Abstract
Uterine fibroids (leiomyomas) are present in >75% of women and can cause serious morbidity. They are by far the leading cause of hysterectomy. Fibroids are a complex mixture of cells that include fibroblasts and smooth muscle cells. Rich in extracellular matrix, they typically arise through somatic mutations, most commonly MED12. Their lack of growth inhibition and their ability to have facets of malignancy yet be histologically and biologically benign provide opportunities to explore basic processes. To date, the mechanisms responsible for growth and development of leiomyomas are an enigma. This review provides an overview of current understanding and future directions for clinical and basic research of fibroids.
Keywords: fibroid, hysterectomy, leiomyoma, pathogenesis, treatment
Clinical Importance
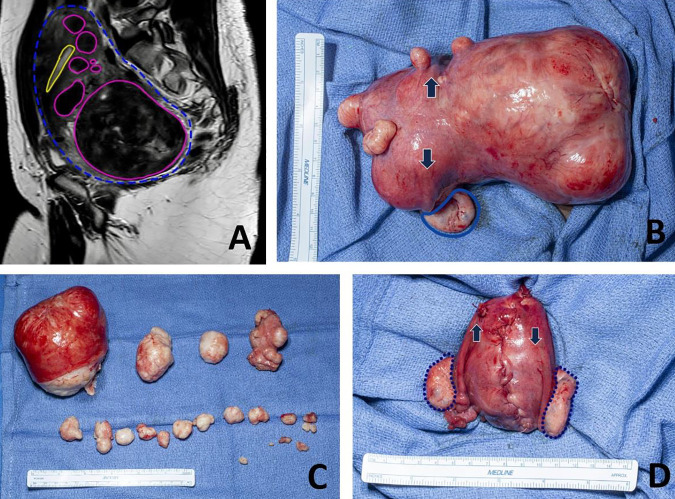
The prevalence of uterine fibroids (e.g., leiomyomas, myomas) reaches more >75% of women worldwide and thus accounts for much gynecologic care (1–3). However, only 25% of these women have fibroid symptoms, which can include heavy or prolonged menstrual bleeding that frequently results in severe iron-deficiency anemia, pelvic pain, extrinsic compression of bowel or bladder, appearance of pregnancy when it does not exist, and reproductive impairment, including infertility and pregnancy complications (FIGURE 1).
FIGURE 1.
The clinical picture of uterine fibroids Although uterine fibroids can be symptomatic, clinical disease is often impressive. A: sagittal view of a single slice of a T2-weighted magnetic resonance image showing the uterus (outlined in green) with multiple fibroids (outlined in blue) and the endometrial cavity (highlighted in yellow). B: the same uterus at the beginning of the myomectomy. The large posterior fibroid is on right in the image. The origin of the fallopian tubes, and thus the normal part of the uterine cavity, is highlighted by the arrows. The left ovary is highlighted in blue, and the right ovary is hidden by the fibroid. C: fibroids surgically excised. D: the uterus after myomectomy, which is a normal size. Both ovaries are outlined in black, and arrows indicate the origin of the fallopian tube. The same ruler is visualized for comparison in B–D.
In addition, fibroids are the leading cause of hysterectomy, accounting for at least one-third of all hysterectomies; in contrast, all types of gynecologic cancers account for <10% (4). This predominance of hysterectomy for benign disease is important not only for economic reasons but also because long-term health risks are increasingly identified after hysterectomy, even with conservation of both ovaries (5).
Fibroids are clinically important during a woman’s reproductive years. They rarely are seen in teenage girls and have a progressive increase in incidence and severity until menopause, which for most women occurs in their early 50s. Thus, most research has focused on the role of the gonadal steroids estrogen and progesterone in fibroid pathogenesis.
Uterine fibroids are a disease with substantial health disparities that most often affect women of African descent (6, 7). In Black women, fibroids are more common, are more severe, have an earlier age at onset, and have different growth patterns. Prusinski Fernung et al. (9) reported that the myometrial stem cell population is expanded in the uteri of African American women compared with White women and that this increase is correlated with fibroid number and size, thus potentially contributing to the biological understanding of these disparities. Black women also have different priorities in fibroid therapies, prefer different sources of fibroid information, and have more impairment of work and home life because of this disease (10). Although most studies have suggested that Latina and Asian women have a risk of fibroids that is similar to White women, little research has been done in this area.
The annual cost of fibroid treatment was estimated in 2010 dollars to be $34 billion in the United States (11). This cost is likely an underestimate because only direct costs for care and pregnancy complications were considered. Loss of work productivity and long-term sequelae of fibroid therapies, including hysterectomies, have not been included in cost estimates.
Biological Factors
Fibroids are well-circumscribed lesions of the uterine myometrium that can range more than several orders of magnitude in size—from microscopic to larger than a 20-cm diameter. New investigative work is changing the classic conception of fibroids as a clonal smooth muscle cell (SMC) neoplasm (12) to a conception of various SMC types (myometrial and vascular), fibroblasts, immune cells, and stem cells (13–16). Some investigative work has proposed that through an initiating event the clonal cell differentiates into both fibroblast and SMC (17); other work challenges that premise. Using single-cell RNA sequencing, investigators have identified 18 different cell types in leiomyoma tumors, and not all these cells express somatic mutations (13).
Extracellular Matrix
An essential component of fibroids is the abundant extracellular matrix (ECM) caused primarily by overexpression of collagen types I and III (18, 19). ECM proteins were once thought to have only passive roles, serving merely as scaffolding proteins, but apparently the ECM has an active part in regulation of the survival, migration, and proliferation of cells (18, 20, 21). Leiomyoma tissues contain an altered collagen III protein-to-collagen I protein ratio compared with myometrium, and the collagen fibrils are highly disorganized, similar to keloids (18, 22).
Gonadal Steroids
Historically, estrogen was thought to be the driver of fibroid biological processes. Then, the primacy of progesterone was shown in both the basic science of estrogen’s key role in induction of the progesterone receptor (PR) and the clinical success of PR modulators for treatment of fibroids while maintaining physiological levels of estradiol (23, 24). Medical therapy for fibroids still relies on modulation of the hypothalamic-pituitary-ovarian axis. Most recent therapies are oral gonadotropin-releasing hormone (GnRH) antagonists with menopause-range add-back of estrogen and progestins to treat fibroids while limiting hypogonadal adverse effects (5, 25).
Fibrosis
Transforming growth factor-β (TGF-β) signaling has been consistently linked to fibrosis and leiomyoma pathology (18, 21, 26). Compared with adjacent myometrium, leiomyomas show a three- to fivefold greater expression of TGF-β3, a 30% higher level of SMAD3 mRNA, and a markedly increased phosphorylation of TGF-β-RII and Smad3 proteins (18, 19, 21, 27, 28). In addition to aberrant activation of the TGF-β signaling pathways in fibroids (29), dysregulated mechanistic pathways have been identified recently. These include the nuclear receptors NR4A1, A2, and A3, which are markedly suppressed in leiomyomas compared with myometrium (19) and act as negative regulators of profibrotic factors, including TGF-β3, SMAD3, and collagens I and VI (19).
Several para-phenyl-substituted diindolylmethane analogs have been developed that specifically activate the NR4A receptors and can target this pathway for therapeutic purposes. In addition, the activin signaling pathway has been shown to regulate ECM production in leiomyoma cells and myofibroblast transformation in myometrial cells (30) through the p38/mitogen-activated protein kinase (MAPK) (30, 31) and sphingosine-1-phosphate (32) signaling pathways. Small-molecule inhibitors of the activin A receptor-like type 1, such as SB-431542 and A-83-01, are available and merit investigation as potential therapies.
Angiogenesis
Leiomyomas produce and secrete various angiogenic factors, including fibroblast growth factors, heparin-binding growth factor, platelet-derived growth factor, vascular endothelial growth factor, and prolactin (33, 34). This abundant supply of angiogenic factors has an important role in neoangiogenesis during the early development and growth of fibroid tumors and likely is a contributing factor to the abnormal uterine bleeding that is one of the main symptoms of leiomyomas. Interestingly, the larger leiomyoma tumors with abundant collagen deposition are less vascular because of the large areas of ECM.
Genetics
Most fibroids arise from somatic mutations within SMCs, with MED12, the Mediator Complex Subunit 12 gene, accounting for up to one-half of all fibroids in most series (35). The high-mobility group AT-hook2 (HMGA2) and collagen IV α5 (COL4A5) are the other two most frequent somatic driver mutations (36). Chromothripsis, multiple concurrent gene rearrangements, and genomic instability also have apparent roles in fibroid pathogenesis (36, 37).
Mutations in fumarate hydratase (FH) are most often autosomal-dominant germline changes and result in fibroids, papillary renal cell cancer, and cutaneous leiomyomas—the triad of hereditary leiomyomatosis and renal cell cancer syndrome (38). However, because fibroids arise independently within the uterus, and thus multiple genetic subtypes of myomas may be present in that uterus, the signaling pathways of all the groups except the FH group have substantial overlap. Therefore, genotype-based therapies have not been developed (39).
Stem Cells
Fibroid initiation is becoming better understood as studies identify populations of progenitor or stem cells (FIGURE 2) (40–43). The identities of leiomyoma stem cells and myometrial stem cells are not completely clear because several different sets of surface markers have been used to isolate and characterize these stem cell populations. They include CD34/CD49 (41), Stro11/CD44 (44), SUSD2, and CD146/CD140b (45) cell surface markers. The results of these studies indicate that the CD34/CD49 and Stro11/CD44 populations are most likely the same. The myometrial stem cells are thought to have an important part in tissue regeneration and may become leiomyoma tumor-initiating stem cells when undergoing somatic mutations, such as MED12. The leiomyoma stem cells remain as a subpopulation within the tumor cells and retain the ability to initiate tumors in mouse xenograft models (41).
FIGURE 2.
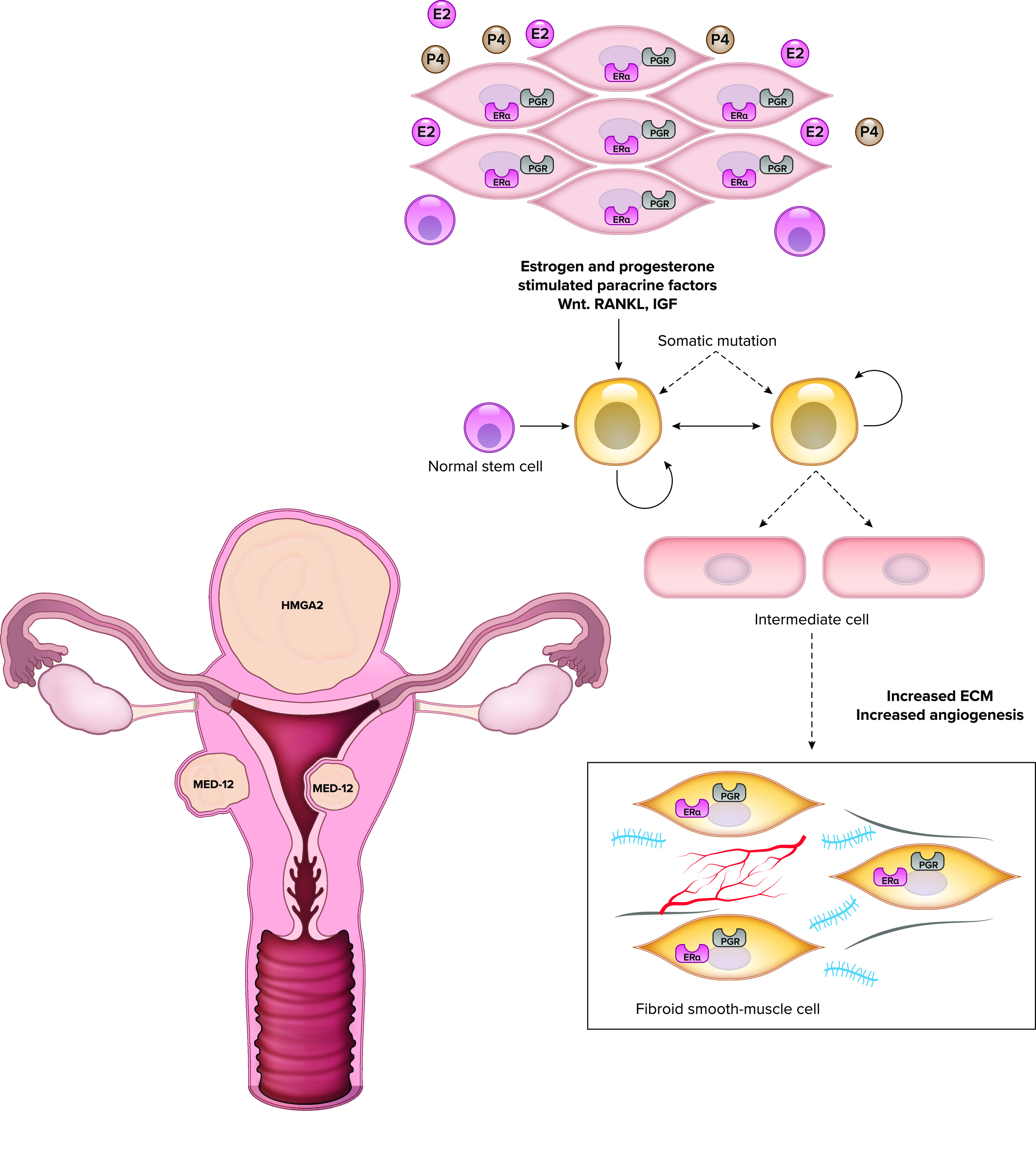
Early pathogenesis of uterine fibroids The initiation of fibroids requires paracrine events among multiple cell types in the uterus. The normal myometrial cells (myocytes pictured in black) contain receptors for the steroids estrogen and progesterone (ER and PR). The uterine stem cells (orange) do not have steroid hormone receptors, but products produced by the myocytes in response to these steroids act on stem cells to promote transformation. Additionally, somatic mutations take place in the stem cells to create the phenotype of intermediate cells (blue). Of note, multiple somatic mutations can take place in a uterus, so that, as shown, the uterus commonly can have 2 fibroids with MED12 mutations and 1 with HMGA2 mutation. Fibroid cells (red) represent the transformed cell type and have increased extracellular matrix (ECM). RANKL, receptor activator of NF-κB; Wnt, wingless related integration site.
Several reports have provided crucial insights regarding the interaction between stem cell populations and other cells to initiate and promote growth of a clinical fibroid. Somatic mutations including MED12 are expressed in the stem cell population and in the SMCs within leiomyoma tumors but not in the associated cells (15, 44). Several studies (46–48) have determined that the receptor activator of NF-κB (RANKL)-RANK, wingless related integration site (Wnt), and IGF2–insulin receptor A (IR-A) signaling pathways are important regulators of proliferation in the leiomyoma stem cell population. RANKL is produced by the differentiated SMCs within the tumor and acts to increase proliferation of the stem cell population. Studies have also determined that leiomyoma stem cells exhibit a more inflammatory phenotype with increased expression of cytokines associated with the T-helper type II pathway (49). Finally, the stem cells undergo increased DNA damage with altered expression of DNA repair genes (9) and dysregulated DNA methylation with hypermethylation of important myometrial genes (50).
MicroRNAs
MicroRNAs (miRNAs) are short, noncoding, single-stranded RNAs that regulate gene expression at the transcriptional and translational levels (51). Alterations in miRNA levels were first described as regulators of malignant tumors but have also been associated with various reproductive diseases, including endometriosis (52). Many miRNAs have been identified in leiomyomas with expression profiles that differ from autologous myometrium (53, 54). Several appear to regulate important aspects of leiomyoma function and phenotype (55).
miR-21 is one of the key overexpressed miRNAs in leiomyomas. It has been shown to promote tumor fibrosis by blocking the inhibitory effects of the Smad7 protein. Thus, it stimulates the TGF-β signaling pathway, increases the expression of TGF-β receptor type 2 (56), increases expression of TGF-β3 in uterine leiomyoma and myometrial cells, and increases collagen, fibronectin, and cell proliferation (57). Fitzgerald et al. (58) also reported that miR-21 levels were higher in leiomyomas and that knockdown of miR-21 in these cells led to increased apoptosis.
Another important leiomyoma miRNA is miR-29. miR-29 is downregulated in fibroids and is a negative regulator of ECM genes, including collagens I, II, and III (8, 59). Moreover, overexpression of miR-29 in leiomyoma cells reduced collagen and fibronectin production, whereas knockdown of this miRNA in myometrial cells led to upregulation of several ECM proteins and a more fibroidlike phenotype (8, 59–61). Finally, let-7 miRNA is downregulated in leiomyomas, which gives investigators insight into the pathogenesis of the HMGA2 subgroup. Exogenous let-7 miRNAs directly repress HMGA2, and the levels of miR-let-7 are negatively correlated with HMGA2 expression (62, 63).
Limitation of Current In Vitro Systems
Studies focusing on leiomyoma molecular mechanisms have relied heavily on the use of primary cultures of human leiomyoma and myometrial cells from surgical tissue specimens. It has become apparent that traditional cell culture systems are not ideal for studying the biology of these cells, particularly if the cells must be passaged in culture, because primary and passaged leiomyoma cell cultures begin to lose expression of important genes, including estrogen receptor (ER) and PR, after only a few days (16, 64). Traditional two-dimensional culture of leiomyoma cells also leads to altered expression of specific genetic mutations and chromosomal rearrangements.
The HMGA2 gene is expressed in uterine leiomyomas with the (12,14)(q15;q24) chromosomal rearrangement but not in autologous myometrium or in karyotypically normal leiomyomas. However, HMGA2 expression was present in primary cultures of karyotypically normal leiomyoma cells and myometrial cells (65). Studies focusing on the MED12 mutation have shown that even during the early phases of in vitro culture, MED12-mutated cells are lost (43, 66).
Establishment of cells as primary two-dimensional cell cultures from fibroids results in a mixed population of cells. Analysis of differential gene expression in the three different cell subtypes within leiomyoma and myometrial cultures showed that genes including TGFβ2, PR, and cellular retinoic acid-binding protein 2 were differentially expressed in the SMCs of leiomyomas but not in the fibroblast cells in these cultures (17). A more recent study (15) identified a substantial proportion of tumor-associated fibroblasts within MED12-mutant leiomyomas. The MED12 mutation was not present in the tumor-associated fibroblasts but only in the SMCs. MED12-mutant leiomyomas also contained more collagen than the HMGA2-overexpressing leiomyomas, likely due to the fibroblasts. These findings suggest that the loss of MED12-expressing cells in culture may be due to preferential growth of fibroblasts in primary cultures and in subsequent passages. Future studies are needed to better understand how the heterogeneity of cell populations within leiomyomas contributes to the phenotype of those tumors.
Because of these limitations, interest has been substantial in the potential use of three-dimensional culture systems. The systems allow for a more physiological tissue structure, recapitulating the interactions between ECM and the heterogeneous cell populations present within leiomyomas. Initial studies used immortalized leiomyoma and myometrial SMCs cultured in collagen gels (67). These SMCs maintained their normal morphological characteristics and differential expression of several profibrotic genes, including TGFβ-3, collagen, fibronectin, and dermatopontin. Subsequently, investigators developed in vitro three-dimensional leiomyoma spheroid culture systems, in which primary cells from leiomyomas or myometrium were cultured on low-attachment plates in mesenchymal stem cell medium. These spheroid cultures can be maintained for up to 7 days and, importantly, maintain expression of ER and PR and differential expression and secretion of collagen (68, 69). Leiomyoma spheroids also have shown low proliferation, increased levels of phosphorylated AKT (protein kinase B), and increased oxidative stress, similar to in vivo tissue (61). The spheroid culture system has been used to study the role of specific circular RNAs and long noncoding RNAs that are expressed differentially between leiomyoma and autologous myometrium (70, 71).
Clinical and Environmental Influences
Lifestyle factors appear to influence clinically important uterine fibroids. Through studies such as the Nurses’ Health Study (72), the Black Women’s Health Study (73), and the Study of Environment, Lifestyle and Fibroids (74), the medical community knows that race/ethnicity, diet, exercise, and contraceptive use influence fibroid risk. However, unlike studies conducted after the Framingham Heart Study, evidence is not currently available about whether modifying risk results in decreased fibroid risk or progression. One currently exciting possibility is that vitamin D deficiency may be associated with fibroid risk, because that outcome would also explain the increased risk of fibroids in women with darker skin. However, modification of risk with vitamin D supplementation would need to be demonstrated.
Hypertension and fibroids have been linked through multiple epidemiological studies, and the fact that they both are diseases of SMC proliferation raises the possibility of a shared pathogenesis (75). One recent study suggested that the angiotensin-converting enzyme (ACE) pathway may be the link, where a large database study showed fibroid risk lessened with clinical ACE inhibitors (76). Similarly, statin use has been linked to decreased fibroid risk (77).
Environmental Toxicants
Endocrine-disrupting chemicals are environmental toxicants that can cause adverse effects on the endocrine system because of their structural similarity to endogenous hormones (78–80). As hormone-dependent neoplasms, fibroids may be targets for the endocrine-disrupting chemicals. Various studies are now accumulating evidence on a role for endocrine-disrupting chemicals in the pathogenesis of uterine leiomyomas (78, 81, 82).
Diethylstilbestrol (DES) is a pharmacological estrogen widely used between the 1940s and 1970s to prevent miscarriages in pregnant women. The National Institute of Environmental Health Sciences Fibroid Study Group (83) reported that women who were born in the era when DES was prescribed and who self-reported prenatal DES exposure had a greater incidence of uterine leiomyomas and had a tendency to develop larger fibroids. Studies performed in rat ELT-3 cells showed that treatment with DES increased cell proliferation in a dose-dependent manner. Coumestrol and naringenin, two phytoestrogens, have also been reported to induce proliferation of ELT-3 cells (84). Early-life exposure to environmental estrogens such as DES or genistein also led to increased tumor incidence and tumor size in Eker rats (85).
There appears to be a link between exposure to endocrine-disrupting chemicals, such as bisphenol A (BPA) and phthalates, and increased risk of leiomyomas. Shen et al. (86) evaluated the amounts of BPA, nonylphenol, and octylphenol in samples of blood and urine from Han Chinese women. The investigators observed that urinary BPA, nonylphenol, and octylphenol concentrations were greater in women who had leiomyomas than in control women. Zhou et al. (137) also quantified the levels of BPA, nonylphenol, and octylphenol in the urine of women with leiomyomas versus control women and found similar results. Zota et al (88) reported a positive association between the urinary levels of the di(2-ethylhexyl) phthalate (DEHP) metabolites MEHHP, MEOHP, and MECPP and the fibroid volume in a patient cohort undergoing hysterectomy. Further epidemiological and mechanistic studies are warranted to validate the associations observed in these studies.
The effects of BPA, nonylphenol, and octylphenol on leiomyoma cells were examined with primary cultures of human leiomyoma cells. Shen et al. (87) treated human leiomyoma cells with estrogen, BPA, nonylphenol, or octylphenol and saw increased proliferation rates compared with untreated cells, along with downregulation of the TGF-β-Smad signaling pathway. They further reported that BPA also increased proliferation of human leiomyoma cells by increasing the expression of ER, IGF-1, and vascular epithelial growth factor. He et al (89) isolated mesenchymal stem cells from uterine leiomyomas and determined that BPA induced cyclooxygenase-2 (COX-2) expression and stimulated both proliferation and migration of leiomyoma stem cells. Kim et al (90) tested the effects of DEHP on human leiomyoma cells in vitro and observed that DEHP-treated cells had greater viability, less apoptosis, and increased expression of COX-2.
The mechanisms by which endocrine-disrupting chemicals affect the development and growth of uterine leiomyomas involves epigenetic effects and DNA damage. Prusinski Fernung et al. (91) analyzed DNA repair capacity and function in myometrial stem cells isolated from adult Eker rats exposed during uterine development to DES. Their results showed that exposure to DES resulted in higher levels of DNA damage and a reduced ability to repair DNA double-strand breaks compared with stem cells from unexposed control rats. In a subsequent study, Elkafas et al (92) investigated whether vitamin D3 could ameliorate the DNA damage response defects in rat myometrial stem cells due to early-life exposure to DES and whether this was a mechanism leading to reduced incidence of uterine fibroids later in life. Their results confirmed that treatment of rats with DES led to significantly reduced expression of key DNA repair proteins in isolated myometrial stem cells and that treatment of these myometrial stem cells with vitamin D3 restored the DNA damage repair signaling network.
Taken together, the results of these studies confirm that endocrine-disrupting chemicals can have direct effects on uterine leiomyoma cells, including stem cells, through epigenetic mechanisms that may promote tumorigenesis. Additional investigations are needed to clarify the molecular pathways involved and to identify potential therapeutic approaches to ameliorate these effects.
Parallels in Physiology
Leiomyomas and Pregnant Myometrium Share Several Characteristics
In an interesting and relatively unexplored characteristic of leiomyomas, leiomyoma SMCs have a differentiated phenotype that is more similar to myometrial SMCs of pregnancy than of nongravid myometrium. Both myometrium of pregnancy and leiomyomas are characterized by increased responsiveness or hypersensitivity to gonadal steroids, particularly estrogen, due to increased expression of steroid hormone receptors. This hypersensitivity leads to increased cell proliferation; increased extracellular matrix production, particularly fibrillar collagens; increased expression of connexin-43; and decreased apoptosis (93–96).
More recent studies have identified additional similarities between leiomyoma SMCs and myometrium of pregnancy, including increased expression of the IGF ligands and signaling (97, 98) and of COX-2 (40, 99) compared with SMCs of nongravid myometrium. Expression of COX-2 was linked to the proliferative capacity of leiomyoma SMCs. Jaffer et al (100) reported that the mammalian target of rapamycin (mTOR) pathway is activated in association with increased proliferation of myometrium during pregnancy. Leiomyomas show a similar activation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway (101). Why leiomyoma SMCs maintain the pregnancy phenotype requires further investigation.
Bypassing the Gonads
Most fibroid pathogenesis is tied to the gonadal steroids, yet direct actions of pituitary hormones may occur (102). Studies have linked both the anterior pituitary (luteinizing hormone) and posterior pituitary (prolactin) to uterine disease through fibroids and the related disorder adenomyosis (83, 103, 104). This evidence is most developed for prolactin, where work has shown that the receptor is present in myometrium and the ligand serves as an SMC mitogen (104)
Unique Features of Leiomyomas
Lack of Growth Inhibition
Unlike most neoplasms, which range in size from millimeters to a few centimeters, fibroids can commonly exceed 20 cm, suggestive that they lack growth inhibition (105). Their location within the peritoneal cavity indicates that growth may escape detection in a way that a thyroid or breast lesion might not. However, the same should be true for lesions of the gastrointestinal tract, which does have leiomyomas but typically not large ones. Likewise, animal models of leiomyomas typically have small nodules and not the large lesions seen in women (106–110) (Table 1). In most cases, the leiomyomas become apparent only in older animals, most likely because of the slow growth and smaller size of the tumor.
Table 1.
Animal models for uterine leiomyomas
| Species | Incidence | Reference |
|---|---|---|
| Gray seals | 65%; occur in older seals ages 22–41 yr | (106) |
| Miniature pigs | 75%; incidence linked to older age (mean 9.7 yr) | (107) |
| Dogs | Linked to age; most common uterine tumor in dogs | (110) |
| Nonhuman primates | Oviductal and uterine leiomyomas; incidence increases with age | (108) |
| Poultry | Oviductal; incidence increases to 75% in aging hens | (109) |
Some Facets of Malignancy, Yet a Benign Disease
A group of lesions typically referred to as leiomyoma variants occupy the interstices of cancer and benign lesions (111). Benign metastasizing leiomyomas are lesions where clonal lesions arising from a uterine source are found in distant sites, with the lung being the most frequent site (112). Intravenous leiomyomatosis occurs when a fibroid spreads through the venous system from the pelvis to the heart, often presenting as dyspnea or an atrial mass; it can occur long after hysterectomy (113). Finally, disseminated peritoneal leiomyomatosis, or leiomyomatosis peritonealis disseminata, clinically resembles ovarian cancer, with nodules throughout the pelvis that are histologically benign and share some of the karyotypic changes found in conventional fibroids (114).
Additional diseases have less clear relationships to fibroids. SMC tumors of undetermined malignant potential and even uterine sarcomas also present as a myometrial mass on imaging but are histologically different (115).
Lymphangioleiomyomatosis (LAM) presents with histologically benign but much more morbid disease in the lung. It is linked to fibroids through mutations in the tuberous sclerosis gene in LAM and the early Eker rat model (116, 117).
Paradox of Growth
Finally, the mechanics of growth in leiomyomas are unclear. By definition, fibroids have a low mitotic index, yet image-based studies showed a median volume growth of 9% over 6 mo and a maximal growth rate of 138% over the same interval (118). Approximately 7% of the fibroids in that study shrunk at least 20%; however, the shrinkage was mainly in the smaller fibroids. Because leiomyomas are rich in ECM, expansion of the matrix component can occur, but studies have suggested that this is not the primary mechanism of their growth.
Future Directions
Surgery and Hormonal Therapies
Fibroid therapy currently is rooted in the nineteenth century model of surgical excision, analogous to the Halstead paradigm of breast cancer. This model has limitations beyond the morbidity of surgery. Although fibroids can be surgically removed or destroyed with various therapies (e.g., radiofrequency ablation, embolization, focused ultrasound), the risk of new fibroids forming is high (119). Nonetheless, both myomectomy (surgical removal of fibroids alone) and uterine artery embolization are highly effective at reducing symptoms and likely are underused (120, 121).
Hysterectomy continues to be a mainstay of therapy because it removes the risk of new disease formation. Yet, increasingly, research has shown substantial downstream risk of hysterectomy even when both ovaries are conserved, including increased cardiovascular risk, mood disorders, and urinary dysfunction (4, 122). Moreover, since long-term risks appear higher in women who have hysterectomy at a younger age, improvement in uterine-sparing therapies is critical (122).
Contraceptive steroids are used to treat early-stage symptoms and especially heavy menstrual bleeding (HMB). They neither treat the symptoms attributable to fibroid size nor allow for pregnancy. Despite their widespread off-label use, little evidence supports use of oral contraceptives for fibroid-related HMB; instead, evidence favors use of localized progestin action in the form of a progestin-releasing intrauterine device (123, 124). Newer therapies still focus on modulation of gonadal steroids.
PR modulators have been widely used outside the United States and have shown great efficacy. In the United States, concerns about rare but sometimes fatal liver toxicity have restricted their use. PR modulators are unlikely to receive approval from the US Food and Drug Administration (125). A new therapeutic possibility is the use of oral GnRH antagonists. The goal of this therapy is to suppress endogenous gonadal steroid hormone production coupled with estrogen and progestin to recreate the steroidal milieu of the early follicular phase and thereby to abrogate fibroid symptoms but not provoke hypogonadal symptoms (5, 25).
Given the near ubiquity of subclinical disease, especially for women of color, prevention of growth may be more successful than targeting clinical lesions (2). However, tools to accurately predict future growth do not exist. Data demonstrate that fibroids can regress, so targeting the regression pathway is another unexplored treatment route (118).
Dietary intervention as a therapeutic approach to leiomyoma treatments has only recently begun to be considered (40, 126). Several different plant-based compounds have been investigated. Studies carried out in quail have tested dietary lycopene, an antioxidant, to slow the growth of leiomyomas and have shown both reduced size and a 40–50% decrease in the incidence of oviductal leiomyomas (127, 128).
Curcumin, a curcuminoid found in turmeric that has strong anti-inflammatory and antioxidant properties, has also been studied. Malik et al (129) found that curcumin treatment of human leiomyoma cells in culture inhibited proliferation, increased apoptosis, and decreased expression of fibronectin production in a dose-dependent manner. In addition, Tsuiji et al (130) confirmed the effects of curcumin on proliferation and apoptosis of cultured ELT-3 leiomyoma cells. These effects of curcumin occurred through activation of peroxisome proliferator-activated receptor-gamma.
Clinical pilot studies and studies in animal models are needed to determine the mechanisms by which such plant-based anti-inflammatory compounds affect the growth of uterine leiomyomas.
Interest is strong in the use of vitamin D3 supplementation as a dietary treatment of leiomyomas. Several studies have reported that serum vitamin D3 levels are inversely correlated with leiomyoma tumor size, and African American women often have vitamin D3 deficiency (131, 132). Vitamin D3 is recognized not only as an antifibrotic agent (103, 133) but also for its anti-inflammatory effects (40). Recent studies by Halder, Al-Hendy, and colleagues (44, 132, 134, 135) have focused on 1,25-hydroxyvitamin D3 supplementation as a dietary intervention for leiomyomas. The investigators reported that 1) the vitamin D receptor activator paricalcitol inhibited leiomyoma tumor formation in Eker rats and 2) vitamin D3 inhibited proliferation and collagen production in cultured human leiomyoma cells (44, 134). Using a mouse xenograft model in which rat ELT-3 cells were injected into mice, these investigators also demonstrated that treatment with oral paricalcitol caused significant size reduction in the leiomyoma tumors that formed (135). Vitamin D3 supplementation of cultured leiomyoma cells also decreased DNA damage by increasing the expression of DNA repair genes (136). Taken together, these studies support that vitamin D3 has strong potential as a dietary intervention therapy for uterine leiomyomas. However, additional in vivo studies and clinical trials are needed.
Summary
Uterine fibroids are a major disease of women, yet the science of the disease has been limited by an overreliance on hysterectomy as treatment. Important strides have been made in understanding the genesis of these tumors and the mechanisms regulating their growth (FIGURE 3). Exploration of facets other than steroid responsiveness should lead to better treatments and prevention strategies. Moreover, distinct lesions such as leiomyoma variants may provide model systems for investigating such fundamental disease processes as malignancy.
FIGURE 3.
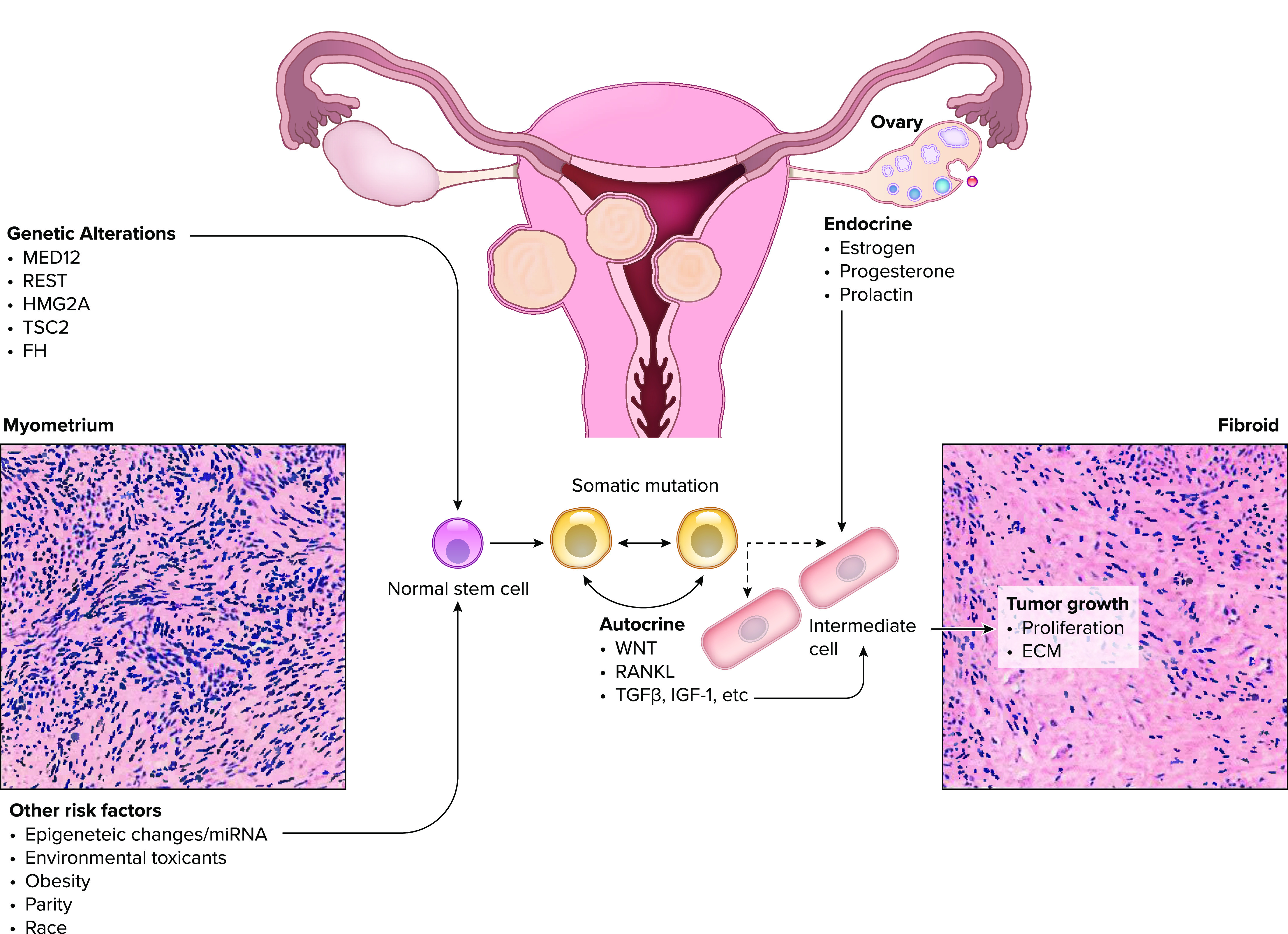
The genetic and environmental factors contributing to initiation and growth of uterine leiomyoma tumors Somatic mutations of the stem cell population are thought to have a major role in initiation of tumors while the subsequent phenotype, and progression is influenced by environmental and epigenetic factors. miRNA, microRNA; RANKL, receptor activator of NF-κB; TGFβ, transforming growth factor β; Wnt, wingless related integration site.
Acknowledgments
We thank Tauseef Shah Bashir for help in creating the figures for this manuscript and Barbara L. Beckel Eppard for expert technical assistance.
R. A. Nowak received funding from the National Institutes of Health related to uterine fibroids (R21 HD085530 and PO1HD057877). E. A. Stewart has received research support from the National Institutes for Health related to uterine fibroids (P50HS023418).
E. A. Stewart has been a consultant for AbbVie, Bayer, ObsEva, and Myovant. She holds a patent for Methods and Compounds for Treatment of Abnormal Uterine Bleeding (US 6440445), which has no commercial activity. She has received royalties from UpToDate and payments for the development of educational content from the Med Learning Group PER, Massachusetts Medical Society, and Peer View. R. A. Nowak has no conflicts of interest, financial or otherwise, to disclose.
E.A.S. and R.A.N. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
References
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 188: 100–107, 2003. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Laughlin-Tommaso SK, Stewart EA. Moving toward individualized medicine for uterine leiomyomas. Obstet Gynecol 132: 961–971, 2018. doi: 10.1097/AOG.0000000000002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 308: 1589–1592, 2005. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 4.Stewart EA, Missmer SA, Rocca WA. Moving beyond reflexive and prophylactic gynecologic surgery. Mayo Clin Proc 96: 291–294, 2021. doi: 10.1016/j.mayocp.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hendy A, Lukes AS, Poindexter AN 3rd, Venturella R, Villarroel C, Critchley HO, Li Y, McKain L, Arjona Ferreira JC, Langenberg AG, Wagman RB, Stewart EA. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med 384: 630–642, 2021. doi: 10.1056/NEJMoa2008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol 210: 194–199, 2014. doi: 10.1016/j.ajog.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sengoba KS, Ghant MS, Okeigwe I, Mendoza G, Marsh EE. Racial/ethnic differences in women's experiences with symptomatic uterine fibroids: a qualitative assessment. J Racial Ethn Health Disparities 4: 178–183, 2017. doi: 10.1007/s40615-016-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril 106: 766–772, 2016. doi: 10.1016/j.fertnstert.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prusinski Fernung LE, Al-Hendy A, Yang Q. A preliminary study: human fibroid Stro-1+/CD44+ stem cells isolated from uterine fibroids demonstrate decreased DNA repair and genomic integrity compared to adjacent myometrial Stro-1+/CD44+. Reprod Sci 26: 619–638, 2019. doi: 10.1177/1933719118783252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 22: 807–816, 2013. doi: 10.1089/jwh.2013.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 206: 211.e1–211.e9, 2012. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science 150: 67–69, 1965. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- 13.Goad J, Rudolph J, Wei JJ, Bulun SE, Chakravarti D, Rajkovic A. Single cell atlas of uterine myometrium and leiomyomas reveals diverse and novel cell types of non-monoclonal origin. bioRxiv 402313, 2020. doi: 10.1101/2020.12.21.402313. [DOI]
- 14.Holdsworth-Carson SJ, Zhao D, Cann L, Bittinger S, Nowell CJ, Rogers PA. Differences in the cellular composition of small versus large uterine fibroids. Reproduction 152: 467–480, 2016. doi: 10.1530/REP-16-0216. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Serna VA, Thomas J, Qiang W, Blumenfeld ML, Kurita T. Subtype-specific tumor-associated fibroblasts contribute to the pathogenesis of uterine leiomyoma. Cancer Res 77: 6891–6901, 2017. doi: 10.1158/0008-5472.CAN-17-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod 12: 187–207, 2006. doi: 10.1093/molehr/gal018. [DOI] [PubMed] [Google Scholar]
- 17.Holdsworth-Carson SJ, Zaitseva M, Girling JE, Vollenhoven BJ, Rogers PA. Common fibroid-associated genes are differentially expressed in phenotypically dissimilar cell populations isolated from within human fibroids and myometrium. Reproduction 147: 683–692, 2014. doi: 10.1530/REP-13-0580. [DOI] [PubMed] [Google Scholar]
- 18.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol 195: 415–420, 2006. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin H, Lo JH, Kim JY, Marsh EE, Kim JJ, Ghosh AK, Bulun S, Chakravarti D. Expression profiling of nuclear receptors identifies key roles of NR4A subfamily in uterine fibroids. Mol Endocrinol 27: 726–740, 2013. doi: 10.1210/me.2012-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lelièvre S, Weaver VM, Bissell MJ. Extracellular matrix signaling from the cellular membrane skeleton to the nuclear skeleton: a model of gene regulation. Recent Prog Horm Res 51: 417–432, 1996. [PMC free article] [PubMed] [Google Scholar]
- 21.Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci 16: 1153–1164, 2009. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer 40: 204–217, 2004. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, Terrill P, Osterloh I, Loumaye E. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 366: 409–420, 2012. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151: 2433–2442, 2010. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlaff WD, Ackerman RT, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, Carr BR, Feinberg EC, Hurtado SM, Kim J, Liu R, Mabey RG Jr, Owens CD, Poindexter A, Puscheck EE, Rodriguez-Ginorio H, Simon JA, Soliman AM, Stewart EA, Watts NB, Muneyyirci-Delale O. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med 382: 328–340, 2020. doi: 10.1056/NEJMoa1904351. [DOI] [PubMed] [Google Scholar]
- 26.Thomas BJ, Kan-O K, Loveland KL, Elias JA, Bardin PG. In the shadow of fibrosis: innate immune suppression mediated by transforming growth factor-beta. Am J Respir Cell Mol Biol 55: 759–766, 2016. doi: 10.1165/rcmb.2016-0248PS. [DOI] [PubMed] [Google Scholar]
- 27.Kuo FT, Fan K, Ambartsumyan G, Menon P, Ketefian A, Bentsi-Barnes IK, Pisarska MD. Relative expression of genes encoding SMAD signal transduction factors in human granulosa cells is correlated with oocyte quality. J Assist Reprod Genet 28: 931–938, 2011. doi: 10.1007/s10815-011-9609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab 86: 913–920, 2001. doi: 10.1210/jc.86.2.913. [DOI] [PubMed] [Google Scholar]
- 29.Saito A, Horie M, Nagase T. TGF-beta signaling in lung health and disease. Int J Mol Sci 19: 2460, 2018. doi: 10.3390/ijms19082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao H, Sin TK, Zhang G. Activin A induces leiomyoma cell proliferation, extracellular matrix (ECM) accumulation and myofibroblastic transformation of myometrial cells via p38 MAPK. Biochem Biophys Res Commun 504: 447–453, 2018. doi: 10.1016/j.bbrc.2018.08.171. [DOI] [PubMed] [Google Scholar]
- 31.Bao H, Sin TK, Zhang G. Activin A induces tumorigenesis of leiomyoma via regulation of p38beta MAPK-mediated signal cascade. Biochem Biophys Res Commun 529: 379–385, 2020. doi: 10.1016/j.bbrc.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 32.Bernacchioni C, Ciarmela P, Vannuzzi V, Greco S, Vannuccini S, Malentacchi F, Pellegrino P, Capezzuoli T, Sorbi F, Cencetti F, Bruni P, Donati C, Petraglia F. Sphingosine 1-phosphate signaling in uterine fibroids: implication in activin A pro-fibrotic effect. Fertil Steril 115: 1576–1585, 2021. [DOI] [PubMed] [Google Scholar]
- 33.Chodankar R, Critchley HO. Biomarkers in abnormal uterine bleeding. Biol Reprod 101: 1155–1166, 2019. doi: 10.1093/biolre/ioy231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sajewicz M, Konarska M, Wrona AN, Aleksandrovych V, Bereza T, Komnata K, Solewski B, Maleszka A, Depukat P, Warchol Ł. Vascular density, angiogenesis and pro-angiogenic factors in uterine fibroids. Folia Med Cracov 56: 27–32, 2016. [PubMed] [Google Scholar]
- 35.Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R, Virolainen E, Böhling T, Koski TA, Launonen V, Sjöberg J, Taipale J, Vahteristo P, Aaltonen LA. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 334: 252–255, 2011. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 36.Mehine M, Kaasinen E, Mäkinen N, Katainen R, Kämpjärvi K, Pitkänen E, Heinonen HR, Bützow R, Kilpivaara O, Kuosmanen A, Ristolainen H, Gentile M, Sjöberg J, Vahteristo P, Aaltonen LA. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med 369: 43–53, 2013. doi: 10.1056/NEJMoa1302736. [DOI] [PubMed] [Google Scholar]
- 37.Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest 125: 3280–3284, 2015. doi: 10.1172/JCI81534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA; Multiple Leiomyoma Consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30: 406–410, 2002. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 39.Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril 102: 621–629, 2014. doi: 10.1016/j.fertnstert.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 40.Barker T, Martins TB, Hill HR, Kjeldsberg CR, Dixon BM, Schneider ED, Henriksen VT, Weaver LK. Vitamin D sufficiency associates with an increase in anti-inflammatory cytokines after intense exercise in humans. Cytokine 65: 134–137, 2014. doi: 10.1016/j.cyto.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Bulun SE, Moravek MB, Yin P, Ono M, Coon JS 5th, Dyson MT, Navarro A, Marsh EE, Zhao H, Maruyama T, Chakravarti D, Kim JJ, Wei JJ. Uterine leiomyoma stem cells: linking progesterone to growth. Semin Reprod Med 33: 357–365, 2015. doi: 10.1055/s-0035-1558451. [DOI] [PubMed] [Google Scholar]
- 42.Commandeur AE, Styer AK, Teixeira JM. Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum Reprod Update 21: 593–615, 2015. doi: 10.1093/humupd/dmv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drosch M, Schmidt N, Markowski DN, Zollner TM, Koch M, Bullerdiek J. The CD24hi smooth muscle subpopulation is the predominant fraction in uterine fibroids. Mol Hum Reprod 20: 664–676, 2014. doi: 10.1093/molehr/gau022. [DOI] [PubMed] [Google Scholar]
- 44.Brakta S, Diamond JS, Al-Hendy A, Diamond MP, Halder SK. Role of vitamin D in uterine fibroid biology. Fertil Steril 104: 698–706, 2015. doi: 10.1016/j.fertnstert.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson AL, George JW, Chatterjee A, Carpenter TJ, Wolfrum E, Chesla DW, Teixeira JM. Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties. Hum Reprod 35: 44–57, 2020. doi: 10.1093/humrep/dez247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikhena DE, Liu S, Kujawa S, Esencan E, Coon JS 5th, Robins J, Bulun SE, Yin P. RANKL/RANK pathway and its inhibitor RANK-Fc in uterine leiomyoma growth. J Clin Endocrinol Metab 103: 1842–1849, 2018. doi: 10.1210/jc.2017-01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Yin P, Kujawa SA, Coon JS 5th, Okeigwe I, Bulun SE. Progesterone receptor integrates the effects of mutated MED12 and altered DNA methylation to stimulate RANKL expression and stem cell proliferation in uterine leiomyoma. Oncogene 38: 2722–2735, 2019. doi: 10.1038/s41388-018-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moravek MB, Yin P, Coon JS 5th, Ono M, Druschitz SA, Malpani SS, Dyson MT, Rademaker AW, Robins JC, Wei JJ, Kim JJ, Bulun SE. Paracrine pathways in uterine leiomyoma stem cells involve insulinlike growth factor 2 and insulin receptor A. J Clin Endocrinol Metab 102: 1588–1595, 2017. doi: 10.1210/jc.2016-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orciani M, Caffarini M, Biagini A, Lucarini G, Delli Carpini G, Berretta A, Di Primio R, Ciavattini A. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int 2018: 1716246, 2018. doi: 10.1155/2018/1716246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Yin P, Xu J, Dotts AJ, Kujawa SA, Coon JS 5th, Zhao H, Shilatifard A, Dai Y, Bulun SE. Targeting DNA methylation depletes uterine leiomyoma stem cell-enriched population by stimulating their differentiation. Endocrinology 161: bqaa143, 2020. doi: 10.1210/endocr/bqaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 9: 402, 2018. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nothnick WB. Non-coding RNAs in uterine development, function and disease. Adv Exp Med Biol 886: 171–189, 2016. doi: 10.1007/978-94-017-7417-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim YJ, Kim YY, Shin JH, Kim H, Ku SY, Suh CS. Variation in microRNA expression profile of uterine leiomyoma with endometrial cavity distortion and endometrial cavity non-distortion. Int J Mol Sci 19: 2524, 2018. doi: 10.3390/ijms19092524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosom Cancer 46: 336–347, 2007. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 55.Ciebiera M, Wlodarczyk M, Zgliczynski S, Lozinski T, Walczak K, Czekierdowski A. The role of miRNA and related pathways in pathophysiology of uterine fibroids—from bench to bedside. Int J Mol Sci 21: 3026, 2020. doi: 10.3390/ijms21083026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noetel A, Kwiecinski M, Elfimova N, Huang J, Odenthal M. microRNA are central players in anti- and profibrotic gene regulation during liver fibrosis. Front Physiol 3: 49, 2012. doi: 10.3389/fphys.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardozo ER, Foster R, Karmon AE, Lee AE, Gatune LW, Rueda BR, Styer AK. MicroRNA 21a-5p overexpression impacts mediators of extracellular matrix formation in uterine leiomyoma. Reprod Biol Endocrinol 16: 46, 2018. doi: 10.1186/s12958-018-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitzgerald JB, Chennathukuzhi V, Koohestani F, Nowak RA, Christenson LK. Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil Steril 98: 726–734.e2, 2012. doi: 10.1016/j.fertnstert.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiang W, Liu Z, Serna VA, Druschitz SA, Liu Y, Espona-Fiedler M, Wei JJ, Kurita T. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology 155: 663–669, 2014. doi: 10.1210/en.2013-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuang TD, Khorram O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil Steril 105: 236–245.e1, 2016. doi: 10.1016/j.fertnstert.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Kim JJ, Li Y, Xie J, Shao C, Wei JJ. Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma. J Mol Med (Berl) 96: 1095–1106, 2018. doi: 10.1007/s00109-018-1682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mello JB, Barros-Filho MC, Abreu FB, Cirilo PD, Domingues MA, Pontes A, Rogatto SR. MicroRNAs involved in the HMGA2 deregulation and its co-occurrence with MED12 mutation in uterine leiomyoma. Mol Hum Reprod 24: 556–563, 2018. doi: 10.1093/molehr/gay037. [DOI] [PubMed] [Google Scholar]
- 63.Peng Y, Laser J, Shi G, Mittal K, Melamed J, Lee P, Wei JJ. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res 6: 663–673, 2008. doi: 10.1158/1541-7786.MCR-07-0370. [DOI] [PubMed] [Google Scholar]
- 64.Severino MF, Murray MJ, Brandon DD, Clinton GM, Burry KA, Novy MJ. Rapid loss of oestrogen and progesterone receptors in human leiomyoma and myometrial explant cultures. Mol Hum Reprod 2: 823–828, 1996. doi: 10.1093/molehr/2.11.823. [DOI] [PubMed] [Google Scholar]
- 65.Gross KL, Neskey DM, Manchanda N, Weremowicz S, Kleinman MS, Nowak RA, Ligon AH, Rogalla P, Drechsler K, Bullerdiek J, Morton CC. HMGA2 expression in uterine leiomyomata and myometrium: quantitative analysis and tissue culture studies. Genes Chromosomes Cancer 38: 68–79, 2003. doi: 10.1002/gcc.10240. [DOI] [PubMed] [Google Scholar]
- 66.Bloch J, Holzmann C, Koczan D, Helmke BM, Bullerdiek J. Factors affecting the loss of MED12-mutated leiomyoma cells during in vitro growth. Oncotarget 8: 34762–34772, 2017. doi: 10.18632/oncotarget.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malik M, Catherino WH. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril 97: 1287–1293, 2012. doi: 10.1016/j.fertnstert.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 68.Vidimar V, Chakravarti D, Bulun SE, Yin P, Nowak R, Wei JJ, Kim JJ. The AKT/BCL-2 axis mediates survival of uterine leiomyoma in a novel 3D spheroid model. Endocrinology 159: 1453–1462, 2018. doi: 10.1210/en.2017-03191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie J, Xu X, Yin P, Li Y, Guo H, Kujawa S, Chakravarti D, Bulun S, Kim JJ, Wei JJ. Application of ex-vivo spheroid model system for the analysis of senescence and senolytic phenotypes in uterine leiomyoma. Lab Invest 98: 1575–1587, 2018. doi: 10.1038/s41374-018-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chuang TD, Rehan A, Khorram O. Functional role of the long noncoding RNA X-inactive specific transcript in leiomyoma pathogenesis. Fertil Steril 115: 238–247, 2021. doi: 10.1016/j.fertnstert.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W, Zhou L, Wang J, Zhang X, Liu G. Circular RNA expression profiling identifies novel biomarkers in uterine leiomyoma. Cell Signal 76: 109784, 2020. doi: 10.1016/j.cellsig.2020.109784. [DOI] [PubMed] [Google Scholar]
- 72.Harris HR, Eliassen AH, Doody DR, Terry KL, Missmer SA. Dietary fat intake, erythrocyte fatty acids, and risk of uterine fibroids. Fertil Steril 114: 837–847, 2020. doi: 10.1016/j.fertnstert.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wise LA, Palmer JR, Rosenberg L, Haddad SA, Ruiz-Narváez EA. FASN, dietary fat intake, and risk of uterine leiomyomata in the Black Women’s Health Study. Fertil Steril 106: 1136–1141, 2016. doi: 10.1016/j.fertnstert.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wise LA. Study of Environment Lifestyle and Fibroids (SELF): advancing the field of fibroid epidemiology. J Womens Health (Larchmt) 24: 862–864, 2015. doi: 10.1089/jwh.2015.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart EA, Borah BJ. Uterine fibroids and hypertension: steps toward understanding the link. J Clin Endocrinol Metab 106: e1039–e1041, 2021. doi: 10.1210/clinem/dgaa829. [DOI] [PubMed] [Google Scholar]
- 76.Fischer NM, Nieuwenhuis TO, Singh B, Yenokyan G, Segars JH. Angiotensin-converting enzyme inhibitors reduce uterine fibroid incidence in hypertensive women. J Clin Endocrinol Metab 106: e650–e659, 2021. doi: 10.1210/clinem/dgaa718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Afrin S, Islam MS, Patzkowsky K, Malik M, Catherino WH, Segars JH, Borahay MA. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am J Obstet Gynecol 223: 733.e1–733.e14, 2020. doi: 10.1016/j.ajog.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amir S, Shah ST, Mamoulakis C, Docea AO, Kalantzi OI, Zachariou A, Calina D, Carvalho F, Sofikitis N, Makrigiannakis A, Tsatsakis A. Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: a review. Int J Environ Res Public Health 18: 1464, 2021. doi: 10.3390/ijerph18041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fucic A, Galea KS, Duca RC, El Yamani M, Frery N, Godderis L, Halldorsson TI, Iavicoli I, Ndaw S, Ribeiro E, Viegas S, Moshammer H. Potential health risk of endocrine disruptors in construction sector and plastics industry: a new paradigm in occupational health. Int J Environ Res Public Health 15: 1229, 2018. doi: 10.3390/ijerph15061229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36: E1–E150, 2015. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canipari R, De Santis L, Cecconi S. Female fertility and environmental pollution. Int J Environ Res Public Health 17: 8802, 2020. doi: 10.3390/ijerph17238802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Street ME, Angelini S, Bernasconi S, Burgio E, Cassio A, Catellani C, Cirillo F, Deodati A, Fabbrizi E, Fanos V, Gargano G, Grossi E, Iughetti L, Lazzeroni P, Mantovani A, Migliore L, Palanza P, Panzica G, Papini AM, Parmigiani S, Predieri B, Sartori C, Tridenti G, Amarri S. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: highlights from a national Italian meeting. Int J Mol Sci 19: 1647, 2018. doi: 10.3390/ijms19061647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baird DD, Kesner JS, Dunson DB. Luteinizing hormone in premenopausal women may stimulate uterine leiomyomata development. J Soc Gynecol Investig 13: 130–135, 2006. doi: 10.1016/j.jsgi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Hunter DS, Hodges LC, Vonier PM, Fuchs-Young R, Gottardis MM, Walker CL. Estrogen receptor activation via activation function 2 predicts agonism of xenoestrogens in normal and neoplastic cells of the uterine myometrium. Cancer Res 59: 3090–3099, 1999. [PubMed] [Google Scholar]
- 85.Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci USA 102: 8644–8649, 2005. doi: 10.1073/pnas.0503218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen Y, Xu Q, Ren M, Feng X, Cai Y, Gao Y. Measurement of phenolic environmental estrogens in women with uterine leiomyoma. PLoS One 8: e79838, 2013. doi: 10.1371/journal.pone.0079838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen Y, Dong YM, Lu Q, Xu J, Wu YT, Yun SS, Ren ML. Phenolic environmental estrogens in urine and blood plasma from women with uterine leiomyoma: epidemiological survey. J Obstet Gynaecol Res 42: 440–445, 2016. doi: 10.1111/jog.12928. [DOI] [PubMed] [Google Scholar]
- 88.Zota AR, Geller RJ, Calafat AM, Marfori CQ, Baccarelli AA, Moawad GN. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril 111: 112–121, 2019. doi: 10.1016/j.fertnstert.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He Y, Zeng Q, Dong S, Qin L, Li G, Wang P. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: a case-control study in China. Asia Pac J Clin Nutr 22: 109–117, 2013. doi: 10.6133/apjcn.2013.22.1.07. [DOI] [PubMed] [Google Scholar]
- 90.Kim HJ, Kim SH, Oh YS, Heo SH, Kim KH, Kim DY, Lee SR, Chae HD. Effects of phthalate esters on human myometrial and fibroid cells: cell culture and NOD-SCID mouse data. Reprod Sci 28: 479–487, 2021. doi: 10.1007/s43032-020-00341-0. [DOI] [PubMed] [Google Scholar]
- 91.Prusinski Fernung LE, Yang Q, Sakamuro D, Kumari A, Mas A, Al-Hendy A. Endocrine disruptor exposure during development increases incidence of uterine fibroids by altering DNA repair in myometrial stem cells. Biol Reprod 99: 735–748, 2018. doi: 10.1093/biolre/ioy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elkafas H, Ali M, Elmorsy E, Kamel R, Thompson WE, Badary O, Al-Hendy A, Yang Q. Vitamin D3 ameliorates DNA damage caused by developmental exposure to endocrine disruptors in the uterine myometrial stem cells of Eker rats. Cells 9: 1459, 2020. doi: 10.3390/cells9061459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andersen J. Comparing regulation of the connexin43 gene by estrogen in uterine leiomyoma and pregnancy myometrium. Environ Health Perspect 108, Suppl 5: 811–815, 2000. doi: 10.2307/3454311. [DOI] [PubMed] [Google Scholar]
- 94.Cesen-Cummings K, Copland JA, Barrett JC, Walker CL, Davis BJ. Pregnancy, parturition, and prostaglandins: defining uterine leiomyomas. Environ Health Perspect 108, Suppl 5: 817–820, 2000. doi: 10.1289/ehp.00108s5817. [DOI] [PubMed] [Google Scholar]
- 95.Cesen-Cummings K, Houston KD, Copland JA, Moorman VJ, Walker CL, Davis BJ. Uterine leiomyomas express myometrial contractile-associated proteins involved in pregnancy-related hormone signaling. J Soc Gynecol Investig 10: 11–20, 2003. [PubMed] [Google Scholar]
- 96.Geimonen E, Boylston E, Royek A, Andersen J. Elevated connexin-43 expression in term human myometrium correlates with elevated c-Jun expression and is independent of myometrial estrogen receptors. J Clin Endocrinol Metab 83: 1177–1185, 1998. doi: 10.1210/jcem.83.4.4695. [DOI] [PubMed] [Google Scholar]
- 97.Burroughs KD, Howe SR, Okubo Y, Fuchs-Young R, LeRoith D, Walker CL. Dysregulation of IGF-I signaling in uterine leiomyoma. J Endocrinol 172: 83–93, 2002. doi: 10.1677/joe.0.1720083. [DOI] [PubMed] [Google Scholar]
- 98.Shynlova O, Tsui P, Dorogin A, Langille BL, Lye SJ. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biol Reprod 76: 571–578, 2007. doi: 10.1095/biolreprod.106.056929. [DOI] [PubMed] [Google Scholar]
- 99.Slater DM, Dennes WJ, Campa JS, Poston L, Bennett PR. Expression of cyclo-oxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod 5: 880–884, 1999. doi: 10.1093/molehr/5.9.880. [DOI] [PubMed] [Google Scholar]
- 100.Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology 150: 4672–4680, 2009. doi: 10.1210/en.2009-0419. [DOI] [PubMed] [Google Scholar]
- 101.Varghese BV, Koohestani F, McWilliams M, Colvin A, Gunewardena S, Kinsey WH, Nowak RA, Nothnick WB, Chennathukuzhi VM. Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci USA 110: 2187–2192, 2013. doi: 10.1073/pnas.1215759110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stewart EA. Gonadotropins and the uterus: is there a gonad-independent pathway? J Soc Gynecol Investig 8: 319–326, 2001. doi: 10.1016/S1071-5576(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 103.Andersson JK, Khan Z, Weaver AL, Vaughan LE, Gemzell-Danielsson K, Stewart EA. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: a pilot study. Acta Obstet Gynecol Scand 98: 1341–1350, 2019. doi: 10.1111/aogs.13632. [DOI] [PubMed] [Google Scholar]
- 104.Nowak RA, Mora S, Diehl T, Rhoades AR, Stewart EA. Prolactin is an autocrine or paracrine growth factor for human myometrial and leiomyoma cells. Gynecol Obstet Invest 48: 127–132, 1999. doi: 10.1159/000010154. [DOI] [PubMed] [Google Scholar]
- 105.Jonas HS, Masterson BJ. Giant uterine tumors: case report and review of the literature. Obstet Gynecol 50: 2s–4s, 1977. [PubMed] [Google Scholar]
- 106.Bredhult C, Bäcklin BM, Bignert A, Olovsson M. Study of the relation between the incidence of uterine leiomyomas and the concentrations of PCB and DDT in Baltic gray seals. Reprod Toxicol 25: 247–255, 2008. doi: 10.1016/j.reprotox.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Ilha MR, Newman SJ, van Amstel S, Fecteau KA, Rohrbach BW. Uterine lesions in 32 female miniature pet pigs. Vet Pathol 47: 1071–1075, 2010. doi: 10.1177/0300985810382522. [DOI] [PubMed] [Google Scholar]
- 108.Kirejczyk S, Pinelli C, Gonzalez O, Kumar S, Dick E Jr, Gumber S. Urogenital lesions in nonhuman primates at 2 National Primate Research Centers. Vet Pathol 58: 147–160, 2021. doi: 10.1177/0300985820971752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Machado SA, Bahr JM, Hales DB, Braundmeier AG, Quade BJ, Nowak RA. Validation of the aging hen (Gallus gallus domesticus) as an animal model for uterine leiomyomas. Biol Reprod 87: 86, 2012. doi: 10.1095/biolreprod.112.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patsikas M, Papazoglou LG, Jakovljevic S, Papaioannou NG, Papadopoulou PL, Soultani CB, Chryssogonidis IA, Kouskouras KA, Tziris NE, Charitanti AA. Radiographic and ultrasonographic findings of uterine neoplasms in nine dogs. J Am Anim Hosp Assoc 50: 330–337, 2014. doi: 10.5326/JAAHA-MS-6130. [DOI] [PubMed] [Google Scholar]
- 111.Stewart EA. Uterine Fibroids: The Complete Guide. Baltimore, MD: Johns Hopkins University Press, 2007, p. 223. [Google Scholar]
- 112.Awonuga AO, Shavell VI, Imudia AN, Rotas M, Diamond MP, Puscheck EE. Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv 65: 189–195, 2010. doi: 10.1097/OGX.0b013e3181d60f93. [DOI] [PubMed] [Google Scholar]
- 113.Quade BJ, Dal Cin P, Neskey DM, Weremowicz S, Morton CC. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol 15: 351–356, 2002. doi: 10.1038/modpathol.3880529. [DOI] [PubMed] [Google Scholar]
- 114.Quade BJ, McLachlin CM, Soto-Wright V, Zuckerman J, Mutter GL, Morton CC. Disseminated peritoneal leiomyomatosis. Clonality analysis by X chromosome inactivation and cytogenetics of a clinically benign smooth muscle proliferation. Am J Pathol 150: 2153–2166, 1997. [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka YO, Nishida M, Tsunoda H, Okamoto Y, Yoshikawa H. Smooth muscle tumors of uncertain malignant potential and leiomyosarcomas of the uterus: MR findings. J Magn Reson Imaging 20: 998–1007, 2004. doi: 10.1002/jmri.20207. [DOI] [PubMed] [Google Scholar]
- 116.Henske EP, McCormack FX. Lymphangioleiomyomatosis—a wolf in sheep’s clothing. J Clin Invest 122: 3807–3816, 2012. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker CL, Hunter D, Everitt JI. Uterine leiomyoma in the Eker rat: a unique model for important diseases of women. Genes Chromosomes Cancer 38: 349–356, 2003. doi: 10.1002/gcc.10281. [DOI] [PubMed] [Google Scholar]
- 118.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, Baird DD. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA 105: 19887–19892, 2008. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2: 16043, 2016. doi: 10.1038/nrdp.2016.43. [DOI] [PubMed] [Google Scholar]
- 120.Hartmann KE, Fonnesbeck C, Surawicz T, Krishnaswami S, Andrews JC, Wilson JE, Velez Edwards DR, Kugley S, Sathe NA. Management of Uterine Fibroids. Comparative Effectiveness Review No. 195 [AHRQ Publication No. 17(18)-EHC028-EF]. Rockville, MD: Agency for Healthcare Research and Quality, 2017. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 121.Manyonda I, Belli AM, Lumsden MA, Moss J, McKinnon W, Middleton LJ, Cheed V, Wu O, Sirkeci F, Daniels JP, McPherson K; FEMME Collaborative Group. Uterine-artery embolization or myomectomy for uterine fibroids. N Engl J Med 383: 440–451, 2020. doi: 10.1056/NEJMoa1914735. [DOI] [PubMed] [Google Scholar]
- 122.Laughlin-Tommaso SK, Khan Z, Weaver AL, Schleck CD, Rocca WA, Stewart EA. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause 23: 121–128, 2016. doi: 10.1097/GME.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstet Gynecol 137: e100–e115, 2021. doi: 10.1097/AOG.0000000000004401. [DOI] [PubMed] [Google Scholar]
- 124.Sangkomkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev 11: CD008994, 2020. doi: 10.1002/14651858.CD008994.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mahase E. Uterine fibroid drug is recalled after case of liver failure requiring transplant prompts EU review. BMJ 368: m1112, 2020. doi: 10.1136/bmj.m1112. [DOI] [PubMed] [Google Scholar]
- 126.Islam MS, Akhtar MM, Ciavattini A, Giannubilo SR, Protic O, Janjusevic M, Procopio AD, Segars JH, Castellucci M, Ciarmela P. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: promising options for prevention and treatment of uterine fibroids? Mol Nutr Food Res 58: 1667–1684, 2014. doi: 10.1002/mnfr.201400134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sahin K, Ozercan R, Onderci M, Sahin N, Gursu MF, Khachik F, Sarkar FH, Munkarah A, Ali-Fehmi R, Kmak D, Kucuk O. Lycopene supplementation prevents the development of spontaneous smooth muscle tumors of the oviduct in Japanese quail. Nutr Cancer 50: 181–189, 2004. doi: 10.1207/s15327914nc5002_8. [DOI] [PubMed] [Google Scholar]
- 128.Sahin K, Ozercan R, Onderci M, Sahin N, Khachik F, Seren S, Kucuk O. Dietary tomato powder supplementation in the prevention of leiomyoma of the oviduct in the Japanese quail. Nutr Cancer 59: 70–75, 2007. doi: 10.1080/01635580701365076. [DOI] [PubMed] [Google Scholar]
- 129.Malik M, Mendoza M, Payson M, Catherino WH. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril 91: 2177–2184, 2009. doi: 10.1016/j.fertnstert.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 130.Tsuiji K, Takeda T, Li B, Wakabayashi A, Kondo A, Kimura T, Yaegashi N. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol Endocrinol 27: 512–517, 2011. doi: 10.3109/09513590.2010.507287. [DOI] [PubMed] [Google Scholar]
- 131.Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology 24: 447–453, 2013. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health 5: 93–100, 2013. doi: 10.2147/IJWH.S38800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zerr P, Vollath S, Palumbo-Zerr K, Tomcik M, Huang J, Distler A, Beyer C, Dees C, Gela K, Distler O, Schett G, Distler JH. Vitamin D receptor regulates TGF-beta signalling in systemic sclerosis. Ann Rheum Dis 74: e20, 2015. doi: 10.1136/annrheumdis-2013-204378. [DOI] [PubMed] [Google Scholar]
- 134.Halder SK, Sharan C, Al-Hendy A. 1,25-Dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod 86: 116, 2012. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halder SK, Sharan C, Al-Hendy O, Al-Hendy A. Paricalcitol, a vitamin d receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod Sci 21: 1108–1119, 2014. doi: 10.1177/1933719114537721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol Sin 40: 957–970, 2019. doi: 10.1038/s41401-018-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou F, Zhang L, Liu A, Shen Y, Yuan J, Yu X, Feng X, Xu Q, Cheng C. Measurement of phenolic environmental estrogens in human urine samples by HPLC-MS/MS and primary discussion the possible linkage with uterine leiomyoma. J Chromatogr B Analyt Technol Biomed Life Sci 938: 80–85, 2013. doi: 10.1016/j.jchromb.2013.08.032. [DOI] [PubMed] [Google Scholar]