurements include gene (PCR, sequencing, genetic links, and familial studies) and protein (histology and immunohistochemical analysis) studies.
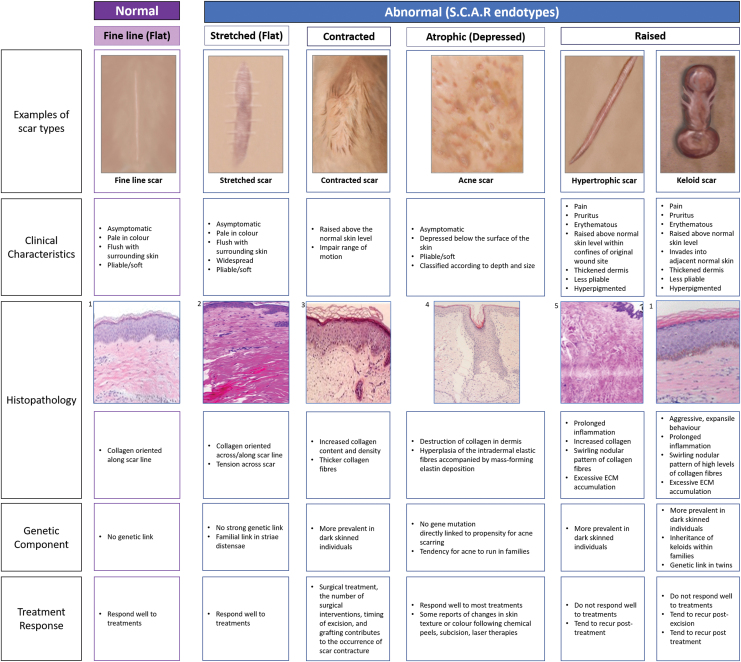
Figure 4.
Scar endotype characteristics. A diagram outlining examples of each scar endotype including the clinical characteristics of each, histopathology, genetic components, and treatment response. Histological image references: (1) Tan et al.,48 (2) Ud-Din et al.,49 (3) Lee et al.,50 (4) Kaminaka et al.,51 and (5) Hu et al.52 Color images are available online.
Summary
In this article, we have proposed criteria for defining scar endotypes and from this we suggest four distinct scar endotypes (Stretched (flat), Contracted, Atrophic (depressed), and Raised), each with specific clinical features and response to treatment. The heterogeneity of skin scarring advocates against the therapeutic approaches still currently used, which can often be one standard approach. Precision medicine approaches, which aim for targeted, tailored, endotype prevention and treatment, rely on detailed definitions of the scar variability across the different phenotypes.
The use of these scar endotypes in research could identify groups of patients who may benefit from specific treatments and lead to scar payment coding, as if someone is allocated to the incorrect treatment for their scar endotype, this would be a waste of time and resources. An attempt to define the patient's endotype before treatment should be made to optimize therapeutic responses based on the different clinical and molecular subsets. Understanding the different endotypes would require a number of approaches including further immunophenotyping and genomics. Studies should include a large number of subjects with similar phenotypic characteristics for each endotype. It will be important to ensure that mechanism identified is unique for one specific endotype and not for all endotypes. Further clinical studies are necessary to test the robustness of these endotype definitions.
Take-Home Messages
Skin scarring should be recognized as not a simple entity but complex with significant variability and heterogeneity in individuals' scar type and individualized response to treatment. Despite these tangible differences in treatment response, to date there has been no clear structured approach in defining the spectrum of skin scarring in relation to clinical behavior, and prediction of outcome post-treatment.
Distinct scarring phenotypes arise mainly owing to genetic and environmental susceptibilities associated with the development of each specific scar endotype. Characteristic features of skin scarring, however, can be objectively and quantitatively evaluated and used as an aid in the theranostic goal-directed management of skin scarring.
We propose that skin scarring consists of distinct endotypes, which is characterized by their specific etiopathologenesis, clinical behavior, and response to therapy. Four distinct scar endotypes can be observed: (1) Stretched (flat), (2) Contracted, (3) Atrophic (depressed), and (4) Raised dermal scarring, which can be abbreviated to S.C.A.R. endotypes.
Each endotype can include subphenotypes and each phenotype can be present in more than one endotype. For instance, raised scarring can be subdivided into hypertrophic and keloid. A structured approach in assessment of all relevant parameters in skin scar evaluation has been presented including: clinical (scar symptoms, and signs) and nonclinical parameters (device measurements of structural, mechanical and physiological properties of scars as well as gene and protein laboratory studies).
Endotype definition may enable identification of novel therapeutic targets and biomarkers, standardize improved scar management approaches, and predict response to therapies more effectively.
The development of more standardized, objective, and quantitative methods to accurately evaluate healing and scarring phenotypes are becoming more established and shown to aid in the evaluation of surgical outcomes validated by gene and protein studies.
Precision medicine approaches, which target tailored, endotype prevention and treatment, rely on detailed definitions of the skin scar endotype heterogeneity across the different phenotypes. Thus, an attempt at the outset to define the patient's endotype before treatment should be made ideally to optimize therapeutic responses based on the above concept.
Abbreviations and Acronyms
- CNVs
copy number variations
- RA
rheumatoid arthritis
- SNPs
single nucleotide polymorphisms
- VSS
Vancouver Scar Scale
Acknowledgments And Funding Sources
We would like to acknowledge the National Institute for Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC) for their support/funding. AB acknowledges the ongoing support for wound healing research, Wound Healing Unit, MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, South Africa. No funding was received for this work.
Author Disclosure and Ghost-Writing
The authors declare that they have no competing financial interests to disclose. The content of this article was expressly written by the authors listed and no ghost-writers were used.
About the Authors
Sara Ud-Din, MSc is a PhD candidate and a researcher in the Plastic and Reconstructive Surgery Research Group at the University of Manchester. Ardeshir Bayat, MBBS, PhD, is a clinician scientist and Reader at the University of Manchester, United Kingdom and Professor at the Division of Dermatology, University of Cape Town and he is corresponding author for this article.
References
- 1. Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ 2003;326:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 2012;26:141–152. [DOI] [PubMed] [Google Scholar]
- 3. Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011;127:355–360. [DOI] [PubMed] [Google Scholar]
- 4. Lefaudeux D, De Meulder B, Loza MJ, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol 2017;139:1797–1807. [DOI] [PubMed] [Google Scholar]
- 5. Opina MT, Moore WC. Phenotype-driven therapeutics in severe asthma. Curr Allergy Asthma Rep 2017;17:10. [DOI] [PubMed] [Google Scholar]
- 6. Thijs JL, Strickland I, Bruijnzeel-Koomen CAFM, et al. Moving toward endotypes in atopic dermatitis: identification of patient clusters based on serum biomarker analysis [published correction appears in J Allergy Clin Immunol 2018;142:714]. J Allergy Clin Immunol 2017;140:730–737. [DOI] [PubMed] [Google Scholar]
- 7. Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016;387:40–52. [DOI] [PubMed] [Google Scholar]
- 8. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335–2348. [DOI] [PubMed] [Google Scholar]
- 9. Blair JPM, Bager C, Platt A, Karsdal M, Bay-Jensen AC. Identification of pathological RA endotypes using blood-based biomarkers reflecting tissue metabolism. A retrospective and explorative analysis of two phase III RA studies. PLoS One 2019;14:e0219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bay-Jensen AC, Platt A, Byrjalsen I, Vergnoud P, Christiansen C, Karsdal MA. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin Arthritis Rheum 2014;43:470–478. [DOI] [PubMed] [Google Scholar]
- 11. Weinblatt ME, Genovese MC, Ho M, et al. Effects of fostamatinib, an oral spleen tyrosine kinase inhibitor, in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol 2014;66:3255–3264. [DOI] [PubMed] [Google Scholar]
- 12. Idriss N, Maibach HI. Scar assessment scales: a dermatologic overview. Skin Res Technol 2009;15:1–5. [DOI] [PubMed] [Google Scholar]
- 13. Beausang E, Floyd H, Dunn KW et al. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg 1998;102:1954–1961. [DOI] [PubMed] [Google Scholar]
- 14. Jumper N, Hodgkinson T, Paus R, Bayat A. Site-specific gene expression profiling as a novel strategy for unravelling keloid disease pathobiology. PLoS One 2017;12:e0172955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suarez E, Syed F, Rasgado TA, Walmsley A, Mandal P, Bayat A. Skin equivalent tensional force alters keloid fibroblast behavior and phenotype. Wound Repair Regen 2014;22:557–568. [DOI] [PubMed] [Google Scholar]
- 16. Agache I, Akdis CA. Endotypes of allergic diseases and asthma: an important step in building blocks for the future of precision medicine. Allergol Int 2016;65:243–252. [DOI] [PubMed] [Google Scholar]
- 17. Hexsel D, Soirefmann M, Porto MD, Schilling-Souza J, Siega C, Dal'Forno T. Superficial dermabrasion versus topical tretinoin on early striae distensae: a randomized, pilot study. Dermatol Surg 2014;40:537–544. [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Deng Y. Ablative fractional CO2 laser for facial atrophic acne scars. Facial Plast Surg 2018;34:205–219. [DOI] [PubMed] [Google Scholar]
- 19. Lee JW, Park SH, Lee SJ, Kim SH, Suh IS, Jeong HS. Clinical impact of highly condensed stromal vascular fraction injection in surgical management of depressed and contracted scars. Aesthetic Plast Surg 2018;42:1689–1698. [DOI] [PubMed] [Google Scholar]
- 20. Ud-Din S, Bayat A. Non-invasive objective devices for monitoring the inflammatory, proliferative and remodelling phases of cutaneous wound healing and skin scarring. Exp Dermatol 2016;25:579–585. [DOI] [PubMed] [Google Scholar]
- 21. Cobb MJ, Chen Y, Underwood RA, Usui ML, Olerud J, Li X. Noninvasive assessment of cutaneous wound healing using ultrahigh-resolution optical coherence tomography. J Biomed Opt 2006;11:064002. [DOI] [PubMed] [Google Scholar]
- 22. Dyson M, Moodley S, Verjee L, Verling W, Weinman J, Wilson P. Wound healing assessment using 20MHz ultrasound and photography. Skin Res Technol 2003;9:116–121. [DOI] [PubMed] [Google Scholar]
- 23. Vardaxis N, Brans T, Boon M, Kreis RW, Marres LM., Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J Anat 1997;190:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horzic M, Bunoza D, Maric K. Contact thermography in a study of primary healing of surgical wounds. Ostomy Wound Manage 1996;42:36–38, 40–42, 44. [PubMed] [Google Scholar]
- 25. Ud-Din S, Foden P, Mazhari M, et al. A double-blind, randomized trial shows the role of zonal priming and direct topical application of epigallocatechin-3-gallate in the modulation of cutaneous scarring in human skin. J Invest Dermatol 2019;139:1680–1690.e16. [DOI] [PubMed] [Google Scholar]
- 26. Ud-Din S, Foden P, Stocking K, et al. Objective assessment of dermal fibrosis in cutaneous scarring, using optical coherence tomography, high-frequency ultrasound and immunohistomorphometry of human skin. Br J Dermatol 2019;181:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ud-Din S, Greaves NS, Sebastian A, Baguneid M, Bayat A. Noninvasive device readouts validated by immunohistochemical analysis enable objective quantitative assessment of acute wound healing in human skin. Wound Repair Regen 2015;23:901–914. [DOI] [PubMed] [Google Scholar]
- 28. Ud-Din S, Perry D, Giddings P, et al. Electrical stimulation increases blood flow and haemoglobin levels in acute cutaneous wounds without affecting wound closure time: evidenced by non-invasive assessment of temporal biopsy wounds in human volunteers. Exp Dermatol 2012;21:758–764. [DOI] [PubMed] [Google Scholar]
- 29. Ud-Din S, McAnelly SL, Bowring A, et al. A double-blind controlled clinical trial assessing the effect of topical gels on striae distensae (stretch marks): a non-invasive imaging, morphological and immunohistochemical study. Arch Dermatol Res 2013;305:603–617. [DOI] [PubMed] [Google Scholar]
- 30. Basson R, Baguneid M, Foden P, Al Kredly R, Bayat A. Functional testing of a skin topical formulation in vivo: objective and quantitative evaluation in human skin scarring using a double-blind volunteer study with sequential punch biopsies. Adv Wound Care (New Rochelle) 2019;8:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greaves NS, Lqbal SA, Morris J, et al. Acute cutaneous wounds treated with human decellularised dermis show enhanced angiogenesis during healing [published correction appears in PLoS One 2015;10:e0121503. Lqbal, Syed A [added]]. PLoS One 2015;10:e0113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greaves NS, Iqbal SA, Hodgkinson T, et al. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen 2015;23:483–494. [DOI] [PubMed] [Google Scholar]
- 33. Ashrafi M, Xu Y, Muhamadali H, et al. A microbiome and metabolomic signature of phases of cutaneous healing identified by profiling sequential acute wounds of human skin: an exploratory study. PLoS One 2020;15:e0229545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith BJ, Nidey N, Miller SF, et al. Digital imaging analysis to assess scar phenotype. Wound Repair Regen 2014;22:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lemperle G, Knapp D, Tenenhaus M. Minimal scar formation after orthopaedic skin incisions along main folding lines. J Bone Joint Surg Am 2019;101:392–399. [DOI] [PubMed] [Google Scholar]
- 36. Cho S, Park ES, Lee DH, Li K, Chung JH. Clinical features and risk factors for striae distensae in Korean adolescents. J Eur Acad Dermatol Venereol 2006;20:1108–1113. [DOI] [PubMed] [Google Scholar]
- 37. Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg 2001;17:263–272. [DOI] [PubMed] [Google Scholar]
- 38. Tredget E, Nedelec B, Scott P, Ghahary A. Hypertrophic scars, keloids, contractures: the cellular and molecular basis for therapy. Surg Clin N Am 1997;77:701–729. [DOI] [PubMed] [Google Scholar]
- 39. Bayat A, Bock O, Mrowietz U, Ollier WE, Ferguson MW. Genetic susceptibility to keloid disease: transforming growth factor beta receptor gene polymorphisms are not associated with keloid disease. Exp Dermatol 2004;13:120–124. [DOI] [PubMed] [Google Scholar]
- 40. Keller R, Belda W, Valente NYS, Rodrigues CJ. Nonablative 1,064-nm Nd: YAG laser for treating atrophic facial acne scars: histologic and clinical analysis. Dermatol Surg 2007;33:1470–1476. [DOI] [PubMed] [Google Scholar]
- 41. Amadeu TP, Braune AS, Porto LC, Desmouliere A, et al. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen 2004;12:169–174. [DOI] [PubMed] [Google Scholar]
- 42. Shih B, Bayat A. Genetics of keloid scarring. Arch Dermatol Res 2010;302:319–339. [DOI] [PubMed] [Google Scholar]
- 43. Brown JJ, Ollier W, Arscott G, et al. Genetic susceptibility to keloid scarring: SMAD gene SNP frequencies in Afro-Caribbeans. Exp Dermatol 2008;17:610–613. [DOI] [PubMed] [Google Scholar]
- 44. Bayat A, Arscott G, Ollier WE, McGrouther DA, Ferguson MW. Keloid disease: clinical relevance of single versus multiple site scars. Br J Plast Surg 2005;58:28–37. [DOI] [PubMed] [Google Scholar]
- 45. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician 2009;80:253–260. [PubMed] [Google Scholar]
- 46. Ud-Din S, Thomas G, Morris J, Bayat A. Photodynamic therapy: an innovative approach to the treatment of keloid disease evaluated using subjective and objective non-invasive tools. Arch Dermatol Res 2013;305:205–214. [DOI] [PubMed] [Google Scholar]
- 47. Ud-Din S, Bayat A. Keloid scarring or disease: unresolved quasi-neoplastic tendencies in the human skin. Wound Repair Regen 2020;28:422–426. [DOI] [PubMed] [Google Scholar]
- 48. Tan KT, McGrouther DA, Day AJ, Milner CM, Bayat A. Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin. J Eur Acad Dermatol Venereol 2011;25:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ud-Din S, McGeorge D, Bayat A. Topical management of striae distensae (stretch marks): prevention and therapy of striae rubrae and albae. J Eur Acad Dermatol Venereol 2016;30:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee SJ, Suh DH, Lee JM, Song KY, Ryu HJ. Dermal remodeling of burn scar by fractional CO2 laser. Aesthetic Plast Surg 2016;40:761–768. [DOI] [PubMed] [Google Scholar]
- 51. Kaminaka C, Uede M, Nakamura Y, Furukawa F, Yamamoto Y. Histological studies of facial acne and atrophic acne scars treated with a bipolar fractional radiofrequency system. J Dermatol 2014;41:435–438. [DOI] [PubMed] [Google Scholar]
- 52. Hu ZC, Tang B, Guo D, et al. Expression of insulin-like growth factor-1 receptor in keloid and hypertrophic scar. Clin Exp Dermatol 2014;39:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]