Abstract
Substantial evidence suggests that 7,12-dimethylbenzanthracene (DMBA)-induced mammary carcinogenesis in mice mimics human breast cancer (BC) in many respects. It has therefore been used extensively to evaluate preventive and therapeutic agents for human BC. Mammary carcinogenesis induced by DMBA administration in female SENCAR (SENsitive to CARcinogen) mice was characterized by histopathological analysis of the mammary glands and alterations to the PI3K/AKT/CDK1 pathway. We recently reported that 2’-hydroxyflavanone (2HF) is a promising diet-derived chemotherapeutic agent that suppresses BC growth in vitro and in vivo by targeting RLIP. The objective of the current study was to investigate the synergistic anti-carcinogenic effects of RLIP inhibition/depletion and 2HF in an in vivo model of DMBA-induced mammary carcinogenesis in SENCAR mice. Mice were given 2HF (50 mg/kg, b.w., orally on alternate days), RLIP-antibody (Rab; 5 mg/kg, b.w., i.p. weekly), RLIP-antisense (RAS; 5 mg/kg, b.w., i.p. weekly), or a combination of 2HF+Rab+RAS. Animals were monitored daily, and 7 days after the first appearance of moribund behavior, tissues were harvested for morphological and immuno-histological analysis. Western-blot analyses were performed to determine expression of anti- and proapoptotic proteins in the mammary glands. Our results reveal that 2HF, RAS, and Rab significantly prevented the carcinogenic effects of DMBA administration in the mammary glands and other organs. Further, mice treated with a combination of 2HF+RAS+Rab exhibited no carcinogenic effect of DMBA as compared to either or the single agent-treated mice. This study demonstrates for the first time the anti-carcinogenic effects of 2HF and RLIP-inhibition/depletion in vivo in a novel DMBA-induced model of BC in SENCAR mice and provides the rationale for further clinical investigation.
Keywords: RLIP, mammary gland, phytochemicals, DMBA, mercapturic acid pathway transporter, chemotherapeutics
One Sentence Summary:
Our results suggest for the first time that 2HF combined with RLIP inhibition/depletion has the potential to more effectively suppress DMBA-induced mammary carcinogenesis in SENCAR mice than either treatment alone.
Introduction
Breast cancer (BC) is the most frequently diagnosed malignancy in women worldwide. Polycyclic aromatic hydrocarbons (PAH) are a well-studied class of environmental contaminants that are implicated as human carcinogens (1–5). Human exposure to PAH can occur in the workplace, through ambient airborne exposure, in the food supply, or through lifestyle exposures such as smoking. This suggests that the most common mode of human exposure is likely through multiple, relatively low-dose exposures, occurring sporadically or chronically. This mode of exposure has been mimicked in classical mammary carcinogenesis models in mice administered 7,12-dimethylbenzanthracene (DMBA) (1–6). However, mechanism underlying the induction of mammary carcinogenesis in rodents exposed to chemical carcinogens is not well understood. The carcinogen is believed to cause a mutational event, referred to as initiation, whereas systemic factors, including hormones and other growth-enhancing factors, are responsible for the promotion of cancer growth and progression (1, 4–6).
Although natural products are a promising addition to current toxic anticancer drugs, there are several major obstacles to the successful use of individual nutritional compounds as preventive or therapeutic agents, including low efficacy and bioavailability. One approach to overcoming these problems is using combinations of nutrients to induce synergistic effects. Given that the human diet consists of multiple nutrients, it is likely that nutrients in the diet act synergistically to provide health benefits. In fact, human diets routinely encompass many biologically active small molecules, and evidence for synergy between dietary compounds is emerging (7). 2’-Hydroxyflavanone (2HF) is a natural compound with chemotherapeutic properties found in consumable citrus fruits (8). Previous studies report that 2HF inhibits the growth of SW620 and HCT116 human colon cancer cells and MCF7 BC cells via induction of caspase-mediated apoptosis (9). 2HF suppresses in vivo lung cancer metastasis in immune deficient nude mice (BALB/c nu/nu mice) and has been found to inhibit proliferation of A549 human lung cancer cells in vitro by arresting them in the G2/M phase of the cell cycle (8, 10).
BC is a myriad of diseases with various phenotypes. Clinically, BCs are subdivided according to estrogen receptor (ER) and oncogenic HER2 (human epidermal growth factor receptor 2) status. Progesterone receptor (PR) is another molecular marker that is used to predict a lack of response to hormone therapy (11). More recent studies using global gene expression profiling with widely available microarray techniques describe distinct molecular subtypes of BC, each defined by a large number of genes (12). These include basal-like, HER2-enriched, normal-like, luminal A, and luminal B subtypes. This classification has been further refined and now includes a set of 50 representative genes known as “PAM50” genes (13). The refined classifications align with the established clinical and histology-based classifications, with basal-like representing ER−/HER2−cancers, HER2 enriched representing ER−/HER2+, and normal-like and luminal A/B subtypes representing ER+. Considering this diversity, it is expected that no single therapeutic agent or dietary supplement will be effective for all malignant subtypes. The protein SERPINB5 (also known as maspin, mammary gland-associated serine protease inhibitor) is a proapoptotic tumor suppressor that is completely suppressed in most BCs but re-expressed upon anticancer treatment (14). In contrast, the protein BIRC5 (also known as survivin), belongs to the inhibitors of apoptosis protein (IAP) family, which is mostly absent from well-differentiated, normal adult tissues, but is over-expressed in nearly all human cancers (15).
RLIP, a stress-responsive, multi-functional protein with multi-specific capacity to transport glutathione-electrophile conjugates (GS-E) and chemotherapeutic agents, is frequently overexpressed in malignant cells (16–21). The inhibition and/or depletion of RLIP is known to exert potent anti-proliferative effects in lung, colon, breast, and kidney cancers and melanoma (16, 22). As yet, no oncogene knockdown has been reported to abrogate spontaneous carcinogenesis in rodent p53 homozygous knockout models. However, pharmacologically mediated partial suppression of RLIP prevents the appearance of lymphoma and other malignancies in p53 knockout homozygous mice (23). Recently, we showed that the combination of 2HF and RLIP-targeting agents exert a synergistic anticancer effect in BC cell lines. RNA sequencing data demonstrated that the expression of genes involved in apoptosis, inhibition of metastasis, and cell adhesion were upregulated upon combination treatment, whereas genes involved in cancer development and progression, metastasis, and cell cycle progression were downregulated (24). RLIP, an oncoprotein, is over-expressed in several solid tumors (16–22). 2HF downregulates RLIP expression and inhibits RLIP-mediated transport of doxorubicin in BC cell lines (24, 25). We also showed that RLIP is essential for tumor formation and that RLIP knockout homozygous (RLIP−/−) mice are resistant to DMBA/phorbol ester-induced skin carcinogenesis (26).
Mammary tumors have been induced by chemical carcinogens in many different strains of mice. DMBA has been found to be the most potent carcinogen and generally results in ~70% incidence in mice (27). In this study, we used an established DMBA-induced SENCAR (SENsitive to CARcinogen) mouse model to assess the in vivo role of RLIP in the mammary carcinogenesis. This paper therefore describes the synergistic effects of the dietary phytochemical 2HF and RLIP-targeting therapy on DMBA-induced mammary tumor incidence in SENCAR mice and on the utility of the SENCAR mice for short-term studies of mammary carcinogenesis.
Materials and methods
Materials
2HF (purity ∼99%), DMBA, horseradish peroxidase (HRP)-conjugated anti-mouse, and anti-rabbit secondary antibodies, and MTT were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against pAKT (S473), PCNA, CD31, Ki67, CDK1, Bcl2, maspin, survivin, Bax, vimentin, and E-cadherin antibodies were purchased from Santa Cruz Biotechnology (Columbus, OH) and Cell Signaling Technologies (Danvers, MA). The avidin/biotin complex detection kit was procured from Vector (Burlingame, CA). Mayer’s hematoxylin, methyl salicylate, and Permount were purchased from Fisher Scientific (Hanover Park, IL). The source of RLIP antibodies was the same as previously described (16).
RLIP76 antisense preparation
Chemically synthesized phosphorothioate DNA in desalted form was purchased from Biosynthesis, Inc., (Lewisville, TX). A 21-nucleotide-long scrambled phosphorothioate DNA was used as a control (16).
Animals
One week after they were received, SENCAR mice (female, 3 weeks old, 002746 – SENCARA/PtJ; The Jackson Laboratory, Sacramento, CA) were randomly divided into seven groups (10 mice/group) for treatment consisting of 1) corn oil, 2) 2HF (50 mg/kg b.w. by oral gavage on alternate days), 3) scrambled antisense (CAS), 4) RLIP-antisense (RAS; 5 mg/kg b.w., i.p. weekly), 5) pre-immune serum (PIS), 6) RLIP-antibody (Rab; 5 mg/kg b.w., i.p. weekly), or 7) a combination of 2HF+RAS+Rab for 2 weeks prior to tumor induction. Mice continued on the corresponding treatment and were weighed every week throughout the study. Starting at six weeks of age, the mice were gavaged with 200 μl of DMBA (1 mg/ml in corn oil) once per week for six consecutive weeks. Animals were monitored daily, and the first day of moribund behavior was recorded. Mice were anesthetized using isoflurane seven days after the first appearance of moribund behavior, and tissues were harvested for morphological, immunohistological, and Western blot analyses. Mammary glands were also examined in a time course to monitor mammary tumor initiation and progression by whole mount staining and by sectioning and hematoxylin and eosin (H&E) staining of paraffin-embedded tissues. All mice in the experiment were euthanized within a week, 14 weeks after the first DMBA application, and a thorough examination was performed. A portion of the tissues was paraffin-embedded for immuno-histology for RLIP, Ki67, CD31, E-cadherin, and vimentin expression and histological evaluation by H&E stain. The use and treatment of SENCAR mice was approved by the Institutional Animal Care and Use Committee (IACUC), City of Hope National Medical Center, and the experiments were conducted in strict compliance with IACUC regulations.
Whole breast mount
The entire intact lower abdominal mammary gland (#4) was dissected, gently spread flat onto a glass slide, briefly dried, and fixed overnight in 10% neutral-buffered formalin. Following fixation, the gland was dehydrated in 70% and then 100% ethanol, and fat was removed by successive baths in xylene. The tissue was rehydrated in baths of 100% then 70% ethanol, rinsed in running tap water, and stained with Mayer’s hematoxylin. The stained gland was then rinsed in running tap water, followed by 1% HCl, and again in running tap water. The duct system within the gland should be stained dark blue. The stained gland was placed in 70% ethanol until photographed using a Leica MZ 10F Stereomicroscope (Chicago, IL). Slides were then placed in methyl salicylate for long term storage (28).
Histopathological examination of tissues for markers of differentiation, proliferation, and angiogenesis
Mice were euthanized by CO2 asphyxiation and their mammary glands and other tissues were evaluated for carcinogenicity. Immediately after euthanasia, blood from the right ventricle was obtained for hematological examination. Tissues from control (corn oil, CAS, and PIS) and experimental (2HF, RAS, Rab, and a combination of 2HF+RAS+Rab) treatment groups were fixed in 10% buffered formalin for 12 h. Paraffin-embedded 5 μm-thick tissues sections were prepared for histological evaluation. H&E staining to assess hyperplasia was performed on paraffin-embedded mammary gland, duodenum, spleen, bone marrow, brain, heart, kidney, liver, and lung sections. Mammary carcinogenicity was classified histologically according to the criteria outlined by Russo (29). The histopathological criteria used to determine malignancy were loss of tubular-alveolar pattern of the normal mammary gland; presence of large epithelial cells with increased nuclear-cytoplasmic ratio and increased frequency of mitosis; stromal response by fibrosis and inflammatory cell infiltration; necrosis and hemorrhage; and evidence of infiltration of surrounding tissues and metastasis.
Histopathologic analysis of proteins, such as E cadherin and vimentin to analyze the epithelial-mesenchymal transition (EMT), CD31 to visualize blood vessels, and Ki67 and RLIP to assess cell proliferation, was performed in mammary gland sections using a universal ABC detection kit (Vector). Immuno-reactivity is evident as a dark brown stain, whereas non-reactive areas display only the background color. Photomicrographs at 40x magnification were acquired using an Olympus DP72 microscope. Percent staining was determined by measuring positive immuno-reactivity per unit area. The intensity of antigen staining was quantified by digital image analysis using DP2-BSW software.
Western blot analysis
Mammary gland tissue lysates were prepared from DMBA-administered mice that received control treatments (corn oil, CAS, or PIS) and experimental treatments (2HF, RAS, Rab, or 2HF+RAS+Rab) and were processed for Western blot analysis, as we described previously (25). Briefly, tissue lysates containing 50 μg protein were resolved by SDS-PAGE and transferred onto nitrocellulose membranes for Western blot analyses. Blots were incubated with primary antibodies and secondary antibodies conjugated with HRP and developed using the enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ). Expression levels of the desired proteins were determined by densitometric scanning of the immuno-reactive bands. Nitrocellulose membranes were stripped for 30 minutes at 50 °C in 62.5 mM Tris–HCl (pH 6.8) containing 2% SDS and 100 mM β-mercaptoethanol. Stripped blots were washed 5 times in TBST, blocked, and reprobed with a different antibody. Equal loading of proteins was confirmed by stripping and re-probing the membranes with β-actin antibodies.
Statistical analysis
The data was evaluated using two-tailed unpaired Student’s t test and results are expressed as the mean ± SD. Changes in tumor size and body weight during the course of the experiments were visualized by scatter plot. The statistical significance of differences between control and treatment groups was determined by ANOVA followed by multiple comparison tests. Differences were considered statistically significant when the p value was < 0.05.
Ethics statement
No human subjects were involved in the present study. All animal studies were approved by the City of Hope Animal Care and Ethics Committee. Any mice showing signs of distress, pain, or suffering due to tumor burden were humanely euthanized.
Results
Recently, we showed that 2HF is a promising diet-derived chemotherapeutic agent that targets RLIP in BC. We also demonstrated the inhibitory effects of 2HF on BC cell growth in vitro and in vivo using an array of BC lines and xenograft mouse models of ER-positive, HER2-positive, and triple negative BC cells. 2HF treatment reduced cell viability and suppressed the migratory and invasive potential of BC cells. Further, 2HF inhibited the expression of RLIP, a stress-defensive and anti-apoptotic protein that is over-expressed in BC cells, and simultaneously reduced proliferation of BC cells (25, 30). The cytotoxic effects of RLIP inhibition via RLIP antibodies or depletion via RLIP antisense on BC cells was also investigated using an MTT assay (25, 30). Here, we used a novel SENCAR mouse model to study the carcinogenic effects of DMBA on the mammary gland. Recently, Siddiqui et al. (31) established a protocol for DMBA administration, depending on the age of the animals and number of applications of the carcinogen, with the aim of investigating the advanced stages of mammary gland carcinogenesis. We used the same protocol as Siddiqui, and the results are presented and discussed regarding the suitability of the DMBA-induced mammary gland carcinogenesis SENCAR mouse model for evaluating the effects of 2HF and RLIP-targeting therapy as preventive and therapeutic agents.
Mouse Growth
Administration of DMBA did not induce acute toxicity in any mice. The body weights of all mice increased up to the end of the experiment. No significant differences in body weight gain were observed among the groups. Ten weeks after the first DMBA application, one mouse in the control treatment group became moribund and was humanely euthanized.
Hematological Results
Knockout or inhibition of RLIP by antisense and/ or antibody administration is known to inhibit the progression of multiple cancers (16–23), decrease insulin resistance, and minimize the risk for metabolic syndrome, obesity, and type II diabetes mellitus (32–34). In this regard, we assessed the effects of 2HF in vivo following oral administration to SENCAR mice to investigate the tolerance and characteristic blood chemistry parameters in comparison to RLIP depletion by antisense. The mice were administered either DMBA, corn oil, 2HF (50 mg/kg b.w. by oral gavage), scrambled antisense, RLIP76 antisense (5 mg/kg. b.w. i.p.), or in combination for six-weeks. The blood was analyzed, and the values of red blood cells (RBC), hemoglobin, hematocrit, and mean corpuscular hemoglobin concentration (MCHC) were slightly lower in the DMBA-administered group than in the control group, indicating a tendency to anemia. On the other hand, the number of white blood cells (WBC) was significantly higher in the DMBA-administered group than in the control group. The number of neutrophils was also significantly higher in the DMBA group, indicating an inflammatory response in animals with DMBA-induced carcinogenesis. 2HF was tolerable and no significant toxic effects were observed on blood cell parameters, including total RBC, WBC, platelet, hemoglobin, and hematocrit values, as compared to RLIP antisense-treated groups and controls (Table 1).
Table 1.
Regulation of blood chemistry parameters in control and DMBA-administered mice, following 2HF and RLIP antisense treatment.
| Blood Profile* | Corn oil (control) |
DMBA | 2HF | DMBA + 2HF |
Scrambled antisense |
RLIP antisense |
DMBA + RLIP antisense |
|---|---|---|---|---|---|---|---|
| RBCs (106/μL) | 8.8 ± 1.8 | 7.2 ± 1.7 | 8.9 ± 2.4 | 8.3 ± 2.1 | 8.5 ± 1.7 | 8.1 ± 1.9 | 7.9 ± 2.2 |
| WBCs (103/μL) | 7.5 ± 1.7 | 10.4 ± 2.4** | 7.3 ± 1.5 | 8.1 ± 2.7 | 8.2 ± 2.1 | 7.8 ± 1.6 | 8.5 ± 2.4 |
| Platelets (103/μL) | 722 ± 129 | 888 ± 154 | 645 ± 127 | 752 ± 129 | 704 ± 132 | 618 ± 112 | 686 ± 138 |
| Hemoglobin (g/dL) | 12.9 ± 1.4 | 10.1 ± 1.8 | 12.5 ± 1.8 | 12.1 ± 2.1 | 12.4 ± 2.1 | 11.3 ± 1.7 | 11.1 ± 1.9 |
| Hematocrit (%) | 38.6 ± 8.7 | 31.2 ± 5.9 | 41.1 ± 3.6 | 40.3 ± 7.2 | 40.2 ± 6.7 | 37.7 ± 4.4 | 36.8 ± 4.7 |
| MCHC (g/dL) | 32.8 ± 3.9 | 30.4 ± 3.1 | 32.1 ± 4.3 | 32.2 ± 2.7 | 32.9 ± 4.2 | 31.6 ± 3.2 | 31.1 ± 2.8 |
| Neutrophil (%) | 21.9 ± 6.1 | 33.7 ± 7.9** | 23.8 ± 4.4 | 24.1 ± 5.2 | 22.8 ± 3.9 | 23.7 ± 3.3 | 23.9 ± 5.3 |
n = 3 mice/group;
Represents significant difference from the control (p < 0.03)
Mammary carcinogenesis
Female SENCAR mice were highly sensitive to the carcinogenic action of DMBA. To determine the effects of DMBA administration on SENCAR mice, seven groups of mice received 200 μg DMBA by oral gavage, once a week for 6 weeks, beginning two weeks after the initiation of treatment with: 1. corn oil, 2. 2HF, 3. CAS, 4. RAS, 5. PIS, 6. Rab, or 7. a combination of 2HF+RAS+Rab. By the end of the experiment, one mouse in the CAS group and one mouse in the PIS group were dead due to non-neoplastic disease. None of the mice died due to acute toxicity. Weight gain was slightly decreased in CAS group, but not significantly different than that of the other groups during treatment with DMBA. Weight gain decreased slightly in the RAS group at week 5, but it recovered to normal quickly after the cessation of treatment. Mice were anesthetized using isoflurane seven days after the first appearance of moribund behavior, and tissues were harvested for morphological, immunohistological, and Western blot analyses. Mammary glands were also examined in a time course to monitor mammary tumor initiation and progression by whole mount staining (Fig. 1) and by sectioning and H&E staining the paraffin-embedded tissues (Fig. 2). The mammary carcinogenesis study was terminated at ~8 weeks after the last DMBA administration.
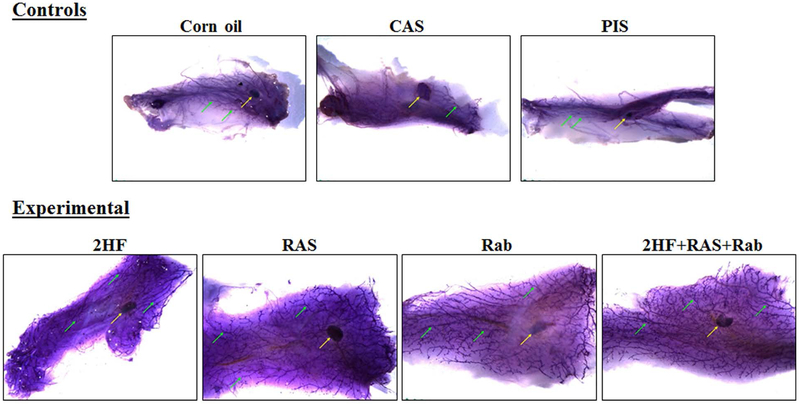
Figure 1. Whole mount preparations of mammary gland fat pads from SENCAR mice.
Mammary gland fat pads, excised from DMBA-administered SENCAR mice that received a control treatment (A, corn oil; B, CAS; C, PIS) or an experimental treatment (D, 2HF; E, RAS; F, Rab; G, a combination of 2HF+RAS+Rab), and stained with Mayer’s hematoxylin. Mammary gland fat pads from the control treatment groups were slightly small and edematous, making it difficult to achieve adequate spread on glass slide. The lymph nodes associated with the fat pads were present in all groups (yellow arrow). The development of the duct system (green arrows) was markedly attenuated in all control treatment groups when compared with any of the experimental treatment groups. Fat pads in all of the experimental treatment groups are similar to each other. The stained glands were placed in 70% ethanol until photographed using a Leica MZ 10F Stereomicroscope (Chicago, IL). Abbreviations: 2HF, 2'-hydroxyflavanone-treated; CAS, scrambled antisense-treated; RAS, RLIP antisense-treated; PIS, preimmune serum-treated; Rab, RLIP antibody-treated.
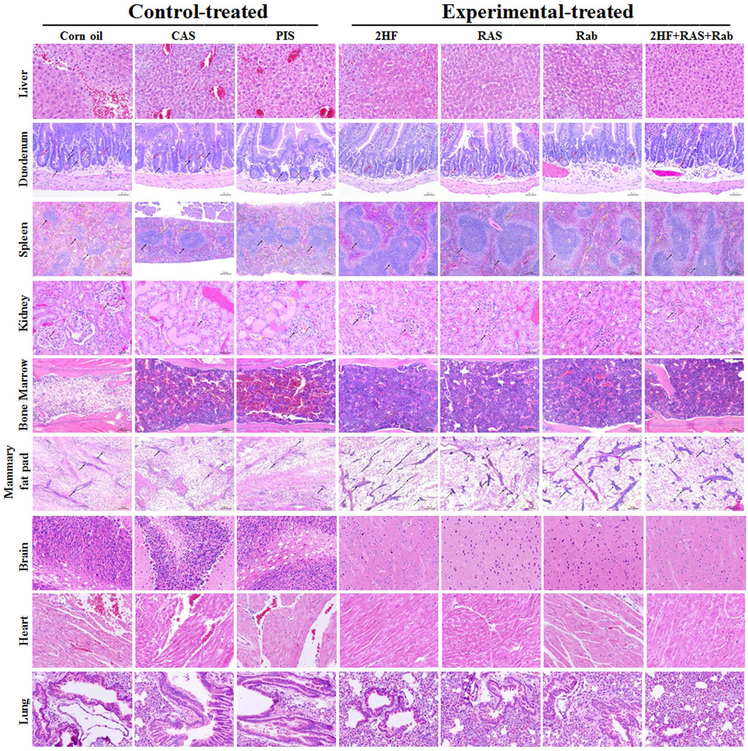
Figure 2. Effect of RLIP-targeting treatments on tissue morphology in DMBA-administered SENCAR mice.
Tissue sections from DMBA-administered female SENCAR mice that received a control treatment (corn oil, CAS, or PIS) or an experimental treatment (2HF, RAS, Rab, or a combination of 2HF+RAS+Rab) were used for H&E staining. Hemorrhagic areas involving the liver, duodenum, spleen, kidney, bone marrow, mammary gland, brain, lung, and heart tissues were observed in the corn oil-, CAS-, and PIS-treated mice whereas tissues from 2HF-, RAS-, Rab-, and combination-treated mice were histologically normal. Representative images at x400 magnification are shown. 2HF, 2'-hydroxyflavanone-treated; CAS, scrambled antisense-treated; RAS, RLIP antisense-treated; PIS, preimmune serum-treated; Rab, RLIP antibody-treated.
Effects of 2HF and RLIP-targeting agents on breast development
DMBA is a known chemical carcinogen that causes DNA damage and induces tumorigenesis in leukemia and cancers of the mammary gland, ovary, and epidermis (35, 36). To examine the role of RLIP in chemical carcinogen-induced neoplasia, we treated female SENCAR mice with DMBA once weekly for 6 consecutive weeks and monitored DMBA-induced carcinogenesis. To determine whether the attenuated mammary ductal growth in DMBA-induced SENCAR mice was due to the function of RLIP in the mammary epithelial cells or an alteration of the systemic endocrine system, we conducted whole mount staining. We further investigated if the carcinogen had any influence on normal mouse breast development by preparing breast whole mounts (Fig. 1). The total length of breast tissue per gram body weight did not differ significantly among the treatment groups with or without DMBA-induced carcinogenesis. Also, the total width of breast tissue per gram body weight was not significantly different between mice that were or were not treated with DMBA. The lymph nodes associated with the fat pads were present in all groups. The development of the duct system was markedly attenuated in all control treatment groups when compared with that in any of the experimental treatment groups, and fat pads in all of the experimental treatment groups were similar. Whole-mount preparations of glands and H&E sections were scored for the presence of hyperplasia and neoplasia. The basic morphological features of mouse tissues were evaluated using H&E stain. The amount of fat and size of adipocytes was reduced in fat pads collected from control treatment groups when compared with those from experimental treatment groups. Additionally, the number of sectioned duct profiles (black arrows) was greatly reduced in the fat pads collected from control-treated mice when compared with in those from mice in any of the experimental treatment groups. The numbers of sectioned duct profiles were similar in mice across all of the experimental treatment groups (Fig. 2). These observations are consistent with observations in the whole mount preparations of mammary gland fat pads (Fig. 1). The amount of lymphoid tissue in the white pulp of the spleen (black arrows) was greatly reduced in control treatment groups when compared with that in the experimental treatment groups. Additionally, numbers of megakaryocytes (yellow arrows) and the amount of hemosiderin (yellow-brown, granular material) were greater in control treatment groups than in the experimental treatment groups. The apparent differences in megakaryocytes and hemosiderin may be relative rather than actual, reflecting the reduced size of spleens in mice receiving the control treatments. Spleens from all of the mice receiving one of the experimental treatments were similar to each other (Fig. 2). Apoptotic cells (black arrows) were commonly observed in the intestinal crypts of control treatment groups but were rarely seen in mice in any of the experimental treatment groups. Additionally, intestinal crypts of mice in control treatment groups contain more mitotic figures (red arrows) and the nuclei of crypt epithelium were enlarged and crowded (suggesting epithelial hyperplasia; yellow arrows) compared to those of mice receiving the experimental treatments (Fig. 2). The cellularity of bone marrow was markedly reduced in mice receiving corn oil treatment. The cellularity of bone marrow was also reduced in mice receiving the CAS and PIS treatments although less so than in the corn oil-treated mice. The bone marrow specimens from mice in the control treatment groups with those from mice in the experimental treatment groups all exhibited cellularity as expected in normal mice (Fig. 2). Glomeruli (black arrows) are marked in all photographs; those in the kidneys of mice receiving experimental treatments (2HF, RAS, or Rab) were morphologically unremarkable. In contrast, mice receiving control treatments (corn oil, CAS, or PIS) exhibited irregular cellularity and mesangial deposits of eosinophilic material in their glomeruli. Additionally, cells of Bowman’s capsule (red arrow) were prominent in the glomeruli of the mice treated with the PIS, and the glomeruli were surrounded by a rim of fibrous tissue. Dilated, protein-filled renal tubules lined with flattened epithelium (yellow arrows) were also seen in kidneys from mice receiving the CAS or PIS control treatments (Fig. 2). Histopathologic analysis of brain, lung, liver, and heart tissues did not reveal obvious histologic differences between groups. The histological appearance of the mammary glands in this study was in agreement with that reported for chemically-induced mammary tumors in other studies [27]. The frequency of mammary tumor incidence in DMBA-administered control treatment groups of SENCAR mice was significantly higher than DMBA-administered groups that received 2HF or RLIP-targeting agents (p < 0.05). The data presented in Figures 1 and 2 demonstrate that 2HF and RLIP-targeting agents significantly reduced the incidence of mammary carcinogenesis in DMBA-administered SENCAR mice. Thus, RLIP plays an autonomous role in the regulation of mammary ductal growth in the mammary epithelium. Taken together, these results indicate that inhibition or depletion of RLIP selectively protects the mammary glands from DMBA-induced carcinogenesis.
The Bcl2 gene family regulates tissue development and tissue homeostasis through the interplay of survival and death factors. Family members are characterized as either anti- or proapoptotic, depending on cellular context. In addition to its anti-apoptotic effect, Bcl2 also inhibits progression through the cell cycle (37, 38). Functional interactions between family members, as well as binding to other cellular proteins, modulate their activities. Mammary gland tissue expresses a number of different Bcl2 relatives, including bcl-x, bax, bak, bad, bcl-w, bfl-1, bcl2 as well as the bcl2 binding protein Bag1. Bcl2 expression in human BCs has been associated with a good prognosis, and decreased Bax expression has been linked to poor clinical outcomes (37). Next, mammary gland tissue from DMBA-administered mice that received controls or experimental treatments were assessed for markers of proliferation, apoptosis, and cell cycle progression by Western blot and immunohistochemical analyses.
RLIP inhibition downregulates the PI3K/Akt/cdk1 pathway in DMBA–induced mammary carcinogenesis
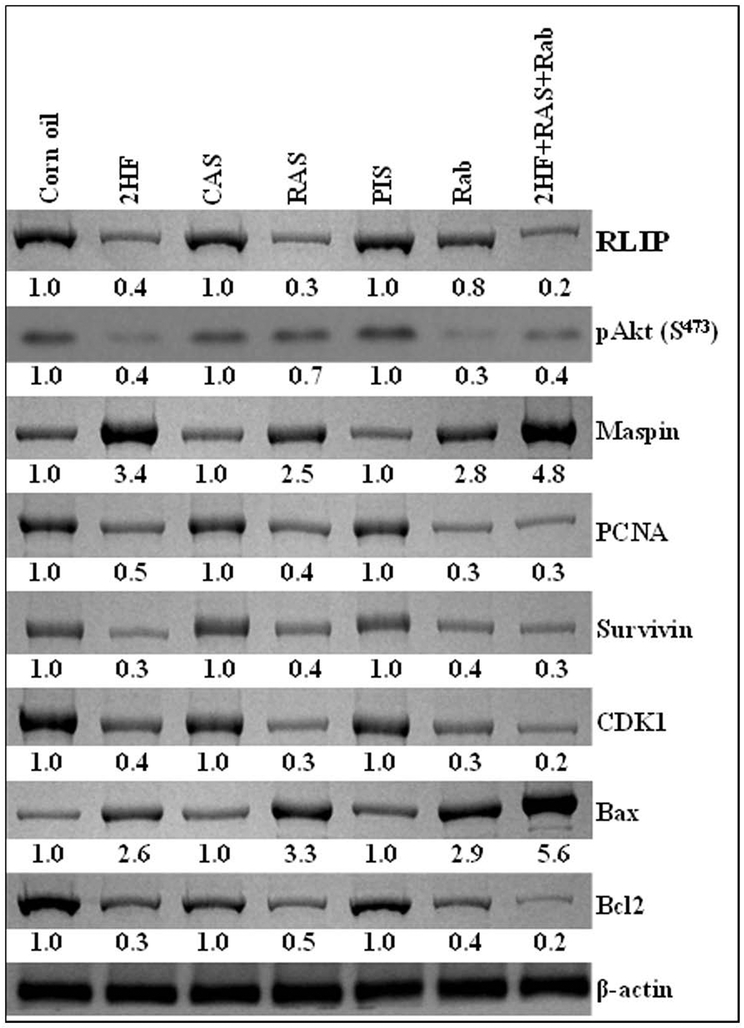
The PI3K/Akt signaling pathway plays a critical role in BC initiation and progression (6). To address the question of whether inhibition of RLIP suppresses DMBA-induced mammary carcinogenesis through alteration of the PI3K/Akt pathway, we examined several essential components of this signaling pathway. Low levels of the pro-apoptotic proteins Bax and maspin were detected in the mammary gland extracts, and much higher levels of the anti-apoptotic proteins Bcl2 and survivin were found in the mammary gland extracts of DMBA-administered mice in the control treatment groups than in those of mice in the experimental treatment groups. Total Akt protein expression in mammary glands tissues did not differ across groups. However, much more of the activated form of Akt, pAkt S473, was observed in the mammary glands tissues of DMBA-administered mice that received control treatment compared with those of mice treated with 2HF or RLIP-targeting agents. These results suggest that RLIP inhibition can suppress Bcl2 and survivin upregulation and inhibit Akt activation in DMBA-administered mammary glands tissues (Fig. 3). The cdk1 protein was also much higher in the mammary gland tissues of DMBA-administered mice that received control treatments than in those that received RLIP-targeting agents (Fig. 3). These results suggest that RLIP is required for PI3K/Akt signaling pathway-stimulated upregulation of cdk1 during DMBA-induced breast carcinogenesis initiation and progression.
Figure 3. Effects of 2HF and RLIP-targeting agents on protein expression in the mammary glands of DMBA-administered SENCAR mice.
Mammary gland fat pads excised from DMBA-administered SENCAR mice that received a control treatment (corn oil, CAS, or PIS) or an experimental treatment (2HF, RAS, Rab, or combination of 2HF+RAS+Rab) were analyzed for changes in the expression of RLIP, pAkt, maspin, PCNA, survivin, CDK1, Bax, and Bcl2. β-actin was used as a loading control. Numbers below the blots represent the fold change in the levels of protein expression, compared to expression in control tissues, as determined by densitometry. 2HF, 2'-hydroxyflavanone-treated; CAS, scrambled antisense-treated; RAS, RLIP antisense-treated; PIS, preimmune serum-treated; Rab, RLIP antibody-treated.
Effect of 2HF and RLIP-targeting agents on markers of cell cycle progression and cell proliferation
The effect of 2HF and RLIP-targeting agents was also examined on markers of cell cycle regulation (cdk1) and cell proliferation (PCNA) in the mammary glands of SENCAR mice upon DMBA-induced carcinogenesis. Both cdk1 and PCNA were up-regulated in the mammary glands of DMBA-administered mice that received control treatments. DMBA-administered mice treated with 2HF and RLIP-targeting agents exhibited significantly lower cdk1 and PCNA expression than mice that received control treatment, and this effect was more prominent in the glands of mice that received combination treatment (Fig. 3). Overall, 2HF and RLIP-targeting agents (antisense and antibodies) had significant effect on protein expression, including reduced expression of the anti-apoptotic protein Bcl2, survivin, cdk1, PCNA, and increased expression of the pro-apoptotic proteins Bax and maspin in the mammary gland tissues of DMBA-administered SENCAR mice. It is very intriguing to observe that the effects of the combination treatment were more pronounced than the effects of any single agent treatment (Fig. 3).
Histological Evaluation
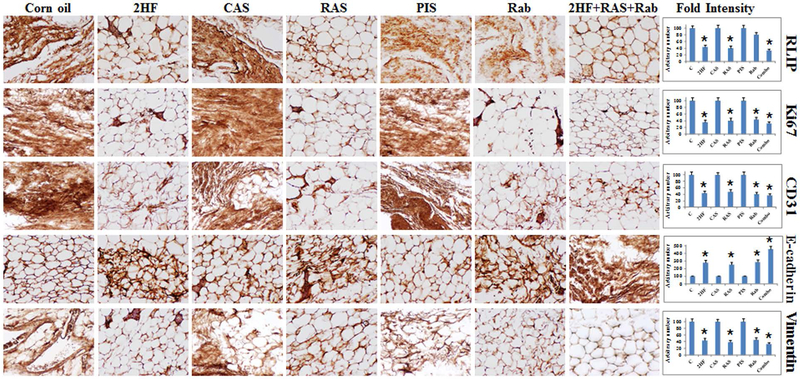
Complete autopsies and both gross and microscopic examinations were performed. Tissues were fixed in 4% paraformaldehyde, blocked in paraffin, sectioned at 5 μm, routinely H&E stained, and examined as indicated in the legend to Figure 2. The immuno-histochemical analysis of the mammary gland tissue sections of mice that received 2HF and RLIP-targeting agent showed lower levels of RLIP, the proliferation marker Ki67, and the angiogenesis marker CD31, as well as higher levels of the epithelial differentiation marker E-cadherin and lower levels of mesenchymal marker vimentin, as compared to sections from mice that received control treatments (Fig. 4). Taken together, our results indicate that 2HF exerts strong anticancer effects in BC by targeting RLIP. In summary, we showed that inhibition/depletion of RLIP in SENCAR mice significantly extended the latency and reduced the frequency of mammary tumor incidence induced by DMBA. RLIP deficiency attenuated the upregulation of pro-apoptotic proteins, as well as the downregulation of anti-apoptotic proteins, which might be underlie the suppression of DMBA-induced mammary carcinogenesis in SENCAR mice. The fact that inhibition/depletion of RLIP suppresses DMBA-induced mammary carcinogenesis in SENCAR mice suggests that RLIP is a potential target for the prevention and treatment of human BC.
Figure 4. Immunohistochemistry of mammary gland tissue from DMBA-administered SENCAR mice.
Immunohistochemistry was performed to detect RLIP, Ki67, CD31, E-cadherin, and vimentin expression in mammary gland tissue isolated from DMBA-administered mice that received a control treatment (corn oil, CAS, or PIS) or an experimental treatment (2HF, RAS, Rab, or 2HF+RAS+Rab). The intensity of antigen staining was quantified by digital image analysis using Pro Plus software. Bars represent mean ± S.E. (n = 5). One representative image for each treatment group is shown. *Statistically significant differences were determined by two-tailed Student’s t tests; p < 0.04, tissues from 2HF-, RAS-, and Rab-treated mice compared with their respective controls. 2HF, 2'-hydroxyflavanone-treated; CAS, scrambled antisense-treated; RAS, RLIP-antisense-treated; PIS, pre-immune serum-treated; Rab, RLIP antibody-treated.
Discussion
Several animal models of BC have been developed to study various aspects of BC biology. Induction of mammary carcinomas by DMBA in female SENCAR mice is one of the most frequently used animal models for the investigation of breast carcinogenesis and mammary tumor treatment (2–5). There is substantial evidence suggesting that the DMBA animal model mimics human BC in terms of tumor histopathology, origination from mammary ductal epithelial cells, dependence on ovarian hormones for tumor development, and altered expression of TGFβ, ERBB2, and cyclin D1 (6). The DMBA-induced breast carcinogenesis model has therefore been used extensively to evaluate preventive and therapeutic agents for human BC. For the present studies using SENCAR mice, DMBA was chosen from a variety of carcinogens known to induce mammary carcinogenesis because it is the most potent (2) and was the carcinogen used in a skin cancer study (39).
Utilizing the SENCAR mouse model, researchers showed that curcumin reduced tumor burden and the number of invasive tumors, and drastically increased the mean latency to the initial tumor in mammary tumor-bearing mice (31). Phytochemicals are non-toxic and have a wide range of biological activity, including anti-inflammatory, anti-proliferative, antioxidant, and anticancer properties. 2HF is a natural phytochemical extracted from oranges that has inhibitory and chemopreventive effects in malignant cell lines (7–10). In our recently published studies, we demonstrated the anticancer effects of 2HF in five BC cell lines, covering distinct receptor expression phenotypes: MDA-MB231 (ER− /PR− /Her2−), SKBR3 (ER− /PR− /Her2+), MCF7 and T47D (ER+ /PR+ /Her2−), and MDA-MB361 (ER+ /PR− /Her2+) (25, 30). We further demonstrated that the anticancer effect of 2HF was mediated through the inhibition/depletion of RLIP function/expression (24, 30). In our previous studies on MCF7, MDA-MB231, and SKBR3 cells, we determined that treating BC cells in vitro with a combination of 2HF and RLIP-targeting agents reflected a similar response in vivo. We therefore extended our studies in an in vivo model of DMBA-induced breast carcinogenesis in SENCAR mice.
The results of the present study demonstrate that treatment with 2HF, RAS, and Rab, individually or in combination, prevents the incidence of tissues injury and mammary carcinogenesis in DMBA-administered SENCAR mice. These findings are consistent with our previous in vitro and in vivo studies that demonstrated significant inhibition of angiogenic and invasive parameters in the human BC MDA-MB231, SKBR3, and MCF7 cell lines. Our previous studies also demonstrated that the anticancer effects of a mixture of 2HF, RAS, and Rab on BC cell lines both in vitro and in vivo was greater than that of the individual agent (30).
We also examined the morphological features of whole breast tissues from animals in different treatment groups (Fig. 1). Whole breast tissues in mice treated with 2HF or RLIP-targeting agents appeared to be more differentiated than those in mice in the control treatment groups. The mammary ducts of control treated animals exhibited less differentiation, with significantly lower density of terminal end buds (TEB), compared to those of animals treated with 2HF, RAS, and/or Rab. Animals receiving 2HF, RAS, and/or Rab treatment had well-differentiated breast tissues and the TEB density similar to that of control, non-DMBA-induced animals. No apparent difference was found in the size (length and width) of normal breast tissue in any group, indicating that the controls vehicles had no effect on breast development.
The pro-apoptotic proteins Bax, Bak and Bad, as well as the cell death-suppressors Bcl-x and Bcl2, are synthesized in the mouse mammary gland. Dynamic changes in the expression profiles of these proteins occur during development, suggesting that these changes may be involved in regulating post-lactational apoptosis (37). The intrinsic apoptotic signaling mediated by Bcl2 family members (e.g., Bax and Bcl2) is an important factor in mammary gland development, as well as in breast carcinogenesis. The interaction between Bax and Bcl2 dictates susceptibility to apoptosis and is implicated in BC prognosis and chemoprevention. Higher Bax:Bcl2 ratios indicate enhanced proapoptotic signaling or lower anti-apoptotic processes. In BC, Bax:Bcl2 ratios are usually low, reflecting low pro-apoptotic rates [40]. In the present study, we noted that Bax expression was lower and Bcl2 was higher in DMBA-administered SENCAR mice than in non-DMBA-induced control mice. Treatment of the DMBA-administered mice with 2HF and RLIP-targeting agent downregulated Bcl2 expression and up-regulated Bax levels (Fig. 3). Survivin, the smallest member (16 kDa) of the inhibitor of the apoptosis protein (IAP) family, which acts not only to inhibit apoptosis but also to control cell cycle progression. Survivin overexpression has been reported in nearly all human cancers, including BC (41, 42). Data presented in Figure 3 indicate that the mammary glands of DMBA-administered mice expressed substantial levels of survivin. These levels were not affected by either CAS or PIS treatment, but 2HF, RAS, and/or Rab treatment caused almost a 50% reduction in survivin expression. Disrupting survivin expression or function in cancer cells has been shown to decrease cell proliferation by enhancing apoptosis. Survivin has been considered an effective target for anticancer strategies in several preclinical and early-phase clinical trials (41–43).
The increase in PCNA during DMBA-induced carcinogenesis is usually accompanied by induction of genes/proteins involved in the cell cycle. An important player in cell cycle regulation is cdk1. It is required twice during the cell cycle, first in the G1 phase for the onset of S phase and again in G2 for the onset of mitosis (44). Increased activity and expression of cdk1 occur in several types of human cancer (45). Treatment of DMBA-administered mice with 2HF and RLIP-targeting agents, separately and in combination, downregulated cdk1 expression (Fig. 3). In summary, the present findings provide a rationale for the studying the effectiveness of 2HF-mediated suppression of RLIP in inhibiting DMBA-induced mammary carcinogenesis in the SENCAR mouse model of BC.
Conclusion
The DMBA-induced SENCAR mouse model of BC is widely used in research, mostly for evaluating preventive and therapeutic agents for human BC. RLIP suppression is the first oncoprotein-suppressive strategy that has resulted in dramatic suppression of spontaneous lymphomagenesis in p53−/− mice (23) and has been shown to suppress the growth, as well as cause regression, of xenografts of various types of solid malignancies (16–22). Present studies demonstrated that the synergistic effects of 2HF and RLIP-targeting agents prevent mammary carcinogenesis in DMBA-administered SENCAR mice. One possible mechanism for the synergistic effects of 2HF and RLIP-targeting agents on mammary carcinogenesis involves the enhanced expression of Bax and the suppression of survivin. Although clinical trials are necessary to assess the antitumor ability of the tested agents in cancer patients, the results of this study suggest strong potential for its use as a therapeutic regimen for inhibiting BC development. Western blot analysis showed that treatment with individual 2HF or RLIP-targeting agents reduced expression of RLIP; inhibited phosphorylation (activation) of Akt and expression of survivin, cdk1, PCNA, and Bcl2; increased levels of Bax and maspin, as compared to control treatments in DMBA-administered mice. In conclusion, simultaneous treatment with 2HF and RLIP-targeting agents inhibited mammary carcinogenesis more effectively (and possibly synergistically) than targeting each molecule alone. These data suggest that the combined treatment could be effective in limiting growth of cancerous cells.
Highlights.
RLIP depletion selectively protects the mammary gland from carcinogenesis induced by DMBA.
RLIP plays a mammary epithelial-autonomous role in the regulation of mammary ductal growth and carcinogenesis.
RLIP may serve as a potential target for the prevention of BC initiation as well as for the treatment of BC progression.
RLIP plays an essential role in mammary carcinogenesis.
Acknowledgments:
This work was supported in part by a Department of Defense grant (W81XWH-16–1-0641) and the Beckman Research Institute of the City of Hope. We thank the personnel in the Animal Resources Center and Pathology Core for their invaluable assistance. The authors are grateful to Prof. Steven Vonderfecht, Director of the Pathology Core Lab, for technical assistance with whole mount preparation and imaging. Research reported in this publication included work performed in the Pathology, Small Animal Imaging, and Biostatistics Cores at City of Hope, which are supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572. We sincerely thank Ravi Salgia, M.D., Ph.D., Professor and Chair of the Department of Medical Oncology at City of Hope, for providing research space and support.
Abbreviations:
- BC
breast cancer
- CAS
control antisense
- DMBA
7,12-dimethylbenzanthracene
- EMT
epithelial-mesenchymal transition
- ER
estrogen receptor
- GSH
glutathione
- GS-E
glutathione-electrophile conjugate
- 2HF
2’-hydroxyflavanone
- PI3K
phosphatidylinositol 3-kinase
- PIS
pre-immune serum
- PR
Progesterone receptor
- Rab
RLIP antibody
- RAS
RLIP antisense
- RLIP
a 76 kDa ral-interacting protein
- SENCAR
sensitive to carcinogenesis
- TNBC
triple negative breast cancer
Footnotes
Conflicts of interest: No conflict of interest exists for any of the authors.
Data availability statement The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Shimkin MB, Gropper L, Thatcher D, Gruenstein M. Hormonal treatment of mammary tumors in rats induced by 3-methylcholanthrene (NSC-21970) and 7,12-dimethylbenz(alpha)anthracene (NSC-408823). Cancer Res 1967; 27: 1284–1288. [PubMed] [Google Scholar]
- [2].Medina D, Butel JS, Socher SH, Miller F. Mammary tumorigenesis in 7,12-dimethylbenz-anthracene treated C57BL x DBA/2f F1mice. Cancer Res 1980; 40: 368–373. [PubMed] [Google Scholar]
- [3].Medina D, Lane HW, Shepherd F. Effect of dietary selenium levels on 7,12-dimethylbenz-anthracene-induced mouse mammary tumorigenesis. Carcinogenesis 1983; 4: 1159–1163. [DOI] [PubMed] [Google Scholar]
- [4].Fischer SM, Conti CJ, Locniskar M, Belury MA, Maldve RE, Lee ML, et al. The effect of dietary fat on the rapid development of mammary tumors induced by 7,12-dimethylbenz(a)anthracene in SENCAR mice. Cancer Res 1992; 52: 662–666. [PubMed] [Google Scholar]
- [5].Qing W-G, Conti CJ, LaBate M, Johnston D, Slaga TJ, MacLeod MC. Induction of mammary cancer and lymphoma by multiple, low oral doses of 7,12-dimethylbenz(a)anthracene in SENCAR mice. Carcinogenesis 1997; 18: 553–559. [DOI] [PubMed] [Google Scholar]
- [6].Kuang S-Q, Liao L, Wang S, Medina D, O’Malley BW, Xu J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen–induced mammary tumorigenesis. Cancer Res 2005; 65: 7993–8002. [DOI] [PubMed] [Google Scholar]
- [7].Cheah YH, Nordin FJ, Sarip R, Tee TT, Azimahtol HL, Sirat HM, et al. Combined xanthorrhizol-curcumin exhibits synergistic growth inhibitory activity via apoptosis induction in human breast cancer cells MDA-MB231. Cancer Cell Int 2009; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hsiao YC, Hsieh YS, Kuo WH, Chiou HL, Yang SF, Chiang WL, et al. The tumor-growth inhibitory activity of flavanone and 2′-OH flavanone in vitro and in vivo through induction of cell cycle arrest and suppression of cyclins and CDKs. J Biomed Sci 2007; 14: 107–119. [DOI] [PubMed] [Google Scholar]
- [9].Shin SY, Kim JH, Lee JH, Lim Y, Lee YH. 2’-Hydroxyflavanone induces apoptosis through Egr-1 involving expression of Bax, p21, and NAG-1 in colon cancer cells. Mol Nutr Food Res 2012; 56: 761–774. [DOI] [PubMed] [Google Scholar]
- [10].Hsiao YC, Kuo WH, Chen PN, Chang HR, Lin TH, Yang WE, et al. Flavanone and 2’-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chem Biol Interact 2007; 167: 193–206. [DOI] [PubMed] [Google Scholar]
- [11].Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 2005; 23: 7721–7735. [DOI] [PubMed] [Google Scholar]
- [12].Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011; 5: 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prat A, Ellis MJ, Perou CM. Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol 2011; 9: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khalkhali-Ellis Z, Christian AL, Kirschmann DA, Edwards EM, Rezaie-Thompson M, Vasef MA, et al. Regulating the tumor suppressor gene maspin in breast cancer cells: a potential mechanism for the anticancer properties of tamoxifen. Clin Cancer Res 2004; 10: 449–454. [DOI] [PubMed] [Google Scholar]
- [15].Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 2006; 5: 1087–1098. [DOI] [PubMed] [Google Scholar]
- [16].Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res 2006; 66: 2354–2360. [DOI] [PubMed] [Google Scholar]
- [17].Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo J-H, Cunningham CC, et al. RLIP76 and Cancer. Clin Cancer Res 2008; 14: 4372–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee S, Wurtzel J, Singhal SS, Awasthi S, Goldfinger LE. RLIP depletion in mice suppresses tumor growth by inhibiting tumor neo-vascularization. Cancer Res 2012; 72: 5165–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Q, Wang JY, Zhang XP, Lv ZW, Fu D, Lu YC, et al. RLIP76 is overexpressed in human glioblastomas and is required for proliferation, tumorigenesis and suppression of apoptosis. Carcinogenesis 2013; 34: 916–926. [DOI] [PubMed] [Google Scholar]
- [20].Zhong W, Owens C, Chandra N, Popovic K, Conaway M, Theodorescu D. RalBP1 is necessary for metastasis of human cancer cell lines. Neoplasia 2010; 12: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang CZ, Yuan P, Xu B, Yuan L, Yang HZ. Liu X. RLIP76 expression as a prognostic marker of breast cancer. Eur Rev Med Pharmacol Sci 2015; 19: 2105–2111. [PubMed] [Google Scholar]
- [22].Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor PJ, Awasthi YC, et al. “Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding protein 1). Cancer Res 2007; 67: 4382–4389. [DOI] [PubMed] [Google Scholar]
- [23].Awasthi S, Tompkins J, Singhal J, Riggs AD, Yadav S, Wu X, et al. RLIP depletion prevents spontaneous neoplasia in TP53 null mice. Proc Natl Acad Sci 2018; 115: 3918–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nagaprashantha L, Singhal J, Li H, Warden C, Liu X, Horne D, et al. 2’-Hydroxyflavanone effectively targets RLIP76-mediated drug transport and regulates critical signaling networks in breast cancer. Oncotarget 2018; 9: 18053–18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Singhal J, Nagaprashantha L, Chikara S, Awasthi S, Horne D, Singhal SS. 2’-Hydroxyflavanone: A novel strategy for targeting breast cancer. Oncotarget 2017; 8: 75025–75037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Singhal SS, Wickramarachchi D, Yadav S, Singhal J, Leake K, Vatsyayan R, et al. Glutathione-conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis. Mol Cancer Ther 2011; 10: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Medina D Mammary tumors. In: Homburger F (ed.). The Mouse in Biomedical Research, Vol. IV., pp. 373–391. New York: Academic Press, Inc., 1982. [Google Scholar]
- [28].Plant I, Stewart MK, Laird DW. Evaluation of mammary gland development and function in mouse models. J Vis Exp 2011; 53: e2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia 2000; 5: 187–200. [DOI] [PubMed] [Google Scholar]
- [30].Singhal J, Chikara S, Horne D, Salgia R, Awasthi S, Singhal SS. 2’-Hydroxyflavanone inhibits in vitro and in vivo growth of breast cancer cells by targeting RLIP76. Mol Carcinogenesis 2018; 57: 1751–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Siddiqui RA, Harvey KA, Walker C, Altenburg J, Xu Z, Terry C, et al. Characterization of synergistic anticancer effects of docosahexaenoic acid and curcumin on DMBA-induced mammary tumorigenesis in mice. BMC Cancer 2013; 13: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Awasthi S, Singhal SS, Yadav S, Singhal J, Vatsyayan R, Zajac E, et al. A central role of RLIP76 in regulation of glycemic control. Diabetes 2010; 59: 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singhal J, Nagaprashantha L, Vatsyayan R, Awasthi S, Singhal SS. RLIP76, a glutathione-conjugate transporter, plays a major role in the pathogenesis of metabolic syndrome. PLoS One 2011; 6: e24688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Singhal SS, Figarola J, Singhal J, Reddy MA, Liu X, Berz D, et al. RLIP76 protein knockdown attenuates obesity due to a high-fat diet. J Biol Chem 2013; 288: 23394–23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Medina D Mammary tumorigenesis in chemical carcinogen-treated mice. Dependence on hormone stimulation for tumorigenesis. J Natl Cancer Inst 1974; 53: 223–226. [DOI] [PubMed] [Google Scholar]
- [36].Medina D, Warner MR. Mammary tumorigenesis in chemical carcinogen-treated mice. Induction of mammary ductal hyperplasias. J Natl Cancer Inst 1976; 57: 331–337. [DOI] [PubMed] [Google Scholar]
- [37].Metcalfe AD, Gilmore A, Klinowska T, Oliver J, Valentijn AJ, Brown R, et al. Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J Cell Sci 1999; 112: 1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schorr K, Li M, Krajewski S, Reed JC, Furth PA. Bcl-2 gene family and related proteins in mammary gland involution and breast cancer. J Mammary Gland Biol Neoplasia 1999; 4: 153–164. [DOI] [PubMed] [Google Scholar]
- [39].Leyton J, Lee ML, Locniskar M, Belury MA, Slaga TJ, Bechtel D, et al. Effects of type of dietary fat on phorbol ester-elicited tumor promotion and other events in mouse skin. Cancer Res 1991; 51: 907–915. [PubMed] [Google Scholar]
- [40].Kumar R, Vadlamudi RK, Adam L. Apoptosis in mammary gland and cancer. Endocrin Related Cancer 2000; 7: 257–269. [DOI] [PubMed] [Google Scholar]
- [41].Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008; 8: 61–70. [DOI] [PubMed] [Google Scholar]
- [42].Kennedy SM, O’Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, et al. Prognostic importance of survivin in breast cancer. Br J Cancer 2003; 88: 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Doolittle H, Moore A, Talbot D. Survivin-directed anticancer therapies – a review of preclinical data and early-phase clinical trials. Eur Oncol 2010; 6: 10–14. [Google Scholar]
- [44].Nurse P, Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature 1981; 292: 558–560. [DOI] [PubMed] [Google Scholar]
- [45].Bartkova J, Zemanova M, Bartek J. Expression of CDK7/CAK in normal tumor cells of diverse histogenic, cell cycle position and differentiation. Int J Cancer 1996; 66: 732–737. [DOI] [PubMed] [Google Scholar]