Abstract
Background
Routine preoperative screening of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with reverse transcriptase-polymerase chain reaction (RT-PCR) may reduce in-hospital SARS-CoV-2 transmission.
Methods
This was a prospective, observational, cohort study. The endpoints were the incidence of asymptomatic patients with positive preoperative RT-PCR results and the incidence and factors associated with postoperative SARS-CoV-2 infection in patients with cancer referred for elective surgery. Patients with elective surgery between May and October 2020 were included. RT-PCR of nasopharyngeal swabs was performed preoperatively for all patients. Postoperative SARS-CoV-2 infection was assessed within 30 postoperative days.
Results
A total of 1636 preoperative screening RT-PCR tests were performed. Of these, 102 (6.2%) cases were positive, and 1,298 surgical procedures were analyzed. The postoperative SARS-CoV-2 infection rate was 0.9%. The length of stay (odds ratio [OR] 1.08; 95% confidence interval [CI] 1.04–1.11; p < 0.001), surgical time (OR 1.004; 95% CI 1.001–1.008; p = 0.023), intensive care unit admission (OR 7.7; 95% CI 2.03–29.28; p = 0.003), and hospital readmissions (OR 9.56; 95% CI 2.50–36.56; p = 0.001) were associated with postoperative coronavirus disease (COVID-19). Using unadjusted and adjusted logistic regression, length of stay (OR 1.08; 95% CI 1.04–1.11; p < 0.001), and readmission (OR 9.02; 95% CI 2.30–35.48; p = 0.002) were independent factors of postoperative COVID-19.
Conclusions
Screening patients preoperatively may reduce in-hospital SARS-CoV-2 transmission. Length of stay and readmission were independently correlated with postoperative COVID-19.
With the ongoing coronavirus disease (COVID-19) pandemic, hospitals are facing major challenges in meeting their patients’ needs, especially those of patients with malignancies.1,2 Strategies have been recommended to curb the high transmissibility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); these include surgery deferral, intentional delay in initiating adjuvant treatment, and less intensive surveillance and follow-up.3 It has been suggested that surgery deferral limits the spread of in-hospital infection, helps to avoid potentially fatal complications in patients with COVID-19 in the perioperative period, and helps to reserve intensive care unit (ICU) beds and hospital resources for patients diagnosed with COVID-19.4,5
Surgery may be the best treatment option for many tumors, especially for early-stage tumors for which surgery is potentially curative and cannot be deferred without risks. Cancer surgery deferral may impact these patients’ outcomes negatively.4,6–8 However, during the perioperative period, patients with cancer and concurrent COVID-19 are more likely to be admitted to ICUs and have a high risk of severe adverse events and death.9,10 Hence, providing a COVID-19-free surgical pathway for these patients may help to reduce the in-hospital spread of SARS-CoV-2 and mitigate the impact of COVID-19 in cancer surgery.7,8,11
Routine preoperative reverse transcriptase-polymerase chain reaction (RT-PCR) testing of asymptomatic patients is effective in detecting most infected individuals in the early infection phase; this strategy may be effective in preventing in-hospital transmission and avoiding postoperative surgical complications.12 Following the greater likelihood for ICU admission and high morbidity and mortality risk of patients with cancer and concurrent COVID-19, this subject has been of increasing interest to researchers, and several guidelines and considerations have been published. However, no prospective study assessing the effectiveness of the recommendations in COVID-19 prevention has been conducted.13–17 This study was designed to assess the impact of systematic preoperative SARS-CoV-2 screening with RT-PCR as part of an institutional protocol for mitigating the postoperative in-hospital transmission of SARS-CoV-2 among patients with cancer awaiting an elective surgery during the COVID-19 pandemic.
Methods
Study Design and Participants
This was a single-center, prospective, observational cohort study. Patients with a pending elective surgery to be performed at Instituto Brasileiro de Controle do Cancer – São Camilo Oncologia, a cancer center in São Paulo, Brazil between May and October 2020, 3 months after the World Health Organization (WHO) declared COVID-19 a pandemic, were included.
RT-PCR of nasopharyngeal swabs was performed for all the patients preoperatively to detect asymptomatic SARS-CoV-2 infection. PCR-negative patients who underwent elective surgeries were included in the study, and the postoperative SARS-CoV-2 infection rate was calculated within 30 days after discharge. Patients were excluded if (1) they were followed up for less than 10 days, (2) underwent previous emergency surgery, or (3) had a previous diagnosis of COVID-19.
Consecutive sampling was performed until 100 PCR-positive patients were detected. A positive SARS-CoV-2 infection was based on the detection of viral RNA by quantitative RT-PCR.
The primary endpoints of the study were the incidence of asymptomatic patients with positive preoperative RT-PCR results and the incidence and factors associated with SARS-CoV-2 infection within 30 postoperative days. The secondary endpoint was the correlation of the number of infected patients and the infected institution’s employees in accordance with the month of surgery.
Data Collection
The following data were collected: age, race (self-reported), sex, number of hospital’s employees with confirmed SARS-COV-2 infection, American Society of Anesthesiologists score, the surgical team responsible for the surgical procedure, operative time, hospital stay, grade of complications according to the Clavien-Dindo Classification,18 type of anesthesia, ICU stay, readmissions (defined as a hospital stay of more than 24 hours after discharge), and type of insurance (private or public). All inserted data were double-checked by seven different investigators.
Preoperative Screening Program
RT-PCR testing was performed for all patients with a pending scheduled elective surgery. According to the institutional protocol, 5 days before the surgery, the patient received a phone call from a trained health professional who administered a questionnaire assessing COVID-19-related symptoms. If the patient was asymptomatic and had no COVID-19 contact history, the patient was invited to undergo quantitative RT-PCR testing of their nasopharyngeal swab for detection of SARS-CoV-2 three days preoperatively; this time interval was the required time for the availability of the test result. One day before the surgery, the patient received a second phone call to communicate the test result and the symptom assessment questionnaire was administered again. On the day of the surgery, the patient presented to the hospital facility 2 hours before the scheduled time for the surgery, and patients were directed to a specific lobby for surgical patients.
Statistical Analysis
Descriptive statistics were calculated for qualitative and quantitative variables. Qualitative variables are presented by absolute and relative frequencies. For quantitative variables, means, medians, standard deviation, and minimum and maximum values were calculated. Unadjusted and adjusted logistic regression analyses were used to identify factors associated with COVID-19. The final model was built using the stepwise backward method, starting with the complete (saturated) model and ending with the statistically significant variables.
The correlation between the number of infected patients and the institution’s employees was assessed using Spearman’s correlation coefficient. The scatter plot was used to present the distribution of these two characteristics. Statistical significance was set at p < 0.05. Analyses were performed using SPSS for Windows v.25 statistical software.
Ethical Considerations
This study was approved by the ethics committee of São Camilo Oncologia Hospital (reference number: 4.084.713, NCT04434261). Informed consent was waived due to the non-interventional nature and absence of changes in the established care routine. However, all patients signed the institution’s informed consent for the RT-PCR test and surgery during the pandemic.
Results
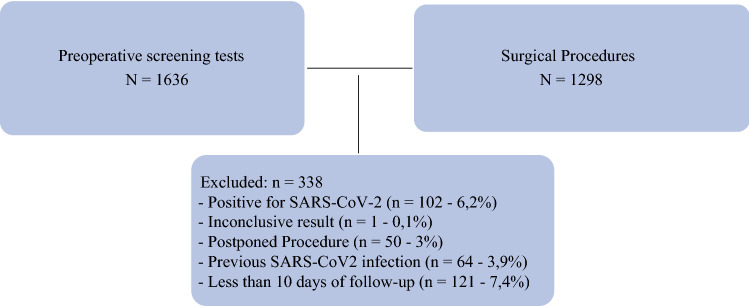
A total of 1,636 preoperative screening RT-PCR tests were performed during the study period. Of these, 102 (6.2%) patients tested positive, 1,533 (93.7%) patients tested negative, and 1 (0.1%) patient had inconclusive results. The following patients were excluded: 50 patients who did not undergo surgery for personal or medical reasons, 64 who were previously diagnosed with COVID-19, and 245 who were followed up for less than 10 days. Ultimately, 1,238 patients were included in the final analysis. Among these, 53 underwent two or more surgeries on different days, resulting in a total of 1,298 surgical procedures (Fig. 1). Clinical and surgical features of the patients are described in Tables 1 and 2, respectively.
Fig. 1.
Patient selection flow chart
Table 1.
Patients’ characteristics
| Variable | n = 1238 | n (%) |
|---|---|---|
| Sex | Female | 987 (79.7) |
| Male | 251 (20.3) | |
| Age (yr) | Mean (SD) | 54.9 (15.3) |
| Median (min–max) | 55 (17–101) | |
| Race (self-reported) | Black | 86 (6.9) |
| White | 932 (75.3) | |
| Mixed | 206 (16.6) | |
| Yellow | 14 (1.1) | |
| Active malignancy | No | 440 (35.5) |
| Yes | 798 (64.5) | |
| Arterial hypertension | No | 778 (62.8) |
| Yes | 460 (37.2) | |
| Diabetes mellitus | No | 1067 (86.2) |
| Yes | 171 (13.8) | |
| Other comorbidity | No | 789 (63.7) |
| Yes | 449 (36.3) | |
| Insurance | Public | 612 (49.4) |
| Private | 626 (50.6) | |
| Surgical team responsible for the surgical procedure | Breast surgery | 359 (29.0) |
| Gynecology | 199 (16.1) | |
| Melanoma, skin, and sarcoma | 145 (11.7) | |
| Vascular | 97 (7.8) | |
| Urology | 130 (10.5) | |
| Head and neck | 121 (9.8) | |
| Thoracic | 27 (2.2) | |
| Reconstructive | 86 (6.9) | |
| Dermatology | 27 (2.2) | |
| Surgical oncology | 32 (2.6) | |
| Neurology | 4 (0.3) | |
| Orthopedics | 10 (0.8) | |
| Pain management | 1 (0.1) |
SD standard deviation; min–max minimum–maximum
Table 2.
Surgical and postoperative features
| Characteristic | n = 1298 | n (%) |
|---|---|---|
| American Society of Anesthesiologists score | I | 439 (33,8) |
| II | 795 (61.2) | |
| III | 64 (4.9) | |
| Operative time (min) | Mean (SD) | 109.6 (96.8) |
| Median (min–max) | 84 (5–825) | |
| Blood transfusion | No | 1276 (98.3) |
| Yes | 22 (1.7) | |
| Type of anesthesia | General with or without regional | 933 (71.8) |
| Regional with or without sedation | 161 (12.4) | |
| Local with or without sedation | 204 (15.7) | |
| Intensive care unit stay | No | 1242 (95.7) |
| Yes | 56 (4.3) | |
| Clavien-Dindo classification of complications | 0 | 925 (71.2) |
| I | 234 (18.1) | |
| II | 73 (5.7) | |
| IIIa | 32 (2.5) | |
| IIIb | 17 (1.3) | |
| IVa | 8 (0.6) | |
| IVb | 2 (0.2) | |
| V | 7 (0.5) | |
| Readmissions | No | 1251 (96.4) |
| Yes | 47 (3.6) |
SD standard deviation; min–max minimum–maximum
Twenty-seven patients presented symptoms of COVID-19 within 30 postoperative days. They were all tested, and 12 (0.9%) were confirmed RT-PCR-positive. One of them died of pulmonary complications; two had severe symptoms and required hospitalization. The remaining nine patients had mild or moderate symptoms and did not require hospitalization.
The 12 individuals with positive postoperative RT-PCR-positive were further analyzed. Among these, eight patients presented with symptoms during the hospital stay or within 5 days of discharge while one patient tested positive 15 days after discharge. The remaining three patients were readmitted due to surgical or clinical complications and tested positive during the readmission period. Ten of 12 patients with positive RT-PCR were women.
There was a significant difference in the length of stay between positive and negative cases. The proportion of Clavien-Dindo grades 0-II complications was significantly higher among negative patients than among positive patients (p = 0.026). Patients diagnosed with COVID-19 postoperatively had longer operative times (p = 0.016). Patients who remained in the ICU and required readmission also were associated with a postoperative positive RT-PCR test. Surgeries performed in May and June had a higher proportion of positive cases than the other months (Table 3).
Table 3.
Clinical outcomes stratified per group, based on COVID-19 postoperative diagnosis
| Variable | n = 1298 | Results for postoperative COVID-19 | p value | |
|---|---|---|---|---|
| Negative | Positive | |||
| n = 1286 | n = 12 | |||
| n (%) | n (%) | |||
| Hospital stay (days) | Mean (SD) | 1.5 (4.2) | 9.5 (17.8) | <0.001a |
| Median (min–max) | 1 (0–63) | 2 (0–58) | ||
| Clavien-Dindo classification | 0 | 917 (99.6) | 5 (0.4) | 0.026b |
| I | 229 (97.9) | 5 (2.1) | ||
| II | 74 (100) | 0 | ||
| III–V | 66 (97.0) | 2 (3.0) | ||
| Operative time (min) | Mean (SD) | 109.0 (96.5) | 175.0 (199.0) | 0.016a |
| Median (min–max) | 84 (5–825) | 162.5 (45–451) | ||
| Blood transfusion | No | 1265 (99.1) | 11 (0.9) | 0.187b |
| Yes | 21 (95.5) | 1 (4.5) | ||
| Type of anesthesia | General with or without regional | 922 (99.1) | 10 (0.9) | 0.632b |
| Regional with or without sedation | 165 (100) | 0 | ||
| Local with or without sedation | 199 (99) | 2 (1) | ||
| Intensive care unit stay | No | 1232 (99.3) | 9 (0.7) | 0.013b |
| Yes | 54 (94.6) | 3 (5.4) | ||
| Readmission | No | 1240 (99.3) | 9 (0.7) | 0.007b |
| Yes | 44 (93.5) | 3 (6.5) | ||
| Month of surgery (2020) | May | 88 (96.7) | 3 (3.3) | 0.010b |
| June | 205 (97.6) | 5 (2.4) | ||
| July | 254 (100) | 0 | ||
| August | 261 (99.2) | 2 (0.8) | ||
| September | 313 (99,7) | 1 (0.3) | ||
| October | 165 (99.4) | 1 (0.6) | ||
SD standard deviation; min–max minimum–maximum
aMann-Whitney test
bFisher exact test
From the regression analysis, length of hospital stay and readmission were independently associated with SARS-CoV-2 infection within 30 postoperative days (Table 4).
Table 4.
Factors associated with postoperative COVID 19 infection. Unadjusted and adjusted logistic regression
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| n = 1298 | OR (95% CI) | p value | OR (95% CI) | p value |
| Hospital stay (days) | 1.07 (1.04–1.11) | < 0.001 | 1.08 (1.04–1.11) | < 0.001 |
| Clavien-Dindo | ||||
| 0–II | 1 | |||
| III–IV–V | 3.79 (0.81–17.66) | 0.090 | ||
| Operative time (min) | 1.004 (1.001–1.008) | 0.023 | ||
| Blood transfusion | ||||
| No | 1 | |||
| Yes | 5.44 (0.67–44.09) | 0.112 | ||
| ICU stay | ||||
| No | 1 | |||
| Yes | 7.70 (2.03–29.28) | 0.003 | ||
| Readmission | ||||
| No | 1 | 1 | ||
| Yes | 9.56 (2.50–36,56) | 0.001 | 9.02 (2.30–35.48) | 0.002 |
| Month of surgery (2020) | ||||
| May | 1 | |||
| June | 0.71 (0.17–3.06) | 0.650 | ||
| July | NA | |||
| August | 0.22 (0.04–1.3) | 0.103 | ||
| September | NA | |||
| October | 0.18 (0.02–1.73) | 0.136 | ||
OR odds ratio; CI confidence interval; ICU intensive care unit; NA not applicable
The number of patients and employees who tested positive within the postoperative period according to the month of surgery is shown in Table 5. Most of the positive postoperative cases (among employees and patients) occurred in May and June; however, there was no significant correlation between the number of infected employees and patients (r = 0.580; p = 0.228). The scatter plot of infected cases is shown in Fig. 2.
Table 5.
Patients and employees with COVID-19 by month (2020)
| Month | Patients (n = 12) | Employees (n = 217) | Spearman correlation coefficient | p value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| May | 3 (25) | 59 (27.2) | 0.580 | 0.228 |
| June | 5 (41.7) | 47 (21.7) | ||
| July | 0 | 36 (16.6) | ||
| August | 2 (16.7) | 29 (13.4) | ||
| September | 1 (8.3) | 24 (11.1) | ||
| October | 1 (8.3) | 22 (10.1) |
Fig. 2.
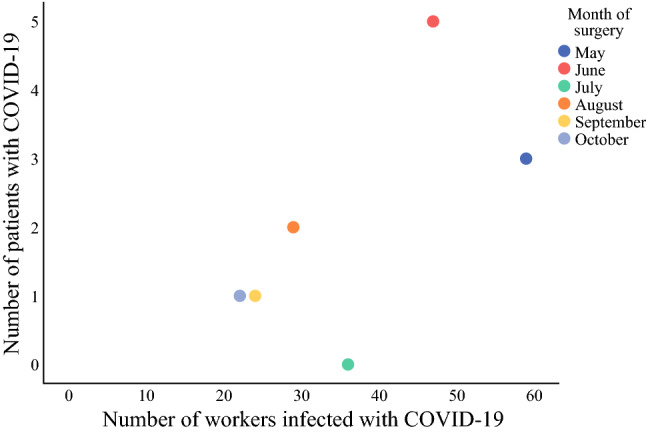
Patients and employees infected by COVID-19 by month
Discussion
This prospective cohort study evaluated the impact of a preoperative SARS-CoV-2 screening test as part of an institutional protocol for the mitigation of postoperative COVID-19 among patients awaiting elective surgery. This study also assessed the incidence and factors associated with postoperative SARS-CoV-2 infection. The rate of positivity was 6.2% in asymptomatic patients, and the rate of postoperative infection was 0.9%.
The rationale for preoperative screening is to minimize the transmission between health professionals and patients; however, it should not be the only strategy for decreasing the spread of COVID-19. To reduce COVID-19 in-hospital transmission, the institution established the following actions:
Avoidance of admission of patients with COVID-19
Transfer of patients with confirmed COVID-19 to a COVID-19 treatment-dedicated hospital facility
Physical separation of wards and ICUs for patients with COVID-19 having different health teams
Exclusive elevator for patients with confirmed or suspected COVID-19
Exclusive operating room for patients with COVID-19 only
Suspension of hospital visits and limited number of visitors, except when extremely necessary
Encouragement of sanitary measures, such as hand washing and wearing of masks, for both patients and healthcare professionals
Mortality and postoperative complications are significantly higher in patients with cancer and concurrent COVID-19. For patients with confirmed COVID-19 requiring surgery, it should be deferred due to the high risk of postoperative complications and nosocomial spread.9–11,19 In our institutional protocol, all patients with COVID-19 symptoms (detected from the questionnaire) or with positive RT-PCR test results will have their surgery deferred by the doctor, and a clinical evaluation will be scheduled on the date of deferral. Postoperative infection should be mitigated as early as possible, given the higher risk of mortality during postoperative recovery.
The effectiveness of routine testing of asymptomatic individuals is unclear.20 However, preoperative screening tests, especially for patients with cancer may detect early infection and contribute to preventing nosocomial transmission among patients and healthcare professionals. RT-PCR of nasopharyngeal swabs maybe 86% sensitive and 96% specific.21 Furthermore, testing asymptomatic patients may be a central component amongst strategies implemented for COVID-19 transmission control.12 Our results suggest that a preoperative screening test detects asymptomatic patients and may reduce postoperative SARS-CoV-2 infection. Hospital stay, readmissions, complications, and ICU stay were associated with postoperative COVID-19. These results may be explained by the patient’s greater exposure to the hospital environment, to the emergency department facilities, and consequently a higher probability of infection. Ten of the 12 patients with postoperative RT-PCR positive tests were women, which may reflect the greater number of gynecological and breast surgeries in our institution. Another interesting finding was the distribution of infected patients and institution employees by month. May and June 2020 were among the months of highest transmissibility in Brazil.22 This finding may be a reflection of COVID-19 community transmission.
The incidence of postoperative SARS-CoV-2 infection in our study was very low, with one reported COVID-19-related death. This patient also had chronic obstructive pulmonary disease, which may have worsened the prognosis. It is noteworthy that between March and May 2020 before implementation of the routine screening in our institution, 104 patients with cancer were infected with SARS-CoV-2, and 43 deaths were registered. Of these patients, 29 were diagnosed during the postoperative period.23 Another Brazilian study reported a similar preoperative detection rate, whereas studies from other countries reported different preoperative and postoperative infection rates. These discrepancies may be due to the difference in how the pandemic affected the different study sites and the pandemic pattern during the study periods.22,24–28 Brazil and the city of Sao Paulo have been heavily affected by the pandemic, and this study was conducted at a time when the country and city were massively affected—the so-called “first wave.”
This study had some limitations. First, there was no control group. Second, different surgical procedures were performed; these could have affected variables such as hospital stay and complications. Third, postoperative testing was not systematic; this could have resulted in missed asymptomatic cases. However, this may have had some strengths, such as its focus on a vulnerable population, the prospective design, and follow-up of patients. In our institution, patients with symptoms or complications were referred to the hospital’s emergency department or attending medical team. If the patient had COVID-19-related symptoms in the postoperative period, he or she was encouraged to undergo RT-PCR tests. Patients were contacted via telephone calls after hospital discharge by the investigators to screen for COVID-19 after hospital discharge.
Screening tests and strategies designed to provide a COVID-19-free surgical pathway may mitigate nosocomial transmission; however, there are still risks associated with surgeries performed during the COVID pandemic, and patients should be informed about those risks.29,30 Nevertheless, even in the face of these unprecedented times, efforts must be made to maintain access to health care and adequate care for patients with cancer. During the study period, vaccines were not available in Brazil; hence the rate of postoperative infection is expected to decrease further with the arrival of vaccines, and this is an interesting topic for future work.
Conclusions
Length of hospital stay and readmission were independent factors for postoperative COVID-19. A combination of routine screening of all individuals preoperatively with other COVID-19 spread-reducing strategies will help to minimize the spread of the virus and may reduce in-hospital SARS-CoV-2 transmission.
Author Contributions
Conception and design: AL, ADY. Collection and assembly of data: CBPP, PD, IKMWH, ALRD, MVMM, ANM, MMV. Data analysis and interpretation: AL, RVML. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Disclosure
There are no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzeng CD, Teshome M, Katz MHG, et al. Cancer surgery scheduling during and after the COVID-19 first wave: the MD Anderson Cancer Center experience. Ann Surg. 2020;272:e106–e111. doi: 10.1097/SLA.0000000000004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet Oncology COVID-19 and cancer: 1 year on. Lancet Oncol. 2021;22:411. doi: 10.1016/S1470-2045(21)00148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett DL, Howe JR, Chang G, et al. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27:1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinde RS, Naik MD, Shinde SR, et al. To do or not to do? A review of cancer surgery triage guidelines in COVID-19 pandemic. Indian J Surg Oncol. 2020;11:1–7. doi: 10.1007/s13193-020-01086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrag D, Hershman DL, Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323:2005–2006. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 9.Collaborative C. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasbey JC, Nepogodiev D, Simoes JFF, et al. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021;39:66–78. doi: 10.1200/JCO.20.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellewell J, Russell TW, Beale R, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021;19:106. doi: 10.1186/s12916-021-01982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilmans G, Chenevas-Paule Q, Muller X, et al. Surgical outcomes after systematic preoperative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) screening. Surgery. 2020;168:209–211. doi: 10.1016/j.surg.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganesh Kumar N, Drolet BC. COVID-19—implications on and of surgical practices: where do we draw the line? Ann Surg. 2020;272:e45–e46. doi: 10.1097/SLA.0000000000004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler J, Finley C, Norell CH, et al. New approaches to cancer care in a COVID-19 world. Lancet Oncol. 2020;21:e339–e340. doi: 10.1016/S1470-2045(20)30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fader AN, Huh WK, Kesterson J, et al. When to operate, hesitate and reintegrate: Society of Gynecologic Oncology surgical considerations during the COVID-19 pandemic. Gynecol Oncol. 2020;158:236–243. doi: 10.1016/j.ygyno.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Yan S, Zhang F, et al. Guidance for safely performing oncologic surgery during the COVID-19 pandemic. Br J Surg. 2020;107:e401–e402. doi: 10.1002/bjs.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haffner MR, Le HV, Saiz AM, et al. Postoperative in-hospital morbidity and mortality of patients with COVID-19 infection compared with patients without COVID-19 infection. JAMA Netw Open. 2021;4:e215697. doi: 10.1001/jamanetworkopen.2021.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan M, Kahwati L, Jahn B, et al. Universal screening for SARS-CoV-2 infection: a rapid review. Cochrane Database Syst Rev. 2020;9:CD013718. doi: 10.1002/14651858.CD013718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floriano I, Silvinato A, Bernardo WM, et al. Accuracy of the polymerase chain reaction (PCR) test in the diagnosis of acute respiratory syndrome due to coronavirus: a systematic review and meta-analysis. Rev Assoc Med Bras. 1992;2020(66):880–888. doi: 10.1590/1806-9282.66.7.880. [DOI] [PubMed] [Google Scholar]
- 22.Aguiar S, Baiocchi G, Duprat JP, et al. Value of preoperative testing for SARS-CoV-2 for elective surgeries in a cancer center during the peak of pandemic in Brazil. J Surg Oncol. 2020;122:1293–1295. doi: 10.1002/jso.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros LA, Magalhães Filho MA, Alves RB, Rebouças CM, Rodrigues CM, Viu MM, et al. Alta mortalidade entre pacientes com câncer e infecção por COVID-19: a experiência de um centro oncológico brasileiro. Einstein (São Paulo) 2021;19:6254. doi: 10.31744/einstein_journal/2021AO6254. [DOI] [Google Scholar]
- 24.Kulle CB, Azamat IF, Vatansever D, et al. Is elective cancer surgery feasible during the lock-down period of the COVID-19 pandemic? Analysis of a single institutional experience of 404 consecutive patients. J Surg Oncol. 2021;123:1495–1503. doi: 10.1002/jso.26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dursun P, Dervisoglu H, Daggez M, et al. Performing gynecologic cancer surgery during the COVID-19 pandemic in Turkey: a multicenter retrospective observational study. Int J Gynaecol Obstet. 2020;151:33–38. doi: 10.1002/ijgo.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nekkanti SS, Vasudevan Nair S, Parmar V, et al. Mandatory preoperative COVID-19 testing for cancer patients-Is it justified? J Surg Oncol. 2020;122:1288–1292. doi: 10.1002/jso.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collaborative C. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis. 2020. [DOI] [PMC free article] [PubMed]
- 28.Ribeiro R, Wainstein AJA, de Castro Ribeiro HS, et al. Perioperative cancer care in the context of limited resources during the COVID-19 pandemic: Brazilian Society of Surgical Oncology Recommendations. Ann Surg Oncol. 2021;28:1289–1297. doi: 10.1245/s10434-020-09098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad NK, Lake R, Englum BR, et al. Increased complications in patients who test COVID-19 positive after elective surgery and implications for pre and postoperative screening. Am J Surg. 2021. [DOI] [PMC free article] [PubMed]
- 30.Kettering CSotORECaMS. Cancer Surgery and COVID19. Ann Surg Oncol. 2020;27: 1713–6. [DOI] [PMC free article] [PubMed]