Abstract
Purpose
To assess whether monocular contrast sensitivity and stereoacuity impairments remain when visual acuity is fully recovered in children with refractive amblyopia.
Methods
A retrospective review of 487 patients diagnosed with refractive amblyopia whose visual acuity improved to 0.08 logMAR or better in both eyes following optical treatment was conducted. Measurements of monocular contrast sensitivity and stereoacuity had been made when visual acuity normalized. All patients had been treated with refractive correction for approximately 2 years following diagnosis. No other treatments were provided. Monocular contrast sensitivity was measured using the CSV-1000E chart for children 6 years of age or younger and a psychophysical technique called the quick contrast sensitivity function in older children. Stereoacuity was measured using the Random Dot Test that includes monocular cues and the Randot Stereoacuity Test that does not have monocular cues.
Results
Statistically significant interocular differences in contrast sensitivity were observed. These differences tended to occur at higher spatial frequencies (12 and 18 cycles per degree). Stereoacuity within the age-specific normal range was achieved by 47.4% of patients for the Random Dot Test and only 23.1% of patients for the Randot Stereoacuity Test.
Conclusions
Full recovery of visual acuity following treatment for refractive amblyopia does not equalize interocular contrast sensitivity or restore normal stereopsis. Alternative therapeutic approaches that target contrast sensitivity and/or binocular vision are required.
Keywords: amblyopia, treated, contrast sensitivity, stereoacuity
The success of amblyopia treatment is judged on amblyopic eye visual acuity (VA) improvement.1,2 However, in addition to impairing monocular high-contrast VA of the affected eye, amblyopia affects a broad range of monocular and binocular visual functions,3 including contrast sensitivity4 and stereopsis.5,6 The effect of amblyopia on binocular vision and stereopsis is particularly important because it contributes to visuomotor function deficits experienced by individuals with amblyopia.7–15 Therefore, when evaluating the functional significance of amblyopia treatment, it is important to consider whether improvements extend beyond amblyopic eye VA.
The gold-standard treatment for amblyopia in children involves correction of refractive error followed by occlusion or penalization of the fellow eye if necessary.16,17 It is well established that this treatment produces clinically significant improvements in amblyopic eye VA.18–22 However, contrast sensitivity and stereopsis deficits may remain. In follow-up studies of children randomized to either occlusion or atropine therapy at younger than age 7 years, the Pediatric Eye Disease Investigator Group (PEDIG) assessed monocular contrast sensitivity using the Pelli–Robson chart at age 10 years and stereoacuity using the Randot Preschool Test at ages 10 and 15 years. Contrast sensitivity was statistically significantly poorer for amblyopic eye versus fellow eye viewing, but the absolute difference was small. Stereopsis impairments were more pronounced with only 18% of children at age 10 years23 and 14% of children at age 15 years18 having stereopsis within the normal range for the Randot Preschool Test (60 arcsec or better).24 Most of the children followed up by PEDIG in these two studies had residual amblyopia, which may have influenced the stereoacuity outcomes. However, a larger follow-up study conducted by the same group revealed impaired stereopsis in a subset of children and teens who had undergone therapy for anisometropic amblyopia even when the amblyopic eye VA deficit had mostly resolved.25
The posttreatment deficits in amblyopic eye contrast sensitivity and stereopsis reported by PEDIG are consistent with other studies of posttreatment visual function in amblyopia. Amblyopic eye contrast sensitivity remains poorer than that of the fellow eye or control eyes26–32 and stereopsis tends to be impaired,27 although this is not always the case.32 Attenuated and delayed pattern reversal visually evoked potentials have also been reported for treated amblyopic eyes relative to fellow eyes, supporting the idea that treatment does not restore normal cortical processing of visual information in amblyopia.33
In this study, we retrospectively evaluated contrast sensitivity and stereopsis in a large group of over 400 individuals with treated refractive amblyopia who no longer had a VA deficit. We hypothesized that contrast sensitivity and stereopsis deficits would be present even when the amblyopic eye VA deficit had fully resolved following standard treatment. We chose to focus on refractive amblyopia because strabismus can impair stereopsis independently from amblyopia, and very few patients with deprivation amblyopia within our patient database achieved normal distance VA in both eyes. The American Academy of Ophthalmology identifies two types of refractive amblyopia: unilateral (anisometropic amblyopia) and bilateral (isoametropic amblyopia).17 We included both types in our retrospective study.
Methods
This study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University.
The retrospective review used the Abnormal Binocular Function and Low Vision Rehabilitation Database at Zhongshan Ophthalmic Center, a major regional eye hospital. The database stores longitudinal measurements of visual function made at each clinical visit for all patients with amblyopia treated in the hospital. Standard operating procedures are followed for all measures to minimize intertester variability. Measurements include routine ocular examination, monocular tumbling E-Early Treatment Diabetic Retinopathy Study (E-ETDRS) distance best-corrected VA (BCVA), stereopsis, cycloplegic refraction, fixation assessment (central or eccentric), and monocular contrast sensitivity function (CSF) assessment. Data for all pediatric patients diagnosed with refractive amblyopia from January 2015 to April 2021 were exported from the database. In addition to clinical and vision measures, the age at first visit to Zhongshan Ophthalmic Center (i.e., the age at which refractive amblyopia was diagnosed) and age when VA had normalized (i.e., the age at which the patient's VA met the study inclusion criteria) were extracted. Some patients may have received prior amblyopia diagnosis and treatment at another clinical site prior to their first visit at Zhongshan Ophthalmic Center.
Anisometropic and isoametropic amblyopia were defined according to the Preferred Practice Pattern from the American Academy of Ophthalmology.16,17 Specifically, anisometropic amblyopia required an interocular difference of >1.5 diopters (D) for sphere and/or >1 D for cylinder along with an interocular BCVA difference of at least 0.2 logMAR. Isoametropic amblyopia was defined as >3 D for sphere or >2 D for cylinder and BCVA worse than the age-specific cutoff values provided within the Preferred Practice Pattern.16 If both the anisometropia and isoametropia criteria were met, patients were classified as isoametropic.
The inclusion criteria for our retrospective review were (1) meeting the criteria for refractive amblyopia at the first visit to Zhongshan Ophthalmic Center, (2) ability to resolve an E on the 0 logMAR line of the E-ETDRS chart (i.e., 0.08 logMAR or better) for each eye following amblyopia treatment at Zhongshan Ophthalmic Center, and (3) ability to cooperate with and understand the CSF and stereoacuity tests. Exclusion criteria were (1) the presence of any other eye disease, including constant, nonalternating, or unequally alternating tropias and visual deprivation; (2) history of previous eye surgery; (3) eccentric fixation; (4) opacity of refracting media; and (5) older than 18 years of age.
Vision Assessment
Visual Acuity
High-contrast (96.9 ± 0.83) logMAR best-corrected distance VA was measured using the ETDRS tumbling E Chart (WEHEN Vision, Guangzhou, Guangdong, China), viewed from a distance of 4 m at a luminance of 200 candelas per square meter (cd/m2). The chart consisted of 5 optotypes per line for a total of 14 lines, decreasing from 1.0 to –0.3 logMAR. Visual acuity was scored per correct letter (0.02 logMAR per letter).
CSF
Monocular CSF measurements were made using the CSV-1000E chart for children aged 6 years or younger and a computerized quick contrast sensitivity (qCSF) psychophysical test for those older than 6 years. The CSV-1000E chart (VectorVision, Dayton, OH, USA) provides an auto-light calibration to maintain a light level of 85 cd/m2 for testing. The chart consists of four rows of grating aperture pairs. Grating spatial frequency varies by row (3, 6, 12, and 18 cycles per degree [cpd]) and contrast varies by column. Patients identify which aperture in a pair contains the grating. The qCSF measurement was performed and analyzed as described by Zheng et al.34 The qCSF measurement generated contrast thresholds at 19 spatial frequencies (equally spaced in log units) together with the cutoff spatial frequency. The area under the log contrast sensitivity function (AULCSF)35 was also calculated for both CSF measurement types. This was done by fitting a third-order polynomial to the threshold data for each spatial frequency.
Stereoacuity
Near stereoacuity was measured using the Random Dot Stereo Acuity Test (Vision Assessment Corporation, Elk Grove Village, IL, USA), and distance stereoacuity was measured using the Randot Stereoacuity Test (Stereo Optical, Inc., Chicago, IL, USA). The tests were administered in accordance with the manufacturer's instructions. For the near Random Dot Stereo Test, patients were tested using sections B and C, which include contour-based circle and symbol targets with disparities ranging from 12.5 to 400 arcsec. Monocular cues are available. The distance Randot Stereoacuity Test includes disparities ranging from 60 to 400 arcsec with no monocular cues. Each measurement was repeated twice. Age-normal performance was defined as meeting the third interquartile locations or the lower limit of published normative data for the relevant age group. For the Random Dot Stereo Acuity Test, normal performance was considered ≤70” at 5 years, ≤50” at 6 and 7 years, ≤30” at 8 and 9 years, and ≤40” at 10 years and older.36 For the Randot Stereoacuity Test, normal performance was defined as ≤200” at 5 years and ≤100” at 6 years and older.37
Fixation
A direct ophthalmoscope YZ6E (66Vision.Tech, Suzhou, China) was used to screen for eccentric fixation.38
Statistical Analysis
The eyes of patients with anisometropic amblyopia were labeled as the fellow eye (FE) and the amblyopic eye (AE). For isoametropic amblyopia, which is bilateral, the eyes were labeled fellow (Iso-FE) and amblyopic (Iso-AE) depending on which had the better BCVA. If there was no interocular difference in BCVA, then the eye with less refractive error was labeled Iso-FE. If there was no interocular difference in refractive error, the labels were assigned randomly (very few cases). Data sets were allocated to one of four groups for analysis according to amblyopia type (anisometropic versus isoametropic) and CSF measurement method (CSV-1000E chart versus qCSF).
Statistical analyses were performed using SPSS version 25 (SPSS, Inc., Chicago, IL, USA) and plots were produced using GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA). Descriptive statistics were used to summarize patient demographics. Categorical variables were expressed as frequencies (percentage) and continuous variables as mean and SD or median and quartiles depending on their distribution. No missing data were reported for age, sex, amblyopia type, BCVA, CSF, or refractive error. Comparisons between paired eyes (FE versus AE or Iso-FE versus Iso-AE) and paired subset groups (at first visit versus at normal VA) were made using the Wilcoxon signed-rank test. Comparisons of stereoacuity between the anisometropic group versus isoametropic group were made using Mann–Whitney U test. Differences were considered significant at P < 0.05.
Results
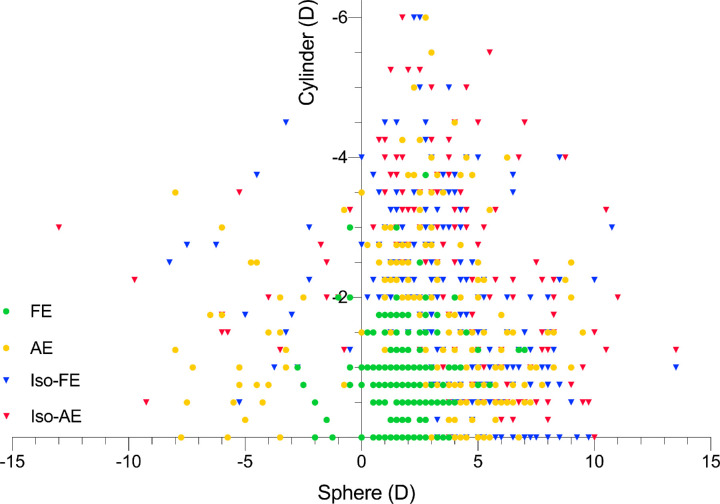
The Abnormal Binocular Function and Low Vision Rehabilitation database contained data from 3677 patients with refractive amblyopia. Of these patients, 487 (269 anisometropic and 218 isoametropic amblyopia) met the study inclusion criteria (Table 1). Thirty-five of these patients met the criteria for both anisometropic and isoametropic amblyopia and were classified as isoametropic. All included patients had been treated with refractive correction only since their first visit to Zhongshan Ophthalmic Center. Treatment duration was approximately 2 years. The range of refractive errors at the time of the first visit to Zhongshan Ophthalmic Center is shown in Figure. The anisometropic and isoametropic groups did not differ significantly in the distribution of sex (χ2 = 0.325, P = 0.569) and age at normal VA (z = –0.271, P = 0.786). Patients with isoametropic amblyopia had a younger age at first visit than patients with anisometropic amblyopia (z = –3.379, P = 0.001).
Table 1.
Summary Demographics for the Anisometropic and Isoametropic Amblyopia Groups
| Characteristic | Anisometropic (n = 269) | Isoametropic (n = 218) | Total (N = 487) |
|---|---|---|---|
| Male | 155 (57.6) | 120 (55) | 275 (56.5) |
| Age at first visit | 6.2 (5.0, 7.9) | 5.6 (4.5, 7.1) | 6 (5.0, 7.5) |
| Age at normal VA | 7.6 (6.5, 9.3) | 7.9 (6.6, 9.0) | 7.7 (6.6, 9.2) |
| Hyperopia | 237 (88.1) | 201 (92.2) | 438 (90) |
| With astigmatism <2 D | 142 (52.8) | 75 (34.4) | 217 (44.6) |
| With astigmatism ≥2 D | 95 (35.3) | 126 (57.8) | 221 (45.4) |
| Myopia | 32 (11.9) | 17 (7.8) | 49 (10) |
| With astigmatism <2 D | 23 (8.6) | 7 (3.2) | 30 (6.2) |
| With astigmatism ≥2 D | 9 (3.3) | 10 (4.6) | 19 (3.9) |
Data are presented as median (quartile 1, quartile 3) for age (years), otherwise as n (%). First visit refers to the patient's first appointment at Zhongshan Ophthalmic Center.
Figure 1.
Spherical refractive error (x-axis) and cylindrical refractive error (y-axis) measured under cycloplegia at the first visit to Zhongshan Ophthalmic Center.
Median contrast sensitivity measured when VA had normalized is shown for spatial frequencies of 3, 6, 12, and 18 cpd along with cutoff spatial frequency and AULCSF in Table 2. Data for younger children tested with the CSV-1000E chart are shown separately from that of older children (>6 years) tested with the qCSF method. Contrast sensitivity was poorer in the amblyopic eye for both amblyopia groups across both age groups, with the largest and statistically significant differences occurring at higher spatial frequencies. We note that the magnitude of interocular contrast sensitivity difference required for clinical significance is currently unknown. A statistically significant interocular VA difference still existed in patients with anisometropia when VA had normalized, but this difference was the equivalent of 1 logMAR letter and therefore not clinically significant (median difference, –0.02 logMAR; interquartile range, –0.04 to 0.00). The same effect was present in patients with isoametropic amblyopia, although this difference is expected because we used VA to classify the fellow and amblyopic eyes in this group of patients with a history of bilateral amblyopia.
Table 2.
Contrast Sensitivity and Visual Acuity for Each Eye When Visual Acuity Had Normalized Following Amblyopia Treatment
| Characteristic | FE | AE | P Value | Iso-FE | Iso-AE | P Value |
|---|---|---|---|---|---|---|
| CSV group | n = 104 | n = 104 | n = 87 | n = 87 | ||
| CS-3 cpd | 1.49 (1.34, 1.63) | 1.49 (1.34, 1.63) | 0.01*,† | 1.63 (1.49, 1.63) | 1.49 (1.49, 1.63) | 0.383 |
| CS-6 cpd | 1.84 (1.70, 1.84) | 1.70 (1.70, 1.84) | 0.005* | 1.84 (1.70, 1.84) | 1.70 (1.70, 1.84) | 0.108 |
| CS-12 cpd | 1.54 (1.40, 1.54) | 1.40 (1.25, 1.54) | <0.001* | 1.40 (1.40, 1.54) | 1.40 (1.40, 1.54) | 0.06 |
| CS-18 cpd | 1.10 (0.96, 1.14) | 0.96 (0.81, 1.10) | <0.001* | 0.96 (0.81, 1.10) | 0.96 (0.81, 1.10) | 0.079 |
| Cutoff SF | 1.28 (1.27, 1.29) | 1.27 (1.26, 1.28) | 0.009* | 1.27 (1.26, 1.29) | 1.27 (1.26, 1.28) | 0.304 |
| AULCSF | 1.24 (1.19, 1.31) | 1.20 (1.15, 1.28) | <0.001* | 1.25 (1.17, 1.33) | 1.21 (1.14, 1.29) | 0.008* |
| LogMAR BCVA | 0.00 (0.00, 0.00) | 0.02 (0.00, 0.04) | <0.001* | 0.00 (0.00, 0.02) | 0.00 (0.00, 0.04) | 0.007* |
| qCSF group | n = 165 | n = 165 | n = 131 | n = 131 | ||
| CS-3 cpd | 1.71 (1.60, 1.83) | 1.73 (1.61, 1.81) | 1.00 | 1.78 (1.67, 1.87) | 1.77 (1.66, 1.87) | 0.242 |
| CS-6 cpd | 1.44 (1.25, 1.63) | 1.42 (1.22, 1.60) | 0.36 | 1.53 (1.36, 1.65) | 1.50 (1.28, 1.66) | 0.04* |
| CS-12 cpd | 1.02 (0.78, 1.25) | 0.93 (0.67, 1.14) | 0.02* | 1.06 (0.85, 1.24) | 0.95 (0.76, 1.19) | 0.01* |
| CS-18 cpd | 0.41 (0.17, 0.67) | 0.26 (0.05, 0.51) | <0.001* | 0.33 (0.15, 0.57) | 0.29 (0.09, 0.50) | 0.003* |
| Cutoff SF | 1.31 (1.22, 1.39) | 1.26 (1.17, 1.34) | <0.001* | 1.29 (1.22, 1.36) | 1.26 (1.19, 1.33) | 0.001* |
| AULCSF | 1.50 (1.34, 1.68) | 1.46 (1.30, 1.64) | 0.06 | 1.57 (1.44, 1.67) | 1.52 (1.37, 1.66) | 0.012* |
| LogMAR BCVA | 0.00 (–0.06, 0.00) | 0.00 (0.00, 0.02) | <0.001* | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.02) | <0.001* |
Data are presented as median (quartile, quartile 3). Comparisons between the FE versus AE and Iso-FE versus Iso-AE were made using the Wilcoxon signed-rank test. Data are split by amblyopia group (anisometropia [FE and AE] and isometropia [Iso-FE and Iso-AE]) as well as type of contrast sensitivity measurement: CVS-1000E (top) and qCSF (bottom). CS, contrast sensitivity; SF, spatial frequency.
Statistically significant difference.
The Wilcoxon signed-rank test indicated a statistically significant difference in the distribution of signed ranks despite the median and interquartile ranges being the same for each eye.
Near and distance stereoacuity measurements made when VA had normalized are shown in Table 3. Over 47% of patients had normal stereoacuity for the Random Dot Test (which includes monocular cues), whereas only 23% had normal distance stereoacuity for the Randot Stereoacuity Test. The distribution of stereoacuity scores did not differ significantly between the anisometropic and isoametropic amblyopia groups for either test (near stereoacuity Random Dot Test, z = –0.624, P = 0.533; distance stereoacuity Randot Stereoacuity Test, z = –0.524, P = 0.601).
Table 3.
Stereoacuity When Visual Acuity Had Normalized
| Characteristic | Total | Anisometropic | Isoametropic |
|---|---|---|---|
| Randot Stereoacuity Test | n = 464 | n = 258 | n = 206 |
| Age-normal | 220 (47.4) | 118 (45.7) | 102 (49.5) |
| Reduced for age | 208 (44.8) | 121 (46.9) | 87 (42.2) |
| Unmeasurable | 36 (7.8) | 19 (7.4) | 17 (8.3) |
| Randot Test | n = 468 | n = 257 | n = 211 |
| Age-normal | 108 (23.1) | 60 (23.3) | 48 (22.7) |
| Reduced for age | 177 (37.8) | 92 (35.8) | 85 (40.3) |
| Unmeasurable | 183 (39.1) | 105 (40.9) | 78 (37.0) |
Data are presented as n (%). See main text for definitions of age-normal stereoacuity.
Stereoacuity data were available for a subset of patients at the time of first visit to Zhongshan Ophthalmic Center and when VA had normalized (Table 4). Near stereoacuity measured using the Random Dot Test improved significantly from first visit to normalization of VA (z = –5.821, P < 0.001) with the number of patients achieving age-normal stereoacuity increasing from 32.6% to 62.5%. The improvement remained significant when the anisometropic (z = –4.555, P < 0.001) and isoametropic (z = –3.642, P < 0.001) groups were considered separately. Distance stereopsis measured using the Randot Stereoacuity Test also improved significantly from first visit to when VA had normalized (all participants, z = –5.551, P < 0.001; anisometropia only, z = –4.778, P < 0.001; isoametropia only, z = –2.852, P = 0.004), although the magnitude of improvement was smaller (15.5% age-normal at first visit versus 26.5% when VA normalized).
Table 4.
Stereoacuity at the Time of First Visit to Zhongshan Ophthalmic Center and When Visual Acuity Had Normalized for a Subset of Patients With Stereoacuity Measurements at Both Time Points
| Near Stereoacuity (Random Dot Test) | ||||||
|---|---|---|---|---|---|---|
| Total (N = 144) | Anisometropic (n = 91) | Isoametropic (n = 53) | ||||
| Characteristic | First Visit | Normal VA | First Visit | Normal VA | First Visit | Normal VA |
| Age-normal | 47 (32.6) | 90 (62.5) | 31 (34.1) | 58 (63.7) | 16 (30.2) | 32 (60.4) |
| Reduced for age | 80 (55.6) | 51 (35.4) | 49 (53.8) | 31 (34.1) | 31 (58.5) | 20 (37.7) |
| Unmeasurable | 17 (11.8) | 3 (2.1) | 11 (12.1) | 2 (2.2) | 6 (11.3) | 1 (1.9) |
| Distance Stereoacuity (Randot Stereoacuity Test) | ||||||
| Total (N = 155) | Anisometropic (n = 101) | Isoametropic (n = 54) | ||||
| Age-normal | 24 (15.5) | 41 (26.5) | 14 (13.9) | 28 (27.7) | 10 (18.5) | 13 (24.1) |
| Reduced for age | 39 (25.2) | 66 (42.6) | 24 (23.8) | 39 (38.6) | 15 (27.8) | 27 (50.0) |
| Unmeasurable | 92 (59.4) | 48 (31.0) | 63 (62.4) | 34 (33.7) | 29 (53.7) | 14 (25.9) |
Data are presented as n (%).
Because our sample had a large age range, we reran our analyses including only patients who were 12 years or younger when their VA normalized (n = 460; 94.5% of the total sample). The pattern of results was the same as the original analysis (Supplementary Analysis).
Discussion
Our retrospective review of a large group of patients with successfully treated refractive amblyopia revealed significant interocular differences in contrast sensitivity, particularly for high spatial frequencies, and prevalent stereoacuity deficits. In addition, no systematic differences in contrast sensitivity or stereoacuity outcomes were apparent for patients with successfully treated anisometropic amblyopia versus those with isoametropic amblyopia.
Our results are consistent with previous observations of residual deficits in monocular and binocular function despite recovery of normal VA following amblyopia treatment.26,29–31,39 Of these residual deficits, the persistent loss of binocular vision may be the most functionally significant as it contributes to motor function impairments40 that are, in turn, associated with reduced self-perception.41 Amblyopia treatments that directly target binocular vision and stereopsis have been developed, and promising initial results have been reported,6,42 including improvements in motor function.15 However, randomized clinical trial outcomes have been mixed43–45 for these new treatments, possibly due to adherence difficulties.46 The current results highlight the importance of continued research into amblyopia treatments that directly target binocular vision and stereopsis.
A large number of patients with refractive amblyopia met our conservative VA criterion for successful treatment (BCVA of 0.08 logMAR or better in each eye) following treatment with spectacles alone for a period of approximately 2 years. Refractive correction is an effective treatment for both anisometropic19,47 and isoametropic amblyopia,20 although the underlying mechanism is not known. It also unknown whether the results of this study would have differed if the patients had been treated with additional therapies such as fellow eye occlusion. However, overall, the available data, including the present results, point to a dissociation between improvements in VA, contrast sensitivity, and stereopsis following treatment in both anisometropic and isoametropic amblyopia. Amblyopia is associated with changes in brain connectivity, white matter microstructure, and functional responses to visual stimuli at multiple stages of the visual pathway.48 It is possible that contrast sensitivity for high spatial frequencies and binocular visual function are more susceptible to these neurologic changes than high-contrast monocular letter acuity even when neural function is improved following treatment.
Our sample included similar numbers of patients with anisometropic and isoametropic amblyopia. Although anisometropic amblyopia is generally more common than isoametropic amblyopia, the difference in prevalence between the two types of amblyopia varies substantially across studies of different populations.17 Studies of children in China have reported prevalence rates ranging from 0.45% to 0.47% for unilateral anisometropic amblyopia and 0.24% to 0.65% for isoametropic amblyopia.49–51 Therefore, our sample likely reflects the similar prevalence of anisometropic and isoametropic amblyopia in children in China.
We observed a large difference in stereoacuity outcomes following treatment depending on the test. Stereoacuity was substantially better for the Random Dot Test than the Randot Stereoacuity Test. We attribute this difference to monocular cues that are present in the Random Dot Test but not the Randot Stereoacuity Test. Several new digital stereopsis tests are being developed for pediatric testing that may provide even more accurate stereoacuity estimates in future studies of amblyopia treatment.52,53
In conclusion, we observed interocular differences in contrast sensitivity and a high risk of impaired stereopsis in a large group of successfully treated patients with refractive amblyopia. Given the importance of stereopsis for motor function, these results indicate the need for the continued development of treatments that directly target binocular vision.
Supplementary Material
Acknowledgments
Supported by National Key Research & Development Project (2020YFC2003905) and National Natural Science Foundation of China (81770954). B. Thompson is supported by the Hong Kong Special Administrative Region Government and InnoHK.
Disclosure: Y. Jia, None; Q. Ye, None; S. Zhang, None; L. Feng, None; J. Liu, None; Z. Xu, None; Y. Zhuang, None; Y. He, None; Y. Zhou, None; X. Chen, None; Y. Yao, None; R. Jiang, None; B. Thompson, None; J. Li, None
References
- 1. Stewart CE, Moseley MJ, Fielder AR.. Defining and measuring treatment outcome in unilateral amblyopia. Br J Ophthalmol. 2003; 87: 1229–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. IRIS50. Amblyopia: Interocular Visual Acuity. San Francisco, California: AAO; 2019. [Google Scholar]
- 3. Hamm LM, Black J, Dai S, Thompson B.. Global processing in amblyopia: a review. Front Psychol. 2014; 5: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hess R, Howell E.. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vis Res. 1977; 17: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 5. Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013; 33: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levi DM, Knill DC, Bavelier D.. Stereopsis and amblyopia: a mini-review. Vis Res. 2015; 114: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engel-Yeger B. Evaluation of gross motor abilities and self perception in children with amblyopia. Disabil Rehabil. 2008; 30(4): 243–248. [DOI] [PubMed] [Google Scholar]
- 8. Grant S, Melmoth D, Morgan M, Finlay A.. Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 9. Grant S, Moseley MJ.. Amblyopia and real-world visuomotor tasks. Strabismus. 2011; 19: 119–128. [DOI] [PubMed] [Google Scholar]
- 10. Grant S, Suttle C, Melmoth D, Conway M, Sloper J.. Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Invest Ophthalmol Vis Sci. 2014; 55: 5687–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niechwiej-Szwedo E, Goltz H, Chandrakumar M, Hirji Z, Crawford J, Wong A.. Effects of anisometropic amblyopia on visuomotor behavior, part 2: visually guided reaching. Invest Ophthalmol Vis Sci. 2011; 52: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly KR, Morale SE, Beauchamp CL, Dao LM, Luu BA, Birch EE.. Factors associated with impaired motor skills in strabismic and anisometropic children. Invest Ophthalmol Vis Sci. 2020; 61: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelly KR, Jost RM, De La, Cruz A, Birch EE.. Multiple-choice answer form completion time in children with amblyopia and strabismus. JAMA Ophthalmol. 2018; 136: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webber A, Wood J, Gole G, Brown B.. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci. 2008; 49: 594–603. [DOI] [PubMed] [Google Scholar]
- 15. Webber AL, Wood JM, Thompson B.. Fine motor skills of children with amblyopia improve following binocular treatment. Invest Ophthalmol Vis Sci. 2016; 57: 4713–4720. [DOI] [PubMed] [Google Scholar]
- 16. Panel. AAoOPOS. Preferred Practice Pattern Guidelines: Amblyopia. American Academy of Ophthalmology; 2012. [Google Scholar]
- 17. Wallace DK, Repka MX, Lee KA, et al.. Amblyopia Preferred Practice Pattern. Ophthalmology. 2018; 125: P105–P142. [DOI] [PubMed] [Google Scholar]
- 18. Repka MX, Kraker RT, Holmes JM, et al.. Atropine vs patching for treatment of moderate amblyopia. JAMA Ophthalmol. 2014; 132: 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheiman M, Hertle R, Beck R, et al.. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005; 123: 437–447. [DOI] [PubMed] [Google Scholar]
- 20. Wallace D, Chandler D, Beck R, et al.. Treatment of bilateral refractive amblyopia in children three to less than 10 years of age. Am J Ophthalmol. 2007; 144: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stewart CE, Moseley MJ, Stephens DA, Fielder AR.. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci. 2004; 45: 3048–3054. [DOI] [PubMed] [Google Scholar]
- 22. Stewart C, Fielder A, Stephens D, Moseley M.. Design of the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Br J Ophthalmol. 2002; 86: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pediatric Eye Disease Investigator Group, Repka MX, Kraker RT, et al.. A randomized trial of atropine vs patching for treatment of moderate amblyopia: follow-up at age 10 years. Arch Ophthalmol. 2008; 126: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birch E, Williams C, Drover J, et al.. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS. 2008; 12: 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallace DK, Lazar EL, Melia M, et al.. Stereoacuity in children with anisometropic amblyopia. J AAPOS. 2011; 15: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Tao L, Zhou Y, Lu Z-L.. Treated amblyopes remain deficient in spatial vision: a contrast sensitivity and external noise study. Vis Res. 2007; 47: 22–34. [DOI] [PubMed] [Google Scholar]
- 27. Simmers A, Gray L, McGraw P, Winn B.. Functional visual loss in amblyopia and the effect of occlusion therapy. Invest Ophthalmol Vis Sci. 1999; 40: 2859–2871. [PubMed] [Google Scholar]
- 28. Rogers GL, Bremer DL, Leguire LE.. The contrast sensitivity function and childhood amblyopia. Am J Ophthalmol. 1987; 104: 64–68. [DOI] [PubMed] [Google Scholar]
- 29. Chatzistefanou KI, Theodossiadis GP, Damanakis AG, Ladas ID, Moschos MN, Chimonidou E.. Contrast sensitivity in amblyopia: the fellow eye of untreated and successfully treated amblyopes. J AAPOS. 2005; 9: 468–474. [DOI] [PubMed] [Google Scholar]
- 30. Oner O, Akca Bayar S, Oto S, Gokmen O, Tekindal MA. Contrast sensitivity in microtropic and anisometropic eyes of successfully treated amblyopes. Turk J Ophthalmol. 2017; 47: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang G, Zhao C, Ding Q, Wang P.. An assessment of the contrast sensitivity in patients with ametropic and anisometropic amblyopia in achieving the corrected visual acuity of 1.0. Sci Rep. 2017; 7: 42043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao W, Jia WL, Chen G, et al.. A complete investigation of monocular and binocular functions in clinically treated amblyopia. Sci Rep. 2017; 7: 10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shawkat F, Kriss A, Timms C, Taylor D.. Comparison of pattern-onset, -reversal and -offset VEPs in treated amblyopia. Eye. 1998; 12, 863–869. [DOI] [PubMed] [Google Scholar]
- 34. Zheng H, Wang C, Cui R, et al.. Measuring the contrast sensitivity function using the qCSF method with 10 digits. Transl Vis Sci Technol. 2018; 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Applegate RA, Howland HC, Sharp RP, Cottingham AJ, Yee RW.. Corneal aberrations and visual performance after radial keratotomy. J Refract Surg. 1998; 14: 397–407. [DOI] [PubMed] [Google Scholar]
- 36. Adler P, Scally AJ, Barrett BT.. Test-retest variability of Randot stereoacuity measures gathered in an unselected sample of UK primary school children. Br J Ophthalmol. 2012; 96: 656–661. [DOI] [PubMed] [Google Scholar]
- 37. Wang J, Hatt SR, O'Connor AR, et al.. Final version of the Distance Randot Stereotest: normative data, reliability, and validity. J AAPOS. 2010; 14: 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper J, Gelfond I, Carlson PE, Campolattaro B, Wang F. Comparison of eccentric fixation measurements using the streak target of an ophthalmoscope and a traditional visuoscopy target. J Pediatr Ophthalmol Strabismus. 2005; 42: 89–96. [DOI] [PubMed] [Google Scholar]
- 39. Hoshi S, Hiraoka T, Kotsuka J, et al.. Functional visual acuity in patients with successfully treated amblyopia: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 40. Webber AL. The functional impact of amblyopia. Clin Exp Optom. 2021; 101: 443–450. [DOI] [PubMed] [Google Scholar]
- 41. Birch EE, Castañeda YS, Cheng-Patel CS, et al.. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmology. 2019; 137: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF.. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013; 23: R308–R309. [DOI] [PubMed] [Google Scholar]
- 43. Brin TA, Chow A, Carter C, Oremus M, Bobier W, Thompson B.. Efficacy of vision-based treatments for children and teens with amblyopia: a systematic review and meta-analysis of randomised controlled trials. BMJ Open Ophthalmol. 2021; 6: e000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Birch EE, Kelly KR, Wang J.. Recent advances in screening and treatment for amblyopia. Ophthalmol Ther. 2021; 10: 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiao S, Angjeli E, Wu HC, et al.. Randomized controlled trial of a dichoptic digital therapeutic for amblyopia. Ophthalmology. 2022; 129: 77–85. [DOI] [PubMed] [Google Scholar]
- 46. Gao TY, Black JM, Babu RJ, et al.. Adherence to home-based videogame treatment for amblyopia in children and adults. Clin Exp Optom. 2021; 104: 773–779. [DOI] [PubMed] [Google Scholar]
- 47. Chen PL, Chen JT, Tai MC, Fu JJ, Chang CC, Lu DW.. Anisometropic amblyopia treated with spectacle correction alone: possible factors predicting success and time to start patching. Am J Ophthalmol. 2007; 143: 54–60. [DOI] [PubMed] [Google Scholar]
- 48. Brown HD, Woodall RL, Kitching RE, Baseler HA, Morland AB.. Using magnetic resonance imaging to assess visual deficits: a review. Ophthalmic Physiol Opt. 2016; 36: 240–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang D, Chen X, Zhu H, et al.. Prevalence of amblyopia and its association with refraction in Chinese preschool children aged 36-48 months. Br J Ophthalmol. 2018; 102: 767–771. [DOI] [PubMed] [Google Scholar]
- 50. Chen X, Fu Z, Yu J, et al.. Prevalence of amblyopia and strabismus in Eastern China: results from screening of preschool children aged 36-72 months. Br J Ophthalmol. 2016; 100: 515–519. [DOI] [PubMed] [Google Scholar]
- 51. Fu J, Li SM, Li SY, et al.. Prevalence, causes and associations of amblyopia in year 1 students in Central China: the Anyang Childhood Eye Study (ACES). Graefes Arch Clin Exp Ophthalmol. 2014; 252: 137–143. [DOI] [PubMed] [Google Scholar]
- 52. Vancleef K, Serrano-Pedraza I, Sharp C, et al.. ASTEROID: a new clinical stereotest on an Autostereo 3D tablet. Transl Vis Sci Technol. 2019; 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hess RF, Ding R, Clavagnier S, et al.. A robust and reliable test to measure stereopsis in the clinic. Invest Ophthalmol Vis Sci. 2016; 57: 798–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.