Abstract
In the United States, Dermacentor variabilis and Dermacentor andersoni are considered key vectors for Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever. Through regional surveillance, a wide diversity of Rickettsia spp. have been documented in D. variabilis, and Dermacentor spp. has been suggested as potential vectors for various other pathogens, including Babesia spp. and Ehrlichia canis. To better define the prevalence and diversity of pathogens in Dermacentor spp. across the United States, 848 ticks collected from dogs and cats in 44/50 states in 2018–2019 were tested by PCR for Rickettsia spp.-specific 17 kDa and ompA gene fragments; a subset of Dermacentor spp. was also tested with PCR, targeting fragments of the 18S and large subunit region rRNA genes of Babesia spp. and 16S rRNA genes of E. canis. Rickettsia spp. was identified in 12.5% (106/848) of ticks. Species detected include Rickettsia montanensis (n = 64 ticks), Rickettsia bellii (n = 15 ticks), Rickettsia rhipicephali (n = 13 ticks), Rickettsia peacockii (n = 8 ticks), Rickettsia amblyommatis (n = 3 ticks), Rickettsia cooleyi (n = 1 tick), and unclassified Rickettsia spp. (n = 2 ticks). Ticks with R. montanensis and R. bellii were submitted from every U.S. region; R. rhipicephali was predominantly detected in ticks from the southern half of the United States, and all R. peacockii-positive ticks were D. andersoni that originated from the Rocky Mountain states. Ehrlichia canis was not detected in any Dermacentor spp., and Babesia conradae was detected in two Dermacentor albipictus. Because most ticks had fed on dogs or cats before submission, these findings do not implicate a given Dermacentor sp. as a primary vector of these agents, but in regard to Rickettsia spp., the data do support other published work showing D. variabilis harbors a diversity of Rickettsia species with unknown implications for animal and human health.
Keywords: Dermacentor variabilis, Dermacentor albipictus, tick, pet, Rickettsia, Babesia
Introduction
Ticks transmit more vector-borne disease agents in the United States than any other arthropod (Parola et al. 2005). Tick-borne rickettsial pathogens are widely distributed across this region and pose a significant health risk to both humans and animals as nontreated, and some treated, cases may end in death (CDC 2004, Parola et al. 2013, Levin et al. 2014, Biggs et al. 2016, Jiang et al. 2021). In the United States, Rocky Mountain spotted fever (RMSF) caused by Rickettsia rickettsii is historically the primary agent of severe rickettsiosis in humans and dogs (Biggs et al. 2016). In humans, disease is characterized by a petechial rash, but infected individuals may initially present with fever, headache, malaise, and myalgia. RMSF in dogs can manifest as fever, lethargy, decreased appetite, and tremors with an occasional maculopapular rash on areas of exposed skin (Levin et al. 2014, Biggs et al. 2016). Classically, Dermacentor variabilis in the eastern United States and Dermacentor andersoni in the western United States were considered the primary vectors for R. rickettsii in the region. However, Rhipicephalus sanguineus sensu lato (s.l.) has been identified as a competent vector in the southwestern United States and Amblyomma americanum has also been implicated as a secondary vector (Demma et al. 2005, Breitschwerdt et al. 2011, Biggs et al. 2016, Levin et al. 2017, Saleh et al. 2021).
Reports and concern for spotted fever group Rickettsia (SFGR) other than R. rickettsii as a significant cause of human disease have grown over the last two decades (Delisle et al. 2016, CDC 2018). For instance, R. philipii (Rickettsia 364D) transmitted by Dermacentor occidentalis in California may cause fever, headache, or a maculopapular rash, while Rickettsia parkeri, transmitted by Amblyomma maculatum across its southeastern range, can cause similar signs in humans (Parola et al. 2013, Paddock et al. 2018, Yoshimizu and Billeter 2018). In addition, a human case report describes rickettsiosis following a D. variabilis bite caused by R. montanensis, and recently, a novel Rickettsia sp. was identified as the cause of fever, lethargy, and thrombocytopenia in three dogs from south central U.S. states (McQuiston et al. 2012, Wilson et al. 2020). As many as 60% of dogs in disease-endemic areas may have evidence of exposure to SFGR (Levin et al. 2014). However, because of the cross-reactivity of various SFGR on routinely used Rickettsia indirect fluorescent antibody (IFA) assays, novel pathogens such as the one recently isolated from dogs, as well as other SFGR, likely go unrecognized (Parola et al. 2005, Wilson et al. 2020). Investigators in several U.S. regions have surveyed D. variabilis for Rickettsia spp. and demonstrated this tick carries a variety of SFGR, and, of particular importance, that R. rickettsii is rarely detected in D. variabilis, which is significant, given this tick is currently considered a primary vector for this pathogen (Table 1).
Table 1.
Representative Reports of Rickettsia spp. detected in Dermacentor variabilis in the United States, 2000–Present
| U.S. region and state | Source(s) of ticks | Rickettsia spp. | Prevalence (%) | References |
|---|---|---|---|---|
| West | ||||
| California | Humans, environment | R. bellii | 88.2–88.4 | Hecht et al. (2019); Osborne et al. (2020) |
| Humans | R. montanensis | 2.2 | Stromdahl et al. (2011) | |
| Environment | R. rhipicephali | 40.0 | Wikswo et al. (2008) | |
| Washington | Environment | R. bellii | 6.8 | Hecht et al. (2019) |
| Environment | R. montanensis | 1.9 | Hecht et al. (2019) | |
| Environment | R. rhipicephali | 1.0 | Hecht et al. (2019) | |
| Midwest | ||||
| Kansas | Humans, environment | R. montanensis | 10.5 | St. John et al. (2016); Hecht et al. (2019) |
| Minnesota | Humans, environment | R. montanensis | 1.6–31.9 | Stromdahl et al. (2001); St. John et al. (2016); Hecht et al. (2019) |
| Missouri | Environment | R. amblyommatis | NR | Santanello et al. (2018) |
| Environment | R. montanensis | NR | Santanello et al. (2018) | |
| Nebraska | Environment | R. amblyommatis | 0.5 | Luedtke et al. (2020) |
| Environment | R. bellii | 0.4 | Luedtke et al. (2020) | |
| Environment | R. montanensis | 3.1 | Luedtke et al. (2020) | |
| North Dakota | Environment | R. montanensis | 4.3 | Hecht et al. (2019) |
| Environment | R. rhipicephali | 4.3 | Hecht et al. (2019) | |
| Wisconsin | Humans | R. montanensis | 0.3–0.6 | Stromdahl et al. (2001); St. John et al. (2016) |
| South | ||||
| Arkansas | Dogs | R. montanensis | 2.2 | Trout Fryxell et al. (2015) |
| Georgia | Environment | R. bellii | 2.8 | Hecht et al. (2019) |
| Domestic animals | R. felis | 0.6 | Stanley and Rhodes (2021) | |
| Environment, domestic animals | R. montanensis | 4.2–14.7 | Hecht et al. (2019); Stanley and Rhodes (2021) | |
| Kentucky | Humans, environment, domestic and wild animals | R. amblyommatis a | 0.8–1.1 | Fritzen et al. (2011); Hecht et al. (2019) |
| Environment | R. bellii | 8.9 | Hecht et al. (2019) | |
| Humans, environment, domestic and wild animals | R. montanensis b | 0.3–4.5 | Fritzen et al. (2011); Pagac et al. (2014); St. John et al. (2016); Hecht et al. (2019) | |
| Humans, environment, domestic and wild animals | R. parkeri c | 0.6–2.4 | Fritzen et al. (2011); Hecht et al. (2019) | |
| Environment | R. rickettsii c | 0.8 | Hecht et al. (2019) | |
| Mississippi | Environment | R. bellii | 2.0 | Hecht et al. (2019) |
| North Carolina | Environment | R. amblyommatis | 29.3 | Kakumanu et al. (2018) |
| Environment | R. bellii | 1.9 | Kakumanu et al. (2018) | |
| Environment | R. canadensis | 1.3 | Kakumanu et al. (2018) | |
| Environment | R. conorii like | 3.6 | Kakumanu et al. (2018) | |
| Environment | R. massiliae | 3.9 | Kakumanu et al. (2018) | |
| Environment | R. montanensis | 7.7 | Kakumanu et al. (2018) | |
| Environment | R. parkeri c | 7.9 | Kakumanu et al. (2018) | |
| Environment | R. rhipicephali | 0.4 | Kakumanu et al. (2018) | |
| Environment | R. rickettsii c | 0.9 | Kakumanu et al. (2018) | |
| Environment | R. typhi | 1.3 | Kakumanu et al. (2018) | |
| Oklahoma | Humans | R. montanensis | 0.3 | Stromdahl et al. (2001) |
| Tennessee | Humans, environment, wild and domestic animals | R. amblyommatis a | 2.5 | Moncayo et al. (2010) |
| Humans, environment, wild and domestic animals | R. montanensis b | 4.0–9.5 | Moncayo et al. (2010); Pagac et al. (2014); St. John et al. (2016) | |
| Virginia | Environment | R. amblyommatis a | 0.04–4.2 | Henning et al. (2014); Hecht et al. (2019) |
| Environment | R. bellii | 1.9 | Hecht et al. (2019) | |
| Humans, environment | R. montanensis | 0.2–1.4 | Stromdahl et al. (2001); Henning et al. (2014); St. John et al. (2016); Hecht et al. (2019) | |
| Environment | R. parkeri c | 0.7 | Henning et al. (2014) | |
| West Virginia | Humans | R. montanensis | NR | St. John et al. (2016) |
| Northeast | ||||
| Connecticut | Humans | R. montanensis | NR | St. John et al. (2016) |
| Maryland | Humans, environment | R. montanensis | 0.5–3.8 | Ammerman et al. (2004); St. John et al. (2016) |
| Humans | R. rickettsii c | 0.02 | Stromdahl et al. (2011) | |
| New Jersey | Humans, environment | R. montanensis | 0.4–1.3 | Stromdahl et al. (2001); St. John et al. (2016); Occi et al. (2020) |
| New York | Environment | R. amblyommatis | 8.3 | Hecht et al. (2019) |
| Environment | R. montanensis | 8.3 | Hecht et al. (2019) | |
| Pennsylvania | Environment | R. bellii | 1.8 | Hecht et al. (2019) |
| Humans, environment | R. montanensis | 0.2–3.6 | St. John et al. 2016; Hecht et al. (2019) | |
| Rhode Island | Humans | R. montanensis | NR | St. John et al. (2016) |
Detection of a given pathogen, alone, does not confirm vector competence.
Reported as Rickettsia amblyommii in one or more reference.
Reported as Rickettsia montana in one or more reference.
Commonly associated with human disease in the United States.
NR, not reported. Prevalence, or data to calculate prevalence, was not included in the reference.
In addition to SFGR, Dermacentor spp. has been implicated as vectors for other pathogens in the United States. Experimentally, D. variabilis can transmit Ehrlichia canis to dogs, causing ehrlichiosis, a potentially life-threatening illness with some dogs showing no sign of infection, while others display evidence of fever, lethargy, anorexia, myalgia, lymphadenopathy, bleeding tendencies, and neurologic abnormalities (Johnson et al. 1998, Little 2010). Also, a rapidly emerging and medically relevant pathogen in the western United States and Canada, Babesia duncani, has caused serious disease in immunocompromised humans, and preliminary research suggests Dermacentor albipictus as one of the tick vectors (Swei et al. 2019, Yang et al. 2021). The winter tick, D. albipictus, is occasionally found on dogs and cats, although it is maintained in nature by wild ungulates (Duncan et al. 2020).
Various Dermacentor spp. is encountered questing in the environment across its range and is commonly collected from dogs and cats (Thomas et al. 2016, Little et al. 2018, Lehane et al. 2020, Saleh et al. 2021). In a survey of ticks infesting dogs and cats across the United States, D. variabilis was the tick species most commonly infesting dogs (Saleh et al. 2019). Recently, D. variabilis was shown to be the predominant Dermacentor spp. infesting pets in the United States, including in the Rocky Mountain states, where this tick was historically considered rare or absent (Duncan et al. 2021). With the potential to spread pathogenic agents coupled with their changing geographic distribution, Dermacentor spp. surveillance and pathogen screening remain warranted (Minigan et al. 2018, Sonenshine 2018, Lehane et al. 2020, Duncan et al. 2021). In addition, pets may be considered sentinels for both ticks and tick-borne pathogens, and those with evidence of disease may indicate a risk to other members of the household (Paddock et al. 2002, Levin et al. 2014). Hence, the objective of this study is to describe the diversity and geographic distribution of potential pathogenic organisms in Dermacentor species ticks removed from dogs and cats across the United States.
Materials and Methods
Tick samples
Dermacentor spp. (n = 848; 544 females, 297 males, and 7 nymphs) from 471 dogs (n = 770 ticks) and 56 cats (n = 78 ticks) from 44/50 states used in this study was obtained and identified through an ongoing national survey of ticks on dogs and cats as previously described (Saleh et al. 2019, Duncan et al. 2021). Ticks were collected in private veterinary practices during the course of normal physical examinations; institutional review board approval was not required. Ticks were selected for Rickettsia spp. testing with a goal of achieving extensive geographic and Dermacentor species representation. Since D. variabilis was identified in all 44 states with Dermacentor spp. submitted, D. variabilis specimens (n = 827) were selected for use to reflect a broad geographic distribution across the four U.S. regions (Northeast, South, Midwest, and West) (Blagburn et al. 1996, Duncan et al. 2021). D. andersoni (n = 12) and D. albipictus (n = 9) were included to diversify the species tested, even though low numbers were submitted. To determine if less commonly identified pathogens were present, a subsample of ticks (n = 150) from the southern United States where canine ehrlichiosis is known to occur was tested for E. canis, and a subset of ticks (n = 189) primarily from the southern United States and from along the Atlantic and Pacific Coasts where babesiosis has been reported or suspected to occur was tested for Babesia species (Beall et al. 2012, Birkenheuer et al. 2020, Little et al. 2021).
Molecular assays
Each evaluated tick was dissected dorsoventrally and all internal contents collected for nucleic acid extraction, which was performed using a commercial blood kit with no modification added (Illustra GenomicPrep Kit; GE Healthcare). Successful extraction was measured by PCR of the partial fragments of an ITS-2 gene and 16S rRNA gene in a previous study (Duncan et al. 2021); there were no failed DNA extraction or notable PCR inhibitor in this study. Rickettsia spp. was detected and identified using previously described and optimized 17-kDa and ompA gene targets (Sumner et al. 2007, Heise et al. 2010). An E. canis-specific PCR targeting a 16S rRNA gene fragment was performed to determine if E. canis was present in the subsample described in the previous paragraph (Chen et al. 1994, Dawson et al. 1996). Babesia spp. primers targeting fragments of 18S and the large subunit region rRNA genes were used for PCR on the subset of ticks described above (Kim et al. 2013, Qurollo et al. 2017). All amplified products were confirmed on a 2% agarose gel, column purified, and sequenced with an ABI 3730 capillary sequencer (Applied Biosystems, Foster City, CA, USA) at the Oklahoma State University Molecular Core Facility (Stillwater, Oklahoma, USA). High-quality electropherograms were verified by visual inspection and sequences were compared to those available in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Data analysis
Nucleic acid alignment and phylogenetic analyses for Rickettsia spp. were conducted using MacVector with Assembler 18.0.0 (MacVector, Inc.). Descriptive statistics (proportions and corresponding exact binomial 95% confidence intervals [CI]) were calculated alongside each reported prevalence with Microsoft Excel. Inferential statistics (chi-squares) were calculated with Microsoft Excel (Microsoft Office Professional Plus 2016) to compare Rickettsia spp. prevalence against tick stage, tick host, and tick U.S. region origin. The level of significance was set at α = 0.05, and Bonferroni correction was applied to multiple comparisons (i.e., U.S. regions). Maps were developed with MapViewer 8.7.752 (Golden Software, LLC, Golden, CO, USA) with geographic regions (Northeast, South, Midwest, and West) as previously established (Blagburn et al. 1996).
Results
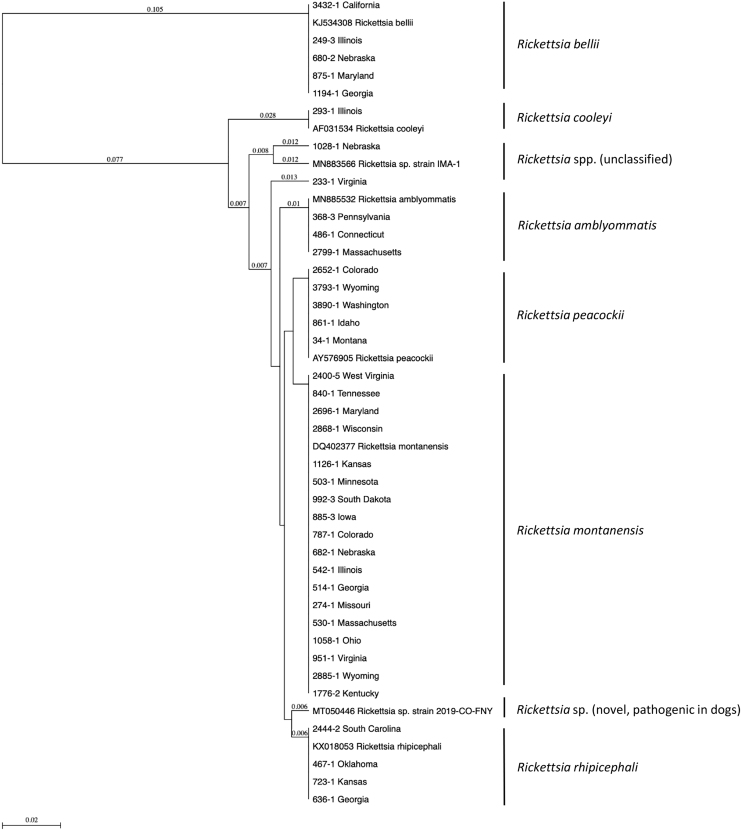
Rickettsia spp. was found in 12.5% (106/848; 95% CI 10.4–14.9) of the ticks tested with 11.9% (98/827; 95% CI 9.8–14.2) and 66.7% (8/12; 95% CI 38.8–86.5) of D. variabilis and D. andersoni, respectively, having detectable Rickettsia species; Rickettsia spp. was not detected in any D. albipictus (n = 9) (Table 1). Out of the 544 female ticks evaluated, 11.9% (65/544; 95% CI 9.5–15.0) had a Rickettsia sp. identified, and similarly, 13.8% (41/297; 95% CI 10.3–18.2) of the male ticks contained a Rickettsia species (chi-squared test, χ2 = 0.44, df = 1, p = 0.5). Rickettsia spp. was not detected in the immature ticks tested (n = 2 D. variabilis and n = 5 D. albipictus). Host (dog versus cat) did not significantly affect prevalence of Rickettsia species in this tick population (chi-squared test, χ2 = 0.01, df = 1, p = 0.9) as 12.5% (96/770; 95% CI 10.3–15.0) of ticks collected from dogs had a Rickettsia sp., while 12.8% (10/78; 95% CI 6.9–22.2) of ticks from cats had a Rickettsia sp. identified. Rickettsia spp. identified include R. montanensis (7.6%; 64/848; 95% CI 5.9–9.5), R. bellii (1.8%; 15/848; 95% CI 1.1–2.9), Rickettsia rhipicephali (1.5%; 13/848; 95% CI 0.9–2.6), R. peacockii (0.9%; 8/848; 95% CI 0.4–1.9), R. amblyommatis (0.4%; 3/848; 95% CI; 0.1–1.1), R. cooleyi (0.1%; 1/848; 95% CI 0.0–0.7), and two unclassified Rickettsia spp. (0.2%; 2/848; 95% CI 0.0–0.9) (Fig. 1). No tick had more than one Rickettsia spp. identified using both gene targets.
FIG. 1.
Phylogenetic relationship of representative Rickettsia spp. Seventeen kilodalton sequences detected in Dermacentor spp. from dogs and cats across the United States. Sequences are identified by tick accession number and state of geographic origin or by accession number of comparator sequences.
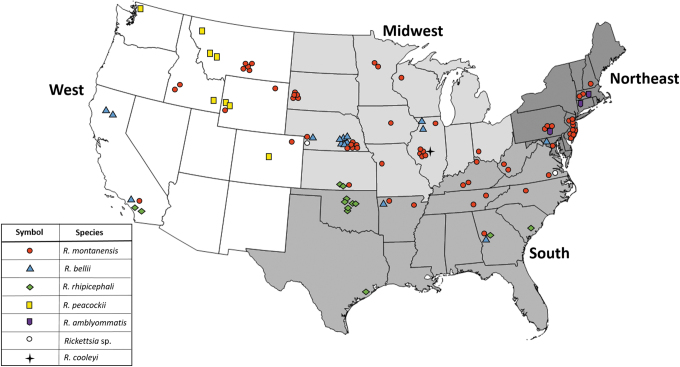
Overall prevalence of Rickettsia spp. in ticks from the four different defined regions (Northeast, South, Midwest, and West) was not significantly different (chi-squared test, χ2 = 7.06, df = 3, p = 0.07) with Rickettsia spp. detected in 13.4% (38/284; 95% CI 9.9–17.9) of Midwest ticks, 13.0% (24/185; 95% CI 8.8–18.6) of ticks from the West, 11.8% (24/203; 95% CI 8.0–17.0) of ticks from the South, and 11.0% (20/181; 95% CI 7.2–16.5) of ticks from the Northeast (Table 2). Across every region, R. montanensis was most often detected in the evaluated ticks. In addition, R. bellii was detected in ticks originating from every region. Rickettsia peacockii was only detected in D. andersoni from the Rocky Mountain states in the West, and no other Rickettsia spp. was present in the evaluated D. andersoni (n = 12). R. rhipicephali was detected in ticks that originated solely in the southern portions of the United States, and the three ticks with R. amblyommatis were submitted from states in the Northeast (Fig. 2).
Table 2.
Prevalence, and Corresponding 95% Confidence Intervals, of Rickettsia Species in Adult Dermacentor spp. Collected from Dogs and Cats in Different Regions of the United States, February 2018 to November 2019
| Rickettsia spp. |
Northeast |
Midwest |
South |
West |
|
|---|---|---|---|---|---|
| Dermacentor variabilis | D. variabilis | D. variabilis | D. variabilis | Dermacentor andersoni | |
| R. amblyommatis a | 3/181 (1.7%) (0.3–5.0) | 0 (0.0–1.6) | 0 (0.0–2.2) | 0 (0.0–2.6) | 0 |
| R. bellii | 1/181 (0.6%) (0.0–3.4) | 9/284 (3.2%) (1.6–6.0) | 2/203 (1.0%) (0.0–3.8) | 3/173 (1.7%) (0.4–5.2) | 0 |
| R. cooleyi | 0 (0.0–2.5) | 1/284 (0.4%) (0.0–2.2) | 0 (0.0–2.2) | 0 (0.0–2.6) | 0 |
| R. montanensis | 16/181 (8.8%) (5.4–14.0) | 25/284 (8.8%) (6.0–12.7) | 12/203 (5.9%) (3.3–10.1) | 11/173 (6.4%) (3.5–11.1) | 0 |
| R. peacockii | 0 (0.0–2.5) | 0 (0.0–1.6) | 0 (0.0–2.2) | 0 (0.0–2.6) | 8/12 (66.7%) (38.8–86.5) |
| R. rhipicephali b | 0 (0.0–2.5) | 2/284 (0.7%) (0.0–2.7) | 9/203 (4.4%) (2.2–8.3) | 2/173 (1.2%) (0.1–4.4) | 0 |
| Unclassified Rickettsia sp. | 0 (0.0–2.5) | 1/284 (0.4%) (0.0–2.2) | 1/203 (0.5%) (0.0–3.0) | 0 (0.0–2.6) | 0 |
| Total | 20/181 (11.0%) (7.2–16.5) | 38/284 (13.4%) (9.9–17.9) | 24/203 (11.8%) (8.0–17.0) | 16/173 (9.2%) (5.7–14.6) | 8/12 (66.7%) (38.8–86.5) |
Dermacentor andersoni (n = 12) was only submitted from the western United States; Rickettsia spp. was not detected in any Dermacentor albipictus (n = 9).
Dogs with Dermacentor variabilis in which Rickettsia amblyommatis was identified were co-infested with Ixodes scapularis (n = 1) or not co-infested with other tick species at the time of presentation (n = 2).
Dogs with D. variabilis in which Rickettsia rhipicephali was identified were co-infested with Amblyomma americanum (n = 3), Amblyomma maculatum (n = 2), I. scapularis (n = 1), and Rhipicephalus sanguineus sensu lato (n = 1), or were not co-infested with other tick species at the time of presentation (n = 8).
FIG. 2.
Geographic distribution of Rickettsia spp. detected in Dermacentor spp. from dogs and cats across the United States; each symbol represents a tick with a detectable Rickettsia spp. (n = 106). Color images are available online.
E. canis was not identified in any of the 150 Dermacentor spp. from 102 dogs (123 ticks) and 21 cats (27 ticks) from 17 states tested. A subset of Dermacentor spp. (n = 189) from 132 dogs (157 ticks) and 26 cats (32 ticks) from 20 states was tested for Babesia spp., and two nymphal D. albipictus had detectable Babesia conradae (1.1%; 2/189; 95% CI 0.0–4.0). The ticks originated from Minnesota and Colorado in October and November, respectively, and were both collected from adult cats.
Discussion
Even though D. variabilis and D. andersoni are considered primary vectors of R. rickettsii, the causative agent of RMSF, we did not detect this pathogen in any Dermacentor tick (n = 848) collected from dogs and cats in 44 U.S. states. Instead, we identified various other SFGR, which support data from other studies reporting a range of SFGR and a low prevalence of R. rickettsii in Dermacentor ticks (Moncayo et al. 2010, Fritzen et al. 2011, Trout Fryxell et al. 2015, Santanello et al. 2018, Dykstra et al. 2020, Francis et al. 2020, Luedtke et al. 2020, Occi et al. 2020). In fact, when identified in D. variabilis, R. rickettsii has a reported prevalence less than 1%; however, this is in contrast to previous reports of greater than 8% of ticks harboring R. rickettsii (Schriefer and Azad 1994, Stromdahl et al. 2011, Kakumanu et al. 2018, Hecht et al. 2019). This change to a lack, or rarity, of R. rickettsii in its primary tick vectors could be explained by evidence suggesting tick endosymbionts have the potential to alter tick physiology and transmission of pathogens as indicated by the inverse relationship between R. rickettsii and R. peacockii in D. andersoni and documentation of R. peacockii infection, excluding R. rickettsii infection in the same tick (Burgdorfer et al. 1981, Narasimhan and Fikrig 2015). Approximately two-thirds (66.7%) of D. andersoni had R. peacockii identified in this study, although only 12 specimens were evaluated. Other studies have documented R. peacockii in 1.3–80% of D. andersoni tested and found no R. rickettsii in those specimens (Burgdorfer et al. 1981, Niebylski et al. 1997, Francis et al. 2020, Lefcort et al. 2020).
For D. variabilis, a similar mechanism may exist as data suggest this tick is unable to maintain two different Rickettsia spp. through transovarial transmission (Macaluso et al. 2002). In addition, other tick genera may be responsible for transmitting the causative agent of RMSF more frequently than originally recognized. In the southwestern United States, the brown dog tick, R. sanguineus s.l., is capable of transmitting R. rickettsii, while Haemaphysalis longicornis and A. americanum can successfully maintain and transmit R. rickettsii under laboratory conditions, although their transmission potential in nature is not fully known (Demma et al. 2005, Labruna et al. 2008, Levin et al. 2017, Stanley et al. 2020). Besides R. rickettsii, other SFGR can cause significant disease in humans and therefore may be contributing to the rise in reported cases of rickettsiosis since many SFGR cross-react on commonly used serologic assays (Moncayo et al. 2010). Two such examples include R. philipii (Rickettsia 364D), the causative agent of Pacific Coast tick fever, transmitted by D. occidentalis along its range in California, and R. parkeri transmitted by A. maculatum in the southeastern United States (Paddock et al. 2010, 2018, Stromdahl et al. 2011, Yoshimizu and Billeter 2018).
In this study, R. montanensis was the most prevalent Rickettsia spp. identified in D. variabilis, with this agent detected in over half (60.4%; 64/106; 95% CI 50.4–69.8) of the Rickettsia-positive ticks. Geographically, R. montanensis was found in ticks collected from every U.S. region and in 26 of the 44 states in which ticks were evaluated. Regional surveys from 22 states across the United States similarly found R. montanensis in D. variabilis (Table 1). Although the pathogenicity of R. montanensis in dogs and cats is currently unknown, mild disease has been induced in laboratory-reared guinea pigs and the agent was identified in a D. variabilis collected from a human before the development of a rash illness (McQuiston et al. 2012, Snellgrove et al. 2021). When considering the case report of human disease attributed to this SFGR, the widespread distribution of R. montanensis reported in this study is noteworthy. Before the human case report, R. montanensis was considered solely a nonpathogenic symbiont of D. variabilis (McQuiston et al. 2012, Parola et al. 2013). In ticks, the transition from endosymbiont to pathogen is not novel as evidenced by the emergence of disease-causing strains of Coxiella following numerous mutations and acquisition of new virulence factors (Duron et al. 2015). A recent example comes from the southern United States where a novel Rickettsia sp. has been identified as a cause of rickettsiosis in dogs (Wilson et al. 2020). Further investigation of intracellular pathogens and their evolution suggests this transition to a medically relevant pathogen may be achieved through inaccurate replication of the nonpathogenic Rickettsia spp. widely prevalent in ticks (Darby et al. 2007, Bonnet et al. 2017).
In total, eight different Rickettsia spp. were identified in this study. Diversity of SFGR appears common in surveys of D. variabilis with as many as 10 species having been found within a population of this tick (Stromdahl et al. 2011, Trout Fryxell et al. 2015, Kakumanu et al. 2018, Hecht et al. 2019). In general, Rickettsia spp. makes up the largest proportion of microbial biomass of D. variabilis (Sanchez-Vicente et al. 2019). Although none of the ticks examined in this study had more than one Rickettsia spp. detected, it has been reported that multiple Rickettsia spp. can infect a single D. variabilis or D. andersoni; however, the transovarial transmission of more than one Rickettsia species may not occur (Macaluso et al. 2002, Carmichael and Fuerst 2010, Kakumanu et al. 2018, Francis et al. 2020). Regardless, detecting the presence of Rickettsia spp. or any other tick-borne pathogen in a given tick does not confirm that species plays a role in transmission; vector competence can only be assessed through experimental transmission studies.
Dermacentor spp. is most well known for their ability to transmit Rickettsia spp., but this genus may also serve as a vector for other medically relevant tick-borne pathogens. For instance, two D. variabilis ticks from Mexico, an endemic region for canine ehrlichiosis, were found to have E. canis, and experimentally, D. variabilis is capable of transmitting E. canis (Johnson et al. 1998, Sosa-Gutierrez et al. 2016). However, none of the Dermacentor tested (n = 150) in this study had detectable E. canis. The ticks in this study were submitted primarily from regions of the United States where canine infection with E. canis is relatively rare, which may have precluded detection of the organism (Beall et al. 2012). Moreover, as with detection alone, proving vector competence in an experimental study does not confirm relevance to transmission in nature. Ixodes scapularis ticks from Louisiana, a state in the southern United States, are capable of transmitting Borrelia burgdorferi in the laboratory, but, due to differences in tick phenology, host preferences, and questing behavior, do not sustain a maintenance system for this agent in the field (Jacobs et al. 2003, Goddard et al. 2015, Ginsberg et al. 2021).
In addition, some agents of babesiosis are suspected to be transmitted by Dermacentor spp. (Kjemtrup and Conrad 2006, Shock et al. 2014, Swei et al. 2019). In a multistate survey, 2.7% of the D. variabilis tested had detectable Babesia spp., and D. albipictus has been documented in the transmission cycle of B. duncani to humans (Shock et al. 2014, Swei et al. 2019). In this study, B. conradae, a potentially fatal agent infecting dogs in the southern United States, was identified in two D. albipictus collected from cats. The majority of reported cases of B. conradae is associated with kennels of coyote-hunting dogs, and although ticks are suspected to play a role in transmission, to date, no tick has been named as the primary vector (Kjemtrup and Conrad 2006, Dear et al. 2018). Our findings suggest further work is needed to elucidate the relationship, if any, between D. albipictus and B. conradae.
This study had several limitations. The ticks examined in this study were collected from pets and may have ingested a blood meal before submission; therefore, the results of this study do not implicate a particular Dermacentor sp. as the primary vector for the pathogens identified. For instance, R. rhipicephali is more often identified in R. sanguineus (s.l.), R. amblyommatis is associated frequently with A. americanum, and R. cooleyi with Ixodes spp., and yet these agents have been found in various tick species (Hayes and Burgdorfer 1979, Moncayo et al. 2010, Fritzen et al. 2011, Barrett et al. 2014). In addition, if multiple Rickettsia spp. were present in a tick, reaction conditions may have favored amplification of the target in greater abundance or failed to amplify some Rickettsia spp. at all, precluding their detection. Similarly, although ticks were available from 44/50 states for analysis, our reliance on passive surveillance may have resulted in inadequate numbers of tick specimens to test from a given state, leading to failure of detection of less common pathogens.
Conclusion
This study highlights the diversity of SFGR in D. variabilis collected from dogs and cats across the United States. The majority of the detected species currently has an unknown pathogenicity in pets, but may induce cross-reacting antibodies on IFA, confounding diagnostic tests. Continued surveillance of tick-borne agents for the sake of veterinary and human health is warranted.
Acknowledgments
The authors thank the veterinary professionals across the United States, who took time to collect and submit ticks for this research. We also thank the Krull-Ewing Laboratory at Oklahoma State University and the National Center of Veterinary Parasitology for their support.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
K.T.D. currently serves as the Boehringer Ingelheim Resident in Veterinary Parasitology with funding provided through the National Center for Veterinary Parasitology (NIH R03 AI149638; NSF OIA 1920946).
References
- Ammerman NC, Swanson KI, Anderson JM, Schwartz TR, et al. Spotted fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg Infect Dis; 2004; 10:1478–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, Little SE, Shaw E. “Rickettsia amblyommii” and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis 2014; 14:20–25. [DOI] [PubMed] [Google Scholar]
- Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, et al. . Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors 2012; 29:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, et al. . Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44. [DOI] [PubMed] [Google Scholar]
- Birkenheuer AJ, Buch J, Beall MJ, Braff J, et al. . Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Vet Parasitol Reg Stud Rep 2020; 22:100471. [DOI] [PubMed] [Google Scholar]
- Blagburn BL, Lindsay DS, Vaughn JL, Ripley NS, et al. . Prevalence of canine parasites based on fecal flotation. Comp Cont Ed Pract Vet 1996; 18:483–509. [Google Scholar]
- Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O. The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front Cell Infect Microbiol 2017; 7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Maggi RB, Lantos PM, et al. . Rickettsia rickettsii transmission by a lone star tick, North Carolina. Emerg Infect Dis 2011; 5:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Hayes SF, Mavros AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: A limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, Anacker RL, eds. Rickettsiae and Rickettsial Diseases. New York, NY: Academic Press, an Imprint of Elsevier, 1981:585–594. [Google Scholar]
- Carmichael JR, Fuerst PA. Molecular detection of Rickettsia bellii, Rickettsia montanensis, and Rickettsia rickettsii in a Dermacentor variabilis tick from nature. Vector Borne Zoonotic Dis 2010; 10:111–115. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fatal cases of Rocky Mountain spotted fever in family clusters—three states, 2003. MMWR Morb Mortal Wkly Rep 2004; 53:407–410. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Rocky Mountain spotted fever (RMSF). 2018. Available at https://www.cdc.gov/rmsf/stats/index.html.
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol 1994; 32:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby AC, Cho N-H, Fuxelius H-H, Westberg J, et al. . Intracellular pathogens go extreme: Genome evolution in the Rickettsiales. Trends Genet 2007; 23:511–520. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Biggie KL, Cookson K, Jenkins S, et al. . Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res 1996; 57:1175–1179. [PubMed] [Google Scholar]
- Dear JD, Owens SD, Lindsay LL, Biondo AW, et al. . Babesia conradae infection in coyote hunting dogs infected with multiple blood-borne pathogens. J Vet Intern Med 2018; 32:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle J, Mendell NL, Stull-Lane A, Bloch KC, et al. . Human infections by multiple spotted fever group Rickettsiae in Tennessee. Am J Trop Med Hyg 2016; 94:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, et al. . Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594. [DOI] [PubMed] [Google Scholar]
- Duncan KT, Clow KM, Sundstrom KD, Saleh MN, et al. . Recent reports of winter tick, Dermacentor albipictus, from dogs and cats in North America. Vet Parasitol Reg Stud Rep 2020; 22:100490. [DOI] [PubMed] [Google Scholar]
- Duncan KT, Saleh MN, Sundstrom KD, Little SE. Dermacentor variabilis is the predominant Dermacentor spp. (Acari: Ixodidae) feeding on dogs and cats throughout the United States. J Med Entomol 2021; 58:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O, Noël V, McCoy KD, Bonazzi M, et al. . The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q Fever pathogen, Coxiella burnetii. PLoS Pathog 2015; 11:e1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra EA, Oltean HN, Kangiser D, Marsden-Haug N, et al. . Ecology and Epidemiology of tickborne pathogens, Washing, USA, 2011-2016. Emerg Infect Dis 2020; 26:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis L, Paddock CD, Dykstra EA, Karpathy SE. Rickettsia and Anaplasma species in Dermacentor andersoni ticks from Washington. Ticks Tick Borne Dis 2020; 11:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzen CM, Huang J, Westby K, Freye JD, et al. . Infection prevalence of common tick-borne pathogens in adult Lone Star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am J Trop Med Hyg 2011; 85:718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Hickling GJ, Burke RL, Ogden NH, et al. . Why Lyme disease is common in the northern US, but rare in the south: The roles of host choice, host-seeking behavior, and tick density. PLoS Biol 2021; 19:e3001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J, Embers M, Hojgaard A, Piesman J. Comparison of tick feeding success and vector competence for Borrelia burgdorferi among immature Ixodes scapularis (Ixodida: Ixodidae) of both southern and northern clades. J Med Entomol 2015; 52:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SF, Burgdorfer W. Ultrastructure of Rickettsia rhipicephali, a new member of the spotted fever group rickettsiae in tissues of the host vector Rhipicephalus sanguineus. J Bacteriol 1979; 137:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JA, Allerdice MEJ, Dykstra EA, Mastel L, et al. . Multistate survey of American dog ticks (Dermacentor variabilis) for Rickettsia species. Vector Borne Zoonotic Dis 2019; 19:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise SR, Elshahed MS, Little SE. Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. J Med Entomol 2010; 47:258–268. [DOI] [PubMed] [Google Scholar]
- Henning TC, Orr JM, Smith JD, Arias JR, et al. . Spotted fever group Rickettsiae in multiple hard tick species from Farifax county, Virginia. Vector Borne Zoonotic Dis 2014; 14:482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MB, Purcell JE, Philipp MT. Ixodes scapularis ticks (Acari: Ixodidae) from Louisiana are competent to transmit Borrelia burgdorferi, the agent of Lyme borreliosis. J Med Entomol 2003; 40:964–967. [DOI] [PubMed] [Google Scholar]
- Jiang J, Farris CM, Yeh KB, Richards AL. International Rickettsia disease surveillance: An example of cooperative research to increase laboratory capability and capacity for risk assessment of rickettsial outbreaks worldwide. Front Med (Lausanne) 2021; 8:622015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Ewing SA, Barker RW, Fox JC, et al. . Experimental transmission of Ehrlichia canis (Rickettsiales: Ehrlichieae) by Dermacentor variabilis (Acari: Ixodidae). Vet Parasitol 1998; 74:277–288. [DOI] [PubMed] [Google Scholar]
- Kakumanu ML, Ponnusamy L, Sutton H, Meshnick SR, et al. . Prevalence of Rickettsia species (Rickettsiales: Rickettsiaceae) in Dermacentor variabilis ticks (Acari: Ixodidae) in North Carolina. J Med Entomol 2018; 55:1284–1291. [DOI] [PubMed] [Google Scholar]
- Kim E-J, Bauer C, Grevelding CG, Quack T. Improved PCR/nested PCR approaches with increased sensitivity and specificity for the detection of pathogens in hard ticks. Ticks Tick Borne Dis 2013; 4:409–416. [DOI] [PubMed] [Google Scholar]
- Kjemtrup AM, Conrad PA. A review of the small canine piroplasms from California: Babesia conradae in the literature. Vet Parasitol 2006; 138:112–117. [DOI] [PubMed] [Google Scholar]
- Labruna MB, Ogrzewalska M, Martins TF, Pinter A, et al. . Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J Med Entomol 2008; 45:1156–1159. [DOI] [PubMed] [Google Scholar]
- Lefcort H, Tsybulnik DY, Browning RJ, Eagle HP, et al. . Behavioral characteristics and endysymbionts of two potential tularemia and Rocky Mountain spotted fever tick vectors. J Vector Ecol 2020; 45:321–332. [DOI] [PubMed] [Google Scholar]
- Lehane A, Parise C, Evans C, Beati L, et al. . Reported county-level distribution of the American dog tick (Acari: Ixodidae) in the contiguous United States. J Med Entomol 2020; 57:131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova GE, Ritter JM, et al. . Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS One 2014; 9:e115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Zemtsova GE, Killmaster LF, Snellgrove A, et al. . Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick Borne Dis 2017; 8:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE. Ehrlichiosis and anaplasmosis in dogs and cats. Vet Clin North Am Small Pract 2010; 40:1121–1140. [DOI] [PubMed] [Google Scholar]
- Little SE, Barrett AW, Nagamori Y, Herrin BH, et al. . Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet Parasitol 2018; 15:15–20. [DOI] [PubMed] [Google Scholar]
- Little SE, Braff J, Place J, Buch J, et al. . Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasit Vectors 2021; 14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke BE, Shaffer JJ, Monrroy E, Willicott CW, et al. . Molecular detection of spotted fever group Rickettsiae (Rickettsiales: Rickettsiaceae) in Dermacentor variabilis (Acari: Ixodidae) collected along the Platte River in south central Nebraska. J Med Entomol 2020; 57:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol 2002; 39:809–813. [DOI] [PubMed] [Google Scholar]
- McQuiston JH, Zemtsova G, Perniciaro J, Hutson M, et al. . Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis 2012; 12:1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigan JN, Hager HA, Peregrine AS, Newman JA. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick Borne Dis 2018; 9:354–362. [DOI] [PubMed] [Google Scholar]
- Moncayo AC, Cohen SB, Fritzen CM, Huang E, et al. . Absence of Rickettsia rickettsii and occurrence of other spotted fever group Rickettsiae in ticks from Tennessee. Am J Trop Med Hyg 2010; 83:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Fikrig E. Tick microbiome: The force within. Trends Parasitol 2015; 31:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski ML, Schrumpf ME, Burgdorfer W, Fischer ER, et al. . Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol 1997; 47:446–452. [DOI] [PubMed] [Google Scholar]
- Occi J, Egizi AM, Goncalves A, Fonseca DM. New Jersey-wide survey of spotted fever group Rickettsia (Proteobacteria: Rickettsiacae) in Dermacentor variabilis and Amblyomma americanum (Acari: Ixodida: Ixodidae). Am J Trop Med Hyg 2020; 103:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CJ, Wakeman-Hill AJ, Loa SE, Crosbie PR, et al. . Rickettsia spp. in five tick species collected in central California. J Med Entomol 2020; 57:1596–1603. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Brenner O, Vaid C, Boyd DB, et al. . Short report: Concurrent Rocky Mountain spotted fever in a dog and its owner. Am J Trop Med Hyg 2002; 66:197–199. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier P-E, Sumner JW, Goddard J, et al. . Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. App Environ Microbiol 2010; 76:2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Yoshimizu MH, Zambrano ML, Lane RS, et al. . Rickettsia species isolated from Dermacentor occidentalis (Acari: Ixodidae) from California. J Med Entomol 2018; 55:1555–1560. [DOI] [PubMed] [Google Scholar]
- Pagac BB, Miller MK, Mazzei MC, Nielsen DH, et al. . Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg Infect Dis 2014; 20:1750–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin Microbiol Rev 2005; 18:719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, Labruna MB, et al. . Update on tick-borne rickettsioses around the world: A geographic approach. Clin Microbiol Rev 2013; 26:657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurollo BA, Archer NR, Schreeg ME, Marr HS, et al. . Improved molecular detection of Babesia infections in animals using a novel quantitative real-time PCR diagnostic assay targeting mitochondrial DNA. Parasit Vectors 2017; 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, et al. . Polymicrobial nature of tick-borne diseases. mBio 2019; 10:e02055–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanello C, Barwari R, Troyo A. Spotted fever group rickettsiae in ticks from Missouri. Ticks Tick Borne Dis 2018; 9:1395–1399. [DOI] [PubMed] [Google Scholar]
- Saleh MN, Allen KE, Lineberry MW, Little SE, et al. . Ticks infesting dogs and cats in North America: Biology, geographic distribution, and pathogen transmission. Vet Parasitol 2021; 294:109392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MN, Sundstrom KD, Duncan KT, Ientile MM, et al. . Show us your ticks: A survey of ticks infesting dogs and cats across the United States. Parasit Vectors 2019; 12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriefer ME, Azad AF. Changing ecology of Rocky Mountain spotted fever. In Sonenshine DE, Mather TN, eds. Ecological Dynamics of Tick-Borne Zoonoses. New York, NY: Oxford University Press, 1994:314–326. [Google Scholar]
- Shock BC, Moncayo A, Cohen S, Mitchell EA, et al. . Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis 2014; 5:373–380. [DOI] [PubMed] [Google Scholar]
- Snellgrove AN, Krapiunaya I, Scott P, Levin ML. Assessment of the pathogenicity of Rickettsia amblyommatis, Rickettsia bellii, and Rickettsia montanensis in a guinea pig model. Vector Borne Zoonotic Dis 2021; 21:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int J Environ Res Public Health 2018; 15:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Gutierrez CG, Vargas-Sandoval M, Torres J, Gordillo-Perez G. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J Vet Sci 2016; 17:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John HK, Adams ML, Masuoka PM, Flyer-Adams JG, et al. Prevalence, distribution, and development of an ecological niche model of Dermacentor variabilis ticks positive for Rickettsia montanensis. Vector Borne Zoonotic Dis 2016; 16:253–263. [DOI] [PubMed] [Google Scholar]
- Stanley HM, Ford SL, Snellgrove AN, Hartzer K, et al. . The ability of the invasive Asian longhorned tick Haemaphysalis longicornis (Acari: Ixodidae) to acquire and transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the agent of Rocky Mountain spotted fever, under laboratory conditions. J Med Entomol 2020; 57:1635–1639. [DOI] [PubMed] [Google Scholar]
- Stanley H, Rhodes DVL. Presence of Rickettsia species in ticks collected from companion animals in northeastern Georgia, United States. Vet Sci 2021; 8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl EY, Evans SR, O'Brien JJ, Gutierrez AG. Prevalence of infection in ticks submitted to the human tick test kit program of the U.S. Army Center for Health Promotion and Preventive Medicine. J Med Entomol 2001; 38:67–74. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis 2011; 11:969–977. [DOI] [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, et al. . Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis 2007; 13:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A, O'Connor KE, Couper LI, Thekkiniath J, et al. . Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int J Parasitol 2019; 49:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JE, Staubus L, Goolsby JL, Reichard MV. Ectoparasites of free-roaming domestic cats in the central United States. Vet Parasitol 2016; 228:17–22. [DOI] [PubMed] [Google Scholar]
- Trout Fryxell RT, Steelman CD, Szalanski AL, Billingsley PM, et al. . Molecular detection of Rickettsia species within ticks (Acari: Ixodidae) collected from Arkansas United States. J Med Entomol 2015; 52:500–508. [DOI] [PubMed] [Google Scholar]
- Wikswo ME, Hu R, Dasch GA, Krueger L, et al. . Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J Med Entomol 2008; 45:509–516. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Breitschwerdt EB, Juhasz NB, Marr HS, et al. . Novel Rickettsia species infecting dogs, United States. Emerg Infect Dis 2020; 26:3011–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Christie J, Köster L, Du A, et al. . Emerging human Babesiosis with “ground zero” in North America. Microorganisms 2021; 9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu MH, Billeter SA. Suspected and confirmed vector-borne rickettsioses of North America associated with human diseases. Trop Med Infect Dis 2018; 3:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]