Abstract
Background
Determining the presence of modifiable risk factors for atrial fibrillation (AF), such as sleep apnea is of clinical importance due to the potential impact targeting these risk factors can have on the progression and burden of AF. Using new digital-based technology is a promising solution to the underreporting of sleep apnea highlighted by academical societies in recent years. The aim of this study is to report the prevalence and severity of sleep apnea in patients with AF and, secondarily, assess the accuracy and feasibility of a new home-screening device for sleep apnea (NightOwl™ by Ectosense).
Methods
DAN-APNO is a cross-sectional study at the Department of Cardiology, Herlev-Gentofte Hospital recruiting patients with AF referred to anticoagulation initiation aged 18 to 90 years without known sleep apnea. At least 150 patients will be recruited and undergo medical history, clinical evaluation, several sleep-apnea questionnaires, and a sleep-recording evaluation for four nights with sleep apnea home-monitoring device NightOwl™. Additionally, the first 20 participants and participants with moderate-severe sleep apnea by screening are referred to cardio-respiratory monitoring (CRM). This clinical evaluation allows the comparison of standard evaluation method and the NightOwl™. Clinical measures include Apnea–Hypopnea Index (AHI), Oxygen Desaturation Index (ODI), pulse rate, as well as questionaries about sleep apnea assessment and the clinical feasibility of the NightOwl™ device. Main outcomes comprise analysis of the prevalence and severity of sleep apnea, and clinical and demographic predictors of moderate and severe sleep apnea. In addition, correlation analyses for accuracy measures between CRM and NightOwl™ will be conducted along with patient ease-of-use and satisfaction questionnaires.
Discussion
This study is limited by selection bias; only patients with atrial fibrillation from anticoagulation clinic is asked to participate, which could limit the generalizability of our results. However, this study aims to test whether a miniaturized simple home-monitoring device for detecting sleep apnea in patients with AF potentially can evaluate sleep apnea more conveniently and easier.
Trial Registration The study is registered the 18-02-2021 at clinicaltrials.gov with registration number: NCT04760002.
Strengths and limitations of this study
This study aims to test whether a miniaturized simple home-monitoring device for detecting sleep apnea in patients with atrial fibrillation potentially can evaluate sleep apnea more conveniently and easier.
It will simultaneously gather extensive clinical characteristics, hoping to be able to identify important predictors for sleep apnea in patients with atrial fibrillation and evaluate the usefulness of implemented questionnaires when screening and evaluating symptom burden for sleep apnea.
This study is limited by selection bias; only patients with atrial fibrillation from anticoagulation clinic is asked to participate, which could limit the generalizability of our results.
This study does not assess the prevalence of sleep apnea by gold standard of polysomnography (PSG) but by home-monitoring device and CRM, therefore there may be higher risk of false negative and false positives.
Background
Sleep apnea refers to intermittent, cyclical cessations or reductions of airflow and is characterized by snoring, oxygen desaturations and brief arousal from sleep resulting in frequent interruption of respiration and sleep. The most prevalent form is obstructive sleep apnea (OSA) with partial or complete collapse of the upper airway, and this form will throughout this protocol be referred to as sleep apnea. The last two decades, OSA has been recognized as an increasingly common condition occurring in up to 17% of males and 10% of females aged between 50 and 70 years [1, 2]. OSA is also a known risk factor for developing and exacerbating multiple cardiovascular diseases including atrial fibrillation (AF) [3–8]. The diagnosis of sleep apnea is typically based on the number of apnea and hypopnea per hour of sleep, titled the Apnea–Hypopnea Index (AHI).
In patients with AF, OSA has been shown to be associated with a higher risk of recurrent AF episodes after procedures restoring the heart rhythm, such as catheter ablation and electrical cardioversion [9, 10]. There is also evidence that OSA are related to the progression of AF [11], have been shown to reduce the effectiveness of rhythm drug therapy, [12] and associated with increased risk of cardiovascular events such as stroke [13]. In total, presence of concomitant OSA and AF is assumed to constitute a higher cardiovascular disease burden and worse symptoms and related to poorer prognosis. OSA is a modifiable risk factor, that can be treated with weight loss, oral appliances, continuous positive airway pressure (CPAP) and surgery. However, randomized clinical trial are yet to establish benefits of sleep apnea treatment in these patients.
To determine presence of sleep apnea patients with AF is therefore clinically important but is often overlooked and the diagnostic work-up is cumbersome, time-consuming and not widely available. The gold standard of sleep apnea diagnosis and AHI classification is a polysomnography (PSG) but sleep apnea is routinely diagnosed with cardiorespiratory monitoring (CRM) [14]. Academical societies have in recent decades advocated that OSA is underreported and unrecognized, and increasing evidence is supporting this statement. In a recent investigation of 1364 patients without OSA undergoing non-cardiac surgery, researchers found a prevalence of 67.6% with 11.2% suffering from severe OSA [15]. Another study of patients with AF (n = 41) the prevalence of OSA was 56% [16], and in similar study of patients with AF (n = 100), 85% had OSA (AHI ≥ 5) and severe OSA was detected in 28% [17].
A common and important clinical consequence of sleep apnea is excessive daytime sleepiness, and this clinical consequence is advocated by the use of questionnaires to assess daytime sleepiness. However, studies have shown most patients with AF report low daytime sleepiness although subsequently polysomnography analysis graded by AHI found sleep apnea present [18]. As such, screening and management of sleep apnea in patients with AF requires new prediction and assessment tools. Possibly, emerging new technologies consisting of miniaturized easily available monitoring devices and accurate algorithm development may help in the evaluation of sleep apnea status in the large number of patients with AF [19]. One of such devices is the NightOwl™, a self-applied home-monitoring device made to detect sleep apnea. It has been validated against PSG in a single-night in-laboratory setting showing promising results but a multi-night assessment of NightOwl™ in a home environment is still to be examined [20]. Potentially, the device could be an easy and inexpensive method to find undiagnosed sleep apnea in patients with AF.
Objectives and hypothesis
To use a home-monitoring device NightOwl™ to screen patients with AF for sleep apnea and thus;
Report the prevalence and severity of sleep apnea in patients with AF.
Assess the accuracy, user-experience, and feasibility of home-screening for sleep apnea in patients with AF.
Hypothesis
In patients with AF, the prevalence of unrecognized OSA is high (around 50%) and home-monitoring test using NightOwl™ is a viable, easy, and accurate test of measuring sleep apnea.
Method and design
Setting
Patients diagnosed with any type of AF referred to anticoagulation initiation at a nurse-run ambulatory will be asked to participate. The ambulatory consists of four daily nurse-led tracks at Department of Cardiology, Herlev-Gentofte University Hospital. In a formal collaboration, Department of Pulmonology, Herlev-Gentofte University Hospital provides work-up with CRM investigation and clinical evaluation of initiating treatment of sleep apnea in patients referred from the study.
Participants
Participants with AF, without known sleep apnea, and indication for anticoagulation will be recruited from the Thrombosis unit. Inclusion and exclusion criteria are shown in Box 1. The following information will be obtained from the participants:
Clinical data captured at first visit: age, sex, body mass index, neck circumference and blood pressure.
Data captured from electronic health system: latest available blood samples, prior health conditions (comorbidities), prior procedures performed such as electrocardiography and echocardiogram, and prior procedures related to AF such as electrical cardioversion and ablation.
Questionnaires for sleep apnea screening and symptomatic burden of sleep apnea: STOP-BANG, Berlin Questionnaire and Epworth Sleepiness Scale (ESS).
Box 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Diagnosis of AF of any type | Known sleep apnea |
| Age < 90 years | Secondary AF |
| Age > 18 years | Professional drivers |
| Severe heart failure (NYHA class III or IV) | |
| Severe chronic obstructive pulmonary disease |
Outcomes
The primary outcome of the study is the prevalence with 95% confidence intervals of sleep apnea in patients with AF detected by NightOwl™. This analysis will be total and stratified by severity of sleep apnea.
The secondary outcome will be an exploratory analysis of predictors of sleep apnea and predictors of moderate and severe sleep apnea in the participants using baseline characteristics and clinical data of the participants.
Other outcomes of interest are;
The correlation between NightOwl™ and CRM diagnostic testing for sleep apnea and the correlation between sleep apnea screening questionnaire for CRM and NightOwl™.
- Evaluation of user-experience and compliance with NightOwl™.
- The evaluation of user-experience and compliance will consist of a survey questionnaire build with help from CATCHET Unified Method for Assessment of Clinical Feasibility (CUMACF) [21]. The purpose of this method is to deliver a standardized way of measuring clinical feasibility of the many new technologies being designed and tested. The clinical feasibility questionnaire is divided into three parts: Usage Adoption, Perceived Usefulness and Usability, and Health Efficacy.
- Inter-variability of NightOwl™ recordings.
- Recordings of four nights will be used to assess any inter-variability between the NightOwl™ recording. Furthermore, the four nights recording can clarify potential night-to-night fluctuations and thereby gives a more precise screening of the presence of sleep apnea.
Study design
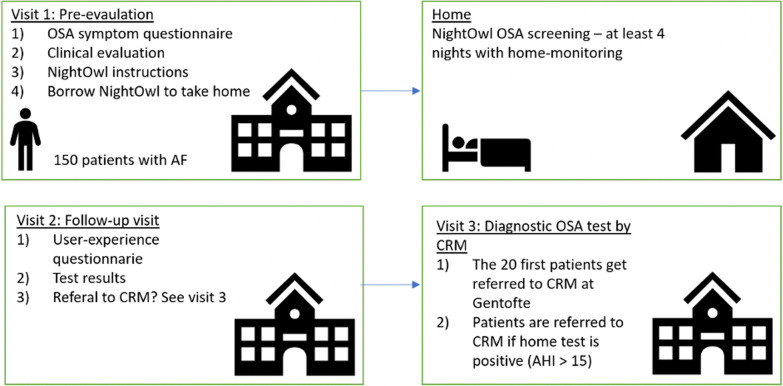
The is a cross sectional study. In total two visits will be planned, with a third visit for the first 20 participants and participants with AHI > 15 included in the study (Fig. 1).
Initial visit with time for clinical evaluation, questionnaire of OSA symptoms, the participant borrows a NightOwl™ and receive device instructions.
Four night of recording with NightOwl™ in home environment.
Follow-up visit for the home-monitoring results and soft node questionnaire.
For the 20 first patients and for all patients where the home test is showing (AHI > 15) a fourth visit at the sleep apnea clinic will be arranged.
Fig. 1.
Design of the study
The time plan for the study inclusion until last patient enrolled are estimated to be approximately 6 months.
The device
The NightOwl™ consists of a small sensor device which is placed on the fingertip and a smartphone app that is connected to an encrypted cloud-based analytics platform within the European Union, the NightOwl™ software (Fig. 2). It is self-applied by attaching the sensor to the fingertip by means of an adhesive patch. The NightOwl™ sensor acquires accelerometer data and reflectance based photoplethysmography (PPG) from which it derives actigraphy (sleep/wake behavior), saturation of peripheral oxygen (SpO2), peripheral artery tone (PAT) and pulse rate, among other features [20]. A PAT analysis derives changes in caliber of arteries elicited by alterations in the contractile activity of vascular smooth muscle and are referred to as changes in arterial tone. The end state of the apnea–hypopnea events are associated with sympathetic activation [22]. Conversely, this autonomic arousal is also associated with nonrespiratory events e.g. the episodic limb movements during sleep, which could lead to a false-positive respiratory event in the PAT analysis. However, by incorporating concurrent analysis of other PPG-derived features such as SpO2 the discrimination amongst type of sympathetic activation associated events are made possible [20].
Fig. 2.

NightOwl™ sensor device
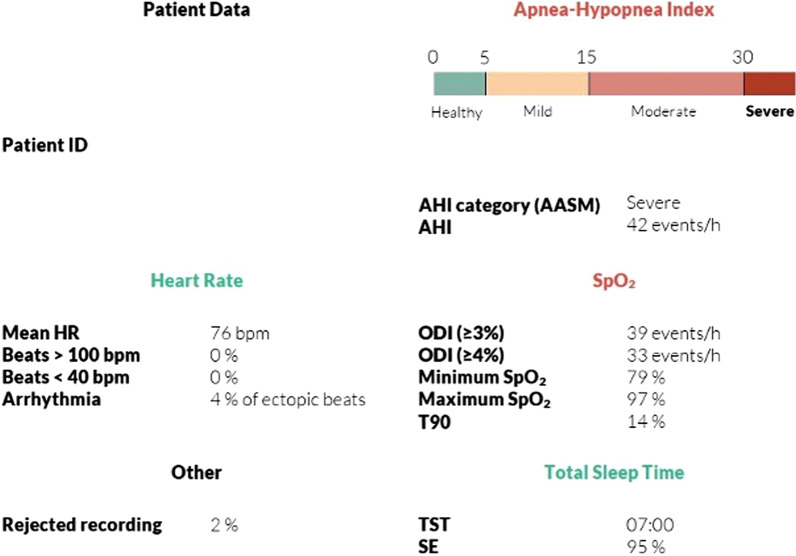
The NightOwl™ software uses an algorithm based on the simultaneous analysis of PPG-derived physiological events, such as PAT, respiratory effort, and SpO2. From these parameters, the software can derive the main clinical parameters used for sleep apnea diagnostics (ODI, AHI among others). As such, the NightOwl™ system can derive all diagnostic parameters recommended by The American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events for home sleep apnea testing [23] (Fig. 3).
Fig. 3.
NightOwl™ Data Output (sample). The NightOwl™ derives all parameters sufficient to diagnose sleep apnea
The product complies with Medical Device Directive 93/42/ECC and amendments 2007/47/EC requirements. The product is CE marked i.e. the product is authorized for clinical use. Instructions of use and complete product description can be found at http://www.ectosense.com/eifu.
Data captured from NightOwl™ comprises data from four sleep evaluations and consists of the parameters AHI, ODI, heart rate and sleep time. The AHI is graded into four different classifications, them being without sleep apnea (AHI < 5), mild sleep apnea (5 < 15), moderate sleep apnea (15 < 30) and severe sleep apnea (> 30). Thus, NightOwl™ defines sleep apnea as AHI > 5. The data captured from the four nights is to be presented in average quantities thereby counteracting potentially variations in the participants’ sleep data from night to night.
Cardiorespiratory monitoring
The CRM for the 20 first participants and for the participants where the home test is showing AHI > 15 (moderate-to-severe sleep apnea) is provided by the Department of Pulmonology, Herlev-Gentofte University Hospital and will quantify the frequency, duration and type of each sleep apnea event (obstructive or central) by measuring AHI, ODI, oximetry distribution and heart rate. Potential treatment for sleep apnea will be at the discretion of the attending sleep apnea physician. Simultaneously with undergoing the CRM, the participants will also be assessed by the NightOwl™ the same night. This simultaneity in the recording by the two different monitoring-methods allows for a direct analysis of the correlation of AHI (and other parameters) measured by CRM and NightOwl™.
Statistical analysis
The prevalence will be calculated by the proportion of participants with sleep apnea out of all participating individuals. The differences between patients with and without a diagnosis of sleep apnea (i.e. AHI > 5) will be assessed by the independent sample t test for continuous variables and the χ2 test for categorical variables. For the participants undergoing CRM, accuracy measurements of the home-monitoring of the NightOwl™ will be conducted by correlation analyses including scatter plots based on AHI measured by CRM versus AHI measured by NightOwl™ and receiver operator characteristics (ROC) with area under the curve (AUC) values.
As an exploratory analysis, a multivariate logistic regression analysis will be performed to test the predictors of a set of factors to detect the presence of any sleep apnea and presence of severe sleep apnea. These include age, body mass index (BMI), sex, neck circumference, European Heart Rhythm Association (EHRA) score (0–4), type of AF (new AF, paroxysmal, persistent AF, permanent AF), prior treatment for AF, blood samples, echocardiographic data and electrocardiogram data. Similar analysis using a multivariate logistic regression will evaluate if commonly used sleep apnea questionnaire can predict presence of sleep apnea in these patients. Correlation analyses will also be conducted of the clinical feasibility questionnaires.
Power calculations
Assuming a prevalence of severe sleep apnea of 10% in the population compared to 4% in the general population, with alpha 0.05 and a power of 80% a sample size of 113 was calculated. We plan to include 150 patients due to drop-outs, and potentially difficulties of patients applying the device.
Missing data
Bias due to missing data will be investigated by comparing baseline characteristics of participants with and without missing values. Depending on the extent of missingness, the predictors of missing values will be identified. Multiple imputation using chained equations shall be considered as part of a sensitivity analysis for missing data in the primary outcome model.
Discussion
This study will estimate the prevalence of unrecognized sleep apnea in patients with AF referred for anticoagulation. Furthermore, we aim to test whether a miniaturized simple home-monitoring device for detecting sleep apnea potentially can evaluate sleep apnea more conveniently and easier. Currently, patients with AF rarely undergo sleep evaluation due to limited resources, lack of standardized evaluation, lack of efficient screening tools, and cumbersome diagnostics. By making it easy and available to evaluate sleep apnea patients with AF, the prevalence of sleep apnea can be determined. Additionally, there is need for better prediction tools and risk scores to identify patients with AF and sleep apnea, by gathering extensive baseline characteristics and clinical data, we hope to be able to identify important predictors for sleep apnea in this population and can evaluate the usefulness of implemented questionnaires in this population when screening and evaluating symptom burden for sleep apnea. In addition, using such a device as the NightOwl™, patients can potentially be more independent of nurse or physician involvement to perform data collection, and clinical assessment and dialogue with patients can be prioritized.
Such cross-sectional studies do not come without limitations. Bias sample selection could apply; only patients with AF from anticoagulation clinic is asked to participate, which could limit the generalizability of our results. We do not assess prevalence of sleep apnea by gold standard of PSG but by CRM, therefore there may be higher risk of false negative and false positives. Using a smart-device such as NightOwl™ also have limitations as the device does not distinguish between central- and obstructive sleep apnea. However, the CRM validation can estimate the prevalence of central- and obstructive sleep apnea in those patients. Additionally, patients can potentially have a high amount of unrecorded data not being able to correctly determine if sleep apnea is present. The four nights of recording should limit this bias. For our secondary outcome of predictors of sleep apnea, we cannot infer causality or temporality since all data is observational and collected at first visit.
Conclusion
DAN-APNO is a cross sectional study intending to determine the prevalence of unrecognized sleep apnea in patients with AF referred for anticoagulation therapy using a miniaturized easy-to-use tool to diagnose sleep apnea. The results gained from this study will likely improve sleep evaluation and potentially inform future clinical guidelines on prevalence and predictors of sleep apnea in patients with AF.
Acknowledgements
Not applicable
Abbreviations
- AF
Atrial Fibrillation
- AUC
Area Under the Curve
- AHI
Apnea-Hypopnea Index
- BMI
Body Mass Index
- CPAP
Continuous positive airway pressure
- CRM
Cardio-Respiratory Monitoring
- CUMACF
CATCHET Unified Method for Assessment of Clinical Feasibility
- EHRA
European Heart Rhythm Association
- ESS
Epworth Sleepiness Scale
- ODI
Oxygen Desaturation Index
- OSA
Obstructive sleep apnea
- PAT
Peripheral Artery Tone
- PPG
Photoplethysmography
- PSG
Polysomnography
- ROC
Receiver Operator Characteristics
- SpO2
Saturation of Peripheral Oxygen
Authors' contributions
MHJ, FD and ML designed the study. ML and FD conceived the original idea. ML supervises the project. MHJ, ML, RRL and VG carried out the implementation. All authors read and approved the final manuscript.
Funding
This work was supported by Helsefonden (grant number: 20-B0269) and Ectosense supplied the home-monitoring devices (NightOwl™). The sponsors will have no influence on the design, protocol, statistical analyses, interpretation of findings, or decision for journal submission.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The project has been approved by The Committees on Health Research Ethics in the Capital Region of Denmark (registration number: H-20047552) and the Danish Data Protection Agency (registration number: P-2021-57).
Participants will be contacted and asked of participation in the research project by a local investigator or a project nurse as part of their standard check at the anticoagulation outpatient clinic at Herlev and Gentofte Hospital. The conversation will include verbal participant information about the research project and about the right of time to consider recruitment. The written participant information will be handed to the participants prior to the verbal information, and it will be handed to the participants by a researcher or a project nurse with thorough knowledge to the project.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea. JAMA. 2003;290(14):1906. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todd K, McIntyre WF, Baranchuk A. Obstructive sleep apnea and atrial fibrillation. Nat Sci Sleep. 2010;2:39–45. doi: 10.2147/nss.s7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 5.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31(2):203–220. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Tung P, Anter E. Atrial fibrillation and sleep apnea: considerations for a dual epidemic. J Atr Fibrillation. 2016;8(6):1283. doi: 10.4022/jafib.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Culebras A. Sleep apnoea and stroke. Stroke Vasc Neurol. 2016;1(4):185–191. doi: 10.1136/svn-2016-000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother. 2018;18(7):523–531. doi: 10.1080/14737175.2018.1489239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108(1):47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 10.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 11.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2015;169(5):647–654.e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Monahan K, Brewster J, Wang L, Parvez B, Goyal S, Roden DM, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012;110(3):369–372. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalgaard F, North R, Pieper K, et al. Risk of major cardiovascukar and neurologic events with obstructive sleep apnea among patients with atrial fibrillation. Am Heart J. 2020;223:65–71. doi: 10.1016/j.ahj.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 15.Chan MTV, Wang CY, Seet E, Tam S, Lai HY, Chew EFF, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788. doi: 10.1001/jama.2019.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broughton C, Piracha S, McGibbon K, Phan T, Allen MB. 15Is sleep apnoea unrecognised in patients with atrial fibrillation? EP Eur. 2018;20(suppl_4):iv7–8. [Google Scholar]
- 17.Abumuamar AM, Dorian P, Newman D, Shapiro CM. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol. 2018;41(5):601–607. doi: 10.1002/clc.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadhim K, Middeldorp ME, Elliot AD, et al. Self-reported daytime sleepiness and sleep-disordered breathing in patients with atrial fibrillation: SNOozE-AF. Can J Cardiol. 2019;35(11):1457–1464. doi: 10.1016/j.cjca.2019.07.627. [DOI] [PubMed] [Google Scholar]
- 19.Rosen IM, Kirsch DB, Chervin RD, Carden KA, Ramar K, Aurora RN, et al. Clinical use of a home sleep apnea test: an American Academy of sleep medicine position statement. J Clin Sleep Med. 2017;13(10):1205–1207. doi: 10.5664/jcsm.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massie F, de Almeida MD, Dreesen P, Thijs I, Vranken J, Klerkx S. An evaluation of the nightowl home sleep apnea testing system. J Clin Sleep Med. 2018;14(10):1791–1796. doi: 10.5664/jcsm.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardram, J. E. CACHET Unified Methodology for Assessment of Clinical Feasibility. Technical report. Copenhagen, Denmark: Copenhagen Center for Health Technology, 2018. Available from http://www.cachet.dk/research/cumacf [pdf]
- 22.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79(2):387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 23.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–6. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.