Abstract
Aims
To determine whether myocardial fibrosis and greyzone fibrosis (GZF) on cardiovascular magnetic resonance (CMR) is associated with ventricular arrhythmias in patients with coronary artery disease (CAD) and a left ventricular ejection fraction (LVEF) >35%.
Methods and results
In this retrospective study of CAD patients, GZF mass using the 3SD method (GZF3SD) and total fibrosis mass using the 2SD method (TF2SD) on CMR were assessed in relation to the primary, combined endpoint of sudden cardiac death, ventricular tachycardia, ventricular fibrillation, or resuscitated cardiac arrest. Among 701 patients [age: 65.8 ± 12.3 years (mean ± SD)], 28 (3.99%) patients met the primary endpoint over 5.91 years (median; interquartile range 4.42–7.64). In competing risks analysis, a GZF3SD mass ≥5.0 g was strongly associated with the primary endpoint [subdistribution hazard ratio (sHR): 17.4 (95% confidence interval, CI 6.64–45.5); area under receiver operator characteristic curve (AUC): 0.85, P < 0.001]. A weaker association was observed for TF2SD mass ≥23 g [sHR 10.4 (95% CI 4.22–25.8); AUC: 0.80, P < 0.001]. The range of sHRs for GZF3SD mass (1–527) was wider than for TF2SD mass (1–37.6).
Conclusions
In CAD patients with an LVEF >35%, GZF3SD mass was strongly associated with the arrhythmic endpoint. These findings hold promise for its use in identifying patients with CAD and an LVEF >35% at risk of arrhythmic events.
Keywords: Cardiovascular magnetic resonance, Coronary artery disease, Sudden cardiac death, Ventricular tachycardia, Ventricular fibrillation, Greyzone scar, Myocardial fibrosis
Graphical Abstract
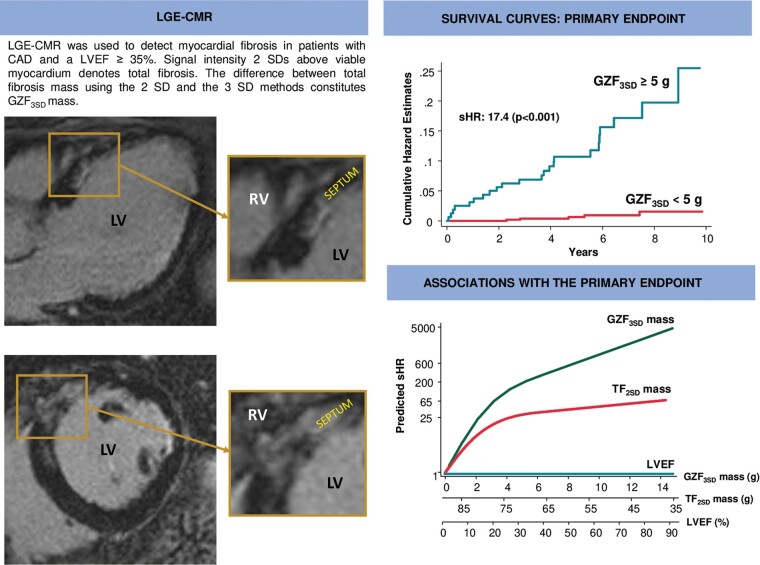
Late gadolinium enhancement cardiovascular magnetic resonance (LGE-CMR) was used to detect myocardial fibrosis in patients with coronary artery disease (CAD). As shown in the horizontal long axis (left upper panel) and short-axis views (left lower panel) of the left ventricle, areas of greyzone fibrosis are typically heterogeneous, often harbouring ‘channels’ in between areas of myocardial fibrosis. As shown in the survival curves (right upper panel), the proportional risk of the primary endpoint increased with increasing greyzone fibrosis mass using the 3SD method (GZF3SD). In comparison to left ventricular ejection fraction (LVEF) and total fibrosis mass, this risk, expressed in terms of subdistribution hazard ratios (sHRs) from competing risks analyses, was much higher for GZF3SD than for total fibrosis mass using the 2SD method, or LVEF (right lower panel). LV, left ventricle; RV, right ventricle.
What’s new?
Greyzone fibrosis mass using the 3SD method (GZF3SD) mass was more strongly associated with ventricular arrhythmias than total fibrosis mass using the 2SD method or left ventricular ejection fraction (LVEF) in coronary artery disease (CAD) patients with an LVEF >35%.
Whether or not appropriately selected patients with CAD and LVEF >35% benefit from implantable cardioverter-defibrillators should be addressed in randomized, controlled trials.
Introduction
Current guidelines recommend implantable cardioverter-defibrillators (ICDs) for the prevention of sudden cardiac death (SCD) in survivors of ventricular fibrillation (VF) or sustained ventricular tachycardia (VT), and for its primary prevention in patients with a left ventricular ejection fraction (LVEF) ≤30% or ≤35%, depending on functional class.1 These cut-offs of LVEF were based on the inclusion criteria of the core ICD trials rather than on a previous validation of LVEF as a predictor of ventricular arrhythmias or benefit from ICDs. Within these cut-offs, there is no doubt that ICDs are effective. However, most primary prevention ICD recipients selected on the basis of an LVEF ≤35% do not receive therapy from the device. On the other hand, most patients succumbing to SCD have an LVEF >35%, both after myocardial infarction (MI) and in the general population.2
Several authorities,2 including the National Heart, Lung and Blood Institute and Heart Rhythm Society,3 have recognized the inability of LVEF to predict arrhythmic events and have called for alternative risk stratification tools. Cardiovascular magnetic resonance (CMR) has emerged as a promising alternative. Myocardial fibrosis (MF), identified using late gadolinium enhancement, has been linked to ventricular arrhythmias in numerous studies.4,5 In addition, the so-called greyzone fibrosis (GZF), an admixture of MF and viable tissue thought to be a substrate for ventricular arrhythmias, identifiable with CMR, has also been shown to be associated with ventricular arrhythmias in clinical studies.6
The recent Cardiovascular Magnetic Resonance—Sudden Cardiac Death (CMR-SCD) study, which included patients with coronary artery disease (CAD) and a wide range of LVEFs, showed that GZF was as a strong predictor of SCD and a composite, arrhythmic endpoint of SCD, resuscitated cardiac arrest, VF or VT.7 It also showed that 49% of SCDs occurred in patients with an LVEF >35%. The present subanalysis of the CMR-SCD study explores whether MF and GZF are associated with arrhythmic events in patients with CAD and an LVEF >35%.
Methods
This is a subanalysis of the Cardiovascular Magnetic Resonance for the prediction of Sudden Cardiac Death (CMR-SCD) study,7 a retrospective study of patients from a single centre (University Hospitals Birmingham, Queen Elizabeth, UK) referred for a CMR scan from July 2010 to August 2017. Approval was obtained from local Ethical Committee and the hospital’s Clinical Audit Department, both of which waived the need for patient consent for retrospectively acquired data. The study conforms with the Declaration of Helsinki.
In the CMR-SCD study,7 clinical documentation and CMR reports were retrospectively screened to determine whether patients satisfied inclusion and exclusion criteria. Inclusion criteria were: CAD, defined as: (i) previous MI, percutaneous coronary intervention or coronary artery bypass grafting; or (ii) coronary angiography showing ≥70% stenosis in ≥1 coronary artery or ≥50% stenosis of the left main stem; or (iii) MF on CMR in an ischaemic distribution; and (iv) LVEF >35% for the purposes of the present subanalysis. Exclusion criteria were as follows: congenital heart disease or adult cardiac disease other than CAD, including valvular disease and channelopathies; a previous cardiac arrest or episode of VT or VF outside the context of a MI; a cardiac implantable electronic device implanted prior to the CMR scan, excluding implantable loop recorders; incomplete follow-up, defined as inability to corroborate from clinical records whether complete information on clinical events was available in our centre or referring centres. Patients who had a cardiac implantable electronic device after the CMR scan were not excluded.
Cardiovascular magnetic resonance
A 1.5 Tesla scanner (MAGNETOM Symphony or Avanto: Siemens, Erlangen, Germany) and a phased array cardiac coil, were used as previously described.7 Late gadolinium enhancement CMR were acquired as short-axis slices and a segmented inversion-recovery sequence 10 min after the intravenous administration of gadolinium contrast (Magnevist® or Gadovist® at 0.15–0.2 mmol/kg). Myocardial fibrosis was quantified using CMR42 software (Circle Cardiovascular Imaging, Calgary, Canada) by an investigator who was blinded to patient outcomes.
The signal threshold vs. reference myocardium technique was used to quantify to quantify total fibrosis mass using the 2SD method (TF2SD) and GZF mass using the 3SD method (GFZ3SD). To this end, remote, nulled myocardium was identified manually as reference. Total fibrosis mass was quantified using a semiautomatic detection algorithm and defined in terms of signal-intensity thresholds above reference myocardium. In the CMR-SCD, GZF3SD was the most consistent measure in predicting SCD and arrhythmic events.7 The difference between TF2SD and the TF using the 3SD method8 constitutes GZF3SD. Both TF2SD and GZF3SD were expressed in grams by multiplying enhanced area by slice thickness. Volume was converted to mass using the myocardial specific gravity of 1.055 g/mL.
Endpoints
The primary endpoint was the composite arrhythmic endpoint of SCD, resuscitated cardiac arrest, sustained VT or VF, or appropriate ICD shock. A ventricular rhythm faster than 100 b.p.m. lasting at least 30 s or requiring termination due to hemodynamic instability, by antitachycardia pacing or shocks was regarded as sustained VT. In patients with defibrillation devices, electrograms were reviewed to determine arrhythmias and to decide whether delivered shocks were appropriate or inappropriate. Only appropriate shocks were considered in the arrhythmic endpoint. In patients without devices, ECG documentation of arrhythmias was required. Reports of an arrhythmia without ECG documentation were discounted. The ancillary endpoint was total mortality. Investigators who were blinded to clinical and imaging data collected clinical outcome data every 6 months from medical records. Event adjudication was undertaken by the investigators 6-monthly, up to May 2020. A national health tracking system that provides vital status data to all hospitals in the UK (SPINE Portal) was used to check survival status. Causes of death were adjudicated on the basis of hospital records and correspondence from primary care physicians. Deaths were classified as unknown if no definitive data was found in hospital or primary care records.
A SCD was defined as a natural, unexpected death due to cardiac causes, heralded by an abrupt loss of consciousness within one hour of the onset of acute symptoms.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD). Normality was tested using the Shapiro–Wilk test. Cumulative incidence curves were used to assess cumulative survival. Cox proportional hazard models were used to assess relative risks. In analyses where the comparator had zero events and the partial likelihood converged to a finite value, Firth’s penalized partial likelihood correction was applied to Cox regression models.9 Fine and Gray proportional subdistribution hazard models10 and the cumulative incidence function were used in competing risks analyses. Death other than SCD and without ventricular arrhythmias was used in competing risk in analyses of the primary endpoint, the first event was used for censoring.
In exploratory analyses, all patients who met the primary endpoint had MF on visual assessment. Absolute mass for both TF2SD and GZF3SD was used in statistical analyses. For the assessment of MF, TF2SD and GZF3SD mass were imputed as zero if there was no MF on visual assessment. In all models, absolute mass was entered as continuous and dichotomous variables, and the latter were defined empirically using the optimal cut-offs associated with the Youden index in receiver operating characteristic analyses, bootstrapped (500 replications) to estimate confidence intervals (CIs). Differences between areas under the receiver operator characteristic curves (AUCs) were assessed using De Long’s test. Variables which achieved a P < 0.10 on univariate analyses for the primary endpoint were included in multivariable models. Statistical analyses were undertaken using Stata15 (StataCorp, Texas; ‘stcrreg’ for Fine and Gray models). The R package (The R Foundation for Statistical Computing, Vienna) ‘coxphf’ (run on R version 3.6.5) was used for Firth’s penalized partial likelihood correction.
Results
The derivation of the CMR-SCD study cohort has been previously described.7 Our focus in the present subanalysis were the 701 patients with an LVEF >35% from the original cohort of 979. As shown in Table 1, patients who met the primary endpoint were more likely to have had a MI (P = 0.001) and obstructed coronary arteries on angiography (P = 0.025). In addition, they had higher left ventricular end-systolic and end-diastolic volumes (both P = 0.001), a lower LVEF, a higher TF2SD mass and a higher GZF3SD mass (both P < 0.001).
Table 1.
Baseline characteristics
| All | Events | No Events | P-value | |
|---|---|---|---|---|
| N | 701 | 28 | 673 | |
| Age, years | 65 ± 12.4 | 66.4 ± 11.8 | 64.9 ± 12.5 | 0.539 |
| Sex (male) | 517 (73.8) | 24 (85.7) | 493 (73.3) | 0.142 |
| Previous cardiac events, n (%) | ||||
| Myocardial infarction | 411 (59.4) | 25 (89.3) | 386 (57.4) | 0.001 |
| Percutaneous coronary intervention | 224 (32.4) | 11 (39.3) | 213 (31.7) | 0.396 |
| Coronary artery bypass | 133 (18.9) | 9 (32.1) | 124 (18.4) | 0.070 |
| Co-morbidities, n (%) | ||||
| Diabetes mellitus | 118 (17.0) | 6 (21.4) | 112 (16.6) | 0.507 |
| Hypertension | 187 (26.9) | 8 (28.6) | 179 (26.6) | 0.817 |
| Coronary angiography | ||||
| Obstructed coronary arteries | 490 (69.9) | 26 (92.9) | 464 (68.9) | 0.025 |
| Unobstructed coronary arteries | 70 (9.99) | 1 (3.57) | 69 (10.3) | |
| Not done | 141 (20.1) | 1 (3.57) | 140 (20.8) | |
| LV CMR volumetric variables | ||||
| Absolute | ||||
| LVEDV, mL | 151.9 ± 51.6 | 206.1 ± 56.9 | 149.7 ± 50.2 | <0.001 |
| LVESV, mL | 72.7 ± 39.8 | 113.3 ± 44.3 | 71.1 ± 38.7 | <0.001 |
| LV mass, g | 137.4 ± 44.5 | 160.2 ± 61.8 | 136.5 ± 43.4 | 0.006 |
| LVEF, % | 54.6 ± 13.4 | 46.8 ± 9.7 | 54.9 ± 13.4 | 0.002 |
| Indexed | ||||
| LVEDVi, mL/m2 | 78.2 ± 26.2 | 98.7 ± 23.4 | 77.4 ± 25.9 | <0.001 |
| LVESVi, mL/m2 | 37.5 ± 20.6 | 54.9 ± 20.1 | 36.8 ± 20.3 | <0.001 |
| LV mass, g/m2 | 72.1 ± 20.5 | 79.6 ± 23.9 | 71.8 ± 20.3 | 0.049 |
| LV CMR fibrosis variables | ||||
| TF2SD mass, g | 15.4 ± 17.3 | 34 ± 19.6 | 14.7 ± 16.8 | <0.001 |
| GZF3SD mass, g | 2.92 ± 2.66 | 6.9 ± 3.3 | 2.76 ± 2.5 | <0.001 |
| Myocardial fibrosis pattern, n (%) | ||||
| Present | 572 (81.6) | 28 (100) | 544 (80.8) | 0.010 |
| Transmural | 357 (50.9) | 21 (75) | 336 (49.9) | 0.009 |
| Subendocardial | 204 (29.1) | 6 (21.4) | 198 (29.4) | 0.362 |
| Mixed | 9 (1.28) | 1 (3.57) | 8 (1.19) | 0.272 |
| Focal | 2 (0.29) | 0 | 2 (0.3) | 0.773 |
CMR, cardiovascular magnetic resonance; GZF3SD, greyzone fibrosis using the 3SD method; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEDVi, left ventricular end-diastolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVESVi,= left ventricular end-systolic volume indexed to body surface area; TS2SD, total fibrosis mass using the 2 SD method.
Variables are mean ± standard deviation or n (%).
All patients had complete follow-up until May 2020. Over a follow-up period of 5.91 years (median; interquartile range 4.42–7.64), 181 (25.8%) patients died, 119 (17.0%) from known causes and 62 (8.84%) from unknown causes. No patient underwent cardiac transplantation or left ventricular assist device implantation. A total of 28 (3.99%) patients met the primary endpoint. The first event within this composite endpoint was SCD in 13 (1.85%) and ventricular arrhythmias in 15 (2.14%) (Table 2).
Table 2.
Events
| Event | N (%) |
|---|---|
| Primary endpoint | |
| Sudden cardiac death, ventricular tachycardia/fibrillation | 28 (3.99) |
| Sudden cardiac death | 13 (1.85) |
| Ventricular tachycardia/ventricular fibrillation | 15 (2.14) |
| Ancillary endpoint | |
| Total mortality | 181 (25.8) |
Variables are expressed as n (%).
Greyzone fibrosis
In receiver-operating characteristic analyses, the AUC for GZF3SD (0.85) with respect to the arrhythmic endpoint was higher than for total fibrosis (0.80) or LVEF (0.68) (Figure 1). For the primary endpoint, a GZF3SD mass ≥5.0 g was associated with a positive predictive value of 14.4% and a negative predictive value of 99.1%. For SCD, a GZF3SD mass ≥5.0 g was associated with a positive predictive value of 6.88% and a negative predictive value of 99.6%.
Figure 1.
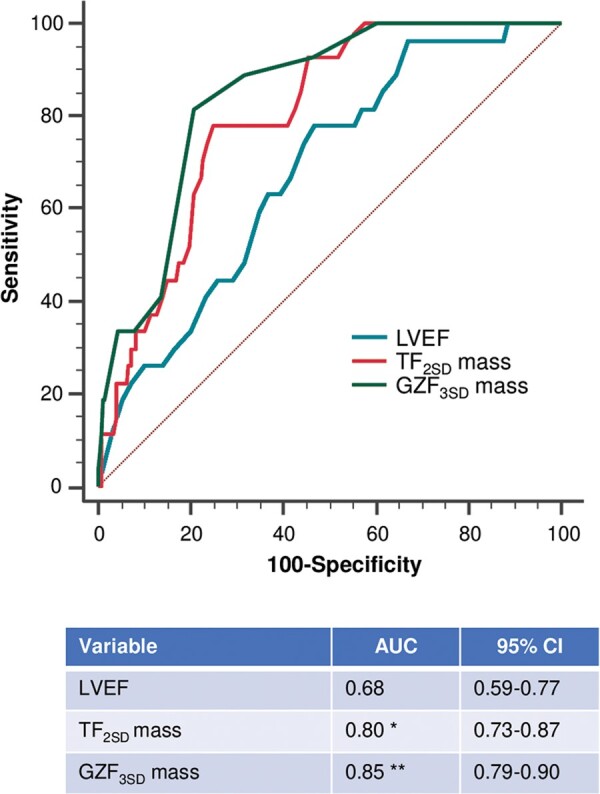
Receiver operator characteristic curves. Graph shows receiver operator characteristic curves for LVEF and myocardial fibrosis measures as predictors of the primary endpoint. *P = 0.008 for comparison between total fibrosis mass using the 2SD method (TF2SD) and LVEF. **P < 0.001 for comparison between greyzone fibrosis using the 3 SD method (GZF3SD) and LVEF; P = 0.034 for comparison between GZF3SD and TF2SD. Differences were assessed using De Long’s test. AUC, area under curve; LVEF, left ventricular ejection fraction.
When death other than SCD and no ventricular arrhythmias was adopted as a competing risk in univariate analyses, GZF3SD mass (per g) was strongly associated with the arrhythmic endpoint [subdistribution hazard ratio (sHR): 1.52, 95% CI 1.37–1.70] (Table 3): compared to GZF3SD <5.0 g, a GZF3SD mass ≥5.0 g was associated with a higher risk of the primary endpoint [hazard ratio (HR) of 17.4 (95% CI 6.64–45.5)] (Figure 2A). As shown in Figure 3A, the range of predicted sHRs for GZF3SD mass was considerably wider than for TF2SD mass, from 1 to 526.6
Table 3.
Univariate and multivariable analyses
| Univariate analyses |
Multivariable analyses |
|||||
|---|---|---|---|---|---|---|
| sHR | 95% CI | P-value | sHR | 95% CI | P-value | |
| Primary endpoint | ||||||
| MF presencea | 12.3 | 1.74–1563 | 0.005 | 10.3 | 1.44–1305 | 0.013 |
| TF2SD mass, per g | 1.04 | 1.03–1.06 | <0.001 | 1.04 | 1.03–10.6 | <0.001 |
| GZF3SD mass, per g | 1.52 | 1.37–1.70 | <0.001 | 1.48 | 1.32–1.67 | <0.001 |
| LVEF, per % | 0.95 | 0.92–0.98 | <0.001 | 0.95 | 0.92–0.98 | 0.003 |
| Total mortality | ||||||
| MF presence | 1.35 | 0.89–2.04 | 0.161 | 1.19 | 0.78–1.81 | 0.412 |
| TF2SD mass, per g | 1.01 | 1.00–1.01 | 0.108 | 1.01 | 1.00–1.02 | 0.060 |
| GZF3SD mass, per g | 1.07 | 1.02–1.03 | 0.006 | 1.08 | 1.02–1.14 | 0.007 |
| LVEF, per % | 0.97 | 0.96–0.98 | <0.001 | 0.98 | 0.97–0.99 | <0.001 |
Results from competing risks analyses with respect to the primary are expressed in terms of subdistribution hazard ratios (sHRs) and 95% confidence intervals (95% CIs). Results for total mortality were derived from Cox proportional hazards models, without correction for competing risks. Detailed results of the multivariable models are shown in Supplementary material online, Tables S2 and S3.
GZF3SD, greyzone fibrosis using the 3SD method; LVEF, left ventricular ejection fraction; MF, myocardial fibrosis; TF2SD, total fibrosis using the 2 SD method.
From Firth’s penalized likelihood method.
Figure 2.
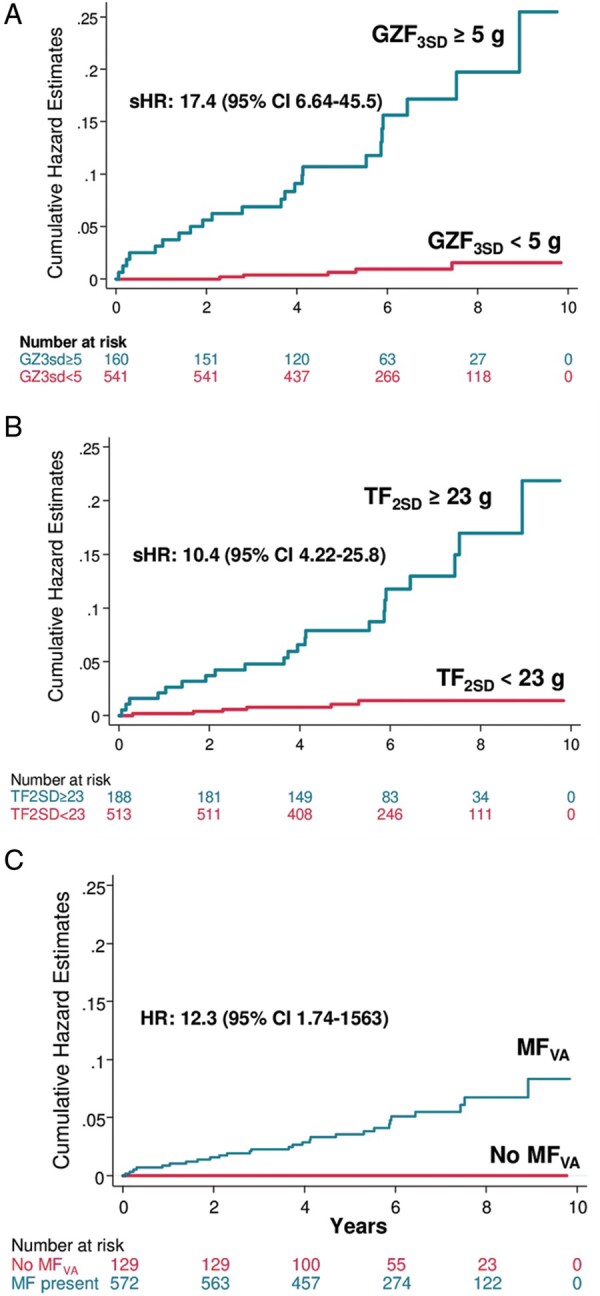
Events according to myocardial fibrosis measures. Cumulative hazards estimates from competing risks analyses for the arrhythmic endpoint, expressed in terms of subdistribution hazard ratios (sHRs) and 95% confidence intervals (CIs). Patients were dichotomized according to (A) greyzone fibrosis mass quantified using the 3SD method (GZF3SD); (B) total fibrosis mass using the 2 SD method (TF2SD); and, (C) presence (MFVA) or absence of myocardial fibrosis on visual assessment. In (C) competing risks analyses were not undertaken for MFVA, given zero events in the comparator group.
Figure 3.

Comparative risks of myocardial fibrosis measures and LVEF. Cubic splines (with 3 knots) showing predicted subdistribution hazard ratios (sSHR) from competing risks analyses for greyzone mass using the 3SD method (GZF3SD), total fibrosis mass using the 2 SD method (TF2SD) and LVEF in relation to the primary end point. Reference values were: 0 g for GZF3SD and TF2SD and 35% for LVEF. LVEF, left ventricular ejection fraction.
In multivariable analyses, GZF3SD mass (per g) predicted the primary endpoint (adjusted HR 1.48; 95% CI 1.32–1.67) after adjustment for a history of MI or coronary artery bypass. (Table 3 and Supplementary material online, Table S2).
In both univariate (HR 1.07, 95% CI 1.02–1.03) and multivariable (adjusted HR 1.08, 95% CI 1.02–1.14) analyses adopting age and sex as covariates, GZF3SD mass emerged as predictor of total mortality (Table 3 and Supplementary material online, Table S3). As shown in Table 4, GZF3SD mass (adjusted HR 1.57; 95% CI 1.38–1.80) was the strongest predictor of the primary endpoint when TF2SD mass and LVEF were included as covariates.
Table 4.
Multivariable analyses: comparison between total fibrosis, greyzone fibrosis, and LVEF
| sHR | 95% CI | P-value | |
|---|---|---|---|
| Primary endpoint | |||
| TF2SD mass, per g | 0.99 | 0.97–1.02 | 0.486 |
| GZF3SD mass, per g | 1.57 | 1.38–1.80 | <0.001 |
| LVEF, per % | 0.99 | 0.95–1.03 | 0.558 |
| Total mortality | |||
| MF presence | 0.91 | 0.57–1.48 | 0.716 |
| TF2SD mass, per g | 0.99 | 0.97–1.00 | 0.055 |
| GZF3SD mass, per g | 1.09 | 0.99–1.21 | 0.066 |
| LVEF, per % | 0.97 | 0.96–0.98 | <0.001 |
Results from competing risks analyses with respect to the primary are expressed in terms of subdistribution hazard ratios (sHRs) and 95% confidence intervals (95% CIs).
GZF3SD, greyzone fibrosis using the 3SD method; LVEF, left ventricular ejection fraction; MF, myocardial fibrosis; TF2SD, total fibrosis using the 2 SD method.
Total fibrosis burden
When death other than SCD and no ventricular arrhythmias was adopted as a competing risk in univariate analyses, TF2SD mass (per g) was strongly associated with the primary endpoint (sHR: 1.04, 95% CI 1.03–1.06) (Table 3): compared to TF2SD mass <23.0 g, a TF2SD mass ≥23.0 g was associated with a higher risk (HR 10.4, 95% CI 4.22–25.8) (Figure 2B). As shown in Figure 3B, predicted sHRs for TF2SD mass ranged from 1 to 37.6.
In multivariable analyses, TF2SD mass predicted the primary endpoint (HR 1.04, 95% CI 1.32–1.67), after adjustment for a history of MI or coronary artery bypass (Table 3 and Supplementary material online, Table S2).
In contrast to GZF3SD, TF2SD failed to emerge as a predictor of total mortality in univariate or multivariable analyses (Table 3 and Supplementary material online, Table S3).
Myocardial fibrosis on visual assessment
All patients who met the primary endpoint had MF on visual assessment. In Cox univariable analyses with bias correction for zero events in the group without MF on visual assessment, MF on visual assessment was strongly associated with the primary endpoint (HR 12.3, 95% CI 1.74–1563, P = 0.005) (Figure 2C).
Left ventricular ejection fraction
As shown in Table 3, LVEF was associated with the primary endpoint in both univariate (sHR: 0.95, 95% CI 0.92–0.98) and multivariable (adjusted sHR: 0.95, 95% CI 0.92–0.98) analyses. The distribution of predicted sHRs for LVEF against the primary endpoint is shown in Figure 3. In addition, LVEF was associated with total mortality in both univariate (HR 0.97, 95% CI 0.96–0.98) and multivariable analyses (adjusted HR 0.98, 95% CI 0.97–0.99) (Table 3 and Supplementary material online, Table S2).
In a subanalysis of patients with an LVEF ≥50% (n = 403), 10 patients met the primary endpoint (SCD in 4, VT in 2 and VF in 2). As shown in Supplementary material online, Table S4, GZF3SD mass predicted the primary endpoint on univariate analyses (HR 1.51, 95% CI 1.35–1.68). A weaker association was observed for LVEF (HR 0.90; 95% CI 0.82–0.99).
Correlations
As expected, GZF3SD correlated strongly with TF2SD (r = 0.83, P < 0.001) (Supplementary material online, Table S5).
Discussion
This is the first study to explore GZF in relation to SCD and ventricular arrhythmias in CAD patients with an LVEF >35%,11 who currently fall outside clinical guideline indications for primary prevention ICD therapy. Several findings have emerged. First, a relatively low proportion (3.99%) met the primary arrhythmic endpoint. Second, all patients succumbing to the primary endpoint had MF on visual assessment. Third, GZF3SD mass was more strongly associated with the primary endpoint than TF2SD mass or LVEF. Last, a GZF3SD mass <5 g, almost excluded a risk of the primary endpoint.
Left ventricular ejection fraction
Currently, primary prevention ICDs are indicated in patients with an LVEF ≤35% or ≤30%, in line with the inclusion criteria of the ICD trials. No trial, however, has explored primary prevention ICDs in patients with LVEF >35%. In this context, we should consider that in the general population, most individuals succumbing to SCD have an LVEF >35%. In the Oregon Sudden Unexpected Death Study, covering a population of 660 486 individuals, the retrospectively assessed LVEF before SCD was ≤35% in 30%, between 36% and 54% in 22% and ≥55% in 48%.2 In other words, 70% of individuals succumbing to SCD in the general population had an LVEF >35%.
On this basis, we may ask whether increasing the cut-off of LVEF could capture more patients at risk of SCD and ventricular arrhythmias. However, LVEF is a poor predictor of SCD and arrhythmic events. Even in ICD recipients with an LVEF ≤35%, the minority receive therapy from the device. In a study of 1729 ICD recipients, the 12-year cumulative incidence were 36% for appropriate therapy.12 In the Detect Long-term Complications after ICD Replacement (DECODE) registry of 332 recipients of cardiac resynchronization with defibrillation devices (primary and secondary prevention; 52% with ischaemic cardiomyopathy), appropriate ICD therapy had occurred in 37% by the time of device replacement.13 In a study of 1151 patients, 68% of whom had ischaemic cardiomyopathy, a first appropriate shock was observed in 23% patients after a mean follow-up of 4.0 years.14 Together, these findings illustrate that a minority of ICD recipients, even with an LVEF ≤35%, do not receive therapy from the implanted ICD. Given the low event rate in patients with an LVEF >35%, simply raising the cut-off of LVEF to capture patients at risk is likely to lead to overtreatment. A parameter other than LVEF would be desirable in patient selection.
Greyzone fibrosis
The early demonstration that GZF provides a substrate for ventricular arrhythmias15 has been corroborated by numerous studies. Using CMR and electroanatomic mapping in patients undergoing VT ablation, Piers et al. showed that critical VT isthmus sites in CAD patients are typically located in close proximity to the transition zone between ‘borderzone’, or greyzone, and transmural scars on CMR.16 The correlation between critical VT isthmus sites and CMR-derived borderzone channels through dense scar areas have also been shown by others.17 Together, these findings suggest that GZF detected on CMR denotes a critical mix between fibrosis and viable myocytes that promotes slow conduction and re-entrant VT. Several small studies6,18–20 have indeed supported a link between GZF and arrhythmic events. Importantly, however, these studies mainly included patients with an LVEF ≤35% implanted with an ICD. The novel finding from the present study is that the association between GZF and ventricular arrhythmias also applies to CAD patients with an LVEF >35%.
In the CMR-SCD study, neither TF2SD mass or GZF3SD mass were associated with total mortality, whereas significant associations were observed in the present subanalysis. A possible explanation is that the original cohort of the CMR-SCD study also included patients with an LVEF ≤35% who are at a higher risk of death from other competing causes, such as heart failure. It is possible, therefore, that the relationship between GZF3SD and total mortality is ‘diluted’ by a low LVEF.
Total fibrosis
Whilst GZF may act as the substrate for VT re-entry circuits, areas of dense MF are also required to create the isthmus. In imaging terms, both GZF and dense MF are required for arrhythmogenesis. It is therefore not surprising that MF, visually assessed and without distinction of GZF from dense scar, predicts arrhythmic events. In this respect, multiple meta-analyses of studies in patients with CAD or non-ischaemic cardiomyopathy points towards an association between the presence of MF on visual assessment and arrhythmic events.5 These findings are supported by the present study, in which all patients meeting the primary endpoint had MF on visual assessment. Moreover, we observed a graded strength of the association between increasing TF2SD mass and arrhythmic events.
Clinical application
The patient population included herein is not currently indicated for primary prevention ICDs. Our findings should not be interpreted as justification for using ICDs in patients with an LVEF >35% and a high GZF3SD mass, as we have only addressed arrhythmic events, rather than ‘benefit’ from ICD therapy. Although the event rate was relatively low, a high GZF3SD mass has the potential for the identification of CAD patients with an LVEF >35% at risk of arrhythmic events.
Speculatively, one could propose offering ICDs to patients with an LVEF >35% and a GZF3SD ≥5 g. In our cohort, this would potentially apply to 160/701 (23%) of CAD patients with an LVEF >35%, which would amount to a substantial increase in ICD implantation beyond current guidelines. However, with reference to all patients included in the CMR-SCD study,7 selecting patients on the basis of a GZF3SD ≥5 g alone, would also reduce potential ICD implantations in the LVEF ≤35% population. Whilst, overall, prescription of ICDs would increase using this strategy, there would be less overtreatment of patients with an LVEF ≤35% and less undertreatment in those with an LVEF >35%. Conversely, one could use the negative predictive value of GZF3SD ≥5 g, which in this study was 99.1% for the primary endpoint and 99.6% for SCD. On this basis, one could be reasonably certain that if GZF is <5 g, the risk of an arrhythmic event is extremely low.
Limitations
Prominent amongst the limitations of the present study is its retrospective nature. However, it reflects ‘real-world’ clinical practice during long-term follow-up. Importantly, this study included patients with complex CAD referred to a tertiary CMR centre, an aspect which may account for the relatively high event rate. Its findings, therefore, may not be generalizable to other populations, particularly asymptomatic patients. Unfortunately, we have no data on New York Heart Association class, atrial rhythm or renal function, all of which are relevant to ventricular arrhythmias and their competing risks. We cannot discount the possibility that these variables may be relevant to arrhythmic risk stratification, either alone or in combination. The absence of these data also precludes comparisons with similar patient cohorts. Clinical application of our findings will require external validation in prospective studies, ideally using an independent endpoint committee. The number of deaths from unknown causes (n = 62) could influence the results, despite competing risks analyses. We have not compared the CMR variables included herein with the numerous other parameters that are known to be of value in arrhythmic risk stratification.3 However, the strength of the observed associations would appear to be on a much higher scale than those observed for other parameters. The study was underpowered for SCD and therefore, we cannot comment on GZF3SD in relation to SCD per se.
Conclusions
In CAD patients with an LVEF >35%, GZF3SD mass was more strongly associated with the primary, arrhythmic endpoint than LVEF or TF2SD mass. These findings are consistent with the observation that GZF is a substrate for ventricular arrhythmias. On this basis, quantification of GZF3SD mass has the potential for identifying patients with CAD and an LVEF >35% at risk of arrhythmic events and, possibly, those who may benefit from ICD therapy.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We are grateful to Medtronic Plc and Boston Scientific for their support in funding this study, in the form of unrestricted educational grants.
Funding
Medtronic Plc provided funding for Dr Abbasin Zegard’s salary as a research fellow and had no participation whatsoever in the study. Boston Scientific provided funding for Dr Tian Qiu as a Senior Statistician and had no participation whatsoever in the study. No additional funding was obtained.
Conflict of interest: F.L. is a consultant with and has received research funding from Medtronic Inc., Boston Scientific, Abbott, Microport and Biotronik. Other authors declare no conflicts of interest.
Data availability
The summary data generated in this research will be shared on reasonable request to the corresponding author.
References
- 1. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018;138:e272–391. [DOI] [PubMed] [Google Scholar]
- 2. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–6. [DOI] [PubMed] [Google Scholar]
- 3. Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO et al. Sudden cardiac death prediction and prevention report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010;122:2335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Disertori M, Rigoni M, Pace N, Casolo G, Masè M, Gonzini L et al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1046–55. [DOI] [PubMed] [Google Scholar]
- 5. Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail 2017;5:28–38. [DOI] [PubMed] [Google Scholar]
- 6. Acosta J, Fernandez-Armenta J, Borras R, Anguera I, Bisbal F, Marti-Almor J et al. Scar characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT study. JACC Cardiovasc Imaging 2018;11:561–72. [DOI] [PubMed] [Google Scholar]
- 7. Zegard A, Okafor O, de Bono J, Kalla M, Lencioni M, Marshall H et al. Myocardial fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol 2021;77:29–41. [DOI] [PubMed] [Google Scholar]
- 8. Yan A, Shayne A, Brown K, Gupta S, Chan C, Luu T et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 2006;114:32–9. [DOI] [PubMed] [Google Scholar]
- 9. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27–38. [Google Scholar]
- 10. Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001;57:114–9. [DOI] [PubMed] [Google Scholar]
- 11. Haghbayan H, Lougheed N, Deva DP, Chan KKW, Lima JAC, Yan AT. Peri-infarct quantification by cardiac magnetic resonance to predict outcomes in ischemic cardiomyopathy: prognostic systematic review and meta-analysis. Circ Cardiovasc Imaging 2019;12:e009156. [DOI] [PubMed] [Google Scholar]
- 12. van der Heijden AC, Borleffs CJ, Buiten MS, Thijssen J, van Rees JB, Cannegieter SC et al. The clinical course of patients with implantable cardioverter-defibrillators: extended experience on clinical outcome, device replacements, and device-related complications. Heart Rhythm 2015;12:1169–76. [DOI] [PubMed] [Google Scholar]
- 13. Narducci ML, Biffi M, Ammendola E, Vado A, Campana A, Potenza DR et al. Appropriate implantable cardioverter-defibrillator interventions in cardiac resynchronization therapy-defibrillator (CRT-D) patients undergoing device replacement: time to downgrade from CRT-D to CRT-pacemaker? Insights from real-world clinical practice in the DECODE CRT-D analysis. Europace 2018;20:1475–83. [DOI] [PubMed] [Google Scholar]
- 14. Seegers J, Conen D, Jung K, Bergau L, Dorenkamp M, Lüthje L et al. Sex difference in appropriate shocks but not mortality during long-term follow-up in patients with implantable cardioverter-defibrillators. Europace 2016;18:1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verma A, Marrouche NF, Schweikert RA, Saliba W, Wazni O, Cummings J et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J Cardiovasc Electrophysiol 2005;16:465–71. [DOI] [PubMed] [Google Scholar]
- 16. Piers SRD, Tao Q, de Riva Silva M, Siebelink H-M, Schalij MJ, van der Geest RJ et al. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging 2014;7:774–84. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Armenta J, Berruezo A, Andreu D, Camara O, Silva E, Serra L et al. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2013;6:528–37. [DOI] [PubMed] [Google Scholar]
- 18. Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2009;2:183–90. [DOI] [PubMed] [Google Scholar]
- 19. de Haan S, Meijers TA, Knaapen P, Beek AM, van Rossum AC, Allaart CP. Scar size and characteristics assessed by CMR predict ventricular arrhythmias in ischaemic cardiomyopathy: comparison of previously validated models. Heart 2011;97:1951–6. [DOI] [PubMed] [Google Scholar]
- 20. Zeidan-Shwiri T, Yang Y, Lashevsky I, Kadmon E, Kagal D, Dick A et al. Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm 2015;12:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The summary data generated in this research will be shared on reasonable request to the corresponding author.