Abstract
Promising preliminary clinical data have stimulated research on the use of the mammalian target of rapamycin (mTOR) inhibitors in lung cancer. AC1LPSZG is an mTOR inhibitor that can significantly reduce the viability in lung adenosquamous carcinoma cell line HTB-178 cells, showing potential benefits in effective control of non-small cell lung carcinomas. In this study, a sensitive LC–MS/MS analytical method for quantification of AC1LPSZG has been developed and optimized to a running time of 3 min per sample. A linear dose–response for quantification was observed over the range of 10–5000 ng/mL in rat plasma with required precision and accuracy. High extraction recovery was achieved in the ranges of 86.87–102.51% at QC levels from rat plasma without significant matrix effect. Stability profile of AC1LPSZG in rat plasma and in extract after protein precipitation suggested that samples should be processed within 6 h after collection and stored at −80 °C until analysis within 30 days. The method was successfully applied to plasma pharmacokinetics (PK) study of AC1LPSZG in rat, showing the plasma drug concentration followed a two-compartment model.
Introduction
Nationally, lung cancer remains the leading cause of cancer deaths in the U.S. with estimated 228,000 new cases diagnosed in 2019 and 21.7% of the five-year survival rate. In general, approximately 84% of malignant types of primary lung cancers are classified as non-small lung cancer (NSCLC) compared with 13% of small cell lung cancer (SCLC) (10). As a rare subtype of NSCLC, adenosquamous carcinoma in the lung shows components of both adenocarcinoma and squamous cell carcinoma, with each comprising at least 10% of the tumor. The prognosis of adenosquamous carcinoma is generally worse than that of other NSCLS in terms of the survival rate (5). Thus, discovery and development of targeted therapies for adenosquamous carcinoma has gained more and more attention in recent years. One of the most promising targets identified in intracellular pro-oncogenic pathways is mammalian target of rapamycin (mTOR), a pathway found to be selectively dysregulated in sizable cases of NSCLC (6).
Activation of the mTOR has crucial impact on cell growth and metabolism that has been implicated in aging and many diseases, including cancer, diabetes and neurological diseases (3, 11, 16). Studies have shown that mTOR signal inhibition blocks tumor cell progression, disrupts angiogenesis and induces apoptosis and autophagy (4). The use of Rapamycin or other mTOR inhibitors, either alone or in combination with other anticancer agents has been evaluated in ongoing clinical trials with isolated successes in subsets of cancer (7). However, development of mTOR inhibitors as anticancer agents with their full therapeutic potential was yet exploited because of unfavorable pharmacokinetic properties (15).
The recently identified compound, AC1LPSZG, has revealed its potential anti-NSCLC effect as a new generation of mTORC1/2 inhibitor. Preliminary data indicated that AC1LPSZG could significantly reduce the viability of the HTB-lung tumor cells in vitro by inhibiting mTOR, while rapamycin did not increase cell death under the same test conditions (2). This study shows the great potential of AC1LPSZG to be a new anti-cancer drug and calls for further preclinical development. AC1LPSZG, whose chemical name is (2E)-3-(4-bromophenyl)-2-(phenylsulfonyl)-N-(pyridine-3-ylmethyl)prop-2-enamide (C21H17BrN2O3S) and the exact mass is 456.0 g/mol is shown in Figure 1. AC1LPSZG was evaluated by Lipinski’s rule of five (RO5), which suggests it is drug-like compound by possessing five hydrogen bond acceptors, one hydrogen bond donor, small molecular weight less than 500 Da and a partition coefficient log P value < 5 predicted from ACD/Labs software.
Figure 1.
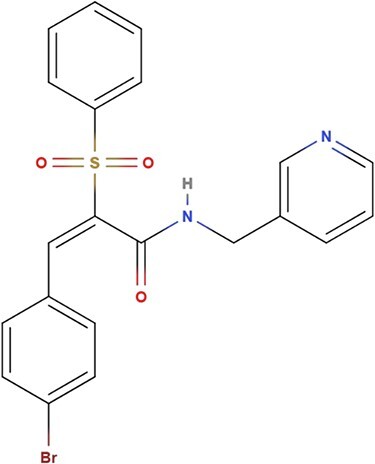
Chemical structure of AC1LPSZG
The objective of the study was to develop and validate a liquid chromatography with tandem mass spectrometry (LC–MS/MS) method for quantitation of AC1LPSZG concentration in biological samples and to apply this method in PK studies. To the best of our knowledge, there is no literature information available for quantitation of AC1LPSZG. Therefore, we conducted series of experiments to analyze its concentrations in biological samples by multiple-reaction monitoring mode (MRM) MS method with an optimized gradient LC program.
Materials and Methods
Chemicals and Materials
AC1LPSZG powder (ACS grade) was ordered from ChemBridge (San Diego, CA). Griseofulvin (as internal standard, IS), formic acid, acetonitrile (ACN), water, DMSO, PEG400, and saline solution were purchased from Millipore Sigma (St. Louis, MO). Transcutol HP was obtained from Gattefosse (Paramus, NJ). Drug-free blank rat plasma was obtained from Innovative Research and stored at −20 °C prior to use. Male Sprague–Dawley rats were purchased from Envigo (Haslett, MI) were included in the study.
Instrument and chromatography
Bioanalytical analysis was carried out on an AB SCIEX API 4000 Qtrap mass spectrometer coupled to Shimadzu 30 AD UPLC system. A Waters ACQUITY UPLC HSS T3 C18 (50 mm x 3 mm i.d., 1.8 μm, 100 Å) reverse phase analytical column controlled at 35 °C was selected to separate ingredients. A linear LC gradient profile was employed using two mobile phases (aqueous mobile phase A: 0.1% formic acid in water, organic mobile phase B: 0.1% formic acid in ACN); The flow rate was fixed at 0.5 mL/min, and 5 μL of processed samples was injected. The LC gradient profile was set at 20% organic mobile phase B for the first 0.5 min and then increased to 100% B at 2 min. It held 100% B for another 0.5 min and then decreased to the initial condition in 0.1 min for column re-equilibrium. The running time was 3 min per sample.
The analyte responses in the eluate were monitored and recorded by MS detector. Area integration, peak area measurement, calculations, and the plotting of the chromatograms were performed by SCIEX MultiQuant software v 3.0.1. Multiple-reaction monitoring mode (MRM) was employed for simultaneous quantification of AC1LPSZG and the IS in a complex mixture by monitoring pairs of m/z values associated to the precursor and its selected product ions, referred as “transitions”.
Preparation of stock solution, calibration standard, quality control, and internal standard
A stock solution of 1 mg/mL AC1LPSZG in ACN was used and stored at −20 °C. Serial dilution of the stock solution was done to make seven calibration and three quality control (QC) standards in blank rat plasma. The final concentrations for each calibration standard samples were 10, 50, 250, 1500, 2500, 4500, and 5000 ng/mL, respectively. Three levels of QC samples were also made at 15 (LQC), 1000 (MQC), and 4000 (HQC) ng/mL. Small molecular compound griseofulvin with matching eluting time was used as the internal standard (IS), which was prepared at 1 mg/mL in ACN and stored at −20 °C. The final IS spiking solution contained 100 ng/mL of griseofulvin in ACN.
Sample Preparation
Extraction of AC1LPSZG from rat plasma was carried out by protein precipitation method (8, 14). Briefly, aliquot of 50 μL of working solution (standards and QCs) or 50 μL PK study sample were spiked in to 500 μL of the final IS spike organic solution, while 50 μL blank rat plasma was made for blank samples. Samples were vortexed for 30 seconds and allowed to sit on ice for 15 min before centrifuging at 13,000 x g for 15 min at 4 °C. Partial supernatant was collected for LC–MS/MS analysis.
Method Validation
The method validation procedures followed the US Food and Drug Administration (FDA) requirement described in its “Guidance for Industry: Bioanalytical Method Validation”, addressing sensitivity, calibration curve, precision, accuracy, recovery, matrix effect, carry-over, and stability of the developed method.
Selectivity and specificity
Blank plasma of 4 rats in PK study was collected to define the selectivity and specificity of the method by evaluating any interference shown at the retention time of AC1LPSZG and IS.
Calibration curve, precision, and accuracy
Establishment of calibration curves with samples at seven points in the range of 10–5000 ng/mL was freshly prepared for accuracy and precision validation. A response factor (R) is calculated with the IS according to Equation:
R = (Ax/AIS)/(Cx/CIS).
Where A is the chromatographic peak area, C is standard concentration, X is the analyte, and IS is the internal standard.
The sensitivity evaluation was built by the measurement of the limit of detection (LOD) and the lower limit of quantitation (LLOQ). The LOD and LLOQ of the method were established with the signal-to-noise ratio (S/N) 3:1 and 10:1 respectively.
The accuracy and precision of intra- or inter-day assays were evaluated by analyzing LLOQ and QC samples in sextuplicate on the same day and repeating on three separate days. The results of accuracy and precision were expressed in relative error from the nominal drug concentration and coefficient of variation, which are generally considered as good target values for methods if they are within ±15%.
Recovery, Matrix effect, and carry-over
The recovery efficacy of AC1LPSZG in extraction procedure was evaluated at LLOQ, LQC, MQC, and HQC levels, where recovery of the IS was kept at the concentration of 100 ng/mL. The response of AC1LPSZG extracted from QC samples (n = 6, each level) was compared to corresponding one after post-extraction of spiked standard samples at the same nominal concentration. The effects of rat plasma on ionization of the analytes were determined after comparison of the response factor from post extracted QC samples (n = 6, each level) to correspondences of the analyte in neat solvent (ACN) at the same nominal concentration. Calculated recovery and matrix effect below 0.85 or above 1.15 would imply existence of poor recovery and matrix effect.
Carry-over was measured by injecting 3 blank samples after five of the ULOQ samples. It would be classified as carry-over effects if the response was more than 20% of that of LLOQ. Experiments were conducted in triplicate.
Stability
Preliminary stability assessments were conducted under five experimental conditions: stock solution, short-term, long-term, freeze–thaw, and autosampler stability. Stock solution stability was evaluated by comparing replicate QC samples with freshly-prepared and 21-day-old stock solutions of AC1LPSZG. Changing of processed sample in autosampler was appraised within 16 h windows. Stability assessments of short-term, long-term, and freeze–thaw were performed with LLOQ and QC samples in sextuplicate. Short-term and long-term matrix stability were determined on bench top at RT for 2, 4, 6, 24 h and at −80 °C for 30 days, respectively. The stability of analyte in matrix for the freeze–thaw cycles was evaluated by comparing samples after three cycles of freeze–thaw at −80 °C and RT with the freshly prepared ones. Samples with the average deviations and relative errors within ±15% of the nominal concentration were concluded to be stable at variety of experimental conditions. The stability result was used to support request for repeat analysis.
Application to a PK study in rat
The PK study was carried out in male Sprague–Dawley rats to evaluate the applicability of the developed method. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Texas Southern University. Rats (275 ± 25 g, n = 4) were housed in a standard controlled environment for 1 week and fasted for 12 h prior to drug administration. AC1LPSZG was dissolved in an optimized cosolvent system containing DMSO: PEG400: Transcutol HP: Saline solution (10:30:30:30, v/v) at 5 mg/mL. Each rat received an intravenous bolus injection of freshly prepared AC1LPSZG cosolvent solution in the jugular vein at a dose of 5 mg/kg. Approximately 200 μL of blood was collected from the jugular vein at 0, 2, 5, 15, 30, 60, 90, 120, 180, 240, 360, and 480 min. Blood samples were centrifuged at 2,000 x g for 15 min within 1 h of collection, and plasma layers were used for sample preparation within 3 h after separation and stored at −80 °C until quantitative analysis using the developed bioanalytical method.
PK Data Analysis
Plasma concentration of AC1LPSZG was used for the PK analysis. Both non-compartmental and compartmental methods were used for analyzing PK parameters with Phoenix WinNonLin software (version 8.1). The clearance (CL), the steady state volume of distribution (Vss), the area under the plasma concentration–time curve (AUC0–∞), the terminal half-life (t1/2), and the mean residence time (MRT) were computed using non-compartmental analysis (NCA) under plasma and IV bolus model, which assumes exponential decay and fits the natural logs of the concentrations values by a straight regression line. Then the clearance (Cl) and the steady state volume of distribution (Vss) were used as initial estimation for compartmental analysis.
For compartmental analysis, primary parameters were obtained by simultaneously fitting observed rat plasma concertation data implementing Phoenix two-compartmental model based on first-order estimation, the maximum likelihood (ML) criterion, and visual inspection. The secondary parameters were calculated under configuration of two-compartmental micro bolus classic modeling. A blow quantifiable limit (BQL) rule set was defined and used when the data contain concentrations that fall below the LLOQ of the assay.
Results
Method Qualification
The QTRAP 4000 mass spectrometer with a TurboIonSpray source was used in the positive mode. The instrument conditions after optimization were as follows: ion source temperature set at 500 °C with a curtain gas flow of 25 psi, nebulizer gas of 30 psi, heater gas of 25 psi, and high collision gas; ion spray voltage set at 5000 V; declustering potential 76 V for both AC1LPSZG and the IS; and collision energy 28 V for AC1LPSZG and 24 V for the IS, respectively. The quantification was performed using the following multiple reaction monitoring (MRM) transitions of the respective [M + H]+ ions: m/z 457.1 → 349.0 for AC1LPSZG, 353.3 → 285.1 for the IS, respectively. Full mass spectra and product ion spectra of AC1LPSZG and the IS are listed in Figure 2. Representative chromatogram of AC1LPSZG [LLOQ, 10 ng/mL] and Griseofulvin (IS) sample are shown in Figure 3. Retention times for AC1LPSZG and IS were 1.28 and 1.52 min, respectively without found interference.
Figure 2.
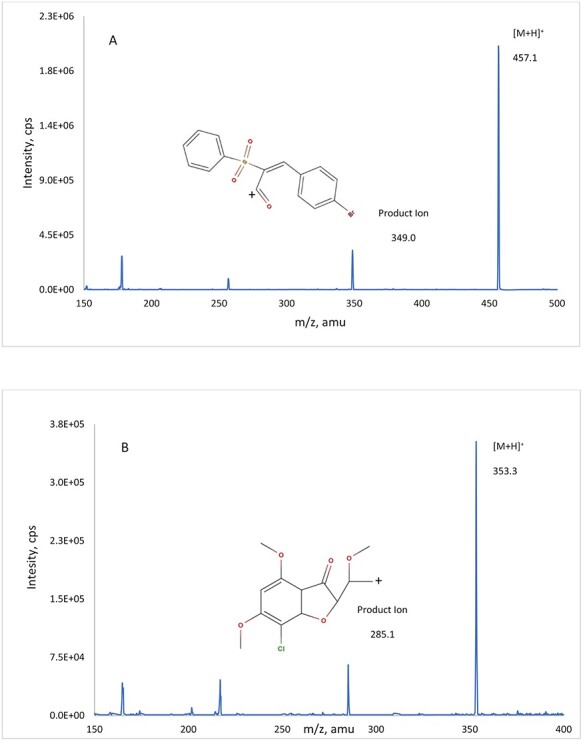
Precursor/product ion spectra and proposed fragmentation pathways for analyte AC1LPSZG (A) and internal standard Griseofulvin (B).
Figure 3.
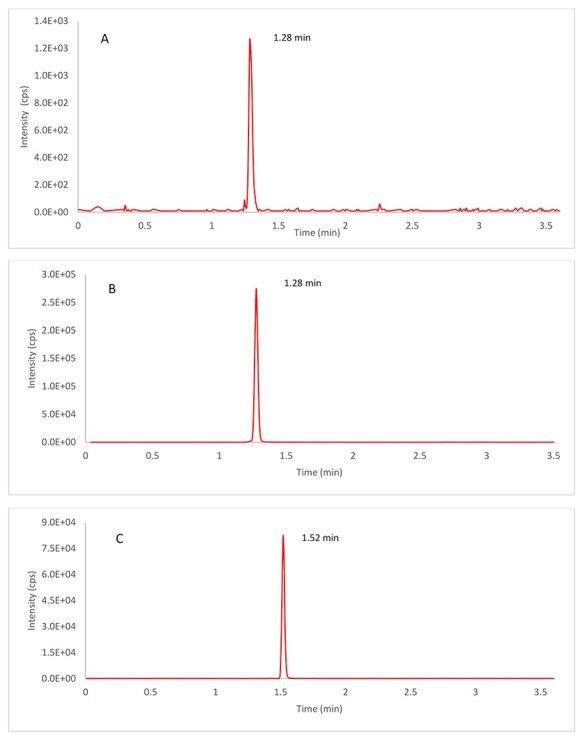
Typical chromatograms of AC1LPSZG and Griseofulvin (IS) are applied to MRM with selected precursor ions and transitions. (A) Lower limit of quantification (LLOQ, 10 ng/mL of AC1LPSZG, (B) plasma sample collected at 2 min after intravenous administration of 5 mg/kg in rat of AC1LPSZG and (C) Griseofulvin with extracted blank matrix.
The result indicated that the LC–MS response is directly proportional to the concentration of AC1LPSZG in plasma within test range. A linear regression using the response factor was weighted by 1/concentration2. The acceptance of the curve was conducted from the coefficient of determination (r2) values for the calibration curves, and the result was ≥0.999 for AC1LPSZG in the range of 10–5000 ng/mL.
The performance of the method was confirmed by evaluating the intra- and inter-day accuracy (% RE) and precision (% CV) for 6 replicates of samples at all three QC and LLOQ levels. The summary of results is presented in Table I. The qualification run met the criteria of acceptance within ±15% of accuracy and precision. Analysis of dilution factor did not perform because the ULOQ of the calibration was 5000 ng/mL, which is more than the level on demand. The carryover of AC1LPSZG and the IS were less than 0.07% and 0.01% of average peak area of LLOQ.
Table I.
Statistics of LLOQ and QC samples from Intra-day and inter-day analysis of AC1LPSZG in rat plasma
| Biological samples | Nominal concentration (ng/mL) | Intra-day (n = 6) | Inter-day (n = 18) | |||||
|---|---|---|---|---|---|---|---|---|
| Observed concentration (mean ± SD) | Accuracy (RE%) | Precision (CV%) | Observed concentration (mean ± SD) | Accuracy (RE%) | Precision (CV%) | |||
| Plasma | 10 | 10.49 ± 0.69 | 4.88 | 6.56 | 9.51 ± 079 | −4.85 | 8.29 | |
| 15 | 15.12 ± 1.14 | 0.81 | 7.51 | 14.86 ± 1.28 | −0.95 | 8.59 | ||
| 1000 | 947.68 ± 63.45 | −5.23 | 6.69 | 1023.74 ± 44.07 | 2.37 | 4.30 | ||
| 4000 | 3928.42 ± 390.36 | −1.79 | 9.94 | 4090.53 ± 223.94 | 2.26 | 5.79 | ||
The extents of the recovery of AC1LPSZG and IS were measured to demonstrate the consistent and reproducible of the method. The extraction recovery and matrix effect from different rat plasma are shown in Table II. Recovery for all QC levels were from 86.87% to 102.51%. Matrix effects were less than 15% from expected for all QC levels, suggesting that the enhancement or suppression signals are negligible.
Table II.
Recovery and matrix effect of AC1LPSZG in rat LLOQ and QC samples
| Biological samples | Nominal concentration (ng/mL) | Matrix effect (%) (n = 6) | Recovery (%) (n = 6) |
|---|---|---|---|
| Plasma | 10 | 7.66 ± 9.93 | 86.87 ± 5.42 |
| 15 | 2.35 ± 6.57 | 102.51 ± 4.98 | |
| 1000 | 10.64 ± 3.09 | 88.00 ± 3.26 | |
| 4000 | 10.98 ± 2.13 | 94.35 ± 9.12 |
The stability of AC1LPSZG under typical storage/handling conditions was accessed and summarized in Table III as the mean remaining percentages of nominal concentration (n = 6; mean ± SD). LLOQ and QC samples in rat plasma can keep integrity at RT for 6 h, while only samples incubated in post extracted matrix can meet the acceptance criteria of ±15% in all short-term, long-term, freeze–thaw and auto-sampler assays, which is identified as subgroup of RPEX in table III. As a result, it is necessary to process pharmacokinetics plasma samples containing AC1LPSZG within 6 h after collection then kept at −80 °C until analysis. Also, stock solution was stable at −4 °C for 21 days.
Table III.
Stability of AC1LPSZG in rat plasma
| Biological samples | Stability test | Nominal Concentration (ng/mL) | Calculated Concentration (ng/mL) | ||
|---|---|---|---|---|---|
| Mean ± SD (n = 6) | CV (%) | RE (%) | |||
| Plasma | auto-sampler (RPEX,16 hr) | 10 | 10.79 ± 0.66 | 6.11 | 7.94 |
| 15 | 14.36 ± 1.29 | 8.98 | -4.30 | ||
| 1000 | 1052 ± 41.66 | 3.96 | 5.26 | ||
| 4000 | 3980.15 ± 123.82 | 3.11 | -0.50 | ||
| short-term (RP, 6 hr, RT) | 10 | 10.09 ± 0.29 | 10.02 | 3.39 | |
| 15 | 15.51 ± 1.55 | 8.22 | 3.15 | ||
| 1000 | 941.07 ± 28.29 | 3.01 | -5.89 | ||
| 4000 | 4027.86 ± 222.85 | 5.53 | 0.70 | ||
| Freeze and thaw (RP, −80 °C to RT) | 10 | 5.21 ± 0.64 | 12.30 | -47.88 | |
| 15 | 10.78 ± 1.56 | 14.48 | -28.12 | ||
| 1000 | 766.65 ± 17.91 | 2.34 | -23.33 | ||
| 4000 | 2986.94 ± 36.76 | 1.23 | -25.33 | ||
| Long-term (RP, −80 °C, 30 days) | 10 | 2.16 ± 0.71 | 48.35 | -78.40 | |
| 15 | 4.34 ± 0.04 | 16.24 | -71.04 | ||
| 1000 | 438.85 ± 46.27 | 10.54 | -56.11 | ||
| 4000 | 1597.18 ± 112.35 | 7.03 | -60.07 | ||
| Long –term (RPEX, −80 °C, 30 days) | 10 | 9.92 ± 0.02 | 0.20 | -0.82 | |
| 15 | 14.46 ± 0.37 | 2.58 | -3.57 | ||
| 1000 | 834.28 ± 39.51 | 4.74 | -16.57 | ||
| 4000 | 3060.42 ± 177.06 | 4.91 | -9.76 | ||
RP, rat plasma; RT, room temperature; RPEX, rat plasma post extraction.
PK application in rats
The LC–MS/MS method was successfully used to quantify AC1LPSZG in plasma samples of male SD rats that received 5 mg/kg single IV dose. The mean plasma-level time curve for AC1LPSZG was illustrated in Figure 4A. Biphasic decay of AC1LPSZG with time suggested that the drug may follow a two-compartment model with initial rapid distributions and subsequent equilibration between tissue and plasma. Table IV shows non-compartmental and two-compartmental parameters performed by WinNonlin.
Figure 4.
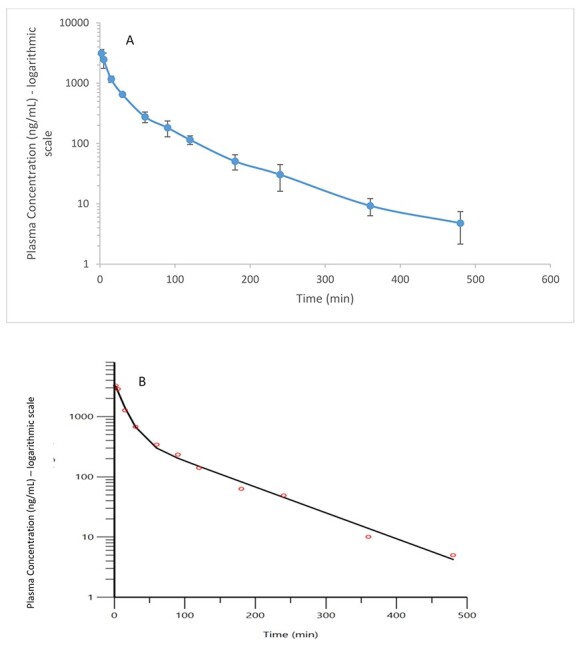
(A) The mean plasma concentration of AC1LPSZG-versus-time semi-log curves after intravenous bolus administration of 5 mg/kg of the drug in rats. Each point value represents the mean and the error bars represent the standard deviation (n = 4). (B) Predicted and observed time-concentration semi-log profiles of AC1LPSZG of one representative rat out of the four. The red circles are observed data. The curves represent the theoretical predictions by two-compartmental model using the input parameters in Table 4.
Table IV.
Non-compartmental and two-compartmental parameters of AC1LPSZG after intravenous administration of a 5 mg/kg dose (mean ± SD, n = 4)
| Parameters | IV (n = 4) | ||
|---|---|---|---|
| Unit | Non-Compartment | Two-compartment | |
| Mean | Mean | ||
| Cl | mL/(min*kg) | 59.9 ± 8.8 | 63.5 ± 8.6 |
| Cl2 | mL/(min*kg) | - | 27.6 ± 10.0 |
| Vss | mL/kg | 3152.4 ± 430.0 | 3624.5.1 ± 379.3 |
| V | mL/kg | - | 1703.3 ± 523.0 |
| V2 | mL/kg | - | 1921.2 ± 150.0 |
| AUC0–∞ | ng·min/mL | 84836.0 ± 11756.5 | 79904.2 ± 10832.0 |
| t1/2 | min | 89.9 ± 35.7 | - |
| t1/2(α) | min | - | 12.0 ± 4.1 |
| t1/2(β) | min | - | 80.4 ± 10.7 |
| MRT | min | 52.8 ± 3.4 | 57.4 ± 3.1 |
CL, central clearance; CL2, intercompartmental clearance; Vss, Steady state volume of distribution; V, volume of distribution of the central compartment; V2, volume of distribution of peripheral compartment; t1/2, terminal phase half-life; t1/2(α), ‘alpha’ distributive half-life; t1/2(β), ‘beta’ phase elimination half-life; AUC0–∞, the area under the plasma drug concentration-time curve; MRT, the mean residence time.
In the study, some concentration measurements below the LLOQ (<10 ng/mL) occurred at the end of the collection interval. So, compartmental analysis using BQL rule was explored, leading to improved accuracy in parameter estimation with lower relative standard error. The two-compartmental PK analysis of AC1LPSZ fits well to our observations, as shown in Figure 4B.
Discussion
The aim of the developed LC–MS/MS method for the quantification of AC1LPSZG was to ensure the reliability for analysis of samples from a preclinical pharmacokinetic study. To obtain a high sensitivity assay, the most intense fragment ion at m/z 349.0 of AC1LPSZG was selected, forming by breakage of the amide bond (–CO–NH–). Also, storage instability of AC1LPSZG in rat plasma may be a result of unexpected hydrolysis of the amide bond through serine hydrolases (SHs), which cleave eater, amide, or thioester bond in small molecules (1, 9). Two remote electron-rich aromatic groups can accelerate this reaction (13). The application of inhibitors to enhance stability of AC1LPSZG was limited because the previous study showed that inhibitors weakly prevented the hydrolytic cleavage (<10%) by SHs in rat plasma (12). Further storage stability studies for AC1LPSZG in blood sample should be conducted in future with pH adjustment in samples, or addition of chemical preservative.
Determination of single-dose PK behavior of AC1LPSZG allows the assessment of the reliability and selectivity of the quantitative bioanalytical method based on LC–MS/MS. The Approach with NCA established the initial exposure characteristics of the drug. The total normalized clearance of AC1LPSZG is 59.9 mL/min/kg when drug elimination occurs by first-order kinetics. If clearance is not changing, then exposure of AC1LPSZG increases linearly with dose. As a determinant of other PK parameters, including half-life, oral bioavailability, and efficacious dose, clearance data may help a medicinal chemist to improve efficiency by modulating physicochemical properties in a chemical series. Together with volume of distribution (Vd), the clearance rate impacts the terminal elimination half-life (t1/2) of 89.9 min. The developed method quantified samples in the linear range up to 6 h, representing more than 4 elimination half-lives of the drug, enough to eliminate > 90% of the administered dose. In addition, Vss scales well with animal species that have body For the purpose of exploring PK variability due to intrinsic factors (e.g., age, sex) and extrinsic factors hereafter, a two-compartmental model with WinNonlin was developed based upon nonlinear regression analysis. The clearance rate in the two-compartmental model is very consistent with that of NCA, while there is variability in the output of Vss between compartmental and noncompartmental analysis since the assumptions used is somewhat different. However, AC1LPSZG with a high Vss of 3624.5.1 mL/kg or 3152.4 mL/kg has a propensity to well distribute in the body or only weight similar to volume, useful for determining dosing regimen in preclinical studies. The two-compartmental PK analysis provided a visual depiction of the rate processes of AC1LPSZG and exhibited how many pharmacokinetic constants are required to express the process properly. In addition, the derivations of generated PK constants are expected to improve developed PK study by optimizing sampling duration, sampling interval, etc.
Conclusion
In this study, a fast, specific and reliable LC–MS/MS method was developed, and applied for the determination of AC1LPSZG in rat plasma, as part of the preclinical drug development process in search of novel anti-NSCLC drug. The bioanalytical method was validated and aligned with FDA guidelines. A simple protein precipitation using a minimal plasma sample of 50 μL and a 3 min running time achieved significant savings on cost and time. The method has a decent LLOQ of 10 ng/mL in plasma. The stability profile of AC1LPSZG indicates PK samples should be prepared within 6 h after collection and stored in −80 °C prior to analysis. A two-compartmental model in rats was rationally constructed using Phoenix WinNonLin and was applied to fit the PK profile of AC1LPSZG in rats. Further investigation of novel formulations of AC1LPSZG and PK study of other administrative routes are undergoing in our research lab for in-depth preclinical development of AC1LPSZG.
Acknowledgement
This study was funded in part by the National Institute of Health’s Research Centers in Minority Institutes Program (RCMI, G12MD007605), Cancer Prevention & Reseach Institute of Texas (CPRIT) Core Facilities Support Awards RP180748 and CPRIT ETRA grant DP150086.
Contributor Information
Yuan Chen, Department of Pharmaceutical and Environmental Health Sciences, College of Pharmacy and Health Sciences, Texas Southern University, Houston, TX, USA.
Xiuqing Gao, Department of Pharmaceutical and Environmental Health Sciences, College of Pharmacy and Health Sciences, Texas Southern University, Houston, TX, USA.
Ritu Gupta, Department of Pharmaceutical and Environmental Health Sciences, College of Pharmacy and Health Sciences, Texas Southern University, Houston, TX, USA.
Jing Ma, Department of Pharmaceutical and Environmental Health Sciences, College of Pharmacy and Health Sciences, Texas Southern University, Houston, TX, USA.
Ruhee Dere, Center for Precision Environmental Health, Baylor College of Medicine, Houston, TX, USA.
Dong Liang, Department of Pharmaceutical and Environmental Health Sciences, College of Pharmacy and Health Sciences, Texas Southern University, Houston, TX, USA.
Huan Xie, Department of Pharmaceutical and Environmental Health Sciences, College of Pharmacy and Health Sciences, Texas Southern University, Houston, TX, USA.
References
- 1. Bradshaw, P.R., Wilson, I.D., Gill, R.U., Butler, P.J., Dilworth, C., Athersuch, T.J.; Metabolic hydrolysis of aromatic amides in selected rat, minipig, and human in vitro systems; Scientific Reports, (2018); 8: 2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cregg, J., Kenyon, C., Zhang, P., Ang, K.-H., Arkin, M.; Compounds and methods for promoting stress resistance. the United States. In (2017April 13).
- 3. Dazert, E., Hall, M.N.; mTOR signaling in disease; Current Opinion in Cell Biology, (2011); 23: 744–755. [DOI] [PubMed] [Google Scholar]
- 4. Faes, S., Santoro, T., Demartines, N., Dormond, O.; Evolving significance and future relevance of anti-Angiogenic activity of mTOR inhibitors in cancer therapy; Cancers, (2017); 9(11): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Filosso, P.L., Ruffini, E., Asioli, S., Giobbe, R., Macri, L., Bruna, M.C. et al. ; Adenosquamous lung carcinomas: A histologic subtype with poor prognosis; Lung Cancer, (2011); 74: 25–29. [DOI] [PubMed] [Google Scholar]
- 6. Fumarola, C., Bonelli, M.A., Petronini, P.G., Alfieri, R.R.; Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer; Biochemical Pharmacology, (2014); 90: 197–207. [DOI] [PubMed] [Google Scholar]
- 7. Hua, H., Kong, Q., Zhang, H., Wang, J., Luo, T., Jiang, Y.; Targeting mTOR for cancer therapy; Journal of hematology & oncology, (2019); 12: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang, S., Bian, X., Sivils, J., Neckers, L.M., Cox, M.B., Xie, H.; Quantification of a new anti-cancer molecule MJC13 using a rapid, sensitive, and reliable liquid chromatography-tandem mass spectrometry method; American journal of modern chromatography, (2014); 1: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 9. Long, J.Z., Cravatt, B.F.; The metabolic serine hydrolases and their functions in mammalian physiology and disease; Chemical Reviews, (2011); 111: 6022–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lung and Bronchus Cancer — Cancer Stat Facts . (n.d.). Retrieved June 16, 2020, from https://seer.cancer.gov/statfacts/html/lungb.html
- 11. Papadopoli, D., Boulay, K., Kazak, L., Pollak, M., Mallette, F., Topisirovic, I. et al. ; mTOR as a central regulator of lifespan and aging. [version 1; peer review: 3 approved]; F1000Research, (2019); 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ratnatilaka Na Bhuket, P., Jithavech, P., Ongpipattanakul, B., Rojsitthisak, P.; Interspecies differences in stability kinetics and plasma esterases involved in hydrolytic activation of curcumin diethyl disuccinate, a prodrug of curcumin; RSC Adv., (2019); 9: 4626–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samaritoni, J.G., Copes, A.T., Crews, D.K., Glos, C., Thompson, A.L., Wilson, C. et al. ; Unexpected hydrolytic instability of N-acylated amino acid amides and peptides; The Journal of Organic Chemistry, (2014); 79: 3140–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu, X., Zhou, Q., Korfmacher, W.A.; Development of a low volume plasma sample precipitation procedure for liquid chromatography/tandem mass spectrometry assays used for drug discovery applications; Rapid Communications in Mass Spectrometry, (2005); 19: 2131–2136. [DOI] [PubMed] [Google Scholar]
- 15. Yuan, R., Kay, A., Berg, W.J., Lebwohl, D.; Targeting tumorigenesis: Development and use of mTOR inhibitors in cancer therapy; Journal of hematology & oncology, (2009); 2: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zoncu, R., Efeyan, A., Sabatini, D.M.; mTOR: From growth signal integration to cancer, diabetes and ageing; Nature Reviews. Molecular Cell Biology, (2011); 12: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


