Abstract
Obesity is associated with higher risks of cardiac arrhythmias. Although this may be partly explained by concurrent cardiometabolic ill-health, growing evidence suggests that increasing adiposity independently confers risk for arrhythmias. Among fat depots, epicardial adipose tissue (EAT) exhibits a proinflammatory secretome and, given the lack of fascial separation, has been implicated as a transducer of inflammation to the underlying myocardium. The present review explores the mechanisms underpinning adverse electrophysiological remodeling as a consequence of EAT accumulation and the consequent inflammation. We first describe the physiological and pathophysiological function of EAT and its unique secretome and subsequently discuss the evidence for ionic channel and connexin expression modulation as well as fibrotic remodeling induced by cytokines and free fatty acids that are secreted by EAT. Finally, we highlight how weight reduction and regression of EAT volume may cause reverse remodeling to ameliorate arrhythmic risk.
Keywords: arrhythmia, epicardial adipose tissue, inflammation, obesity
INTRODUCTION
Atrial fibrillation is the most common arrhythmia globally and its impact on healthcare systems is forecast to increase with an aging population (1, 2). Although ventricular arrhythmias are comparably less common, they are nonetheless associated with significant morbidity and higher risk of mortality (3). With better understanding of the mechanisms underpinning arrhythmogenesis and ability to manage arrhythmias, there has been increasing interest in preventative measures to reduce their prevalence.
Among the cardiovascular risk factors, there has been a particular focus on adiposity and obesity in generating a proarrhythmic substrate (4). Obesity is an economically burdensome pandemic, which has been associated with increased arrhythmic risk (5). It is a multifaceted syndrome that often coexists with cardiometabolic perturbation, such as hypertension and obstructive sleep apnea, which may themselves confer increased risk of arrhythmias (6–8). Nevertheless, there is now compelling evidence linking adipose tissue volume independently with arrhythmic risk and weight loss with arrhythmia-free survival (9–11). Although obesity is characterized by excess whole body adiposity, visceral adipose tissue (VAT) has been shown to be more detrimental to health than its subcutaneous counterpart (12). Consistent with this, epicardial adipose tissue (EAT) has been implicated in arrhythmogenesis owing to its intimate relationship with the underlying myocardium (13, 14).
In this review, we first outline the classification of adipose tissue and the anatomy, physiology, and pathophysiology of epicardial adipose tissue (EAT). We then summarize the evidence for its role as a transducer of inflammation to the underlying myocardium and outline the mechanisms by which it may induce an arrhythmogenic substrate. Finally, we review the evidence for the reversibility of the proarrhythmic substrate associated with obesity with weight reduction strategies.
EPICARDIAL ADIPOSE TISSUE: HISTOLOGY, ANATOMY, AND PHYSIOLOGY
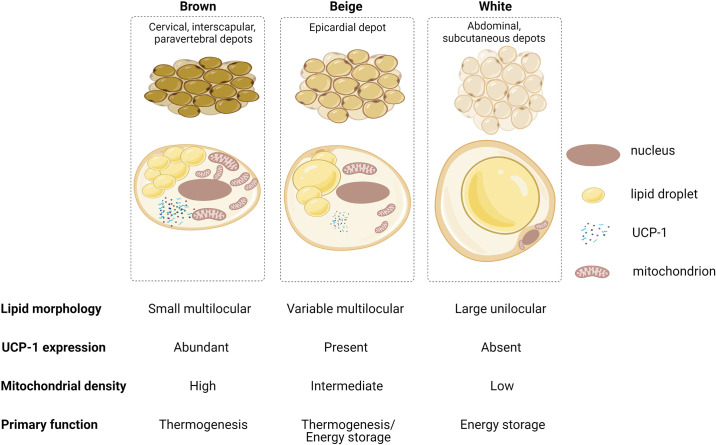
Adipose tissue can be broadly categorized as white, beige, or brown (Fig. 1). White adipose tissue (WAT) is widely distributed throughout the body as either subcutaneous or visceral fat. Its main function is that of energy storage, and hence, its adipocytes consist of a single lipid droplet of triglycerides, giving it a characteristic yellow color, with minimal space for mitochondria, which may be thin, elongated, and often variable in number (15). WAT also secretes a range of hormones, cytokines, complement and growth factors that have both endocrine and paracrine effects on adjacent and distant tissues (16). On the other hand, brown adipose tissue (BAT) is commonly located in cervical, supraclavicular, axillary, paravertebral, mediastinal, and upper abdominal regions and has an antagonistic role to WAT; it dissipates energy through thermogenesis (15, 17). The cellular composition of BAT reflects its function, in that there is abundance of mitochondria within the adipocytes giving it a brown hue, and triglycerides are stored as small vacuoles. BAT expresses uncoupling protein-1 (UCP-1), which has been implicated in nonshivering thermogenesis by uncoupling mitochondrial oxidative phosphorylation. It also has a denser microcirculation and sympathetic innervation owing to a greater oxygen demand and its need to respond to thermogenesis (15). Because of this unique property, BAT is widely found in small mammals and newborns in which the high surface area-to-volume ratio predisposes them to heat loss. In adulthood, BAT has a minimal role in thermoregulation as core body temperature is regulated via other mechanisms, such as muscle shivering and insulation with WAT.
Figure 1.
Characteristics of brown, beige, and white adipose tissue. Brown adipose tissue (BAT) is mainly located in cervical, interscapular, supraclavicular, and paravertebral regions. Brown adipocytes contain several small vacuolated lipid droplets, contain multiple mitochondria that give them characteristic color, and express uncoupling protein-1 (UCP-1) in relative abundance. These features reflect their primary function in nonshivering thermogenesis. By contrast, white adipose tissue (WAT) is more widely distributed subcutaneously and viscerally. WAT adipocytes typically contain a single large lipid droplet with few mitochondria and minimal UCP-1 and serve as an energy store. Beige adipose tissue, of which epicardial fat is example, is an intermediate phenotype characterized by multiple loculated lipid droplets with a mitochondrial density and UCP-1 expression that is greater than WAT but less than BAT. Beige adipose tissue contributes to thermogenesis as well as serving as an energy store. Created with BioRender.com, and published with permission.
Beige adipose tissue (BeAT), also called “brite” adipocytes, is an intermediate phenotype that is characterized by WAT and BAT features. In contrast to WAT and BAT, BeAT develops postnatally and is inducible. It typically develops within the subcutaneous WAT from a distinct preadipocyte population (18) or via transdifferentiation of white adipocytes (19, 20), i.e., “browning” of WAT. Like BAT, BeAT exhibits multilocular lipid droplets and UCP-1 expression and contains a dense population of mitochondria and therefore functionally regulates energy balance and thermogenesis (Fig. 1). Wu et al. (18) showed multilocular beige adipocytes can develop from a subset of unilocular preadipocyte upon noradrenergic stimulation, characterized by an enhanced expression of thermogenic genes such as UCP-1. However, despite its similarities to BAT (Fig. 1), BeAT exhibits a unique molecular signature. This includes, for example, expression of Tbx1 and molecules involved in the lipid metabolism and inflammatory response pathways (Slc27a1 and CD137, respectively) (18).
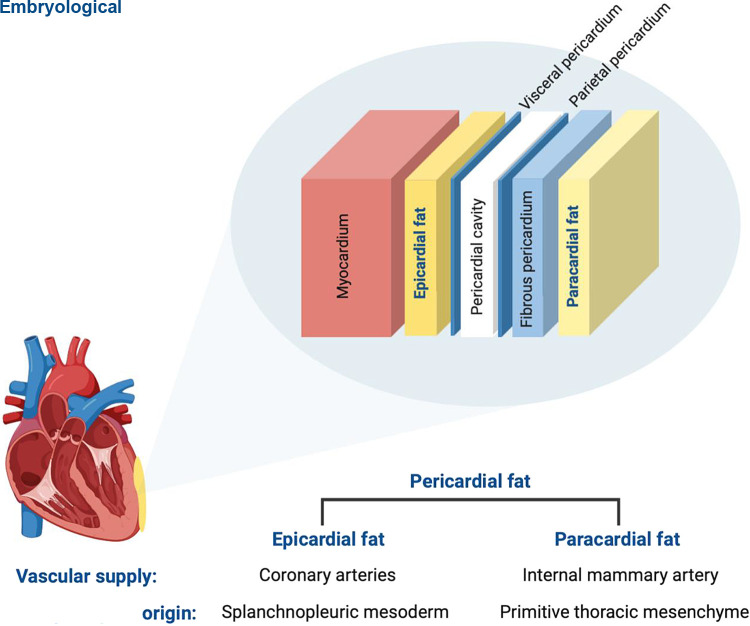
Epicardial adipose tissue (EAT) is a white visceral fat depot that uniquely exhibits BeAT features (21–23). Like BAT, EAT expresses UCP-1 and contains small multilocular adipocytes reflecting the transformation of WAT-derived precursor cells into mature thermogenic “brown-like” adipocytes (21). EAT is localized deep to the visceral pericardium, i.e., the epicardium, and therefore is in direct contact with the underlying myocardium due to a lack of a fascial boundary (Fig. 2). It can cover up to 80% of the heart surface and contributes up to 20% of the whole cardiac mass despite being primarily distributed in the atrioventricular and interventricular grooves (24, 25). EAT is unique among other cardiac adipose tissue depots as it shares the same embryological origin and circulation with the myocardium: both structures are derived from the splanchnic mesoderm and receive their blood supply from the coronary arteries (26). The anatomical proximity of EAT to myocardium and shared circulation has led to a growing body of evidence supporting a paracrine role of EAT in the development of cardiac disease. Although this evidence is compelling, inconsistent definitions of cardiac adiposity between studies is a limitation to the extrapolation of evidence between fat volume and arrhythmias. Pericardial fat is often used interchangeably with EAT, although the latter strictly refers to adipose tissue deep to the parietal pericardium. On the other hand, pericardial fat is the combination of EAT and adipose tissue external to the parietal pericardium, i.e., paracardial fat (Fig. 2).
Figure 2.
The anatomy of epicardial adipose tissue (EAT) in relation to the myocardium and surrounding structures. EAT lies deep to the visceral pericardium (i.e., the epicardium) and in direct contact with myocardial tissue. It derives from the splanchnopleuric mesoderm and draws its vascular supply from the coronary arteries. By contrast, paracardial fat refers to adipose tissue external to the parietal pericardium. Its origin is from the primitive thoracic mesenchyme and is perfused by the internal mammary artery. Pericardial fat refers to EAT and paracardial adipose tissue. Created with BioRender.com, and published with permission.
EAT has a physiological role of protecting the myocardium against potential stressors. Because of its elastic and compressible properties, it protects the coronary arteries from mechanical forces generated by arterial pulsation and myocardial contraction (27). Unlike other fat depots, EAT has a uniquely higher rate of lipogenesis and lipolysis and therefore acts as a local energy store of free fatty acids (FFAs) and also as a buffer against the lipotoxic effects of excess FFAs (28, 29). It also functions as an endocrine organ secreting a range of adipokines (26, 30, 31). In this way, EAT has been shown to modulate proliferation of vascular smooth muscle cells and vascular contractility while also exhibiting antiapoptotic and antioxidant effects (32–35). Given that low levels of UCP-1 have been linked with atrial fibrillation (AF) (36), it has been postulated that EAT, by expressing UCP-1, may also protect against hypothermia-induced fatal arrhythmia (23).
EAT AS A PATHOPHYSIOLOGICAL INFLAMMATORY SUBSTRATE
Under pathological conditions, EAT undergoes a phenotypic shift from a protective neighbor to an inflammatory substrate. For example, EAT in patients with coronary artery disease (CAD) exhibit a greater proinflammatory profile than in non-CAD counterparts (37). Both mRNA expression and protein levels of proinflammatory cytokines such as tumor necrosis factors-α (TNF-α), interleukin (IL)-6, and monocyte chemoattractant protein (MCP-1) have been shown to be upregulated, and anti-inflammatory adiponectin to be downregulated, after adjustment for concurrent hypertension, diabetes, dyslipidemia, and body mass index (BMI) (38–42). Consistent with the rise in MCP-1, histological analysis of EAT from CAD patients exhibited infiltration by M1 macrophages and CD68+ cells (43).
The shift toward a proinflammatory EAT secretome has also been observed in type 2 diabetes mellitus (T2DM), with a reduction in adiponectin and increase in expression of MCP-1 and CD68+ cells compared with nondiabetic counterparts (44). The transcriptome of EAT in T2DM patients has also been shown to primarily consist of genes coding for cytokine production and leucocyte recruitment, such as TNF-α, IL-6, and chemokine receptors CXCR1 and CXCR2 (45). By contrast, a study by Teijeira-Fernandez et al. (46) demonstrated no difference in the adipokine profiles of EAT in T2DM and non-T2DM patients. This may be explained by an older and more overweight non-T2DM cohort in which subclinical insulin resistance may have masked any differences in adipokine profiles between the two groups (46).
Pharmacotherapy in CAD and T2DM has been shown to reverse the inflammatory phenotype of EAT. In a study by Parisi et al. (47), 40 mg atorvastatin, an HMG-CoA reductase inhibitor, daily for a mean duration of 24 mo in CAD was associated with a lower EAT volume and a less inflammatory profile of the EAT secretome among patients undergoing cardiothoracic surgery. Consistent with this, in vitro application of 2 μM atorvastatin on EAT for 24 h resulted in selective suppression of proinflammatory adipokines (47). Similarly, among T2DM patients taking 25 mg pioglitazone, a thiazolidinedione, for a mean duration of 24 mo, there was reduced local expression of proinflammatory IL-1β in EAT compared with those without treatment (48). Likewise, 6-h incubation of EAT in vitro with 100 μM dapagliflozin, a reversible inhibitor of sodium-glucose cotransporter 2, resulted in decreased IL-8, CCL2, and CCL5 concentrations (49). Despite the evidence for EAT adopting a proinflammatory state in the context of cardiometabolic perturbation and its reversal with pharmacological modulation, whether this is a cause or consequence of concurrent disease processes remains contentious.
EPICARDIAL VERSUS SUBCUTANEOUS ADIPOSE TISSUE SECRETOMES
Several studies have also demonstrated that EAT exhibits a more proinflammatory profile compared with other fat depots (50–53). Mazurek et al. (50) first demonstrated mRNA and protein levels of IL-1β, IL-6 and TNF-α were greater in EAT compared with subcutaneous adipose tissue (SAT) in paired samples harvested from CAD patients. EAT was also associated with a higher concentration of macrophages, mast cells and T lymphocytes. Similarly, Baker et al. (52) showed fivefold lower concentrations of adiponectin and threefold higher resistin concentrations, as well as a greater expression of IL-6, leptin, and CD45 in EAT compared with SAT. Although these findings are consistent with Mazurek et al. (50), EAT and SAT biopsies in this study were unpaired, i.e., harvested from different patient cohorts which may confound comparisons. Addressing this by harvesting paired EAT and SAT during cardiothoracic surgery, Fain et al. (51) showed that EAT expresses a greater concentration of inflammatory adipokines, such as IL-1β, MCP-1, leptin, and TNF-α compared with sternal, abdominal and leg SAT. Adipocytes isolated from EAT and cultured in vitro also demonstrated an adipokine imbalance that favors proinflammatory pathways (54). More recently, the inflammatory characteristic of EAT has been demonstrated to have a genetic basis with 383 genes encoding cytokine-receptor interaction, focal adhesion, and complement cascades (55). Consistent with this, EAT from overweight and obese individuals exhibited a greater expression of IL-1, IL-6, TNF-α, and IFN-γ that was not observed in SAT or serum, independent of concurrent coronary artery disease or hypertension status (56).
OBESITY AND ARRHYTHMIC RISK
Obesity is characterized by excess adiposity and represents a chronic low-grade systemically proinflammatory state. Adipocytes hypertrophy with increasing adiposity creating a hypoxic environment and inducing genetic mutations that results in up- and downregulation of pro- and anti-inflammatory mediators, respectively (57, 58). Consistent with other examples of enhanced inflammatory state, obesity has been associated with increased risk of atrial and ventricular arrhythmias (59, 60). For example, AF risk may increase as much as 65% in individuals with BMI >30 kg/m2 compared with normal BMI (18.5–24.9 kg/m2), translating to a 4% increased risk for every unit increment in BMI (59, 60). This increased propensity for AF may be explained by the electrophysiological remodeling as a consequence of atrial volume expansion, conduction abnormalities, and increased expression of profibrotic mediators that contribute to generating a proarrhythmogenic substrate (61, 62). These changes are reversible with weight loss, as demonstrated in an obese ovine model in which 30% weight reduction reduced atrial pressure and fibrosis and improved conduction via increased expression of connexin-43 (Cx43) (10). In addition to systemic obesity, electrostructural remodeling has been shown to be modulated by localized adiposity, whereby changes were most pronounced in regions adjacent to EAT, implicating it with arrhythmogenesis (14).
EAT AND CARDIAC ARRHYTHMIAS
Given that EAT is a metabolically active depot that is intimately related to the myocardium, there are several lines of evidence that implicate EAT with arrhythmogenesis. This was first demonstrated by Thanassoulis et al. (63) in the Framingham Heart study in which AF risk increased by almost 30% for every 1-SD increment in volume of pericardial fat. Furthermore, AF burden correlated with the pericardial fat volume; pericardial fat in persistent AF was 23% greater than in paroxysmal AF (64). A meta-analysis of 63 observational studies encompassing 352,275 participants also reported that 1-SD increment in the volume of EAT was associated with a 2.2-fold higher risk of persistent AF compared with paroxysmal AF (4). Furthermore, EAT has been demonstrated to be a better predictor of AF risk than other measures of adiposity, including BMI, body surface area, waist circumference, waist-to-hip ratio, intrathoracic fat, and abdominal VAT (4, 63–65). EAT volume has also been shown to be as much as 34% greater in patients with recurrent AF than those in sinus rhythm (66) with several other studies implicating EAT as an independent predictor of AF recurrence following radiofrequency- and cryoballoon-based ablation (66–70). Indeed, epicardial fat and pericardial fat confer comparable incremental risks of AF (31% and 30% per standard deviation increase between EAT and pericardial adipose tissue volume, respectively) (63, 71). Furthermore, EAT volume correlates with serum CRP in those with recurrent AF suggesting inflammation as the mediator between EAT and arrhythmic risk (70). These lines of evidence suggest that adipose tissue that is in direct contact with the myocardium contributes to a proarrhythmic substrate and that it may do so by paracrine mechanisms.
On the other hand, the evidence linking EAT volume with ventricular arrhythmogenesis is conflicting. For instance, EAT thickness positively correlates with the ventricular ectopy burden (72, 73) and is predictive of QT interval prolongation (defined as >450 ms), ventricular tachycardia/fibrillation risk in the context of heart failure, and ventricular tachycardia recurrence postablation (74–76). By contrast, others do not report an association between EAT volume and QTc interval (77, 78) but rather that EAT thickness better correlates with PR interval prolongation (79) and P wave, rather than QT, dispersion (80).
DIFFERENCES IN EAT AND CARDIAC ARRHYTHMIAS BETWEEN SEXES
Epicardial adiposity is influenced by a number of factors aside from sex, such as obesity status and concurrent cardiometabolic ill health (22, 30, 81). It is therefore unsurprising that there are conflicting reports for whether EAT volume is greater in either sex. Some studies have reported higher EAT volume in females (24, 82). By contrast, it has also been shown to be as much as 29% greater in males undergoing cardiothoracic surgery compared with females (83). EAT in males from a similar cohort also revealed reduced expression of the cardioprotective adipokine adiponectin compared with females (84). Nevertheless, it is possible that differences in EAT volume between sexes may at least partly explain the higher age-adjusted lifetime AF risk in males compared with females (26.0 vs. 23.0%) (85, 86). Nevertheless, the incidence of AF in females increases postmenopause, resulting in higher burden of AF in females aged over 70 yr compared with males (74 vs. 58%) (87). Increased susceptibility to AF in this population could be mediated by an increased EAT volume after menopause and with increasing age (88, 89), specifically in the periatrial region, which may adversely alter the electrophysiological properties of the atria (90). The arrhythmogenicity associated with aging and EAT has also been demonstrated in rodents; expression of adiponectin in EAT decreases throughout the life span of female rats from 6 to 26 mo (91). It is therefore plausible that the differential hormonal profile between sexes and their changes with age, particularly in females, may contribute to arrhythmic risk.
ELECTROPHYSIOLOGICAL CHANGES INDUCED BY EAT
In vitro studies support a paracrine effect of EAT in generating an arrhythmic substrate. Lin et al. (92) studied the electrophysiological changes in rabbit left atrial (LA) myocytes induced by either epicardial, retrosternal, abdominal adipocytes or adipocyte-conditioned media for 2–4 h. When compared with control, adipocyte-incubated myocytes demonstrated prolonged action potential duration (APD) at 90% repolarization (APD90), suggesting a shared arrhythmogenic potential among different fat depots. Moreover, EAT adipocyte-incubated myocytes displayed modulation of ionic currents, which included increased late sodium (INa) and L-type calcium (ICaL) currents and decreased delayed rectifier potassium (IKr) and inward rectifier potassium currents (IK1), all of which were consistent with prolonged APD (92). Furthermore, myocytes incubated in adipocyte-conditioned media exhibited prolonged APD, supporting a paracrine mechanism of action (92). Although this effect was modest in comparison to adipocyte-myocyte coculture, the duration of incubation with conditioned media was relatively short and may have underestimated this effect. Microscopic observation of adipocyte-myocyte coculture rarely revealed direct contact between adipocytes and myocytes suggesting that a paracrine mechanism of action underpinning electrophysiological modulation was more likely than an electrotonic interaction between the two cell types. A similar APD-prolonging effect in rabbit LA myocytes was observed in the context of heart failure (HF) whereby incubation of LA myocytes with HF EAT resulted in early and delayed afterdepolarizations, as well as increased spontaneous activity (93). Ventricular myocytes incubated with EAT also demonstrated APD prolongation at 90% (APD90), 50% (APD50), and 20% (APD20) repolarization compared with cells not exposed to EAT, in addition to having a more positive resting membrane potential whereby threshold for depolarization is reached earlier and facilitating triggered activity (94). Furthermore, the inhomogeneous distribution of EAT around the myocardium may result in APD heterogeneity and thereby generate an arrhythmogenic substrate enabling reentry (94).
More recently, Nalliah et al. (95) showed a differential effect between murine pericardial versus subcutaneous fat on cell electrophysiology in vitro. HL-1 atrial cardiomyocytes incubated with pericardial fat-conditioned media for 24 h recorded slower conduction velocities and longer activation times than cells exposed to inguinal SAT. Analyzes of their respective secretomes showed that pericardial fat had an abundance of proteins associated with disruption of intermyocyte adhesion, modulation of cellular metabolism, and inflammation compared with the relatively anti-inflammatory proteome of inguinal SAT (95). This was consistent with observations of connexin40 lateralization in cells incubated with pericardial versus subcutaneous fat and explains the conduction slowing observed in the study. The relatively proinflammatory proteome of pericardial fat is also consistent with the paracrine effect of cardiac adiposity in arrhythmogenesis.
Electrotonic interactions between adipocytes and myocytes may also alter electrophysiological function of the myocardium. For example, Ten Sande et al. (96) showed that monolayers of neonatal rat ventricular myocytes incubated with human and rat adipose-derived stromal cells exhibited slower conduction than cells treated with their respective conditioned media. However, given that adipose tissue includes adipocytes and stromal cells, both of which synthesize and secrete adipokines, it is possible that conditioned media from the stromal cell fraction alone may underestimate the paracrine effect of whole adipose tissue. Interestingly, porcine adipose-stromal cell conditioned media exerted a similar degree of conduction slowing as the stromal-adipocyte coculture in the same experimental settings, supporting a species-specific adverse paracrine effect of adipose tissue on adjacent myocardium (96).
MECHANISMS UNDERLYING ADVERSE ELECTROPHYSIOLOGICAL REMODELING DUE TO EAT
Ion Channel Modulation by Cytokines
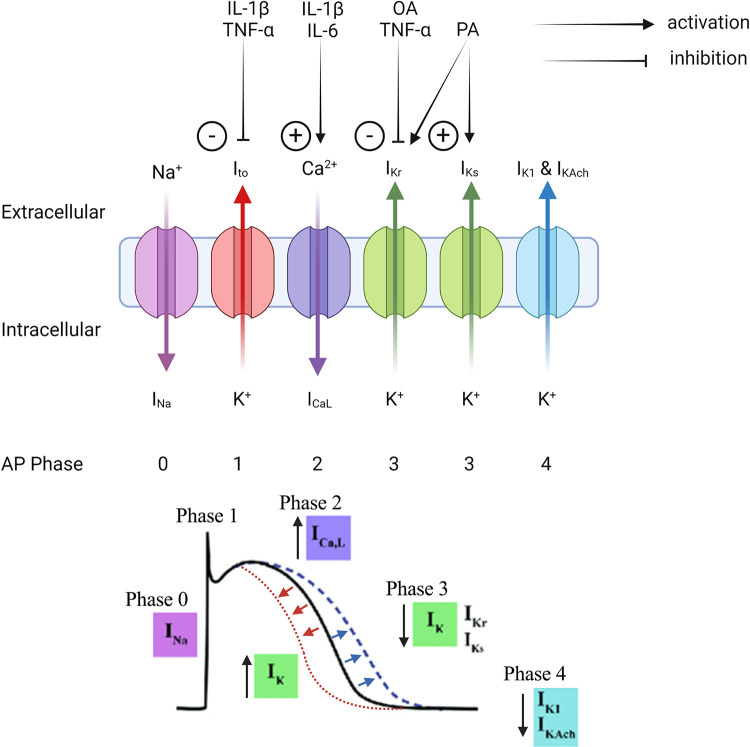
Given the considerable evidence supporting local and systemic inflammation in inducing a proarrhythmic substrate (97–106), several in vitro studies have demonstrated how cytokine-mediated ion channel modulation may underpin this phenomenon (Fig. 3). Although much of this evidence is not derived from the context of EAT, cytokines that are known to be actively secreted by EAT, such as IL-1β, TNF-α, and IL-6, have been shown to alter ion channel function and may mediate part of the increased arrhythmogenicity associated with increasing adiposity (56) (Fig. 4).
Figure 3.
Hypothetical mechanisms by which epicardial adipose tissue (EAT)-derived inflammatory cytokines and free fatty acids modulate ion channels resulting in action potential (AP) duration shortening and prolongation. EAT secretes IL-1β, TNF-α, IL-6, oleic acid (OA), and palmitic acid (PA), which exert a paracrine effect on the ion channels expressed in the membranes of myocardial cells. The activation of L-type calcium channels (ICaL) and inhibition of fast (IKr) and slow (IKs) rectifying potassium channels results in action potential prolongation (dashed blue line). By contrast, activation of the rectifying potassium currents by PA shortens action potential duration (dashed red line). INa, sodium current; IK1, inward rectifier potassium current; Ito, transient outward potassium current; IKAch, acetylcholine-activated inward-rectifying potassium current. Created with BioRender.com, and published with permission.
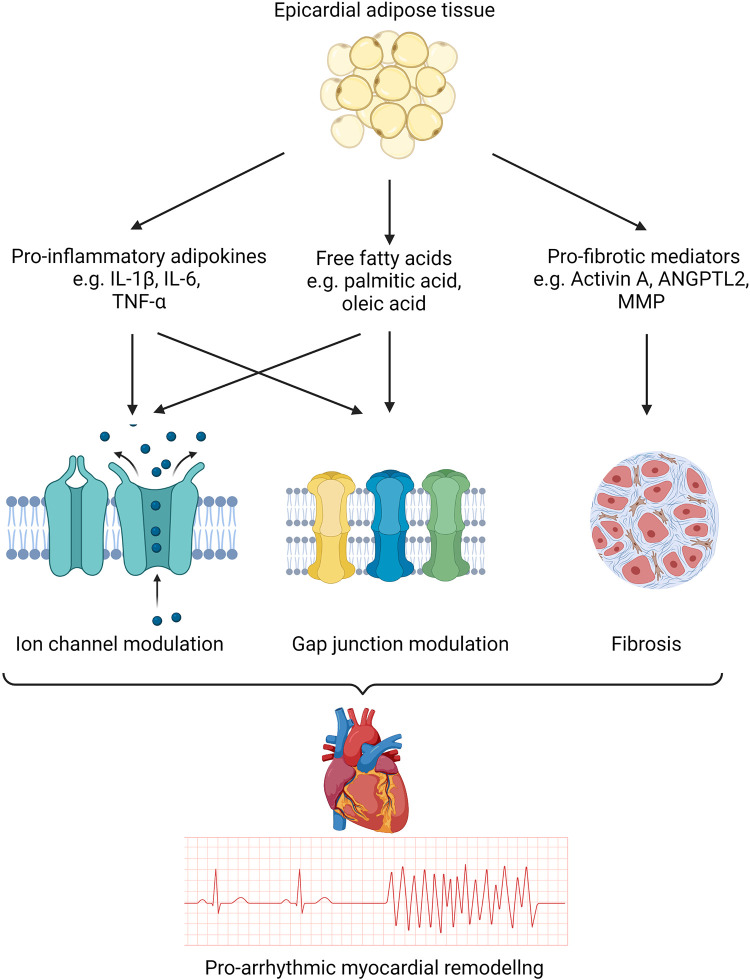
Figure 4.
Mechanisms underpinning electrophysiological remodeling associated with epicardial adipose tissue (EAT). EAT is a rich source of proinflammatory cytokines and free fatty acids that modulate ion channels and gap junction proteins. Additionally, profibrotic mediators have been shown to induce structural remodeling. These mechanisms result in delayed conduction and repolarization heterogeneity within the myocardium that renders it vulnerable to arrhythmias. ANGPTL2, angiopoietin-like 2; MMP, matrix metalloproteinase. Created with BioRender.com, and published with permission.
For instance, IL-1β causes APD90 prolongation that may be explained by increased ICa2+ current (107). Reduced transient outward potassium current (Ito) and altered Ca2+ homeostasis in the sarcoplasmic reticulum that increases Ca2+ spark and prolongs APD have been shown to increase propensity for spontaneous depolarization and triggered activity (108). TNF-α and IL-1β synergistically increase sarcoplasmic reticulum (SR) Ca2+ leak and reduce SR Ca2+ content, which increases the frequency of spontaneous Ca2+ waves (109). Transgenic mice overexpressing TNF-α exhibit elevated diastolic Ca2+ concentration and a prolonged Ca2+ transient, resulting in slow heterogenous conduction and consequently a functional line of conduction block (110). IL-6 treatment in guinea pig ventricular myocytes has also been shown to alter APD, an effect that was reversed with an antagonist (111).
However, much of the evidence for cytokine-mediated ion channel modulation comes from murine models. Although the currents that contribute to rapid depolarization (phase 0) and resting membrane potential (phase 4), INa and IK1, respectively, are common among mice and humans, those determining AP morphology differ significantly. ICaL is weaker in murine cardiomyocytes resulting in an almost absent plateau phase (phase 2) (112, 113). In mice, repolarization is driven by Ito, IK,slow and a noninactivating steady-state current (Iss), whereas in humans it is primarily mediated by the rapid and slow delayed outward rectifier potassium currents (IKr and IKs) (112–114). Potassium currents equivalent to IK,slow and Iss have not yet been identified in the human ventricles, and IKr and IKs have minimal roles in the murine cardiomyocytes. Thus, murine studies showing APD prolongation via modulation of IK,slow would not be expected to be replicated in human cardiomyocytes. The effect of cytokines on IKr and IKs is also less well characterized (113, 115). This may limit the extrapolation of the effects of EAT on ion channel function in humans.
Ion Channel Modulation by Free Fatty Acids
In addition to inflammatory cytokines, FFAs secreted by EAT exert an arrhythmogenic influence on the underlying myocardium via direct modulation of APD (Fig. 4). EAT, like other fat depots, is a rich source of saturated, monounsaturated, and polyunsaturated FFAs, with a greater content of eicosapentanoic and arachidonic acids compared with SAT (116). In contrast to the APD-prolonging effect of cytokines, FFAs have been shown to have inconsistent effects on APD duration. Aromolaran et al. (117) demonstrated that high-fat-diet (HFD) in guinea pigs shortened APD90 and APD30 and suggested that some of this APD shortening may be mediated by FFAs secreted from EAT. Whole cell patch clamp of atrial myocytes showed that 1-h incubation with palmitic acid (PA), a saturated FFAs, enhanced both IKr and IKs, which reduced APD90 and APD30 by 46 and 30%, respectively (117). Haim et al. (118) showed similar results in murine ventricular myocytes exposed to PA recorded Ito and IKs currents up to 20% greater than control, resulting in APD90, APD50, and APD20 shortening. These effects were reversible with pharmacological antagonism of the potassium channels consistent with FFA-induced augmentation of repolarizing currents. O’Connell et al. (119) similarly demonstrated shortened APD30 in ovine LA myocytes after 24-h exposure to PA, although this did not alter APD50 or APD80. Optical imaging of LA myocytes incubated with stearic acid (saturated FFA) revealed altered T-tubular architecture and reduced ICaL, perhaps explaining APD shortening.
When compared with the shortening of APD observed with PA, studies have shown inconsistent effects of unsaturated FFA oleic acid (OA) on APD. For instance, incubation of atrial myocytes with OA in one study showed reduced IKr functionality and consequently prolonged APD90 and APD30 by 14 and 42%, respectively (117). On the other hand, Lin et al. (120) showed 24-h exposure to OA shortened APD90 and APD50 in HL-1 myocytes and reported higher incidence of delayed afterdepolarization compared with control cells. These electrophysiological changes were attributed to intracellular dysregulation of calcium and sodium ions, reflected by larger calcium transients and calcium stores in the sarcoplasmic reticulum, as well as attenuated sodium-calcium exchanger and sodium-potassium pump currents. Consistent with the ionic current modulation, OA-treated cells displayed higher expression of sarcoplasmic reticular Ca2+-ATPase and calmodulin kinase II but lower expression of the sodium-potassium ATPase than control myocytes (120).
More recently, Aromolaran (121) showed that PA- and OA-induced changes in Ito and IKr in guinea pigs can be attenuated with the inhibition of phosphoinositide 3-kinase (PI3K) and serine-threonine protein phosphatase-2 (PP2A), respectively. In addition to this, dietary fish oils have shown to prevent electrical remodeling and hence susceptibility to triggered activities via modulation of the potassium currents (122, 123). This had the additional benefits of preventing structural remodeling in volume overload and heart failure, which could have an antiarrhythmic effect. Therefore, the current body of evidence suggests possibilities of therapeutically treating and preventing electrophysiological changes that are induced by lipotoxicity and obesity.
Although these studies provide insights into the modulation of ionic currents in a lipotoxic environment, the contrasting effects of FFA on APD may stem from differences in animal models, including atrial versus ventricular myocytes, as well as the duration of FFA exposure. Furthermore, despite the opposing effects of cytokines and FFAs, both APD prolongation and shortening facilitate reentry and delayed afterdepolarizations, respectively. It is therefore likely that EAT-derived cytokines and FFAs contribute to spatial heterogeneities of repolarization, although the predominant mechanism underpinning arrhythmogenesis in epicardial obesity remains to be elucidated.
Gap Junction Modulation
In addition to ionic modulation, inflammatory mediators and FFAs have been associated with the alteration of gap junctions (Fig. 4). Gap junctions are primarily located at the intercalated disks and facilitate electrical coupling between adjacent cardiomyocytes. They consist of two hemichannels or connexons, each of which is formed by six ion-channel proteins called connexins. Connexin40 (Cx40), -43 (Cx43), and -45 (Cx45) are the most common in the human heart. These are primarily found in gap junctions in the atrial, ventricular, and specialized conducting tissue.
The relationship between altered connexin expression and propensity for atrial and ventricular arrhythmias is well established (124–126). More recent studies have demonstrated a relationship between connexin expression and BMI. For example, weight loss of 30% in obese ovine model has been associated with increased expression of Cx43 and concurrent improvements in conduction velocity, decreased conduction heterogeneity, and reduced AF susceptibility (10). Given that obesity and BMI correlate with EAT volume (127), it is possible that this phenomenon is the consequence of EAT attenuation. Indeed, Egan Benova et al. (128) showed that weight gain and an increase in EAT volume following high-sucrose diet was associated with downregulation of Cx43 and greater inducibility of ventricular arrhythmias. Chronic HFD also alters connexin distribution from intercalated disks to the longitudinal membrane of cardiomyocytes, and this “lateralization” was shown to correlate with higher EAT volume and slower conduction velocity (95, 129).
Cytokines secreted by EAT, such as IL-6 and TNF-α, have been shown to cause gap junctional changes in experimental settings. For instance, Lazzerini et al. (130) demonstrated an inverse relationship between circulating concentrations of IL-6 protein, and Cx40 and Cx43 mRNA in patients undergoing cardiothoracic surgery. Moreover, increasing concentrations of IL-6 were associated with greater P wave dispersion, a predictor of AF (131). An in vitro study showed reduced Cx40 and Cx43 expression in HL-1 atrial myocytes incubated with IL-6 for 24–48 h, an effect that was reversed by preincubation with monoclonal antibody against IL-6 (130). Transgenic mice overexpressing TNF-α also demonstrated Cx40 downregulation that is associated with slower atrial conduction and increased incidence of supraventricular arrhythmias (132). Although a 90-min exposure of TNF-α in guinea pig heart did not suppress Cx43 protein expression, it was nonetheless associated with its distribution away from intercalated disks (132, 133). Furthermore, TNF-α exposure altered ephaptic coupling, in which conduction relies on the close spatial arrangement of adjacent cardiomyocytes and high density of sodium channels in the sarcolemma (133). Conduction via this mechanism occurs as depolarization of myocyte sequesters sodium ions in the cleft, rendering the voltage of the cleft more negative and creating a steep voltage gradient between the adjacent myocytes. This stimulates the opening of sodium channels in the adjacent myocyte allowing propagation of electrical activity from one myocyte to the other. In this way, narrow perinexi enable cardiomyocytes to remain electrically coupled in instances where gap junction coupling may be compromised (134, 135). TNF-α has been shown to widen the perinexus between adjacent cardiomyocytes and consequently reduce ephaptic coupling resulting in slower conduction velocity (133). Indeed, perinexal width has been shown to be greater in patients with AF (136). Along these lines, other inflammatory proteins such as vascular endothelial growth factor also disrupt gap junctions resulting in conduction slowing predisposing normal hearts to arrhythmias (137). Langendorff-perfused guinea pig heart preparations have also been used to investigate the effects of perinexal width variation on arrhythmia susceptibility and how this may be modulated by extracellular sodium and calcium concentrations experimentally (138–140). It is therefore possible that the inflammatory secretome of EAT may exert proarrhythmic effects by modulating both gap junction and ephaptic coupling.
FFAs also modulate connexin expression with OA and arachidonic acid shown to inhibit gap junctional coupling within hours of exposure in neonatal rat cardiac myocytes (141, 142). This was rapidly reversed with washing the cells in a FFA-free solution, suggesting the effect is most likely to be due to an extracellular component activating membrane receptors (141, 142) and subsequently initiating an intracellular signaling pathway mediated by protein kinases via G protein-coupled receptors (143, 144). A 24-h incubation with OA has been shown to activate protein kinase C in rat cardiomyocytes which consequently induced posttranslational phosphorylation of connexins and hence the disintegration of gap junctions (145). Furthermore, protein kinase A, PI3K, AKT, Src, and MEK1/2 have been shown to similarly modulate connexin functionality following FFA exposure (146–149). FFA-activated protein kinases also modulate adherens junctions (150), which comprise catenin and cadherin proteins that maintain the integrity of intercalated disks (151). Neonatal rat cardiomyocytes treated with OA result in activation of the PKCα/PKCɛ-Fyn signaling cascades and consequently tyrosine phosphorylation of β-catenin and p120 catenin (150). This in turn results in decreased binding of β-catenin with α-catenin and p120 catenin with N-cadherin, causing adherens junctions to become unstable and gap junction disassembly because of disruption of catenin-ZO-1-Cx43 complex (152).
Fibrotic Remodeling
Fibrotic remodeling in the myocardium provides a substrate for reentry as it produces tortuous paths of conduction and concurrently slows macroscopic conduction through the myocardium. Mahajan et al. (62) demonstrated atrial fibrosis increases with adiposity and provided evidence for EAT as a source of profibrotic cytokines that are responsible for structural remodeling. Indeed, Nalliah et al. (95) also demonstrated the association between EAT volume and degree of myocardial fibrosis and Abe et al. (153) provided histological evidence of a greater degree of fibrosis in LA adjacent to EAT compared with tissue that was not associated with EAT (16.5 vs. 8.4%) and that this may be explained by paracrine mechanisms . Severity of atrial fibrosis positively correlated with EAT fibrosis, meanwhile fibrotic EAT was infiltrated with inflammatory macrophages that were positioned between the adipocytes and the myocardium, suggesting involvement of local inflammation in the induction of fibrotic remodeling (153). IL-1β, IL-6, TNF-α, and MCP-1, which are actively secreted by EAT, have been associated with atrial collagen deposition and the degree of atrial fibrosis was positively correlated with fibrotic markers such as platelet-derived growth factor-BB and matrix metalloproteinase (MMP)-2 (153). Angiopoietin-like 2 (ANGPTL2), which is also found in EAT, also positively correlated with the local concentrations of MMP2 and MMP9, as well as atrial collagen level, implicating its mediation of myocardial fibrosis (153, 154). Other studies have demonstrated that MMP2 and MMP7 can induce interstitial fibrosis and that MMP2 is positively correlated with EAT volume as well as AF severity (155, 156). Moreover, connective tissue growth factor (cTGF) is more highly expressed in EAT of AF patients compared with those in sinus rhythm (157). Local concentrations of cTGF also strongly correlated with atrial fibrosis and were an independent predictor of AF occurrence, suggesting EAT may be the source of cTGF facilitating atrial remodeling (157).
Activin A, a member of the transforming growth factor-β (TGF-β) superfamily, is also abundantly synthesized in EAT and has been associated with fibrotic remodeling of the atria (158). Organo-culture of rat atria treated with activin A for a week at a concentration of 2 ng/mL resulted in increased production of collagen types I, III, and VI and marked global fibrosis (158). Furthermore, preincubation with anti-activin A antibodies prevented fibrotic remodeling induced by EAT-conditioned media. EAT-conditioned media and activin A induced comparable changes in atrial gene expression, specifically increased TGF-β1 and TGF-β2, suggesting that EAT-induced atrial fibrosis in this study was primarily facilitated by activin A (158).
Fatty Infiltration
Aside from paracrine effects, epicardial adipocyte infiltration into the myocardium may separate myocardial fibers resulting in conduction slowing or block that would facilitate reentrant arrhythmias. This is not dissimilar to the fibro-fatty infiltration observed with inherited cardiomyopathies (159). For example, Venteclef et al. (158) showed that myocardial fibrosis was more profound in areas of EAT filtration into atrial and ventricular tissue in vitro, and that this occurred at the interface between adipocytes and the surrounding myocardium as well as neighboring tissue. These effects were unique to EAT and were not observed with SAT. Mahajan et al. (62) showed EAT infiltration into the posterior left atria of chronically obese sheep was associated reduced endocardial voltage. In addition to conduction slowing, greater conduction heterogeneity and fractionated electrograms, the atria of obese sheep were also more dilated and pressure overloaded compared with their nonobese counterparts (62). Similar conduction abnormalities associated with obesity have been shown to colocalize with EAT in humans and may be attributed to atrial fatty infiltration (14). As well as greater EAT volume, atria of obese adults exhibit a global reduction in conduction velocity, greater electrogram fractionation, and reduced voltage, which correlate more strongly with EAT volume than global measures of obesity, such as BMI. (14) Vulnerability for AF as a consequence of epicardial fatty filtration has also been demonstrated in obese canines with or without rapid atrial pacing to induce AF (160). Interestingly, epicardial fatty infiltration occurred following rapid atrial pacing to induce AF, even in nonobese animals, and correlated with interstitial fibrosis (160). A lower negative resting membrane potential, smaller action potential amplitude, and longer repolarization time as well as augmented transient outward potassium current and reduced L-type calcium and inward rectifier potassium currents have all been observed with cardiomyocytes connected to epicardial adipose tissue and may explain the vulnerability for arrhythmias as a consequence of fatty infiltration (94).
WEIGHT REDUCTION REDUCES EAT VOLUMES AND REVERSES PROARRHYTHMIC REMODELING
Given the evidence supporting EAT as a source of a proarrhythmic secretome, it follows that its regression may reduce arrhythmic risk. EAT volume has been shown to decrease with weight reduction achieved by lifestyle modification and surgical intervention. The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) registry demonstrated an association between weight loss and EAT volume, such that a 2.3% reduction in EAT was recorded among participants achieving >5% body weight loss (161). However, <5% whole body weight loss was not associated with significant changes in EAT volume (162). Specific weight loss programs via low-calorie diet targeted at obese patients also demonstrated similar pattern of EAT change (81, 163). Iacobellis et al. (81) showed that a 6-mo 900 kcal/day diet resulted in 20% whole body weight loss and reduced EAT thickness by 32%. Kim et al. (163) showed a more modest calorie restriction of 1547 kcal/day over 12 wk was sufficient to result in significant changes in body weight (−11.0%) and EAT thickness (−17.2%) as well as visceral abdominal adiposity. An hour of aerobic exercise achieving 60–70% of maximal predicted heart rate three times a week for 12 wk in obese individuals has been shown to result in almost 9% reduction in EAT thickness (164). Weight loss following bariatric surgery has been shown to regress EAT volumes by as much as 24%, although results may vary according to intervention (14.6% with Roux-en-Y gastric bypass vs. 5.3% with sleeve gastrectomy) (165, 166).
Correspondingly, recent studies have demonstrated reduced propensity for arrhythmias following weight loss. In a randomized controlled trial Abed et al. (167) showed calorie restriction and low intensity exercise resulted in a greater weight reduction than lifestyle advice over a median follow-up of 15 mo (14.3 kg vs. 3.6 kg). More importantly, patients restricting calorie intake and undertaking exercise recorded lesser burden and severity of AF than those receiving lifestyle advice (167). The reduction in arrhythmia burden has been reported up to 2 yr following AF ablation; cardiometabolic risk factor management was associated with arrhythmia-free survival in 32.9 and 87% of patients undergoing single and multiple ablations, respectively, compared with 9.7 and 17.8% in those receiving standard care (168). Long-term monitoring of weight managed AF patients in the LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort) study showed a dose-response relationship between weight loss and arrhythmia-free survival; >10% weight loss was associated with a sixfold greater likelihood of arrhythmia-free survival than those recording <10% weight loss (169). These effects were more marked in those achieving consistent and progressive weight reduction and were partly reversed with weight gain. A fluctuation in body weight of >5% following lifestyle modification conferred a twofold higher risk of AF (169). The same cohort of patients in the LEGACY study were subsequently investigated in the REVERSE-AF (PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation) study to assess the progression and possible reversal of AF with sustained weight loss (11). The study reported 88% of patients with >10% weight loss reversed from persistent to paroxysmal AF, compared with 49 and 26% in groups achieving 3–9% and <3% weight loss, respectively (11). Therefore, given that EAT volume regresses with weight reduction, lifestyle modification represents a noninvasive and inexpensive intervention by which arrhythmias and its risk can be managed.
Despite such compelling evidence for weight reduction for the management of AF, it is unclear whether similar benefits are conferred for ventricular arrhythmias. A meta-analysis comprising 7,197 patients demonstrated prolonged QT interval and QT dispersion in overweight and obese individuals compared with normal weight and that this reversed by 25.77 ms and 13.47 ms with weight loss (170). Given that QT interval and QT dispersion are metrics by which ventricular arrhythmic risk is determined (171–175), it is plausible that the benefits of weight loss may extend to arrhythmias of ventricular origin, although this remains to be determined.
CONCLUSIONS
Given that the association between adiposity and arrhythmic risk is established, there has been increasing interest in understanding the mechanisms underlying this relationship and the interventions by which the risk can be mitigated. EAT is a rich source of proinflammatory cytokines and FFA, which, owing to its anatomical proximity, renders the myocardium vulnerable to arrhythmias via paracrine mechanisms that modulate ionic currents and gap junctions and induces fibrotic remodeling. These effects are, however, reversible with aggressive risk factor management and lifestyle modification. Although much of this evidence comes from studying AF, in vitro studies suggest that similar mechanisms also underpin a higher risk ventricular arrhythmia in obesity. Prospective clinical studies exploring these effects and those of reverse remodeling following weight reduction are required to affirm these findings.
GRANTS
This work was supported by British Heart Foundation Grant RG/16/3/32175 (to F. S. Ng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.H.K.P., T.H., and C.S.L. conceived and designed research; K.H.K.P., T.H., and C.S.L. prepared figures; K.H.K.P., T.H., and C.S.L. drafted manuscript; K.H.K.P., T.H., and F.S.N. edited and revised manuscript; K.H.K.P., T.H., C.S.L., and F.S.N. approved final version of manuscript.
REFERENCES
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129: 837–847, 2014. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 34: 2746–2751, 2013. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khurshid S, Choi SH, Weng LC, Wang EY, Trinquart L, Benjamin EJ, Ellinor PT, Lubitz SA. Frequency of cardiac rhythm abnormalities in a half million adults. Circ Arrhythm Electrophysiol 11: e006273, 2018. doi: 10.1161/circep.118.006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, Pathak RK, Wong DT, Selvanayagam JB, Sanders P, Clarke R. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol 9: e004378, 2016. doi: 10.1161/circep.116.004378. [DOI] [PubMed] [Google Scholar]
- 5.Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, Twomey D, Ganesan AN, Rangnekar G, Roberts-Thomson KC, Lau DH, Sanders P. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol 1: 139–152, 2015. doi: 10.1016/j.jacep.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 45: 187–192, 2005. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation 109: 594–600, 2004. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 49: 565–571, 2007. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 9.Donnellan E, Wazni O, Kanj M, Hussein A, Baranowski B, Lindsay B, Aminian A, Jaber W, Schauer P, Saliba W. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol 12: e007598, 2019. [ doi: 10.1161/circep.119.007598. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Wood JP, Manavis J, Samuel CS, Patel KP, Finnie JW, Alasady M, Kalman JM, Sanders P. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 7: 630–641, 2021. [ doi: 10.1016/j.jacep.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JM, Linz D, McEvoy RD, Abhayaratna WP, Kalman JM, Lau DH, Sanders P. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 20: 1929–1935, 2018. doi: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]
- 12.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48, 2007. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan R, Wong CX. Obesity and metabolic syndrome in atrial fibrillation: cardiac and noncardiac adipose tissue in atrial fibrillation. Card Electrophysiol Clin 13: 77–86, 2021. doi: 10.1016/j.ccep.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, Carbone A, Teo K, Agbaedeng T, Linz D, de Groot JR, Kalman JM, Lau DH, Sanders P. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol 4: 1529–1540, 2018. doi: 10.1016/j.jacep.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology 58: 15–23, 2012. [PMC doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 16.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9: 191–200, 2013. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thoonen R, Hindle AG, Scherrer-Crosbie M. Brown adipose tissue: the heat is on the heart. Am J Physiol Heart Circ Physiol 310: H1592–H1605, 2016. doi: 10.1152/ajpheart.00698.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253, 2010. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 20.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 21.Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab 98: E1448–E1455, 2013. doi: 10.1210/jc.2013-1265. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11: 363–371, 2015. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 23.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY, Carter RA, Robbins T, Wolford D, Samaha J. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 94: 3611–3615, 2009. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 24.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, Bordi C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 13: 313–316, 2004. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol 76: 414–418, 1995. doi: 10.1016/S0002-9149(99)80116-7. [DOI] [PubMed] [Google Scholar]
- 26.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 153: 907–917, 2007. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Prati F, Arbustini E, Labellarte A, Sommariva L, Pawlowski T, Manzoli A, Pagano A, Motolese M, Boccanelli A. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J 24: 329–336, 2003. doi: 10.1016/S0195-668X(02)00426-8. [DOI] [PubMed] [Google Scholar]
- 28.Sacks HS, Fain JN. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol Physiol 38: 879–887, 2011. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 29.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B 94: 225–232, 1989. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2: 536–543, 2005. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 31.Xu A, Vanhoutte PM. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol 302: H1231–H1240, 2012. doi: 10.1152/ajpheart.00765.2011. [DOI] [PubMed] [Google Scholar]
- 32.Fésüs G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727, 2007. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res 78: 376–384, 2008. doi: 10.1093/cvr/cvn034. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280: 18341–18347, 2005. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 35.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol 292: H1655–H1663, 2007. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 36.He Y, Ma N, Tang M, Jiang ZL, Liu H, Mei J. The differentiation of beige adipocyte in pericardial and epicardial adipose tissues induces atrial fibrillation development. Eur Rev Med Pharmacol Sci 21: 4398–4405, 2017. [PubMed] [Google Scholar]
- 37.Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, Sinagra G, Zingone B, Alfieri O, Ferrero E, Maseri A, Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol 298: H746–H753, 2010. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 38.Sahasrabuddhe AV, Pitale SU, Sivanesan SD, Deshpande PK, Deshpande SP, Daiwile A. Pathogenic gene expression of epicardial adipose tissue in patients with coronary artery disease. Indian J Med Res 151: 554–561, 2020. doi: 10.4103/ijmr.IJMR_1374_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 32: 268–274, 2008. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 40.Shibasaki I, Nishikimi T, Mochizuki Y, Yamada Y, Yoshitatsu M, Inoue Y, Kuwata T, Ogawa H, Tsuchiya G, Ishimitsu T, Fukuda H. Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul Pept 165: 210–217, 2010. doi: 10.1016/j.regpep.2010.07.169. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Jiao Z, Wang L, Sun Z, Wei Y, Wang X, Xia D. Roles of human epicardial adipose tissue in coronary artery atherosclerosis. J Huazhong Univ Sci Technol Med Sci 30: 589–593, 2010. doi: 10.1007/s11596-010-0547-9. [DOI] [PubMed] [Google Scholar]
- 42.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 288: H2031–H2041, 2005. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 43.Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, Nishio C, Higashida M, Nakaya Y, Kitagawa T, Sata M. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J 52: 139–142, 2011. doi: 10.1536/ihj.52.139. [DOI] [PubMed] [Google Scholar]
- 44.Bambace C, Sepe A, Zoico E, Telesca M, Olioso D, Venturi S, Rossi A, Corzato F, Faccioli S, Cominacini L, Santini F, Zamboni M. Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessels 29: 42–48, 2014. doi: 10.1007/s00380-012-0315-9. [DOI] [PubMed] [Google Scholar]
- 45.Camarena V, Sant D, Mohseni M, Salerno T, Zaleski ML, Wang G, Iacobellis G. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr Metab Cardiovasc Dis 27: 739–750, 2017. doi: 10.1016/j.numecd.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Salgado-Somoza A, Martinez-Comendador JM, Gonzalez-Juanatey JR. Diabetic and nondiabetic patients express similar adipose tissue adiponectin and leptin levels. Int J Obes (Lond) 34: 1200–1208, 2010. doi: 10.1038/ijo.2010.30. [DOI] [PubMed] [Google Scholar]
- 47.Parisi V, Petraglia L, D'Esposito V, Cabaro S, Rengo G, Caruso A, Grimaldi MG, Baldascino F, De Bellis A, Vitale D, Formisano R, Ferro A, Paolillo S, Davin L, Lancellotti P, Formisano P, Perrone Filardi P, Ferrara N, Leosco D. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int J Cardiol 274: 326–330, 2019. doi: 10.1016/j.ijcard.2018.06.106. [DOI] [PubMed] [Google Scholar]
- 48.Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, Wolford D, Samaha J. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care 34: 730–733, 2011. doi: 10.2337/dc10-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Díaz-Rodríguez E, Agra RM, Fernández ÁL, Adrio B, García-Caballero T, González-Juanatey JR, Eiras S. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res 114: 336–346, 2018. doi: 10.1093/cvr/cvx186. [DOI] [PubMed] [Google Scholar]
- 50.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108: 2460–2466, 2003. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 51.Fain JN, Sacks HS, Bahouth SW, Tichansky DS, Madan AK, Cheema PS. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism 59: 379–1386, 2010. doi: 10.1016/j.metabol.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 5: 1, 2006. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salgado-Somoza A, Teijeira-Fernández E, Fernández AL, González-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 299: H202–H209, 2010. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- 54.Gruzdeva O, Uchasova E, Dyleva Y, Borodkina D, Akbasheva O, Antonova L, Matveeva V, Belik E, Ivanov S, Sotnikov A, Kozyrin K, Brel N, Sinitsky M, Karetnikova V, Kokov A, Bychkova E, Pecherina T, Barbarash O. Adipocytes directly affect coronary artery disease pathogenesis via induction of adipokine and cytokine imbalances. Front Immunol 10: 2163, 2019. doi: 10.3389/fimmu.2019.02163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzgibbons TP, Lee N, Tran KV, Nicoloro S, Kelly M, Tam SK, Czech MP. Coronary disease is not associated with robust alterations in inflammatory gene expression in human epicardial fat. JCI Insight 4: e124859, 2019. doi: 10.1172/jci.insight.124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyas V, Blythe H, Wood EG, Sandhar B, Sarker SJ, Balmforth D, Ambekar SG, Yap J, Edmondson SJ, Di Salvo C, Wong K, Roberts N, Uppal R, Adams B, Shipolini A, Oo AY, Lawrence D, Kolvekar S, Lall KS, Finlay MC, Longhi MP. Obesity and diabetes are major risk factors for epicardial adipose tissue inflammation. JCI Insight 6: e145495, 2021. doi: 10.1172/jci.insight.145495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 58.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol 302: H2148–H2165, 2012. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 59.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol 55: 2319–2327, 2010. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA 292: 2471–2477, 2004. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 61.Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, Sanders P. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 10: 90–100, 2013. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 62.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 66: 1–11, 2015. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 63.Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol 3: 345–350, 2010. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol 56: 784–788, 2010. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 65.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC, Wittert GA, Abhayaratna WP, Worthley SG, Sanders P. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol 57: 1745–1751, 2011. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 66.Tsao HM, Hu WC, Wu MH, Tai CT, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Sheu MH, Chang CY, Chen SA. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am J Cardiol 107: 1498–1503, 2011. doi: 10.1016/j.amjcard.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 67.Kim TH, Park J, Park JK, Uhm JS, Joung B, Lee MH, Pak H. Pericardial fat volume is associated with clinical recurrence after catheter ablation for persistent atrial fibrillation, but not paroxysmal atrial fibrillation: an analysis of over 600-patients. Int J Cardiol 176: 841–846, 2014. doi: 10.1016/j.ijcard.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Chao TF, Hung CL, Tsao HM, Lin YJ, Yun CH, Lai YH, Chang SL, Lo LW, Hu YF, Tuan TC, Chang HY, Kuo JY, Yeh HI, Wu TJ, Hsieh MH, Yu WC, Chen SA. Epicardial adipose tissue thickness and ablation outcome of atrial fibrillation. PLoS One 8: e74926, 2013. doi: 10.1371/journal.pone.0074926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kocyigit D, Gurses KM, Yalcin MU, Turk G, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Hazirolan T, Tokgozoglu L, Oto MA, Ozer N, Aytemir K. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. J Cardiovasc Comput Tomogr 9: 295–302, 2015. doi: 10.1016/j.jcct.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Canpolat U, Aytemir K, Yorgun H, Asil S, Dural M, Özer N. The impact of echocardiographic epicardial fat thickness on outcomes of cryoballoon-based atrial fibrillation ablation. Echocardiography 33: 821–829, 2016. doi: 10.1111/echo.13193. [DOI] [PubMed] [Google Scholar]
- 71.Bos D, Vernooij MW, Shahzad R, Kavousi M, Hofman A, van Walsum T, Deckers JW, Ikram MA, Heeringa J, Franco OH, van der Lugt A, Leening MJ. Epicardial fat volume and the risk of atrial fibrillation in the general population free of cardiovascular disease. JACC Cardiovasc Imaging 10: 1405–1407, 2017. doi: 10.1016/j.jcmg.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Tam WC, Lin YK, Chan WP, Huang JH, Hsieh MH, Chen SA, Chen YJ. Pericardial fat is associated with the risk of ventricular arrhythmia in Asian patients. Circ J 80: 1726–1733, 2016. doi: 10.1253/circj.CJ-16-0047. [DOI] [PubMed] [Google Scholar]
- 73.Kırış A, Turan OE, Kırış G, İlter A, Öztürk M, Aydın M, Kaplan Ş, Kutlu M, Gedikli Ö. The relationship between epicardial fat tissue thickness and frequent ventricular premature beats. Kardiol Pol 73: 527–532, 2015. doi: 10.5603/KP.a2015.0025. [DOI] [PubMed] [Google Scholar]
- 74.Yılmaz AS, Çinier G, Çırakoğlu ÖF, Çetin M. Epicardial adipose tissue predicted prolonged QTc interval in patients with arterial hypertension. Clin Exp Hypertens 43: 230–236, 2021. doi: 10.1080/10641963.2020.1847131. [DOI] [PubMed] [Google Scholar]
- 75.Wu CK, Tsai HY, Su MY, Wu YF, Hwang JJ, Tseng WY, Lin JL, Lin LY. Pericardial fat is associated with ventricular tachyarrhythmia and mortality in patients with systolic heart failure. Atherosclerosis 241: 607–614, 2015. doi: 10.1016/j.atherosclerosis.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 76.Sepehri Shamloo A, Schoene K, Stauber A, Darma A, Dagres N, Dinov B, Bertagnolli L, Hilbert S, Müssigbrodt A, Husser D, Bollmann A, Hindricks G, Arya A. Epicardial adipose tissue thickness as an independent predictor of ventricular tachycardia recurrence following ablation. Heart Rhythm 16: 1492–1498, 2019. doi: 10.1016/j.hrthm.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Quisi A, Şentürk SE, Harbalıoğlu H, Baykan AO. The relationship between echocardiographic epicardial adipose tissue, P-wave dispersion, and corrected QT interval. Turk Kardiyol Dern Ars 46: 471–478, 2018. doi: 10.5543/tkda.2018.01578. [DOI] [PubMed] [Google Scholar]
- 78.Jhuo SJ, Hsieh TJ, Tang WH, Tsai WC, Lee KT, Yen HW, Lai WT. The association of the amounts of epicardial fat, P wave duration, and PR interval in electrocardiogram. J Electrocardiol 51: 645–651, 2018. doi: 10.1016/j.jelectrocard.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Hung WC, Tang WH, Wang CP, Lu LF, Chung FM, Lu YC, Hsu CC, Tsai IT, Jhuo SJ, Lai WT, Lee YJ, Ya TH. Increased epicardial adipose tissue volume is associated with PR interval prolongation. Clin Invest Med 38: E45–52, 2015. doi: 10.25011/cim.v38i1.22575. [DOI] [PubMed] [Google Scholar]
- 80.Çiçek Y, Doğan S, Durakoğlugil ME, Balcıoğlu AS, Erdoğan T, Şatıroğlu Ö, Karadağ Z, Duman H, Bostan M. The relationship between epicardial adipose tissue and P wave and QT dispersions. Turk Kardiyol Dern Ars 43: 621–629, 2015. doi: 10.5543/tkda.2015.47598. [DOI] [PubMed] [Google Scholar]
- 81.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 16: 1693–1697, 2008. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 82.Reiner L, Mazzoleni A, Rodriguez FL. Statistical analysis of the epicardial fat weight in human hearts. AMA Arch Pathol 60: 369–373, 1955. [PubMed] [Google Scholar]
- 83.Mancio J, Pinheiro M, Ferreira W, Carvalho M, Barros A, Ferreira N, Vouga L, Ribeiro VG, Leite-Moreira A, Falcao-Pires I, Bettencourt N. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int J Cardiol 249: 419–425, 2017. doi: 10.1016/j.ijcard.2017.09.178. [DOI] [PubMed] [Google Scholar]
- 84.Iglesias MJ, Eiras S, Piñeiro R, López-Otero D, Gallego R, Fernández AL, Lago F, González-Juanatey JR. [Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. Findings in patients undergoing cardiac surgery]. Rev Esp Cardiol 59: 1252–1260, 2006. doi: 10.1157/13096596. [DOI] [PubMed] [Google Scholar]
- 85.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114: 119–125, 2006. [Erratum in Circulation 114: e498, 2006]. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 86.Ehdaie A, Cingolani E, Shehata M, Wang X, Curtis AB, Chugh SS. Sex differences in cardiac arrhythmias: clinical and research implications. Circ Arrhythm Electrophysiol 11: e005680, 2018. [DOI] [PubMed] [Google Scholar]
- 87.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 386: 154–162, 2015. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keller KM, Howlett SE. Sex differences in the biology and pathology of the aging heart. Can J Cardiol 32: 1065–1073, 2016. doi: 10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 89.Zoico E, Rubele S, De Caro A, Nori N, Mazzali G, Fantin F, Rossi A, Zamboni M. Brown and beige adipose tissue and aging. Front Endocrinol (Lausanne) 10: 368, 2019. doi: 10.3389/fendo.2019.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim JS, Shin SY, Kang JH, Yong HS, Na JO, Choi CU, Kim SH, Kim EJ, Rha SW, Park CG, Seo HS, Oh DJ, Hwang C, Kim YH, Lim HE. Influence of sex on the association between epicardial adipose tissue and left atrial transport function in patients with atrial fibrillation: a multislice computed tomography study. J Am Heart Assoc 6: e006077, 2017. doi: 10.1161/jaha.117.006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fei J, Cook C, Blough E, Santanam N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis 212: 488–494, 2010. doi: 10.1016/j.atherosclerosis.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin YK, Chen YC, Chen JH, Chen SA, Chen YJ. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic Res Cardiol 107: 293, 2012. doi: 10.1007/s00395-012-0293-1. [DOI] [PubMed] [Google Scholar]
- 93.Lin YK, Chen YC, Chang SL, Lin YJ, Chen JH, Yeh YH, Chen SA, Chen YJ. Heart failure epicardial fat increases atrial arrhythmogenesis. Int J Cardiol 167: 1979–1983, 2013. doi: 10.1016/j.ijcard.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 94.Lu YY, Huang SY, Lin YK, Chen YC, Chen YA, Chen SA, Chen YJ. Epicardial adipose tissue modulates arrhythmogenesis in right ventricle outflow tract cardiomyocytes. Europace 23: 970–977, 2021. [ doi: 10.1093/europace/euaa412. [DOI] [PubMed] [Google Scholar]
- 95.Nalliah CJ, Bell JR, Raaijmakers AJ, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, Lui E, Sim CB, Larobina M, O'Keefe M, Goldblatt J, Royse A, Lee G, Porrello ER, Watt MJ, Kistler PM, Sanders P, Delbridge LM, Kalman JM. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol 76: 1197–1211, 2020. doi: 10.1016/j.jacc.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 96.Ten Sande JN, Smit NW, Parvizi M, van Amersfoorth SC, Plantinga JA, van Dessel PF, de Bakker JM, Harmsen MC, Coronel R. Differential mechanisms of myocardial conduction slowing by adipose tissue-derived stromal cells derived from different species. Stem Cells Transl Med 6: 22–30, 2017. doi: 10.5966/sctm.2015-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation 108: 3006–3010, 2003. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 98.Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, Benn M. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol 56: 789–795, 2010. doi: 10.1016/j.jacc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 99.Patel KH, Jones TN, Sattler S, Mason JC, Ng FS. Proarrhythmic electrophysiological and structural remodeling in rheumatoid arthritis. Am J Physiol Heart Circ Physiol 319: H1008–H1020, 2020. doi: 10.1152/ajpheart.00401.2020. [DOI] [PubMed] [Google Scholar]
- 100.Klein RM, Vester EG, Brehm MU, Dees H, Picard F, Niederacher D, Beckmann MW, Strauer BE. Z Kardiol 89, Suppl 3: 24–35, 2000. [PubMed] [Google Scholar]
- 101.Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 235: 39–41, 1976. [PubMed] [Google Scholar]
- 102.Shahreyar M, Fahhoum R, Akinseye O, Bhandari S, Dang G, Khouzam RN. Severe sepsis and cardiac arrhythmias. Ann Transl Med 6: 6, 2018. doi: 10.21037/atm.2017.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 96: 3542–3548, 1997. doi: 10.1161/01.CIR.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 104.Seemann A, Boissier F, Razazi K, Carteaux G, de Prost N, Brun-Buisson C, Mekontso Dessap A. New-onset supraventricular arrhythmia during septic shock: prevalence, risk factors and prognosis. Ann Intensive Care 5: 27, 2015.doi: 10.1186/s13613-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 306: 2248–2254, 2011. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care 18: 688, 2014. doi: 10.1186/s13054-014-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li YH, Rozanski GJ. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc Res 27: 525–530, 1993. doi: 10.1093/cvr/27.3.525. [DOI] [PubMed] [Google Scholar]
- 108.Monnerat G, Alarcón ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O, Malan D, Travassos LH, Sepúlveda M, Burgos JI, Vila-Petroff M, Dutra FF, Bozza MT, Paiva CN, Carvalho AB, Bonomo A, Fleischmann BK, de Carvalho AC, Medei E. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun 7: 13344, 2016. [Erratum in Nat Commun 12: 7261, 2021]. doi: 10.1038/ncomms13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 47: 378–386, 2010. doi: 10.1016/j.ceca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.London B, Baker LC, Lee JS, Shusterman V, Choi BR, Kubota T, McTiernan CF, Feldman AM, Salama G. Calcium-dependent arrhythmias in transgenic mice with heart failure. Am J Physiol Heart Circ Physiol 284: H431–H441, 2003. doi: 10.1152/ajpheart.00431.2002. [DOI] [PubMed] [Google Scholar]
- 111.Aromolaran AS, Srivastava U, Alí A, Chahine M, Lazaro D, El-Sherif N, Capecchi PL, Laghi-Pasini F, Lazzerini PE, Boutjdir M. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One 13: e0208321, 2018. doi: 10.1371/journal.pone.0208321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaese S, Verheule S. Cardiac electrophysiology in mice: a matter of size. Front Physiol 3: 345, 2012. doi: 10.3389/fphys.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sabir IN, Killeen MJ, Grace AA, Huang CL. Ventricular arrhythmogenesis: insights from murine models. Prog Biophys Mol Biol 98: 208–218, 2008. doi: 10.1016/j.pbiomolbio.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 114.Nerbonne JM. Studying cardiac arrhythmias in the mouse–a reasonable model for probing mechanisms? Trends Cardiovasc Med 14: 83–93, 2004. doi: 10.1016/j.tcm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Salama G, Baker L, Wolk R, Barhanin J, London B. Arrhythmia phenotype in mouse models of human long QT. J Interv Card Electrophysiol 24: 77–87, 2009. doi: 10.1007/s10840-008-9339-6. [DOI] [PubMed] [Google Scholar]
- 116.Hjelmgaard K, Eschen RB, Schmidt EB, Andreasen JJ, Lundbye-Christensen S. Fatty acid composition in various types of cardiac adipose tissues and its relation to the fatty acid content of atrial tissue. Nutrients 10: 1506, 2018. doi: 10.3390/nu10101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aromolaran AS, Colecraft HM, Boutjdir M. High-fat diet-dependent modulation of the delayed rectifier K(+) current in adult guinea pig atrial myocytes. Biochem Biophys Res Commun 474: 554–559, 2016. doi: 10.1016/j.bbrc.2016.04.113. [DOI] [PubMed] [Google Scholar]
- 118.Haim TE, Wang W, Flagg TP, Tones MA, Bahinski A, Numann RE, Nichols CG, Nerbonne JM. Palmitate attenuates myocardial contractility through augmentation of repolarizing Kv currents. J Mol Cell Cardiol 48: 395–405, 2010. doi: 10.1016/j.yjmcc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Connell RP, Musa H, Gomez MS, Avula UM, Herron TJ, Kalifa J, Anumonwo JM. Free fatty acid effects on the atrial myocardium: membrane ionic currents are remodeled by the disruption of T-tubular architecture. PLoS One 10: e0133052, 2015. doi: 10.1371/journal.pone.0133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin YK, Chen YC, Kao YH, Tsai CF, Yeh YH, Huang JL, Cheng CC, Chen SA, Chen YJ. A monounsaturated fatty acid (oleic acid) modulates electrical activity in atrial myocytes with calcium and sodium dysregulation. Int J Cardiol 176: 191–198, 2014. doi: 10.1016/j.ijcard.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 121.Aromolaran AS. Mechanisms of electrical remodeling in lipotoxic guinea pig heart. Biochem Biophys Res Commun 519: 639–644, 2019. doi: 10.1016/j.bbrc.2019.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Den Ruijter HM, Verkerk AO, Schumacher CA, Houten SM, Belterman CN, Baartscheer A, Brouwer IA, van Bilsen M, de Roos B, Coronel R. A diet rich in unsaturated fatty acids prevents progression toward heart failure in a rabbit model of pressure and volume overload. Circ Heart Fail 5: 376–384, 2012. doi: 10.1161/CIRCHEARTFAILURE.111.963116. [DOI] [PubMed] [Google Scholar]
- 123.Kitamura K, Shibata R, Tsuji Y, Shimano M, Inden Y, Murohara T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am J Physiol Heart Circ Physiol 300: H1814–H1821, 2011. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]
- 124.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res 92: 218–225, 2011. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 125.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS, Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 125: 216–225, 2012. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kato T, Iwasaki YK, Nattel S. Connexins and atrial fibrillation: filling in the gaps. Circulation 125: 203–206, 2012. doi: 10.1161/CIRCULATIONAHA.111.075432. [DOI] [PubMed] [Google Scholar]
- 127.Aitken-Buck HM, Moharram M, Babakr AA, Reijers R, Van Hout I, Fomison-Nurse IC, Sugunesegran R, Bhagwat K, Davis PJ, Bunton RW, Williams MJ, Stiles MK, Jones PP, Coffey S, Lamberts RR. Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte 8: 412–420, 2019. doi: 10.1080/21623945.2019.1701387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Egan Benova T, Viczenczova C, Szeiffova Bacova B, Knezl V, Dosenko V, Rauchova H, Zeman M, Reiter RJ, Tribulova N. Obesity-associated alterations in cardiac connexin-43 and PKC signaling are attenuated by melatonin and omega-3 fatty acids in female rats. Mol Cell Biochem 454: 191–202, 2019. doi: 10.1007/s11010-018-3463-0. [DOI] [PubMed] [Google Scholar]
- 129.Meng T, Cheng G, Wei Y, Ma S, Jiang Y, Wu J, Zhou X, Sun C. Exposure to a chronic high-fat diet promotes atrial structure and gap junction remodeling in rats. Int J Mol Med 40: 217–225, 2017. doi: 10.3892/ijmm.2017.2982. [DOI] [PubMed] [Google Scholar]
- 130.Lazzerini PE, Laghi-Pasini F, Acampa M, Srivastava U, Bertolozzi I, Giabbani B, Finizola F, Vanni F, Dokollari A, Natale M, Cevenini G, Selvi E, Migliacci N, Maccherini M, Boutjdir M, Capecchi PL. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J Am Heart Assoc 8: e011006, 2019. doi: 10.1161/jaha.118.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perez MV, Dewey FE, Marcus R, Ashley EA, Al-Ahmad AA, Wang PJ, Froelicher VF. Electrocardiographic predictors of atrial fibrillation. Am Heart J 158: 622–628, 2009. doi: 10.1016/j.ahj.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Sawaya SE, Rajawat YS, Rami TG, Szalai G, Price RL, Sivasubramanian N, Mann DL, Khoury DS. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol 292: H1561–H1567, 2007. doi: 10.1152/ajpheart.00285.2006. [DOI] [PubMed] [Google Scholar]
- 133.George SA, Calhoun PJ, Gourdie RG, Smyth JW, Poelzing S. TNFα modulates cardiac conduction by altering electrical coupling between myocytes. Front Physiol 8: 334, 2017. doi: 10.3389/fphys.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ivanovic E, Kucera JP. Localization of Na(+) channel clusters in narrowed perinexi of gap junctions enhances cardiac impulse transmission via ephaptic coupling: a model study. J Physiol 599: 4779–4811, 2021. doi: 10.1113/JP282105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei N, Tolkacheva EG. Interplay between ephaptic coupling and complex geometry of border zone during acute myocardial ischemia: effect on arrhythmogeneity. Chaos 30: 033111, 2020. doi: 10.1063/1.5134447. [DOI] [PubMed] [Google Scholar]
- 136.Raisch TB, Yanoff MS, Larsen TR, Farooqui MA, King DR, Veeraraghavan R, Gourdie RG, Baker JW, Arnold WS, AlMahameed ST, Poelzing S. Intercalated disk extracellular nanodomain expansion in patients with atrial fibrillation. Front Physiol 9: 398, 2018. doi: 10.3389/fphys.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mezache L, Struckman HL, Greer-Short A, Baine S, Györke S, Radwański PB, Hund TJ, Veeraraghavan R. Vascular endothelial growth factor promotes atrial arrhythmias by inducing acute intercalated disk remodeling. Sci Rep 10: 20463, 2020. doi: 10.1038/s41598-020-77562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu X, Hoeker GS, Blair GA, King DR, Gourdie RG, Weinberg SH, Poelzing S. Hypernatremia and intercalated disc edema synergistically exacerbate long-QT syndrome type 3 phenotype. Am J Physiol Heart Circ Physiol 321: H1042–H1055, 2021. doi: 10.1152/ajpheart.00366.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.King DR, Entz M 2nd, Blair GA, Crandell I, Hanlon AL, Lin J, Hoeker GS, Poelzing S. The conduction velocity-potassium relationship in the heart is modulated by sodium and calcium. Pflugers Arch 473: 557–571, 2021. doi: 10.1007/s00424-021-02537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.George SA, Hoeker G, Calhoun PJ, Entz M 2nd, Raisch TB, King DR, Khan M, Baker C, Gourdie RG, Smyth JW, Nielsen MS, Poelzing S. Modulating cardiac conduction during metabolic ischemia with perfusate sodium and calcium in guinea pig hearts. Am J Physiol Heart Circ Physiol 316: H849–H861, 2019. doi: 10.1152/ajpheart.00083.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hii CS, Ferrante A, Schmidt S, Rathjen DA, Robinson BS, Poulos A, Murray AW. Inhibition of gap junctional communication by polyunsaturated fatty acids in WB cells: evidence that connexin 43 is not hyperphosphorylated. Carcinogenesis 16: 1505–1511, 1995. doi: 10.1093/carcin/16.7.1505. [DOI] [PubMed] [Google Scholar]
- 142.Hirschi KK, Minnich BN, Moore LK, Burt JM. Oleic acid differentially affects gap junction-mediated communication in heart and vascular smooth muscle cells. Am J Physiol Cell Physiol 265: C1517–C1526, 1993. doi: 10.1152/ajpcell.1993.265.6.C1517. [DOI] [PubMed] [Google Scholar]
- 143.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 144.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 145.Huang YS, Tseng YZ, Wu JC, Wang SM. Mechanism of oleic acid-induced gap junctional disassembly in rat cardiomyocytes. J Mol Cell Cardiol 37: 755–766, 2004. doi: 10.1016/j.yjmcc.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 146.De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell 18: 34–46, 2007. doi: 10.1091/mbc.e06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Figueroa V, Sáez PJ, Salas JD, Salas D, Jara O, Martínez AD, Sáez JC, Retamal MA. Linoleic acid induces opening of connexin26 hemichannels through a PI3K/Akt/Ca(2+)-dependent pathway. Biochim Biophys Acta 1828: 1169–1179, 2013. doi: 10.1016/j.bbamem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 148.Popp R, Brandes RP, Ott G, Busse R, Fleming I. Dynamic modulation of interendothelial gap junctional communication by 11,12-epoxyeicosatrienoic acid. Circ Res 90: 800–806, 2002. doi: 10.1161/01.RES.0000015328.20581.D6. [DOI] [PubMed] [Google Scholar]
- 149.Radosinska J, Bacova B, Knezl V, Benova T, Zurmanova J, Soukup T, Arnostova P, Slezak J, Gonçalvesova E, Tribulova N. Dietary omega-3 fatty acids attenuate myocardial arrhythmogenic factors and propensity of the heart to lethal arrhythmias in a rodent model of human essential hypertension. J Hypertens 31: 1876–1885, 2013. doi: 10.1097/HJH.0b013e328362215d. [DOI] [PubMed] [Google Scholar]
- 150.Hsu KL, Fan HJ, Chen YC, Huang YS, Chen CH, Wu JC, Wang SM. Protein kinase C-Fyn kinase cascade mediates the oleic acid-induced disassembly of neonatal rat cardiomyocyte adherens junctions. Int J Biochem Cell Biol 41: 1536–1546, 2009. doi: 10.1016/j.biocel.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 151.Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol 4: 834–839, 1992. doi: 10.1016/0955-0674(92)90108-O. [DOI] [PubMed] [Google Scholar]
- 152.Wu JC, Tsai RY, Chung TH. Role of catenins in the development of gap junctions in rat cardiomyocytes. J Cell Biochem 88: 823–835, 2003. doi: 10.1002/jcb.10390. [DOI] [PubMed] [Google Scholar]
- 153.Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, Saito S, Fukui A, Shinohara T, Yufu K, Nakagawa M, Hijiya N, Moriyama M, Shimada T, Miyamoto S, Takahashi N. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 15: 1717–1727, 2018. doi: 10.1016/j.hrthm.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 154.Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab 25: 245–254, 2014. doi: 10.1016/j.tem.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 155.Boixel C, Fontaine V, Rücker-Martin C, Milliez P, Louedec L, Michel JB, Jacob MP, Hatem SN. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol 42: 336–344, 2003. doi: 10.1016/S0735-1097(03)00578-3. [DOI] [PubMed] [Google Scholar]
- 156.Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune M, Mano H, Sonoda K, Hiro T, Nikaido M, Hirayama A. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ Arrhythm Electrophysiol 5: 676–683, 2012. doi: 10.1161/CIRCEP.112.971200. [DOI] [PubMed] [Google Scholar]
- 157.Wang Q, Xi W, Yin L, Wang J, Shen H, Gao Y, Min J, Zhang Y, Wang Z. Human epicardial adipose tissue cTGF expression is an independent risk factor for atrial fibrillation and highly associated with atrial fibrosis. Sci Rep 8: 3585, 2018. doi: 10.1038/s41598-018-21911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clément K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 36: 795–805a, 2015. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 159.Anumonwo JM, Herron T. Fatty infiltration of the myocardium and arrhythmogenesis: potential cellular and molecular mechanisms. Front Physiol 9: 2, 2018. doi: 10.3389/fphys.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Otsuka N, Okumura Y, Arai M, Kurokawa S, Nagashima K, Watanabe R, Wakamatsu Y, Yagyu S, Ohkubo K, Nakai T, Hao H, Takahashi R, Taniguchi Y, Li Y. Effect of obesity and epicardial fat/fatty infiltration on electrical and structural remodeling associated with atrial fibrillation in a novel canine model of obesity and atrial fibrillation: a comparative study. J Cardiovasc Electrophysiol 32: 889–899, 2021. doi: 10.1111/jce.14955. [DOI] [PubMed] [Google Scholar]
- 161.Nakazato R, Rajani R, Cheng VY, Shmilovich H, Nakanishi R, Otaki Y, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Wong ND, Shaw LJ, Budoff M, Rozanski A, Berman DS, Dey D. Weight change modulates epicardial fat burden: a 4-year serial study with non-contrast computed tomography. Atherosclerosis 220: 139–144, 2012. doi: 10.1016/j.atherosclerosis.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jonker JT, de Mol P, de Vries ST, Widya RL, Hammer S, van Schinkel LD, van der Meer RW, Gans RO, Webb AG, Kan HE, de Koning EJ, Bilo HJ, Lamb HJ. Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology 269: 434–442, 2013. doi: 10.1148/radiol.13121631. [DOI] [PubMed] [Google Scholar]
- 163.Kim MK, Tanaka K, Kim MJ, Matuso T, Endo T, Tomita T, Maeda S, Ajisaka R. Comparison of epicardial, abdominal and regional fat compartments in response to weight loss. Nutr Metab Cardiovasc Dis 19: 760–766, 2009. doi: 10.1016/j.numecd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 164.Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol (1985) 106: 5–11, 2009. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]
- 165.Kokkinos A, Alexiadou K, Liaskos C, Argyrakopoulou G, Balla I, Tentolouris N, Moyssakis I, Katsilambros N, Vafiadis I, Alexandrou A, Diamantis T. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes Surg 23: 31–38, 2013. doi: 10.1007/s11695-012-0743-8. [DOI] [PubMed] [Google Scholar]
- 166.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol 99: 1242–1245, 2007. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 167.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 310: 2050–2060, 2013. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 168.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 64: 2222–2231, 2014. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 169.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 65: 2159–2169, 2015. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 170.Omran J, Firwana B, Koerber S, Bostick B, Alpert MA. Effect of obesity and weight loss on ventricular repolarization: a systematic review and meta-analysis. Obes Rev 17: 520–530, 2016. doi: 10.1111/obr.12390. [DOI] [PubMed] [Google Scholar]
- 171.Ahnve S. QT interval prolongation in acute myocardial infarction. Eur Heart J 6, Suppl D: 85–95, 1985. doi: 10.1093/eurheartj/6.suppl_d.85. [DOI] [PubMed] [Google Scholar]
- 172.Braschi A, Abrignani MG, Francavilla VC, Francavilla G. Novel electrocardiographic parameters of altered repolarization in uncomplicated overweight and obesity. Obesity (Silver Spring) 19: 875–881, 2011. doi: 10.1038/oby.2010.252. [DOI] [PubMed] [Google Scholar]
- 173.Fu GS, Meissner A, Simon R. Repolarization dispersion and sudden cardiac death in patients with impaired left ventricular function. Eur Heart J 18: 281–289, 1997. doi: 10.1093/oxfordjournals.eurheartj.a015232. [DOI] [PubMed] [Google Scholar]
- 174.Glancy JM, Garratt CJ, Woods KL, de Bono DP. QT dispersion and mortality after myocardial infarction. Lancet 345: 945–948, 1995. doi: 10.1016/S0140-6736(95)90697-5. [DOI] [PubMed] [Google Scholar]
- 175.Turrini P, Corrado D, Basso C, Nava A, Bauce B, Thiene G. Dispersion of ventricular depolarization-repolarization: a noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 103: 3075–3080, 2001. doi: 10.1161/01.CIR.103.25.3075. [DOI] [PubMed] [Google Scholar]