Keywords: bile acid synthesis, GATA-4, neonatal, oxysterols, premature infants
Abstract
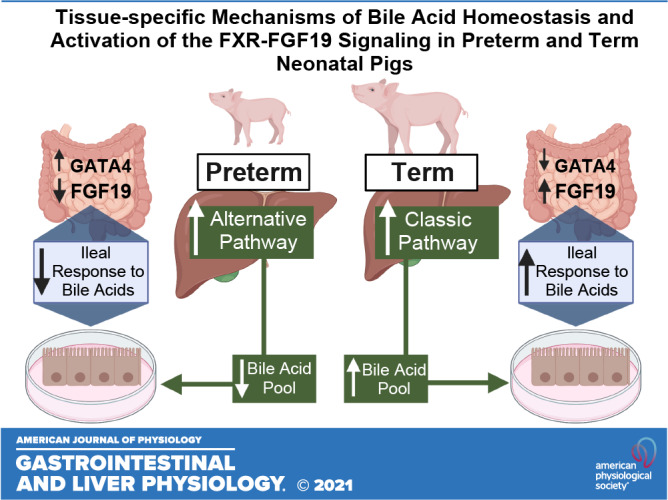
The tissue-specific molecular mechanisms involved in perinatal liver and intestinal farnesoid X receptor (FXR)-fibroblast growth factor 19 (FGF19) signaling are poorly defined. Our aim was to establish how gestational age and feeding status affect bile acid synthesis pathway, bile acid pool size, ileal response to bile acid stimulation, genes involved in bile acid-FXR-FGF19 signaling and plasma FGF19 in neonatal pigs. Term (n = 23) and preterm (n = 33) pigs were born via cesarean section at 100% and 90% gestation, respectively. Plasma FGF19, hepatic bile acid and oxysterol profiles, and FXR target gene expression were assessed in pigs at birth and after a bolus feed on day 3 of life. Pig ileal tissue explants were used to measure signaling response to bile acids. Preterm pigs had smaller, more hydrophobic bile acid pools, lower plasma FGF19, and blunted FXR-mediated ileal response to bile acid stimulation than term pigs. GATA binding protein 4 (GATA-4) expression was higher in jejunum than ileum and was higher in preterm than term pig ileum. Hepatic oxysterol analysis suggested dominance of the alternative pathway of bile acid synthesis in neonates, regardless of gestational age and persists in preterm pigs after feeding on day 3. These results highlight the tissue-specific molecular basis for the immature enterohepatic bile acid signaling via FXR-FGF19 in preterm pigs and may have implications for disturbances of bile acid homeostasis and metabolism in preterm infants.
NEW & NOTEWORTHY Our results show that the lower hepatic bile acid synthesis and ileum FXR-FGF19 pathway responsiveness to bile acids contribute to low-circulating FGF19 in preterm compared with term neonatal pigs. The molecular mechanism explaining immature or low-ileum FXR-FGF19 signaling may be linked to developmental patterning effects of GATA-4.
INTRODUCTION
Premature birth is a phenomenon that affects at least 11% of total births, equating to 15 million births worldwide (1). Many of these infants have inadequate growth and development, and this is more common in extremely premature infants (2–5). The ability to restore a normal growth trajectory by optimizing nutrition is limited by their intolerance to oral feeding and poor gut capacity for digestion, especially of fat. Another process critical for fat digestion is the hepatic synthesis and biliary secretion of bile acids that function as detergents to solubilize fatty acids released by intraluminal lipase action and facilitate fat absorption. The bile acid pool size and synthesis rates increase during the final trimester of gestation and the perinatal period (6). Studies with stable isotopes of [2H]cholic acid showed that preterm neonates have a smaller bile acid pool than their term counterparts, which coupled with a smaller population of lipolysis enzymes, may profoundly limit their ability to digest and absorb lipid (7–9). The molecular basis for reduced bile acid synthesis and pool size in preterm infants is unknown.
Bile acids not only facilitate lipid digestion and absorption but they also function as ligands for multiple receptors, including the farnesoid X nuclear receptor (FXR), the Takeda G protein receptor (TGR5) membrane receptor, and sphingosine-1-phosphate receptor 2 (S1PR2) (10). A key FXR target gene involved in bile acid homeostasis is fibroblast growth factor 19 (FGF19), a member of the endocrine family of fibroblast growth factors (11). FGF19 is produced by luminal bile acid activation of FXR in ileal epithelial cells and circulates to the liver where it downregulates the hepatic expression of the rate-limiting enzyme cytochrome P450 section 7 family A member 1 (CYP7A1) involved in the classic pathway of bile acid synthesis. Several authors have suggested extrahepatic, systemic functions of FGF19 in metabolism and skeletal muscle growth (11–13). However, despite the evidence of FGF19 functions in adult human and mouse models, there is a paucity information about FGF19 actions during early neonatal development.
Early evidence of the developmental regulation of FGF19 showed that plasma FGF19 concentrations increase 10-fold between birth and 4 mo of age and are lower in growth-restricted infants (14). In contrast, a study in preterm infants showed a decline in circulating FGF19 with advancing postnatal age (15). Studies in neonatal pigs also reported developmental increases in the circulating concentration and ileal expression of FGF19 (16), consistent with increased ileal FGF15 in neonatal mice (17). GATA binding protein 4 (GATA-4) is a transcription factor implicated in the regulation of regional patterns in target gene expression along the proximal to distal intestine and has been suggested as an underlying mechanism regulating FXR activity and, therefore, FGF19 transcription. (18). Specifically, GATA-4 has been shown to inhibit FGF15 (the rodent ortholog of FGF19).
The preterm pig is unique in that is offers the advantage to model the developmental physiology of preterm infants at ∼30-wk gestation (19). Pigs also express FGF19 in both the liver and intestine, like humans, whereas rodents produce the FGF15 ortholog only in the intestine (20, 21). Our recent studies in neonatal pigs also showed a developmental increase in plasma FGF19 concentrations in the first week after birth but showed a strikingly lower FGF19 level in pigs born preterm compared with term (20). Taken together, the findings of low bile acid pool size and low FGF19 in neonates, especially in preterm pigs, suggests that the tissue-specific feedback regulation of bile acid homeostasis between the intestine and liver may be developmentally regulated. The aim of the current study was to extend our previous finding in preterm and term pigs, quantify the relationship between bile acid pool size and plasma FGF19, and identify the molecular basis of liver bile acid synthesis and transport, as well as intestinal FXR-FGF19 signaling.
METHODS
Cesarean Section
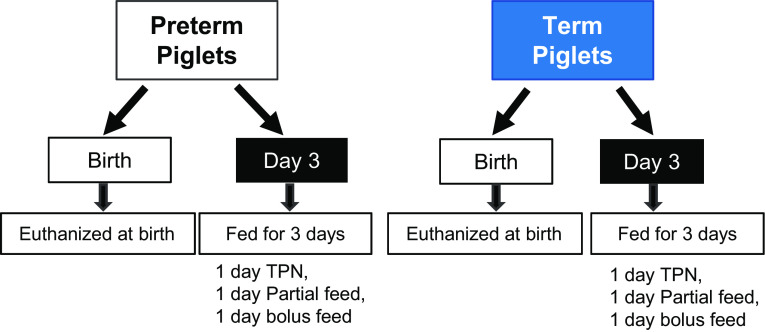
The protocol for this study was approved by the Baylor College of Medicine Institutional Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals produced by the National Research Council. Pregnant Yorkshire and Duroc sows were obtained from a commercial swine farm and delivered to the United States Department of Agriculture/ Agriculture Research Service (USDA/ARS) Children’s Nutrition Research Center (Houston, TX). Sows were allowed ad libitum access to feed and water until surgery. Cesarean sections were performed on gestational days 106 (preterm) and 114 (term) as described previously (22–24). After newborn pigs were removed and recovered, blood was obtained from the uterine vasculature to obtain maternal plasma and the sow was euthanized using a commercial pentobarbital solution (SomnaSol, Henry-Schein Animal Health, Dublin, OH) (see study design in Fig. 1).
Figure 1.
Diagram of study design. TPN, total parenteral nutrition.
Surgery, Clinical Care, and Nutrition
One-half of the newborn pigs were euthanized using a commercial pentobarbital solution immediately after birth (BIRTH), and tissues were harvested, snap-frozen in liquid nitrogen, and stored at −80°C for later analysis. The remaining piglets (day 3) were surgically implanted with a jugular venous catheter and orogastric Tygon feeding tube (French) to facilitate enteral feeding as previously described (22, 23). These pigs received 16 mL/kg maternal plasma to provide passive immunity and were housed in individual cages under a 12-h light-dark cycle at 31°C–32°C. Pigs received 50% of their full nutritional requirements (240 kcal, 13 g amino acid, 25 g glucose, 5 g lipid, and 240 mL fluid) via total parental nutrition at a rate of 5 mL fluid/kg/h for 12 h. After 12 h, parental nutrition was reduced to 2.5 mL/kg/h and the pigs were enterally fed 20 mL/kg commercial sow’s milk replacer (Liqui-wean, Heritage Animal Health, Hawarden, IA) every 4 h, for 24 h. Pigs received a final bolus enteral feed (40 mL/kg) on day 3. Pigs were euthanized using a commercial pentobarbital (SomnaSol, Henry-Schein Animal Health, Dublin, OH) solution 4 h after feeding.
Tissue collection.
After euthanasia, gallbladder contents were collected via aspiration. Livers were dissected and weighed before subsampling. Proximal colon and small intestine luminal contents were collected and snap-frozen in liquid nitrogen. Jejunum and ileum were dissected, weighed, and appropriately subsampled. Subsamples of liver, jejunum, ileum, hypothalamus, longissimus dorsi, and gallbladder were snap-frozen in liquid nitrogen and subsequently stored at −80°C until analysis.
Plasma Assays FGF19, Insulin, and Glucose
Blood samples were collected immediately at birth before piglets were euthanized. In pigs that were managed until day 3, blood was collected immediately after catheter placement during surgery on day 1, before bolus feeding on day 3, then 1, 2, 3, and 4 h post bolus feed. Blood samples were then processed to yield serum and plasma which were stored at −80° until analysis. Plasma fibroblast growth factor 19 (FGF19) was measured using a Porcine FGF19 ELISA kit (RayBiotech). Plasma glucose and insulin were measured in plasma using commercial ELISAs (Mercodia, Salem, NC and Thermo Scientific, Waltham, MA).
Bile Acid and Oxysterol Analysis
Samples of plasma, liver and intestinal tissue, and contents of gallbladder, small intestine, and proximal colon were analyzed for total bile acid content using an enzymatic colorimetric kit (Genway, San Diego, CA). Bile acid content relative to body weight was calculated for the liver, gallbladder contents, and total small intestine by multiplying the measured bile acid level by organ weight and dividing by body weight. Bile acid profiles on liver samples were analyzed by liquid chromatography-mass spectrometry (LC-MS/MS; Q-Exactive Orbitrap, Thermo Scientific) with a heated electrospray ionization (HESI) probe using internal standards of d9-chenodeoxycholic acid (CDCA) and d9-glyco-CDCA (Cambridge Isotopes, Andover, MA) to determine free and conjugated bile acid concentrations by reverse isotope dilution as described previously (25). Bile acid pool hydrophobicity was estimated by multiplying bile acid species fractions by hydrophobicity indices estimated by Heuman (26).
For oxysterol analysis, 500 mg tissue were homogenized with 200 μL methanol, 400 μL dichloromethane, and 100 μL LCMS grade water and mixed with an appropriate amount of internal standard mixture [d7-22(S)hydroxy cholesterol and d7-7α-hydroxycholeste-3-one]. Samples were centrifuged and the dichloromethane layer was collected and dried under nitrogen vacuum. This was reconstituted with methanol and LCMS grade water and vortexed. Oasis HLB solid phase extraction (SPE) Cartridges (Waters) were primed with 800 µL methanol and 600 µL LCMS grade water with 0.1% formic acid. Samples were then passed through the columns, the eluent discarded, and the column then washed with 600 μL LCMS grade water with 0.1% formic acid and then with 600 μL hexane. The oxysterol fraction was then eluted from the SPE Cartridge with butyl acetate. This fraction was then dried under vacuum and dissolved in mobile phase in preparation for analysis by LCMS. Chromatographic separation of oxysterols was achieved using Accela 1250 liquid chromatograph and a 150 × 4.6 mm, 2.7 u Ascentis Express C-18 (Sigma-Aldrich) analytical column maintained at 40°C. The mobile phase consisted of methanol (5 mM ammonium acetate) and water (5 mM ammonium acetate). Mass spectrometry analysis was performed using a Thermo Q-Exactive Focus high-resolution mass spectrometer equipped with the atmospheric pressure chemical ionization probe in full-scan mode.
Tissue Gene Expression
Quantitative real-time PCR was performed on samples of distal ileum, proximal jejunum, hypothalamus, liver, gallbladder, and skeletal muscle (longissimus dorsi) tissue. Total RNA was isolated using TRIzol reagent (Invitrogen) and a commercial kit (Qiagen). Total RNA analyzed for concentration and quality using a spectrophotometer (NanoDrop, Thermo Scientific) and subsequently diluted to 250 ng/μL. Reverse transcription was done using a commercial kit (Applied Biosystems). Real-time PCR was performed on a Bio-Rad CFX96 using PowerUp SYBR Green Master Mix (Applied Biosystems). Relative mRNA expression within each tissue was quantified relative to β-actin using the 2−ΔΔCT method. Relative mRNA expression was compared among tissues using mean β-actin expression within each gestational age (preterm vs. term) and day of sample collection (birth vs. day 3) using the 2−ΔΔCT method (see Table 1 for primers).
Table 1.
PCR primers
| Primer | Forward | Reverse |
|---|---|---|
| β-ACTIN | GGACCTGACCGACTACCTCA | GCGACGTAGCAGAGCTTCTC |
| CYP7A1 | GAAAGAGAGACCACATCTCGG | GAATGGTGTTGGCTTGCGAT |
| CYP27A1 | ACTGAAGACCGCCGATGAAAC | CAAAGGCGAATCAGGAAGGG |
| CYP4A21 | TTTTCCCGCTTGAGGAGTGC | ACTCGGTCTGTGTGTTGATGGA |
| CYP7B1 | AATACTTCCTCCCTTCTGCCC | GGGCAGAAGGGAGGAAGTATT |
| NTCP | ACTTTCGGAAACCTAAGGGACT | AAGAGCTTGCCCAGTGCAAAG |
| ILBP | CTCCTGCCTCATCCTTC | CATCGTAGTTCTTCTACTC |
| OST-α | TGTACAAGAACACTCGCTGC | GAACACACACACTATCGTGGG |
| ASBT | ATAATGGGATGCTGTCCAGG | TAGATTAAGAGGCACAGCGG |
| BSEP | TTTCATTCAGCGCCTGACCA | ACTCCAATGAGAGGGCTGAC |
| FGF19 | CTGGGCCGCACGTGCACTAC | GGGCCCGTCTGAGTGGATGCG |
| SHP | GCCTACCTGAAAGGGACCAT | CAACGGGTGTCAAGCCTTTA |
| FXR | TTTGTGTCGTTTGCGGAGAC | GTTGCCCCCATTTTTACACTTG |
| KI67 | AGTCTGTAAGGAAAGCCACCC | ACAAAAGCCAAGCAGACAGG |
| SOX9 | CGGTTCGAGCAAGAATAAGC | GTAATCCGGGTGGTCCTTCT |
| FGFR1 | GGTTGTAGCAGTATTGCAG | CGGACCTCTATATGTCATTG |
| FGFR4 | CTACAAGAAAAGAAGCAACG | TGTAGACTCTGTCAAACAAG |
| KLB | AAGGCCTTCCAAGACTACGC | GCGCTCGTAGATGTCACTCA |
| GATA-4 | CGGGAAGAAACTTGAAGTCG | TCCCATAAAATCCAGCACCT |
ASBT, apical sodium-dependent bile acid transporter; BSEP, bile salt export pump; CYP7A1, cytochrome P450 section 7 family A member 1; CYP27A1, cytochrome P450 family 27 subfamily A member 1; CYP4A21, cytochrome P450 family 4 subfamily A member 21; CYP7B1, cytochrome P450, family 7, subfamily B, member 1; FGF19, fibroblast growth factor 19; FGFR1, fibroblast growth factor receptor 1; FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; GATA-4, GATA binding protein 4; ILBP, ileal lipid binding protein; KI67, antigen KI-67; NTCP, Na-taurocholate cotransporting polypeptide; OST-α, organic solute transporter α; SHP, small heterodimer partner; SOX9, SRY-box transcription factor 9; KLB, β-klotho.
Liver tissue immunoblot analysis.
Western immunoblotting of frozen liver tissue was performed as described previously (27). The extracts (50 μg protein/lane) were separated via 10% SDS-PAGE; transferred to nitrocellulose membranes; and after blocking with 5% nonfat milk in tris-buffered saline (20 mM Tris, 150 mM NaOH, pH 7.4), incubated with a primary antibody diluted in 5% nonfat milk in Tris-buffered saline + 0.1% Tween-20. The membranes were probed with anti-cholesterol 7-hydroxylase [cholesterol 7-hydroxylase (CYP7A1)] (1:1,000, rabbit polyclonal antibody, Catalog Number RP1079; Boster Biological Technology, Pleasanton, CA), which produced a single band at approximately molecular weight 55 kDa. The loading control was anti-β-actin (1:10,000 dilution, mouse monoclonal antibody, Catalog Number A5316; Sigma-Aldrich, St. Louis, MO). Membranes were incubated with a secondary antibody (goat anti-mouse IgG-HRP, 1:5,000, Santa Cruz Biotechnology, Dallas, TX). Signal was developed by ECL-plus (Amersham Biosciences, Piscataway, NJ) and then detected by Bio-Rad Chemi Doc (Bio-Rad Laboratories, Hercules, CA), and the image was quantified by ImageQuant 5.0 software (Molecular Dynamics, Sunnyvale, CA). We expressed the abundances of specific target proteins relative to that of β-actin measured after stripping and reprobing membranes. The membranes were then incubated in the appropriate secondary horseradish peroxidase (HRP)-conjugated antibodies (Santa Cruz Biotechnology). The membrane was visualized on a CCD camera using a ChemiDoc XRS system (Bio-Rad). Gels were run with extracts from individual pigs in 6–8 lanes per group and relative abundance of band densities for each pig were used for statistical analysis of group means.
Intestinal Explant Culture
Ileal tissue from preterm (n = 7) and term (n = 8) pigs was collected immediately after euthanasia and placed in ice-cold sterile saline. Each tissue was divided into proximal, middle, and distal sections, which were opened along the mesenteric edge and were further divided into 15, 40- to 50-mg pieces. Each piece was placed into a well of a 6-well culture plate and incubated in a 3 mL basal medium consisting of culture media (Dulbecco modified Eagle’s medium, Sigma-Aldrich), 10% heat-inactivated fetal calf serum (Thermo Fisher), 100 μg/mL penicillin, 100 μg/mL streptomycin, and protease inhibitor (Sigma). Three explants per pig were treated with 100 μM chenodeoxycholic acid (CDCA), 15 μM obeticholic acid (OCA, Intercept Pharmaceuticals), or 15 μM DMSO (CTL) and incubated at 37°C for 2, 4, or 6 h. Immediately after incubation, media was collected and each explant was blotted dry, weighed, placed in TRIzol (Invitrogen) and stored at −80°C until RNA extraction and subsequent RT-PCR analysis. Media samples were stored at −20°C, then freeze-dried, reconstituted with 300 μL RNA-pure water, and analyzed for FGF19 protein by ELISA as described in Plasma Assays FGF19, Insulin, and Glucose. Explant experiments were performed using ileal tissue as described above from term (n = 4) neonatal piglets to assess the FXR agonism of HCA and HDCA. Tissues were incubated at 37°C in the basal medium as earlier, with added 15 μM DMSO (CTL), 15 μM OCA (OCA), 100 μM CDCA (CDCA), 100 μM HCA (HCA), and 100 μM HDCA (HDCA).
Hepatocyte Spheroid Cell Culture
Hepatocytes were isolated from term pigs and frozen at −80°C as described previously (28). Frozen hepatocytes were gently thawed and suspended in Matrigel (Corning) (330 cells/μL). The cell/Matrigel mixture was plated in a 96-well plate in 15 μL droplets and allowed to polymerize, inverted at 37°C for 5 min. Spheroids were maintained for at least 24 h at 37°C in complete media (DMEM, Thermo Fisher Scientific). Half of media in each well was replaced with serum-free media every 48 h. Spheroids were treated with DMSO (CTL), 50 µM hyocholic acid (HCA), 50 µM chenodeoxycholic acid (CDCA), 50 µM hyodeoxycholic acid (HDCA), 50 µM HCA and 50 µM CDCA(CDCA + HCA), and 50 µM HDCA and 50 µM CDCA (CDCA + HDCA) for 24 h and then RNA was extracted as described in Tissue Gene Expression.
Statistical Analysis
Data are presented as means ± SE. Statistical analysis was performed using R. Analysis of data generated from term and preterm piglets at birth and day 3 was analyzed by a two-way ANOVA with main effects of gestational age (GA) and day of life (Day) or time (Time). Data generated from explant and hepatocyte experiments were also analyzed by a two-way ANOVA with main effects of gestational age (GA) and treatment (TRT). CYP7A1 was regressed against plasma FGF19 at the time of tissue collection using Prism GraphPad 9.2.0. Tukey’s test was used for posttest separation of means. Plasma data and sow data were analyzed using Student’s t tests. Differences were considered statistically significant at P < 0.05.
RESULTS
Plasma FGF19 is Higher in Term Pigs but Plasma Bile Acids and Insulin Are Similar
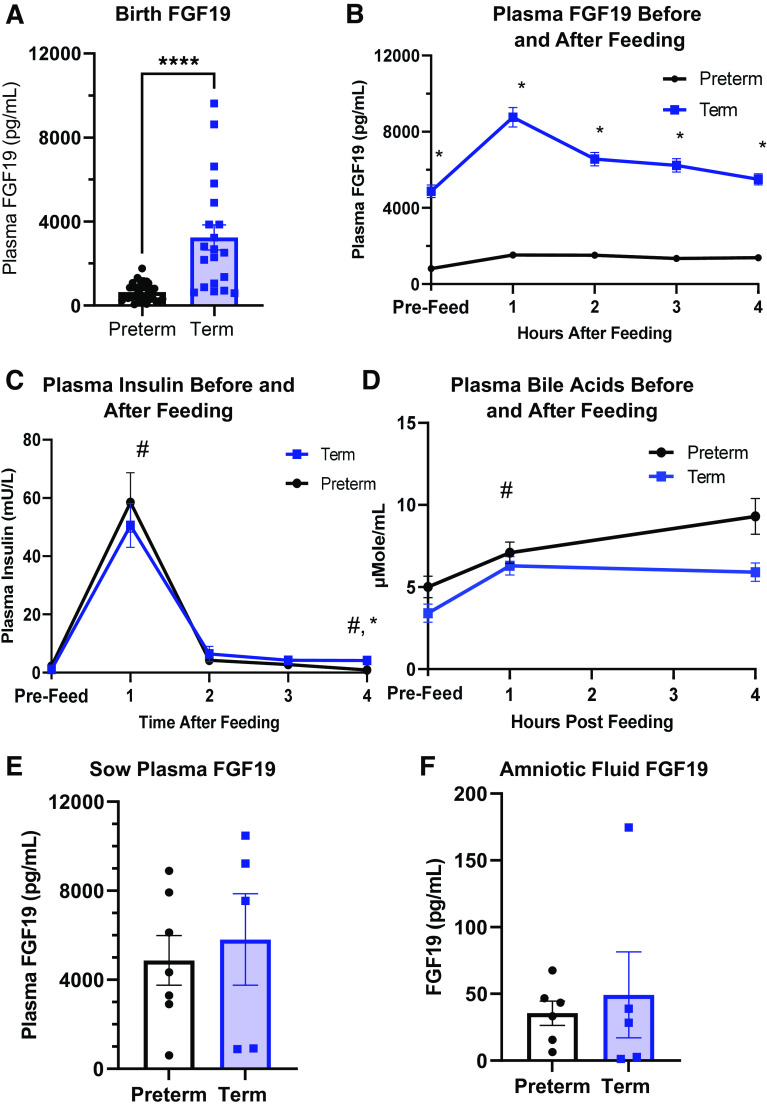
Plasma FGF19 was measured in both term and preterm pigs at birth and on day 3 of life after a bolus meal (Fig. 2, A and B). Plasma FGF19 at birth was approximately 10-fold higher in term (P < 0.001) than in preterm pigs. FGF19 levels remained higher in term pigs before and after feeding on day 3 than in preterm pigs (P < 0.001). On day 3 after a bolus feeding, plasma FGF19 concentration increased 1 h, but this was not statistically significant [P (GA × Time) = 0.124], whereas in preterm pigs, FGF19 concentrations remained relatively unchanged. Plasma bile acid levels (Fig. 2D) were similar in preterm and term pigs immediately before bolus enteral feeding and similarly increase [P (Time) < 0.05] 1 h after feeding. However, at 4 h post enteral feed, plasma bile acid levels in term pigs was higher than preterm [P (GA × Time) < 0.05]. In both term and preterm pigs, plasma insulin levels (Fig. 2C) increased from prefeeding levels to the 1 h postfeeding levels and then decreased to prefeed levels from 2 to 4 h postfeed. Plasma FGF19 in sows (Fig. 2E) was higher than what was observed in piglets but was similar across gestational status. Amniotic fluid (Fig. 2F) contained trace amounts of FGF19 protein in similar levels in both preterm and term gestational age.
Figure 2.
Plasma fibroblast growth factor 19 (FGF19), bile acids, and insulin in piglets at birth and after feeding and sow plasma and amniotic fluid FGF19. A: plasma FGF19 levels were approximately 10 times greater in term pigs than in preterm pigs at birth (n = 33 preterm and 23 term). B: plasma FGF19 levels were greater in term pigs on day 3 [P (GA) <0.001] (preterm, n = 17; term, n =13), but levels within gestational age (GA) groups were similar across time postfeeding [P (GA × Hour) >0.05]. C: total bile acid level in plasma of preterm and term pigs before and after feeding on day 3 of life (preterm, n = 17; term, n =13). D: plasma insulin levels in preterm and term pigs before and after feeding on day 3 of life [P (Hour) < 0.05, P (GA) >0.05] (preterm, n = 14; term, n = 13). E: plasma levels of FGF19 in sows immediately before cesarean section at term or preterm gestation (n = 5–7). F: FGF19 levels in amniotic fluid collected during cesarean section (n = 5–6) of term and preterm piglets. Data are shown as means ± SE. #P (Hour) <0.05; *P (GA) < 0.05; ****P (GA) <0.0001.
Total Bile Acid Pool Size is Lower in Preterm Than in Term Pigs
Total bile acid levels were measured in three different tissue pools: liver tissue and gallbladder contents, small intestinal tissue and luminal contents, and total combined tissue bile acid levels which includes liver, gallbladder contents, small intestinal tissue and contents, and proximal colon contents, expressed relative to pig body weight (Fig. 3A). Total bile acid levels in the liver and gallbladder were higher in term than in preterm piglets at birth and on day 3 [P (GA × Day) <0.01]. Small intestinal bile acids were low at birth [P (GA) >0.05] but similarly increased at day 3 in both preterm and term piglets [P (Day) >0.05]. The total bile acid pool was similar in preterm and term piglets at birth but higher in term pigs on day 3 [P (GA × Day) = 0.019].
Figure 3.
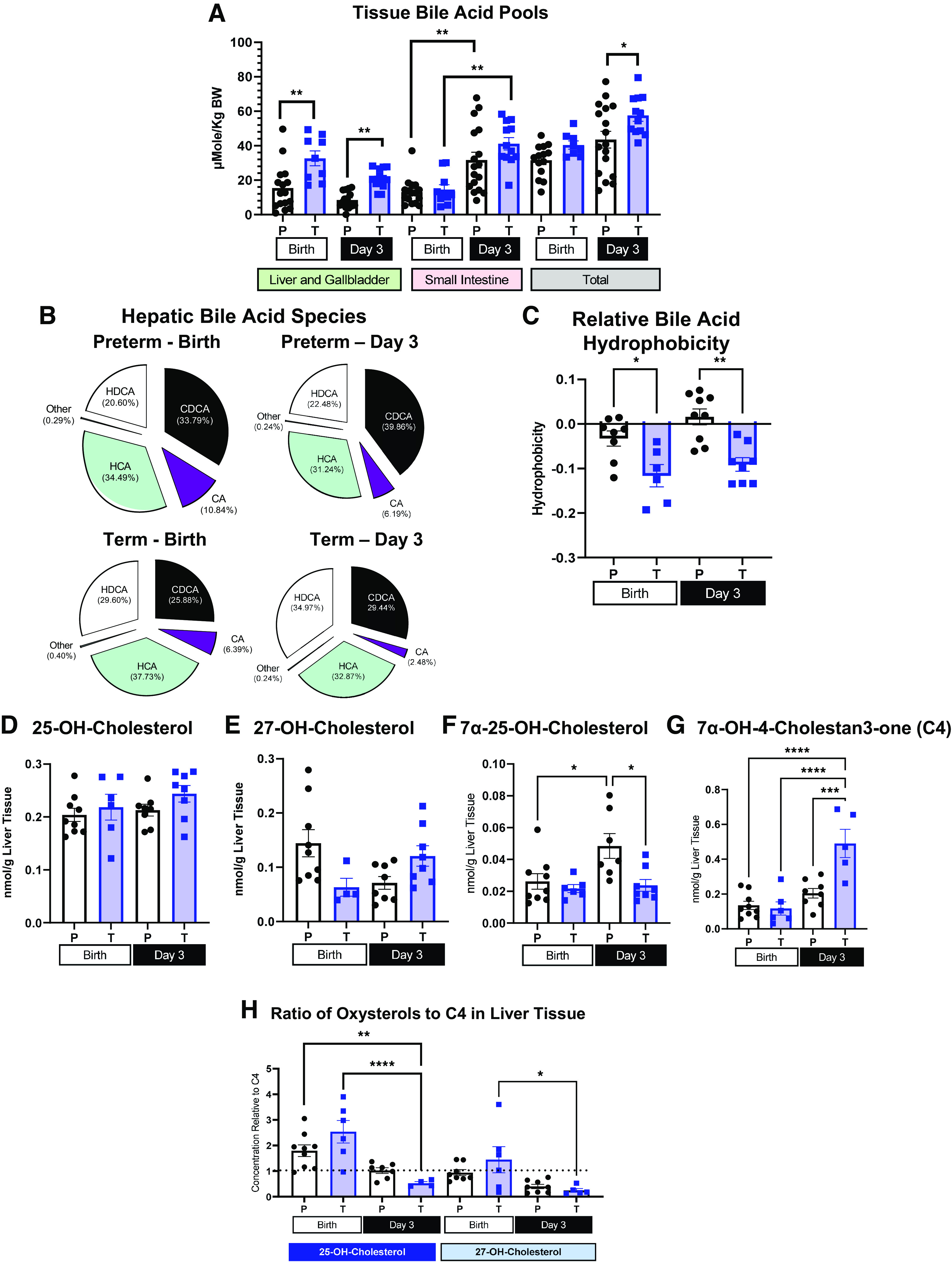
Tissue total bile acid pool, relative bile acid species, estimate of hydrophobicity of bile acid pool, oxysterol content, and relative abundance in liver tissue. A: total bile acid content of liver and gallbladder contents, small intestine tissue, and luminal contents and total (liver and gallbladder contents, small intestinal tissue and luminal contents, and luminal contents of proximal colon) tissue bile acid pool in preterm (P) and term (T) pigs at birth and day 3 (Preterm-Birth, n = 16, Preterm-Day 3, n = 17, Term-Birth, n = 10, Term-Day 3, n = 13) represented by means ± SE. B: proportion of bile acid species, HDCA (hyodeoxycholic), HCA (hyocholic acid), CDCA (chenodeoxycholic acid), CA (cholic acid), and other (lithocholic acid and ursodeoxycholic acid) in livers of preterm (P) and term (T) piglets at birth and day 3 (Preterm-Birth, n = 9, Preterm-Day 3, n = 9, Term-Birth, n = 6, Term-Day 3, n = 8). C: estimate of relative hepatic bile acid pool hydrophobicity in liver tissue of preterm (P) and term (T) pigs at birth and day 3 (Preterm-Birth, n = 9, Preterm-Day 3, n = 9, Term-Birth, n = 6, Term-Day 3, n = 8). D–G: concentration (nmol/g liver tissue) of 25-OH-cholesterol (D), 27-OH-cholesterol (E), 7α-25-dihydroxy-cholestrol (F), 7α-OH-cholesten-3-one (C4) (G) in preterm (P) and term (T) pigs at birth and day 3. H: ratio of 25-OH-cholesterol and 27-OH-cholesterol to 7α-OH-cholesten-3-one (C4) in liver tissue. Preterm-Birth, n = 9, Preterm-Day 3, n = 9, Term-Birth, n = 6, Term-Day 3, n = 5. Data are shown as means ± SE. *P (GA × Day) < 0.05), ***P (GA × Day) < 0.001, ****P (GA × Day) < 0.0001. BW, body weight.
The profile of conjugated and unconjugated bile acids was measured in the liver of preterm and term pigs at birth and after feeding on day 3 of life (Table 2). The majority of bile acids were glycine conjugated in both term and preterm pigs, but the overall proportion of glycine-conjugated bile acids was higher in term than preterm pigs [P (GA × Day) < 0.05]. Levels of conjugated and unconjugated bile acids were combined by bile acid species and presented in Fig. 3B. Figure 3B shows the relative proportion of conjugated and unconjugated cholic acid, chenodeoxycholic acid, hyocholic acid, and hyodeoxycholic acid in term and preterm livers at birth and after feeding on day 3. Lithocholic and ursodeoxycholic acids were found in negligible amounts relative to other bile acids and are, therefore, represented as “Other” in each pie chart. Cholic acid levels, regardless of conjugation status, were highest at birth in preterm pigs and lowest on day 3 in term piglets [P (GA × Day) < 0.05)]. There was also a higher proportion of conjugated and unconjugated hyodeoxycholic acid in term liver tissue compared with preterm liver on day 3 [P (GA × Day) < 0.05]. However, there was higher conjugated and unconjugated CDCA in preterm compared with term livers on day 3 [P (GA × Day) < 0.05]. The estimated hepatic bile acid pool hydrophobicity (Fig. 3C) was higher in preterm pigs at birth and day 3 compared with term pigs at birth and day 3 [P (GA × Day) < 0.05].
Table 2.
Hepatic bile acid profile
| Preterm Birth | Term Birth | Preterm Day 3 | Term Day 3 |
P
|
|||
|---|---|---|---|---|---|---|---|
| GA | Day | GA × Day | |||||
| CA | BDL | BDL | 0.635 ± 0.022 | 0.075 ± 0.001 | n/a | n/a | n/a |
| CDCA | 0.194 ± 0.030 | 0.062 ± 0.001 | 2.055 ± 0.113 | 0.151 ± 0.020 | 0.150 | 0.120 | 0.208 |
| HCA | 0.085 ± 0.001 | BDL | 2.550 ± 0.130 | 0.458 ± 0.130 | 0.256 | 0.069 | 0.256 |
| HDCA | 0.392 ± 0.070 | 0.120 ± 0.030 | 3.380 ± 0.182 | 1.996 ± 0.800 | 0.564 | 0.043 | 0.646 |
| DCA | BDL | BDL | BDL | BDL | n/a | n/a | n/a |
| LCA | 0.007 ± 0.001 | BDL | BDL | BDL | n/a | n/a | n/a |
| UDCA | BDL | BDL | BDL | 1.076 ± 0.290 | n/a | n/a | n/a |
| Total UN-CONJ | 0.666 ± 0.095 | 0.181 ± 0.035 | 8.054 ± 4.193 | 2.884 ± 1.065 | 0.298 | 0.059 | 0.394 |
| G-CA | 8.56 ± 1.39 | 6.80 ± 1.01 | 5.32 ± 0.45 | 3.93 ± 1.24 | 0.142 | 0.014 | 0.878 |
| G-CDCA | 20.53 ± 4.06 | 19.99 ± 3.52 | 29.31 ± 0.22 | 35.91 ± 6.24 | 0.373 | 0.015 | 0.447 |
| G-HCA | 33.82 ± 7.39 | 41.58 ± 6.13 | 26.440 ± 2.01 | 47.115 ± 1.09 | 0.074 | 0.821 | 0.422 |
| G-DHCA | 18.93 ± 3.95 | 32.541 ± 5.55 | 18.460 ± 2.95a | 42.547 ± 3.82b | <0.001 | 0.348 | 0.233 |
| G-DCA | BDL | BDL | BDL | BDL | n/a | n/a | n/a |
| G-LCA | 0.127a ± 0.001 | 0.184 ± 0.002b | 0.122 ± 0.001 | 0.150 ± 0.010 | <0.001 | 0.075 | 0.151 |
| G-UCDA | 0.176 ± 0.030 | 0.350 ± 0.004 | 0.185 ± 0.030 | 0.272 ± 0.080 | 0.029 | 0.288 | 0.176 |
| Total G-CONJ | 85.65 ± 17.70 | 101.45 ± 9.87 | 79.80 ± 6.30 | 129.85 ± 12.02 | 0.040 | 0.566 | 0.305 |
| T-CA | 3.44 ± 0.65 | 1.83 ± 0.24 | 1.75 ± 0.26 | 1.19 ± 0.38 | 0.026 | 0.016 | 0.288 |
| T-CDCA | 20.82 ± 4.65 | 16.27 ± 2.36 | 15.21 ± 1.89 | 17.13 ± 3.64 | 0.741 | 0.457 | 0.397 |
| T-HCA | 9.60 ± 3.18 | 12.25 ± 5.24 | 7.779 ± 0.89 | 15.81 ± 3.97 | 0.133 | 0.889 | 0.465 |
| T-HDCA | 6.69 ± 1.76 | 9.29 ± 1.90 | 6.46 ± 1.12 | 12.77 ± 1.84 | 0.013 | 0.445 | 0.309 |
| T-DCA | BDL | BDL | BDL | BDL | n/a | n/a | n/a |
| T-LCA | 0.003 ± 0.001 | 0.234 ± .020 | BDL | 0.027 ± 0.001 | n/a | n/a | n/a |
| Total T-CONJ | 42.70 ± 10.96 | 39.79 ± 7.57 | 31.19 ± 3.16 | 46.91 ± 9.45 | 0.421 | 0.723 | 0.306 |
| Total | 129.02 ± 28.52 | 141.42 ± 16.89 | 119.05 ± 11.15 | 179.64 ± 29.82 | 0.125 | 0.647 | 0.346 |
Data are shown as means ± SE in units—nmol/g liver tissue. Bile acid profile (nmol/g liver tissue), including unconjugated (UN-CONJ), taurine conjugated (T-CONJ), and glycine conjugated (G-CONJ) species, of liver tissue of preterm (P) and term (T) pigs at birth and day 3 (Preterm-Birth, n = 9, Preterm-Day 3, n = 9, Term-Birth, n = 6, Term-Day 3, n = 8). BDL, below detectable limits; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GA, gestational age; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; n/a, not applicable; UDCA, ursodeoxycholic acid. a,bP (GA × Day) < 0.05.
Hepatic Oxysterol Profile Shows Higher Reliance on Alternative Pathway in Preterm Pigs
Levels of oxysterols from the alternative pathway of bile acid synthesis 27-OH-cholesterol, 25-OH cholesterol, and 7α-25-dihydroxy-cholestrol are presented in Fig. 3, D–F. Levels of 25-OH- and 27-OH-cholesterol were similar in preterm and term pigs; however, the level of 7α-25-dihydroxy-cholestrol was greater in preterm pigs on day 3 [P (GA × Day) < 0.05]. 7α-OH-4-cholesten-3-one (C4) (Fig. 3G) is an intermediate in the classic pathway of bile acid synthesis, and C4 levels were highest in term pigs on day 3 of life [P (GA × Day) < 0.05]. The relative activity of the alternative pathway compared with the classic pathway was compared by calculating the ratio of alternative pathway intermediates to C4 in preterm and term pigs at birth and day 3 (Fig. 3H). The ratios of 25-OH- and 27-OH-cholesterol to C4 was higher in preterm pigs at birth and day 3 and term pigs at birth compared with term pigs at day 3 of life, indicating a greater reliance on the alternative pathway in term pigs at birth, and preterm pigs from birth to day 3 compared with term pigs after feeding on day 3 [P (GA × Day) < 0.05].
Bile Acid Synthesis Enzyme Gene Expression
Differing hepatic bile acid and oxysterol profiles prompted analysis of the relative expression of bile acid synthesis enzymes in the liver. CYP7A1 is the rate-limiting step in hepatic bile acid synthesis via the classic pathway, which predominates bile acid synthesis in healthy adult liver tissue (29). In healthy adult liver tissue, the expression of CYP7A1 is normally downregulated by FGF19 (30), yet we found that the expression across gestational age and day of life was similar, in spite of markedly different plasma FGF19 concentrations (Fig. 4A). In addition, the relative abundance of CYP7A1 did not differ in liver of preterm or term piglets on day 3 (Fig. 4K). Cytochrome P450 family 27 subfamily A member 1 (CYP27A1) is normally expressed intra- and extrahepatically; it is the first step of the alternative pathway in mitochondria in intra- and extrahepatic tissues and catalyzes side chain oxidation intrahepatically in the classic pathway. Expression levels of CYP27A1 (Fig. 4F) were similar in pigs of different gestational ages and on different days of life [P (GA × Day) > 0.05]. Cytochrome P450 family 3 sub A member 29 (CYP3A29) is the porcine homolog of cytochrome P450 family 3 sub A member 4, which catalyzes the first step of an additional subpath in the alternative bile acid synthesis pathway (31). CYP3A29 expression (Fig. 4G) did not differ in the liver tissue of preterm and term pigs at birth and day 3 [P (GA × Day) >0.05]. The cytochrome P450, family 7, subfamily B, member 1 (CYP7B1) gene encodes an enzyme unique to the alternative bile acid synthesis pathway that can occur in both liver and in peripheral tissues (29). The relative expression of CYP7B1 in liver tissue (Fig. 4H) was similar across gestational age and day of life in neonatal piglets [P (GA × Day) > 0.05]. Cytochrome P450 family 4 subfamily A member 21 (CYP4A21) catalyzes the formation of hyocholic acid from chenodeoxycholic acid. The relative expression of CYP4A21 (Fig. 4I) was higher in term pigs at birth than term day 3 or preterm pigs at birth [P (GA × Day) < 0.05]. This observation supports the finding of higher levels of hyocholic acid species in term pigs at birth, compared with cholic acid or CDCA. The relative expression of all bile acid synthetic genes in preterm and term pigs during the perinatal period demonstrated that there is a much higher overall expression of CYP27A1 and CYP4A21 than CYP7A1 and CYP7B1 (Fig. 4J).
Figure 4.
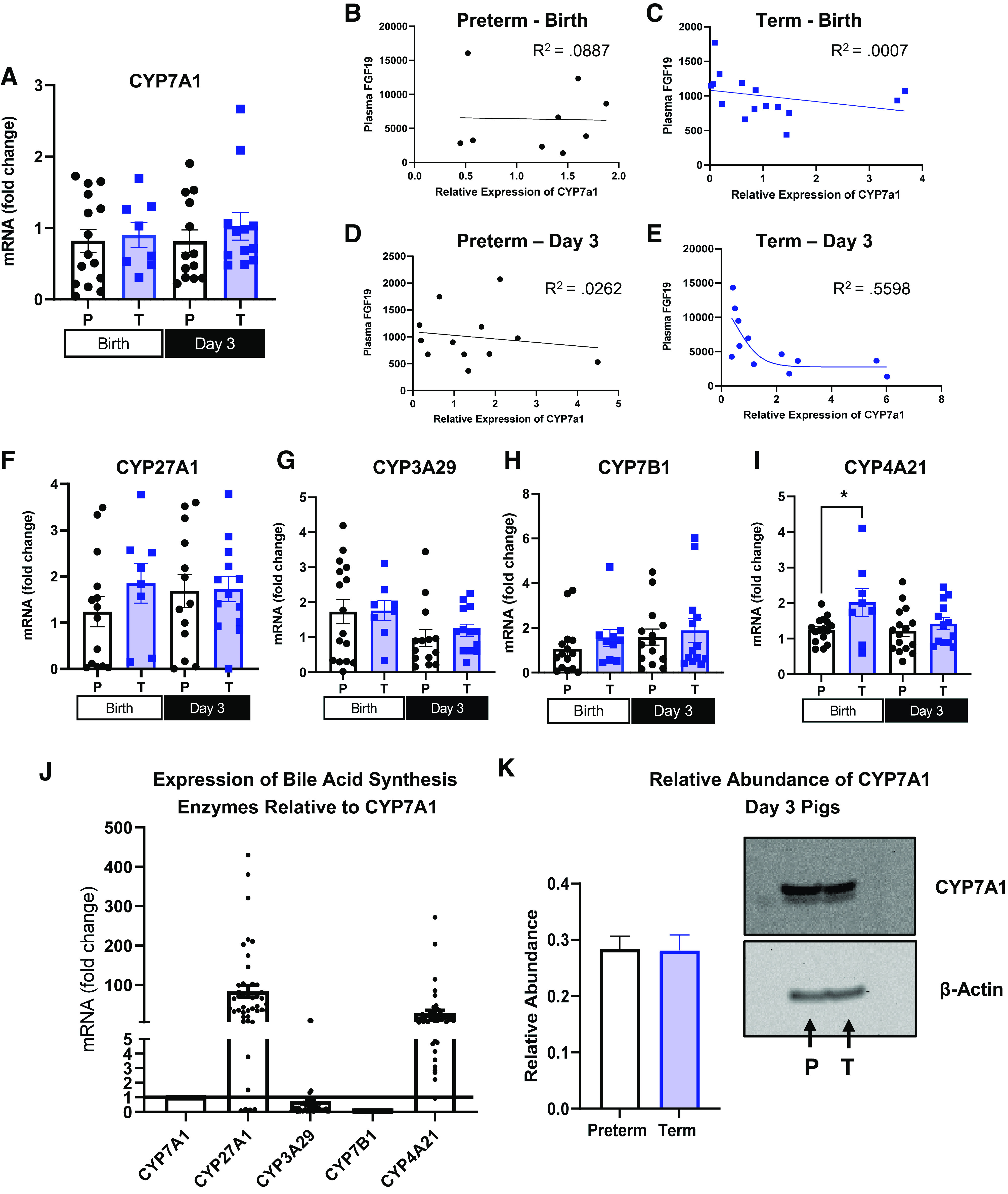
Relative expression of bile acid synthesis enzymes in liver and regression of plasma fibroblast growth factor 19 (FGF19) and expression of cytochrome P450 section 7 family A member 1 (CYP7A1). A and F–I: relative mRNA expression of CYP7A1, CYP3A29, CYP27A1, CYP7A1 and CYP4A21 in the liver tissue of term and preterm pigs at birth and on day 3 (Preterm-Birth, n = 16, Preterm-Day 3, n = 17, Term-Birth, n = 10, Term-Day 3, n = 13). J: overall mRNA expression of CYP7B1, CYP27A1, and CYP4A21 relative to CYP7A1 in neonatal pigs (n= 56). B–E: regression analysis of plasma FGF19 and relative hepatic expression of CYP7A1 in preterm pigs harvested at birth (B) (n = 16), term pigs harvested at birth (C) (n = 10), preterm pigs harvested on day 3 (D) (n = 17), and term pigs harvested on day 3 (E) (n = 17). K: protein abundance of CYP7A1 expressed relative to β-actin quantified from preterm (n = 17) and term (n = 17) liver homogenates (left) and representative immunoblots from pooled liver homogenates from pigs on day 3 of life (P = Preterm; T = Term) (right). Data are shown as means ± SE. *P (GA × Day) < 0.05. CYP3A29, cytochrome P450 family 3 sub A member 29; CYP27A1, cytochrome P450 family 27 subfamily A member 1; CYP4A21, cytochrome P450 family 4 subfamily A member 21; CYP7B1, cytochrome P450, family 7, subfamily b, member 1.
Plasma FGF19 Regression with CYP7A1
Plasma FGF19 was regressed against the relative expression of CYP7A1 (Fig. 4, B–E), because we expected a negative relationship between FGF19 and the expression of CYP7A1, yet there was no correlation in either term or preterm pigs at birth (R2 = 0.00074 and 0.008) or preterm pigs at day 3 (R2 = 0.026). However, in term pigs on day 3, there was a significant negative correlation between FGF19 and hepatic CYP7A1 expression (R2 = 0.5598).
Bile Acid Transporter Gene Expression
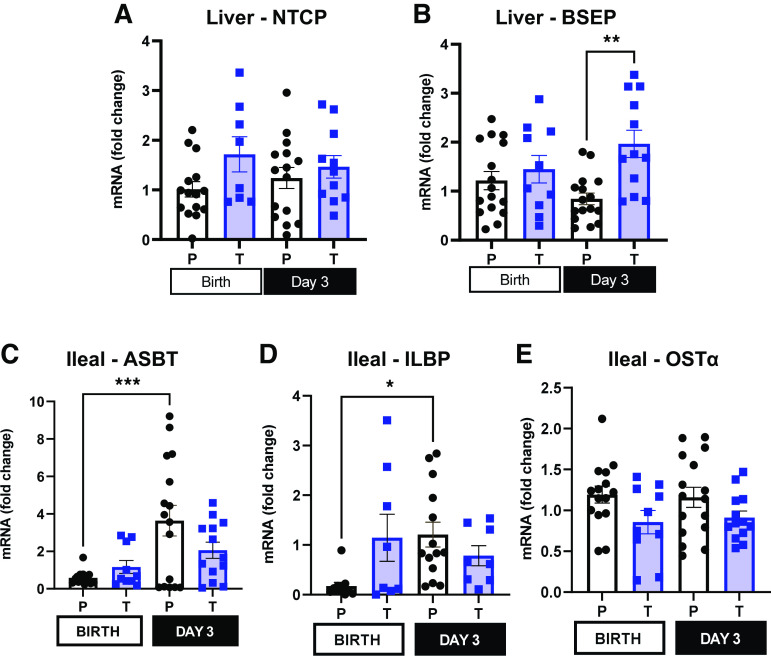
Another cellular process that can influence the bile acid pool size in term and preterm piglets is the relative expression of bile acid transporters. The relative expression of transcellular hepatic bile acid transporter Na-taurocholate cotransporting polypeptide (NTCP) (Fig. 5A) was similar in preterm and term pigs at birth and on day 3 [P (GA × Day) >0.05]. However, the expression of bile salt export pump (BSEP) (Fig. 5B) responsible for hepatobiliary transport of bile acids was higher in term pigs after feeding on day 3 [P (GA × Day) <0.01]. The finding of increased BSEP expression may explain the higher gut bile acid pool observed in term pigs on day 3.
Figure 5.
Bile acid transporter expression in liver and ileum. A and B: relative mRNA expression of Na-taurocholate cotransporting polypeptide (NTCP) and bile salt export pump (BSEP) in liver tissue of term and preterm pigs at birth and on day 3. C–E: relative mRNA expression of apical sodium-dependent bile acid transporter (ASBT), ileal lipid binding protein (ILBP), organic solute transporter α (OST-α) in ileal tissue of term and preterm pigs at birth and on day 3 of life (Preterm-Birth, n = 16, Preterm-Day 3, n = 17, Term-Birth, n = 10, Term-Day 3, n = 13). Data are shown as means ± SE. *P (GA × Day) < 0.05), **P (GA × Day) < 0.01, ***P (GA × Day) < 0.001.
In the gut, apical sodium-dependent bile acid transporter (ASBT) is responsible for transporting bile acids into ileal enterocytes from the intestinal lumen. The relative expression of ASBT was lower in preterm pigs at birth, compared with day 3 [P (GA × Day) < 0.001], but the expression in term pigs was similar, regardless of day (Fig. 5C). Ileal lipid binding protein (ILBP), a cytoplasmic protein that is responsible for intraepithelial transport of bile acids in the intestine (32), is more abundantly expressed in ileal tissue (Fig. 5D) of preterm pigs on day 3 compared with preterm pigs at birth [P (GA × Day) < 0.05]. Ileal organic solute transporter α (OST-α) facilitates basolateral transport of bile acids out of ileal enterocytes. The relative ileal expression of OST-α (Fig. 5E) was similar in pigs of different gestational ages and on different days of life.
Hepatic Spheroid and Ileal Explant Bile Acid FXR Agonism
We performed cell culture experiments to measure hepatic and ileal tissue responsiveness of FXR to HCA and HDCA, since these are primary bile acids found in pigs. The relative expression of bile acid transporters and synthesis enzymes in hepatic spheroids treated with HCA, HDCA, and CDCA is presented in Fig. 6, A–D. CDCA treatment resulted in higher expression of BSEP (Fig. 6A) and OST-α (Fig. 6B), but HCA and HDCA treatment had no effect compared with control. The expression of CYP7A1 (Fig. 6C) was downregulated by the addition of CDCA. However, the addition of HCA and HDCA did not affect CYP7A1 expression, either alone or in addition to CDCA, yet CYP27A1 (Fig. 6D) was not affected by bile acid treatment. In ileal explants (Fig. 6E), FGF19 expression was increased 2- and 2.4-fold, respectively, after 4-h treatment with OCA and CDCA [P (TRT) < 0.05]. However, the FGF19 expression was not different between explants treated with either HCA or HDCA compared with control [P (TRT) > 0.05]. Thus, HCA and HDCA dominate the bile acid pools of both preterm and term pigs, but these experiments confirmed that HCA and HDCA are poor FXR agonists.
Figure 6.
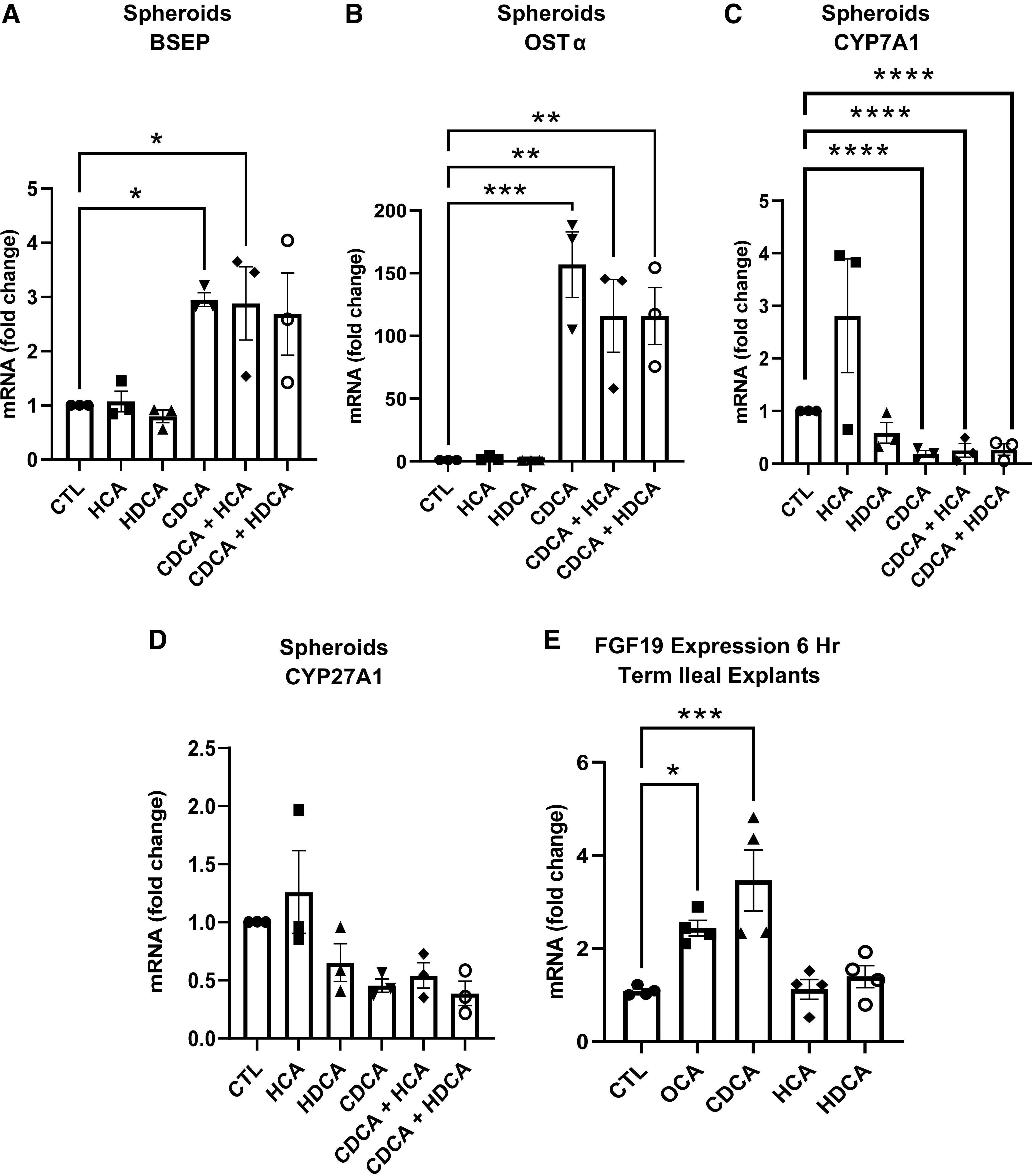
Hepatic spheroid and ileal explant bile acid farnesoid X receptor (FXR) agonism. A–D: relative mRNA expression of BSEP, OSTα, CYP7A1, and CYP27A1 in hepatic spheroids treated with DMSO (CTL), 50 µM hyocholic acid (HCA), 50 µM hyodeoxycholic acid (HDCA), 50 µM chenodeoxycholic acid (CDCA), 50 µM hyocholic acid and 50 µM chenodeoxycholic acid (CDCA + HCA), and 50 µM hyodeoxycholic acid and 50 µM chenodeoxycholic acid (CDCA + HDCA). E: relative expression of fibroblast growth factor 19 (FGF19) in ileal explants from term piglets incubated for 6 h in control (CTL, 15 µM DMSO), 15 µM obeticholic acid (OCA), 100 µM CDCA, 100 µM HCA, or 100 µM hyodeoxycholic acid (n =4). Hepatic spheroids were made from hepatocytes isolated from 3 term piglets at day 2 of age. Data are shown as means ± SE. *P (GA × Day) < 0.05, **P (GA × Day) < 0.01, ***P (GA × Day) < 0.001, ****P (GA × Day) < 0.0001. BSEP, bile salt export pump; CYP7A1, cytochrome P450 section 7 family A member 1; CYP27A1, cytochrome P450 family 27 subfamily A member 1; OST-α, organic solute transporter α.
Intestinal FXR Target Gene Expression
The intestinal expression of small heterodimer partner (SHP) and FGF19 was examined to assess direct downstream signaling of the farnesoid X receptor (FXR), the transcription factor signaled by bile acids in the intestine and responsible for transcription of signaling molecules involved in bile acid regulation. The expression of FGF19 (Fig. 7, A and B) was similar in both the jejunum and ileum in preterm and term pigs at birth [P (GA × Day) > 0.05]. However, after feeding on day 3, the expression of FGF19 was lower in preterm piglets in both jejunum and ileum [P (GA × Day) < 0.05]. SHP expression (Fig. 7, C and D) was highest in the term jejunum on day 3 after bolus feeding, compared with preterm day 3 pigs and term pigs harvested at birth [P (GA × Day) < 0.05]. However, expression of SHP was similar in the ileum.
Figure 7.
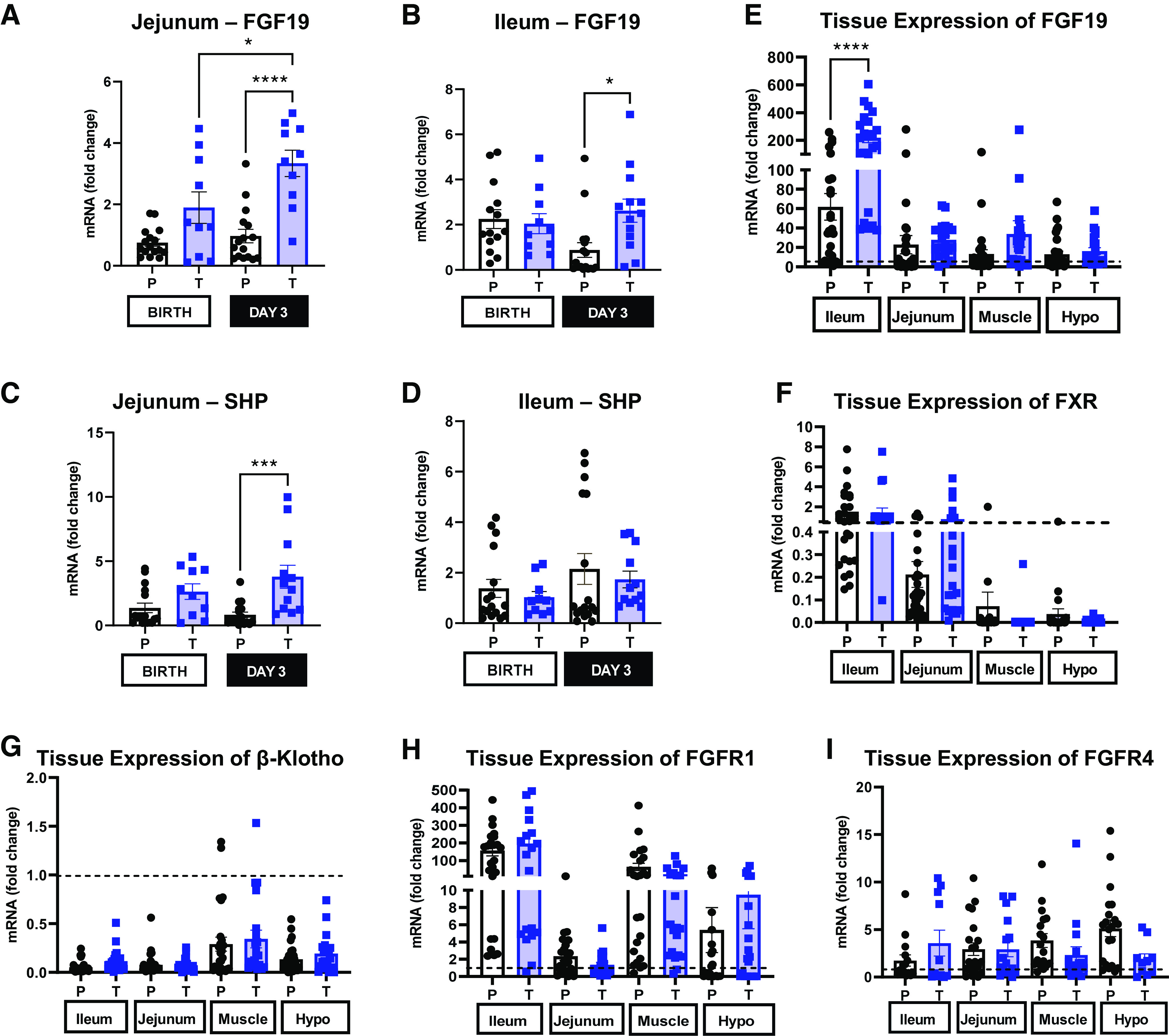
Relative expression of fibroblast growth factor 19 (FGF19) and small heterodimer partner (SHP) in jejunum and ileum, and tissue expression of FGF19, farnesoid X receptor (FXR), β-klotho, fibroblast growth factor receptor 1 (FGFR1) and FGFR4 relative to liver. A–D: relative mRNA expression of FGF19 and SHP in the ileum and jejunum of term and preterm pigs at birth and on day 3 (Preterm-Birth, n = 16, Preterm-Day 3, n = 17, Term-Birth, n = 10, Term-Day 3, n = 13). E–I: tissue mRNA expression of FGF19, FXR, β-klotho, FGFR1, and FGFR4 in ileum, jejunum, muscle, and hypothalamus in term and preterm pigs expressed relative to liver expression; the dashed line is set to 1 and represents hepatic expression [P (Tissue) <0.05)]. Preterm, n = 33, Term, n= 23. All graphs are means ± SE. Data are shown as means ± SE. *P (GA × Day) < 0.05), ***P (GA × Day) < 0.001, ****P (GA × Day) < 0.0001.
Relative tissue expression of FGF19 and FXR (Fig. 7, E and F) in preterm and term pigs at birth and day 3 was measured in specific tissues. FGF19 expression is highest in the distal ileum [P (Tissue) > 0.001], but it is also expressed in the jejunum, muscle, and hypothalamus. The relative FGF19 expression in all these was greater in the term ileum than in the preterm ileum [P < 0.05 (GA × Tissue)], but relative FGF19 expression was similar in term and preterm piglets in jejunum, muscle, and hypothalamus relative to the liver [P > 0.05 (GA)]. Relative tissue expression of FXR is highest in the distal ileum, jejunum, and liver but similar across gestational age [P > 0.05 (GA)] and day [P < 0.05 (Day)]. FXR is expressed to a lesser degree in the skeletal muscle and hypothalamus compared with the distal ileum, jejunum, and liver.
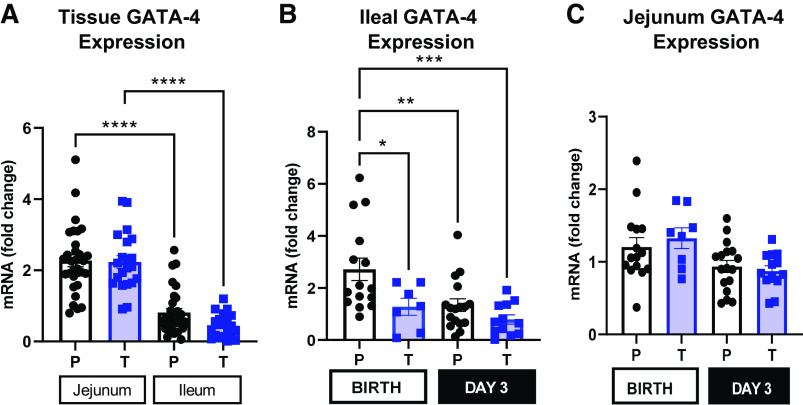
Intestinal GATA-4 Expression
GATA-4 expression (Fig. 8A) was higher in the jejunum than in the ileum of both preterm and term pigs [P (Tissue × GA) < 0.001]. However, the relative expression of GATA-4 in ileal tissue (Fig. 8B) was twofold higher in preterm pigs at birth compared with preterm pigs on day 3, as well as term pigs on day 3 [P (GA × Day) < 0.01]. Relative expression of GATA-4 in jejunum tissue (Fig. 8C) was similar across different gestational ages and days [P (GA × Day) > 0.05].
Figure 8.
Intestinal GATA binding protein 4 (GATA-4) expression. A: relative tissue expression of GATA-4 in jejunum and ileum of term and preterm pigs to localize the tissue of primary expression [n (Preterm) = 33; n (Term) = 23; P (Tissue × GA) <0.05]. B and C: relative expression of GATA-4 in jejunum and ileal tissue of term (T) and preterm (P) pigs at birth and on day 3 (Preterm-Birth, n = 16, Preterm-Day 3, n = 17, Term-Birth, n = 10, Term-Day 3, n = 13). All graphs are means ± SE. *P (GA × Day) < 0.05, **P (GA × Day) < 0.01, ***P (GA × Day) < 0.001, ****P (GA × Day) < 0.0001.
Ex Vivo Ileal Tissue Responsiveness to Bile Acids
To measure the tissue responsiveness to specific bile acids, tissue explants derived from ileal tissue collected from term and preterm pigs were cultured for 2, 4, and 6 h in the presence of obeticholic acid (OCA, a potent FXR agonist) and CDCA (a primary bile acid in pigs and humans). The primary measure was FGF19 explant tissue expression and culture media FGF19 protein concentration. In explants incubated for 2 h, FGF19 expression was four- to fivefold higher in term than in preterm ileal explant tissue but did not statistically differ by bile acid treatment (Fig. 9A). At 4 h (Fig. 9B), the basal expression of FGF19 in control explants was 6.4-fold higher in tissue from term pigs compared with preterm. Importantly, in term pigs, the FGF19 expression from both CDCA and OCA-treated 4 h explants was 10- and 13-fold higher than control explants [P (TRT) < 0.05)], yet FGF19 expression was unaffected by bile acid treatment in explants from preterm pigs [P (TRT) > 0.05]. At 6 h (Fig. 9C), the FGF19 expression in term explants treated with OCA for 6 h was ninefold higher and those treated with CDCA was eightfold higher than control [P (TRT) < 0.05)]. FGF19 protein (Fig. 9D) was highest in media from explants incubated with OCA for 6 h [P (TRT × GA] <0.05], compared with OCA-treated preterm tissue, and nontreated term tissue. There were no detectable levels of FGF19 in medium from explants incubated for 2 or 4 h.
Figure 9.
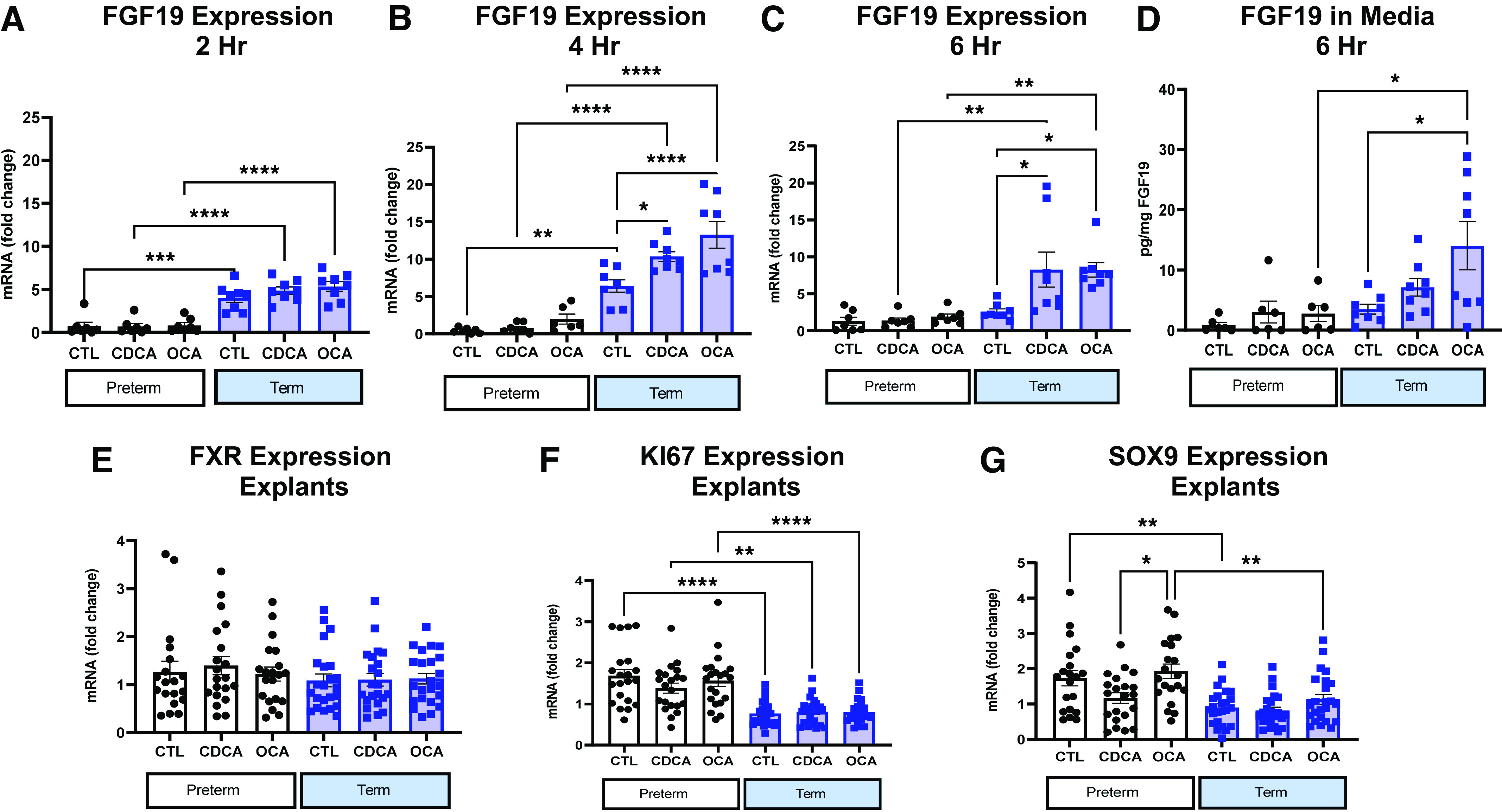
Fibroblast growth factor 19 (FGF19) protein secretion and gene expression in ileal explant model. A–C: relative mRNA expression of FGF19 in ileal explants incubated in control (CTL), chenodeoxycholic acid (CDCA), or obeticholic acid (OCA) for 2 (B), 4 (C), and 6 (D) h (Preterm, n = 7, Term, n = 8) at 37°C. D: FGF19 protein in media from term and preterm ileal explants incubated in CTL (15 µM DMSO), 100 µM CDCA, or 15 µM OCA incubated for 6 h at 37°C (Preterm, n = 7, Term, n = 8). E–G: relative mRNA expression of farnesoid X receptor (FXR; E), KI67 (F), and SRY-box transcription factor 9 (SOX9; G) in 2, 4, and 6 h explants from term (n = 21) and preterm (n = 24) pigs. All graphs are means ± SE. *P (GA × Day) < 0.05), **P (GA × Day) < 0.01, ***P (GA × Day) < 0.001, ****P (GA × Day) < 0.0001.
Relative expression of FXR (Fig. 9E) was similar across explants, regardless of gestational age of the originating tissue, bile acid treatment, or incubation time [P (GA × TRT) > 0.05]. Antigen KI-67 (KI67) expression (Fig. 9F) was greater in preterm explants at all incubation timepoints regardless of treatment or incubation time. Preterm untreated and explants treated with OCA had twofold greater expression of SRY-box transcription factor 9 (SOX9; Fig. 9G) than term explants or preterm explants treated with CDCA [P (GA × TRT) < 0.05].
Tissue FGF19 Receptor Profiles
Fibroblast growth factor receptor 1 (FGFR1; Fig. 7H) and fibroblast growth factor receptor 4 (FGFR4; Fig. 7I) are the primary cellular receptors that mediate FGF19 signaling (33) along with coreceptor β-klotho (Fig. 7G). Relative expression of receptors in ileum, jejunum, muscle, and hypothalamus relative to liver was similar in preterm and term pigs [P (GA × Tissue) > 0.05]. FGFR1 is primarily expressed in the muscle, ileum, and the hypothalamus [P (tissue) < 0.05] and expressed to a lesser degree in the jejunum. The expression of FGFR4 was similar in the ileum, jejunum, muscle, and hypothalamus relative to the liver [P (Tissue) > 0.05]. As expected, β-klotho expression was highest in the liver [P (Tissue) < 0.05] compared with the ileum, jejunum, muscle, and hypothalamus.
DISCUSSION
The aim of the current study was to quantify the relationship between bile acid pool size and plasma FGF19 and identify the underlying tissue-specific molecular basis of gut-liver FXR-FGF19 signaling in preterm and term pigs. Our previous report showing the markedly lower circulating FGF19 in preterm compared with term pigs implied a rapid perinatal upregulation of gut FXR-FGF19 activity. Yet, the molecular characterization of this important signaling pathway is poorly understood in species, such as humans and pigs, which express FGF19 rather than the FGF15 ortholog. Our results show that the size of the hepatic bile acid pool was significantly lower (∼50%) in preterm than term pigs, both at birth and 3 days postnatal. We also show that the gut bile acid pool increased significantly (∼2-fold) between birth and 3 days of age with feeding in both preterm and term pigs, but our explant culture model showed that ileal tissue in preterm pigs is significantly less responsive to bile acid than term pigs.
Our finding of a smaller hepatic bile acid pool in preterm compared with term pig is consistent with previous reports in infants. Watkins et al. (34) used stable isotope kinetics of duodenal bile samples to show that pool size and synthesis of both CA and CDCA in preterm infants were 30%–50% lower than in term infants (35, 36). Fetal bile acid pools are smaller than those measured in neonatal and adult humans, rodents, dogs, and sheep (37–40). This follows from the limited necessity of bile acids for fat digestion in the fetus, since the fat content of swallowed amniotic fluid is low relative to the high-fat content of colostrum and milk consumed at birth. Regardless of gestational age and feeding, the bile acid pool was dominated by CDCA, HCA, and HDCA with low amounts of CA and other secondary bile acids.
The classical pathway is responsible for >90% of bile acid synthesis, whereas the alternative pathway likely evolved to generate and dispose of oxysterols as part of cholesterol homeostasis (41). The alternative pathway begins with the hydroxylation of cholesterol by CYP27A1 (41). CYP27A1 resides on the inner mitochondrial membrane of a variety of cell types. However, the enzymes necessary to catalyze the final steps in bile acid formation in the alternative pathway are only found in hepatocytes. Thus, intermediate oxysterols need to be transported out of the cell of origin and into a hepatocyte, limiting the overall efficiency of the pathway in bile acid production (29). Conversely, the classical pathway solely occurs in the hepatocytes and is, therefore, more efficient. However, some investigators (41–43) assert that the alternative pathway is the dominant bile acid synthesis pathway in cases of liver disease and in perinatal period.
Bile acid pools are compartmentalized and influenced by both hepatic bile acid synthesis and gallbladder storage, as well as enterohepatic bile acid absorption and recycling from the gut. Expression of most bile acid synthesis enzymes (CYP7A1, CYP7B1, and CYP27A1) was similar in preterm and term pigs at birth and on day 3. The level of C4, a classic pathway-specific oxysterol, was low in term pigs at birth and low in preterm pigs at birth and day 3. The ratio of alternative pathway intermediates to C4 was greater in term pigs at birth and in preterm pigs at birth and on day 3, confirming a greater reliance on the alternative pathway in all pigs at birth, which persisted in preterm pigs to day 3 of life. The fact that the alternative pathway may be less efficient than the classic pathway may explain why bile acid synthesis rates and bile acid pools are lower and smaller, respectively, in neonates relative to adults and even slower and smaller in preterm neonates (8, 34).
In the assessment of the machinery underlying enterohepatic recirculation of bile acids, the relative expression of hepatic basolateral bile acid transporter NTCP was similar in preterm and term pigs, regardless of age. However, the hepatic expression of major canalicular efflux transporter BSEP expression was lower in preterm pigs on day 3, which coupled with lower hepatic bile acid pool size, led to lower hepatobiliary flow and gut bile acid pools. Despite this, intestinal ASBT and ILBP, but not FGF19 expression, increased in preterm pigs from birth to day 3. This is in contrast to studies in mice of different ages by Cui et al. (40) that demonstrated that the expression of ileal OST-α and hepatic NTCP was lower in mice 2 days preterm compared with mice after term birth.
A key finding from this study was that the cultured ileum FGF19 expression is less responsive to bile acids in preterm than term pigs. This suggests that combined actions of lower hepatic bile acid synthesis and intestinal bile acid responsiveness contributed to the lower circulating FGF19 concentrations in preterm compared with term pigs. This observation would appear to be linked to the regional patterning of intestinal function that occurs during fetal and neonatal development. The patterning of the small intestinal epithelium along its cephalocaudal axis establishes three functionally distinct regions: duodenum, jejunum, and ileum. This maturation of cephalocaudal axis is necessary to establish the enterohepatic recycling of bile acids, as bile acid synthesis is upregulated for the digestion of fat in the milk-fed newborn. The establishment of the functional difference between duodenum, jejunum, and ileum largely relies on the differential expression of transcription factors such as GATA-4 (44). Thompson et al. (18) demonstrated that the knock-in expression of GATA-4 in the ileum of mice repressed the transcription of ileum-enriched genes and enhanced the transcription of jejunum-enriched genes. GATA-4 specifically represses the expression of FGF15 (the murine homolog to FGF19) in rodents (18). In our pigs, the expression of GATA-4 was similar in the jejunum at birth and on day 3, regardless of gestational age, and relatively lower in the ileum as shown previously (16). However, GATA-4 expression was higher in the ileum of preterm than term pigs at birth and higher than both preterm and term pigs at day 3. Thus, GATA-4 expression was highest in the most developmentally immature intestinal tissue with lower FGF19 expression and secretion in response to bile acid stimulation. This implicates GATA-4 as a transcriptional inhibitory mechanism that regulates perinatal intestinal FXR-FGF19 signaling.
The immaturity of preterm ileal FXR-FGF19 signaling activity may have developmental implications for preterm bile acid homeostasis and growth. Intestinal FGF19 functions as a primary negative feedback signal to reduce bile acid synthesis by reducing the expression of CYP7A1, the rate-limiting enzyme of the classical pathway of bile acid synthesis (11). The finding of higher bile acid pool size and circulating FGF19 in term compared with preterm pigs is at odds with the concept that FGF19 mainly functions to suppress hepatic bile acid synthesis. Regression analysis in the preterm and term pigs showed no association between plasma FGF19 and CYP7A1 in the preterm pigs, whereas negative correlation existed in the term pigs by day 3. This is consistent with our previous report (20) showing a negative correlation exists in pigs, but the majority of the pigs sampled were greater than 5 days old (20). These results suggest that the FGF19-CYP7A1 enterohepatic negative feedback loop is developmentally upregulated after birth as adaptation to extrauterine life. The mechanism underlying this phenomenon is unclear, but we speculate that it could be due to diminished activity of the hepatocellular FGFR4/β-klotho signal transduction pathway and/or the translation of the CYP7A1 mRNA. Moreover, our results suggest that because the alternative pathway of bile acid synthesis may dominate in neonates, that negative feedback via FGF19-CYP7A1 is less effective to reduce total bile acid synthesis.
FGF19 has additional functions beyond the inhibition of hepatic CYP7A1 (45). Benoit et al. (12) demonstrated that treatment of adult mice with recombinant human FGF19 (rhFGF19) resulted in higher food intake, lower body weight, larger myofibers, and increased grip strength. In addition, when rhFGF19 was given to mice treated with high levels of dexamethasone, it was protective against glucocorticoid-induced sarcopenia (12). Other cell culture work has demonstrated that FGF19 treatment can prevent palmitate-induced atrophy by protecting mitochondria from overload and prevention of insulin resistance (46). Beyond skeletal muscle, FGF19 has also been studied for its capacity to improve glucose homeostasis via interactions with the central nervous system and reducing hepatic gluconeogenesis and glycogenolysis (47). Therefore, although the poor responsiveness of the preterm gastrointestinal (GI) tract to bile acid stimulation may serve to functionally preserve bile acid synthesis, there may be additional FGF19-mediated developmental and metabolic consequences to low levels of FGF19 expression and secretion.
An additional objective of this study was to localize the expression of the FGF19 receptors, including FGFR1 and FGFR4, and β-klotho, their heterodimer partner, that have been closely linked to FGF19 action in effector organs (47). Although FGF19 expression was primarily localized to the distal ileum, lower level expression was also found in the muscle and hypothalamus. Extra-ileal expression of FGF19 has not been widely discussed in the rodent model or human literature; however, detectable levels of FGF19 were found in rectus abdominus muscles of women with gestational diabetes mellitus (48). In addition, work in early-gestation embryos has demonstrated that FGF19 functions as a growth factor outside the gut, including the brain and skeletal system (49), consistent with effects reported in mice. It is possible that the extra-ileal expression of FGF19 in neonates may be a remnant of fetal development (16, 20). The pattern of extra-ileal expression of FGF19 was not matched by FXR, suggesting lack of functional FXR-FGF19 signaling. The relative expression of FGFR1 was primarily observed in the skeletal muscle and hypothalamus. Previous work has largely localized the expression of FGFR1 to adipose tissue (33). FGF19 receptor signal transduction is dependent on colocalization with β-klotho (50). In this study, β-klotho expression primarily occurred in the liver but was detectable in the muscle and hypothalamus, indicating the potential for extrahepatic signal transduction. FGFR4 is primarily expressed not only in the liver, consistent with previous reports (11), but also in the distal ileum and to a lesser degree in the jejunum, muscle, or hypothalamus. A limitation of this study is that we primarily measured mRNA expression rather than protein expression in tissues. It is possible that protein expression does not correlate with mRNA expression, just as protein function may not always correlate with protein expression.
Conclusions
Our results show that the lower hepatic bile acid synthesis and ileum FXR-FGF19 pathway responsiveness to bile acids contribute to low-circulating FGF19 in preterm compared with term neonatal pigs. This finding was despite the fact that the preterm bile acid pool has a greater proportion of CDCA, is more hydrophobic, and should produce a higher FXR agonism in the preterm gut, but this was limited by the underlying immaturity in the intestinal bile acid responsiveness. The molecular mechanism explaining immature or low-ileum FXR-FGF19 signaling may be linked to developmental patterning effects of GATA-4, yet the mechanism for low-hepatic bile acid synthesis is less clear. The dominance of oxysterol intermediates from alternative versus classic pathway bile acid synthesis and the lack of demonstrable feedback between FGF19 and CYP7A1 expression suggest that the alternative pathway of bile acid synthesis may predominate in term pigs at birth and persist in preterm piglets after enteral feeding on day 3. We also show the tissue distribution of FGF19 and FGF19 receptor expression providing a basis to exploring the metabolic effects of FGF19 beyond the gut-liver axis. Future studies are ongoing to explore whether the diminished circulating FGF19 concentration in preterm pigs affects neonatal growth and development.
GRANTS
This work was supported in part by federal funds from the United States Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 3092-51000-060-01, and grants from the National Institutes of Health Grant DK-094616 (to D. G. Burrin) and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338). C. Vonderohe was supported by T32 DK007664 and G. Guthrie was supported by K01 DK129408.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
C.V., B.S., S.C., H.D., and D.G.B. conceived and designed research; C.V., G.G., B.S., S.C., and D.G.B. performed experiments; C.V., G.G., B.S., S.C., H.D., and D.G.B. analyzed data; C.V., G.G., B.S., S.C., H.D., and D.G.B. interpreted results of experiments; C.V. and G.G. prepared figures; C.V. and G.G. drafted manuscript; C.V., G.G., B.S., and D.G.B. edited and revised manuscript; C.V., G.G., B.S., S.C., H.D., and D.G.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the veterinarians and animal care attendants from Baylor College of Medicine’s Center for Comparative Medicine for assistance with animal housing and care in support of this study.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379: 2162–2172, 2012. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Kozuki N, Katz J, Christian P, Lee ACC, Liu L, Silveira MF, Barros F, Tielsch JM, Schmiegelow C, Sania A, Roberfroid D, Ndyomugyenyi R, Mullany LC, Mongkolchati A, Huybregts L, Humphrey J, Fawzi W, Baqui AH, Adair L, Oddo VM, Black RE; Child Health Epidemiology Reference Group Preterm Birth–SGA Working Group. Comparison of US birth weight references and the international fetal and newborn growth consortium for the 21st century standard. JAMA Pediatr 169: e151438, 2015. doi: 10.1001/jamapediatrics.2015.1438. [DOI] [PubMed] [Google Scholar]
- 3.Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 1: e26–e36, 2013. [Erratum in Lancet Glob Health 1: e76, 2013]. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JG, Baer RJ, Partridge JC, Kuppermann M, Franck LS, Rand L, Jelliffe-Pawlowski LL, Rogers EE. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics 138: e20154434, 2016. doi: 10.1542/peds.2015-4434. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID 3rd, Watterberg KL, Saha S, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lester R. The development of bile acid synthesis. Mead Johnson Symp Perinat Dev Med 11: 22–29, 1977. [PubMed] [Google Scholar]
- 7.Watkins JB. Bile acid metabolism and fat absorption in newborn infants. Pediatr Clin North Am 21: 501–512, 1974. doi: 10.1016/s0031-3955(16)33005-x. [DOI] [PubMed] [Google Scholar]
- 8.Watkins JB, Järvenpää AL, Szczepanik-Van Leeuwen P, Klein PD, Rassin DK, Gaull G, Raiha NC. Feeding the low-birth weight infant: V. Effects of taurine, cholesterol, and human milk on bile acid kinetics. Gastroenterology 85: 793–800, 1983. doi: 10.1016/0016-5085(83)90427-4. [DOI] [PubMed] [Google Scholar]
- 9.Watkins JB, Ingall D, Szczepanik P, Klein PD, Lester R. Bile-salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technic. N Engl J Med 288: 431–434, 1973. doi: 10.1056/NEJM197303012880902. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med 21: 702–714, 2015. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Markan KR, Potthoff MJ. Metabolic fibroblast growth factors (FGFs): mediators of energy homeostasis. Semin Cell Dev Biol 53: 85–93, 2016. doi: 10.1016/j.semcdb.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoit B, Meugnier E, Castelli M, Chanon S, Vieille-Marchiset A, Durand C, Bendridi N, Pesenti S, Monternier PA, Durieux AC, Freyssenet D, Rieusset J, Lefai E, Vidal H, Ruzzin J. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med 23: 990–996, 2017. doi: 10.1038/nm.4363. [DOI] [PubMed] [Google Scholar]
- 13.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145: 2594–2603, 2004. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Infantes D, Gallego-Escuredo JM, Díaz M, Aragones G, Sebastiani G, López-Bermejo A, de Zegher F, Domingo P, Villarroya F, Ibáñez L. Circulating FGF19 and FGF21 surge in early infancy from infra- to supra-adult concentrations. Int J Obes (Lond) 39: 742–746, 2015. doi: 10.1038/ijo.2015.2. [DOI] [PubMed] [Google Scholar]
- 15.Memon N, Griffin IJ, Lee CW, Herdt A, Weinberger BI, Hegyi T, Carayannopoulos MO, Aleksunes LM, Guo GL. Developmental regulation of the gut-liver (FGF19-CYP7A1) axis in neonates. J Matern Fetal Neonatal Med 33: 987–992, 2018. doi: 10.1080/14767058.2018.1513483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavaldà-Navarro A, Pastor JJ, Mereu A, Villarroya F, Ipharraguerre IR. Developmental regulation of the intestinal FGF19 system in domestic pigs. Am J Physiol Gastrointest Liver Physiol 314: G647–G654, 2018. doi: 10.1152/ajpgi.00312.2017. [DOI] [PubMed] [Google Scholar]
- 17.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48: 2664–2672, 2007. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Thompson CA, Wojta K, Pulakanti K, Rao S, Dawson P, Battle MA. GATA4 is sufficient to establish jejunal versus ileal identity in the small intestine. Cell Mol Gastroenterol Hepatol 3: 422–446, 2017. doi: 10.1016/j.jcmgh.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci 91: 4713–4729, 2013. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith V, Jiang Y, Thymann T, Sangild P, Maj M, Manjarin R, Burrin D. Rapid postnatal upregulation of intestinal farnesoid X receptor-fibroblast growth factor 19 signaling in premature pigs. J Pediatr Gastroenterol Nutr 70: e94–e99, 2020. doi: 10.1097/MPG.0000000000002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour SL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol 269: 264–275, 2004. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Robinson JL, Smith VA, Stoll B, Agarwal U, Premkumar MH, Lau P, Cruz SM, Manjarin R, Olutoye O, Burrin DG, Marini JC. Prematurity reduces citrulline-arginine-nitric oxide production and precedes the onset of necrotizing enterocolitis in piglets. Am J Physiol Gastrointest Liver Physiol 315: G638–G649, 2018. doi: 10.1152/ajpgi.00198.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoneim N, Bauchart-Thevret C, Oosterloo B, Stoll B, Kulkarni M, de Pipaon MS, Zamora IJ, Olutoye OO, Berg B, Wittke A, Burrin DG. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS One 9: e106888, 2014. doi: 10.1371/journal.pone.0106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Call L, Stoll B, Oosterloo B, Ajami N, Sheikh F, Wittke A, Waworuntu R, Berg B, Petrosino J, Olutoye O, Burrin D. Metabolomic signatures distinguish the impact of formula carbohydrates on disease outcome in a preterm piglet model of NEC. Microbiome 6: 111, 2018. doi: 10.1186/s40168-018-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Call L, Molina T, Stoll B, Guthrie G, Chacho S, Plat J, Robinson J, Lin S, Vonderohe C, Mohammad M, Kunichoff D, Cruz S, Lau P, Premkumar M, Nielsen J, Fang Z, Olutoye O, Thymann T, Britton R, Salgild P, Burrin D. Parenteral lipids shape gut bile acid pools and microbiota profiles in the prevention of cholesasis in preterm pigs. J Lipid Res 61: 7, 2020. doi: 10.1194/jlr.RA120000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res 30: 719–730, 1989. doi: 10.1016/S0022-2275(20)38331-0. [DOI] [PubMed] [Google Scholar]
- 27.Ng K, Stoll B, Chacko S, Saenz de Pipaon M, Lauridsen C, Gray M, Squires EJ, Marini J, Zamora IJ, Olutoye OO, Burrin DG. Vitamin E in new-generation lipid emulsions protects against parenteral nutrition-associated liver disease in parenteral nutrition-fed preterm pigs. JPEN J Parenter Enteral Nutr 40: 656–671, 2016. doi: 10.1177/0148607114567900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guthrie G, Tackett B, Stoll B, Martin C, Olutoye O, Burrin DG. Phytosterols synergize with endotoxin to augment inflammation in Kupffer cells but alone have limited direct effect on hepatocytes. JPEN J Parenter Enteral Nutr 42: 37–48, 2018. doi: 10.1177/0148607117722752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40: 539–551, 2004. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Li X. The FGF metabolic axis. Front Med 13: 511–530, 2019. doi: 10.1007/s11684-019-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda A, Miyazaki T, Ikegami T, Iwamoto J, Maeda T, Hirayama T, Saito Y, Teramoto T, Matsuzaki Y. Cholesterol 25-hydroxylation activity of CYP3A. J Lipid Res 52: 1509–1516, 2011. doi: 10.1194/jlr.M014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Praslickova D, Torchia EC, Sugiyama MG, Magrane EJ, Zwicker BL, Kolodzieyski L, Agellon LB. The ileal lipid binding protein is required for efficient absorption and transport of bile acids in the distal portion of the murine small intestine. PLoS One 7: e50810, 2012. doi: 10.1371/journal.pone.0050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shan Z, Alvarez-Sola G, Uriarte I, Arechederra M, Fernández-Barrena MG, Berasain C, Ju C, Avila MA. Fibroblast growth factors 19 and 21 in acute liver damage. Ann Transl Med 6: 257, 2018. doi: 10.21037/atm.2018.05.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watkins JB, Szczepanik P, Gould JB, Klein P, Lester R. Bile salt metabolism in the human premature infant: preliminary observations of pool size and synthesis rate following prenatal administration of dexamethasone and phenobarbital. Gastroenterology 69: 706–713, 1975. doi: 10.1016/S0016-5085(19)32473-4. [DOI] [PubMed] [Google Scholar]
- 35.Chmielewska A, Farooqi A, Domellof M, Ohlund I. Lean tissue deficit in preterm infants persists up to 4 months of age: results from a Swedish longitudinal study. Neonatology 117: 80–87, 2020. doi: 10.1159/000503292. [DOI] [PubMed] [Google Scholar]
- 36.Yamato Y, Kimura A, Inoue T, Kurosawa T, Kato H. Fetal bile acid metabolism: analysis of urinary 3β-monohydroxy-Δ5 bile acid in preterm infants. Biol Neonate 80: 19–25, 2001. doi: 10.1159/000047114. [DOI] [PubMed] [Google Scholar]
- 37.Hardy KJ, Hoffman NE, Mihaly G, Sewell RB, Smallwood RA. Bile acid metabolism in fetal sheep; perinatal changes in the bile acid pool. J Physiol 309: 1–11, 1980. doi: 10.1113/jphysiol.1980.sp013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smallwood RA, Lester R, Plasecki GJ, Klein PD, Greco R, Jackson BT. Fetal bile salt metabolism. II. Hepatic excretion of endogenous bile salt and of a taurocholate load. J Clin Invest 51: 1388–1397, 1972. doi: 10.1172/JCI106934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lester R. Bile acid metabolism in the fetus and newborn. Ciba Found Symp 70: 99–115, 1979. doi: 10.1002/9780470720530.ch6.[120241] [DOI] [PubMed] [Google Scholar]
- 40.Cui JY, Aleksunes LM, Tanaka Y, Fu ZD, Guo Y, Guo GL, Lu H, Zhong XB, Klaassen CD. Bile acids via FXR initiate the expression of major transporters involved in the enterohepatic circulation of bile acids in newborn mice. Am J Physiol Gastrointest Liver Physiol 302: G979–G996, 2012. doi: 10.1152/ajpgi.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandak WM, Kakiyama G. The acidic pathway of bile acid synthesis: not just an alternative pathway. Liver Res 3: 88–98, 2019. doi: 10.1016/j.livres.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura A, Yamakawa R, Ushijima K, Fujisawa T, Kuriya N, Kato H, Inokuchi T, Mahara R, Kurosawa T, Tohma M. Fetal bile acid metabolism during infancy: analysis of 1 beta-hydroxylated bile acids in urine, meconium and feces. Hepatology 20: 819–824, 1994. doi: 10.1002/hep.1840200408. [DOI] [PubMed] [Google Scholar]
- 43.Shoda J, Mahara R, Osuga T, Tohma M, Ohnishi S, Miyazaki H, Tanaka N, Matsuzaki Y. Similarity of unusual bile acids in human umbilical cord blood and amniotic fluid from newborns and in sera and urine from adult patients with cholestatic liver diseases. J Lipid Res 29: 847–858, 1988. doi: 10.1016/S0022-2275(20)38479-0. [DOI] [PubMed] [Google Scholar]
- 44.Walker EM, Thompson CA, Battle MA. GATA4 and GATA6 regulate intestinal epithelial cytodifferentiation during development. Dev Biol 392: 283–294, 2014. doi: 10.1016/j.ydbio.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schumacher JD, Guo GL. Pharmacologic modulation of bile acid-FXR-FGF15/FGF19 pathway for the treatment of nonalcoholic steatohepatitis. Handb Exp Pharmacol 256: 325–357, 2019. doi: 10.1007/164_2019_228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun YN, Yang ZX, Ren FZ, Fang B. FGF19 alleviates palmitate-induced atrophy in C2C12 cells by inhibiting mitochondrial overload and insulin resistance. Int J Biol Macromol 158: 401–407, 2020. doi: 10.1016/j.ijbiomac.2020.04.186. [DOI] [PubMed] [Google Scholar]
- 47.Gadaleta RM, Moschetta A. Metabolic messengers: fibroblast growth factor 15/19. Nat Metab 1: 588–594, 2019. doi: 10.1038/s42255-019-0074-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Xu S, Ding W, Zhu C, Deng S, Qiu X, Wang Z. Decreased placental and muscular expression of the fibroblast growth factor 19 in gestational diabetes mellitus. J Diabetes Investig 10: 171–181, 2019. doi: 10.1111/jdi.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H, Li J, Zhang D, Zhou X, Xie J. Role of the fibroblast growth factor 19 in the skeletal system. Life Sci 265: 118804, 2021. doi: 10.1016/j.lfs.2020.118804. [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, Schlessinger J. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553: 501–505, 2018. doi: 10.1038/nature25010. [DOI] [PMC free article] [PubMed] [Google Scholar]