SUMMARY
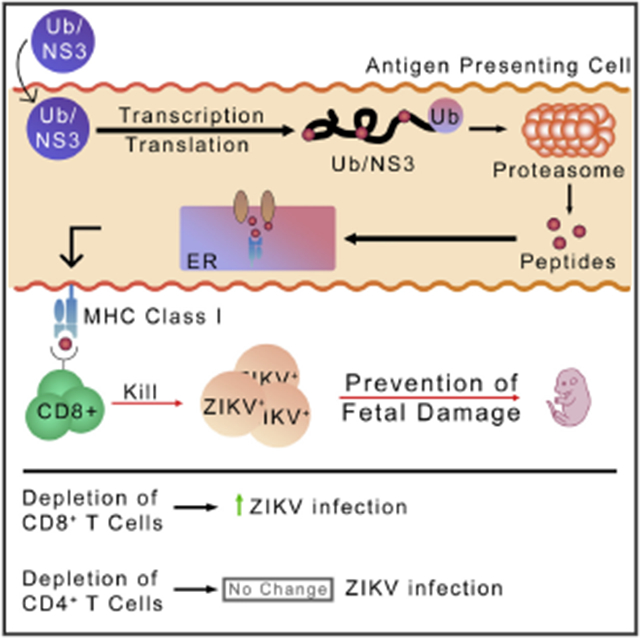
As vaccine-induced non-neutralizing antibodies may cause antibody-dependent enhancement of Zika virus (ZIKV) infection, we test a vaccine that induces only specific cytotoxic T lymphocytes (CTLs) without specific antibodies. We construct a DNA vaccine expressing a ubiquitinated and rearranged ZIKV non-structural protein 3 (NS3). The protein is immediately degraded and processed in the proteasome for presentation via major histocompatibility complex (MHC) class I for CTL generation. We immunize Ifnar1−/− adult mice with the ubiquitin/NS3 vaccine, impregnate them, and challenge them with ZIKV. Our data show that the vaccine greatly reduces viral titers in reproductive organs and other tissues of adult mice. All mice immunized with the vaccine survived after ZIKV challenge. The vaccine remarkably reduces placenta damage and levels of pro-inflammatory cytokines, and it fully protects fetuses from damage. CD8+ CTLs are essential in protection, as demonstrated via depletion experiments. Our study provides a strategy to develop safe and effective vaccines against viral infections.
Graphical Abstract
In brief
Non-neutralizing antibodies have been shown to enhance viral infection in flaviviruses. Gambino et al. sought a vaccine strategy that protects against ZIKV without inducing antibodies. They demonstrate that a ZIKV vaccine inducing solely NS3-specific CTLs provides full protection against ZIKV challenge and protects against fetal damage in pregnant mice.
INTRODUCTION
Dengue virus (DENV) and Zika virus (ZIKV) belong to the family Flaviviridae (Lazear and Diamond, 2016). DENV has four serotypes that differ by 30%–35%, with their viral envelope (E) protein sequences differing from ZIKV E protein only by 41%–46% (Screaton et al., 2015). DENV infection usually does not cause severe symptoms but may lead to life-threatening complications such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (Khetarpal and Khanna, 2016). Primary infection by DENV leads to lifelong immunity to the infecting serotype, but not to the other serotypes (Murphy and Whitehead, 2011). Secondary infection by other serotypes is often responsible for DHF and DSS (Kalayanarooj, 2011). It is believed that the antibodies generated during primary DENV infection are unable to neutralize another serotype of the virus during secondary infection. These non-neutralizing antibodies instead bind to the virus and opsonize it into monocytes and macrophages via Fc-receptor-mediated endocytosis, leading to antibody-dependent enhancement (ADE) of infection. Similar to DENV infection, ZIKV infection causes mild symptoms, if any, such as fever, myalgia, arthralgia, headache, conjunctivitis, and thrush in most infected people. However, severe symptoms such as microcephaly and other neurological abnormalities have been associated with ZIKV infection (Brasil et al., 2016; Cao-Lormeau et al., 2016; Driggers et al., 2016). Furthermore, it has been shown that human monoclonal antibodies generated from DENV-infected subjects cross-reacted with ZIKV (Priyamvada et al., 2016). Importantly, DENV-specific antibodies have been shown to enhance ZIKV pathogenesis and ZIKV-induced microcephaly-like syndrome in mice (Bardina et al., 2017; Dejnirattisai et al., 2016; Rathore et al., 2019). In addition, maternally acquired ZIKV-specific antibodies enhanced DENV infection and heightened disease states in mice (Fowler et al., 2018). It has also been demonstrated that preexisting high-antibody titers to DENV were associated with reduced risk of ZIKV infection and symptoms in humans (Rodriguez-Barraquer et al., 2019). Although it is unclear if the antibodies to DENV enhance ZIKV infection or provide protection against ZIKV infection, it may be prudent to design a vaccine that does not induce antibodies that may enhance either disease. Furthermore, vaccination with Dengvaxia, which expresses the precursor of membrane (prM) and E proteins from four serotypes of DENV, has led to more hospitalization of DENV-uninfected children than infected ones (a possible consequence of ADE) (Dans et al., 2018; Halstead, 2017; Martínez-Vega et al., 2017). This alarming result has prompted us to design a vaccine that does not induce potentially disease-enhancing antibodies.
A conventional vaccine is expected to induce neutralizing antibodies. However, it also induces non-neutralizing antibodies, which are responsible for ADE of DENV or ZIKV infection when neutralizing antibodies wane (Dejnirattisai et al., 2016; Langerak et al., 2019; Shim et al., 2019). It is very difficult to induce only neutralizing antibodies without non-neutralizing antibodies. We hypothesize that a ZIKV vaccine that elicits only ZIKV-specific cytotoxic T lymphocytes (CTLs), but not ZIKV-specific antibodies, will prevent ZIKV infection without the risk of ADE-mediated consequences. It is unknown whether CTLs are sufficient for full protection against a viral infection. It is traditionally believed that both neutralizing antibodies and CTLs are essential for prevention of a viral infection. Because humans mount significant T cell responses to ZIKV nonstructural protein 1 (NS1), NS3, and NS5 (Delgado et al., 2018; Herrera et al., 2018a, 2018b) and dominant CTL responses to NS3 and NS5 (Edupuganti et al., 2017; El Sahly et al., 2018; Waggoner et al., 2017), we designed a vaccine that targets ZIKV NS3. Furthermore, we used a ubiquitination and gene rearrangement strategy to enhance the degradation of NS3 in the proteasome with the goal of inducing only NS3-specific CTLs. We tested the efficacy of this vaccine in protection against ZIKV challenge in animal models.
RESULTS
TCI ZIKV vaccine design
Great efforts have been made to develop vaccines to prevent ZIKV infection. These vaccine candidates range from live-attenuated viruses to viral vectors, mRNA, and DNA (Abbink et al., 2016, 2017; Brault et al., 2017; Bullard et al., 2018; Dowd et al., 2016; Gaudinski et al., 2018; Griffin et al., 2017; Grubor-Bauk et al., 2019; Jagger et al., 2019; Larocca et al., 2016, 2019; Pardi et al., 2017; Richner et al., 2017a, 2017b; Van Rompay et al., 2019; Shan et al., 2017; Tebas et al., 2017). In several of these studies, prM and E proteins have been targeted, albeit with considerably differing amino acid sequences across strains. A predictive epitope analysis found that all CD8+ T cell epitopes on the NS3 amino acid sequence were conserved across 54 different ZIKV genomes (Dar et al., 2016; Mirza et al., 2016). Normally, the NS3 protein is covalently bonded to NS2B, an anchor protein that functions as a cofactor to promote the productive folding and activity of NS3 (Phoo et al., 2016). The N-terminal region of the NS3 protein encodes for a serine protease, while the latter region encodes for a helicase. This ZIKV NS2B-NS3pro complex is responsible for the cleavage of the ZIKV polyprotein precursor and the generation of the other proteins in the ZIKV viral genome (Hilgenfeld et al., 2018; Lei et al., 2016; Li et al., 2017; Phoo et al., 2018). As the NS3 protein is essential for the functionality of every other ZIKV viral protein, and its CD8+ T cell epitopes are conserved across 54 different ZIKV genomes (Dar et al., 2016), this protein serves as an attractive target for vaccine therapy. Thus, we used NS3 as the target protein in our T-cell-inducing (TCI) ZIKV vaccine.
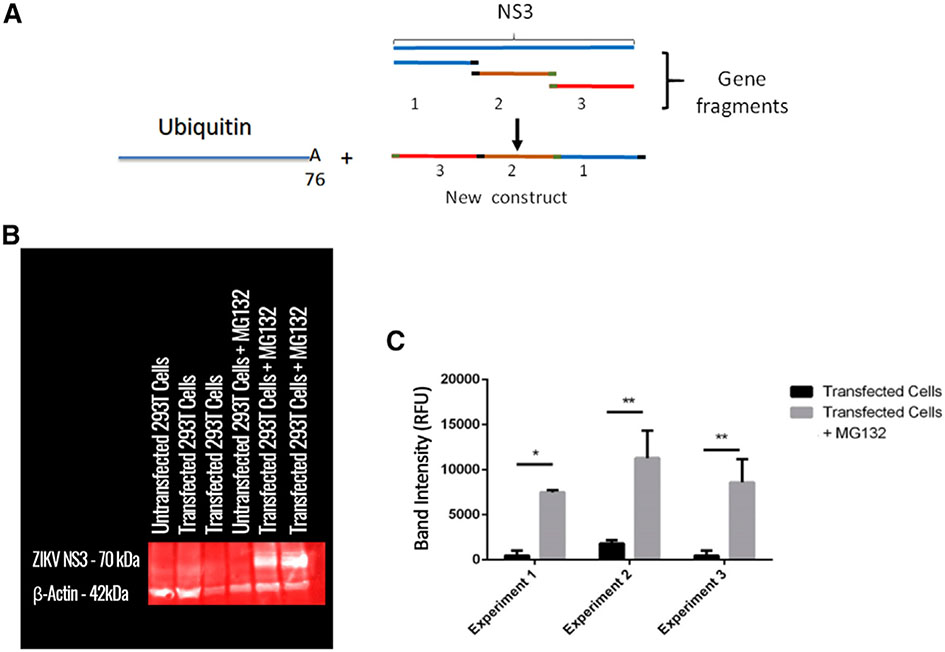
First, we split the NS3 gene (PRVABC59/2015 strain of ZIKV in Homo sapiens) into three parts and rearranged them (Figure 1A) in order to disrupt NS3’s viral functions. Furthermore, this rearrangement may produce an unstable protein, which will be likely targeted to the proteasome for degradation. In constructing this sequence, the 30 nucleotide bases before and after any cleaved sites were placed back to preserve any CTL epitopes that may have been disrupted. The open reading frame (ORF) encoding for a mouse/human monomer of ubiquitin (Ub) was placed immediately upstream of the rearranged NS3 DNA sequence. A glycine at the 76th residue was modified to encode an alanine to enhance the stability of the Ub/NS3 complex (Figure 1A), which has been shown to promote protein degradation in the proteasome (Rodriguez et al., 1997). In order to ensure efficient transcription, a Kozak sequence was placed upstream of this combined Ub/NS3 sequence. The rearranged Ub/NS3 sequence was inserted into pVAX1, an FDA-approved vector for use in DNA vaccines in humans.
Figure 1. ZIKV TCI vaccine design and antigen expression.
(A) Schematic diagram of plasmid design. The gene sequence encoding for the NS3 protein was split into three parts (denoted 1, 2, and 3). The 30 nucleotide bases before and after any cleaved sequence were placed in front of each region to preserve any epitopes that may have been disrupted. The ORF gene encoding for a mouse/human monomer of ubiquitin (Ub) was placed immediately upstream of the rearranged NS3 sequence. A glycine at the 76th residue was modified to encode an alanine to enhance the stability of the Ub/NS3 complex.
(B) 293T cells were transfected with the rearranged Ub/NS3 plasmid overnight. The cells were allowed to stably express plasmid for 36 h. After this period, MG132 was added overnight. The cell lysate was analyzed via western blot for the expression of NS3 and beta-actin. A representative blot from three experiments is shown.
(C) Total band density of western blot was quantified using ImageJ and analyzed using GraphPad (Prism). The data are represented as mean ± SEM (n = 2 for each experiment, three experiments total). *p < 0.05; **p < 0.01.
To determine the expression of the rearranged NS3, the plasmid DNA was used to transfect 293T cells. RNA was isolated, and RNA reverse transcription was conducted. Quantitative reverse transcription PCR (qRT-PCR) was conducted using primers specific to the Ub/NS3 sequence. NS3 gene transcription was confirmed (Figures S1A and S1B). We further determined expression of the NS3 protein. Naturally, there comes a problem with visualizing a protein that is innately ubiquitinated. It is widely known that many proteins are targeted for degradation by covalent ligation to ubiquitin (Schrader et al., 2009). Therefore, any protein that is innately ubiquitinated is targeted for immediate destruction. To address this issue, we used proteasome inhibitor MG132. Thus, 293T cells were transfected overnight with the plasmid DNA delivered in polyethylenimine (PEI) transfection reagent. The 293T cells were allowed to stably express the transfected protein for 36 h. After this period, cells were treated with 50 μM MG132 overnight. A western blot was then conducted to determine the production of the rearranged Ub/NS3 protein (Figure 1B), and band intensity was quantified via ImageJ (Figure 1C). In transfected cells not treated with MG132, there is very low band intensity at 70 kDa, indicating that the Ub/NS3 protein is either not translated or it is targeted for immediate degradation. When cells are treated with MG132, however, band intensity increases tremendously. These data not only confirm the translation of the Ub/NS3 protein, but by inhibiting the proteasome, also demonstrate that the translated protein would have been targeted for immediate degradation otherwise (Figure 1C).
ZIKV TCI-DNA vaccine protected female pregnant BALB/c mice and their fetuses against ZIKV challenge
To investigate the efficacy of our ZIKV TCI-DNA vaccine in protecting pregnant mothers and their fetuses against ZIKV infection, we first tested the immunogenicity of this vaccine in immunocompetent mice, as interferon (IFN)-α/β receptor (IF-NAR)-knockout mice (the current mouse model for ZIKV challenge) may have a reduced ability to generate CTLs. Thus, we immunized immunocompetent female BALB/c mice (non-lethal to ZIKV infection) with TCI-DNA in the presence of Imiquimod adjuvant, ZIKV E ectodomain, EDI/II peptides in the presence of aluminum hydroxide (Alum) and monophosphoryl lipid A (MPL) adjuvants, or PBS. We used E ectodomain (Aviva Systems Biology) as a control immunogen because it is expected to induce E-protein-specific antibodies that may result in ADE. E ectodomain should be recognized by B cells and processed in antigen-presenting cells for presentation to CD4+ and CD8+ T cells. The peptide control immunogen (EDI/II peptides) is made of long peptides that contain major histocompatibility complex (MHC)-class-I-restricted epitopes including H-2 Db, Kb-, and Kd-restricted epitopes (no MHC-class-II-restricted epitopes), which are confirmed in animal studies. EDI/II peptides are expected to induce only CTLs but are less immunogenic than our TCI vaccine. Ten days after the second immunization, the female mice were mated with males. It took 2–3 weeks for the mice to be impregnated. After the female mice were impregnated (embryonic day [E]5–E7), they were injected with anti-IFNAR1 antibody (to make the mice susceptible to ZIKV by blocking IFNARs) (Gorman et al., 2018; Morrison and Diamond, 2017; Wang et al., 2019). One day later, mice were challenged with a high dose of ZIKV (R103451, 2 × 105 plaque-forming unit [PFU]) and examined for morphological changes of uteri 6 days after. Mouse placenta and amniotic fluid were also evaluated for ZIKV titers via plaque-forming assay (Figure S2A).
The uteri (E11–E13) from TCI-DNA and E-ectodomain-immunized pregnant BALB/c mice exhibited normal morphology without obvious fetal death, indicating complete protection against uteri damage and fetal death; in contrast, EDI/II-peptide- or PBS-injected pregnant mice had either slightly or severely damaged uteri with reduced size, indicating fetal death and incomplete protection against ZIKV infection (Figure S3A). Investigation of viral titers in ZIKV-infected placenta and amniotic fluid revealed undetectable or significantly lower titer of ZIKV in placenta (Figure S3B) and amniotic fluid (Figure S3C) of mice receiving TCI-DNA vaccine than in those of mice receiving E ectodomain, EDI/II peptides, or PBS. In addition, there were significant but slightly lower viral titers in the placenta of mice immunized with E ectodomain than in those of mice injected with PBS (Figure S3B). Collectively, these results suggest that the TCI-DNA vaccine completely protected immunocompetent pregnant mice and their fetuses against high-dose challenge of ZIKV R103451, a human strain responsible for the recent ZIKV outbreaks (Tai et al., 2018). Because we challenged the pregnant mice with ZIKV >30 days after the last immunization, we concluded that the protection was due to the vaccine-induced immune memory.
ZIKV TCI-DNA vaccine protected ZIKV-susceptible adult or pregnant Ifnar1−/− mice and their fetuses against two different strains of ZIKV challenge
Because there is no disease pathology in wild-type adult mice challenged with the ZIKV, we used a lethal mouse model to test our vaccine efficacy. Thus, we immunized Ifnar1−/− mice, an IFNAR-deficient mouse model lethal to ZIKV (Marzi et al., 2018; Tai et al., 2018, 2019a), with TCI-DNA, ZIKV E ectodomain, EDI/II peptides, or PBS as described above and performed the following three challenge experiments (Figure S2A).
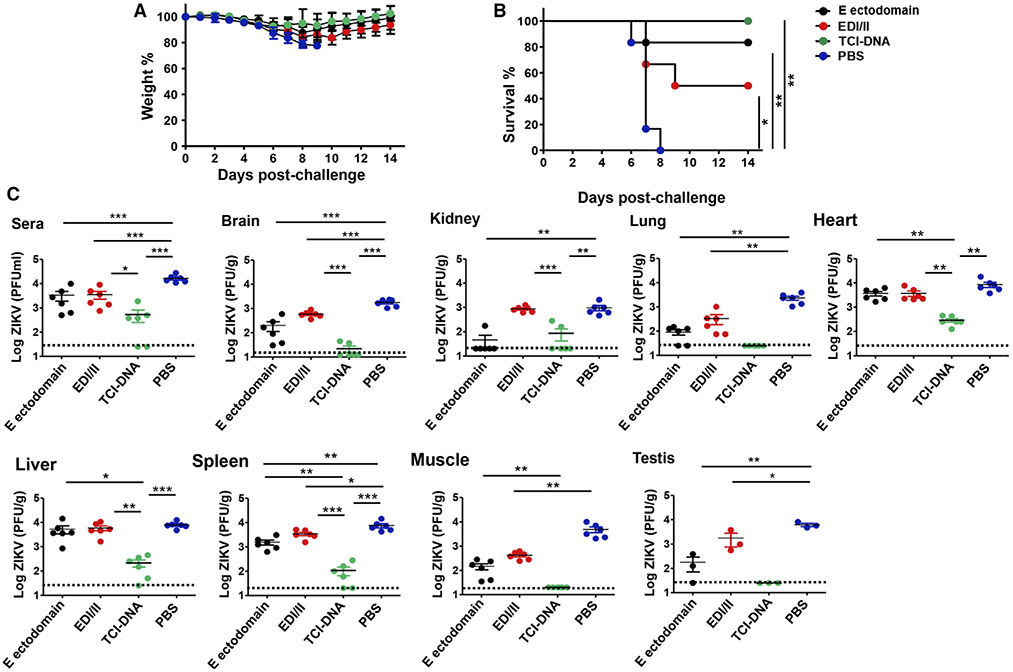
First, we challenged IFNAR-deficient immunized adult (male and female) mice with ZIKV (strain R103451, 103 PFU) 10 days post-last dose and monitored their weight and survival changes for 14 days. We found that mice immunized with TCI-DNA had only slight weight loss during days 7–9 post-challenge, followed by constantly increased weight until 14 days (Figure 2A), and all mice from this group survived ZIKV challenge (Figure 2B). In contrast, the mice immunized with E ectodomain or EDI/II peptides showed reduced survival rates (to about 83% and 50%, respectively), and their weight either slightly or moderately decreased (Figures 2A and 2B). Mice injected with PBS had continuously decreased weight, and all mice died at 8 days post-challenge (Figures 2A and 2B). These data confirm complete protection of TCI-DNA vaccine against ZIKV-caused death and weight loss.
Figure 2. ZIKV TCI-DNA vaccine protected adult male and female Ifnar1−/− mice against ZIKV challenge with complete survival and reduced viral titers, including reproductive organs.
Equal numbers of male and female Ifnar1−/− mice were immunized with ZIKV E ectodomain, EDI/II peptides, TCI-DNA, or PBS control, and sera were collected at 10 days post-second immunization.
(A and B) At 13 days post-second immunization, the mice were challenged (i.p.) with ZIKV (strain R103451, 103 PFU/mouse) and recorded for weight (A) and survival (B) daily for 14 days (n = 6).
(C) In a separate experiment, the immunized Ifnar1−/− mice were challenged with ZIKV (strain PAN2016, 103 PFU/mouse), and 6 days later, sera and tissues (including brain, kidney, lung, heart, liver, spleen, muscle, and testis) were collected for detection of viral titers using plaque-forming assay. The detection limit was 25 PFU/ml (for sera), 12.5 PFU/g (for brain), 20 PFU/g (for kidney, spleen, and muscle), and 25 PFU/g (for lung, heart, liver, and testis).
The data are represented as mean ± SEM (n = 3 for testis and n = 6 for other groups). *p < 0.05; **p < 0.01; ***p < 0.001.
Second, we challenged immunized adult (male and female) Ifnar1−/− mice with ZIKV PAN2016 (103 PFU/mouse), another human strain causing recent ZIKV outbreaks (Gao et al., 2019; Tai et al., 2018), and measured ZIKV titers in sera and tissues via plaque-forming assay 6 days later. We noted undetectable viral titers in the lung, muscle, and testis of mice immunized with TCI-DNA, and viral titers in the sera and most tissues of these mice were also significantly lower than in those of mice immunized with E ectodomain, EDI/II peptides, or PBS (Figure 2C). In addition, ZIKV titers in sera, brain, lung, spleen, muscle, or testis of mice receiving E ectodomain or EDI/II peptides were also significantly lower than in those of mice injected with PBS (Figure 2C). These data indicate that the TCI-DNA vaccine significantly enhanced this protection, resulting in undetectable or significantly decreased viremia and viral titers in key organs, including reproductive organs such as testis.
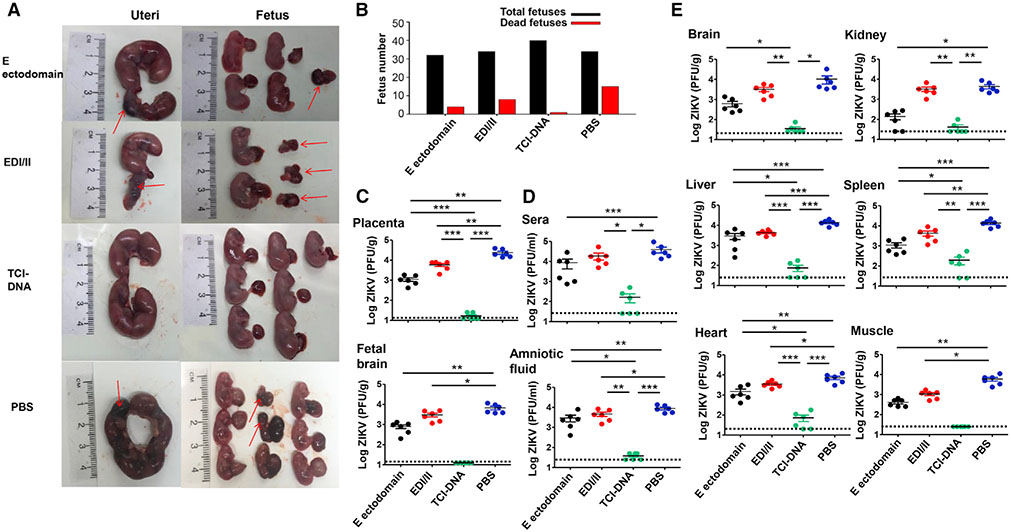
Third, immunized female Ifnar1−/− mice were mated with male Ifnar1−/− mice for pregnancy (E5–E7) and were challenged with ZIKV (strain R103451; 104 PFU/mouse) as we did for BALB/c mice. Mice were then examined for morphological changes of uteri and fetuses, as well as viral titers in sera, amniotic fluid, fetal brain, and tissues at 6 days post-challenge. The uteri from TCI-DNA-immunized pregnant Ifnar1−/− mice had intact morphology and normal fetuses without demise, whereas the uteri from mice immunized with E ectodomain or EDI/II peptides exhibited slight or severe damage, with moderate and severe fetal resorption or fetal death in uteri, respectively (Figure 3A). Different from other groups, almost all fetuses survived from uteri of TCI-DNA-immunized pregnant mice after ZIKV challenge (Figure 3B). Importantly, viral titers in the placenta, amniotic fluid, and fetal brain (Figures 3C and 3D) of mice receiving the TCI-DNA vaccine were either undetectable or significantly lower than in those of mice receiving E ectodomain, EDI/II peptides, or PBS. Also, there were significantly lower titers of ZIKV in the placenta, amniotic fluid, and fetal brain of mice immunized with E ectodomain and EDI/II peptides than in those of mice injected with PBS (Figures 3C and 3D). Notably, ZIKV titers in the muscle, brain, heart, liver, and spleen of mice immunized with TCI-DNA were undetectable or significantly lower than in those of mice immunized with E ectodomain, EDI/II peptides, or PBS, and viral titers in the sera and kidney of TCI-DNA-immunized mice were also significantly lower than in those of mice receiving EDI/II peptides or PBS (Figures 3C and 3D). Moreover, the results also denoted significantly lower viral titers in the sera and tissues (kidney, heart, liver, spleen, and muscle) of mice vaccinated with E ectodomain than in those of mice injected with PBS (Figures 3C and 3D).
Figure 3. ZIKV TCI-DNA vaccine protected female pregnant Ifnar1−/− mice and their fetuses against ZIKV challenge.
Female Ifnar1−/− mice were immunized with ZIKV E ectodomain, EDI/II peptides, TCI-DNA, or PBS control and mated with male Ifnar1−/− mice for pregnancy at day 10 post-second immunization. The pregnant mice (E10–E12) were challenged with ZIKV (strain R103451, 104 PFU/mouse), and 6 days later, uteri and fetuses were collected to evaluate morphological changes as well as sera, body fluid, and tissues (including placenta) to measure viral titers using plaque-forming assay. Placenta was also observed for apoptosis and vascular damage and examined for inflammatory cytokines and chemokines as described below.
(A and B) Representative image of morphology of uteri (E16–E18) and fetuses from pregnant mice challenged with ZIKV at E10-E12 (A), among which total numbers and dead fetuses from each group are shown (B).
(C–E) Viral titers in placenta, fetal brain (C), as well as sera and amniotic fluid (D) and tissues (including brain, kidney, liver, spleen, heart, and muscle) (E), 6 days post-challenge. The detection limit was 12.5 PFU/g (for placenta and fetal brain), 20 PFU/g (for heart), 25 PFU/ml (for sera and amniotic fluid), and 25 PFU/g (for brain, kidney, liver, spleen, and muscle).
The data are represented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001.
Collectively, the above data confirm that the TCI-DNA vaccine completely protected adult or pregnant Ifnar1−/− mice and their fetuses against two different strains of ZIKV infection.
ZIKV TCI-DNA vaccine prevented ZIKV-caused apoptosis, vascular damage and inflammation, and ZIKV-associated ADE
ZIKV infection may cause apoptosis and fetal blood vessel damage in placenta, leading to severe inflammation with increased cytokines and chemokines (He et al., 2018; Miner et al., 2016; Rabelo et al., 2018; Souza et al., 2016; Tripathi et al., 2017). It is reported that ZIKV E protein, including fusion loop (FL) region, may induce cross-reactive antibodies that enhance ZIKV or DENV infection, resulting in ADE (Barba-Spaeth et al., 2016; Barbosa et al., 2018; Fowler et al., 2018).
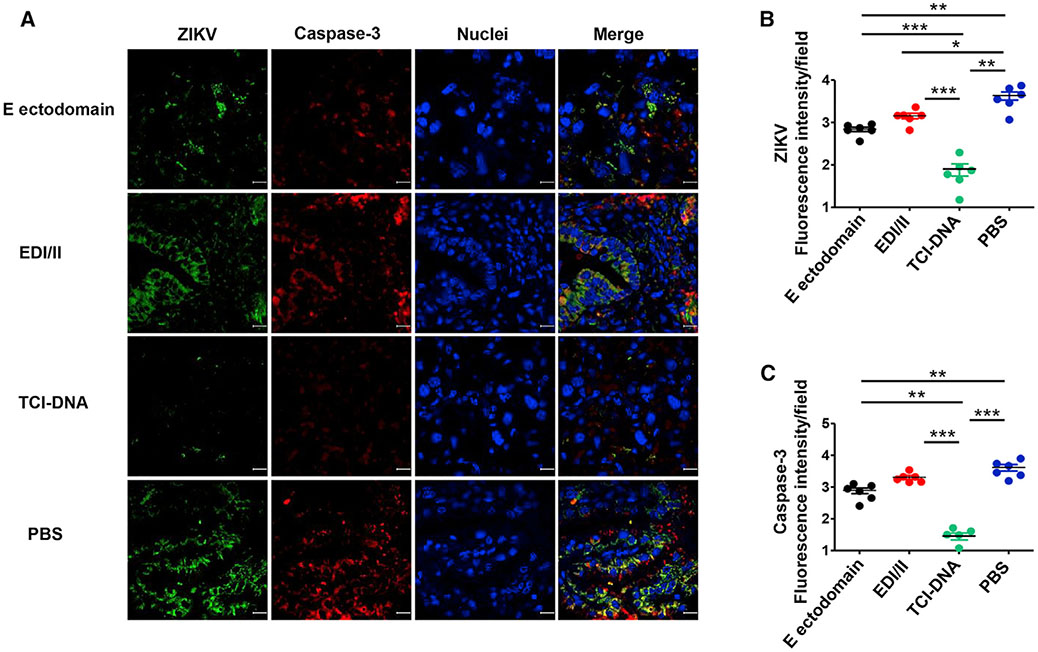
To evaluate whether the ZIKV TCI-DNA vaccine can prevent apoptosis and vasculature damage associated with ZIKV, we performed immunofluorescence staining using placental tissues of vaccine-immunized and ZIKV-challenged pregnant Ifnar1−/− mice (as described above) and stained for active caspase-3, an apoptotic marker (Yuan et al., 2017), and vimentin, a marker for fetal blood vessels and fetal capillary endothelium (Miner et al., 2016; Sapparapu et al., 2016). There were significantly lower numbers of caspase-3+ and ZIKV+ signals in the placenta of mice immunized with the TCI-DNA vaccine than in those of mice immunized with E ectodomain, EDI/II, or PBS (Figures 4A-4C), suggesting nearly no ZIKV-associated cell death in the TCI-DNA-immunized mouse placenta. In contrast, there was moderate to strong staining of caspase-3 and ZIKV in the placenta from other groups (E ectodomain, EDI/II, or PBS) (Figures 4A-4C), illustrating that E ectodomain or EDI/II may not fully prevent ZIKV-related cell death. Furthermore, there was strong staining for vimentin in the placenta of mice immunized with TCI-DNA (Figure S4A), with significantly higher numbers of vimentin+ signals than those of mice receiving E ectodomain, EDI/II, or PBS (Figures S4B and S4C), suggesting intact vasculature in the TCI-DNA-vaccinated mouse placenta but partially or completely damaged vasculature in the placenta of other groups.
Figure 4. ZIKV TCI-DNA vaccine prevented ZIKV-caused apoptosis in placenta of female pregnant Ifnar1−/− mice.
Placenta collected from above ZIKV-challenged pregnant (E10–E12) Ifnar1−/− mice was stained for activated form of caspase-3 (an apoptotic marker) by immunofluorescence assay.
(A) Representative images of immunofluorescence staining of activated caspase-3 in placenta. ZIKV (green), activated caspase-3 (red), and nuclei (blue) were stained with anti-ZIKV antibody, anti-active caspase-3 antibody, and DAPI, respectively. The images were magnified at 63×, with a scale bar of 10 μm.
(B and C) Quantification of ZIKV+ (B) and activated caspase-3+ (C) staining in (A) by ImageJ software.
The data are presented as mean ± SEM of fluorescence intensity for ZIKV+ or caspase-3+ staining in each field (n = 6; n indicates numbers of images from different placentas). *p < 0.05; **p < 0.01; ***p < 0.001.
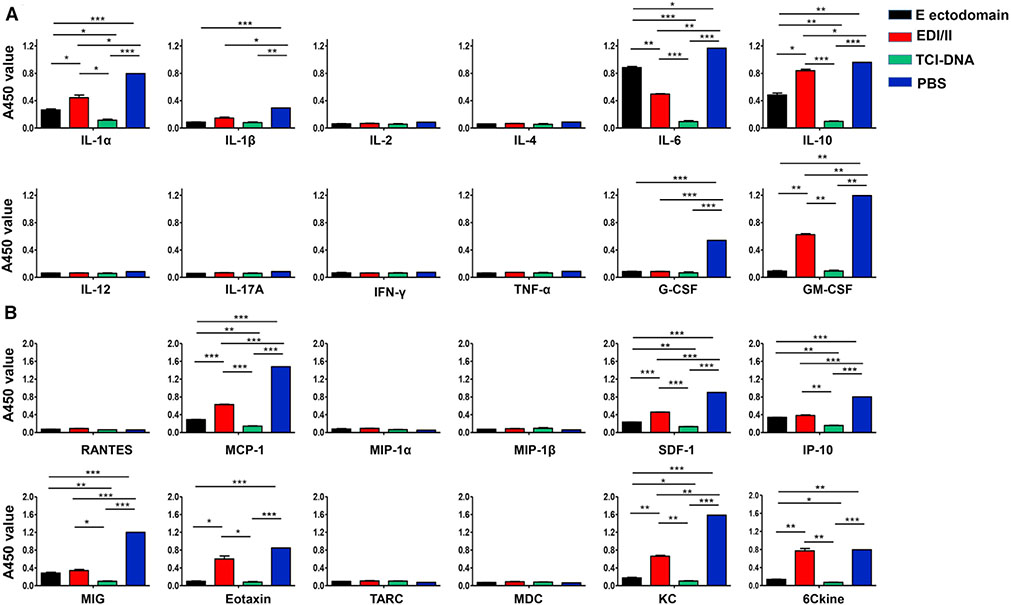
To examine whether ZIKV TCI-DNA vaccine can prevent ZIKV-caused inflammation, we measured inflammatory cytokines and chemokines in the placenta of immunized and ZIKV-infected pregnant Ifnar1−/− mice using Multi-Analyte ELISArray kits. Significantly lower levels of cytokines (interleukin [IL]-1α, IL-1β, IL-6, IL-10, G-CSF, and GM-CSF) (Figure 5A) and chemokines (MCP-1, SDF-1, IP-10, MIG, Eotaxin, KC, and 6Ckine) (Figure 5B) were identified in the TCI-DNA-immunized mouse placenta than in the placenta of mice immunized with EDI/II, E ectodomain or PBS. These results indicate that TCI-DNA is effective in preventing ZIKV-associated inflammation.
Figure 5. ZIKV TCI-DNA vaccine prevented ZIKV-caused inflammation in placenta of female pregnant Ifnar1−/− mice.
Placentas collected from above challenged pregnant (E10–E12) Ifnar1−/− mice were examined for inflammatory cytokines (A) and chemokines (B) by Mouse Inflammatory Cytokines Multi-Analyte ELISArray Kit and Mouse Common Chemokines Multi-Analyte ELISArray Kit, respectively. The data are presented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001.
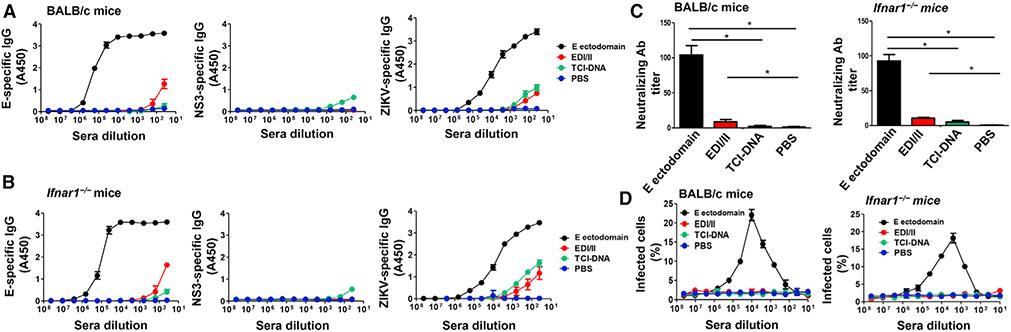
To investigate whether the ZIKV TCI-DNA vaccine may induce ZIKV-specific antibodies and, if so, whether these antibodies can cause ZIKV-associated ADE, we measured antibodies by ELISA and plaque-forming assay and tested for ADE in vitro using immunized sera of BALB/c and Ifnar1−/− mice. Clearly, no or a very low level of immunoglobulin G (IgG) antibodies was detected against ZIKV E ectodomain, NS3 peptides, and/or ZIKV lysates in the sera of both mice immunized with TCI-DNA (Figures 6A and 6B), and there were no neutralizing antibodies detected against ZIKV infection in these sera (Figure 6C). Moreover, similar to the sera collected from PBS-injected mice, TCI-DNA-immunized mouse sera did not present any ADE (Figure 6D). In contrast, ZIKV E ectodomain elicited high-level E- and ZIKV-specific IgG antibodies with neutralizing activity against ZIKV infection, exhibiting strong ADE of ZIKV infection (Figure 6). However, the ADE showed up only when the sera was diluted until the neutralizing activity was gone. These data demonstrate that TCI-DNA did not induce ZIKV E-specific antibodies but only elicited very weak NS3-specific antibody responses, eliminating the possibility of causing ZIKV-associated ADE.
Figure 6. ZIKV TCI-DNA vaccine induced low to no ZIKV-, E-, and NS3-specific antibodies without ADE effect.
Mouse sera collected at 10 days post-second immunization were detected for ZIKV E-, NS3-, and ZIKV-specific IgG antibody, neutralizing antibodies, and ADE of ZIKV infection. ZIKV strain R103451 was used for the neutralization and ADE tests.
(A and B) ELISA was used for detection of IgG antibody specific to ZIKV E ectodomain, NS3 peptides, and ZIKV (R103451 strain) in sera of BALB/c (A) or Ifnar1−/− (B) mice immunized with ZIKV E ectodomain, EDI/II peptides, TCI-DNA, or PBS control. IgG antibody titers are presented as positively detectable endpoint serum dilutions.
(C and D) Detection of neutralizing antibodies (C) by plaque-reduction neutralization test (PRNT) and ADE (D) by flow cytometry-based assay in sera of immunized BALB/c (left) or Ifnar1−/− (right) mice. Neutralizing antibody titers are presented as 50% PRNT titer (PRNT50) of 2-fold serially diluted sera. The ADE is presented as percent of infected cells, which was calculated based on fluorescence signals in the presence or absence of serially diluted sera.
The data are expressed as mean ± SEM (n = 6). *p < 0.01.
Collectively, the above results confirm that the ZIKV TCI-DNA vaccine demonstrated the ability to prevent ZIKV-associated apoptosis, vascular damage, and inflammation without leading to ADE.
ZIKV TCI-DNA-vaccine-induced CD8+ T cells play a key role in protecting adult or pregnant mice and their fetuses against ZIKV infection
Since ZIKV TCI-DNA induced very low to no antibody responses, we then tested whether the T cell responses elicited by this vaccine were essential in protecting against ZIKV infection. As such, we performed the following two experiments (Figure S2B).
First, we immunized immunocompetent male and female BALB/c mice with TCI-DNA or PBS control and then depleted their CD4+ and CD8+ T cells, respectively (using anti-CD4 or anti-CD8a antibody), followed by ZIKV challenge. There were minimal numbers of CD4+ or CD8+ T cells in the peripheral blood cells and splenocytes of anti-CD4- or anti-CD8a-treated, TCI-DNA-, or PBS-immunized mice compared to those of mice treated with isotype antibody control (Figures S5A and S5B), confirming complete depletion of CD4+ or CD8+ T cells in these mice. Compared to PBS control, the mice immunized with TCI-DNA have significantly more CD8+ T cells, as seen in the mice receiving anti-CD4 or isotype control antibody (no CD8+ T cell depletion) (Figures S5A and S5B). In contrast, the mice immunized with TCI-DNA have similar numbers of CD4+ T cells as control mice, as seen in the mice receiving anti-CD8a or isotype control antibody (no CD4+ T cell depletion) (Figures S5A and S5B). In addition, high viral titers were detected in the sera and tissues (lung, eye, and muscle) of mice immunized with the TCI-DNA vaccine, and CD8+ T cells depleted, which were significantly higher than those of TCI-DNA-immunized mice injected with anti-CD4 or isotype control antibody (no CD8+ T cell depletion) (Figure S5C). In contrast, there were no significant differences in viral titers in the mice immunized with TCI-DNA who received either anti-CD4 (CD4+ T cell depletion) or isotype control antibody (Figure S5C). These data suggest that CD8+ T cells in TCI-DNA-immunized adult mice are essential in the prevention of ZIKV infection.
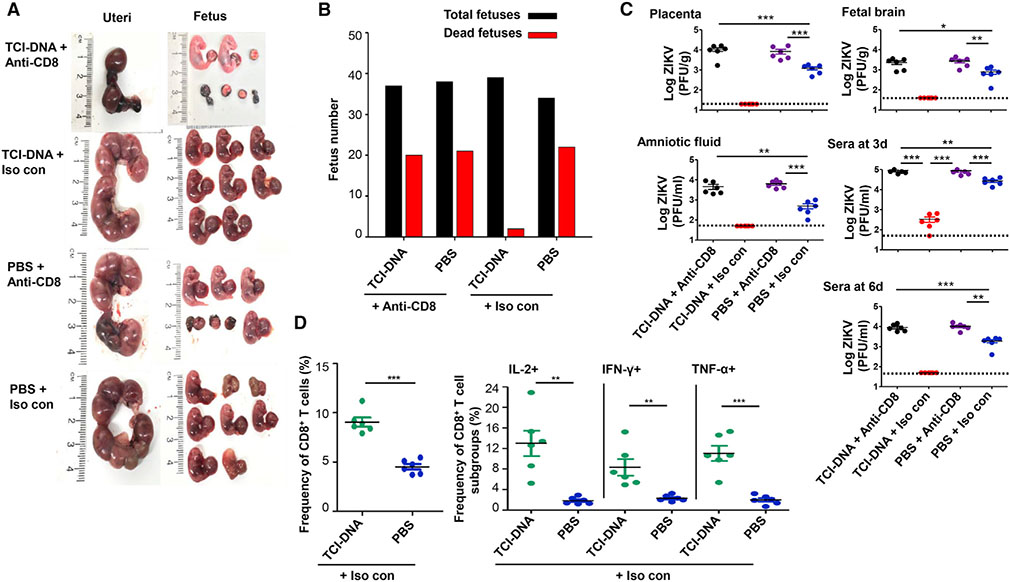
Second, we evaluated the role of CD8+ T cells induced by TCI-DNA in protecting pregnant mice and their fetuses against ZIKV challenge. As such, we immunized female BALB/c mice with TCI-DNA or PBS control, mated them with male BALB/c mice for pregnancy, and then depleted their CD8+ T cells before ZIKV challenge. Depletion of CD8+ T cells was confirmed by flow cytometry analysis in the whole blood of mice 6 h before and 3 days after ZIKV challenge.
We compared the uteri status, fetal damage, and virus titers in the mice with or without CD8+ T cell depletion. In the mice immunized with the TCI-DNA vaccine, we found significantly damaged uteri and severe fetal demise when their CD8+ T cells were depleted, whereas intact uteri and fetuses without any damage were found when the mice were injected with isotype control antibody (no CD8+ T cell depletion) (Figure 7A). The mice receiving PBS and injected with anti-CD8a or isotype control antibody exhibited different degrees of uteri damage and/or fetal death (Figure 7A). In addition, at least half of the fetuses died in the uteri of CD8+ T-cell-depleted mice, whereas almost all fetuses survived in those of TCI-DNA-immunized mice receiving isotype control antibody (Figure 7B). Moreover, CD8+ T cell depletion led to significantly increased ZIKV titers in the placenta, amniotic fluid, and fetal brain, as well as day 3 or day 6 post-infection (p.i.) in sera (Figure 7C) in the TCI-DNA-immunized pregnant mice and their fetuses. These data showed enhanced infection of TCI-DNA-vaccinated pregnant mice to ZIKV after their CD8+ T cells were depleted.
Figure 7. CD8+ T cell immune responses induced by ZIKV TCI-DNA vaccine played a key role in protecting pregnant mothers and their fetuses against ZIKV infection.
Female BALB/c mice were immunized with ZIKV TCI-DNA or PBS control for two doses and mated with male BALB/c mice for pregnancy at 10 days post-second immunization. The pregnant (E10–E12) mice were then injected (i.p.) with anti-CD8a (for depleting CD8+ T cells) or IgG2a isotype control (i.e., Iso con; without depleting CD8+ T cells) antibody (200 μg/mouse) for three times (−2, −1, and 3 days p.i.). One day before challenge, the mice were also injected with anti-IFNAR1 blocking antibody (for depleting type I IFN; 2 mg/mouse) and then infected with ZIKV (strain R103451, 106 PFU/mouse).
(A–C) Six days post-challenge, the mice were euthanized, recorded for morphology of uteri and fetuses (A), and counted for dead and total fetuses (B), and viral titers were determined by plaque-forming assay in placenta, amniotic fluid, and fetal brain, as well as sera collected at 3 and 6 days post-challenge (C). The detection limit was 20 PFU/g (for placenta), 40 PFU/g (for fetal brain), and 50 PFU/ml (for sera and amniotic fluid). Six days post-challenge, splenocytes were isolated from the mice injected with isotype control antibody (i.e., Iso con) and analyzed for ZIKV-specific CD8+ T cell responses by flow cytometry analysis.
(D) Quantification of the frequencies of CD8+ T cells (left), as well as IL2+, IFN-γ+, and tumor necrosis factor (TNF)-α secretion in CD8+ T cells (right) in splenocytes.
The data are represented as mean ± SEM (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001.
We further evaluated ZIKV-specific CD8+ T cell responses using flow cytometry analysis in the TCI-DNA-immunized and ZIKV-challenged pregnant mice without CD8+ T cell depletion (i.e., mice receiving isotype antibody). To do this, splenocytes were isolated from these mice 6 days post-challenge and stimulated with ZIKV NS3 overlapping peptides. Remarkably, TCI-DNA elicited ZIKV-specific CD8+ T cell responses (Figure 7D, left), being able to secrete high-level IL-2, IFN-γ, and TNF-α cytokines; however, PBS control induced only a background level of these cytokines (Figure 7D, right).
Collectively, the above data demonstrate that ZIKV TCI-DNA-vaccine-induced CD8+ T cells play an essential role in protecting adult, pregnant mice and their fetuses against ZIKV infection and/or ZIKV-associated fetal damage and death.
DISCUSSION
Since a ZIKV vaccine targeting prM and E proteins may induce ADE, investigators have tested an innovative approach to use NS1 as a ZIKV vaccine target antigen for the prevention of ZIKV infection (Brault et al., 2017; Grubor-Bauk et al., 2019; Rodriguez et al., 1997). Immunization with ZIKV NS1 expressed by an MVA vector protected immunocompetent mice against a lethal intracerebral dose of ZIKV challenge (Brault et al., 2017). However, it is unclear whether the protection was dependent on T cells or antibodies. It has been shown that T cell immunity induced by a DNA vaccine expressing ZIKV NS1 played a crucial role in the early control of viral titers in immunocompetent mice (Grubor-Bauk et al., 2019); however, the contribution of NS1-specific antibodies in controlling the viremia throughout the infection was not ruled out in that study. Furthermore, the NS1 vaccine did not provide full protection for ZIKV-susceptible Ifnar−/− mice because most of the immunized mice did not survive the challenge (only about 20% survival rate) with ZIKV (Grubor-Bauk et al., 2019). Both studies have not yet determined whether NS1-specific immune responses are sufficient for the prevention of ZIKV from transmission through placenta to fetuses and consequent damage.
We have made a vaccine that expressed Ub and rearranged ZIKV NS3 protein to generate CTLs without ADE-mediating antibodies. This NS3 design is expected to lead to rapid degradation in the proteasome. Our western blot result showed negligible amounts of the rearranged NS3 protein in the cells transfected with the plasmid expressing the NS3. In contrast, large amounts of NS3 can be seen with a proteasome inhibitor, indicating that the Ub/rearranged NS3 protein is rapidly degraded in the proteasome. We did not expect that large numbers of NS3-specific antibodies would be induced due to a lack of intact NS3, which was confirmed by ELISA. There was not much concern that even small numbers of antibodies to NS3 were induced, as NS3 is not on ZIKV virion surface. Indeed, sera from mice immunized with our vaccine did not show any ADE activity. As expected, the vaccine induced a strong NS3-specific CTL response. This is most likely due to the use of Ub upstream of the rearranged NS3 sequence, which promotes protein degradation in the proteasome, where antigenic peptides are loaded on MHC class I to induce CTL response (Rodriguez et al., 1997). Furthermore, we rearranged the NS3 sequence to avoid potential harmful effects of an intact NS3 protein. Although transfection of 293T cells with a plasmid expressing non-rearranged ZIKV NS3 did not show any obvious toxic effects, this strategy could be used to construct vaccines expressing viral proteins with harmful activity such as an oncoprotein HPV E6/7.
This study demonstrated that a ZIKV vaccine inducing NS3-specific CTLs provided full protection against ZIKV challenge, particularly against fetal damage in pregnant mice. Our data suggest that the CTLs controlled the virus infection in the placenta so that virus-induced inflammation was greatly reduced and fetal damage was prevented. Our data indicate that CTLs alone are sufficient to provide protection against viral infection.
It is believed that neutralizing antibodies and CTLs were needed to clear viruses (Grifoni et al., 2017; Hassert et al., 2019; Osuna and Whitney, 2017; Regla-Nava et al., 2018). The most devastating consequence of ZIKV infection is congenital syndrome, which may develop in DENV-endemic countries. DENV and ZIKV are very similar and share CTL epitopes, as determined in Ifnar1−/− HLA-transgenic mice (Wen et al., 2017b). It has been shown that DENV-specific antibodies enhance transmission of ZIKV from mother to fetuses (Brown et al., 2019; Langerak et al., 2019; Rathore et al., 2019) and that these antibodies cross-reacted with ZIKV but did not neutralize the virus (Bardina et al., 2017; Dejnirattisai et al., 2016). The passive transfer experiments showed no role for DENV-immune sera in the protection against ZIKV challenge (Grifoni et al., 2017; Regla-Nava et al., 2018; Wen et al., 2017a). However, it has been shown that preexisting high-antibody titers to DENV were associated with a reduced risk of ZIKV infection and symptoms in humans (Rodriguez-Barraquer et al., 2019). Thus, it is unclear if the DENV-specific antibodies promote or prevent ZIKV infections (cross-neutralizing antibodies, if present, may prevent ZIKV infection). It is unknown if DENV-specific CTLs are sufficient in protecting against ZIKV infection. In mice, both CD8+ T cell depletion and adaptive transfer of CD8+ T cell experiments demonstrated that the ZIKV protection was provided principally by DENV-specific CTLs (Grubor-Bauk et al., 2019; Huang et al., 2017; Elong Ngono and Shresta, 2019; Regla-Nava et al., 2018; Wen et al., 2017a). However, it is unclear if DENV immunity prevents or reduces fetal ZIKV infection in humans. It has been shown that there is an 11%–13% risk of microcephaly born to Brazilian women infected with ZIKV during pregnancy, particularly in the first trimester of pregnancy (de Araújo et al., 2018; Brady et al., 2019; Johansson et al., 2016; Regla-Nava et al., 2018). A recent study showed that the ZIKV-induced microcephaly occurred in the DENV-immune mothers (Reynolds et al., 2020), suggesting that T cell immunity does not provide sufficient cross-protection among ZIKV and DENV serotypes. The protection may be dependent on the numbers of cross-reacting CTLs and neutralizing antibodies (if any) at the time of ZIKV infection. It is likely that DENV-immune individuals presenting large numbers of CTLs cross-reacting with ZIKV and high titers of neutralizing antibodies cross-reacting with ZIKV will be protected, whereas those with small numbers of CTLs and low titers of neutralizing antibodies cross-reacting with ZIKV will suffer from congenital syndromes such as microcephaly. Thus, it is safe to design a vaccine expressing NS3 from ZIKV and DENV to induce a large number of memory CTLs to prevent against their infections.
Vaccines for humans usually induce neutralizing antibodies, an expected goal for many vaccines against infectious agents. However, these vaccines also induce non-neutralizing antibodies. Non-neutralizing antibodies may also protect the host from diseases such as HIV, CMV, and influenza (Alter and Barouch, 2018; Nelson et al., 2018; Sutton et al., 2017; Winarski et al., 2019) via antibody-mediated cell cytotoxicity, antibody-dependent cell phagocytosis, and antibody-dependent complement-mediated lysis (Jegaskanda, 2018; Jegaskanda et al., 2013; van der Lubbe et al., 2018; Winarski et al., 2019). The non-neutralizing antibodies, however, can augment the infection of viruses such as influenza virus and coronaviruses in addition to DENV and ZIKV (Bardina et al., 2017; Shim et al., 2019; Vanderven et al., 2018; Wang et al., 2014). The most alarming concern is that the DENV vaccine, Dengvaxia, has led to more severe consequences in individuals without DENV infection than in those with prior DENV infection, most likely due to ADE (Halstead, 2017; Martínez-Vega et al., 2017). Furthermore, non-neutralizing or suboptimal antibodies may also cause tissue damage, as anti-SARS-CoV full-length spike IgG antibodies induced by a vaccine have caused acute lung inflammation via activating macrophages by Fcγ receptor (FcγR) (Liu et al., 2019). This raised concerns if anti-SARS-CoV-2 full-length spike protein induced by vaccines would enhance lung inflammation when the neutralizing antibodies wane over time. Our study demonstrates that a vaccine inducing solely CTLs can fully protect from viral infection, which provides a strategy for developing safe and effective vaccines for viral infections without inducing antibody-mediated pathology.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for the supporting data, resources, and reagents should be directed to and will be fulfilled upon request by the lead contact, Liang Qiao (lqiao@luc.edu).
Materials availability
Plasmids generated in this study are stored in the lab of L.Q. and are available upon request. No samples have been submitted/banked in a public facility.
Data and code availability
This study did not generate datasets/code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Four-to-10-week-old female BALB/c mice and 4-6-week-old male and female interferon-α/β receptor (IFNAR)-deficient (Ifnar1−/−) mice were used in the studies. The animal studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals, National Research Council (National Academy of Sciences, 2011). The protocols were approved by the Institutional Animal Care and Use Committee of the New York Blood Center (Permit Numbers: 344 and 345).
Cell lines
Vero E6, K562 and 293T cells were purchased from ATCC. The Vero E6 were cultured with DMEM containing 1% carboxymethyl cellulose and 2% FBS, at 37°C with 5% CO2. The 293T and K562 cells were cultured with DMEM containing 10% FBS at 37°C with 5% CO2.
Viruses
Zika virus (human strain R103451 (2015/Honduras)) and Zika virus (human strain PAN2016 (2016/Panama)) were from BEI.
METHOD DETAILS
Construction of the vaccine plasmid
ZIKV NS3 sequence was rearranged and a ubiquitin monomer sequence was added to the front of the rearranged NS3 (see Results section). A Kozak sequence was placed ahead of the Ub/NS3 open reading frame to ensure efficient transcription of the plasmid. Upstream the Kozak sequence, linker DNA as well as an EcoRI restriction site was placed to facilitate proper cloning of the gene segment into the vector of interest. Downstream the rearranged Ub/NS3 gene sequence, a stop codon was placed along with linker DNA and a NotI restriction site. This nucleotide sequence was ordered through the GeneArt program (Thermo Fisher Scientific). The NS3 gene was delivered in a proprietary Thermo Fisher Scientific vector in a lyophilized form. Plasmid DNA was resuspended in Molecular Biology Grade water (Fisher Scientific) to a concentration of approximately 200 ng/uL. DNA was digested with FastDigest (FD) EcoRI and NotI for 15 min in a 37°C heat block. The digested DNA was then loaded directly onto a 1 % Agarose gel and allowed to run for 40 min at 80V. The 2.2 kb Ub/NS3 DNA was exposed to 440 nm UV light in the imaging room and excised. The gel slice was placed into a clear, 1.5 mL microcentrifuge tube and the purified DNA was extracted using a QIAquick Gel Extraction Kit (QIAGEN). This gene fragment was stored in a −20°C freezer as the pVAX1 vector (Thermo Fisher Scientific) was digested with FD EcoRI and FDNotI, run on a gel, and extracted using the same kit. The Ub/NS3 gene fragment was ligated using a 3:1 ratio of insert DNA (Ub/NS3) to vector DNA (pVAX1) at room temperature in the presence of T4 DNA ligase.
Bacterial transformation
Previously made Chemically Competent DH5α E. coli was removed from −80°C freezer and thawed on ice for 25 min. Ligated Ub/NS3/pVAX1 was introduced to the E. coli and remained on ice for 30 more min. Heat shock transformation occurred by placing the tube into a 42°C heat block for 45 s and back on ice for 2 min. 1 mL of SOC media was added to the E. coli and was grown in a shaking incubator at 37°C for 45 min. The E. coli was then spun down at 9,000 rpm for 2 min, resuspended in 200 μL SOC media, and streaked on an LB Kanamycin (Kan+) plate. Once dry, the plate was inverted and incubated overnight at 37°C. Colonies from the plate were inoculated into 2 mL LB Kan+ media, and a Miniprep was conducted using a QIAprep Spin Miniprep Kit according to the manufacturer’s instructions (QIAGEN). Plasmid was subsequently re-digested with FD EcoRI and NotI. Upon observance of correct band digestion, the plasmid was sent out for sequencing (ACGT, Inc) for confirmation of correct insertion.
Plasmid transfection
293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10% Fetal Bovine Serum (FBS) and 1% Penicillin/Streptomycin. Cells were split upon reaching 90% confluence (every 2-3 days). Upon sufficient generation of 293T cells, 10 μg of Ub/NS3/pVAX1 plasmid was transfected using PEI transfection reagent overnight. Media was removed the next morning and cells were cultured in DMEM. For subsequent experiments using proteasome inhibitor, MG132 was added to cell culture medium in a 1:1,000 ratio overnight at either 12, 36, or 60 h after transfection.
qRT-PCR
After 293T cells were transfected as described above, purification of total RNA was conducted using a RNeasy Mini Kit according to the manufacturer’s instructions (QIAGEN). First-Strand cDNA was then synthesized using a GoScript Reverse Transcription System according to the manufacturer’s instructions (Promega). In a PCR tube, the RNA harvested from transfected 293T cells was incubated with NS3-specific primers along with GAPDH primers (negative control) in a heat block at 70°C for 5 min. Tubes were immediately placed on ice for 10 min. RNA/primer mix was mixed with GoScript Reverse Transcription mix in a 3:1 ratio for each reaction. Tubes were placed on a heat block at 25°C for 5 min, then placed on a heat block at 42°C for 1 h. Reverse transcriptase was inactivated by placing tubes in a heat block at 70°C for 15 min. The qRT-PCR was conducted using iTaq Universal SYBR Green Supermix (Bio-Rad) using NS3-specific primers as well as GAPDH specific primers (negative control). Fold induction was measured as 2−ΔΔCT.
Western blot
293T cells were cultured, transfected, and treated with proteasome inhibitor as described above. After overnight treatment with 50 mM MG132, 293T cells were treated with 0.25% trypsin for 5 min at 37°C in 5% CO2. 293T cells were resuspended in DMEM, centrifuged at 1,200 rpm for 5 min, and cell pellets were resuspended in RIPA buffer with freshly added proteinase inhibitor. Total protein concentration was calculated via Bradford Assay (Bio-Rad) and 6x SDS Loading Buffer was added to 20 μg of total protein. Protein was denatured at 100°C for 10 min and then placed on ice for 2-3 min. Proteins were run on a 12% polyacrylamide gel for 20 min at 80V and for 40 minutes at 120V, or until the dye line reached the bottom of the gel. Bands from the polyacrylamide gel were transferred to a nitrocellulose membrane using an iBlot Gel Transfer Device (Thermo Fisher Scientific). Membrane was blocked in 5% blocking buffer (5% non-fat milk in PBS) for 1 h. Membrane was washed in PBS-Tween 20 (PBS-T) and blocked in 5% blocking buffer containing a 1:1,000 dilution of anti-ZIKV NS3 antibody (Genetex) overnight. The next day, the membrane was washed with PBS-T for 20 min, and the membrane was blocked in 5% blocking buffer with secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody for 1 h. Membrane was washed in PBS-T for 20 min, and then exposed to 10 mL Chemiluminescent substrate for 3 min devoid of light. Proteins were visualized using a FluorChem E system (ProteinSimple).
Immunization of mice with vaccines
ZIKV E ectodomain, EDI/EDII peptides, and TCI-DNA vaccines were used to immunize mice as previously described (Tai et al., 2019a). Briefly, groups of six female BALB/c (4-6-week old), female Ifnar1−/− (4-week old), or mixed-sex Ifnar1−/− (3 female and 3 male, 5-6-week old) mice were intramuscularly (i.m.) immunized with ZIKV E ectodomain (Aviva Systems Biology; 10 μg/mouse) or EDI/EDII peptides (50 μg/mouse) in the presence of Alum (500 μg/mouse) and MPL (10 μg/mouse) adjuvants (InvivoGen), or with TCI-DNA (10 μg/mouse) in the presence of Imiquimod adjuvant (20 μg//mouse, InvivoGen). Mice injected with PBS were included as a control. The immunized mice were boosted once at three weeks, and sera were collected 10 days post-last dose to detect IgG antibodies, neutralizing antibodies, and/or ADE. The immunized mice were further processed for subsequent challenge experiments as described below. In two additional experiments, groups of five to six male or female BALB/c mice (6-10-week old) were immunized with TCI-DNA (10 μg/mouse) or PBS (control), depleted for CD4+ or CD8+T cells, and subjected to subsequent challenge experiments, as described in Figure S2.
ELISA
ZIKV-, E-, or NS3-specific IgG antibodies were detected by ELISA in the immunized mouse sera (Du et al., 2016; Tai et al., 2019a). Briefly, ELISA plates were pre-coated with ZIKV E ectodomain (1 μg/ml), NS3 peptides, or ZIKV (human strain R103451 (2015/Honduras))-infected Vero E6 cell lysates at 4°C overnight and blocked with PBS-T containing 2% non-fat milk at 37°C for 2 h. After three washes with PBS-T, the plates were then sequentially incubated at 37°C for 1 h with serially diluted mouse sera and HRP-conjugated anti-mouse IgG-Fab antibody (1:5,000, Sigma). The substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma) was added to the plates, and the reaction was stopped after addition of 1N H2SO4. Absorbance at 450 nm (A450) was measured using ELISA plate reader (Tecan).
In vitro ADE assay
ADE potentially induced by immunized mouse serum antibodies was measured in K562 cells using a flow cytometry-based assay (de Alwis et al., 2014). Briefly, 100 PFU of ZIKV (strain R103451) was mixed with sera at 4-fold serial dilutions, and incubated at 37°C for 1 h. The virus-serum mixture was then added to K562 cells (5 × 104/well), and incubated at 37°C for 2 h in DMEM containing 10% FBS and 1% Penicillin/Streptomycin, followed by washing the cells with fresh DMEM, and culturing them for 3 days. The cells were then fixed, permeabilized, and sequentially stained with mouse anti-flavivirus 4G2 monoclonal antibody (mAb, 2 μg/ml) and FITC-conjugated anti-mouse antibody (1:100, Biolegend). The percent of infected cells was calculated based on the fluorescence signals in the presence or absence of serially diluted mouse sera.
ZIKV challenge studies and evaluation of vaccine efficacy in mice
The following five experiments were designed to evaluate the efficacy of vaccines in the immunized mice (Figure S2; Richner et al., 2017a; Tai et al., 2019a). (1) At 13 days post-last dose of E ectodomain, EDI/EDII peptides, TCI-DNA vaccine, or PBS control, Ifnar1−/− mice (male and female) were challenged (I.P.) with ZIKV (human strain R103451; 103 PFU; 200 μl/mouse), and investigated for survival and weight daily for 14 days. (2) Immunized Ifnar1−/− mice were challenged (I.P.) with ZIKV (human strain PAN2016 (2016/Panama), 103 PFU/mouse; 200 ml/mouse) as in (1), and 6 days later, sera and tissues were collected for detection of viral titers by plaque-forming assay (described below). (3) Ten days post-last dose of E ectodomain, EDI/EDII peptides, TCI-DNA, or PBS control, female BALB/c and Ifnar1−/− mice were mated with respective naive male mice for pregnancy. The pregnant mice (E5-E7 for BALB/c, and E10-E12 for Ifnar1−/− mice) were challenged (I.P.) with ZIKV (strain R103451, 2 × 105 PFU/mouse for BALB/c, and 104 PFU/mouse for Ifnar1−/− mice). Six days p.i., sera and tissues of adult mice, as well as placenta, amniotic fluid, and/or fetal brain, were collected for detection of viral titers, and uteri and/or fetuses were collected for analysis of morphological changes. (4) Ten days post-last dose of TCI-DNA or PBS control, male and female BALB/c mice were injected (I.P.) with or without anti-mouse-CD4 (IgG2b) mAb, anti-mouse-CD8a (IgG2b) mAb (200 μg/mouse) or IgG2b isotype control mAb (Bio X Cell) at −2, −1, and 1 days post-ZIKV challenge, and the mice were (I.P.) challenged with ZIKV (strain R103451, 2 × 105 PFU/mouse). The challenged mice were sacrificed at 3 days p.i., peripheral blood cells and splenocytes were evaluated for CD4+ and CD8+ T cell depletion by flow cytometry analysis, and sera and tissues were examined for viral titers by plaque-forming assay. (5) Ten days post-last dose of TCI-DNA or PBS control, female BALB/c mice were mated with naive male BALB/c mice for pregnancy. The pregnant mice (E10-E12) were injected (I.P.) with or without anti-CD8a (IgG2a) mAb (200 μg/mouse, for TCI-DNA) or IgG2a isotype control mAb (for PBS) (Bio X Cell) at −2, −1, and 3 days post-ZIKV challenge, and peripheral blood cells (collected at 6 h before infection and 3 days p.i.) and splenocytes (collected at 6 days p.i.) were evaluated for CD8+ T cell depletion by flow cytometry analysis (Zhong et al., 2017). The pregnant mice with or without CD8+ T cell depletion were then (I.P.) challenged with ZIKV (strain R103451, 2 × 105 PFU/mouse). Sera (collected 3 and 6 days p.i.), amniotic fluid, and tissues (placenta and fetal brain) (collected 6 days p.i.) were detected for viral titers by plaque-forming assay, fetuses and uteri (collected 6 days p.i.) were observed for morphological analysis, and splenocytes (collected 6 days p.i.) were used to detect ZIKV-specific CD8+ T cell responses by flow cytometry analysis, as described below. For all groups in (1) to (5), the challenged mice with greater than 25% weight loss and significant clinical symptoms were humanely euthanized. For all BALB/c mice with ZIKV challenge, an anti-IFNAR1 blocking mAb (MAR1-5A3, 2 mg/mouse; Leinco) were injected into mice 1 day before ZIKV infection.
Plaque reduction neutralization test (PRNT) and plaque-forming assay
PRNT and plaque-forming assays were performed to detect neutralizing antibodies in the immunized mouse sera and viral titers in the challenged mice, respectively (Tai et al., 2018, 2019a, 2019b). For PRNT assay, ZIKV (strain R103451, 100 PFU/mouse) was incubated with 2-fold serially diluted mouse sera at 37°C for 1.5 h, which was added to Vero E6 cells and culture of the cells at 37°C for 1 h. The cells were further overlaid with DMEM containing 1% carboxymethyl cellulose and 2% FBS, and cultured at 37°C for 4-5 days, followed by staining with crystal violet (0.5%). 50% PRNT titers (PRNT50) were calculated based on 2-fold serial dilutions of individual mouse serum at 50% plaque reduction using the CalcuSyn computer program (Chou, 2006; Tai et al., 2019a). For plaque-forming assay, sera, amniotic fluid, and tissue lysates of ZIKV-challenged mice were serially diluted and transferred to Vero E6 cells, followed by similar procedures as described above. Viral titers were calculated as PFU/g or PFU/ml of test samples.
Immunofluorescence staining
This was performed as previously described (Tai et al., 2019b). Briefly, maternal placental tissues were harvested from the challenged pregnant Ifnar1−/− mice, and fixed in 4% paraformaldehyde (MilliporeSigma), which were further embedded in paraffin and sectioned. For vimentin staining, the deparaffinized tissue slides were blocked with 2% BSA (MilliporeSigma) for 30 min at room temperature, and then sequentially incubated with ZIKV EDIII-specific human mAb (ZV-64, 1:200, Absolute Antibody) and rabbit anti-vimentin antibody (1:300, Abcam). For caspase-3 staining, the tissue slides were fixed and permed with FIX and PERM Cell Permeabilization Kit (Thermo Fisher Scientific), before being blocked as described above and incubated sequentially with ZV-64 and rabbit anti-active caspase-3 antibody (1:200, Abcam). The slides were washed with PBS, and incubated for 1 h at room temperature with anti-human FITC (1:300, Sigma; for ZIKV)- or anti-rabbit Alexa Fluor® 647 (1:300, Abcam; for vimentin and caspase-3)-conjugated antibodies, which were counter-stained for nuclei for 5 min with DAPI (4′,6-diamidino-2-phenylindole, 300 nM; Thermo Fisher Scientific), and mounted in a Mounting Medium (VectaMount Permanent; Vector Laboratories). The stained slides were imaged on a confocal microscope (Zeiss LSM 880), and prepared for images using ZEN software. The fluorescence signals were quantified by ImageJ software for relative intensity (particle analysis) as described previously (Tai et al., 2019b).
Detection of inflammatory cytokines and chemokines
Maternal placental tissues collected from the challenged pregnant Ifnar1−/− mice were measured for inflammatory cytokines and chemokines using Mouse Inflammatory Cytokines Multi-Analyte ELISArray Kit and Mouse Common Chemokines Multi-Analyte ELISArray Kit (QIAGEN) (Tai et al., 2019b). Briefly, ELISA plates were pre-coated with cytokine or chemokine capture antibodies, followed by incubation with tissue lysates for 2 h at room temperature. After three washes, the plates were sequentially incubated with detection antibody for 1 h, and Avidin-HRP conjugates for 30 min at room temperature, followed by incubation with development and stop solutions, respectively. A450 value was measured using ELISA plate reader (Tecan).
Flow cytometry
Flow cytometry analysis was performed to evaluate CD4+ and CD8+ T cell depletion and ZIKV-specific CD8+ T cell responses in the challenged mice (Guerrero et al., 1986; Zhang et al., 2016). For analysis of CD4+ and CD8+ depletion in whole blood and splenocytes, peripheral blood cells and splenocytes were treated with 1 × Red Blood Cell Lysis Buffer (Biolegend), and stained with FITC-anti-mouse CD4 or PerCP-Cy5.5-anti-mouse CD8 antibody (BD Biosciences), followed by flow cytometry analysis using BD LSRFortessa 4 system. For analysis of ZIKV-specific CD8+ T cell responses, the above-treated splenocytes (2 × 106 cells/well) were incubated with ZIKV NS3 overlapping peptides (0.25 nM/peptide, final concentration 5 μg/ml) in the presence of 5 μg/ml brefeldin A (Biolegend), and cultured at 37°C for 5 h. After stimulation, the cells were washed with PBS and stained for surface marker using PerCP/Cy5.5 anti-mouse CD8a. After fixation and permeabilization, the cells were stained for intracellular markers using FITC-anti-mouse IL-2, PE-anti-mouse IFN-γ, and Brilliant Violet 421-anti-mouse TNF-α antibodies (BD Biosciences), followed by analysis using flow cytometry as described above.
QUANTIFICATION AND STATISTICAL ANALYSIS
All values are expressed as mean with standard error (SEM). Statistical significance of antibody titers among different groups was calculated using the Mann-Whitney test. Statistical significance of viral titers, fluorescence intensity, cytokines and chemokines, as well as CD8+ T cell responses, among various groups was calculated using a Student’s two-tailed t test. Statistical significance of survival rates among different groups was calculated using the Log-Rank test. The data were analyzed using GraphPad Prism Statistical Software. *, ** and *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD4 (IgG2b) antibody | Bio X Cell | BP0003-1; RRID:AB_1107636 |
| Anti-mouse CD8a (IgG2b) antibody | Bio X Cell | BP0061; RRID:AB_1125541 |
| Rat IgG2b isotype control antibody | Bio X Cell | BP0090; RRID:AB_1107780 |
| Anti-mouse-CD8a (IgG2a) antibody | Bio X Cell | BE0004-1; RRID:AB_1107671 |
| Rat IgG2a isotype control antibody | Bio X Cell | BE0089; RRID:AB_1107769 |
| Anti-mouse IFNAR1 (MAR1-5A3) antibody | Leinco | I-401; RRID:AB_2491621 |
| ZIKV EDIII-specific human antibody, ZV-64 | Absolute Antibody | Ab00811-10.0 |
| Rabbit anti-vimentin antibody | Abcam | Ab92547; RRID:AB_10562134 |
| Rabbit anti-active caspase-3 antibody | Abcam | Ab2302; RRID:AB_302962 |
| FITC-anti-human antibody | Sigma | 9512-1mL; RRID:AB_259808 |
| Anti-rabbit Alexa Fluor® 647 antibody | Abcam | Ab150075; RRID:AB_2752244 |
| FITC-anti-mouse antibody | Biolegend | 406001; RRID:AB_315029 |
| Anti-flavivirus 4G2 antibody | BEI Resources | NR-50327 |
| PerCP-Cy5.5-anti-mouse CD8 antibody | BD Biosciences | 551162; RRID:AB_394081 |
| FITC-anti-mouse IL-2 antibody | BD Biosciences | 554427; RRID:AB_395385 |
| FITC-anti-mouse CD4 antibody | BD Biosciences | 553729; RRID:AB_395013 |
| PE-anti-mouse IFN-γ antibody | BD Biosciences | 554412; RRID:AB_395376 |
| Brilliant Violet 421-anti-mouse TNF-α antibody | Biolegend | 506328; RRID:AB_2562902 |
| HRP-conjugated anti-mouse IgG-Fab antibody | Sigma | a9917-1mL; RRID:AB_258476 |
| Anti-ZIKV NS3 antibody | Genetex | GTX133309; RRID:AB_2756864 |
| Bacterial and virus strains | ||
| Zika virus (human strain R103451 (2015/Honduras)) | BEI Resources | NR-50355 |
| Zika virus (human strain PAN2016 (2016/Panama)) | BEI Resources | NR-50210 |
| Chemicals, peptides, and recombinant proteins | ||
| Zika virus full-length envelope (E) protein | Aviva Systems Biology | OPMA04848 |
| Zika virus EDI/EDII peptides (see below peptide 1-5) | BEI Resources | NR-50553 |
| Peptide 1: IGVSNRDFVEGMSGG | BEI Resources | N/A |
| Peptide 2: TWVDVVLEHGGCVTV | BEI Resources | N/A |
| Peptide 3: MAQDKPTVDIELVTT | BEI Resources | N/A |
| Peptide 4: VDRGWGNGCGLFGKG | BEI Resources | N/A |
| Peptide 5: VVLGSQEGAVHTALA | BEI Resources | N/A |
| Zika virus NS3 overlapping peptides (see below peptide 6-15) | GenScript | N/A |
| Peptide 6: AETDEDHAHWLEARM | GenScript | N/A |
| Peptide 7: HAHWLEARMLLDNIY | GenScript | N/A |
| Peptide 8: ARMLLDNIYLQDGLI | GenScript | N/A |
| Peptide 9: NIYLQDGLIASLYRP | GenScript | N/A |
| Peptide 10: GLIASLYRPEADKVA | GenScript | N/A |
| Peptide 11: YRPEADKVAAIEGEF | GenScript | N/A |
| Peptide 12: KVAAIEGEFKLRTEQ | GenScript | N/A |
| Peptide 13: GEFKLRTEQRKTFVE | GenScript | N/A |
| Peptide 14: TEQRKTFVELMKRGD | GenScript | N/A |
| Peptide 15: FVELMKRGDLPV | GenScript | N/A |
| Alum (Aluminum hydroxide gel) | InvivoGen | vac-alu-250 |
| MPL (Monophosphoryl Lipid A) | InvivoGen | vac-mpla |
| Imiquimod | InvivoGen | vac-imq |
| Tween-20 | Sigma | P1379-1L |
| Non-fat milk | Bio-Rad | 1706404 |
| TMB (3,3′,5,5′-tetramethylbenzidine) | Sigma | T0440-1L |
| DMEM cell culture medium | Thermo Fisher Scientific | 12430062 |
| Fetal bovine serum (FBS) | ATLANTA biologicals | S11550 |
| Penicillin/Streptomycin | Thermo Fisher Scientific | 15140163 |
| Carboxymethyl cellulose | Sigma | M0512-250G |
| Crystal violet | Sigma | C0775-100G |
| DAPI (4′,6-diamidino-2-phenylindole) | Thermo Fisher Scientific | 62247 |
| Red Blood Cell Lysis Buffer | Biolegend | 420301 |
| Brefeldin A | Biolegend | 420601 |
| Molecular Biology Grade Water | Fisher | BP2819100 |
| FastDigest EcoRI | Thermo Fisher Scientific | FD0274 |
| FastDigest NotI | Thermo Fisher Scientific | FD0593 |
| Certified Molecular Biology Agarose | Bio-Rad | 1613102 |
| T4 DNA Ligase | New England Biolabs | M0202T |
| DH5α E. coli | In House | N/A |
| SOC Medium | Sigma | S1797-100mL |
| Kanamycin Solution | Sigma | K0254 |
| Polyethylenimine Solution | Millipore Sigma | P3143 |
| (R)-MG132 | Millipore Sigma | M8699-1MG |
| Trypsin-EDTA (0.25%), phenol red | Thermo Fisher Scientific | 25200056 |
| Bio-Rad Protein Assay Dye Reagent | Bio-Rad | 5000006 |
| Concentrate | ||
| 12% Mini-PROTEAN TGX Precast Protein | Bio-Rad | 4561043 |
| Gels | ||
| SuperSignal West Pico PLUS | Thermo Fisher Scientific | 34579 |
| Chemiluminescent Substrate | ||
| Critical commercial assays | ||
| FIX and PERM Cell Permeabilization Kit | Thermo Fisher Scientific | GAS004 |
| Mouse Inflammatory Cytokines Multi- | QIAGEN | 336161 |
| Analyte ELISArray | ||
| Mouse Common Chemokines Multi-Analyte | QIAGEN | 336161 |
| ELISArray | ||
| Qiaquick Gel Extraction Kit | QIAGEN | 28704 |
| Qiaprep Spin Miniprep Kit | QIAGEN | 27104 |
| RNeasy Mini Prep Kit | QIAGEN | 74104 |
| GoScript Reverse Transcription System | Promega | A5003 |
| iTaq Universal SYBRGreen Supermix | Bio-Rad | 1725121 |
| Experimental models: cell lines | ||
| K562 | ATCC | CCL-243 |
| Vero E6 | ATCC | CRL-1586 |
| Experimental models: organisms/strains | ||
| BALB/c mice | The Jackson Laboratory | 651 |
| Interferon-α/β receptor-deficient (Ifnar1−/−) mice | The Jackson Laboratory | 32045-JAX |
| Software and algorithms | ||
| GraphPad Prism 5 | Graphpad Software | N/A |
| SigmaPlot 13.0 | SigmaPlot Software | N/A |
| ZEN 2 | Zen Software | N/A |
| ImageJ | NIH, USA | N/A |
Highlights.
Vaccine of modified ZIKV NS3 induces specific CTLs with little antibody response
The vaccine fully protects adult mice against ZIKV and greatly reduces viral titers
The vaccine protects fetuses against ZIKV challenge and reduces placental damage
Depletion of CD8+ T cells abrogates vaccine protection in animal models
ACKNOWLEDGMENTS
This research was supported by NIH grant R21AI137790 to L.D., NIH grant R21AI140210 to L.Q., and intramural fund of the New York Blood Center (NYB616 and NYB673). ZIKV strains (R103451 and PAN2016) were obtained from BEI Resources.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.109107.
DECLARATION OF INTERESTS
There are two patents pending: one US-based and one internationally. US Application No. 62/970,592; PCT International Application No. PCT/US2021/16654. Title: ZIKA VIRUS IMMUNOGENIC COMPOSITIONS.
REFERENCES
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, et al. (2016). Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353, 1129–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, Kirilova M, Peterson R, Li Z, Nanayakkara O, et al. (2017). Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci. Transl. Med 9, eaao4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, and Barouch D (2018). Immune Correlate-Guided HIV Vaccine Design. Cell Host Microbe 24, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. (2016). Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536, 48–53. [DOI] [PubMed] [Google Scholar]
- Barbosa M, Joshi RS, Garg P, Martin-Trujillo A, Patel N, Jadhav B, Watson CT, Gibson W, Chetnik K, Tessereau C, et al. (2018). Identification of rare de novo epigenetic variations in congenital disorders. Nat. Commun 9, 2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. (2017). Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Osgood-Zimmerman A, Kassebaum NJ, Ray SE, de Araújo VEM, da Nóbrega AA, Frutuoso LCV, Lecca RCR, Stevens A, Zoca de Oliveira B, et al. (2019). The association between Zika virus infection and microcephaly in Brazil 2015-2017: An observational analysis of over 4 million births. PLoS Med. 16, e1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. (2016). Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med 375, 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Domi A, McDonald EM, Talmi-Frank D, McCurley N, Basu R, Robinson HL, Hellerstein M, Duggal NK, Bowen RA, and Guirakhoo F (2017). A Zika vaccine targeting NS1 protein protects immunocompetent adult mice in a lethal challenge model. Sci. Rep 7, 14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Singh G, Acklin JA, Lee S, Duehr JE, Chokola AN, Frere JJ, Hoffman KW, Foster GA, Krysztof D, et al. (2019). Dengue virus immunity increases Zika virus-induced damage during pregnancy. Immunity 50, 751–762.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard BL, Corder BN, Gorman MJ, Diamond MS, and Weaver EA (2018). Efficacy of a T Cell-biased adenovirus vector as a Zika virus vaccine. Sci. Rep 8, 18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. (2016). Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387, 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC (2006). Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev 58, 621–681. [DOI] [PubMed] [Google Scholar]
- Dans AL, Dans LF, Lansang MAD, Silvestre MAA, and Guyatt GH (2018). Controversy and debate on dengue vaccine series-paper 1: review of a licensed dengue vaccine: inappropriate subgroup analyses and selective reporting may cause harm in mass vaccination programs. J. Clin. Epidemiol 95, 137–139. [DOI] [PubMed] [Google Scholar]
- Dar H, Zaheer T, Rehman MT, Ali A, Javed A, Khan GA, Babar MM, and Waheed Y (2016). Prediction of promiscuous T-cell epitopes in the Zika virus polyprotein: An in silico approach. Asian Pac. J. Trop. Med 9, 844–850. [DOI] [PubMed] [Google Scholar]
- de Alwis R, Williams KL, Schmid MA, Lai CY, Patel B, Smith SA, Crowe JE, Wang WK, Harris E, and de Silva AM (2014). Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 10, e1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, Valongueiro S, de Albuquerque MFPM, Braga C, Filho SPB, et al. ; investigators from the Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco (2018). Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect. Dis 18, 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. (2016). Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol 17, 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado FG, Torres KI, Castellanos JE, Romero-Sánchez C, Simon-Lorière E, Sakuntabhai A, and Roth C (2018). Improved Immune Responses Against Zika Virus After Sequential Dengue and Zika Virus Infection in Humans. Viruses 10, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. (2016). Rapid development of a DNA vaccine for Zika virus. Science 354, 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, et al. (2016). Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med 374, 2142–2151. [DOI] [PubMed] [Google Scholar]
- Du L, Tai W, Yang Y, Zhao G, Zhu Q, Sun S, Liu C, Tao X, Tseng CK, Perlman S, et al. (2016). Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat. Commun 7, 13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edupuganti S, Natrajan MS, Rouphael N, Lai L, Xu Y, Feldhammer M, Hill C, Patel SM, Johnson SJ, Bower M, et al. (2017). Biphasic Zika Illness With Rash and Joint Pain. Open Forum Infect. Dis 4, ofx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sahly HM, Gorchakov R, Lai L, Natrajan MS, Patel SM, Atmar RL, Keitel WA, Hoft DF, Barrett J, Bailey J, et al. (2018). Clinical, Virologic, and Immunologic Characteristics of Zika Virus Infection in a Cohort of US Patients: Prolonged RNA Detection in Whole Blood. Open Forum Infect. Dis 6, ofy352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A, and Shresta S (2019). Cross-reactive T cell immunity to dengue and Zika viruses: New insights into vaccine development. Front. Immunol 10, 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AM, Tang WW, Young MP, Mamidi A, Viramontes KM, McCauley MD, Carlin AF, Schooley RT, Swanstrom J, Baric RS, et al. (2018). Maternally acquired Zika antibodies enhance dengue disease severity in mice. Cell Host Microbe. 24, 743–750.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Tai W, Wang N, Li X, Jiang S, Debnath AK, Du L, and Chen S (2019). Identification of novel natural products as effective and broad-spectrum anti-Zika virus inhibitors. Viruses 11, 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, Berkowitz N, Mendoza F, Saunders JG, Novik L, et al. ; VRC 319; VRC 320 study teams (2018). Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet 391, 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, Caine EA, Zaitsev K, Begley MC, Weger-Lucarelli J, Uccellini MB, Tripathi S, Morrison J, Yount BL, Dinnon KH 3rd., et al. (2018). An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 23, 672–685.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin BD, Muthumani K, Warner BM, Majer A, Hagan M, Audet J, Stein DR, Ranadheera C, Racine T, De La Vega MA, et al. (2017). DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat. Commun 8, 15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Pham J, Sidney J, O’Rourke PH, Paul S, Peters B, Martini SR, de Silva AD, Ricciardi MJ, Magnani DM, et al. (2017). Prior dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol 91, 1469–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubor-Bauk B, Wijesundara DK, Masavuli M, Abbink P, Peterson RL, Prow NA, Larocca RA, Mekonnen ZA, Shrestha A, Eyre NS, et al. (2019). NS1 DNA vaccination protects against Zika infection through T cell-mediated immunity in immunocompetent mice. Sci. Adv 5, eaax2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero JM, Goberna R, Molinero P, Jimenez J, and Calvo JR (1986). Interaction of a bovine thymic peptide extract with vasoactive intestinal peptide (VIP) receptors. Biosci. Rep 6, 579–584. [DOI] [PubMed] [Google Scholar]
- Halstead SB (2017). Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 35, 6355–6358. [DOI] [PubMed] [Google Scholar]
- Hassert M, Harris MG, Brien JD, and Pinto AK (2019). Identification of protective CD8 T cell responses in a mouse model of Zikavirus infection. Front. Immunol 10, 1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Chen J, Zhu X, An S, Dong X, Yu J, Zhang S, Wu Y, Li G, Zhang Y, et al. (2018). NLRP3 inflammasome activation mediates Zika virus-associated inflammation. J. Infect. Dis 217, 1942–1951. [DOI] [PubMed] [Google Scholar]
- Herrera BB, Tsai WY, Brites C, Luz E, Pedroso C, Drexler JF, Wang WK, and Kanki PJ (2018a). T Cell Responses to Nonstructural Protein 3 Distinguish Infections by Dengue and Zika Viruses. MBio 9, e00755–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera BB, Tsai WY, Chang CA, Hamel DJ, Wang WK, Lu Y, Mboup S, and Kanki PJ (2018b). Sustained Specific and Cross-Reactive T Cell Responses to Zika and Dengue Virus NS3 in West Africa. J. Virol 92, e01992–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R, Lei J, and Zhang L (2018). The structure of the Zika virus protease, NS2B/NS3(pro). Adv. Exp. Med. Biol 1062, 131–145. [DOI] [PubMed] [Google Scholar]
- Huang H, Li S, Zhang Y, Han X, Jia B, Liu H, Liu D, Tan S, Wang Q, Bi Y, et al. (2017). CD8+ T cell immune response in immunocompetent mice during Zika virus infection. J. Virol 91, e00900–e00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger BW, Dowd KA, Chen RE, Desai P, Foreman B, Burgomaster KE, Himansu S, Kong WP, Graham BS, Pierson TC, and Diamond MS (2019). Protective efficacy of nucleic acid vaccines against transmission of Zika virus during pregnancy in mice. J. Infect. Dis 220, 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegaskanda S (2018). The Potential role of Fc-receptor functions in the development of a universal influenza vaccine. Vaccines (Basel) 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, and Kent SJ (2013). Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J. Immunol 190, 1837–1848. [DOI] [PubMed] [Google Scholar]
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, and Hills SL (2016). Zika and the risk of microcephaly. N. Engl. J. Med 375, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayanarooj S (2011). Clinical manifestations and management of Dengue/DHF/DSS. Trop. Med. Health 39, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetarpal N, and Khanna I (2016). Dengue fever: Causes, complications, and vaccine strategies. J. Immunol. Res 2016, 6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerak T, Mumtaz N, Tolk VI, van Gorp ECM, Martina BE, Rockx B, and Koopmans MPG (2019). The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLoS Pathog. 15, e1007640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, et al. (2016). Vaccine protection against Zika virus from Brazil. Nature 536, 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca RA, Mendes EA, Abbink P, Peterson RL, Martinot AJ, Iampietro MJ, Kang ZH, Aid M, Kirilova M, Jacob-Dolan C, et al. (2019). Adenovirus vector-based vaccines confer maternal-fetal protection against Zika virus challenge in pregnant IFN-alphabetaR(−/−) mice. Cell Host Microbe. 26, 591–600.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, and Diamond MS (2016). Zika virus: New clinical syndromes and its emergence in the Western Hemisphere. J Virol. J. Virol 90, 4864–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Hansen G, Nitsche C, Klein CD, Zhang L, and Hilgenfeld R (2016). Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science 353, 503–505. [DOI] [PubMed] [Google Scholar]
- Li Y, Phoo WW, Loh YR, Zhang Z, Ng EY, Wang W, Keller TH, Luo D, and Kang C (2017). Structural characterization of the linked NS2B-NS3 protease of Zika virus. FEBS Lett. 591, 2338–2347. [DOI] [PubMed] [Google Scholar]
- Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, et al. (2019). Anti-spike IgG causes servere acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4, 4–123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vega RA, Carrasquila G, Luna E, and Ramos-Castañeda J (2017). ADE and dengue vaccination. Vaccine 35, 3910–3912. [DOI] [PubMed] [Google Scholar]
- Marzi A, Emanuel J, Callison J, McNally KL, Arndt N, Chadinha S, Martellaro C, Rosenke R, Scott DP, Safronetz D, et al. (2018). Lethal Zika virus disease models in young and older iInterferon alpha/beta receptor knock out mice. Front. Cell. Infect. Microbiol 8, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. (2016). Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza MU, Rafique S, Ali A, Munir M, Ikram N, Manan A, Salo-Ahen OM, and Idrees M (2016). Towards peptide vaccines against Zika virus: Immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci. Rep 6, 37313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE, and Diamond MS (2017). Animal models of Zika virus infection, pathogenesis, and immunity. J. Virol 91, e00009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, and Whitehead SS (2011). Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol 29, 587–619. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences (2011). The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals, Fletcher Cameron H. and Crossgrove Ruth, eds. (National Academies Press; ). [Google Scholar]
- Nelson CS, Huffman T, Jenks JA, Cisneros de la Rosa E, Xie G, Vandergrift N, Pass RF, Pollara J, and Permar SR (2018). HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. USA 115, 6267–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna CE, and Whitney JB (2017). Nonhuman primate models of Zikavirus infection, immunity, and therapeutic development. J. Infect. Dis 216, S928–S934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. (2017). Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoo WW, Li Y, Zhang Z, Lee MY, Loh YR, Tan YB, Ng EY, Lescar J, Kang C, and Luo D (2016). Structure of the NS2B-NS3 protease from Zika virus after self-cleavage. Nat. Commun 7, 13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoo WW, Zhang Z, Wirawan M, Chew EJC, Chew ABL, Kouretova J, Steinmetzer T, and Luo D (2018). Structures of Zika virus NS2B-NS3 protease in complex with peptidomimetic inhibitors. Antiviral Res. 160, 17–24. [DOI] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. (2016). Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 113, 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo K, Souza LJ, Salomão NG, Oliveira ERA, Sentinelli LP, Lacerda MS, Saraquino PB, Rosman FC, Basílio-de-Oliveira R, Carvalho JJ, and Paes MV (2018). Placental inflammation and fetal injury in a rare Zika case associated with Guillain-Barre Syndrome and abortion. Front. Microbiol 9, 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore APS, Saron WAA, Lim T, Jahan N, and St John AL (2019). Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci. Adv 5, eaav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh AT, Wang YT, Nguyen AT, Salgado R, Mamidi A, Kim K, Diamond MS, and Shresta S (2018). Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat. Commun 9, 3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CJ, Watber P, Santos CNO, Ribeiro DR, Alves JC, Fonseca ABL, Bispo AJB, Porto RLS, Bokea K, de Jesus AMR, et al. (2020). Strong CD4 T Cell Responses to Zika Virus Antigens in a Cohort of Dengue Virus Immune Mothers of Congenital Zika Virus Syndrome Infants. Front. Immunol 11, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. (2017a). Modified mRNA vaccines protect against Zika virus infection. Cell 168, 1114–1125.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, Himansu S, Caine EA, Nunes BTD, Medeiros DBA, et al. (2017b). Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170, 273–283.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez F, Zhang J, and Whitton JL (1997). DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J. Virol 71, 8497–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barraquer I, Costa F, Nascimento EJM, Nery N, Castanha PMS, Sacramento GA, Cruz J, Carvalho M, De Olivera D, Hagan JE, et al. (2019). Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 363, 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Bin Cao, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. (2016). Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader EK, Harstad KG, and Matouschek A (2009). Targeting proteins for degradation. Nat. Chem. Biol 5, 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton G, Mongkolsapaya J, Yacoub S, and Roberts C (2015). New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol 15, 745–759. [DOI] [PubMed] [Google Scholar]
- Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, Wakamiya M, Tesh RB, Barrett AD, Wang T, et al. (2017). A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med 23, 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim BS, Kwon YC, Ricciardi MJ, Stone M, Otsuka Y, Berri F, Kwal JM, Magnani DM, Jackson CB, Richard AS, et al. (2019). Zika virus-immune plasmas from symptomatic and asymptomatic individuals enhance Zika pathogenesis in adult and pregnant mice. MBio 10, e00758–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza BS, Sampaio GL, Pereira CS, Campos GS, Sardi SI, Freitas LA, Figueira CP, Paredes BD, Nonaka CK, Azevedo CM, et al. (2016). Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci. Rep 6, 39775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton TC, Lamirande EW, Bock KW, Moore IN, Koudstaal W, Rehman M, Weverling GJ, Goudsmit J, and Subbarao K (2017). In vitro neutralization is not predictive of prophylactic efficacy of broadly neutralizing monoclonal antibodies CR6261 and CR9114 against lethal H2 influenza virus challenge in mice. J. Virol 91, e01603–e01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W, He L, Wang Y, Sun S, Zhao G, Luo C, Li P, Zhao H, Fremont DH, Li F, et al. (2018). Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses. Emerg. Microbes Infect 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W, Chen J, Zhao G, Geng Q, He L, Chen Y, Zhou Y, Li F, and Du L (2019a). Rational Design of Zika virus subunit vaccine with enhanced efficacy. J. Virol 93, e02187–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W, Voronin D, Chen J, Bao W, Kessler DA, Shaz B, Jiang S, Yazdanbakhsh K, and Du L (2019b). Transfusion-transmitted Zika virus infection in pregnant mice leads to broad tissue tropism with severe placental damage and fetal demise. Front. Microbiol 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, et al. (2017). Safety and immunogenicity of an anti-Zika virus DNA vaccine. N. Engl. J. Med Published online October 4, 2017. 10.1056/nejmoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Balasubramaniam VR, Brown JA, Mena I, Grant A, Bardina SV, Maringer K, Schwarz MC, Maestre AM, Sourisseau M, et al. (2017). A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 13, e1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe JEM, Huizingh J, Verspuij JWA, Tettero L, Schmit-Tillemans SPR, Mooij P, Mortier D, Koopman G, Bogers WMJM, Dekking L, et al. (2018). Mini-hemagglutinin vaccination induces cross-reactive antibodies in pre-exposed NHP that protect mice against lethal influenza challenge. NPJ Vaccines 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay KKA, Keesler RI, Ardeshir A, Watanabe J, Usachenko J, Singapuri A, Cruzen C, Bliss-Moreau E, Murphy AM, Yee JL, et al. (2019). DNA vaccination before conception protects Zika virus-exposed pregnant macaques against prolonged viremia and improves fetal outcomes. Sci. Transl. Med 11, eaay2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderven HA, Wragg K, Ana-Sosa-Batiz F, Kristensen AB, Jegaskanda S, Wheatley AK, Wentworth D, Wines BD, Hogarth PM, Rockman S, and Kent SJ; INSIGHT FLU005 Pilot Study Writing Group (2018). Anti-influenza hyperimmune immunoglobulin enhances Fc-functional antibody immunity during human influenza infection. J. Infect. Dis 218, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner JJ, Rouphael N, Xu Y, Natrajan M, Lai L, Patel SM, Levit RD, Edupuganti S, and Mulligan MJ (2017). Pericarditis Associated With Acute Zika Virus Infection in a Returning Traveler. Open Forum Infect. Dis 4, ofx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, Chen KH, Liu FT, Liu WT, Chen YM, and Huang JC (2014). Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun 451, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tai W, Zhang X, Zhou Y, Du L, and Shen C (2019). Effects of Adjuvants on the Immunogenicity and Efficacy of a Zika Virus Envelope Domain III Subunit Vaccine. Vaccines (Basel) 7, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Elong Ngono A, Regla-Nava JA, Kim K, Gorman MJ, Diamond MS, and Shresta S (2017a). Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun 8, 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, and Shresta S (2017b). Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol 2, 17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winarski KL, Tang J, Klenow L, Lee J, Coyle EM, Manischewitz J, Turner HL, Takeda K, Ward AB, Golding H, and Khurana S (2019). Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc. Natl. Acad. Sci. USA 116, 15194–15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, Ji X, Xu YP, Li G, Li C, et al. (2017). A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358, 933–936. [DOI] [PubMed] [Google Scholar]
- Zhang N, Channappanavar R, Ma C, Wang L, Tang J, Garron T, Tao X, Tasneem S, Lu L, Tseng CT, et al. (2016). Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell. Mol. Immunol 13, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Zhai Y, Bu P, Shah S, and Qiao L (2017). Papilloma-pseudovirus eradicates intestinal tumours and triples the lifespan of ApcMin/+ mice. Nat. Commun 8, 15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets/code.