Abstract
Purpose
Dry eye–induced chronic ocular pain is also called ocular neuropathic pain. However, details of the pathogenic mechanism remain unknown. The purpose of this study was to elucidate the pathogenic mechanism of dry eye–induced chronic pain in the anterior eye area and develop a pathophysiology-based therapeutic strategy.
Methods
We used a rat dry eye model with lacrimal gland excision (LGE) to elucidate the pathogenic mechanism of ocular neuropathic pain. Corneal epithelial damage, hypersensitivity, and hyperalgesia were evaluated on the LGE side and compared with the sham surgery side. We analyzed neuronal activity, microglial and astrocytic activity, α2δ–1 subunit expression, and inhibitory interneurons in the trigeminal nucleus. We also evaluated the therapeutic effects of ophthalmic treatment and chronic pregabalin administration on dry eye–induced ocular neuropathic pain.
Results
Dry eye caused hypersensitivity and hyperalgesia on the LGE side. In the trigeminal nucleus of the LGE side, neuronal hyperactivation, transient activation of microglia, persistent activation of astrocytes, α2δ–1 subunit upregulation, and reduced numbers of inhibitory interneurons were observed. Ophthalmic treatment alone did not improve hyperalgesia. In contrast, continuous treatment with pregabalin effectively ameliorated hypersensitivity and hyperalgesia and normalized neural activity, α2δ–1 subunit upregulation, and astrocyte activation.
Conclusions
These results suggest that dry eye–induced hypersensitivity and hyperalgesia are caused by central sensitization in the trigeminal nucleus with upregulation of the α2δ–1 subunit. Here, we showed that pregabalin is effective for treating dry eye–induced ocular neuropathic pain even after chronic pain has been established.
Keywords: dry eye, corneal hyperalgesia, voltage-gated calcium channel α2δ–1 subunit, pregabalin
Dry eye exhibits a variety of clinical manifestations, including discomfort, pain, and surface damage.1 With a prevalence of approximately 5% to 30%, dry eye is a common disease.2–4 Risk factors for dry eye have increased in recent years because of the increased use of contact lenses and work involving visual display terminal use.5 Chronic pain caused by dry eye is sometimes described as “corneal pain without stain” or “phantom cornea.”6 Many patients whose symptoms do not match the clinical findings in the anterior segment of the eye experience significantly reduced quality of life because of dry eye–induced chronic pain.1,7–9 However, because there is currently no effective treatment, the present study was conducted to facilitate establishment of a treatment based on the pathogenic mechanism of the disease.
Noxious stimuli to the cornea are received by nociceptor fibers such as the corneal and ciliary nerves and conducted through the trigeminal ophthalmic nerve branch and trigeminal ganglion to the trigeminal nucleus in the bridge of the brainstem, where the signals are transmitted to second-order neurons. Nociceptive signals are finally transmitted to the somatosensory cortex and perceived as pain or unpleasant dysesthesia.
Chronic pain caused by corneal epithelial damage in the anterior segment of the eye has been referred to as “ocular neuropathic pain.”1,7 Patients with ocular neuropathic pain present with photoallodynia, symptoms of burning, irritation, dryness, and grittiness in the eyes.1,7 When chronic dry eye patients present with ocular surface pain after recovery from corneal epithelial damage, persistent pain under ocular anesthesia, corneal hypersensitivity, or allodynia, it has been suggested that the neuropathic pain may have developed at the level of the trigeminal nerve or upper neurons. However, the mechanism underlying dry eye–induced ocular neuropathic pain remains unknown.
Neuropathic pain is defined by the International Association for the Study of Pain as “pain caused by a lesion or disease of the somatosensory nervous system.”5 A previous study investigated the pathogenic mechanism of neuropathic pain in the dorsal root ganglion and spinal dorsal horn (SDH) using an animal neuropathy model.10 In the SDH, activated microglia are reportedly involved in the onset of neuropathic pain.11 On the other hand, activated astrocytes have been shown to play critical roles in maintaining neuropathic pain.12
Voltage-gated Ca2+ channels consist of a pore-forming α1 subunit and accessory β and α2δ subunits. The α2δ subunits play essential roles in membrane transport and Ca2+ channel function,13,14 and among the four subtypes of the α2δ subunit family, α2δ–1 is ubiquitously expressed.15 In a previous study using a mouse spinal nerve ligation model, we found that the α2δ–1 subunit protein level is significantly increased in the dorsal horn because of up-regulated expression in the dorsal root ganglion on the injured side of the spinal cord.16 It has also been reported that α2δ–1 transgenic mice develop tactile allodynia.17 Therefore the α2δ–1 subunit is thought to play an essential role in the development of neuropathic pain.13,14
Gabapentinoids have been tried to treat ocular neuropathic pain symptoms.18 Pregabalin, a member of the gabapentinoid family, is reportedly effective in relieving neuropathic pain by attenuating voltage-gated calcium channels, excitatory amino acid transporters, potassium channels, N-methyl-D-aspartate receptors, and inflammation.19 However, details of the mechanism linking the development of neuropathic pain of corneal origin in the trigeminal nerve with the effects of pregabalin remain to be elucidated. Therefore we planned this study with the aim of elucidating the pathogenic mechanism of dry eye–induced chronic pain in the anterior eye area to facilitate development of a therapeutic strategy based on the underlying pathophysiology.
Methods
Animals
This study was approved by the Toho University Animal Care and User Committee and conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals approved by Toho University and the “ARVO Statement on the Use of Animals in Ophthalmic and Vision Research.” Six-week-old male Sprague-Dawley rats (125–150 g, Sankyo Labo Service Corp., Tokyo, Japan) were used in the study.
Lacrimal Gland Excision
Under inhalation anesthesia using isoflurane (3%), the exorbital and infraorbital lacrimal glands on the left side were excised. We designated this as the LGE side.20 The right lateral lacrimal gland was sutured without removal and designated the sham side. Carprofen (Zoetis, Parsippany, NJ, USA) was administered subcutaneously once daily for three days (5 mg/kg) for postoperative analgesia.20 In accordance with the “ARVO Statement on the Use of Animals in Ophthalmic and Vision Research,” unilateral LGE was performed to minimize pain and discomfort. We confirmed that visual function was not impaired on the sham side. To check whether the bilateral dry eye model conforms ARVO Statement, we compared the phenotypic analysis results of the bilateral dry eye model with those of control-sham rats that underwent unilateral sham surgery. We confirmed that the sham surgery did not induce corneal hypersensitivity or hyperalgesia (Supplementary Fig. S1). We also confirmed that the results of phenotypic analysis of the sham surgery side of our bilateral dry eye model rat were similar to those of the sham side of control-sham rats. Thus we confirmed that the sham side of the bilateral dry eye model does not suffer damage, pain, or discomfort.
Phenotype Analysis in Dry Eye Model Rats
Tear Measurements
Tear fluid volume was measured by the length of color change occurring on cotton phenol red threads (Zone-Quick; FCI Ophthalmic, Pembroke, MA, USA) placed in the lateral canthus of the eye for 30 seconds.20
Blinking Measurements
To assess corneal hypersensitivity, the frequency of blinking was measured the day before surgery, the day after surgery, and once per week thereafter. Rats were placed in a 24 × 45 × 20 cm (L × W × H) chamber and allowed to habituate for 15 minutes. The number of spontaneous blinks in each eye was quantified for five minutes by an observer using a manual counter.20 The number of blinks was measured twice at the same time on the same day, and it was confirmed that there was no deviation in the number of observations between the two measurements. The number of blinks was measured between 2:00 and 4:00 PM to account for diurnal variations.
Eye Wiping Behavior
To assess corneal pain sensitivity, eye wipe responses were examined. After a 10-minute acclimation period, the total time each rat spent wiping the eye in response to administration of 5 M NaCl solution was recorded for three minutes.20 Because the administration of 5 M NaCl solution can induce corneal epithelial damage, we carefully measured the duration of eye wipe behavior only once per measurement.
Fluorescein Staining
The extent of corneal damage was assessed by corneal fluorescein staining. Flores 0.7 mg ophthalmic test paper (Ayumi, Tokyo, Japan) was applied to the cornea for one minute under isoflurane anesthesia. After washing out excess fluorescein dye, the eyes were examined under cobalt blue light from an ophthalmic slit lamp handheld model scope (KOWA, Inc., Aichi, Japan). The degree of staining was scored on a 0- to 4-point grading scale: score 0, absence of punctate fluorescein staining; score 1, one-eighth or less of the corneal surface exhibited staining; score 2, one-eighth to one-fourth of the corneal surface exhibited staining; score 3, one-fourth to one-half of the corneal surface exhibited staining; score 4, greater than one-half of the corneal surface was stained.20
Tracing the Trigeminal Ganglion of Corneal Origin
Under inhalation anesthesia with isoflurane (3%), the cornea was epithelially abraded to a diameter of approximately 3 mm. Next, 7.5 µL of Cholera Toxin Subunit B Alexa Fluor 488 Conjugate (Invitrogen, Carlsbad, CA, USA) at 1 mg/mL was applied to the cornea for 10 minutes. One week after treatment, trigeminal ganglia were identified using confocal microscopy.
Eye Drop Treatment
Eye drops (0.1% hyaluronic acid eye drops; Santen, Osaka, Japan) were administered four times to both eyes during the daytime for local treatment of corneal epithelial damage in rats eight weeks after LGE surgery. During the night, Vaseline (FUJIFILM Wako Pure Chemical, Osaka, Japan) was applied as an ophthalmic ointment. Recovery of corneal epithelial damage was confirmed by fluorescein staining after one and two weeks of eye drop treatment.
Chronic Pregabalin Administration
Under inhalation anesthesia with isoflurane (3%), an osmotic micropump (Alzet; Durect Corp., Cupertino, CA) filled with either 0.9% saline solution (saline control group) or 1% pregabalin (Lyrica; Pfizer Inc., New York, NY, USA) dissolved in saline solution (pregabalin treatment group) was implanted in the subcutaneous space of each rat. The pump delivered approximately 10 mg/kg of pregabalin or an equivalent volume of 0.9% saline solution per day. Systemic administration of this dose was shown to provide analgesic effects without inducing fatigue or other side effects in a rat neuropathic pain model.21,22 Considering the pharmacokinetics of pregabalin in rats,23,24 we chose continuous subcutaneous administration using an osmotic pump to approximate the pharmacokinetics of oral administration in humans.25
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). The Student's t test was used to compare differences between two groups, and Dunnett's multiple comparison test was used to compare differences among three or more groups. A P value <0.05 was considered statistically significant. The remaining methods are available in the Supplementary Materials.
Results
Time Course of Hypersensitivity and Hyperalgesia Progression After LGE
We first evaluated the time course of the progression of dry eye–induced corneal hypersensitivity and hyperalgesia. The amount of tear fluid on the LGE side dropped within one week after surgery (hereinafter referred to as “1w”) and remained at a low level for up to eight weeks (“8w”) after surgery. In contrast, there was no change on the sham side (Fig. 1A). Corneal epithelial damage on the LGE side, evaluated by fluorescein staining, was scored as a value of 2 at 1w and then progressed to a score of 4 at 3w and thereafter. In contrast, the sham side had a score of 1 at 4w (Fig. 1B, Supplementary Fig. S2).
Figure 1.
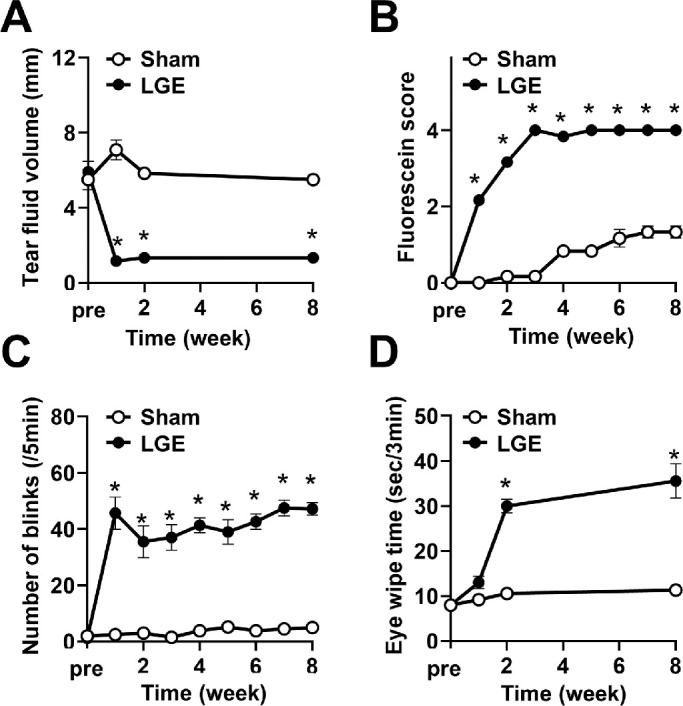
LGE gave rise to corneal epithelial damage, hypersensitivity, and hyperalgesia progression. (A) Change in the tear fluid volume in LGE rats. The “pre” in the abscissa axis indicates the day previous to the LGE surgery. (B) Time-dependent progression of corneal epithelial damage evaluated by fluorescein staining scoring in LGE rats. (C) Progression of hypersensitivity in LGE rats evaluated by the number of blinks. (D) The time course of hyperalgesia progression in LGE rats evaluated by eye wipe response. Each point represents the mean ± SEM (n = 9). *P < 0.05 versus the sham side (Student's t-test).
Corneal sensory hypersensitivity, measured as the number of blinks, increased continuously from 1w to 8w after LGE surgery, whereas there was no change on the sham side (Fig. 1C). Corneal hyperalgesia was assessed by time spent wiping the eye in response to osmotic stimulation. Persistent hyperalgesia developed at 2w to 8w on the LGE side, but there was no change in corneal hyperalgesia on the sham side (Fig. 1D). The slight increase in the fluorescein staining score on the sham side (Fig. 1B) was similar to the above results of control-sham surgery rats (Supplementary Fig. S1). Therefore it was considered to be due to the age-dependent changes and the particulates generated from solid food or wood tips but not the effect of LGE surgery. In addition, the effect of wiping together with the LGE side with both paws might be involved as the frequency of eye wiping increased on the LGE side (Fig. 1D).
These results indicate that corneal hypersensitivity appeared at 1w, hyperalgesia at 2w, and maximal corneal epithelial damage occurred by 3w after LGE surgery in the dry eye model rats.
Dry Eye Enhanced Neuronal Activity and Upregulated the Voltage-Gated Calcium Channel α2δ–1 Subunit in the Trigeminal Nucleus
We hypothesized that dry eye induces hyperactivation of the primary corneal nerve and facilitates excitatory synaptic transmission via upregulation of the voltage-gated calcium channel α2δ–1 subunit (hereinafter referred to as “α2δ–1 subunit”), which in turn activates the second-order trigeminal neurons, leading to hypersensitivity and hyperalgesia. To test this hypothesis, we analyzed neuronal activity and expression of the α2δ–1 subunit in the trigeminal nucleus, where corneal primary and second-order neurons form synapses.
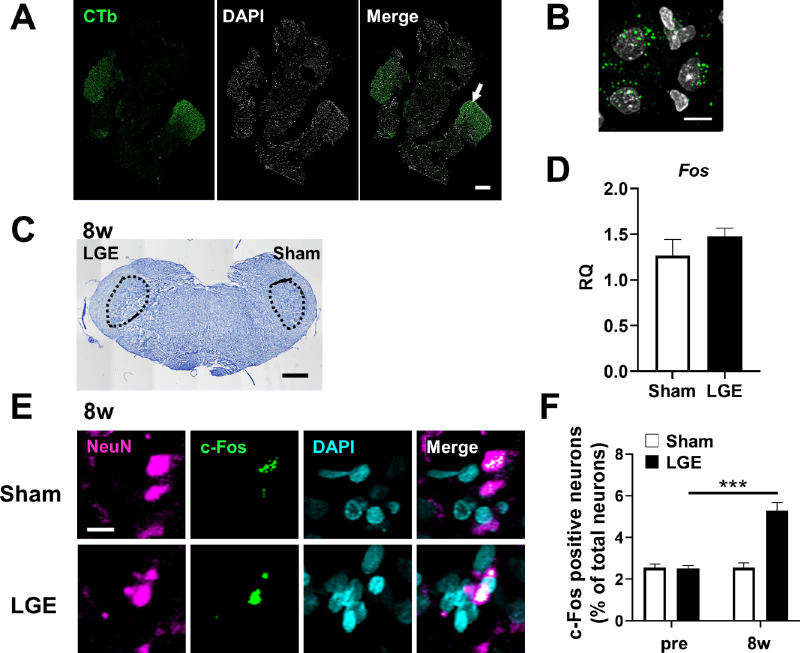
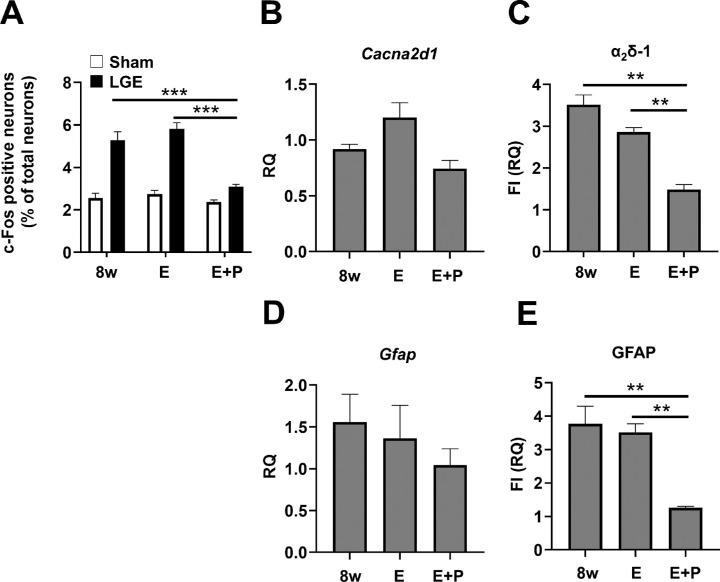
To identify the region for analysis, we first identified the cornea-derived trigeminal nerve in the trigeminal ganglion and its projections in the trigeminal nucleus. The cornea-derived trigeminal ganglion was identified by retrograde labeling with a cholera toxin tracer (Fig. 2A). Under high magnification, trigeminal cell bodies of corneal origin were identified based on the accumulation of fluorescently labeled cholera toxin tracer molecules (Fig. 2B). In the trigeminal nucleus, the Vi/Vc (trigeminal subnucleus interpolaris/caudalis transition zone), the area indicated by the dotted line in Figure 2C, was identified. As a phenotypic analysis indicated persistent development of sensory and pain hypersensitivity at 8w, we evaluated the neuronal activity at that time point. There was no significant difference between the sham and LGE sides in the level of mRNA encoding Fos (the c-Fos gene as a marker of neuronal activity) in the trigeminal nucleus (Fig. 2D). Immunostaining of the trigeminal nucleus to detect local c-Fos protein expression was then conducted. Neurons were identified based on NeuN staining, and neurons double positive for c-Fos and NeuN were considered activated neurons (Fig. 2E). The percentage of c-FOS-positive neurons among all neurons in the trigeminal nucleus was low before surgery (hereafter designated as “pre”), without a significant difference between the sham and LGE sides. By 8w, however, a greater percentage of neurons were activated on the LGE side than on the sham side (Fig. 2F). These results indicate that dry eye enhances neuronal activity in the trigeminal nucleus.
Figure 2.
Identification of the cornea-derived trigeminal ganglion and trigeminal nucleus, and evaluation of the neuronal activity. (A) Fluorescence images of the trigeminal ganglion of corneal origin identified by cholera toxin tracing. The cholera toxin-labeled neurons (CTb; green), DAPI staining (white), and the merged images. Scale bar: 300 µm. (B) Higher magnification image of the arrowed area in A. Scale bar: 10 µm. (C) Nissl staining image of the trigeminal nucleus (Vi/Vc), shown in the dotted area, of the LGE rat at 8w. Scale bar: 1mm. (D) The mRNA level of c-Fos in the trigeminal nucleus of LGE rats at 8w on the sham and LGE sides. RQ (relative quantification) was calculated as a relative value to the Sham side. Each bar graph represents the mean ± SEM (n = 3). (E) Immunofluorescence images of trigeminal nuclei at 8w. NeuN (magenta), c-Fos (green), and DAPI (cyan) indicate neurons, the activated neurons, and nuclei, respectively. Scale bar: 5 µm. (F) The percentage of c-Fos–positive neurons in the trigeminal nucleus at pre and 8w. The vertical axis shows the percentage of c-Fos positive neurons to the total neurons. Each bar graph represents the mean ± SEM (n = 3). ***P < 0.001 versus sham side (Dunnett's multiple comparison test).
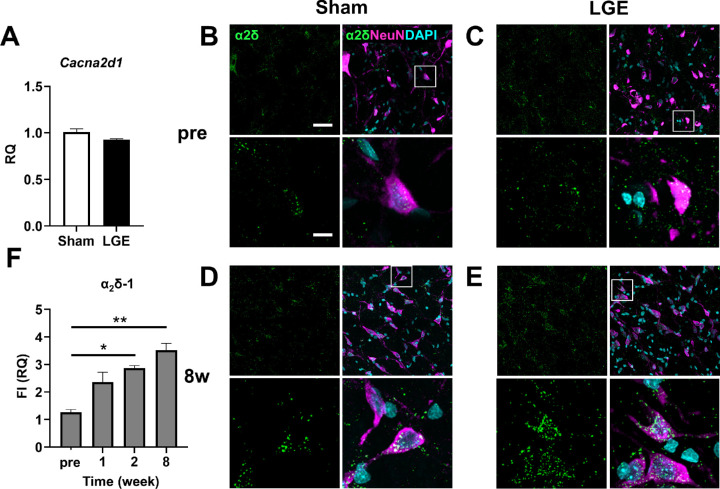
We then analyzed the expression level of the α2δ–1 subunit in the trigeminal nucleus. At 8w, there was no significant difference in the mRNA level of Cacna2d1 (which encodes the α2δ–1 subunit) between the sham and LGE sides (Fig. 3A). In immunostaining, the α2δ–1 subunit exhibited a granular signal. Before surgery, there was no difference in localization of the α2δ–1 subunit or signal intensity between the sham and LGE sides (Figs. 3B, 3C). In contrast, at 8w, α2δ–1 subunit signals were significantly higher on the LGE side than on the sham side (Figs. 3D, 3E). We also evaluated the level of α2δ–1 subunit protein at the following time points: pre; at 1w, when hypersensitivity first appeared; at 2w, when significant pain was observed; and at 8w. The α2δ–1 subunit protein level began to increase at 1w, and significant increases were observed at 2w and 8w (Fig. 3F).
Figure 3.
In the trigeminal nucleus of LGE rats, the α2δ–1 subunit protein expression was increased on the LGE side at 2w and 8w. (A) The mRNA level of Cacna2d1, encoding the α2δ–1 subunit, in the trigeminal nucleus at 8w. Each bar graph represents the mean ± SEM (n = 3). (B, C) Immunofluorescence images of trigeminal nuclei on the sham side (B) and the LGE side (C) at pre. α2δ (green), NeuN (magenta), and DAPI (cyan) indicate α2δ–1, neurons, and nuclei, respectively. Scale bars: 30 µm (upper panel); 10 µm (lower panel). (D, E) Immunofluorescence images of trigeminal nuclei on the sham side (D) and the LGE side (E) at 8w. (F) Summary of the expression levels of α2δ–1 in the trigeminal nucleus of pre, 1w, 2w, and 8w. The vertical axis shows the RQ value of total fluorescence intensity (FI) on the LGE side normalized to that on the Sham side in the same section from the same animal. Each bar graph represents the mean ± SEM (n = 3). *P < 0.05, **P < 0.01 versus sham side (Dunnett's multiple comparison test).
The time course of progression of corneal hyperalgesia coincided with the upregulation of α2δ–1 subunit expression, and enhanced neuronal activity was observed at 8w. These results suggest that dry eye caused neural hyperactivity and hyperalgesia via upregulation of α2δ–1 subunit expression.
Dry Eye Induced a Reduction in Inhibitory Interneurons in the Trigeminal Nucleus
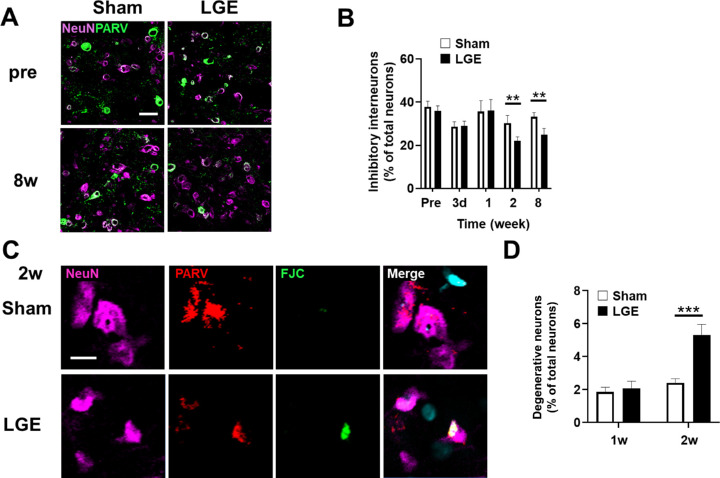
In the trigeminal nucleus of rats, inhibitory interneurons prevent over-excitation associated with sensory nerve inputs from the eyes, whiskers, and mouth. We hypothesized that the pathogenic mechanism of hypersensitivity and hyperalgesia involves downregulation of inhibitory interneurons in the trigeminal nucleus. Therefore we analyzed inhibitory interneurons in the trigeminal nucleus. Cells double immunopositive for NeuN and PARV were counted as inhibitory interneurons (Fig. 4A). Although there was no significant difference between the sham and LGE sides in terms of the percentage of inhibitory interneurons among all neurons in the trigeminal nucleus up to 1w, the percentage was significantly lower on the LGE side compared with the sham side at 2w and 8w (Fig. 4B). Based on these results, we postulated that the loss of inhibitory interneurons may be due to neurodegeneration. We identified degenerated neurons using FJC staining at 1w and 2w, before and after the reduction in the number of inhibitory neurons. FJC-positive, NeuN-positive, and PARV-positive cells were counted as degenerated inhibitory interneurons (Fig. 4C). There was no significant difference in the percentage of degenerated inhibitory interneurons among all neurons between the sham and LGE sides. In contrast, at 2w, the percentage of degenerated inhibitory interneurons was significantly higher on the LGE side compared with the sham side (Fig. 4D). These results suggest that dry eye–induced hypersensitivity and hyperalgesia involve a degeneration-related decrease in the number of inhibitory interneurons in the trigeminal nucleus.
Figure 4.
The number of inhibitory interneurons was reduced on the LGE side at 2w and 8w. (A) Immunofluorescence images of NeuN (magenta) and parvalbumin (PARV; green) in the trigeminal nucleus of LGE rats on the sham and LGE sides at pre and 8w. Neurons double positive for NeuN and PARV were counted as inhibitory interneurons. Scale bar: 30 µm. (B) The percentage of inhibitory interneurons to the total neurons in the trigeminal nucleus of pre, 3d, 1w, 2w and 8w. Each bar graph represents the mean ± SEM (n = 3). **P < 0.01 versus sham side (Student's t test). (C) Immunofluorescence images of trigeminal nuclei of LGE rats. FJC (green), NeuN (magenta), PARV (red) indicates the degenerated neurons, neurons, and inhibitory interneurons, respectively. Scale bar: 5 µm. (D) Summary of the percentage of denervated neurons at 1w and 2w. Each bar graph represents the mean ± SEM (n = 3). ***P < 0.001 versus sham side (Dunnett's multiple comparison test).
Microglia and Astrocyte were Activated in Different Time Course by Dry Eye in the Trigeminal Nucleus
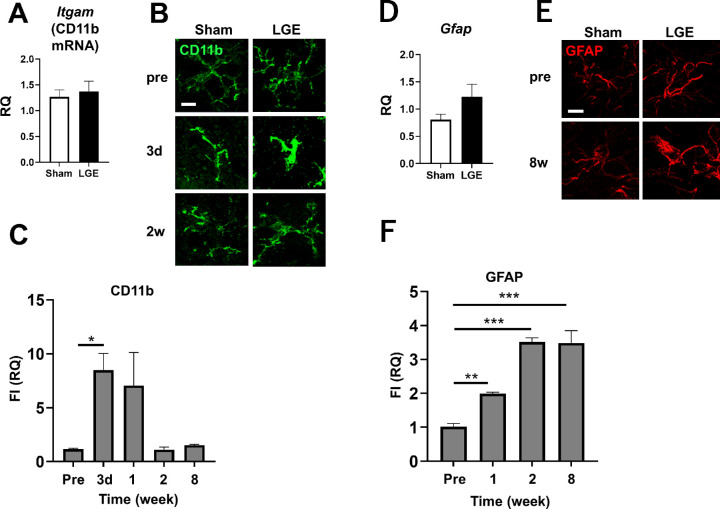
We hypothesized that dry eye–induced enhancement of pain signaling in the trigeminal nucleus involves the activation of glial cells such as microglia and astrocytes. Activation of microglia and astrocytes was therefore evaluated by quantifying the expression levels of mRNAs encoding respective markers at 8w. There was no significant difference at 8w in the mRNA level of Itgam (which encodes the microglial marker CD11b) in trigeminal nucleus between the LGE and sham sides (Fig. 5A). There was no difference in the intensity or shape of the immunostaining CD11b signal in the trigeminal nucleus between the sham and LGE sides in the pre-analysis, and the signal was uniformly distributed with small somatic cells with thin, branched projections. At three days after surgery (hereinafter referred to as “3d”), almost no change in the shape of microglia on the sham side was observed. On the LGE side, however, the microglia assumed an enlarged ameboid shape with short and thick processes, and the fluorescence intensity (FI) was significantly increased. Activation of the microglia subsided by 2w (Fig. 5B). The CD11b expression level on the LEG side, normalized to that on the sham side, was significantly enhanced and peaked (approximately eightfold) at 3d. At 2w and 8w, microglial activation returned to the preoperative level (Fig. 5C).
Figure 5.
Activation of microglia and astrocytes evaluated by the expression levels of CD11b and GFAP, respectively, in the trigeminal nucleus of LGE rats. (A) The mRNA level of CD11b in the trigeminal nucleus on the sham and LGE sides at 8w. (B) Immunofluorescence images of CD11b (green) in the trigeminal nucleus at pre, 1w and 2w. (C) Summary of CD11b protein expression at pre, 3d, 1w, 2w, and 8w. The vertical axis indicates the RQ value of total FI on the LGE side normalized to the sham side in the same section obtained from the same animal. (D) The mRNA level of GFAP in the trigeminal nucleus at 8w. (E) Representative images of GFAP (red) immunostaining in the trigeminal nucleus at pre and 8w. Scale bar: 5 µm. (F) Summarized results of GFAP protein expression at pre, 1w, 2w, and 8w. The vertical axis shows the RQ value of total fluorescence expression on the LGE side normalized to the sham side in the same section obtained from the same animal. Each bar graph represents the mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus the Sham side (Dunnett's multiple comparison test).
Astrocyte activation was analyzed by quantifying the expression level of mRNA encoding the marker GFAP (Gfap gene) in the trigeminal nucleus. At 8w, there was no significant difference in GFAP expression between the LGE and sham sides (Fig. 5D). Preoperatively, there was no difference in immunostaining of GFAP in the trigeminal nucleus between the sham and LGE sides. In contrast, at 8w, staining volume and FI were enhanced on the LGE side compared with the sham side (Fig. 5E). We also evaluated the reactive astrocytes on the LGE side relative to the sham side. Compared with the preoperative level, the level of GFAP protein on the LGE side had doubled by 1w and increased 3.5-fold from 2w through 8w (Fig. 5F). In the trigeminal nucleus on the LGE side, microglial activation peaked at 3d, and astrocyte activation began at 2w and persisted thereafter, suggesting that activation of microglia and astrocytes is involved in the onset and persistence, respectively, of hypersensitivity and hyperalgesia.
Treatment with Pregabalin, but not Ophthalmic Treatment, Ameliorated the Hypersensitivity and Hyperalgesia Induced by Dry Eye
Phenotypic analysis of dry eye model rats showed that hypersensitivity and hyperalgesia chronically persisted through 8w. Our results suggest that dry eye–induced chronic pain involves central sensitization via upregulation of α2δ–1 subunit expression and activation of both microglia and astrocytes. Therefore we investigated whether pregabalin treatment at 8w, after chronic pain was established, would ameliorate hypersensitivity and hyperalgesia. To improve the corneal epithelial damage, ophthalmic treatment (hyaluronic acid drops four times per day and ophthalmic ointment treatment at night) was first performed for two weeks beginning at 8w (designated as “E”). After ophthalmic treatment, continuous treatment with either pregabalin or saline solution was carried out for another two weeks (designated as “E + S” and “E + P”, respectively). Improvement of hypersensitivity and hyperalgesia associated with each treatment was evaluated by measuring the number of blinks and eye wipe responses at 8w, after E, and after E + S or E + P. Although hypersensitivity improved slightly with E + S, more significant improvement was observed with E + P (Fig. 6A). Treatment with E + S was not effective for hyperalgesia. In sharp contrast, E + P significantly improved hyperalgesia (Fig. 6B). These results show that pregabalin treatment effectively improved hypersensitivity and hyperalgesia, whereas the improvement in epithelial damage was not sufficient for complete recovery.
Figure 6.
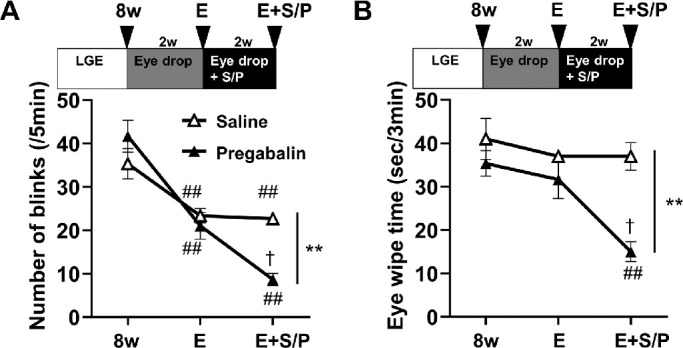
Effects of ophthalmic treatment and continuous subcutaneous administration of pregabalin on the dry eye–induced hypersensitivity and hyperalgesia in rats. Eye drop treatment only improved hypersensitivity, whereas chronic pregabalin administration further improved hypersensitivity and hyperalgesia. (A) Hypersensitivity in 8w, E, and E + S/P groups was evaluated by the number of blinks. The data for 8w were replotted from Figure 1C for comparison. (B) Hyperalgesia was measured by the eye wipe response in 8w, E, and E + S/P groups. The data for 8w are the same as those presented in Figure 1D. Each bar graph represents the mean ± SEM (n = 3). ##P < 0.01 versus 8w group (Dunnett's multiple comparison test). †P < 0.05 versus E group (Dunnett's multiple comparison test). **P < 0.01 versus E + S group (Dunnett's multiple comparison test).
Pregabalin and Ophthalmic Treatment Suppressed Neurological Activity, Increased α2δ–1 Subunit Expression, and Activated Astrocytes in the Trigeminal Nucleus, but not Ophthalmic Treatment Alone
We evaluated the therapeutic effect of E + P on the pathological mechanism in the trigeminal nucleus in comparison with 8w. Neuronal activity, evaluated as the percentage of c-Fos–positive neurons among all neurons in the trigeminal nucleus, did not differ significantly between rats pretreated at 8w versus those treated with E. In contrast, treatment with E + P ameliorated the effect on neuronal activity on the LGE side to the same extent as on the sham side (Fig. 7A). The level of α2δ–1 subunit mRNA expression did not differ significantly different between the 8w, E, and E + P groups (Fig. 7B). In addition, the level of α2δ–1 subunit protein in the trigeminal nucleus did not differ significantly between the 8w and E groups but was significantly reduced on the LGE side in the E + P group to a level almost the same as that on the sham side (Fig. 7C). We quantified the level of α2δ–1 subunit mRNA in the trigeminal ganglion using samples from three rats and found that the level of α2δ–1 subunit mRNA was markedly reduced in the E + P group compared with the 8w group, whereas there was no difference between the 8w and E groups (Supplementary Fig. S3). With regard to astrocyte activation, there was no significant difference between the 8w, E, and E + P groups (Fig. 7D). Interestingly, when evaluated based on GFAP protein expression level, astrocyte activation was significantly suppressed by E + P treatment on the LGE side to a level similar to that of the sham side, whereas there was no significant difference between the 8w and E groups (Fig. 7E). These results suggest that pregabalin treatment alleviated hyperalgesia by inhibiting neuronal activation, α2δ–1 subunit upregulation, and astrocyte activation.
Figure 7.
Chronic pregabalin administration normalized the neuronal activity, upregulation of α2δ–1 subunits, and astrocyte activation. (A) Neuronal activity was assessed by the expression of c-Fos in the trigeminal nucleus of 8w, E, and E + S/P groups. The vertical axis indicates the percentage of c-Fos positive neurons to total number of neurons. The identical data presented in Figure 2F were replotted for comparison. (B) The mRNA level of α2δ–1 in the trigeminal nucleus at 8w, E, and E + P. The data for 8w are the same as those shown in Figure 3A. (C) The expression levels of α2δ–1 in the trigeminal nucleus of the 8w, E and E + P. The vertical axis shows the RQ value of the total FI of α2δ–1 standardized on the Sham side. The data for 8w were replotted from Figure 3F. (D) Real-time PCR results of GFAP in the trigeminal nucleus of the 8w, E and E + P. The data for 8w are the same as those presented in Figure 5D. (E) The expression levels of GFAP in the trigeminal nucleus of the 8w, E and E + P. The vertical axis indicates the total FI of GFAP standardized on the Sham side. The data for 8w were replotted from Figure 5F. Each bar graph represents the mean ± SEM (n = 3). **P < 0.01, ***P < 0.001 versus 8w (Dunnett's multiple comparison test).
Discussion
A number of clinical reports have suggested dry eye involves central sensitization with neuropathic pain-like symptoms; thus development of appropriate therapeutic strategies has become an urgent issue.1,5–9,26 At the animal model level, the mechanism of peripheral sensitization of the cornea has been elucidated.27–30 Although electrophysiological studies have also suggested the involvement of central sensitization,31 the molecular mechanism in relation to dry eye–induced chronic pain has not been elucidated, and therefore treatments based on the pathological mechanism are not yet available.
In our dry eye model, hypersensitivity of the cornea appeared at 1w, hyperalgesia appeared at 2w, and the symptoms persisted thereafter (Figs. 1C, 1D). The time course of symptom progression was similar to that previously reported in a rat LGE model study.20 Sensory and pain hypersensitivity persisted long term and remained even after the corneal epithelial damage was cured by local treatment (Fig. 6A), suggesting the involvement of central sensitization and neuropathic pain. Thus the clinical symptoms of dry eye, such as persistent hypersensitivity and hyperalgesia after recovery of corneal damage, could be reproduced in dry eye model rats. It has been reported that invasion and inflammation of the unilateral cornea can cause symptoms in the contralateral eye.32 In our preliminary study, the number of blinks did not differ between the contralateral sham side and a unilateral sham surgery control group, suggesting that unilateral LGE may not have affected the contralateral sham side. However, because the change in the LGE side was evaluated relative to the sham side, the degree of the changes on the LGE side may have been partially masked.
The time course and pregabalin sensitivity of neural activation and upregulation of α2δ–1 subunit expression were very similar to the results of corneal hyperalgesia assessed as eye wipe time (Figs. 1D, 6B), suggesting that upregulation of α2δ–1 subunit expression is a critical event in dry eye–induced hypersensitivity and hyperalgesia (Figs. 3F, 7C, 8A). As for the origin of the α2δ–1 subunit upregulated in the trigeminal nucleus, it was considered to be derived from primary neurons but not second-order neurons or interneurons, because the level of α2δ–1 subunit mRNA expression on the LGE side was significantly increased in the trigeminal ganglion but not in the trigeminal nucleus (Fig. 3A). These results may reflect the accumulation of α2δ–1 subunit in primary neurons in response to sustained pain signal input to primary neurons by dry eye and the accumulation of α2δ–1 subunit in nerve endings of primary neurons projecting into the trigeminal nucleus via axonal and membrane transport, which is consistent with the mechanism reported for SDH.16,33
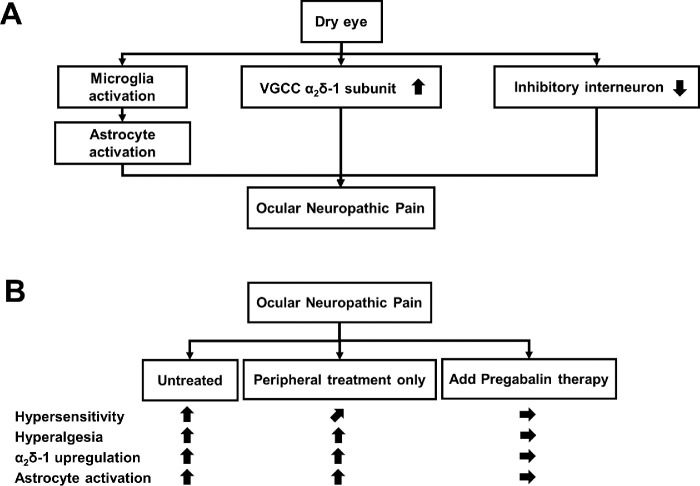
Figure 8.
Schematic representation of the summary of this study. (A) Dry eye causes ocular neuropathic pain, involving activation of microglia and astrocytes, up-regulation of α2δ–1 subunits, and reduced number of inhibitory interneurons in the trigeminal nucleus. (B) For the established ocular neuropathic pain, the treatment of corneal epithelial damage alone only slightly improved hypersensitivity but not hyperalgesia. In contrast, pregabalin administration markedly improved hypersensitivity and hyperalgesia via normalized neural activity, α2δ–1 subunit upregulation, and astrocyte activation.
In addition, the number of inhibitory interneurons on the LGE side decreased after 2w (Fig. 4A). A decrease in the number of inhibitory interneurons in the SDH has been proposed as a causative factor in neuropathic pain.34–36 Taken together, our results suggest that a decrease in the number of inhibitory interneurons in the trigeminal nucleus is involved in dry eye–induced neuropathic pain (Fig. 8A).
Microglia on the LGE side of the trigeminal nucleus exhibited transient activation at 3d and 1w (Fig. 5C). Microglial activation has been implicated as a trigger for the onset of neuropathic pain.11 Our results indicate that microglial activation in the early stages is involved in the onset of neuropathic pain and central sensitization of the trigeminal nucleus (Fig. 8A). In contrast, activation of astrocytes on the LGE side of the trigeminal nucleus started at 1w after LGE surgery, peaked at 2w, and was maintained through 8w (Fig. 5F). Sustained activation of astrocytes is reportedly responsible for the maintenance of neuropathic pain symptoms.12 Our results indicate that the sustained activation of astrocytes in dry eye is involved in the maintenance of neuropathic pain symptoms. We propose that dry eye causes ocular neuropathic pain in the trigeminal nucleus by activating microglia and astrocytes, upregulating the α2δ–1 subunit, and decreasing the number of inhibitory interneurons, resulting in hypersensitivity and hyperalgesia (Fig. 8A).
Based on the above results, we sought to develop a therapeutic strategy that would address this pathological mechanism and investigated the effect of pregabalin treatment. Pregabalin acts on the α2δ–1 subunit to inhibit excitatory synaptic transmission and synaptic remodeling.14,19,21 In the present study, pregabalin reduced the expression of α2δ–1 subunit and the number of c-FOS-positive cells that reflect neuronal activity, suggesting that pregabalin alleviates hyperalgesia via the suppression of synaptic transmission between the corneal primary and second-order neurons in the trigeminal nucleus. Interestingly, pregabalin treatment suppressed the activation of astrocytes in the trigeminal nucleus (Fig. 7F), which may have resulted from the normalization of neuronal activity due to normalization of α2δ–1 subunit expression (Figs. 7C, Supplementary Fig. S3). The mechanism underlying this effect should be elucidated in a future study. The results of pregabalin treatment in the present study provide theoretical support for reports indicating that NSAIDs are ineffective for dry eye–induced ocular neuropathic pain19 and that gabapentinoids exert therapeutic effects.19,27,37,38 Taken together, our results suggest that pregabalin treatment normalizes the level of α2δ–1 subunit protein in the trigeminal nucleus by inhibiting the production and transport of α2δ–1 subunit from the trigeminal ganglion to the nerve terminals in the trigeminal nucleus, thus restoring normal activity of corneal nerves and astrocytes (Fig. 8B). We demonstrated that pregabalin is effective for dry eye–induced chronic ophthalmic pain even after chronic pain has been established. Therefore the application of pregabalin in actual clinical practice is highly anticipated. In the present study, we clarified part of the molecular mechanism of dry eye–induced chronic corneal pain and demonstrated that pregabalin treatment is a promising therapeutic strategy based on the underlying pathological mechanism.
Supplementary Material
Acknowledgments
The authors thank Shin-ichi Morimoto for technical advice on model animal production and analysis of α2δ–1 subunit expression. The authors thank Takashi Suzuki, Takashi Itokawa, Yukinobu Okajima, and Koji Kakisu for clinical discussions.
Supported by the Toho University School of Medicine Project Research Fund (no. 19–10, 20–11) to Y.T. and by JSPS KAKENHI Grant Number JP19K09961 to Y.H.
Disclosure: Y. Tei, None; Y. Mikami, None; M. Ito, None; T. Tomida, None; D. Ohshima, None; Y. Hori, Alcon (F), Santen Pharmaceutical Co., Ltd. (F), Senju Pharmaceutical Co., Ltd. (F); S. Adachi-Akahane, None
References
- 1. Ebrahimiadib N, Yousefshahi F, Abdi P, Ghahari M, Modjtahedi BS.. Ocular neuropathic pain: an overview focusing on ocular surface pains. Clin Ophthalmol. 2020; 14: 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawashima M, Yamatsuji M, Yokoi N, et al.. Screening of dry eye disease in visual display terminal workers during occupational health examinations: the Moriguchi study. J Occup Health. 2015; 57: 253–258. [DOI] [PubMed] [Google Scholar]
- 3. Uchino M, Uchino Y, Dogru M, et al.. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014; 157: 294–300. [DOI] [PubMed] [Google Scholar]
- 4. Uchino M, Nishiwaki Y, Michikawa T, et al.. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011; 118: 2361–2367. [DOI] [PubMed] [Google Scholar]
- 5. Loeser JD, Treede RD.. The Kyoto protocol of IASP basic pain terminology. Pain. 2008; 137: 473–477. [DOI] [PubMed] [Google Scholar]
- 6. Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea? J Refract Surg. 2007; 23: 598–602. [DOI] [PubMed] [Google Scholar]
- 7. Rodrnyhsl P, Borsook D.. Ocular neuropathic pain. Br J Ophthalmol. 2016; 100: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belmonte C, Nichols JJ, Cox SM, et al.. TFOS DEWS II pain and sensation report. Ocul Surf. 2017; 15: 404–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galor A, Levitt RC, Felix ER, et al.. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2015; 29: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaggi AS, Jain V, Singh N.. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011; 25: 1–28. [DOI] [PubMed] [Google Scholar]
- 11. Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig. 2016; 7: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao YJ, Ji RR.. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010; 7: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alles SRA, Smith PA.. Etiology and pharmacology of neuropathic pain. Pharmacol Rev. 2018; 70: 315–347. [DOI] [PubMed] [Google Scholar]
- 14. Alles SRA, Cain SM, Snutch TP.. Pregabalin as a pain therapeutic: beyond calcium channels. Front Cell Neurosci. 2020; 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis SB, Williams ME, Ways NR, et al.. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988; 241: 1661–1664. [DOI] [PubMed] [Google Scholar]
- 16. Morimoto S, Ito M, Oda S, Sugiyama A, Kuroda M, Adachi-Akahane S.. Spinal mechanism underlying the antiallodynic effect of gabapentin studied in the mouse spinal nerve ligation model. J Pharmacol Sci. 2012; 118: 455–466. [DOI] [PubMed] [Google Scholar]
- 17. Li CY, Zhang XL, Matthews EA, et al.. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006; 125: 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA.. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019; 1: CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verma V, Singh N, Singh Jaggi A.. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol. 2014; 12: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meng ID, Barton ST, Mecum NE, Kurose M.. Corneal sensitivity following lacrimal gland excision in the rat. Invest Ophthalmol Vis Sci. 2015; 56: 3347–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao Y, Wang H, Chiang CY, Dostrovsky JO, Sessle BJ.. Pregabalin suppresses nociceptive behavior and central sensitization in a rat trigeminal neuropathic pain model. J Pain. 2013; 14: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiso T, Watabiki T, Tsukamoto M, et al.. Pharmacological characterization and gene expression profiling of an L5/L6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience. 2008; 153: 492–500. [DOI] [PubMed] [Google Scholar]
- 23. Bender G, Gosset J, Florian J, et al.. Population pharmacokinetic model of the pregabalin-sildenafil interaction in rats: application of simulation to preclinical PK-PD study design. Pharm Res. 2009; 26: 2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Löscher W, Brandt C.. High seizure frequency prior to antiepileptic treatment is a predictor of pharmacoresistant epilepsy in a rat model of temporal lobe epilepsy. Epilepsia. 2010; 51: 89–97. [DOI] [PubMed] [Google Scholar]
- 25. Kim KH, Lim SH, Shim CR, et al.. Development of a novel controlled-release tablet of pregabalin: formulation variation and pharmacokinetics in dogs and humans. Drug Des Devel Ther. 2020; 14: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galor A, Moein HR, Lee C, et al.. Neuropathic pain and dry eye. Ocul Surf. 2018; 16: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J.. What causes eye pain? Curr Ophthalmol Rep. 2015; 3: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masuoka T, Yamashita Y, Nakano K, et al.. Chronic tear deficiency sensitizes transient receptor potential vanilloid 1-mediated responses in corneal sensory nerves. Front Cell Neurosci. 2020; 14: 598678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. González-González O, Bech F, Gallar J, Merayo-Lloves J, Belmonte C.. Functional properties of sensory nerve terminals of the mouse cornea. Invest Ophthalmol Vis Sci. 2017; 58: 404–415. [DOI] [PubMed] [Google Scholar]
- 30. Bereiter DA, Rahman M, Thompson R, Stephenson P, Saito H.. TRPV1 and TRPM8 channels and nocifensive behavior in a rat model for dry eye. Invest Ophthalmol Vis Sci. 2018; 59: 3739–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rahman M, Okamoto K, Thompson R, Katagiri A, Bereiter DA.. Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain. 2015; 156: 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luna C, Quirce S, Aracil-Marco A, et al.. Unilateral corneal insult also alters sensory nerve activity in the contralateral eye. Front Med. 2021; 8: 767967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bauer CS, Nieto-Rostro M, Rahman W, et al.. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009; 29: 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scholz J, Broom DC, Youn DH, et al.. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005; 25: 7317–7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeilhofer HU, Benke D, Yevenes GE.. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012; 52: 111–133. [DOI] [PubMed] [Google Scholar]
- 36. Coull JA, Boudreau D, Bachand K, et al.. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003; 424: 938–942. [DOI] [PubMed] [Google Scholar]
- 37. Yoon HJ, Kim J, Yoon KC.. Treatment response to gabapentin in neuropathic ocular pain associated with dry eye. J Clin Med. 2020; 9.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paik DW, Lim DH, Chung TY.. Effects of taking pregabalin (Lyrica) on the severity of dry eye, corneal sensitivity and pain after laser epithelial keratomileusis surgery [published online ahead of print December 10, 2020]. Br J Ophthalmol, 10.1136/bjophthalmol-2020-317570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.