Abstract
Infiltration of maternal peripheral leukocytes into the uterine tissues is a critical event occurring prior to, during and after term labor (TL). Here we investigate the contribution of uterine smooth muscle (myometrium) and pregnant endometrium (decidua) to the inflammatory process during human TL. We hypothesize that labor-related physiological inflammation is orchestrated by uterine-secreted cytokines, which dually activate the uterine vascular endothelium and maternal leukocytes to promote their adhesion and infiltration into the uterus. Using Luminex and ELISA assays we examine a full range of cytokines (45 proteins) in media conditioned by primary decidual and myometrial cells from TL and term not in labor (TNL) women. The effect of conditioned media on the activation of human uterine microvascular endothelial cells (UtMVEC) was measured by qPCR, and on peripheral leukocytes, by flow cytometry. Transendothelial migration of calcein-labeled primary leukocytes towards media was assessed by fluorometry. Stromal decidual cells secrete significantly higher levels of multiple cytokines compared to myometrial cells (P<0.05), and significantly more cytokines during TL than TNL. These cytokines activate UtMVEC through the upregulation of cell adhesion molecule VCAM-1, and peripheral leukocytes – by upregulation of CD11b. Furthermore, multiple cytokines secreted from the TL decidua and myometrium significantly increase migration of granulocytes, monocytes, and lymphocytes compared to TNL (P<0.05), which was blocked by a Broad Spectrum Chemokine Inhibitor (FX125L). These data reveal the critical role for decidual- and myometrial-secreted cytokines in the activation of inflammatory pathways leading to labor. We suggest that these pathways represent targets for therapeutic intervention during PTL.
Keywords: uterus, term labor, myometrium, decidua, Broad Spectrum Chemokine Inhibitor, cytokines, immune cells
Introduction
Human birth is a complex event driven by endocrine, mechanical, and inflammatory signals that converge to cause an increase in uterine contractility that aid in delivery of the baby. In all mammals the uterus remains quiescent throughout gestation, but at term transforms to develop powerful contractions that expel the mature fetus (1, 2). Advances in research have led to a better understanding of the multifactorial process of human labor, yet the precise physiologic mechanisms that trigger term labor (TL) contractions remain elusive. Studies suggest that spontaneous TL in human is characterized by an increased abundance of inflammatory proteins (cytokines) that are secreted by the fetal membranes, cervix, and uterine tissues, i.e. decidua (pregnant endometrium) and smooth muscle (myometrium) (3–9). It is believed that these cytokines activate circulating maternal peripheral leukocytes (3, 4), as well as the uterine vascular endothelium (10) to allow for leukocyte infiltration into the different uterine compartments (11).
A primary site for the infiltration of maternal peripheral immune cell is the decidua, the innermost layer of the uterus that contributes to the maternal-fetal interface and provides an environment that supports blastocyst implantation and embryonic/fetal development (3, 12, 13). Throughout gestation the decidua modulates immune cell interactions in the surrounding uterine compartments, primarily the myometrium and cervix (4–6, 11, 18). At term, the decidua and activated decidual leukocytes induces a cascade of inflammation culminating in the production of large amounts of prostaglandins (PGs), that promotes myometrial activation and contractility (19). In addition, the myometrium also acts as an immune regulatory tissue by modulating the synthesis and release of cytokines, and providing a strong chemotactic stimulus for leukocyte infiltration (20, 21). However, prior studies have focused on one particular uterine tissue type, thus a comprehensive model of to how these complex interactions mediate labor-associated inflammatory pathways has not been fully elucidated. In particular, the cytokine interactions between the myometrium and decidua are incompletely understood. Moreover, to date no human in vitro comparative analysis exists that simultaneously assessed the multiple cytokines secreted by myometrial and decidual cells.
Recruitment of immune cells from the peripheral circulation to the site of inflammation in the reproductive tract is mediated by a family of soluble cytokines, called chemokines, which also activate specific leukocyte subsets (22–36). Targeting cytokine signaling to prevent pathologic uterine activation in high-risk pregnant women may represent a useful therapeutic approach for PTB prevention. Recently a new class of drugs that can simultaneously block multiple chemokine signalling pathways, called Broad Spectrum Chemokine Inhibitors (BSCIs) has been developed (36). The ability of BSCIs to improve different diseases (e.g. allergic asthma, surgical adhesion formation, rheumatoid arthritis, HIV replication, and endometriosis) has been demonstrated in animal models (36, 37–44). Our own in vivo animal studies showed that prophylactic administration of the BSCI was able to prevent PTL induced by Lipopolysaccharide (LPS) in pregnant mice (37) and by Group B Streptococcus (GBS) in pregnant monkeys (45). Though, the exact mechanism by which FX125L was able to fully block PTL in the pregnant macaque model remains to be determined.
We hypothesize that labor-related physiological inflammation is orchestrated by uterine-secreted pro-inflammatory cytokines, which dually activate the uterine vascular endothelium and maternal leukocytes to promote their adhesion and infiltration into the uterus. To test this hypothesis, we developed an in vitro system to 1) examine the full profile of cytokines/chemokines secreted by human decidua and myometrium in preparation for labor; 2) determine whether maternal peripheral leukocytes, and uterine vascular endothelium are activated by secreted cytokines/chemokines, leading to increased trans-endothelial migration of immune cells into the uterine tissues. 3) We also used this complex in vitro model to test whether BSCI (FX125L) can block the migration of maternal leukocytes through the uterine endothelium in response to cytokines, as one of the possible mechanisms to prevent labour contractions.
Methods
Ethics:
This study was carried out in accordance with the protocol approved by the Research Ethics Board, Sinai Health System REB# 02–0061A and REB# 04–0024E. All subjects donating myometrial biopsies and/or placental tissues for research gave written informed consent in accordance with the Declaration of Helsinki. All research using human tissues was performed in a class II certified laboratory by qualified staff trained in biological and chemical safety protocols, and in accordance with Health Canada guidelines and regulations.
Tissue Collection:
After receiving written consent, whole placentae and myometrium biopsies from healthy women undergoing term not in labor elective cesarean sections (TNL, placenta: N=19, myometrium: N=17) or spontaneous term labor (TL, placenta: N=7, myometrium: N=6) were collected and transferred from the operating theatre to the laboratory (See Supplemental Table 1 “Patients Demographics”). After the delivery of fetus and placenta, a small 1.5 cm sliver of myometrium was collected from the upper margin of the uterine incision made in the lower uterine segment in both laboring and non-laboring women. Decidual samples were collected from the maternal surface of the placenta and fetal membranes (detailed protocol in (46)).
Primary decidual cell isolation (described in (46)):
Whole placentae were placed in the fume hood and decidua paretalis was gently scraped off the maternal side of the placenta and associated chorioamniotic membranes with a cell scraper and collected in Hanks Balanced Salt Solution with Ca2+ and Mg2+ (HBSS+/+). Decidual tissue was washed twice and enzymatically digested for 20 min at 37°C in a shaking water bath in an enzymatic solution containing HBSS−/−, Fetal Bovine Serum (FBS, Wisent), collagenase 2 (2 mg/ml, Sigma-Aldrich), trypsin inhibitor (0.1 mg/ml, Sigma-Aldrich), DNase 1 (0.15 mg/ml, Sigma-Aldrich), Bovine Serum Albumin (BSA, 1 mg/ml, Wisent). The cells were pelleted and re-suspended in RPMI media containing 2% FBS; red blood cells were lysed using Erythrocyte Lysis Buffer (Qiagen, Hilden, Germany) for 20 minutes on ice. The cells were washed again, re-suspended in complete growth media (RPMI supplemented with 10% FBS), and seeded at 5 million cells/10 cm tissue culture plate. The cells were grown in 8% oxygen, 5% CO2 balanced N2 tri gas incubator at 37°C.
Primary myometrial cell isolation (described in (47)):
Myometrial biopsies were collected in HBSS+/+ supplemented with 1% Penicillin/Streptomycin (Pen/Strep, Lonza, Basel, Switzerland). Biopsies were mechanically separated using small scissors and 1mm pieces were enzymatically digested in buffer containing collagenase 2 (1 mg/ml, Sigma-Aldrich, ON, Canada), BSA (1 mg/ml, Wisent), DNase 1 (0.15 mg/ml, Sigma-Aldrich), trypsin inhibitor (0.1 mg/ml, Sigma-Aldrich). The cells were centrifuged at 250g for 15 minutes, washed and re-suspended in DMEM (Invitrogen) containing 20% FBS (Wisent) + 1% Pen/Strep (Lonza), seeded in a 10 cm tissue culture plate (Eppendorf) and grown until confluent.
Conditioned media (CM) collection:
For decidual cell cultures, cells were cultured for 48 hours, then washed with HBSS−/− to remove growth medium/serum and then incubated with 10 ml of RPMI serum free media (SFM, supplemented with sodium pyruvate, non-essential amino acids [ThermoFisher Scientific, MA, USA], Insulin-Transferrin-Selenium [ITS, Invitrogen]) for 48 hours. Myometrial cells were cultured until 80–90% confluent, with media changed every two days, washed and serum starved for 48 hours using serum free DMEM (DMEM, Pen/Strep, ITS). Both types of CM (decidual/DCM and myometrial/MCM) were collected, centrifuged for 7 minutes at 4000 rpm at 4°C, supplemented with 5mM L-Glutamine, aliquoted and stored in −20°C freezer until future use.
Multiplex cyto/chemokine assay:
Forty-five different human cytokines were screened in the TNL MCM and DCM using the 5-plex and 40-plex panels of the Bio-Plex Pro Human Chemokine Assays (Bio-Rad, CA, USA) and 27 different human cytokines were screened in TL DCM and MCM using 27-plex Pro Human Cytokine Assays (Bio-Rad) following manufacturer’s instruction manual. The list of human cytokines analyzed in three assays is listed in Supplemental Table 2–4. Samples and standards were run in duplicate. All washing stages were performed using a HydroFlex microplate magnetic washer (Tecan Group Ltd., Switzerland). Data was acquired on the Bio-Plex 200 System with High-Throughput Fluidics. For each cytokine, standard curves were generated on the five-parameter logistic regression model. Once cytokine concentrations were calculated, outliers were excluded using the Grubbs method (Graphpad.com).
Leukocyte activation marker expression by flow cytometry
To determine the activation of peripheral leukocytes (monocytes, lymphocytes and granulocytes) by DCM treatment, flow cytometry was used to assess the expression of CD11b protein marker (integrin αM) as described in (48). Direct immunofluorescence staining of peripheral blood using a lyse/no-wash procedure was applied. Whole peripheral blood collected from 2nd trimester pregnant women was incubated with TNL DCM and TL DCM (1:10 ratio) at 37°C for 1 hour, serum-free RPMI media was used as a negative control. The treated blood was incubated with blocking solution (Serum Free Protein Block, Dako, CA) and specific fluorophore-conjugated antibodies for 30 minutes at 4°C in the dark: pan-leukocyte marker CD45 and markers of leukocyte sub-populations: monocytes (CD14), granulocytes (CD15), and lymphocytes, T helper cells (CD4), cytotoxic T cells (CD8), as well as CD11b specific antibodies as previously described (49). Fixable viability dyes eFluor 450 (for monocytes and granulocytes) and eFluor 506 (for lymphocytes) (1:1000 dilution, eBioscience) were incubated with the antibodies. Freshly prepared FACS Lyse solution (450 μl, BD Biosciences, CA) were added to each tube and incubated for 15 min prior to data acquisition by Gallios flow cytometer (Beckman Coulter, CA, USA). Fluorescence was measured by Median Fluorescence Intensity (MFI). Flow-Set Fluorospheres (Beckman-Coulter) were used for each experiment to ensure identical voltage settings between experiments. CompBead Plus Particles (anti-mouse Ig, BD) were used as single stain controls for each fluorophore to calibrate the Gallios detectors in the initial experiment as well as provide compensation calculation in all experiments. FMO controls were prepared for each treatment to accurately define cell population gating.
Human endothelial cells:
Primary human uterine microvascular endothelial cells UtMVEC-myo were purchased from Promocell (#C-12296, Heidelberg, Germany) and cultured in endothelial growth media MV (EGM-MV, Promocell) according to manufacturer’s instructions. The endothelial cells remained in culture until confluent (48–72 hours). Cells were used from passage 1 to a maximum of passage 8.
Uterine endothelial cell adhesion molecule (CAM) expression analysis by qRT-PCR:
UtMVEC cells were seeded on 6-well tissue culture plates pre-coated with gelatin at density 250,000 cells/well. After 48 hours, the cells were serum-starved with 0.1% ITS-A overnight. The following day the monolayer was incubated with serum-free media (negative control), TL DCM or TNL DCM for 6 hours. UtMVEC cells were washed twice with ice-cold PBS, RNA was extracted using QIAzol (Qiagen, MD, USA); RNA purification was performed using miRNeasy Mini Kit (Qiagen) following manufacturers’ instructions. RNA concentration was measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., USA). cDNA was generated using the iScript gDNA Clear cDNA Synthesis kit (Bio-Rad) following manufacturers’ instructions. Previously published specific primers for cell adhesion molecules ICAM-1, VCAM-1, PECAM-1 and SELE and housekeeping genes, YWHAZ, SDHA and TBP, were used to assess CAM gene expression (46) (Supplemental Table 5). qRT-PCR reactions were performed on the CFX384 Real-Time System C1000 Thermal Cycler (Bio-Rad) using SYBR chemistry. A cycle threshold (Ct) value was recorded for each sample. The Ct value is defined as the number of amplification cycles required to detect a fluorescent signal (SYBR green) that exceeds background levels. Ct values are inversely proportional to mRNA levels within each sample. PCR reactions were set up in technical triplicates and the mean of the 3 Ct values was calculated and analyzed using the CFX Manager software 3.1 (Bio-Rad). The cycling protocol is described as follows: initial denaturation at 95°C for 20 sec followed by 40 cycles of denaturation at 95°C for 5 sec and annealing/extension at 54°C for 20 sec. A comparative Ct method (ΔΔCt method) was applied to the raw Ct values to find relative gene expression; all data are expressed as fold change compared to controls.
Immune cell isolation:
Three populations of peripheral leukocytes (neutrophils, monocytes, and lymphocytes) were isolated from whole blood of pregnant women in the second trimester (16–18 gestational weeks) collected into EDTA-containing blood collection tubes. Monocytes and lymphocytes were isolated separately using the RosetteSep Human Total Monocyte Enrichment Cocktail and the RosetteSep Human Total Lymphocyte Enrichment Cocktail (both from Stemcell Technologies, BC, CA) following manufacturer’s instructions. Following isolation, the monocytes and lymphocytes were resuspended in RPMI media. Primary human neutrophils were isolated by Histopaque double density gradient method, consisting of HISTOPAQUE®−1119 and HISTOPAQUE®−1077 (Sigma-Aldrich, MO, USA). Leukocytes were collected, washed twice, re-suspended in DMEM and counted via Trypan Blue hemocytometry.
Transendothelial migration assays:
Prior to migration experiment, UtMVEC cells were seeded in 3-μm and 8-μm cell culture inserts and cultured for 48 hours. Confluence was assessed by testing for leakage of Trypan blue/BSA reagent. Confluent endothelial cells in the inserts were then stimulated/primed for 2 hours with DCM or MCM generated from TNL/TL patients (N=3–7). Maternal neutrophils, monocytes, and lymphocytes were labeled with calcein-AM (as described earlier in (48)). Neutrophils and lymphocytes (3-μm inserts) or monocytes (8-μm inserts) were loaded into the upper well of UtMVEC-coated inserts at 200,000 cells/well and incubated for 1 hour (neutrophils) or 16 hours (monocytes and lymphocytes) and allowed to transmigrate to the lower wells towards MCM/DCM or negative and positive controls. Serum-free media was used as a negative control, IL-8 (100ng/ml) – as positive control for neutrophil transmigration, CCL14 (100ng/ml) – as positive control for monocyte transmigration, CCL19 (25ng/ml) – positive control for lymphocyte transmigration. Transmigrated calcein-labeled human leukocytes were collected from the bottom chamber and quantified by fluorescence at 490/520 nm on spectrophotometer calculating OD with reference to a standard curve generated by serial dilution of calcein-labelled leukocytes.
BSCI treatment:
(1) Primary maternal neutrophils isolated from peripheral blood of pregnant women were incubated with increasing concentrations of BSCI (100–500nM) and trans-endothelial migration assays was performed using TL DCM (N=4) or serum-free media (SFM, negative control) for 1 hour. (2) Cytotoxic or cytolytic effect of BSCI on primary maternal leukocytes was determined: neutrophils, monocytes, and lymphocytes were isolated from peripheral blood of pregnant women (16–18 gestational weeks, N=3–5), treated with TL DCM with or without BSCI (400nM) and incubated for 2 and 16 hours. Immune cells were stained with 0.4% Trypan Blue solution and viable cells were counted using hemocytometer. Flow cytometry was used to confirm the absence of toxic effect of BSCI on primary leukocytes from pregnant women (16 gestational weeks, N=1) and non-pregnant blood (N=1). Blood was incubated with TL DCM and treated with BSCI for 2, 6, and 24 hours. Peripheral leukocytes identified by CD45, were stained with a fluorophore-conjugated antibody cocktail specific for leukocyte subpopulations – anti-CD14 (monocyte), anti-CD15 (granulocyte), and anti-CD3 (T lymphocytes) and live/dead stain.
(3) To investigate the effectiveness of BSCI in blocking DCM- and MCM-mediated leukocyte transmigration, isolated primary human neutrophils, monocytes, lymphocytes, and UtMVEC monolayers were pre-incubated (“primed”) for 2 hours prior to trans-endothelial migration assay with DCM or MCM generated from TL patients (N=4–7 per group) or DCM/MCM supplemented with BSCI (400nM). Calcein-labeled leukocytes (200,000 cells/well) were loaded into endothelial-coated inserts, and allowed to transmigrate for 1 hour (neutrophils) or 16 hours (monocytes and lymphocytes) towards TL DCM /TL MCM. Results are shown as number of primary leukocytes transmigrated through membrane insert/well.
Statistical Analysis
The normality of all data sets was determined by the Shapiro-Wilk test. For the analysis of two groups, paired t-tests was performed for normally distributed data and the Mann-Whitney U test was utilized for nonparametric data, as specified in each analysis section. One-way ANOVA with Bonferroni’s post-test was employed to determine significance between data sets comprised of more than two groups (TEM assay). Data are presented as mean±SD. Significance level was set at p<0.05. Statistical analysis was performed using Prism 8.0 software (GraphPad Software Inc., CA, USA). *p<0.05, **p<0.01, ***p<0.001.
Results
Primary human decidual cells are a greater source of cytokines and chemokines than myometrial cells at term prior to labor
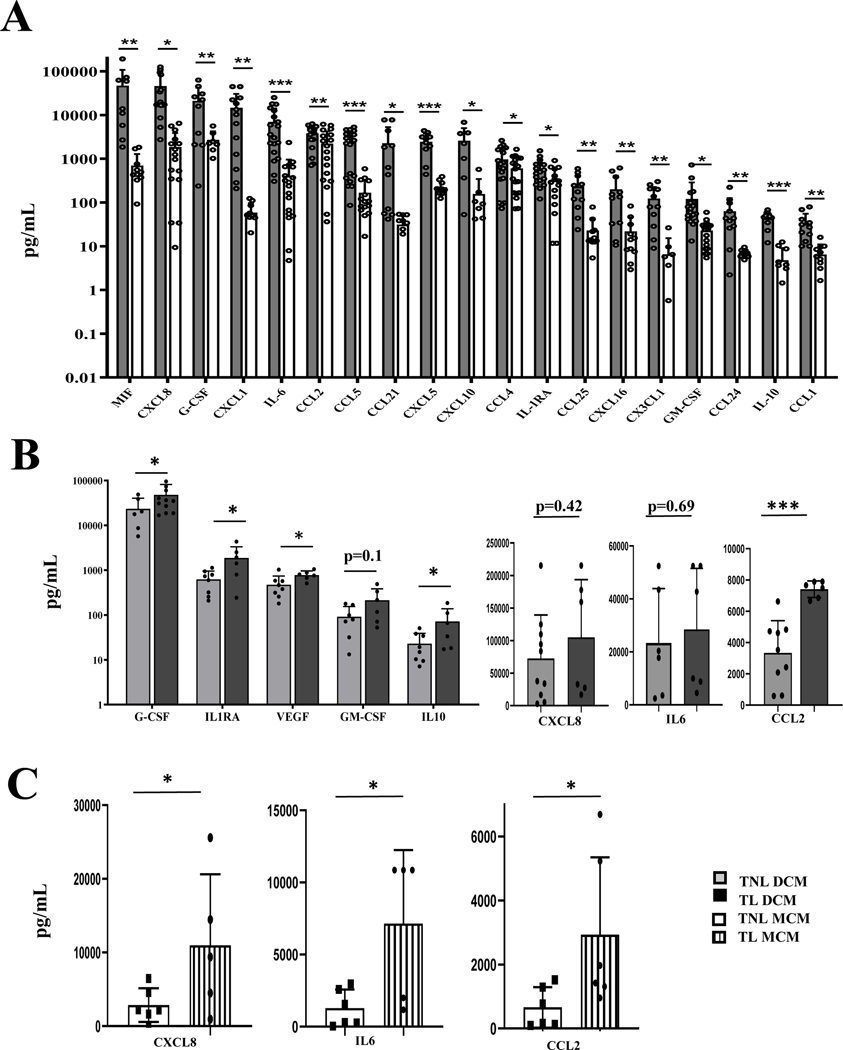
After isolation, primary human myometrial and decidual cells were characterized by immunocytochemistry (ICC) and flow cytometry as we previously described (46–47). Our experimental design excludes leukocyte-secreted cytokines as the presence of immune cells is not supported in culture, resulting in their removal during the culturing process. This allows for an accurate assessment of the secretory phenotype of decidualized stromal cells and its comparison to myometrial cells. Culture media conditioned by primary myometrial or decidual cells from non-laboring tissues (TNL DCM, N=11 and TNL MCM, N=10) were analyzed by multiplex assays to detect the secretion of 45 cytokines, chemokines and growth factors (Figure 1A). All proteins were detected in the DCM, although a quarter of them (including IFNγ, IL(Interleukin)1β, 2, 4, CCL11, 15, 17, 27) were at the limit of detection by Luminex assay. Of the 45 analytes, 19 cytokines were significantly (P<0.05) higher in TNL DCM compared to TNL MCM, listed below from highest to lowest expression: MIF, CXCL8, G-CSF, CXCL1, IL6, CCL2, CCL5/RANTES, CCL21, CXCL5, CXCL10/IP10, CCL4/MIP1β, CXCL16, CX3CL1, GM-CSF, IL1RA, CCL25, CCL24, IL10, CCL1. The majority of the significantly elevated cytokines induce leukocyte migration via chemotactic gradients (CXCL1, CXCL5, CXCL8, CXCL10, CXCL16, CCL1, CCL2, CCL4, CCL5, CCL21, CCL24, CCL25, CX3CL1), function as pro-inflammatory modulators (IL6, MIF), anti-inflammatory proteins (IL1RA and IL10), or act as leukocyte growth factors (G-CSF, GM-CSF). Pro-inflammatory cytokine MIF (Macrophage migration Inhibitory Factor, 76.2±32.9 ng/mL), major neutrophil chemoattractant CXCL8 (aka IL8, 46.0±12.4 ng/mL), G-CSF (Granulocyte Colony Stimulating Factor/CSF3, 34.6±12.6 ng/mL), and CXCL1 (aka GROα, 17.2±4.6 pg/mL) exhibited the highest concentrations in DCM. In MCM, almost half of analytes were not detected by Luminex; of those that were detected, G-CSF, CCL2 (Monocyte Chemoattractant Protein 1, major monocytic chemokine) and CXCL8 exhibited the highest concentrations. We concluded that prior to TL stromal decidual cells are a greater source of cytokines and chemokines than myometrial cells.
Figure 1. Comparative characteristics of cytokine levels released by primary decidual and myometrial cells from pregnant women at term and during labor.
(A) Multiplex analysis of cytokine secreted by primary human decidual cells compared to primary myometrial cells from term not-in-labor patients. Culture media conditioned (CM) by primary decidual and myometrial cells isolated from myometrial biopsies and decidua samples from term not-in-labor (TNL) women was collected and analyzed by the 5-plex and 40-plex Panels of the Bio-Plex Pro Human Chemokine Assays as instructed by the manufacturer. White bars represent cytokines detected in the myometrial conditioned media (TNL MCM, N=11), whereas black bars represent cytokines detected in the decidual conditioned media (TNL DCM, N=11). Absolute concentration values (pg/ml) are presented as mean ± SD on a logarithmic scale. Statistical significance (p<0.05) was determined by unpaired t-tests between DCM and MCM groups for each cytokine (IL-6, MIF, G-CSF, GM-CSF, CXCL1, CXCL5, CXCL8, CXCL10, CXCL16, CCL1, CCL2, CCL4, CCL5, CCL21, CCL24, CCL25, CX3CL1, IL-1RA and IL-10). *p<0.05, **p<0.01, ***p<0.001 comparing DCM to MCM group.
(B) Multiple analysis of cytokines secreted by human decidual cells during term labor (TL) compared to term not in labor (TNL). Conditioned media was collected from primary cells isolated from decidua samples of TNL and TL deliveries. TNL DCM was compared to TL DCM using 27-plex panels of the Bio-plex Pro Human Cytokine Assay as instructed by the manufacturer. Protein levels of CXCL8, CCL2 and IL6 were higher than the capabilities of the Luminex assay, thus they were re-analyzed using specific ELISAs. Grey bars represent cytokines detected in the TL DCM (N=6), whereas black bars represent cytokines detected in the TNL DCM (N=9). Absolute concentration values (pg/ml) are presented as mean ± SD on a logarithmic scale. Statistical significance (p<0.05) was determined by unpaired t-tests between two DCM groups for each cytokine (G-CSF, GM-CSF, VEGF, IL-1RA, IL-10, CXCL8, CCL2 and IL6).
C) Changes in pro-inflammatory cytokine (IL6), and chemokine (CCL2, CXCL8) protein expression in human myometrial cells during term labor (TL) compared to term not in labor (TNL). Supernatants were collected after 48 hours and analyzed by ELISA. Protein concentrations are presented as absolute concentration values (pg/ml) are presented as mean ± SD. Statistical significance (p<0.05) was determined by unpaired t-tests, *p<0.05, **p<0.01, ***p<0.001.
Increased chemokine secretion in laboring decidua and myometrium
Next, using multiplex assay we examined whether the labor process per se can influence the cytokine profile of primary human decidual and myometrial cells. We analyzed 27 cytokines secreted by cells isolated from quiescent or actively contracting uteri, to identify which ones were changed during TL. In DCM five of the 27 analyzed cytokines were significantly upregulated by labor: CCL2 (p=0.01), G-CSF (p=0.01), IL1RA (p=0.03), IL10 (p=0.05) and VEGF (p=0.03) (Figure 1B). G-CSF, a cytokine involved in immune cell survival, proliferation, differentiation and function, was the highest secreted protein in both TNL and TL DCM groups (TL: 54.0±15.3 ng/mL; TNL: 27.0±6.0 ng/mL, Figure 1B). Protein levels of anti-inflammatory cytokines IL1RA (TL: 1877 ± 541 pg/mL vs TNL: 622±111 pg/mL), and IL10 (TL: 71.6±24.6 pg/mL vs TNL: 23±5.3 pg/mL) were significantly higher during TL compared to TNL (Figure 1B). Protein levels of CXCL8, CCL2 and IL6 were higher than the capabilities of the Luminex assay, thus they were re-analyzed using specific ELISAs. All three cytokines - CXCL8 (TL: 104.9±33.1 ng/mL; TNL: 72.4±20.1 ng/mL), IL6 (TL: 28.5±8.6 ng/mL; TNL: 23.4±7.7 ng/mL) and CCL2 (TL: 7410±197 pg/mL; TNL: 3336±648 pg/mL) were increased in laboring human decidua, however only the increase in levels of CCL2 was significant (TL vs TNL, p=0.004, Figure 1B, right panel), while CXCL8 and IL6 did not reach significance due to high variability between samples.
From twenty seven proteins secreted by primary human myometrial cells the majority were only marginally changed during TL compared to TNL. Similar to DCM, anti-inflammatory cytokines IL1RA was found to be up-regulated in MCM during labor (TL: 1141±248 pg/mL vs TNL: 536±81 pg/mL, p<0.05). Three cytokines (CXCL8, CCL2, and IL6) were analyzed by ELISA, all significantly increased in human myometrium during TL: CXCL8 (TL: 10174±4175 pg/mL vs TNL: 2852±854 pg/mL, p=0.01), IL6 (TL:7148±2038 pg/mL vs TNL: 1281±478 pg/mL, p=0.02) and CCL2 (TL: 2862±914 pg/mL vs TNL: 660±236 pg/mL, p=0.05) (Figure 1C). These results indicate that during active labour contractions secretion of pro- and anti-inflammatory cytokines by human decidual and myometrial cells is intensified.
Decidual-driven activation of endothelial and leukocyte adhesion molecules
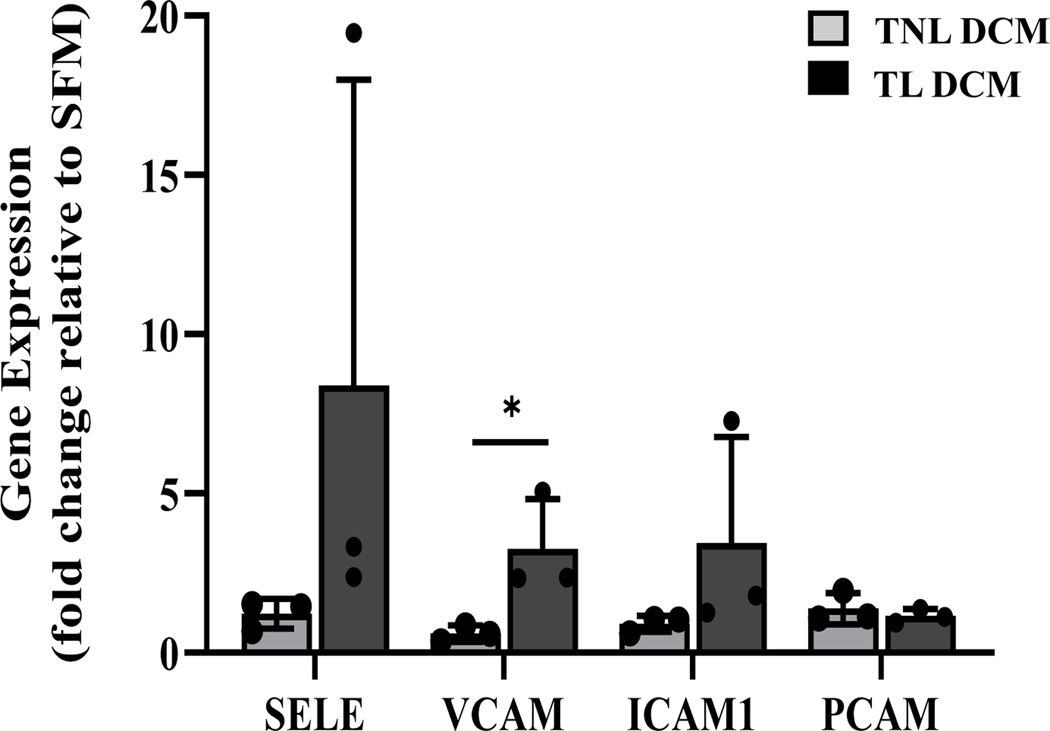
In order for leukocytes to migrate through the vascular endothelium, cell adhesion molecules must be expressed on the surface of endothelial cells to facilitate the tethering, rolling, adherance and leukocyte-endothelial interaction (49–51). The greater secretion of chemokines by decidual compared to myometrial cells led us to hypothesize that decidua drive activation of endothelial and leukocyte cell adhesion molecules on the uterine vasculature which accelerates trans-endothelial leukocyte extravasation during TL. To test this hypothesis, confluent human microvascular endothelial cells UtMVEC-myo were treated with SFM (control), TNL DCM, or TL DCM for 6 hours (N=6). Following incubation, the cells were analyzed by qRT-PCR to investigate the mRNA expression of four cell adhesion molecules: ICAM-1, VCAM-1, PECAM-1 and SELE (gene encoding E-selectin). As seen in Figure 2, the gene expression of VCAM-1 (expressed as fold change relative to serum-free media control) is significantly upregulated on UtMVECs in response to TL DCM treatment compared to TNL, with a 5.4 increase in fold change (p=0.04). While SELE (6.88 fold increase, p=0.25) and ICAM-1 (3.81 fold increase, p=0.26) mRNA levels were higher following exposure to DCM from TL compared to TNL cultures, they were not significantly different due to a high variability between human gestational samples. No changes were observed with PECAM-1 expression in response to DCM treatment.
Figure 2. Cytokines secreted by laboring human decidua activates uterine microvascular endothelial cells through enhanced cell adhesion molecule mRNA expression.
UtMVEC-myo cells were treated with decidua conditioned media generated from term not-in-labor (TNL DCM, solid grey bar) or term laboring (TL DCM, solid black bar) patients (N=3/group) for 6 hours and RNA was extracted for qRT-PCR analysis. Serum free media (SFM, solid white bar) was used as a negative control, denote by dashed line. Statistical significance (*, p<0.05) was determined by unpaired t-tests between DCM groups (TNL and TL). Data presented as mean ± SD.
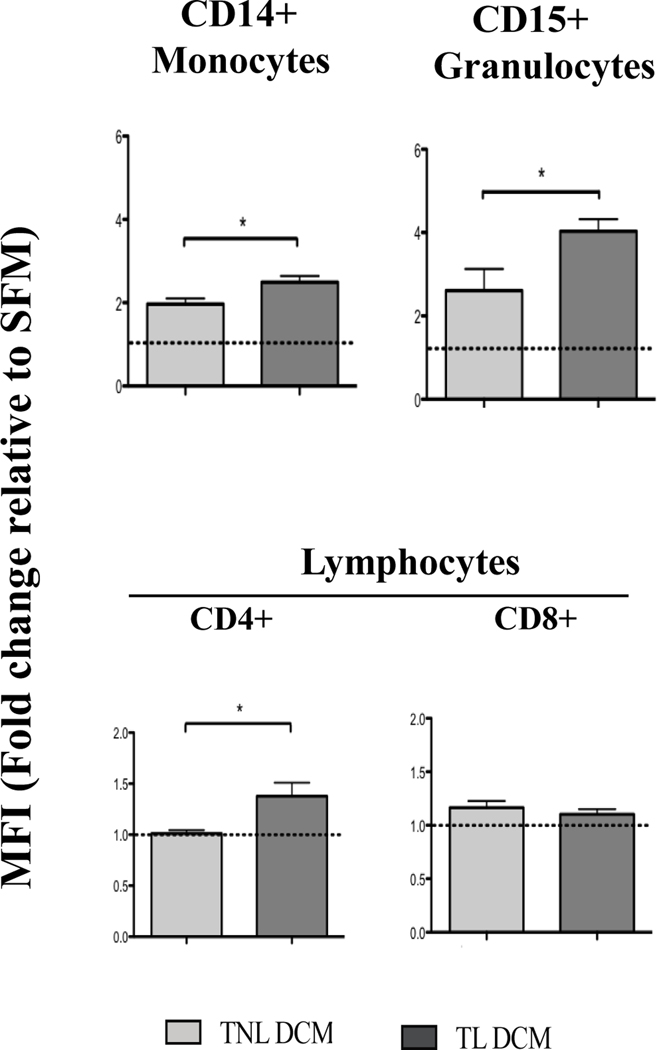
In addition to CAM expression on the vasculature endothelium, peripheral leukocytes must similarly be activated to express adhesion molecules facilitating endothelial-leukocyte bond. CD11b (integrin αM) has been shown to be upregulated on peripheral leukocytes during TL and PTL (10, 49, 52); thus we studied how treatment with DCM influences expression of this CAM on primary leukocytes. Whole blood was treated with TNL DCM/TL DCM or SFM (negative control), and stained with monocyte-specific (anti-CD14), granulocyte-specific (anti-CD15), lymphocyte specific (anti-CD3, CD4, CD8) and anti-CD11b antibodies as described earlier (48). The expression of activation marker CD11b, measured relative to SFM, was significantly upregulated on monocytes (1.27 fold increase in MFI, p=0.023, granulocytes (1.55 fold increase, p=0.04) and CD4+ T lymphocytes (1.36 fold increase, p=0.02) in response to TL DCM compared to TNL DCM (Figure 3).
Figure 3. Cytokines secreted by laboring human decidua significantly upregulate the protein expression of activation markers on peripheral leukocytes from pregnant women.
Whole peripheral blood collected from healthy women in their 2nd trimester of pregnancy was incubated with media conditioned by primary decidual cells from TNL and TL deliveries. Light gray bars denote TNL DCM (N=6), dark gray bars denote TL DCM (N=6). Blood cells were stained with fluorophore-conjugated Abs specific for leukocyte subpopulations: CD14 (monocyte), CD15 (granulocyte), CD4 (T-helper lymphocytes) and CD8 (cytotoxic T-lymphocytes), activation markers CD11b, CD44 and CD55, and analyzed by flow cytometry. Serum free media (SFM, solid white bar) was used as a negative control, denote by dashed line. Statistical significance (*, p<0.05) was determined by unpaired t-tests between DCM groups (TNL and TL). Data presented as mean ± SEM.
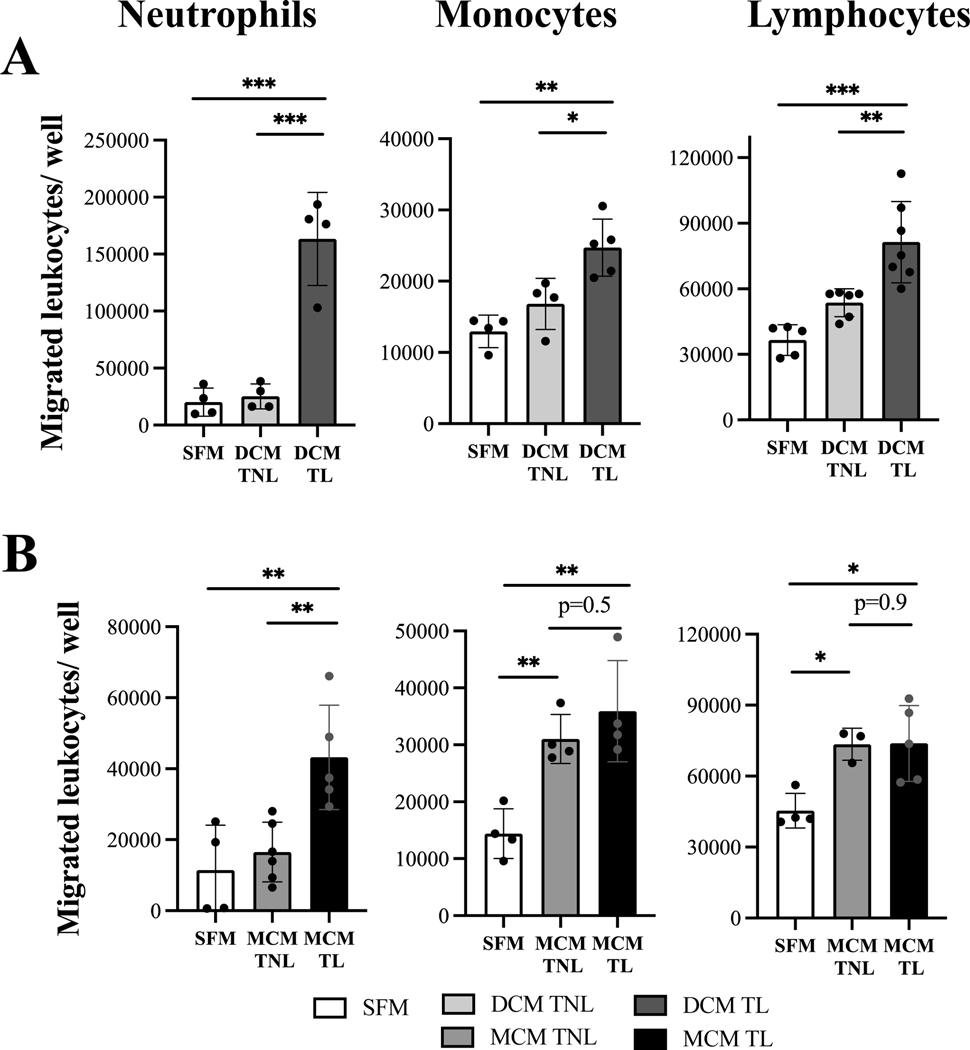
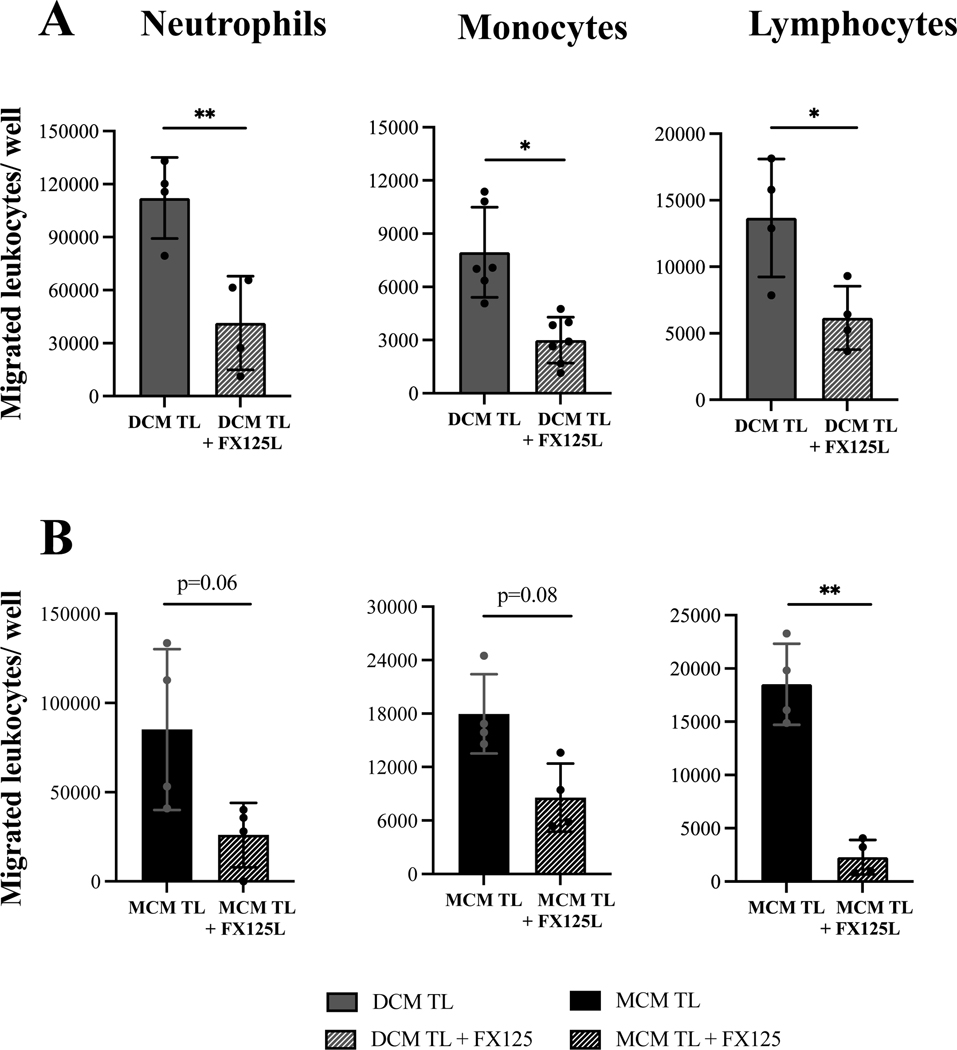
Laboring decidual and myometrium conditioned media stimulate trans-endothelial migration of maternal peripheral leukocyte.
To assess the chemoattractant ability of decidual and myometrial cytokines to activate and recruit maternal peripheral leukocytes, trans-endothelial migration assays were performed. Three sub-populations of human primary leukocytes (granulocytes, monocytes, and lymphocytes) isolated from peripheral blood of pregnant women at mid-gestation were first tested to inspect their ability to transmigrate towards specific chemokines – IL-8 (100 ng/ml, p<0.05) for neutrophils, CCL14 (100 ng/ml, p<0.05) for monocytes and CCL19 (25 ng/ml, p<0.05) for lymphocytes, as compared to negative control (SFM, Supplemental Figure 1). Next, the transmigration of peripheral leukocytes towards media enriched with cytokines secreted by gestational uterine tissues was examined. Remarkably, media from non-labouring decidual cells did not stimulate an increase in trans-endothelial migration of any cell population, while media generated by non-labouring myometrial cells significantly stimulated transmigration of monocytes (2.15 fold increase, p<0.01), and lymphocytes (1.62 fold increase, p<0.01) above the control (serum-free media) (Figure 4). Importantly, we found that media produced by cells from labouring decidua stimulated significantly higher leukocyte transmigration as compared to media from non-labouring decidual cells: neutrophils (8.1 vs 1.2 fold increase, p<0.001), monocytes (1.9 vs 1.3 fold increase, p<0.05), and lymphocytes (2.2 vs 1.5 fold increase, p<0.01). Similarly, media produced by labouring myometrial cells also stimulated significantly higher number of neutrophils as compared to non-labouring myometrium (3.8 vs 1.4 fold increase, p<0.05), however for lymphocytes and monocytes the transmigration potential was similar for labouring and non-labouring cells (Figure 4B). Notably, 4.8 times more primary human neutrophils migrated in response to TL DCM (median=178514) as compared to TL MCM (median=37435), while numbers of monocytes (25259 vs 32764) and lymphocytes (75307 vs 73619) were comparable. Importantly, while labouring decidua and myometrium both enhance transmigration of all immune cells, before labour only myometrium generated a strong chemotactic signal capable to attract immune cells, and this signal was equal in strength to signal for PBMCs in both labouring uterine tissues.
Figure 4: Term laboring conditioned media facilitates trans-endothelial migration of peripheral human leukocytes as compared to term not-in-labor samples.
Confluent hUtMVEC-Myo cells grown on membrane inserts were stimulated for 2 hours with either decidua or myometrium conditioned media (DCM/MCM) generated from term not-in-labor (TNL, solid grey bar) and term laboring (TL, solid black bar) patients (N=3–7/group). Primary human neutrophils, monocytes, and lymphocytes pre-treated with (A) either TNL DCM or TL DCM, (B) either TNL MCM or TL MCM, were labeled with calcein-AM, loaded into endothelial-coated membrane inserts (diameter of pore 3 μm and 8 μm, 200,000 leukocytes/well) and allowed to transmigrate for 1–16 hours towards corresponding DCM/MCM. Results are shown as number of primary leukocytes transmigrated through membrane inserts /well. Serum free media (SFM, solid white bar) was used as a negative control. Values are presented shown in a box plot as median with the interquartile range. Data was analyzed by one-way ANOVA. * p<0.05, ** p<0.01, *** p<0.001 compared to TNL group or SFM control.
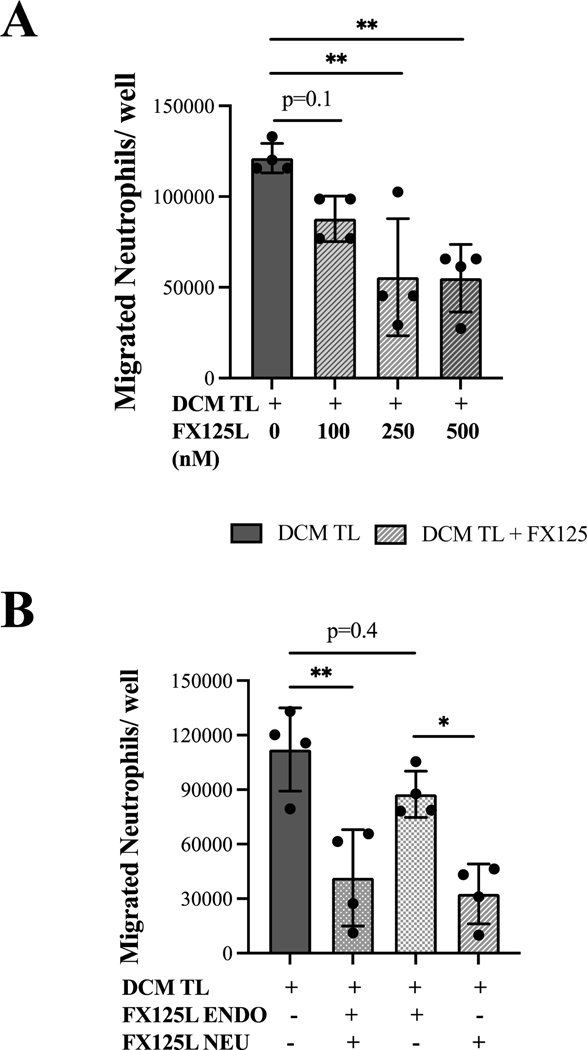
The BSCI (FX125L) inhibits the chemoattractant ability of term laboring uterine tissues.
To assess if chemokine-mediated uterine inflammation could be prevented, we investigated the effectiveness of BSCI in blocking DCM and MCM mediated leukocyte transmigration. First, we established the effective dose of BSCI in trans-endhotelial migration assays (N=3) using primary neutrophils and hUtMVEC-Myo stimulated with TL DCM and increasing concentration of BSCI drug (100–500nM). Neutrophils were loaded into the upper well of endothelial-coated inserts, and allowed to transmigrate towards TL DCM ± BSCI for 1 hour (Figure 5A). Concentrations of BSCI between 250–500nM was able to decrease transmigration by approximately 50%, thus working concentration was chosen as 400nM. Next, the viability of primary neutrophils, monocytes, and lymphocyte after treatment with BSCI was assessed by the Trypan blue staining and flow cytometry (Supplemental Figure 2A,B). Using flow cytometry, peripheral leukocytes were identified by CD45 (pan-leukocyte marker), CD14 (monocytes), CD15 (granulocytes), and CD3 (T lymphocytes). Isolated leukocytes were incubated in DCM with BSCI (400nM) for 2–24 hours, and the effect of drug on viability of immune cells was assessed. Vital staining and immunophenotyping showed an unaltered number of live immune cells after 18–24 hours incubation with BSCI, indicating a complete absence of toxic effect of drug (Supplemental Figure 2B). To identify the major target cell type for BSCI, we pre-incubated the endothelial cells in the insert (upper chamber) with MCM or DCM supplemented with BSCI (400nM) prior to placement of the neutrophils in the insert; in a separate experiments, BSCI was incubated with isolated neutrophils prior to trans-endothelial migration, and with both, endothelial cells and neutrophils. Our results show that pre-treatment of neutrophils alone and both neutrophils and endothelial cells with BSCI efficiently decreased their migration capability, while pre-treatment of endothelial cells alone not significantly reduced neutrophil migration (Figure 5B). All subsequent experiments were carried out after pre-treatment of both cell types with BSCI.
Figure 5: Trans-endothelial migration of primary human neutrophils is inhibited by the BSCI (FX125L).
(A) The effective dose of FX125L was determined after stimulation of 2 hours of human primary neutrophils and human uterine microvascular endothelial cells (hUtMVEC-Myo) with media conditioned by decidual cells isolated from term laboring patients (TL DCM, solid black bar, N=4) and TL DCM supplemented with increasing concentrations of FX125L (100–500nM, patterned grey bars). Neutrophils were loaded into the upper well of endothelial-coated inserts (200,000 cells/well, diameter of pore 3 μm), and allowed to transmigrate towards TL DCM ± FX125L for 1 hour. (B) To establish the inhibitory specificity of FX125L effect we pre-incubated for 2 hours primary neutrophils (NEU) and/or monolayers of uterine microvascular endothelial cells (hUtMVEC-Myo, ENDO) with TL DCM (solid black bar, N=4) ± FX125L (400nM, patterned grey bars). Neutrophils (200,000 cells/well) were loaded into the upper well of ENDO-coated inserts, and allowed to transmigrate towards media for 1 hour. Results are shown as number of primary neutrophils transmigrated through membrane inserts/well. All data was standardized to the negative control (serum free media). Pre-stimulation of neutrophils alone was as effective in inhibiting leukocyte migration as pre-stimulation of both neutrophils and endothelial cells, however the pre-stimulation of endothelial cells alone had no effect on the decrease of migration. Statistical significance was determined by one-way ANOVA. *p<0.05,**p<0.01 compared to TL DCM.
To examine the ability of BSCI to block myometrial or decidual stimuli during TL, we performed trans-endothelial migration assay where the lower chamber contained DCM/MCM with BSCI. UtMVEC monolayers and isolated neutrophils/monocytes/lymphocytes were pre-treated for 2h (“primed”) with DCM/MCM + BSCI prior to seeding in the upper trans-well. Incubation with BSCI significantly (p<0.05) inhibited trans-endothelial migration of neutrophils, monocytes, and lymphocytes in response to TL DCM by 67%, 64%, and, 55% respectively (Figure 6A). Trans-endothelial migration in response to TL MCM was also inhibited by 70%, 52%, and 88% respectively, however only lymphocyte transmigration was significantly reduced by BSCI (p<0.01), whilst neutrophils (p=0.06) and monocytes (p=0.08) migration showed a strong trend that did not reach significance due to a high variability between individual MCM samples (Figure 6B).
Figure 6: The BSCI (FX125L) inhibits labor-associate leukocyte migration.
Isolated primary human neutrophils, monocytes, and lymphocytes, and uterine microvascular endothelial cells (UtMVEC) monolayers were pre-incubated for 2 hours prior to trans-endothelial migration assay with either decidua or myometrium conditioned media (DCM/MCM) generated from TL patients (solid black bar, N=4–7 per group) or DCM/MCM supplemented with FX125L (400nM, grey oblique box). Leukocytes (200,000 cells/well) were loaded into endothelial-coated inserts, and allowed to transmigrate for 1 hour (neutrophils) or 16 hours (monocytes and lymphocytes) towards TL DCM/MCM. Results are shown as number of primary leukocytes transmigrated through membrane insert/well. All data was standardized to negative control (serum free media). Statistical significance was determined by paired t-tests, *p<0.05, **p<0.01, ***p<0.001.
Discussion
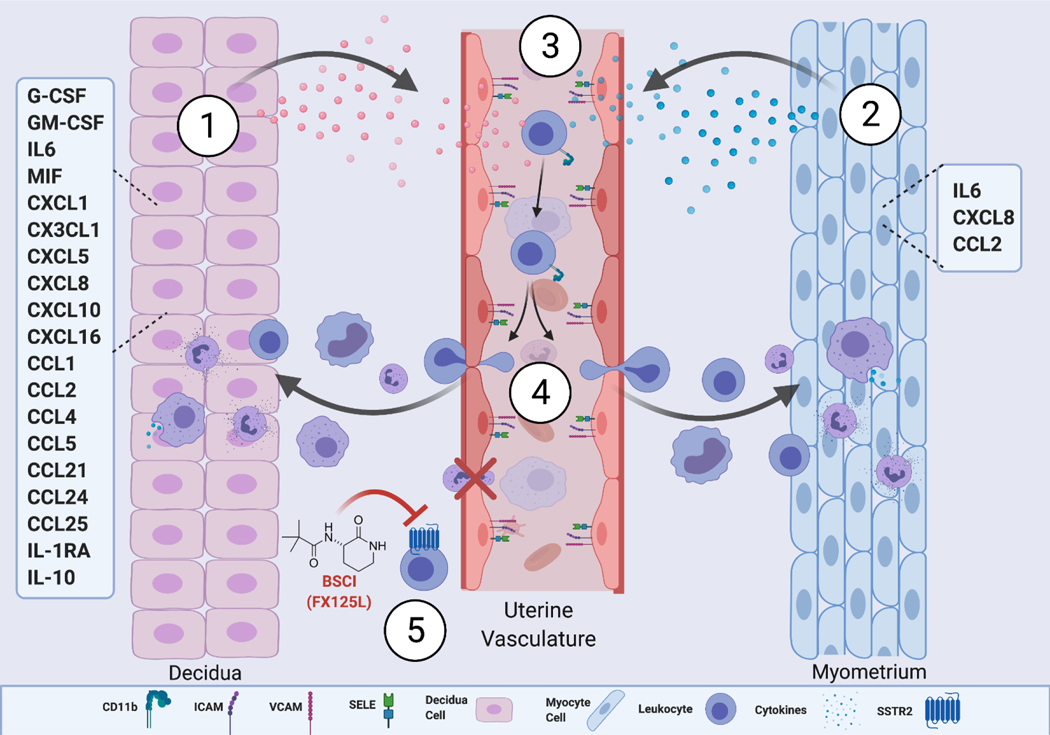
Prior studies of the immunologic cascade of human labour have been typically limited to single tissues without consideration of the complex physiologic interactions necessary for recruitment of leukocytes into the uterus (53). Moreover, to date no human in vitro direct comparative analysis exists to simultaneously examine multiple cytokines secreted by two primary uterine compartments before and during parturition. In this study, we modeled the contributions of cytokines to evaluate how decidual and myometrial cells independently drive the inflammatory processes of labour. We present evidence that the uterine decidua has a high chemotactic and activation potential that effectively recruits peripheral blood leukocytes, due to the enhanced secretion of multiple cytokines. At the same time many strategies that restrict maternal immune cell access to the decidua and suppress local activation of potentially harmful immune components have been identified at the maternal-fetal interface. This includes epigenetic silencing of key T cell-attracting inflammatory chemokine genes in decidual stromal cells (54) to prevent rejection of the fetus bearing foreign paternal antigens, while simultaneously maintaining immunity against pathogenic microbes (55). However pregnant myometrium, despite producing fewer secreted cytokines, recruited similar levels of immune cells. We further directly demonstrate that during labour the uterine signals from both decidual and myometrial cells intensify transmigration of leukocytes, which could be inhibited by novel BSCIs compound FX125L, a potential therapeutic for preterm birth (see conceptual model, Figure 7).
Figure 7: Model of decidual/myometrial activation of the inflammatory pathway during human term labor.
(1) The laboring decidua significantly secretes a milieu of pro-inflammatory cytokines, chemokines, and growth factors (IFNy, G-CSF, MCP1, CXCL1, TNFa, IL1B, IP10 etc.) (2) The laboring myometrium significantly secretes pro-inflammatory cytokines, chemokines (IL6, CXCL8, and CCL2). (3) Secreted cytokines/chemokines enter the maternal peripheral microvasculature and activate the expression of CAMs (ICAM, VCAM-1, and E-selectin) on the uterine vascular endothelium, and increase the expression of integrins (CD11b, CD55, and CD44) on maternal peripheral leukocytes (monocytes, granulocytes and lymphocytes), allowing increased leukocyte adhesion to the uterine vascular endothelium, (4) which subsequently promote their trans-endothelial migration into the uterine tissue, amplifying the inflammatory signal through cytokine/chemokine secretion to further the progression of labor. (5) The Broad-Spectrum Chemokine Inhibitor (FX125L), a possible anti-inflammatory therapeutic, acts on maternal leukocytes to prevent their activation and trans-endothelial migration, and subsequent infiltration into the uterine tissue, thus preventing labor onset. Illistration created using Biorender.com.
During the third trimester of pregnancy, the myometrium and decidua secrete multiple pro-inflammatory cytokines, chemokines, and growth factors which contribute to the labor cascade. We have demonstrated previously that decidual leukocyte infiltration precedes myometrial infiltration in both a pregnant rat and pregnant mouse models, suggesting sequential temporal activation in response to increased cytokine secretion (13). To examine the contribution of individual human uterine tissues’ to the labor process, we used here primary cells isolated from pregnant decidua and myometrium. Forty-five cytokines secreted by these two adjacent uterine compartments were analysed simultaneously by a multiplex assay. Our data indicate that concentrations of 19 chemokines, pro-inflammatory cytokines, and growth factors are significantly higher (10–1000 fold) in media conditioned by TNL decidua compared to TNL myometria. These cytokines are essential players during leukocyte extravasation into the uterus and function as chemoattractants for the monocytes (CCL2, CCL4, CXCL10, CX3CL1), neutrophils (CXCL1, CXCL8) or lymphocytes (CCL5, CXCL16), pro-inflammatory (MIF, IL6), and anti-inflammatory (IL1RN, IL10) agents or factors stimulating immune cell proliferation (GCSF, GMCSF) (23). CXCL8 and CCL2 are canonic α- and β-chemokines stimulating migration of immune cells in different tissues (24). The increase in IL6 can support the differentiation of monocytes to macrophages or activation of resident tissue macrophages (56). It regulates leukocyte infiltration by suppressing neutrophil recruitment in favor of monocytes (57) as a result of increased secretion of specific chemokines (i.e. CCL2) (56, 57). Data presented here assert that normal human gestation is characterized by physiologic inflammation detectable in both uterine compartments, decidua and myometrium, as a result of the secretion of multiple pro-inflammatory cytokines and chemokines, which aids in the progression to labor. Human decidua, located at the maternal-fetal interface, likely plays a greater immunomodulatory role in the labor cascade compared to the myometrium, through increased cytokine production and active leukocyte infiltration, which was suggested previously by animal studies (13, 14). In case of systemic infection with tropism of decidual invasion, pro-inflammatory cytokines however may play a role in the pathogenesis of infection-induced fetal waistages (55).
Next, we characterized proteins secreted from term pregnant human uterine tissues before and after labor onset and found differential expression of multiple inflammatory biomarkers. For instance, our multiplex data revealed that TL is associated with increased inflammation of uterine tissues, as we detected significant difference in concentrations of CXCL8, CCL2 and IL6 cytokines responsible for leukocyte migration and propagation of inflammation in the myometrial compartment (37), while only CCL2 was significantly increased in decidua. These in vitro results reinforce our previous ex vivo analysis of multiple cytokines in bulk human myometrial tissues obtained during spontaneous TL compared to TNL samples: we reported that IL1RA, GCSF were significantly increased, while CXCL8, CCL2 and IL6 exhibited the largest fold-increase in laboring as compared to non-laboring myometrium (47). Others reported that protein levels of IL6 and CXCL8 were significantly increased during human term parturition in the lower uterine segment (3, 4) and IL6 - in plasma (58, 59). Importantly, in this current study GCSF, a cytokine regulator of myeloid progenitor cell proliferation and differentiation, exhibits the highest level of secreted protein in both DCM groups, and in MCM. We also found increased levels of IL1RA protein in media conditioned by laboring human decidua as compared to non-labouring. We suggest that increased secretion of IL1RA may potenitate the generation of M2 macrophages as part of a protective immune response to promote homeostasis and uterine tissue renewal during post-partum involution.
Circulating leukocytes express integrin molecules (CD11b/CD18, aka αMβ2) and LFA-1 on their surface, which enables their transmigration into different tissues (52, 60–63). It is known that intercellular adhesion molecule ICAM-1 is up-regulated in the uterine endothelium during human TL, which allows binding of activated CD11b-expressing leukocytes (10). We and others reported earlier that during TL there is an increase in the expression of CD11b on circulating granulocytes and monocytes isolated from peripheral blood of pregnant women which indicates their increased ability to migrate towards a chemotactic signal produced by uterine tissues (49, 64, 65). However, the exact mechanism of leukocyte activation during gestation is not fully understood. Our current study shows that multiple secreted cytokines were able to activate CD11b protein on CD15+ granulocytes and CD14+ monocytes (as compared to SFM); moreover media conditioned by cells isolated from laboring decidua significantly induced CD11b expression on granulocytes, monocytes, and CD4+ lymphocytes compared to non-laboring decidua. Thus we suggest that CD11b may facilitate the recruitment of immune cells into uterine tissue during TL. Previously data published by our group showed that expression of CD11b was increased on granulocytes and monocytes after incubation with media, conditioned by stretched human myometrial cells that contained multiple inflammatory cytokines and chemokines, which support this assumption (48). Earlier we have demonstrated in vivo by immunohistology enhanced infiltration of maternal peripheral immune cells into labouring human uterine tissues (47). Here we present the mechanism driving this infiltration during human labour: primary leukocytes isolated from peripheral blood of pregnant women significantly increase transmigration through utrerine endothelial cells towards laboring samples compared to non-laboring samples. We suggest that this infiltration of maternal peripheral white blood cells induces uterine inflammation and the subsequent generation of uterotonic agonists (in decidua) and activation of CAP genes (myometrium) (37).
Earlier histologic studies revealed higher myometrial expression of ICAM-1, VCAM-1, and E-selectin during human parturition, mainly in the endothelium (10, 66); however, the cause of such activation was not explored. Given the crucial role for leukocyte-endothelial interaction, we examined the effect of decidual secreted cytokines on the expression of CAMs on uterine microvascular endothelial cells. Elevated CAM expression on endothelium can increase the likelihood of its interaction with circulating leukocytes. Our current results demonstrate that media conditioned by laboring decidua was able to up-regulate CAMs on human uterine microvascular endothelial cells, likely due to a synergistic effect of multiple secreted cytokines. Furthermore, these data perfectly complement our previous in vitro results using conditioned media from stretched human myometrial cells, containing multiple secreted cytokines, which activated uterine endothelial cells by significantly upregulating cell adhesion molecules (E-selectin, ICAM-1 and VCAM-1) (48).
Numerous studies were undertaken in the past decade to predict targets for drug development and novel therapies that would prevent PTB in high-risk pregnant women. Leukocytes are an active component of the maternal immune system; therefore, they can provide relatively accessible means to interrupt the inflammatory pathway leading to inflammation and labor initiation. Here we examined a BSCI (FX125L) and found that it significantly decreased the trans-migratory potential of leukocyte moving towards laboring DCM and MCM. It has been reported that FX125L binds to the cell-surface somatostatin receptor type 2 (SSTR2), which results in a suppression of chemokine signaling without affecting chemokine receptors (70–73). Somatostatin (SST) is a cyclic neuropeptide hormone that functions as an important mediator between the nervous and the immune systems (71–73), which acts through specific Gi-protein-coupled receptors SSTR1–5 (74, 75). Previous studies have suggested the presence of SSTRs protein at the cell surface and mRNA in PBMC-derived activated monocytes (76, 77). SST regulates the immune system responses through inhibition of the synthesis and release of multiple cytokines, macrophage viability, phagocytosis, and natural killer cell activity (76). Partial agonism of BSCI to SSTR2 has been hypothesized to dampen directional signals from the chemokine receptors leaving cells effectively “blinded” to the chemokine gradient (44, 47). Therefore, we proposed that while multiple cytokines in the media conditioned by primary myometrial and decidual cells were able to activate SSTRs (i.e., SSTR2) on the surface of maternal leukocytes, BSCI, aka FX125L, targetes this inflammatory cascade by blocking immune cell transmigration. In support of this idea, we have recently reported the effective use of FX125L in an in vivo non-human primate model of labor and found that administration of FX125L blocks uterine inflammation, myometrial contractions, prevents infection (GBS)-induced PTL, prolonging gestation (45). We suggest that FX125L may represent a novel therapeutic approach in the treatment of PTL in women at risk for PTB.
Of note, there are some limitations to our study. Firstly, we were unable to obtain uterine tissues from women in PTL to examine cytokine secretion, and to prove that BSCI can block uterine infiltration responsible for PTB, which could have strengthened the conclusions drawn from the term labouring decidua and myometrium. Secondly, myometrium and decidua does not come from the same patients; moreover, we were limited in the amount of myometrial tissues collected during TL, which restricted number of samples used, and precluded us from reaching a statistical significance in some experiments. Next, we did not account neither for potential phenotypic changes associated with the culture time from cell isolation until DCM/MCM collection, nor for the lack of immune cells eliminated by our culture conditions. The in vitro conditions from cultured decidual stromal cells and myocytes cannot fully recapitulate the complex in vivo interactions between leukocytes and stromal cells occurring in term pregnant uterine tissues. Lastly, our previous studies utilizing a mouse model of gestation showed temporal migration of leukocytes into the myometrium and decidua, with an initial influx of macrophages prior to labour and a subsequent infiltration of monocytes and neutrophils during active labor and further postpartum (15, 16). Due to the limited time points in which uterine samples can be collected from human pregnancies (TL and TNL), and the restrictions imposed by the use of the specific list of cytokines included in the commercially available multiplex assay, the data presented here cannot fully address this pattern in humans. For instance, the analysis of the M-CSF (Monocyte Colony Stimulating Factor/CSF1), the prototypical macrophage growth factor produced by a variery of stromal cells, in future studies is necessary as it may regulate the homeostatic expansion of macrophages specifically within myometrial tissue layer as compared to decidua (78).
In summary, the results presented in this paper demonstrate in vitro for the first time the contribution of individual tissues in each stage of the inflammatory cascade occurring during human TL. We propose a molecular mechanism linking physiologic uterine inflammation and leukocyte infiltration, which precedes labor onset: upregulation of decidual and myometrial cytokines and chemokines that leads to increased endothelial and leukocyte activation, and leukocyte trans-endothelial migration (summarized in Figure 7). Furthermore, we suggest that targeting the inflammatory pathway prior to myometrial activation and contraction, may prove to be more effective at preventing PTL. Identified here inhibitory effects of FX125L on human leukocyte transmigration, together with our previous in vivo results of the prevention of infection induced PTL in non-human primates supports the idea that BSCI (FX125L) may have potential as a tocolytic in the prevention of PTB in women.
Supplementary Material
Key points.
Laboring decidua secretes pro-inflammatory cytokines, chemokines, and growth factors.
Non-laboring myometrium secrets multiple chemokines for leukocyte infiltration.
Broad-Spectrum Chemokine Inhibitor prevents activation of maternal leukocytes.
Acknowledgments
The authors thank the donors, and the Research Centre for Women’s and Infants’ Health BioBank at the Lunenfeld-Tanenbaum Research Institute, and the Department of Obstetrics & Gynecology, at Sinai Health System particularly the staff of the Labor and Delivery Unit for their support for the human specimens used in this study. We would also like to thank Dr. Vanya Peltekova (Ontario Institute for Cancer Research, Toronto) for her assistance with the Luminex assays, Anna Dorogin and Rathesh Balendran for technical assistance with cell culture. We thank Dr. David Fox (Warwick University, UK) for the generous gift of the BSCI.
Funding
This work was supported by the Burroughs Wellcome Fund (#1013759) to S.L., O.S. and K.A.W. This study also used resources supported by the Canadian Institute of Health Research Foundation Grant to S.L. (CIHR: FDN-143262).
Grant support: This work was supported by the grant from Burroughs Wellcome Fund (#1013759). This study also used resources provided by the Canadian Institute of Health Research (FDN-143262).
Footnotes
Conflict of Interests
The authors declare no conflicts of interest
References
- 1.Challis JRG, Lye SJ, Gibb W, Whittle W, Patel F, and Alfaidy N. 2001. Understanding Preterm Labor. Ann. N. Y. Acad. Sci. 943: 225–234. [DOI] [PubMed] [Google Scholar]
- 2.Shynlova O, Tsui P, Jaffer S, and Lye SJ. 2009. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol 144: S2–S10. [DOI] [PubMed] [Google Scholar]
- 3.Osman I, Young A, Jordan F, Greer IA, and Norman JE. 2006. Leukocyte Density and Proinflammatory Mediator Expression in Regional Human Fetal Membranes and Decidua Before and During Labot at Term. J. Soc. Gynecol. Investig 13: 97–103. [DOI] [PubMed] [Google Scholar]
- 4.Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, and Nelson S. 2009. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol 200: 104.e1–104.e11. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Lopez N, Tanaka S, Zaeem Z, Metz GA, and Olson DM. 2013. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC Pregnancy ChildB. 13: S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, and Vadillo-Ortega F. 2013. Evidence for a Role for the Adaptive Immune Response in Human Term Parturition. Am. J. Reprod. Immunol 69: 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, and Arenas-Hernandez M. 2014. Immune cells in term and preterm labor. Cell. Mol. Immunol 11: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams Waldorf KM, Singh N, Mohan AR, Young RC, Ngo L, Das A, Tsai J, Bansal A, Paolella L, Herbert BR, Sooranna SR, Gough GM, Astley C, Vogel K, Baldessari AE, Bammler TK, MacDonald J, Gravett MG, Rajagopal L, and Johnson MR. 2015. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am. J. Obstet. Gynecol 213: 830.e1–830.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephen GL, Lui S, Hamilton SA, Tower CL, Harris LK, Stevens A, and Jones RL. 2014. Transcriptomic Profiling of Human Choriodecidua During Term Labor: Inflammation as a Key Driver of Labor. Am. J. Reprod. Immunol 73: 36–55. [DOI] [PubMed] [Google Scholar]
- 10.Ledingham MA, Thomson AJ, Jordan F, Young A, Crawford M, and Norman JE. 2001. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet. Gynecol 97: 235–42. [DOI] [PubMed] [Google Scholar]
- 11.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA and Norman JE. 2020. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum. Reprod 1: 229–36. [PubMed] [Google Scholar]
- 12.Grigsby PL, Novy MJ, Waldorf KMA, Sadowsky DW, and Gravett MG. 2009. Choriodecidual Inflammation: A Harbinger of the Preterm Labor Syndrome. Reprod. Sci 17: 85–94. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, and Jones RL. 2012. Macrophages Infiltrate the Human and Rat Decidua During Term and Preterm Labor: Evidence That Decidual Inflammation Precedes Labor1. Biol. Reprod 39: 1–9. [DOI] [PubMed] [Google Scholar]
- 14.Shynlova O, Dorogin A, Li Y, and Lye S. 2014. Inhibition of infection‐mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J. Cell. Mol. Med 18: 1816–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, and Lye SJ. 2012. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J. Cell. Mol. Med 17: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, and Lye SJ. 2013. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J. Cell. Mol. Med 17: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, Ahn H, Done B, Hassan SS, Hsu C-D, Tarca AL, Sanchez-Torres C, and Gomez-Lopez N. 2019. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J. Immunol 202: 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokstrom H, Brannstrom M, Alexandersson M, and Norstrom A. 1997. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum. Reprod 12: 586–590. [DOI] [PubMed] [Google Scholar]
- 19.Norwitz ER, Starkey PM, Bernal AL, and Turnbull AC. 1991. Identification by flow cytometry of the prostaglandin-producing cell populations of term human decidua. J. Endocrinol 131: 327–334. [DOI] [PubMed] [Google Scholar]
- 20.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, and Adashi EY. 2005. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J. Matern. Fetal Neonatal Med 17: 365–373. [DOI] [PubMed] [Google Scholar]
- 21.Shynlova O, Tsui P, Dorogin A, and Lye SJ. 2008. Monocyte Chemoattractant Protein-1 (CCL-2) Integrates Mechanical and Endocrine Signals That Mediate Term and Preterm Labor. J. Immunol 181: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 22.García-Velasco JA, and Arici A. 1999. Chemokines and human reproduction. Fertil. Steril 71: 983–993. [DOI] [PubMed] [Google Scholar]
- 23.Orsi NM, and Tribe RM. 2008. Cytokine Networks and the Regulation of Uterine Function in Pregnancy and Parturition. J. Neuroendocrinol 20: 462–469. [DOI] [PubMed] [Google Scholar]
- 24.Kayisli UA, Mahutte NG, and Arici A. 2002. Uterine Chemokines in Reproductive Physiology and Pathology. Am. J. Reprod. Immunol 47: 213–221. [DOI] [PubMed] [Google Scholar]
- 25.Griffith JW, Sokol CL, and Luster AD. 2014. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol 32: 659–702. [DOI] [PubMed] [Google Scholar]
- 26.Chen K, Bao Z, Tang P, Gong W, Yoshimura T, and Wang JM. 2018. Chemokines in homeostasis and diseases. Cell. Mol. Immunol 15: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay CR 2001. Chemokines: immunology’s high impact factors. Nat. Immunol 2: 95–101. [DOI] [PubMed] [Google Scholar]
- 28.Salamonsen LA, Zhang J, and Brasted M. 2002. Leukocyte networks and human endometrial remodelling. J. Reprod. Immunol 57: 95–108. [DOI] [PubMed] [Google Scholar]
- 29.Choi W-T, and An J. 2011. Biology and clinical relevance of chemokines and chemokine receptors CXCR4 and CCR5 in human diseases. Exp. Biol. Med 236: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Song I, Yun H, Jo D, and Kim S. 2014. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World J. Gastroenterol 20: 1681–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan L, Anderson GM, DeWitte M, and Nakada MT. 2006. Therapeutic potential of cytokine and chemokine antagonists in cancer therapy. Eur. J. Cancer 42: 793–802. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Ceska M, Avila C, Mazor M, Behnke E, and Lindley I. 1991. Neutrophil attractant/activating peptide-1 / interleukin-8 in term and preterm parturition. Am. J. Obstet. Gynecol 165: 813–820. [DOI] [PubMed] [Google Scholar]
- 33.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, and Adashi EY. 2005. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J. Matern. Fetal Neonatal Med 17: 365–373. [DOI] [PubMed] [Google Scholar]
- 34.Shynlova O, Lee Y-H, Srikhajon K, and Lye SJ. 2012. Physiologic Uterine Inflammation and Labor Onset. Reprod. Sci 20: 154–167 [DOI] [PubMed] [Google Scholar]
- 35.Pearce BD, Garvin SE, Grove J, Bonney EA, Dudley DJ, Schendel DE, and Thorsen P. 2008. Serum macrophage migration inhibitory factor in the prediction of preterm delivery. Am. J. Obstet. Gynecol 199: 46.e1–46.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, and Vadillo-Ortega F. 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol 80: 122–131. [DOI] [PubMed] [Google Scholar]
- 37.Grainger DJ, and Reckless J. 2003. Broad-spectrum chemokine inhibitors (BSCIs) and their anti-inflammatory effects in vivo. Biochem. Pharmacol 65: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 38.Naidu BV, Farivar AS, Woolley SM, Grainger D, Verrier ED, and Mulligan MS. 2004. Novel broad-spectrum chemokine inhibitor protects against lung ischemia-reperfusion injury. J. Heart Lung Transplant 23: 128–134. [DOI] [PubMed] [Google Scholar]
- 39.Berkkanoglu M, Zhang L, Ulukus M, Cakmak H, Kayisli UA, Kursun S, and Arici A. 2005. Inhibition of chemokines prevents intraperitoneal adhesions in mice. Hum. Reprod 20: 3047–3052. [DOI] [PubMed] [Google Scholar]
- 40.Grainger DJ, and Lever AML. 2005. Blockade of chemokine-induced signalling inhibits CCR5-dependent HIV infection in vitro without blocking gp120/CCR5 interaction. Retrovirology. 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox DJ, Reckless J, Wilbert SM, Greig I, Warren S, and Grainger DJ. 2005. Identification of 3-(Acylamino)azepan-2-ones as Stable Broad-Spectrum Chemokine Inhibitors Resistant to Metabolism in Vivo. J. Med. Chem 48: 867–874. [DOI] [PubMed] [Google Scholar]
- 42.Fox DJ, Reckless J, Lingard H, Warren S, and Grainger DJ. 2009. Highly Potent, Orally Available Anti-inflammatory Broad-Spectrum Chemokine Inhibitors. J. Med. Chem 52: 3591–3595. [DOI] [PubMed] [Google Scholar]
- 43.Reckless J, Tatalick LM, and Grainger DJ. 2001. The pan-chemokine inhibitor NR58–3.14.3 abolishes tumour necrosis factor-alpha accumulation and leucocyte recruitment induced by lipopolysaccharide in vivo. Immunology. 103: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miklos S, Mueller G, Chang Y, Bouazzaoui A, Spacenko E, Schubert TEO, Grainger DJ, Holler E, Andreesen R, and Hildebrandt GC. 2009. Preventive usage of broad spectrum chemokine inhibitor NR58–3.14.3 reduces the severity of pulmonary and hepatic graft-versus-host disease. Int. J. Hematol 89: 383–397. [DOI] [PubMed] [Google Scholar]
- 45.Coleman M, Orvis A, Wu T-Y, Dacanay M, Merillat S, Ogle J, Baldessari A, Kretzer NM, Munson J, Boros-Rausch AJ, Shynlova O, Lye S, Rajagopal L, and Adams Waldorf KM. 2020. A Broad Spectrum Chemokine Inhibitor Prevents Preterm Labor but Not Microbial Invasion of the Amniotic Cavity or Neonatal Morbidity in a Non-human Primate Model. Front. Immunol 11: 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farine T, Parsons M, Lye S, and Shynlova O. 2018. Isolation of Primary Human Decidual Cells from the Fetal Membranes of Term Placentae. J. Vis. Exp 134: 57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikhajon K, Shynlova O, Preechapornprasert A, Chanrachakul B, and Lye S. 2014. A New Role for Monocytes in Modulating Myometrial Inflammation During Human Labor. Biol. Reprod 91: 1. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y-H, Shynlova O, and Lye SJ. 2014. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell. Mol. Immunol 12: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Shynlova O, Sabra S, Bang A, Briollais L, and Lye SJ. 2017. Immunophenotyping and activation status of maternal peripheral blood leukocytes during pregnancy and labour, both term and preterm. J. Cell Mol. Med 21: 2386–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schenkel AR, Mamdouh Z, and Muller WA. 2004. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol 5: 393–400. [DOI] [PubMed] [Google Scholar]
- 51.Petri B, and Bixel MG. 2006. Molecular events during leukocyte diapedesis. FEBS J. 273: 4399–4407. [DOI] [PubMed] [Google Scholar]
- 52.Sadik CD, Kim ND, and Luster AD. 2011. Neutrophils cascading their way to inflammation. Trends Immunol. 32: 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon R, Bonney EA, Condon J, Mesiano S, and Taylor RN. 2016. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum. Reprod. Update 22: 535–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nancy P, Tagliani E, Tay C-S, Asp P, Levy DE, and Erlebacher A. 2012. Chemokine Gene Silencing in Decidual Stromal Cells Limits T Cell Access to the Maternal-Fetal Interface. Science. 336: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaturvedi V, Ertelt JM, Jiang TT, Kinder JM, Xin L, Owens KJ, Jones HN, and Way SS. 2015. CXCR3 blockade protects against Listeria monocytogenes infection–induced fetal wastage. J. Clin. Investig 125: 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, and Olson DM. 2010. Interleukin-6 Is an Essential Determinant of On-Time Parturition in the Mouse. Endocrinology. 151: 3996–4006. [DOI] [PubMed] [Google Scholar]
- 57.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, and Jones SA. 2001. IL-6 and Its Soluble Receptor Orchestrate a Temporal Switch in the Pattern of Leukocyte Recruitment Seen during Acute Inflammation. Immunity. 14: 705–714. [DOI] [PubMed] [Google Scholar]
- 58.Cierny JT, Unal ER, Flood P, Rhee KY, Praktish A, Olson TH, and Goetzl L. 2014. Maternal inflammatory markers and term labor performance. Am. J. Obstet. Gynecol 210: 447.e1–447.e6. [DOI] [PubMed] [Google Scholar]
- 59.Neal JL, Lamp JM, Lowe NK, Gillespie SL, Sinnott LT, and McCarthy DO. 2015. Differences in inflammatory markers between nulliparous women admitted to hospitals in preactive vs active labor. Am. J. Obstet. Gynecol 212: 68.e1–68.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wengner AM, Pitchford SC, Furze RC, and Rankin SM. 2008. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 111: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luppi P, Haluszczak C, Trucco M, and Deloia JA. 2002. Normal Pregnancy is Associated with Peripheral Leukocyte Activation. Am. J. Reprod. Immunol 47: 72–81. [DOI] [PubMed] [Google Scholar]
- 62.Baumann R, Casaulta C, Simon D, Conus S, Yousefi S, and Simon H-U. 2003. Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria‐dependent death pathway. FASEB J. 17: 2221–2230. [DOI] [PubMed] [Google Scholar]
- 63.Williams MR, Azcutia V, Newton G, Alcaide P, and Luscinskas FW. 2011. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 32: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan M, Jordan F, McInnes IB, Harnett MM, and Norman JE. 2009. Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol. Hum. Reprod 15: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luppi P, Haluszczak C, Betters DM, Richard CA, Trucco M, and Deloia J. 2012. Monocytes are progressively activated in the circulation of pregnant women. J. Leukoc. Biol 72: 874–84. [PubMed] [Google Scholar]
- 66.Winkler M, Ruck P, Horny H-P, Wehrmann M, Kemp B, Kaiserling E, and Rath W. 1998. Expression of cell adhesion molecules by endothelium in the human lower uterine segment during parturition at term. Am. J. Obstet. Gynecol 178: 557–561. [DOI] [PubMed] [Google Scholar]
- 67.Elekes K, Helyes Z, Kereskai L, Sándor K, Pintér E, Pozsgai G, Tékus V, Bánvölgyi Á, Németh J, Szűts T, Kéri G, and Szolcsányi J. 2008. Inhibitory effects of synthetic somatostatin receptor subtype 4 agonists on acute and chronic airway inflammation and hyperreactivity in the mouse. Eur. J. Pharmacol 578: 313–322. [DOI] [PubMed] [Google Scholar]
- 68.Davide C, Caroline N, and Paola B. 2005. Multiple Signalling Transduction Mechanisms Differentially Coupled to Somatostatin Receptor Subtypes: A Current View. J Enzyme Inhib. 1: 265–279. [Google Scholar]
- 69.Olias G, Viollet C, Kusserow H, Epelbaum J, and Meyerhof W. 2004. Regulation and function of somatostatin receptors. J. Neurochem 89: 1057–1091. [DOI] [PubMed] [Google Scholar]
- 70.Elliott DE, Li J, Blum AM, Metwali A, Patel YC, and Weinstock JV. 1999. SSTR2A is the dominant somatostatin receptor subtype expressed by inflammatory cells, is widely expressed and directly regulates T cell IFN-γ release. Eur. J. Immunol 29: 2454–2463. [DOI] [PubMed] [Google Scholar]
- 71.Grainger DJ 2008. Anti-inflammatory compositions and combinations, WO2009074794A2 - Google Patents. Google.com. [Google Scholar]
- 72.Royall S, and Fox D. 2016. Chemistry tipping the biological seesaw. studylib.net. [Google Scholar]
- 73.Armani C, Catalani E, Balbarini A, Bagnoli P, and Cervia D. 2006. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J. Leukoc. Biol 81: 845–855. [DOI] [PubMed] [Google Scholar]
- 74.Krantic S. 2000. Peptides as regulators of the immune system: emphasis on somatostatin. Peptides. 21: 1941–1964. [DOI] [PubMed] [Google Scholar]
- 75.Lichtenauer-Kaligis E, van Hagen P, Lamberts S, and Hofland L. 2000. Somatostatin receptor subtypes in human immune cells. Eur. J. Endocrinol S21–S25. [DOI] [PubMed] [Google Scholar]
- 76.Dalm VASH, van Hagen PM, van Koetsveld PM, Achilefu S, Houtsmuller AB, Pols DHJ, van der Lely A-J, Lamberts SWJ, and Hofland LJ. 2003. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am. J. Physiol. Endocrinol. Metab 285: E344–E353. [DOI] [PubMed] [Google Scholar]
- 77.Lichtenauer-Kaligis E, Dalm V, Oomen S, Mooij D, van Hagen P, Lamberts S, and Hofland L. 2004. Differential expression of somatostatin receptor subtypes in human peripheral blood mononuclear cell subsets. Eur. J. Endocrinol 565–577. [DOI] [PubMed] [Google Scholar]
- 78.Tagliani E, Shi C, Nancy P, Tay C-S, Pamer EG, and Erlebacher A. 2011. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J. Exp. Med 208: 1901–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.