Abstract
Introduction
Lactate dehydrogenase (LDH) is an important oxidoreductase in the anaerobic metabolic pathway. The role of LDH in arterial stiffness (AS) and 10-year cardiovascular disease (CVD10) risk has not been established.
Methods
This retrospective, cross-sectional, and observational study evaluated the relationships between the LDH level and AS and CVD10 risk in 12,597 health-examined people (6988 men and 5609 women; mean age, 49.49 years) in China. Brachial–ankle pulse wave velocity (baPWV) was used to estimate AS. The Framingham CVD 10-year risk prediction model was used to calculate the CVD10 risk score.
Results
In both sexes, an increased LDH level was associated with increased AS and CVD10 (men: β = 0.032, P < 0.001; women: β = 0.025, P < 0.001). Half of the population with a high LDH level (≥172 U/L) showed significantly increased AS and CVD10 risk score. Men and women with baPWV ≥1400 cm/s had a higher LDH level, and the latter was significantly different from that of the group with baPWV <1400 cm/s (men: 176.93±30.99 vs 173.00±33.36, P < 0.001; women: 189.10±34.20 vs 171.39±31.08, P < 0.001). In both sexes, a higher level of LDH was noted in groups with higher CVD10 risk score (men: 176.65±32.51 vs 172.94±32.46, P < 0.001; women: 202.51±44.05 vs 175.73±32.39, P < 0.001).
Discussion
An increased LDH level may be associated with AS and CVD10 risk. The LDH level could be a new predictor of AS and CVD10 risk in health-examined populations.
Keywords: lactate dehydrogenase, arterial stiffness, 10-year cardiovascular disease risk, brachial–ankle pulse wave velocity, Framingham CVD 10-year risk prediction model
Introduction
Models of arterial stiffness (AS) and 10-year cardiovascular risk (CVD10) are used widely to assess the likelihood of cardiovascular diseases (CVDs) and to provide evidence for CVD prevention.1–3 The AS degree can be assessed using brachial–ankle pulse wave velocity (baPWV). CVD10 can be calculated using the Framingham CVD Risk score.4
Lactate dehydrogenase (LDH) is an important enzyme in the anaerobic metabolic pathway. This enzyme is present in plants and animals. It is present ubiquitously in multifarious tissues, and serves as an important “checkpoint” of gluconeogenesis and DNA metabolism.5 It is often used to diagnose myocardial infarction, tissue injury, and some types of malignant tumors. The LDH level can be used to aid the diagnosis of myocardial infarction.6,7 Komolafe et al found that serum levels of C-reactive protein, procalcitonin, and LDH could be used to aid the diagnosis of pancreatic necrosis.8 European phase-I trials have shown a high level of LDH to be an independent diagnostic factor in patients with sarcoma and other mesenchymal tumors.9 However, the role of LDH in AS and CVD10 risk has not been established.
We explored the relationship between AS and CVD10 risk and LDH level. We wished to: (i) clarify the effect of LDH on predicting AS and CVD10 risk; (ii) try to explain how LDH could be used for the early prevention of arteriosclerosis and cardiovascular events.
Methods
Ethical Approval of the Study Protocol
This study was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments. The study protocol was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2018-SR-175) in Nanjing, China. Written informed consent was obtained from all participants. Data on personal identification were anonymized and de-identified before analyses.
Study Population
The study cohort comprised 12,597 individuals (6988 men and 5609 women; mean age, 49.49 ± 10.02 years; range, 18–92 years) who visited the Health Management Center of the First Affiliated Hospital of Nanjing Medical University for a health check-up from January 2017 to December 2020. People were excluded if they had a prior history of malignancy, cardiovascular disease, uremia, or liver cirrhosis.
Participants underwent routine clinical examination, including medical history, physical examination, baPWV, and completion of the Framingham Cardiovascular Risk Questionnaire.
Measurements
After an overnight fast, samples of venous blood from the anterior cubital vein were taken while participants were resting in the sitting position. We used vacuum tubes containing ethylenediamine tetra-acetic acid or serum separation gel to collect blood samples. After blood sampling, routine hematology and biochemistry tests (total cholesterol (TC), triglycerides (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), LDH, fasting blood glucose, alkaline phosphatase (ALP)) were undertaken. The glycated hemoglobin (HbA1c) level was determined by ion-exchange high-performance liquid chromatography.
After resting peacefully for 10 min, systolic blood pressure (sBP) and diastolic blood pressure (dBP) were measured using the same automatic digital sphygmomanometer placed on the upper arm at heart level. The mean value of two measurements was used for dBP and sBP, respectively. We measured height and body weight according a protocol described previously10 and then calculated the body mass index (BMI).
baPWV
After resting for 5–10 min, participants lay supine on a test bed with their hands at the sides of their body while keeping body muscles relaxed and breathing normally. baPWV was measured by an arteriosclerosis tester (VP-1000; Omron, Tokyo, Japan). Balloon markers for the upper arms and lower legs were aligned with the brachial artery and medial sides of the lower legs, respectively. An electrocardiography sensor was placed in the left second intercostal area. The dBP and sBP of limbs were measured simultaneously. baPWV was taken as the average value measured on both sides. A “low baPWV” group (<1400 cm/s (4333 men and 4035 women)) and “high baPWV” group (≥1400 cm/s (2655 men and 1574 women)) were created.
CVD10 Risk
CVD10 risk was assessed according to the Framingham CVD 10-year risk prediction model11 based on stratified scores for sex, age, BP, TC level, HDL-C level, and smoking history. CVD10 risk <10% was defined as the “low-risk” group (4057 men and 131 women). Those with CVD10 risk >10% were considered to be in the “medium–high risk” group (2931 men and 5478 women).
Statistical Analyses
SPSS 20.0 (IBM, Armonk, NY, USA) was used to establish a database. The Shapiro–Wilk normal test was undertaken on the distribution of all indicators. Indicators conforming to a normal distribution are represented by the mean ± standard deviation, and the t-test was used for comparison between two groups. Data with a non-normal distribution are represented by the median (quartiles) [M(P25, P75)], and the comparison between groups was done by the Mann–Whitney U-test. Enumeration data are expressed as rates, and the chi-square test was used for comparison between two groups. To assess the significance of cardiovascular risk factors on AS and increased CVD10 risk, stepwise multivariate regression analyses were carried out. The final model was determined using Pin < 0.05 and Pout > 0.10. A standardized coefficient (β) and P-values are presented. P < 0.05 was considered significant.
Results
To study the new objective markers of AS and CVD10 risk, we collected the data of 12,597 individuals who underwent physical examination at the health management center of our hospital from January 2017 to December 2019 (6988 men and 5609 women), and collected their various test indicators for statistical analyses.
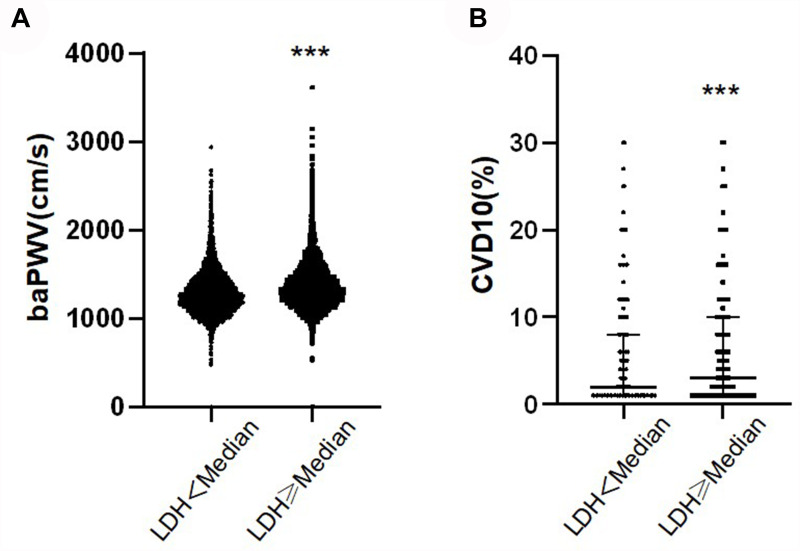
To determine the association between an increased LDH value and baPWV and CVD10 risk score, we divided participants into two groups based on the median LDH value. The high-LDH group was accompanied by high baPWV (P < 0.001) and CVD10 risk score (P < 0.001) (Table 1). Figure 1 shows the distribution of baPWV and CVD10 risk score between the two groups.
Table 1.
Clinical Characteristics of Different LDH Levels
| LDH<Median (172 IU/L) (n=6286) | LDH≥Median (172 IU/L) (n=6311) | P | |
|---|---|---|---|
| Male,n | 3568 (56.76) | 3420 (54.19) | 0.004 |
| Age,y | 47.81±9.56 | 51.16±10.20 | 0.000 |
| Smoking,n | 1199 (19.07) | 1175 (18.62) | 0.191 |
| BMI,kg/m2 | 24.24±3.12 | 25.06±3.28 | 0.008 |
| sBP,mmHg | 124.13±16.58 | 129.88±17.74 | 0.000 |
| dBP,mmHg | 76.54±11.36 | 78.93±11.38 | 0.766 |
| uric acid,umol/L | 330.18±86.67 | 335.93±87.27 | 0.769 |
| ALP,U/L | 79.40±22.35 | 88.25±25.50 | 0.000 |
| TC,mmol/L | 5.27±1.01 | 5.47±1.11 | 0.000 |
| TG,mmol/L | 1.73±1.26 | 1.83±1.48 | 0.055 |
| HDL-C,mmol/L | 1.33±0.30 | 1.35±0.32 | 0.019 |
| LDL-C,mmol/L | 3.27±0.75 | 3.40±0.80 | 0.000 |
| FBG,mmol/L | 5.52±1.26 | 5.54±1.29 | 0.726 |
| HbA1c,% | 5.61±0.76 | 5.68±0.74 | 0.242 |
| baPWV,cm/s | 1293.75±239.54 | 1375.13±263.85 | 0.000 |
| CVD10,% | 3 (1,8) | 4 (1,10) | 0.000 |
Figure 1.
baPWV and CVD10 of different LDH levels. (A) Comparison of baPWV between two groups with different LDH levels. (B) Comparison of CVD10 between two groups with different LDH levels. ***P < 0.001.
Stepwise regression analysis was employed to analyze baPWV and CVD10 risk scores and various clinical indices for the entire study cohort, respectively. This approach enabled determination of the association between each factor and AS and risk of cardiovascular events. Significant associations with baPWV were found for sex, age, smoking, BMI, sBP, dBP, as well as levels of uric acid, ALP, LDH, TC, HDL-C, LDL-C, fasting glucose, and HbA1c. The TG level alone did not show a significant association with baPWV (Table 2). Sex, age, smoking, BMI, sBP, as well as levels of ALP, LDH, TC, TG, HDL-C, LDL-C, and fasting glucose were correlated significantly with increased CVD10 risk score. However, no significant association was found between dBP, uric-acid level, and HbA1c level and CVD10 risk score (Table 2).
Table 2.
Factors Associated with baPWV or CVD10 (Stepwise Multiple Regression Analysis)
| baPWV | CVD10 | |||
|---|---|---|---|---|
| Standardized Coefficient (β) | P | Standardized Coefficient (β) | P | |
| Male,n | −0.062 | 0.000 | −0.289 | 0.000 |
| Age,y | 0.366 | 0.000 | 0.409 | 0.000 |
| Smoking,n | 0.018 | 0.008 | 0.446 | 0.000 |
| BMI,kg/m2 | −0.115 | 0.000 | −0.032 | 0.000 |
| sBP,mmHg | 0.402 | 0.000 | 0.161 | 0.000 |
| dBP,mmHg | 0.090 | 0.000 | −0.012 | 0.124 |
| Uric acid,umol/L | 0.065 | 0.000 | −0.009 | 0.152 |
| ALP,U/L | 0.033 | 0.000 | −0.062 | 0.000 |
| LDH,U/L | 0.032 | 0.000 | 0.025 | 0.000 |
| TC,mmol/L | 0.092 | 0.000 | 0.204 | 0.000 |
| TG,mmol/L | 0.005 | 0.630 | 0.028 | 0.000 |
| HDL-C,mmol/L | −0.047 | 0.000 | −0.151 | 0.000 |
| LDL-C,mmol/L | −0.068 | 0.002 | −0.035 | 0.042 |
| FBG,mmol/L | 0.080 | 0.000 | −0.044 | 0.000 |
| HbA1c,% | 0.030 | 0.004 | −0.013 | 0.125 |
Comparison of LDH values by cutoffs of baPWV (1400 cm/s) or CVD10 risk score (10%) was done for men and women, respectively (Table 3). For men and women, age, BMI, sBP, dBP, as well as levels of ALP, LDH, TC, TG, LDL-C, fasting glucose, and HbA1c with baPWV ≥1400 cm/s were higher than those of men and women with baPWV <1400 cm/s (P < 0.001 or P < 0.05) (Table 3). In men, there was no significant difference in terms of smoking, uric-acid level, or HDL-C level between the two groups (P > 0.05) (Table 3). Among women, the high-baPWV group carried a higher prevalence of smoking, but the prevalence was not significantly different between the two different baPWV-level groups. (P = 0.649) (Table 3). We divided participants into low-risk and medium–high-risk groups for cardiovascular events based on CVD10 risk score (Table 4). Except for the level of uric acid, there was no significant difference between the two groups of men (P = 0.108). Other cardiovascular risk factors were significantly different between the two groups with different CVD10 risk scores in men and women (P < 0.001) (Table 4).
Table 3.
Clinical Characteristics of the Subjects According to baPWV
| Male | Female | |||||
|---|---|---|---|---|---|---|
| baPWV <1400cm/s (n=4333) | baPWV ≥1400cm/s (n=2655) | P | baPWV <1400cm/s (n=4035) | baPWV ≥1400cm/s (n=1574) | P | |
| Age,y | 46.07±9.00 | 53.84±9.53 | 0.000 | 46.82±8.74 | 58.38±8.41 | 0.000 |
| Smoking,n | 1465(33.81) | 894(33.67) | 0.906 | 11(0.25) | 5(0.32) | 0.649 |
| BMI,kg/m2 | 25.46±3.03 | 25.75±3.03 | 0.000 | 23.10±3.00 | 24.54±3.11 | 0.000 |
| sBP,mmHg | 123.55±12.84 | 138.38±15.90 | 0.000 | 117.40±14.61 | 141.99±16.92 | 0.000 |
| dBP,mmHg | 77.65±9.64 | 86.38±10.57 | 0.000 | 70.87±9.67 | 81.02±10.23 | 0.000 |
| Uric acid,umol/L | 379.07±73.95 | 378.96±79.31 | 0.952 | 268.99±59.28 | 293.22±66.63 | 0.000 |
| ALP,U/L | 83.21±23.40 | 87.50±21.96 | 0.000 | 77.69±25.17 | 94.80±25.44 | 0.000 |
| LDH,U/L | 173.00±33.36 | 176.93±30.99 | 0.000 | 171.39±31.08 | 189.10±34.20 | 0.000 |
| TC,mmol/L | 5.32±1.04 | 5.41±1.13 | 0.001 | 5.28±1.01 | 5.66±1.12 | 0.000 |
| TG,mmol/L | 1.97±1.54 | 2.18±1.64 | 0.000 | 1.31±0.82 | 1.80±1.20 | 0.000 |
| HDL-C,mmol/L | 1.23±0.26 | 1.23±0.27 | 0.448 | 1.49±0.32 | 1.42±0.30 | 0.000 |
| LDL-C,mmol/L | 3.36±0.77 | 3.40±0.81 | 0.048 | 3.21±0.74 | 3.49±0.80 | 0.000 |
| FBG,mmol/L | 5.48±1.09 | 6.04±1.79 | 0.000 | 5.17±0.72 | 5.71±1.48 | 0.000 |
| HbA1c,% | 5.58±0.68 | 5.90±1.01 | 0.000 | 5.46±0.47 | 5.86±0.86 | 0.000 |
Table 4.
Clinical Characteristics of the Subjects According to CVD10
| Male | Female | |||||
|---|---|---|---|---|---|---|
| CVD10 <10 (n=4057) | CVD10 ≥10 (n=2931) | P | CVD10 <10 (n=5478) | CVD10 ≥10 (n=131) | P | |
| Age,y | 44.41±7.80 | 55.41±8.79 | 0.000 | 49.54±9.57 | 71.76±6.87 | 0.000 |
| Smoking,n | 629(15.50) | 1730(59.02) | 0.000 | 12(0.22) | 3(2.29) | 0.000 |
| BMI,kg/m2 | 25.34±3.06 | 25.90±2.95 | 0.000 | 23.44±3.06 | 26.23±3.22 | 0.000 |
| sBP,mmHg | 125.80±13.71 | 133.86±17.28 | 0.000 | 123.50±18.20 | 157.90±15.23 | 0.000 |
| dBP,mmHg | 79.49±10.45 | 83.01±11.08 | 0.000 | 73.56±10.78 | 80.34±11.18 | 0.000 |
| Uric acid,umol/L | 380.27±73.48 | 377.31±79.39 | 0.108 | 274.81±61.33 | 316.61±87.57 | 0.000 |
| ALP,U/L | 83.24±21.72 | 87.22±23.54 | 0.000 | 82.10±26.28 | 98.92±25.86 | 0.000 |
| LDH,U/L | 172.94±32.46 | 176.65±32.51 | 0.000 | 175.73±32.39 | 202.51±44.05 | 0.000 |
| TC,mmol/L | 5.20±1.00 | 5.57±1.13 | 0.000 | 5.38±1.05 | 5.88±1.26 | 0.000 |
| TG,mmol/L | 1.86±1.30 | 2.31±1.87 | 0.000 | 1.43±0.95 | 2.15±1.41 | 0.000 |
| HDL-C,mmol/L | 1.25±0.27 | 1.20±0.25 | 0.000 | 1.48±0.32 | 1.34±0.25 | 0.000 |
| LDL-C,mmol/L | 3.27±0.74 | 3.53±0.81 | 0.000 | 3.28±0.76 | 3.65±0.91 | 0.000 |
| FBG,mmol/L | 5.53±1.20 | 5.92±1.66 | 0.000 | 5.30±0.99 | 6.20±1.81 | 0.000 |
| HbA1c,% | 5.57±0.72 | 5.88±0.94 | 0.000 | 5.56±0.61 | 6.26±1.11 | 0.000 |
Discussion
Our cross-sectional study showed that the LDH level was positively associated with baPWV and CVD10 risk, and independent of classical cardiovascular risk factors. These effects persisted even after adjustment for drugs that alter blood-vessel function, such as antihypertensive drugs, anti-DM drugs, and cholesterol-lowering drugs.
The serum LDH level is closely related to the occurrence of CVDs and cardiovascular events.12–14 A recent meta-analysis revealed that the incidence of cardiovascular events and mortality in patients with coronavirus disease-19 was associated with an increased serum level of LDH.15 In addition, LDH is involved in lysosome activation to regulate autophagy,16 participates in tumor occurrence, and can be used as a therapeutic target for acute liver failure.17 Compared with the studies mentioned above, our study reveals the effect of the serum LDH level on vascular sclerosis and the risk of future CVD in people with preclinical CVD. We defined “high” baPWV >1400 cm/s, which has been shown to be associated with higher serum LDH levels in men and women. A “high” Framingham CVD Risk score was defined as an estimated risk >10% for a 10‐year CVD event, which was classified as low risk (<10%) and medium–high risk (≥10%).18 Recently, the use of statins or aspirin has been proposed to prevent future morbidity and mortality from CVD based on risk estimation of a 10‐year CVD event.19,20
LDH consists of two subunits: heart (H) and muscle (M). LDH activity in heart and muscle is hundreds-of-times higher than that in normal serum. Hence, if the myocardium, skeletal muscle, or other tissues are damaged, the LDH level in serum is increased significantly (especially the levels of LDH1 and LDH2). In our study, the serum LDH level was correlated with AS and CVD10, which may be explained by two main biological mechanisms. First, atherosclerotic lesions are systemic, so coronary arteries have different degrees of sclerosis, which affects the myocardial blood supply.21–23 This scenario will not cause myocardial infarction, but will cause different degrees of myocardial ischemia and damage to myocardial cells. Simultaneously, a certain concentration of LDH will be released into blood. The increased incidence of cardiovascular disease after arteriosclerosis was accompanied by increased LDH levels, suggesting that increased LDH levels also increased the risk of CVD10, which was consistent with the results of this study. Second, the high-risk factors of CVD (eg, smoking, hypertension, DM) affect the function of the cardiovascular system to varying degrees, which results in different degrees of cell damage in tissues (eg, muscle) and organs (eg, heart, liver, kidney).24,25 LDH present in various cell types is released into blood, thereby resulting in an increase in the serum LDH level. If the LDH level increases to a certain extent, it indicates serious tissue lesions, myocardial infarction, liver failure, or damage to other important organs, which is consistent with our data showing the LDH level to be positively correlated with CVD10 and baPWV.
Our study had two main limitations. First, we did not assess the effects of baPWV (eg, heart rate, peripheral arterial disease, hypertension, DM, atrial fibrillation). Second, variables which may affect the value of baPWV and CVD10 were not adjusted, which may have had an impact on the relationship between the LDH level and baPWV, and LDH level and CVD10.
Conclusions
The serum LDH level of healthy people was positively correlated with CVD10 and baPWV. The serum LDH level may be a new objective biological indicator to predict CVD and cardiovascular events, and provide a new basis for early intervention of CVD.
Acknowledgments
We appreciate the help and support from all individuals who took part in this study.
Funding Statement
This study was funded by the Social Development Project of Jiangsu Province (BE2016787), Social Development Project of Jiangsu Province (BE2016787), and Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJB320010).
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments. The study protocol was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2018-SR-175) in Nanjing, China. Written informed consent was obtained from all participants. Data on personal identification were anonymized and de-identified before analyses.
Author Contributions
All authors contributed to data analyses, drafting or revising the manuscript, agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this study.
References
- 1.Guo W, Zhu W, Wu J, et al. Triglyceride glucose index is associated with arterial stiffness and 10-year cardiovascular disease risk in a Chinese population. Front Cardiovasc Med. 2021;8:585776. doi: 10.3389/fcvm.2021.585776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128(7):864–886. doi: 10.1161/CIRCRESAHA.121.318061 [DOI] [PubMed] [Google Scholar]
- 3.Kruszyńska E, Łoboz-rudnicka M, Palombo C, et al. Carotid artery stiffness in metabolic syndrome: sex differences. Diabetes Metab Syndr Obes. 2020;25(13):3359–3369. doi: 10.2147/DMSO.S262192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petruzzo M, Reia A, Maniscalco GT, et al. The Framingham cardiovascular risk score and 5-year progression of multiple sclerosis. Eur J Neurol. 2021;28(3):893–900. doi: 10.1111/ene.14608 [DOI] [PubMed] [Google Scholar]
- 5.Farhana A, Lappin SL. Biochemistry, Lactate dehydrogenase. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January, 2021. PMID: 32491468. [PubMed] [Google Scholar]
- 6.Szunerits S, Mishyn V, Grabowska I, Boukherroub R. Electrochemical cardiovascular platforms: current state of the art and beyond. Biosens Bioelectron. 2019;15(131):287–298. doi: 10.1016/j.bios.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 7.Agarkov NM, Markelov MY, Markelova EA, Lutai YA, Volkov PS. The information content and predictive value of cardiac markers in myocardial infarction in the elderly. Adv Gerontol. 2020;33(1):82–86. [PubMed] [Google Scholar]
- 8.Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev. 2017;4(4):CD012645. doi: 10.1002/14651858.CD012645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassier PA, Polivka V, Judson I, et al. Outcome of patients with sarcoma and other mesenchymal tumours participating in Phase I trials: a subset analysis of a European Phase I database. Ann Oncol. 2014;25(6):1222–1228. doi: 10.1093/annonc/mdu108 [DOI] [PubMed] [Google Scholar]
- 10.NCD Risk Factor Collaboration (NCD-RisC). Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396(10261):1511–1524. doi: 10.1016/S0140-6736(20)31859-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn C, Kim J, Bae W. The Framingham risk score, diet, and inflammatory markers in Korean men with metabolic syndrome. Nutr Res Pract. 2012;6(3):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai C, Li Q, May HI, et al. Lactate dehydrogenase A governs cardiac hypertrophic growth in response to hemodynamic stress. Cell Rep. 2020;32(9):108087. doi: 10.1016/j.celrep.2020.108087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong SC, Kim JJ, Kim YH, Kim IS, Han JW. Serum lactate dehydrogenase activity and its isoenzyme patterns in patients with pectus excavatum. J Thorac Dis. 2019;11(10):4349–4356. doi: 10.21037/jtd.2019.09.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallam MY, El-Gowilly SM, El-Mas MM. Cardiac and brainstem neuroinflammatory pathways account for androgenic incitement of cardiovascular and autonomic manifestations in endotoxic male rats. J Cardiovasc Pharmacol. 2021;77(5):632–641. doi: 10.1097/FJC.0000000000000993 [DOI] [PubMed] [Google Scholar]
- 15.Shoar S, Hosseini F, Naderan M, Mehta JL. Meta-analysis of cardiovascular events and related biomarkers comparing survivors versus non-survivors in patients with COVID-19. Am J Cardiol. 2020;135:50–61. doi: 10.1016/j.amjcard.2020.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brisson L, Bański P, Sboarina M, et al. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell. 2016;30(3):418–431. doi: 10.1016/j.ccell.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Ferriero R, Nusco E, De Cegli R, Carissimo A, Manco G, Brunetti-Pierri N. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J Hepatol. 2018;69(2):325–335. doi: 10.1016/j.jhep.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn C, Kim J, Bae W. The Framingham risk score, diet, and inflammatory markers in Korean men with metabolic syndrome. Nutr Res Pract. 2012;Jun(3):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(6):396–404. doi: 10.7326/0003-4819-150-6-200903170-00008 [DOI] [PubMed] [Google Scholar]
- 20.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. doi: 10.1016/j.vph.2015.11.083 [DOI] [PubMed] [Google Scholar]
- 22.Kingwell BA, Ahimastos AA. Arterial stiffness and coronary ischemic disease. Adv Cardiol. 2007;44:125–138. [DOI] [PubMed] [Google Scholar]
- 23.Duprez DA, Cohn JN. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 2007;9(2):139–144. doi: 10.1007/s11883-007-0010-y [DOI] [PubMed] [Google Scholar]
- 24.Cipolla MJ, Liebeskind DS, Chan SL. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38(12):2129–2149. doi: 10.1177/0271678X18800589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leńska-Mieciek M, Korczak-Kowalska G, Bocian K, Fiszer U. Pentosidine, advanced glycation end product, in acute ischaemic stroke patients with and without atrial rhythm disturbances. Neurol Neurochir Pol. 2020;54(4):323–328. doi: 10.5603/PJNNS.a2020.0042 [DOI] [PubMed] [Google Scholar]