Abstract
Hemorrhage is a leading cause of battlefield and civilian trauma deaths. Several pain medications, including fentanyl, are recommended for use in the prehospital (i.e., field setting) for a hemorrhaging solider. However, it is unknown whether fentanyl impairs arterial blood pressure (BP) regulation, which would compromise hemorrhagic tolerance. Thus, the purpose of this study was to test the hypothesis that an analgesic dose of fentanyl impairs hemorrhagic tolerance in conscious humans. Twenty-eight volunteers (13 females) participated in this double-blinded, randomized, placebo-controlled trial. We conducted a presyncopal limited progressive lower body negative pressure test (LBNP; a validated model to simulate hemorrhage) following intravenous administration of fentanyl (75 µg) or placebo (saline). We quantified tolerance as a cumulative stress index (mmHg·min), which was compared between trials using a paired, two-tailed t test. We also compared muscle sympathetic nerve activity (MSNA; microneurography) and beat-to-beat BP (photoplethysmography) during the LBNP test using a mixed effects model [time (LBNP stage) × trial]. LBNP tolerance was not different between trials (fentanyl: 647 ± 386 vs. placebo: 676 ± 295 mmHg·min, P = 0.61, Cohen’s d = 0.08). Increases in MSNA burst frequency (time: P < 0.01, trial: P = 0.29, interaction: P = 0.94) and reductions in mean BP (time: P < 0.01, trial: P = 0.50, interaction: P = 0.16) during LBNP were not different between trials. These data, the first to be obtained in conscious humans, demonstrate that administration of an analgesic dose of fentanyl does not alter MSNA or BP during profound central hypovolemia, nor does it impair tolerance to this simulated hemorrhagic insult.
Keywords: cerebral tissue oxygenation, opioids, respiration, sympathoexcitatory
INTRODUCTION
Hemorrhage, and associated cardiovascular collapse, is a leading cause of battlefield and civilian trauma death (1–4). To this point, 91% of the potentially survivable battlefield casualties during the recent Iraq and Afghanistan conflicts were hemorrhage related (5). Hemorrhagic injuries result in profound central hypovolemia due to reduced venous return. This consequently leads to reductions in perfusion pressure, tissue hypoperfusion, and hypoxia. To preserve blood flow to vital organs such as the heart and brain, perfusion pressure is maintained by upregulation of the sympathetic nervous system, leading to increases in heart rate, cardiac contractility, and systemic vascular resistance (4, 6, 7). These acute physiological mechanisms are essential for survival. Although pain management in such a setting is important, it is crucial that any analgesic administered does not interfere with these critical compensatory responses. Any agents that reduce an individual’s ability to tolerate a hemorrhagic insult would increase the likelihood of multiorgan failure and potentially death.
For the last few decades, opioids have been the medication of choice for the majority of civilian prehospital trauma systems (8). Consistent with civilian settings, the Committee on Tactical Combat Casualty Care (CoTCCC) for combat medics (i.e., the military) has established guidelines for choosing which analgesic to administer depending on injury type and severity (9, 10). As such, fentanyl is designated to be administered on the battlefield if a soldier is 1) experiencing moderate to severe pain, 2) is not in circulatory shock or respiratory distress, and 3) is not expected to develop either condition (9, 10). A limitation of these recommendations is the reliance on research in anesthetized animals – i.e., studies on analgesic doses of fentanyl on hemorrhage tolerance in conscious humans have not been completed. In addition, recent experimental evidence in Sprague-Dawley rats has revealed that fentanyl administration impairs the microcirculatory response to hemorrhage (11). Thus, these preclinical findings suggest that fentanyl may interfere with compensatory responses during a hemorrhagic insult. Therefore, research is warranted to understand to what extent an analgesic dose of fentanyl affects tolerance to a hemorrhagic injury in conscious humans, as well as the physiological mechanisms that contribute to such a response.
Thus, the aim of this study was to examine the extent to which an analgesic dose of fentanyl influences tolerance capacity to a hemorrhagic injury in conscious humans, in addition to assessing the associated compensatory mechanisms. Based on preclinical evidence that fentanyl may have adverse effects on compensatory responses, we hypothesized that fentanyl would impair the capacity for conscious humans to tolerate a simulated hemorrhagic insult.
METHODS
The methodology of this study precisely mirrors our previous investigation addressing a similar question using an analgesic dose of ketamine (12).
Ethical Approval
All participants were informed by oral and written communication of the purpose, procedures, and risks of the study before providing written informed consent. The protocol and consent were approved by the following Institutional Review Boards for Human Subjects: United States Army Medical Research & Material Command Human Research Protection Office, University of Texas Southwestern Medical Center (IRB No. 092017-069), and Texas Health Presbyterian Hospital Dallas. This research study was registered at ClinicalTrial.gov (identifier NCT04136548) and conformed to standards set by the Declaration of Helsinki.
Participants
Forty volunteer civilians free of any known cardiovascular, metabolic, or neurological diseases were enrolled in this study. Of these individuals, five were excluded during the screening process, six participants were lost due to follow-up or scheduling conflicts, and one participant withdrew from the study, resulting in data from 28 subjects analyzed. Inclusion criteria included: age between 18 and 45 yr, body mass ≥65 kg, and body mass index <35 kg/m2 at screening. Exclusion criteria included current or recent (within past 3 yr) nicotine use, and current use of pain modifying or antihypertensive medications. We instructed participants to refrain from the following before each of the two experimental trials: water (2 h), eating (6 h), caffeine (12 h), alcohol (24 h), over-the-counter cold or allergy medication (36 h), NSAIDs (24 h), aspirin (36 h), and exercise for 24 h before the experimental trials.
Lower Body Negative Pressure Tolerance Testing
LBNP tolerance testing is a commonly utilized experimental technique to elicit progressive central hypovolemia, similar to that of a hemorrhage event (13, 14). In this model, blood is redistributed to the lower extremities by application of subatmospheric pressure to the lower body, effectively reducing venous return and central venous pressure (i.e., central hypovolemia) (15, 16). An incremental LBNP protocol was used to determine maximum tolerance to central hypovolemia. This incremental protocol was conducted by applying 40 mmHg of negative pressure for 3 min, with increases of 10 mmHg of negative pressure every 3 min, with a maximum of 100 mmHg of negative pressure, until test termination. Termination of LBNP was based on at least one of the following criteria: 1) continued reports by the subject of feeling faint and/or nauseous; 2) a rapid decline in blood pressure resulting in systolic blood pressure less than or equal to 80 mmHg; and/or 3) a relative bradycardia accompanied with narrowing of pulse pressure.
A cumulative stress index (CSI: mmHg·min) was used to quantify maximum tolerance to the progressive LBNP. This index is determined mathematically by summing the product of the negative pressure and time, in minutes (or fraction of min), for each stage [e.g., (40 mmHg·3 min) + (50 mmHg·3 min) + (60 mmHg·3 min), etc.] until test termination (17).
Experimental Design and Protocol
The experiment design used for this study was a double-blinded (i.e., both participant and researcher responsible for determining the termination of the LBNP test), randomized, placebo-controlled crossover investigation; the medical personnel (anesthesiologist and nurse) administering the medication were aware of the trial arm. Participants completed a preliminary screening visit and two experimental trials—placebo (PBO) and fentanyl (FEN) administration in randomized order—separated by a minimum of 48 h. The preliminary visit consisted of informed consent, a medical screening, and familiarization with the LBNP device and testing procedures. For female adults, we conducted the two experimental visits during the same phase of the participant’s menstrual cycle or timing of oral contraceptive use.
The experimental trials for each subject were initiated at the same time of day and performed in a temperature-controlled room. The protocol and approximate times for the two experimental trials are illustrated in Fig. 1. After instrumentation and a quiet resting period, we obtained baseline data. This baseline period was followed by two pain assessments, one evoked by immersion of a hand in ice-water for 2 min and the other via a digital algometer. The algometer assessment did not occur until after hemodynamic responses had returned to baseline following ice-water hand immersion. Next, a bolus dose (administered over 5 s) of the analgesic (75 µg intravenous fentanyl citrate, Hospira, Inc., Lake Forest, IL) or placebo (normal saline 0.9% solution) was administered via venous catheter infusion. The fentanyl dose was selected to match the plasma fentanyl concentrations and resultant analgesia attained with the 800 µg dose of oral transmucosal fentanyl (18–22), consistent with the US Army’s CoTCCC guidelines (9). One minute after fentanyl or placebo administration, the aforementioned progressive LBNP tolerance test was performed until the onset of syncopal signs or symptoms. Thirty minutes after the first fentanyl or placebo administration, a second administration of the same agent and dose that was previously administered on that day was again administered, followed by a reperformance of the pain assessments. The purpose of repeated (i.e., two) drug administrations per trial was to ensure that the subject obtains the peak plasma concentration of fentanyl at the time of the LBNP testing and during the pain assessments. The effects of fentanyl on the cardiovascular responses and pain ratings in response to ice-water immersion are presented in the companion paper (23).
Figure 1.

Experimental protocol timeline of each experimental day. After instrumentation, participants rested quietly for 10 min for baseline data collection and then assessments of pain (via algometer and the cold pressor test). Participants then received the drug (fentanyl)/placebo for that specific trial, followed 1 min later by lower body negative pressure (LBNP) testing and recovery. Thirty minutes after first administration, the same medication (or placebo) was again administered followed by pain assessments. Afterward, the subject was monitored for a minimum of 60 min to allow for the effects of the drug to subside.
Instrumentation
Before data collection, we confirmed the absence of dehydration with a urine specific gravity measurement of <1.025 utilizing a digital refractometer (Atago, Japan). In addition, we conducted a urine drug screen to rule out recreational drug usage (US Diagnostics, Inc., Huntsville, AL). For females, a negative urine pregnancy test was confirmed before the testing.
We obtained all measurements from participants in the supine position on a patient bed. Heart rate was measured from an electrocardiogram (ECG, Agilent, Munich, Germany). Noninvasive arterial blood pressure (BP) was continuously measured on a beat-to-beat basis utilizing photoplethysmography (Finometer Pro, FMS, Amsterdam, The Netherlands). ECG triggered BP was also obtained via auscultation of the brachial artery (SunTech Medical Instruments, Raleigh, NC). Transcranial Doppler was used to measure blood velocity in the middle cerebral artery (MCAv, Spencer Technologies, Redmond, WA). Near-infrared spectroscopy (NIRS, Moor Instruments Inc., Wilmington, DE) was used to estimate cerebral oxygen saturation (ScO2) within the frontal cortex. Respiratory rate and the partial pressure of end-tidal carbon dioxide (EtCO2) were acquired from a nasal cannula connected to a capnograph (9004 Capnocheck Plus; Smiths Medical International Ltd, Watford, Herts, UK).
A finger pulse oximeter with capabilities to extrapolate continuous beat-by-beat recordings of arterial flow waveforms was used to determine the physiological reserve to compensate for reductions in central blood volume (Flashback Technologies, Boulder, CO). Subsequently, we quantified compensatory reserve using a machine learning algorithm, resulting in a compensatory reserve index (CRI) (24). Briefly, this index is a normalized value on a scale of 0–1, with “1” reflecting the maximal capacity of the summation of compensatory physiological mechanism required during central blood volume deficits and “0” implicating imminent cardiovascular instability and decompensation (25).
A venous catheter was placed for fentanyl/placebo infusion. In addition, we measured plasma catecholamine concentrations from blood samples drawn into a Lithium Heparin spray-coated tube (BD Vacutainer, Oakville, ON, CA) and immediately suspended in ice and spun (2,000 g for 10 min) within 60 min, with the plasma stored at −80°C until analysis. Blood samples were sent and analyzed at a biochemistry laboratory using high-performance liquid chromatography (ARUP Laboratories, Salt Lake City, UT). Muscle sympathetic nerve activity (MSNA) was directly assessed via ultrasound-guided radial microneurography. Briefly, a tungsten recording microelectrode was inserted into a radial nerve of the upper arm and a reference microelectrode was inserted ≤3 cm from the recording electrode (26). The nerve recording was amplified (80–90,000×), bandpass filtered (700–2,000 Hz), rectified, and integrated (time constant 0.11 s) using a nerve traffic analyzer (Nerve Traffic Analyzer, model 662c-4; University of Iowa, Bioengineering, Iowa City, IA).
Data Analysis
All continuously streamed data were sampled at 625 Hz on a 16-channel data acquisition system (Biopac, Santa Barbara, CA). Analysis of the cardiovascular and respiratory measurements were determined and compared at the following time points: baseline, T1: 60 s before the start of LBNP, which equated to 60 s after the first fentanyl or placebo administration, T2: the final 60 s of 40 mmHg LBNP, T3: the final 60 s of 50 mmHg LBNP, and T4: the final 20 s before LBNP termination. MSNA measurements were also analyzed at the final 60 s of each of the aforementioned time points.
Mean arterial pressure [MAP = (diastolic BP + diastolic BP + systolic BP) ÷ 3] was calculated from brachial auscultation and is reported for baseline data. Finometer-derived MAP was analyzed throughout LBNP due to the dynamic nature of the cardiovascular measurements induced by this stress. Venous blood samples were drawn at the following time points: baseline, at the end of the second stage of LBNP (−50 mmHg if completed) and immediately after LBNP.
Although blind to condition, one experienced investigator (JW) visually inspected the sympathetic neurograms on a beat-to-beat basis to determine the presence/absence of MSNA bursts using Ensemble (Elucimed, Wellington, New Zealand). MSNA analysis was conducted in accordance with recent guidelines (27) using the following criteria: 1) >3:1 signal-to-noise ratio, 2) burst morphology consistent with MSNA bursts, and 3) a pulse-synchronous signal. MSNA was quantified as burst frequency (bursts/min) and burst incidence (burst/100 heartbeats). MSNA burst amplitude and area were not assessed for two reasons, both of which are associated with the lack of confirmation of identical microelectrode positions between trials: 1) MSNA was recorded from contralateral radial nerves due to the proximity in time (less than 28 days) of the experimental visits and 2) there were often LBNP-related shifts in the neurogram within each visit. Therefore, it would not be appropriate to estimate the MSNA burst amplitude or area for this protocol given that, even with burst amplitude and area normalization, alterations in baseline characteristics of the neurogram have a large influence on these recorded parameters throughout the protocol (27, 28).
Statistical Analysis
All values are presented as means ± standard deviation (SD). A power analysis based on prior LBNP tolerance testing was used for sample size estimates (29). To test the study hypothesis, with a study power of 0.80 at an adjusted α of 0.015, a sample size of n = 15 was estimated based on estimated effect size of 0.95. To enable comparisons for the primary variable of interest (LBNP tolerance) within each sex, this estimated sample size was nearly doubled. For all other comparison’s (e.g., cardiovascular and sympathetic data), males and females were pooled together to gain insight into the underlying mechanisms (e.g., MSNA).
Paired, two-tailed t tests were performed on baseline measurements between the two experimental trials (for data that did not pass the Shapiro-Wilk normality test, P > 0.05, a Wilcoxon paired rank test was utilized). Paired, two-tailed t tests were utilized to compare the differences in CSI between the two conditions (fentanyl vs. placebo); this was repeated within each sex. To account for drop-outs due to variations in LBNP tolerance time to presyncope, a mixed effects model [time (LBNP stage) × trial] was used to compare the cardiovascular, respiratory, and sympathetic variables at the distinct time points noted above (T1, T2, T3, and T4) between fentanyl and placebo trials. If a significant interaction was observed, post hoc multiple comparisons were performed utilizing paired t tests with a Holm-Bonferroni correction. All analyses and graphing were performed using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA). Statistical significance was accepted at P < 0.05.
RESULTS
Twenty-eight participants, 15 males and 13 females, completed both fentanyl and placebo trials. These participants (n = 28) presented with the following descriptive characteristics (means ± SD): White (n = 22), Hispanic (n = 2), Asian (n = 3), Black (n = 1); age, 28 ± 7 yr (range 20–45 yr); height, 174 ± 7 cm (range 161–189 cm); body mass, 79 ± 11 kg (range 65–110 kg); and BMI 26 ± 3 kg/m2 (range 22–34 kg/m2). Spot urine specific gravity upon arrival at the laboratory was not different between trials (FEN: 1.014 ± 0.010 vs. PBO: 1.014 ± 0.010; P = 0.6535). For the female participants, testing occurred during follicular phase of the menstrual cycle for n = 3, luteal phase n = 3, n = 1 participant had undergone a partial hysterectomy, n = 2 participants utilized an intrauterine device (unreported menstrual cycle phase), n = 3 utilized oral contraceptives, and n = 1 was unreported.
Environmental testing conditions between the two experimental days were not different: temperature (FEN: 23.8 ± 1.2; PBO: 23.6 ± 0.1°C, P = 0.4147) and relative humidity (FEN: 40 ± 8; PBO: 39 ± 9%, P = 0.4011). Resting baseline measurements before the administration of fentanyl and placebo are presented in Table 1. In comparing experimental visits at this resting baseline, HR, MAP, TCD, NIRS, MCAv, plasma epinephrine concentration, plasma norepinephrine concentration, and MSNA measurements were not different between days.
Table 1.
Cardiovascular and sympathetic measurements at resting baseline on separate days before fentanyl or placebo administration
| Fentanyl | Placebo | P Values | |
|---|---|---|---|
| HR, beats/min | 63 ± 13 | 63 ± 14 | 0.3637 |
| Brachial MAP, mmHg | 87 ± 7 | 88 ± 8 | 0.7405 |
| Brachial SBP, mmHg | 118 ± 11 | 118 ± 12 | 0.5917 |
| Brachial DBP, mmHg | 71 ± 7 | 72 ± 8 | 0.7835 |
| MCAv, cm/s | 61 ± 15 | 60 ± 12 | 0.9368 |
| ScO2, % oxygen saturation | 59 ± 13 | 58 ± 12 | 0.9368 |
| Plasma [E], pg/mL | 22 ± 16 | 24 ± 18 | 0.5978 |
| Plasma [NE], pg/mL | 234 ± 132 | 212 ± 90 | 0.8673 |
| MSNA burst frequency, bursts/min | 14 ± 8 | 15 ± 9 | 0.6842 |
| MSNA burst incidence, bursts/100 heartbeats | 23 ± 12 | 25 ± 18 | 0.4852 |
| RR, breaths/min | 14 ± 3 | 13 ± 4 | 0.3137 |
| End-tidal CO2, mmHg | 45 ± 5 | 45 ± 6 | 0.8096 |
Values are means ± SD. DBP, diastolic blood pressure; [E], epinephrine concentration; HR, heart rate; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; MSNA, muscle sympathetic nerve activity; [NE], norepinephrine concentration; RR, respiratory rate; SBP, systolic blood pressure; ScO2, frontal cortex oxygen saturation. Paired t test used for baseline comparisons.
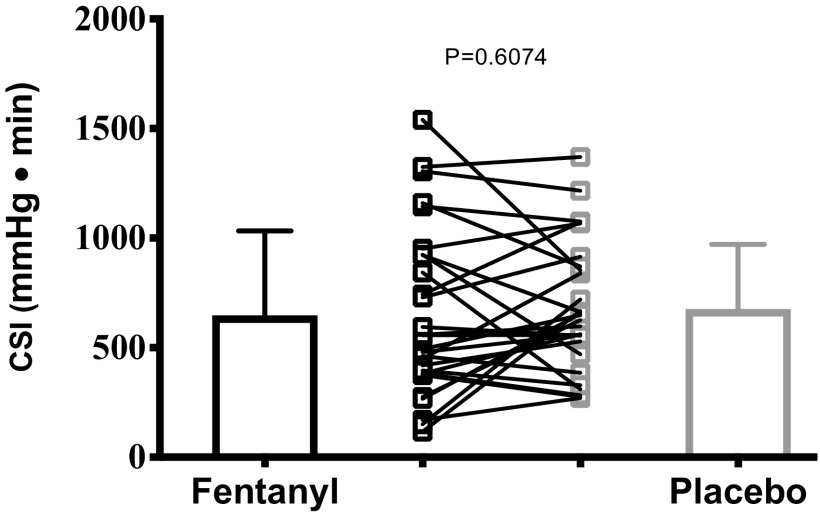
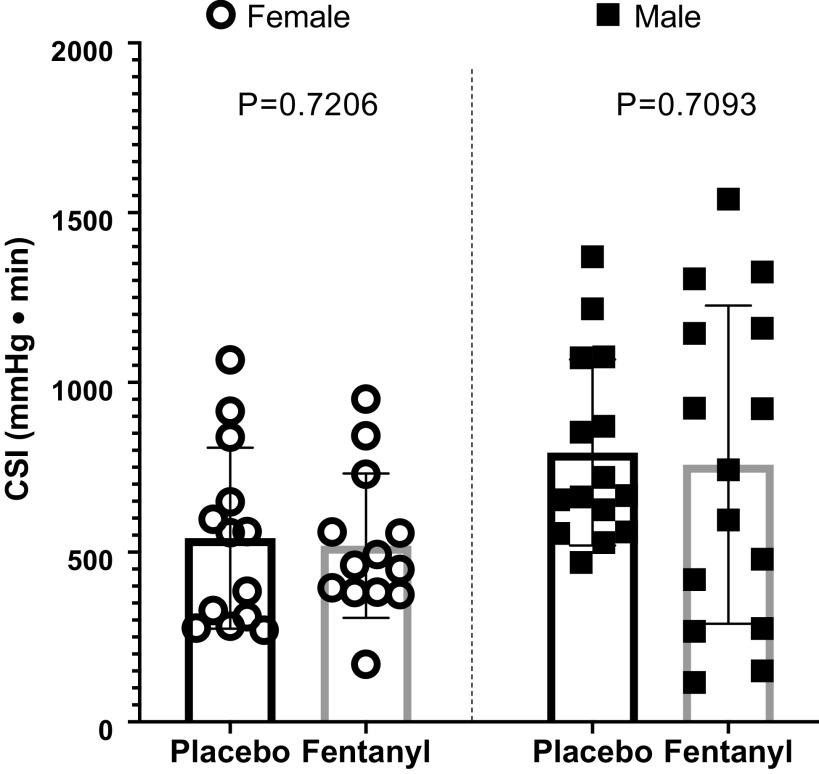
The primary outcome measure, LBNP tolerance capacity expressed as CSI, was not different between trials (paired t test, FEN: 647 ± 386 vs. PBO: 676 ± 295 mmHg·min, P = 0.6074, Cohen’s d = 0.08) and is detailed in Fig. 2. Partitioned by sex, these outcomes were consistent, revealing LBNP tolerance being not different between trials in males (paired t test, FEN: 758 ± 469 vs. PBO: 793 ± 274 mmHg·min, P = 0.7206) and females (paired t test, FEN: 519 ± 213 vs. PBO: 541 ± 267 mmHg·min, P = 0.7093). These comparisons are illustrated in Fig. 3.
Figure 2.
Group mean (±SD) comparison of lower body negative pressure tolerance capacity expressed as cumulative stress index (CSI) following fentanyl vs. placebo administration (n = 28; paired, two-tailed t test analysis).
Figure 3.
Comparisons of cumulative stress index (CSI) partitioned by sex. Data expressed as group means ± SD (female n = 13, male n = 15; paired, two-tailed t test analysis).
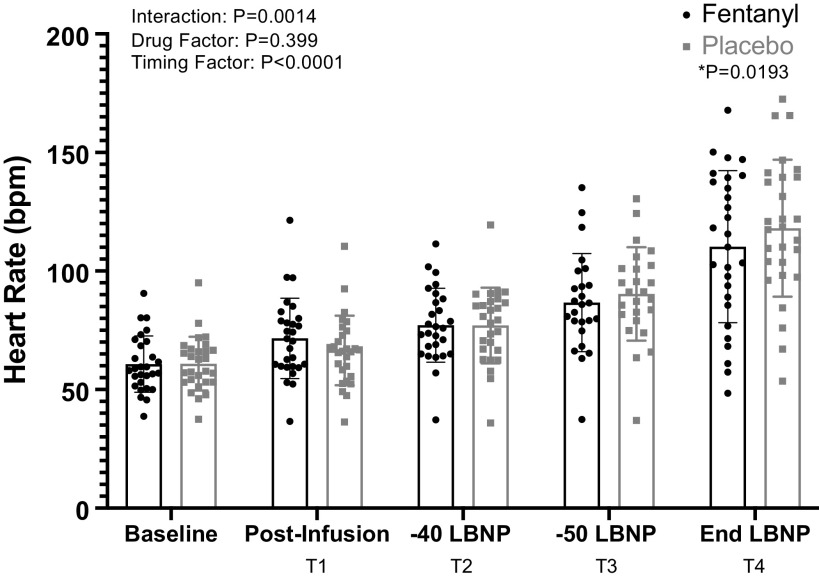
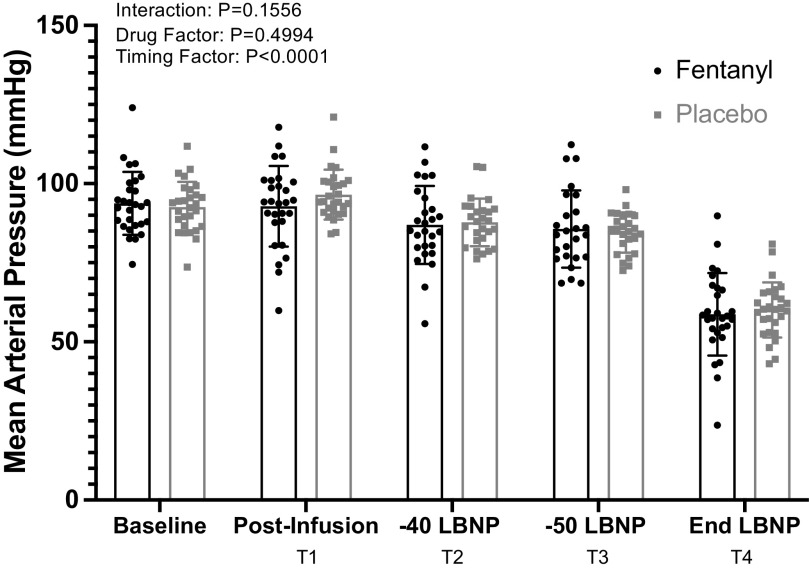
A significant drug × time interaction was identified for heart rate (Fig. 4, P = 0.0014) but not for MAP (Fig. 5, P = 0.1556). Further analysis with post hoc multiple comparisons (Holm-Bonferroni correction) revealed significantly higher HRs for the PBO trial at the end of LBNP (T4) relative to the same time point for the fentanyl trial. Sympathetic responses after drug and placebo administration are depicted in Fig. 6. Although a significant time effect was determined with the mixed effects analysis, there was not a significant interaction or drug effect. The sample sizes for HR and MAP were n = 28 at all time points of the analysis. The sample sizes obtained and analyzed as paired MSNA recordings were baseline: n = 21, T1: n = 20; T2: n = 16; T3: n = 15; and T4: n = 16.
Figure 4.
Group mean (±SD) data showing heart rate responses during fentanyl vs. placebo administration [baseline n = 28, T1 n = 28, T2 n = 27, T3 n = 25, T4 n = 28; mixed-effect model analysis with post hoc Holm-Bonferroni corrected paired t test, baseline data were not included in analysis]. *Significant differences.
Figure 5.
Group mean (±SD) data showing Finometer-derived mean arterial pressure during fentanyl vs. placebo administration. Baseline n = 28, T1 n = 28, T2 n = 27, T3 n = 25, T4 n = 28; mixed-effect model ANOVA, baseline data were not included in analysis.
Figure 6.
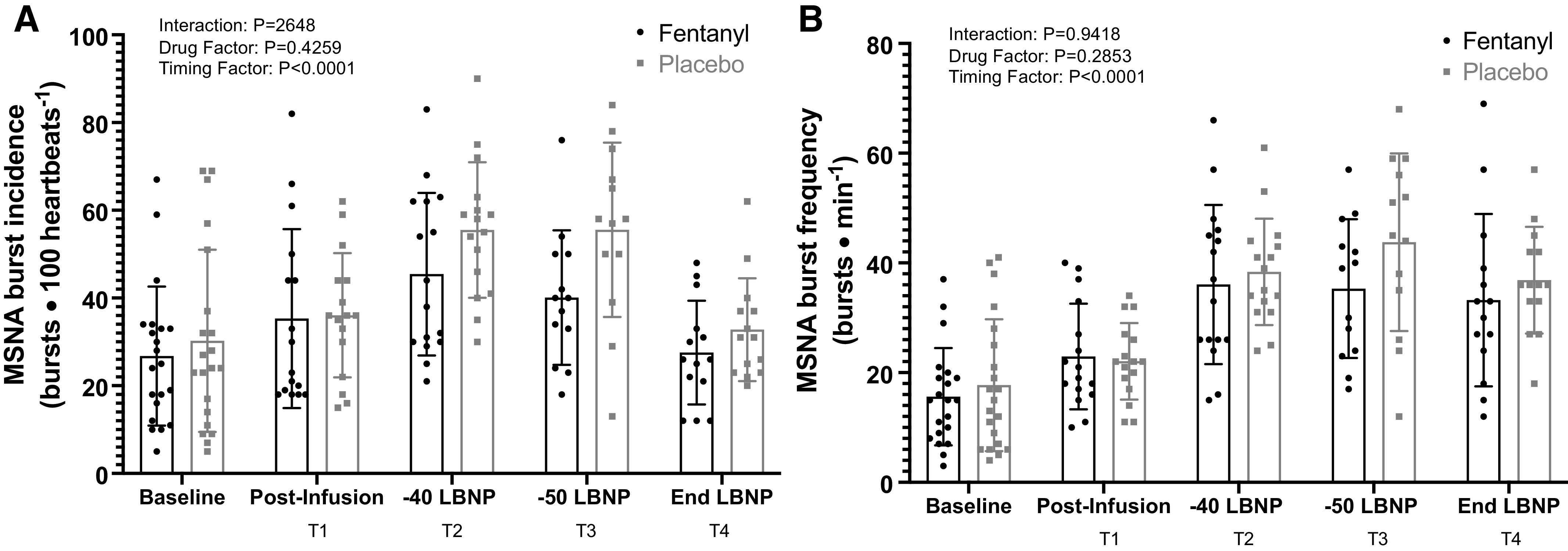
Group mean (±SD) data showing muscle sympathetic nerve activity (A: burst incidence; B: burst frequency) during fentanyl vs. placebo administration. Baseline n = 21, T1 n = 20, T2 n = 16, T3 n = 15, T4 n = 16; mixed-effect model ANOVA, baseline data were not included in analysis.
Hemodynamic (except for HR and MAP) data are reported in Table 2. A significant interaction (mixed effects model, P < 0.0001) is reported for end-tidal CO2, with significantly greater end-tidal CO2 values during fentanyl administration at T3 (P = 0.0195) and T4 (P = 0.0006). Significant differences in time effects (P < 0.0001) and drug administration (P = 0.0074) were revealed for respiratory rate. Significant time effects were revealed for all variables aside from plasma epinephrine (P < 0.05). The sample sizes analyzed for these variables are as follows: MCAv – T1: n = 20; T2: n = 18; T3: n = 16; and T4: n = 18; ScO2 – T1: n = 25; T2: n = 23; T3: n = 21; and T4: n = 24; CRI – T1: n = 25; T2: n = 26; T3: n = 23; and T4: n = 24; plasma epinephrine and norepinephrine concentration – T3: n = 20 and T4: n = 23; respiration – T1: n = 25; T2: n = 26; T3: n = 22; and T4: n = 26; EtCO2 – T1: n = 26; T2: n = 26; T3: n = 24; and T4: n = 24.
Table 2.
Hemodynamic responses throughout fentanyl vs. placebo administration
| Time Points |
P Values |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1: Pre-LBNP (postinfusion) |
T2: −40 mmHg LBNP |
T3: −50 mmHgLBNP |
T4: End LBNP |
Interaction | Drug factor | Timing factor | |||||
| FEN | PBO | FEN | PBO | FEN | PBO | FEN | PBO | ||||
| MCAv, cm/s | 64 ± 18 | 62 ± 14 | 61 ± 18 | 62 ± 14 | 58 ± 20 | 52 ± 15 | 43 ± 15 | 41 ± 10 | 0.1042 | 0.1681 | <0.0001 |
| ScO2, % oxygen saturation | 59 ± 14 | 59 ± 12 | 59 ± 12 | 59 ± 11 | 59 ± 12 | 57 ± 12 | 55 ± 11 | 53 ± 12 | 0.5379 | 0.7800 | <0.0001 |
| CRI (AU) | 0.91 ± 0.13 | 0.92 ± 0.04 | 0.70 ± 0.21 | 0.59 ± 0.22 | 0.43 ± 0.22 | 0.41 ± 0.20 | 0.31 ± 0.20 | 0.27 ± 0.17 | 0.0833 | 0.1666 | <0.0001 |
| Plasma [E], pg/mL | 111 ± 71 | 86 ± 103 | 174 ± 208 | 119 ± 213 | 0.4667 | 0.1511 | 0.1299 | ||||
| Plasma [NE], pg/mL | 425 ± 190 | 462 ± 207 | 811 ± 635 | 675 ± 563 | 0.1425 | 0.3854 | 0.0201 | ||||
| Systolic blood pressure, mmHg | 127 ± 18 | 132 ± 13 | 116 ± 18 | 118 ± 14 | 113 ± 16 | 112 ± 12 | 75 ± 15 | 77 ± 12 | 0.1587 | 0.5846 | <0.0001 |
| Diastolic blood pressure, mmHg | 71 ± 10 | 74 ± 6 | 70 ± 9 | 71 ± 5 | 70 ± 10 | 69 ± 5 | 49 ± 12 | 50 ± 8 | 0.1162 | 0.6032 | <0.0001 |
| RR, breaths/min | 14 ± 4 | 15 ± 4 | 11 ± 3 | 12 ± 4 | 11 ± 4 | 13 ± 3 | 15 ± 5 | 16 ± 5 | 0.8946 | 0.0074 | <0.0001 |
| End-tidal CO2, mmHg | 44 ± 5 | 44 ± 5 | 42 ± 7 | 41 ± 5 | 43 ± 8* | 39 ± 6 | 37 ± 10* | 31 ± 7 | <0.0001 | 0.0370 | <0.0001 |
Values are means ± SD. CRI, compensatory reserve index; [E], epinephrine concentration; FEN, fentanyl trial; MCAv, middle cerebral artery velocity; MSNA, muscle sympathetic nerve activity; [NE], norepinephrine concentration; PBO, placebo trial; RR, respiratory rate; ScO2, frontal cortex oxygen saturation. Mixed effects model used for statistical analysis. *Statistical differences in end-tidal CO2 compared with PBO with Holm–Bonferroni multiple comparisons post hoc testing, P < 0.05.
DISCUSSION
The novel finding of this investigation is that in conscious humans, intravenous administration of an analgesic dose of fentanyl does not compromise tolerance to a simulated hemorrhagic insult. In fact, the difference in average LBNP tolerance was statistically indistinguishable (Cohen’s d = 0.08). Furthermore, this finding was observed within both male and female adults.
Our findings countered the hypothesis that fentanyl impairs hemorrhagic tolerance in conscious humans. This finding was unanticipated as Xiang et al. (11) recently demonstrated in Sprague-Dawley rats that intravenous fentanyl administration impairs the microcirculatory response to hemorrhage. Specifically, in response to hemorrhage, when the rats received fentanyl, the peripheral vasoconstriction was not only diminished but was also replaced with vasodilation (11). This observation suggested that with fentanyl administration, inappropriate volume shunting would reduce critical blood flow to vital organs, adversely altering tolerance capacity to hemorrhage. However, the results of the present investigation in conscious humans do not complement those preclinical findings in anesthetized rats and revealed that an analgesic dose of intravenous fentanyl does not impair tolerance capacity during simulated hemorrhage.
The underlying physiological determinants of tolerance capacity during LBNP were, for the most part, not different between the fentanyl and placebo experimental trials, including indices of cerebral blood flow and cerebral tissue oxygenation that contributes to LBNP tolerance (30). The most notable measurement, BP, was comparable throughout the two different experimental conditions (Fig. 5). The observed responses are noteworthy as they contribute toward the knowledge gap of whether fentanyl compromises BP compensation during hemorrhage, which is implied by the US Army’s CoTCCC guidelines. The impaired vasomotor activity demonstrated by the previously mentioned study by Xiang et al., and other studies showing vasodilatory effects of opioids, highlights the importance of our findings in humans (31, 32).
Low tolerance to hypovolemia is proposed to be associated with the inability to rapidly constrict the peripheral vasculature (33). Therefore, any circumstance that impairs vasoconstriction may be detrimental to hemorrhagic tolerance. The present investigation provides experiment evidence that one of the controllers contributing to vasoconstriction, peripheral sympathetic outflow, is not modified during simulated hemorrhage following intravenous fentanyl. To our knowledge, in humans, this interplay between sympathetic nervous system responses to opioids and the competing compensatory responses to hemorrhage has not been previously elucidated.
One physiological variable that was altered following fentanyl administration was respiratory rate. Consistent with previous research on the suppressive effects of fentanyl on respiration (11, 20, 34, 35), our findings demonstrate a slight depression of respiratory rate, and subsequent increase in end-tidal CO2, throughout LBNP. Despite the statistical differences in this measurement between trials, the numerical differences were minimal, i.e., a decrease of one to two breaths per minute for the fentanyl trial. As such, this difference was unlikely to influence LBNP tolerance. Therefore, within this cohort of participants and this specific dose of fentanyl, respiratory distress was not induced.
The data from this study also indicated an effect on HR in individuals given fentanyl (Fig. 4), fitting the pattern of prior reports (36–40); namely, fentanyl attenuated increases in HR at the end of LBNP. The precise mechanism for this effect, especially during presyncopal limited LBNP, is not clear. That said, in a recent study, fentanyl causes a downward shift in the operating point of the carotid-cardiac baroreflex, resulting in a lower heart rate at a comparable carotid sinus pressure with fentanyl administration (41). It has also been demonstrated that giving fentanyl increases vagal activation (42). Both of these responses may contribute to the observed effects in HR. Regardless of the cause, it appears these alterations to HR do not influence the tolerance capacity to simulated hemorrhage. Along these lines, other indicators of tolerance capacity to central hypovolemia, including middle cerebral artery blood velocity, related indices of cerebral tissue oxygenation, and an aggregate of physiological variables, i.e., compensatory reserve index, were not different between the two experimental trials.
Recently, our research team demonstrated that administration of an analgesic dose of ketamine does not compromise tolerance to progressive central hypovolemia (12). Comparably, an analgesic dose of fentanyl also does not compromise tolerance to an identical simulation of hemorrhage. These combined observations indicate that commonly used opioid and nonopioid analgesics are both potentially suitable in the prehospital setting during a hemorrhagic insult. With this insight that these two analgesics are not detrimental to hemorrhage tolerance, other factors associated with their use need to be considered. One glaring aspect that needs considerable attention is the side effects of these drugs at the administered doses. For example, ketamine given at subanesthetic doses can induce hallucinations (43). Regarding the present investigation, we observed nausea in some of the participants following fentanyl administration. This nausea was not the transient nausea often induced by the LBNP chamber that subsides quickly after the negative pressure is removed, but rather lasted well after testing that on three occasions, resulted in vomiting well after the end of the experimental procedures. Unfortunately, the reports on nausea were descriptive in nature and the exact intensity and duration were not quantified in this study. It will be important in future studies to weigh these side effects, as well as long term effects of opioid addiction, of the differing analgesics used in the prehospital setting.
Similar to our ketamine investigation (12), there was a high degree of individual variability in the effect of fentanyl on tolerance to a simulated hemorrhage. A little less than half of the participants in this study experienced an improvement in LBNP tolerance after receiving fentanyl, with the others demonstrating a worsening (Fig. 2). However, such an analysis ignores normal variability in LBNP tolerance. With that in mind, LBNP tolerance is highly repeatable, though there remains some variability (44, 45). For example, 21 of 28 participants, after receiving fentanyl, fell within the intraindividual variability of ∼1 LBNP stage or ∼3 min. Thus, ∼25% of the assessed cohort exhibited an effect of fentanyl on LBNP tolerance (both improved and reduced tolerance) outside of an expected normal variation. Unfortunately, from the obtained cardiovascular and sympathetic variables, we were not able to identify a mechanism explaining why these individuals exhibited improvements or decrements in hemorrhage tolerance to fentanyl.
It should be pointed out that the participants recruited for this study closely represent military soldiers (i.e., age and BMI) deployed and wounded in the battlefield (46). Therefore, the findings of this study are limited to a population that is relatively young and healthy. Caution is advised in extending these findings to individuals with chronic medical conditions/comorbidities. With these limitations in mind, the findings of this study may provide useful information in advising the CoTCCC guidelines. Currently, the guidelines express that fentanyl should be avoided if a casualty is in circulatory or respiratory distress (9, 10). Counter to these recommendations, our data suggest that there is no evidence that giving fentanyl (at the administered dose) would be detrimental, and thus can be considered during these conditions.
Perspectives and Significance
These data, the first to be obtained in conscious males and females, demonstrate that administration of an analgesic dose of fentanyl does not compromise tolerance to progressive central hypovolemia. These findings may inform medical professionals for the selection of the most suitable analgesic medication in the prehospital setting during a hemorrhagic insult.
GRANTS
This research was supported by the Department of Defense – United States Army W81XWH1820012 (to C. G. Crandall), National Institutes of Health (NIH) F32HL154559 (to J. C. Watso), and NIH F32HL154565 (to L. N. Belval).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H., J.C.W., and C.G.C. conceived and designed research; M.H., J.C.W., L.N.B., F.A.C., M.F., C.P.J., J.M.H., and C.G.C. performed experiments; M.H., J.C.W., F.A.C., and C.P.J. analyzed data; M.H., J.C.W., C.H.-L., and C.G.C. interpreted results of experiments; M.H. prepared figures; M.H. drafted manuscript; M.H., J.C.W., L.N.B., F.A.C., M.F., C.P.J., J.M.H., C.H.-L., and C.G.C. edited and revised manuscript; M.H., J.C.W., L.N.B., F.A.C., M.F., C.P.J., J.M.H., C.H.-L., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the study volunteers for their participation. We thank all study volunteers for their participation. In addition, we thank research nurses Naomi Kennedy, Margot Morris, and Ileana Hill for their contributions to this project. All experiments took place at the Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas, TX.
REFERENCES
- 1.Hoyt DB, Dutton RP, Hauser CJ, Hess JR, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Bouillon B. Management of coagulopathy in the patients with multiple injuries: results from an international survey of clinical practice. J Trauma 65: 755–764, 2008. doi: 10.1097/TA.0b013e318185fa9f. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med 149: 55–62, 1984. [PubMed] [Google Scholar]
- 3.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185–193, 1995. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Crandall CG, Rickards CA, Johnson BD. Impact of environmental stressors on tolerance to hemorrhage in humans. Am J Physiol Regul Integr Comp Physiol 316: R88–R100, 2019. doi: 10.1152/ajpregu.00235.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 73: S431–437, 2012. [Erratum in J Trauma Acute Care Surg 74: 706, 2013]. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 6.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol Heart Circ Physiol 260: H305–H318, 1991. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 7.Evans RG, Ventura S, Dampney RA, Ludbrook J. Neural mechanisms in the cardiovascular responses to acute central hypovolaemia. Clin Exp Pharmacol Physiol 28: 479–487, 2001. doi: 10.1046/j.1440-1681.2001.03473.x. [DOI] [PubMed] [Google Scholar]
- 8.Bronsky ES, Koola C, Orlando A, Redmond D, D’Huyvetter C, Sieracki H, Tanner A, Fowler R, Mains C, Bar-Or D. Intravenous low-dose ketamine provides greater pain control compared to fentanyl in a civilian prehospital trauma system: a propensity matched analysis. Prehosp Emerg Care 23: 1–8, 2019. doi: 10.1080/10903127.2018.1469704.[29775117] [DOI] [PubMed] [Google Scholar]
- 9.Butler FK, Kotwal RS, Buckenmaier CC 3rd, Edgar EP, O’Connor KC, Montgomery HR, Shackelford SA, Gandy JV 3rd, Wedmore IS, Timby JW, Gross KR, Bailey JA. A triple-option analgesia plan for tactical combat casualty care: TCCC guidelines change 13-04. J Spec Oper Med 14: 13–25, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Butler FK Jr, Holcomb JB, Shackelford S, Barbabella S, Bailey JA, Baker JB, Cap AP, Conklin CC, Cunningham CW, Davis M, DeLellis SM, Dorlac WC, DuBose JJ, Eastridge B, Fisher AD, Glasser JJ, Gurney J, Jenkins DA, Johannigman J, King DR, Kotwal RS, Littlejohn LF, Mabry RL, Martin MJ, Miles EA, Montgomery HR, Northern DM, O'Connor KC, Rasmussen TE, Riesberg JC, Spinella PC, Stockinger Z, Strandenes G, Via DK, Weber MA. Advanced resuscitative care in tactical combat casualty care: TCCC guidelines change 18-01:14 October 2018. J Spec Oper Med 18: 37–55, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Xiang L, Calderon AS, Klemcke HG, Scott LL, Hinojosa-Laborde C, Ryan KL. Fentanyl impairs but ketamine preserves the microcirculatory response to hemorrhage. J Trauma Acute Care Surg 89 (Suppl 2): S93.–, 2020. doi: 10.1097/TA.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 12.Huang M, Watso JC, Moralez G, Cramer MN, Hendrix JM, Yoo JK, Badrov MB, Fu Q, Hinojosa-Laborde C, Crandall CG. Low-dose ketamine affects blood pressure, but not muscle sympathetic nerve activity, during progressive central hypovolemia without altering tolerance. J Physiol 598: 5661–5672, 2020. doi: 10.1113/JP280491. [DOI] [PubMed] [Google Scholar]
- 13.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol (1985) 96: 1249–1261, 2004. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, Chung KK, Cap AP, Convertino VA. Validation of lower body negative pressure as an experimental model of hemorrhage. J Appl Physiol (1985) 116: 406–415, 2014. doi: 10.1152/japplphysiol.00640.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper VL, Hainsworth R. Carotid baroreceptor reflexes in humans during orthostatic stress. Exp Physiol 86: 677–681, 2001. doi: 10.1113/eph8602213. [DOI] [PubMed] [Google Scholar]
- 16.Goswami N, Blaber AP, Hinghofer-Szalkay H, Convertino VA. Lower body negative pressure: physiological effects, applications, and implementation. Physiol Rev 99: 807–851, 2019. doi: 10.1152/physrev.00006.2018. [DOI] [PubMed] [Google Scholar]
- 17.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84: 1016–1023, 1991. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 18.Streisand JB, Varvel JR, Stanski DR, Le Maire L, Ashburn MA, Hague BI, Tarver SD, Stanley TH. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology 75: 223–229, 1991. doi: 10.1097/00000542-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Tucker AP, Kim YI, Nadeson R, Goodchild CS. Investigation of the potentiation of the analgesic effects of fentanyl by ketamine in humans: a double-blinded, randomised, placebo controlled, crossover study of experimental pain[ISRCTN83088383]. BMC Anesthesiol 5: 2, 2005. doi: 10.1186/1471-2253-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronoff GM, Brennan MJ, Pritchard DD, Ginsberg B. Evidence-based oral transmucosal fentanyl citrate (OTFC) dosing guidelines. Pain Med 6: 305–314, 2005. doi: 10.1111/j.1526-4637.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 21.Darwish M, Kirby M, Robertson P Jr, Tracewell W, Jiang JG. Absolute and relative bioavailability of fentanyl buccal tablet and oral transmucosal fentanyl citrate. J Clin Pharmacol 47: 343–350, 2007. doi: 10.1177/0091270006297749. [DOI] [PubMed] [Google Scholar]
- 22.Christrup LL, Foster D, Popper LD, Troen T, Upton R. Pharmacokinetics, efficacy, and tolerability of fentanyl following intranasal versus intravenous administration in adults undergoing third-molar extraction: a randomized, double-blind, double-dummy, two-way, crossover study. Clin Ther 30: 469–481, 2008. doi: 10.1016/j.clinthera.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Watso JC, Huang M, Belval L, Cimino Iii FA, Jarrad CP, Hendrix JM, Hinijosa-Laborde C, Crandall CG. Low-dose fentanyl reduces pain perception, muscle sympathetic nerve activity responses, and blood pressure responses during the cold pressor test. Am J Physiol Regul Integr Comp Physiol 321: R000–R000, 2021. doi: 10.1152/ajpregu.00218.2021.[34851729] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Convertino VA, Grudic G, Mulligan J, Moulton S. Estimation of individual-specific progression to impending cardiovascular instability using arterial waveforms. J Appl Physiol (1985) 115: 1196–1202, 2013. doi: 10.1152/japplphysiol.00668.2013. [DOI] [PubMed] [Google Scholar]
- 25.Convertino VA, Wirt MD, Glenn JF, Lein BC. The compensatory reserve for early and accurate prediction of hemodynamic compromise: a review of the underlying physiology. Shock 45: 580–590, 2016. doi: 10.1097/SHK.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 26.Moralez G, Jouett NP, Tian J, Zimmerman MC, Bhella P, Raven PB. Effect of centrally acting angiotensin converting enzyme inhibitor on the exercise-induced increases in muscle sympathetic nerve activity. J Physiol 596: 2315–2332, 2018. doi: 10.1113/JP274697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macefield VG. Recording and quantifying sympathetic outflow to muscle and skin in humans: methods, caveats and challenges. Clin Auton Res 31: 59–75, 2021. doi: 10.1007/s10286-020-00700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlader ZJ, Crandall CG. Normothermic central hypovolemia tolerance reflects hyperthermic tolerance. Clin Auton Res 24: 119–126, 2014. doi: 10.1007/s10286-014-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kay VL, Rickards CA. The role of cerebral oxygenation and regional cerebral blood flow on tolerance to central hypovolemia. Am J Physiol Regul Integr Comp Physiol 310: R375–R383, 2016. doi: 10.1152/ajpregu.00367.2015. [DOI] [PubMed] [Google Scholar]
- 31.Xiang L, Lu S, Fuller W, Aneja A, Russell GV, Jones LB, Hester R. Impaired blood pressure recovery to hemorrhage in obese Zucker rats with orthopedic trauma. Am J Physiol Heart Circ Physiol 302: H340–H348, 2012. doi: 10.1152/ajpheart.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs C, Ertmer C, Rehberg S. Effects of vasodilators on haemodynamic coherence. Best Pract Res Clin Anaesthesiol 30: 479–489, 2016. doi: 10.1016/j.bpa.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Xiang L, Hinojosa-Laborde C, Ryan KL, Rickards CA, Convertino VA. Time course of compensatory physiological responses to central hypovolemia in high- and low-tolerant human subjects. Am J Physiol Regul Integr Comp Physiol 315: R408–R416, 2018. doi: 10.1152/ajpregu.00361.2017. [DOI] [PubMed] [Google Scholar]
- 34.Peng PW, Sandler AN. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 90: 576–599, 1999. doi: 10.1097/00000542-199902000-00034. [DOI] [PubMed] [Google Scholar]
- 35.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94: 825–834, 2005. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 36.Patschke D, Brückner JB, Eberlein HJ, Hess W, Tarnow J, Weymar A. Effects of althesin, etomidate and fentanyl on haemodynamics and myocardial oxygen consumption in man. Can Anaesth Soc J 24: 57–69, 1977. doi: 10.1007/BF03006813. [DOI] [PubMed] [Google Scholar]
- 37.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol Bethesda Md 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HNM, Garcia VP, Rocha NG, Secher NH, Nobrega ACL. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J Physiol 594: 715–725, 2016. doi: 10.1113/JP271335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidhu SK, Weavil JC, Rossman MJ, Jessop JE, Bledsoe AD, Buys MJ, Supiano MS, Richardson RS, Amann M. Exercise pressor reflex contributes to the cardiovascular abnormalities characterizing: hypertensive humans during exercise. Hypertension 74: 1468–1475, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW, Amann M. Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. J Physiol 596: 1373–1384, 2018. doi: 10.1113/JP275465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vettorello M, Colombo R, De Grandis CE, Costantini E, Raimondi F. Effect of fentanyl on heart rate variability during spontaneous and paced breathing in healthy volunteers. Acta Anaesthesiol Scand 52: 1064–1070, 2008. doi: 10.1111/j.1399-6576.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 43.Powers AR 3rd, Gancsos MG, Finn ES, Morgan PT, Corlett PR. Ketamine-induced hallucinations. Psychopathology 48: 376–385, 2015. doi: 10.1159/000438675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goswami N, Lackner HK, Grasser EK, Hinghofer-Szalkay HG. Individual stability of orthostatic tolerance response. Acta Physiol Hung 96: 157–166, 2009. doi: 10.1556/APhysiol.96.2009.2.2. [DOI] [PubMed] [Google Scholar]
- 45.Howden R, Tranfield PA, Lightfoot JT, Brown SJ, Swaine IL. The reproducibility of tolerance to lower-body negative pressure and its quantification. Eur J Appl Physiol 84: 462–468, 2001. doi: 10.1007/s004210100398. [DOI] [PubMed] [Google Scholar]
- 46.DeBruyne NF. American War and Military Operations Casualties: Lists and Statistics (RL32492). Congressional Research Service, Washington, DC, 2017. [Google Scholar]