Abstract
The Diplostomidae Poirier, 1886 is a large, globally distributed family of digeneans parasitic in intestines of their definitive hosts. Diplostomum and Tylodelphys spp. are broadly distributed, commonly reported, and the most often sequenced diplostomid genera. The majority of published DNA sequences from these genera originated from larval stages only, which typically cannot be identified to the species level based on morphology alone. We generated partial large ribosomal subunit (28S) rRNA and cytochrome c oxidase subunit 1 (cox1) mtDNA gene sequences from 14 species/species-level lineages of Diplostomum, six species/species-level lineages of Tylodelphys, two species/species-level lineages of Austrodiplostomum, one species previously assigned to Paralaria, two species/species-level lineages of Dolichorchis and one unknown diplostomid. Our DNA sequences of 11 species/species-level lineages of Diplostomum (all identified to species), four species/species-level lineages of Tylodelphys (all identified to species), Austrodiplostomum compactum, Paralaria alarioides and Dolichorchis lacombeensis originated from adult specimens. 28S sequences were used for phylogenetic inference to demonstrate the position of P. alarioides and Dolichorchis spp. within the Diplostomoidea and study the interrelationships of Diplostomum, Tylodelphys and Austrodiplostomum. Our results demonstrate that two diplostomids from the North American river otter (P. alarioides and a likely undescribed taxon) belong within Diplostomum. Further, our results demonstrate the non-monophyly of Tylodelphys due to the position of Austrodiplostomum spp., based on our phylogenetic analyses and morphology. Furthermore, the results of phylogenetic analysis of 28S confirmed the status of Dolichorchis as a separate genus. The phylogenies suggest multiple definitive host-switching events (birds to otters and among major avian groups) and a New World origin of Diplostomum and Tylodelphys spp. Our DNA sequences from adult digeneans revealed identities of 10 previously published lineages of Diplostomum and Tylodelphys, which were previously identified to genus only. The novel DNA data from this work provide opportunities for future comparisons of larval diplostomines collected in ecological studies.
Keywords: Diplostomidae, Molecular phylogeny, 28S, cox1, Diplostomum, Tylodelphys
1. Introduction
The Diplostomidae Poirier, 1886 is a large, globally distributed family of digeneans, typically parasites in the intestines of their tetrapod definitive hosts. At present, the family includes 42 genera split among four subfamilies (Niewiadomska, 2002; Heneberg et al., 2020). The type-genus Diplostomum von Nordmann, 1932 (subfamily Diplostominae Poirier, 1886) is highly speciose and globally distributed (Shigin, 1986, 1993; Galazzo et al., 2002; Niewiadomska, 2010; Behrmann-Godel, 2013; Georgieva et al., 2013; Locke et al., 2015; Gordy et al., 2016; Hoogendoorn et al., 2020). Members of Diplostomum have been the focus of numerous studies related to their ecology, host-parasite relationships, systematics and taxonomy (e.g., Shigin, 1986, 1993; Galazzo et al., 2002; Karvonen et al., 2004, 2006; Seppälä et al., 2008; Locke et al., 2010a,b, 2015; Niewiadomska, 2010; Georgieva et al., 2013; Pérez-del-Olmo et al., 2014; Kudlai et al., 2017; Enabulele et al., 2018; Rudko et al., 2018; Hoogendoorn et al., 2020; Vivas Muñoz et al., 2021). Complete mitochondrial genome sequences have been generated and studied for the type-species Diplostomum spathaceum (Rudolphi, 1819), Diplostomum pseudospathaceum Niewiadomska, 1984, Diplostomum ardeae Dubois, 1969 and Diplostomum baeri (Dubois, 1937) (Brabec et al., 2015; Landeryou et al., 2020; Locke et al., 2020). Brabec et al. (2015) demonstrated discordance between phylogenies generated from complete mitochondrial genomes and the nuclear rRNA operons of Diplostomum spp. in relation to other members of the order Diplostomida Olson, Cribb, Tkach, Bray & Littlewood, 2003 and Plagiorchiida La Rue, 1957.
The systematic and taxonomic history of Diplostomum is rather complex, with its composition varying greatly among authors (e.g., Dubois, 1968, 1982; Shigin, 1993; Niewiadomska, 2010). Recent molecular phylogenetic studies of Diplostomum (e.g., Galazzo et al., 2002; Locke et al., 2010a, 2015; Georgieva et al., 2013; Faltýnková et al., 2014; Pérez-del-Olmo et al., 2014; Selbach et al., 2015; Kudlai et al., 2017; Soldánová et al., 2017; Gordy and Hanington, 2019; Hoogendoorn et al., 2020) have revealed the presence of numerous species or species-level lineages of Diplostomum. However, most sequences originate from larval specimens, which often cannot be accurately identified morphologically to species. This prevents the resolution of the complex taxonomy and systematics of Diplostomum (e.g., Hoogendoorn et al., 2020). Previous studies have used molecular tools to reveal that some species of Diplostomum are distributed in multiple biogeographic realms (Locke et al., 2015, 2020; Hoogendoorn et al., 2020).
Close relationships between members of Diplostomum and two other genera of the Diplostominae, Tylodelphys Diesing, 1850 and Austrodiplostomum Szidat & Nani, 1951 have been repeatedly demonstrated using molecular phylogenies (e.g., Locke et al., 2015, 2018; Selbach et al., 2015; García-Varela et al., 2016; Blasco-Costa and Locke, 2017; Achatz et al., 2019b–d, 2020, 2021; Pelegrini et al., 2019; Sereno-Uribe et al., 2019a, b; Heneberg et al., 2020; Hoogendoorn et al., 2020; Tkach et al., 2020). Members of these three genera utilize a wide range of fish species as second intermediate hosts and typically parasitize fish-eating birds as adults (e.g., Gibson, 1996; Niewiadomska, 2002; Dronen, 2009; Locke et al., 2010a, b, 2015; Georgieva et al., 2013; Rosser et al., 2016a, b). Importantly, some members of these genera are well-known agents of fish diseases, often causing ocular diplostomiasis (e.g., Inchausty et al., 1997; McCloughlin 2016; Rosser et al., 2016a).
In contrast to the well-studied members of Diplostomum, species of Paralaria Kraus, 1914, parasitic in New World river otters as adults, have received little attention (Kraus, 1914; Dubois, 1944, 1968). Kraus (1914) established Paralaria for Paralaria clathrata (Diesing, 1850), the type-species, and his newly described Paralaria pseudoclathrata (Kraus, 1914). In the concept of Dubois (1938, 1968, 1970, 1982) Paralaria was a subgenus of Alaria Schrank, 1788 and included species parasitic in mammals other than otters. Paralaria is considered a valid, separate genus in the most recent revision of the Diplostomoidea Poirier, 1886 (see Niewiadomska, 2002).
Members of the small genus Dolichorchis Dubois, 1961, also a member of the Diplostominae, are rarely reported parasites of birds in the Afrotropical, Australasian, Indomalayan and Neotropical realms (Dubois, 1968; Niewiadomska, 2002; Lunaschi and Drago, 2006). Historically, this taxon was considered as either a subgenus of Diplostomum (e.g., Dubois, 1968) or as an independent genus (Niewiadomska, 2002).
More than 1,000 cox1 sequences of Diplostomum, Tylodelphys and Austrodiplostomum are currently available in GenBank (19 February 2021), whereas no DNA sequence data have been published for Paralaria or Dolichorchis. Despite the recent surge in molecular systematic and ecological studies on Diplostomum and its close relatives Tylodelphys and Austrodiplostomum, DNA sequence data are available for only 19 nominal species identified based on adult morphology (e.g., Galazzo et al., 2002; Locke et al., 2010a, b, 2015, 2018, 2020; Georgieva et al., 2013; Pérez-del-Olmo et al., 2014; Chibwana et al., 2015; Sereno-Uribe et al., 2019a, b; Hoogendoorn et al., 2020; Heneberg and Sitko, 2021). Less than 6% of the DNA sequence data for Diplostomum spp. currently available in GenBank originates from adult specimens (Hoogendoorn et al., 2020).
In the present study, we generated sequences of the large ribosomal subunit (28S) rRNA and cytochrome c oxidase 1 (cox1) mtDNA genes from 14 species/species-level lineages of Diplostomum from birds, otter, fish and snails collected in the Nearctic, Neotropics and Palaearctic, six species/species-level lineages of Tylodelphys from birds and fish collected in the Nearctic, Neotropics and Palaearctic, two species/species-level lineages of Austrodiplostomum from birds collected in the Palaearctic and Neotropics, two species of Dolichorchis from birds in the Neotropics, one species of Paralaria from otter collected in the Nearctic and an as-yet unidentified diplostomid from a bird in the Neotropics. Sixteen of the 26 studied taxa were identified to the species level based on adult morphology. We used DNA sequence data to explore the interrelationships of these taxa, determine phylogenetic relationships and re-evaluate their systematic placement.
2. Materials and methods
2.1. Morphological study
Adult diplostomids were obtained from the intestines of a variety of avian and mammalian hosts and larval diplostomids were collected from a variety of snail and fish species in Europe as well as North and South America (Table 1). Live digeneans were briefly rinsed in saline, heat-killed with hot water and fixed in 70% ethanol. Dead digeneans were immediately fixed in 70% or 95% ethanol. Specimens for light microscopy were stained with aqueous alum carmine according to Lutz et al. (2017) and studied using a differential interference contrast optics equipped Olympus BX51 compound microscope (Olympus Corp., Tokyo, Japan). Morphological vouchers were deposited in the collection of the H. W. Manter Laboratory, University of Nebraska, Lincoln and Parasitology Collection at the University of Wisconsin - Stevens Point, U.S.A. (Table 1).
Table 1.
Hosts, geographic origin, GenBank and museum accession numbers of diplostomine taxa used in this study. Site of infection is provided for specimens collected from fish intermediate hosts.
| Digenean taxa | Host species | Geographical origin | Museum No. | GenBank accession numbers |
|
|---|---|---|---|---|---|
| 28S | coxl | ||||
|
| |||||
| Austrodiplostomum compactum | Phalacrocorax brasilianus | Brazil (Pantanal) | HWML-216519 | MZ314149 | MZ323246 |
| Austrodiplostomum sp. VVT1 | Pelecanus onocrotalus | Ukraine (Odessa oblast) | HWML-216520 | MZ314150 | MZ323247 |
| Diplostomidae gen. sp. VVT1 | Tigrisoma lineatum | Brazil (Pantanal) | – | MZ314151 | MZ323248 |
| Diplostomum alarioidesa | Lontra canadensis | U.S.A. (Mississippi) | HWML-216521 | MZ314152 | MZ323249 |
| Diplostomum alascense n. comb. | Mergus merganser | U.S.A. (Minnesota) | HWML-216522 | MZ314153 | MZ323250 |
| Diplostomum gavium | Gavia immer | U.S.A. (North Dakota) | HWML-216523 | MZ314154 | MZ323251 |
| D. gavium | Hypentelium nigricans (eye) | U.S.A. (Minnesota) | – | MZ314155 | MZ323252 |
| D. gavium | Lymnaea stagnalis | U.S.A. (North Dakota) | – | MZ314156 | MZ323253 |
| D. gavium | Lymnaea sp. | U.S.A. (North Dakota) | – | MZ314157 | MZ323254,MZ323255 |
| D. gavium | Stagnicola elodes | U.S.A. (North Dakota) | – | MZ314158 | MZ323256 |
| Diplostomum huronense | Larus delawarensis | U.S.A. (Illinois) | UWSP-P-8634 | MZ314159 | MZ323257 |
| D. huronense | Larus delawarensis | U.S.A. (North Dakota) | – | – | MZ323258,MZ323259 |
| D. huronense | Larus dominicanus | Chile (Concepción) | HWML-216524 | MZ314160 | MZ323260 |
| D. huronense | Leucophaeus pipixcan | U.S.A. (North Dakota) | – | – | MZ323261 |
| Diplostomum indistinctum | Larus argentatus | U.S.A. (North Dakota) | – | MZ314161 | MZ323262 |
| D. indistinctum | Larus delawarensis | U.S.A. (North Dakota) | HWML-216525 | MZ314162–MZ314164 | MZ323263 |
| D. indistinctum | Leucophaeus pipixcan | U.S.A. (North Dakota) | – | – | MZ323264 |
| D. indistinctum | Recurvirostra americana | U.S.A. (North Dakota) | – | MZ314165 | MZ323265,MZ323266 |
| D. indistinctum | Stagnicola elodes | U.S.A. (North Dakota) | – | MZ314166 | MZ323267 |
| Diplostomum marshalli | Tringa melanoleuca | U.S.A. (North Dakota) | HWML-216526 | MZ314167 | MZ323268 |
| Diplostomum pseudospathaceum | Chroicocephalus genei | Ukraine (Kherson oblast) | HWML-216527 | MZ314168 | MZ323269 |
| Diplostomum rauschi | Chroicocephalus genei | Ukraine (Kherson oblast) | – | MZ314169 | MZ323270 |
| D. rauschi | Hydroprogne caspia | Ukraine (Kherson oblast) | HWML-216521–HWML-216521 | – | MZ323271,MZ323272 |
| Diplostomum scudderi | Lophodytes cucullatus | U.S.A. (North Dakota) | HWML-216530 | MZ314170 | MZ323273 |
| Diplostomum spathaceum | Chroicocephalus genei | Ukraine (Kherson oblasl) | – | – | MZ323274 |
| D. spathaceum | Larus argentatus | Ukraine (Kherson oblasl) | HWML-216533-HWML-216535 | MZ314171 | MZ323275–MZ323277 |
| D. spathaceum | Larus cachinnans | Ukraine (Chemihiv oblasl) | HWML-216531 | MZ314172 | MZ323278–MZ323280 |
| D. spathaceum | Spatula querquedula | Ukraine (Kherson oblasl) | HWML-216532 | – | MZ323281 |
| Diplostomum sp. VVT1 | Lymnaea stagnalis | U.S.A. (Minnesota) | – | MZ314173, MZ314174 | MZ323282,MZ323283 |
| Diplostomum sp. VVT1 | Umbra limi (brain) | U.S.A. (Minnesota) | – | MZ314175 | MZ323284,MZ323285 |
| Diplostomum sp. VVT2 | Lepomis cyanellus (skin) | U.S.A. (Minnesota) | – | – | MZ323286 |
| Diplostomum sp. VVT2 | Lepomis gibbosus (eye) | U.S.A. (Minnesota) | – | – | MZ323287 |
| Diplostomum sp. VVT2 | Perca flavescens (skin) | U.S.A. (Minnesota) | – | MZ314176 | MZ323288,MZ323289 |
| Diplostomum sp. VVT2 | Perca flavescens (eye) | U.S.A. (Minnesota) | – | – | MZ323290–MZ323293 |
| Diplostomum sp. VVT3 | Lontra canadensis | U.S.A. (Wisconsin) | UWSP-P-8635–8637 | MZ314177 | MZ323294 |
| Diplostomum sp. VVT4 | Lymnaea stagnalis | U.S.A. (Minnesota) | MZ314178 | MZ323295 | |
| Diplostomum sp. VVT5 | Egretta caerulea | U.S.A. (Mississippi) | HWML-216536 | MZ314179 | MZ323296 |
| Dolichorchis lacombeensis | Ardea cocoi | Brazil (Pantanal) | HWML-216537 | MZ314180 | MZ323297 |
| Do. lacombeensis | Busarellus nigricollis | Brazil (Pantanal) | – | MZ314181 | MZ323298 |
| Dolichorchis sp. VVT1 | Phimosus infuscatus | Brazil (Pantanal) | – | MZ314182 | MZ323299 |
| Tylodelphys cf. americana | Jabiru mycteria | Brazil (Pantanal) | HWML-216538 | MZ314183, MZ314184 | MZ323300,MZ323301 |
| Tylodelphys conifera | Podiceps grisegena | U.S.A. (Minnesota) | HWML-216539 | MZ314185 | MZ323302 |
| Tylodelphys immer | Gavia immer | U.S.A. (North Dakota) | HWML-216540 | MZ314186 | MZ323303 |
| Tylodelphys robrauschi n. comb. | Podilymbus podiceps | U.S.A. (Minnesota) | HWML-216541 | MZ314187 | MZ323304 |
| Tylodelphys scheuringi | Lepomis gibbosus (eye) | U.S.A. (Minnesota) | – | – | MZ323305 |
| T. scheuringi | Lepomis macrochirus (eye) | U.S.A. (Minnesota) | – | – | MZ323306 |
| T. scheuringi | Umbra limi (brain) | U.S.A. (Minnesota) | – | MZ314188 | MZ323307 |
| Tylodelphys sp. VVT1 | Ambystoma talpoideum | U.S.A. (Mississippi) | – | MZ314189 | MZ323308 |
Formerly included in Paralaria.
Museum abbreviations: HWML, Harold W. Manter Laboratory, U.S.A.; UWSP – PARA, University of Wisconsin - Stevens Point Parasitology Collection, U.S.A.
Different authors referred to the two distinct body parts in diplostomoideans as prosoma/opisthosoma, or forebody/hindbody, or anterior/posterior segments. The latest revision by Niewiadomska (2002) in the “Keys to the Trematoda” used the terms forebody and hindbody for these body parts, whereas a different meaning was given to the same terms in chapters on all other distome digeneans, which was somewhat confusing. To avoid confusion, we use the terms prosoma and opisthosoma (e.g. Achatz et al., 2019a, c; Tkach et al., 2020) to reflect the fact that these parts of the body in diplostomoideans are not segments (e.g. unlike segments or proglottides in cestodes) and the terms forebody and hindbody are universally used to designate the parts of body posterior and anterior to the ventral sucker in distome digeneans. Our use of this terminology is also consistent with its use in similar situations among other invertebrates, e.g. arachnids.
2.2. Molecular study
Genomic DNA was extracted following the protocol described by Tkach and Pawlowski (1999) or using a ZR Genomic DNA Tissue Micro Prep kit (Zymo Research, Irvine, California, U.S.A.) following the manufacturer’s protocol. A fragment of the nuclear ribosomal 28S rRNA gene was amplified by PCR using the forward primer digL2 (5’-AAG CAT ATC ACT AAG CGG-3’) and reverse primer 1500R (5’-GCT ATC CTG AGG GAA ACT TCG-3’) (Tkach et al., 2003). Fragments of cox1 were amplified by PCR using the forward primers Plat-diploCOX1F (5’-CGT TTR AAT TAT ACG GAT CC-3’), Cox1_Schist_5’ (5’-TCT TTR GAT CAT AAG CG-3’), Dipl_Cox_5’ (5’-ACK TTR GAW CAT AAG CG-3’) and BS_CO1_INT_F (5’-ATT AAC CCT CAC TAA ATG ATT TTT TTY TTT YTR ATG CC-3’) with reverse primers Plat-diploCOX1R (5’-AGC ATA GTA ATM GCA GCA GC-3’), acox650R (5’-CCA AAA AAC CAA AAC ATA TGC TG-3’), JB5 (5’-AGC ACC TAA ACT TAA AAC ATA ATG AAA ATG-3’), Dipl650R (5’-CCA AAR AAY CAR AAY AWR TGY TG-3’), Dipl_Cox_3’ (5’-WAR TGC ATN GGA AAA AAA CA-3’) and BS_CO1_INT_R (5’-TAA TAC GAC TCA CTA TAA AAA AAA MAM AGA AGA RAA MAC MGT AGT AAT-3’) (Lockyer et al., 2003; Derycke et al., 2005; Moszczynska et al., 2009; Kudlai et al., 2015; Achatz et al., 2019a, in press). PCR amplifications were performed in a total volume of 25 μl using GoTaq G2 DNA Polymerase from Promega (Madison, Wisconsin, U.S.A.) according to the manufacturer’s instructions and using an annealing temperature of 53 °C for nuclear rDNA amplifications and 45 °C for cox1 amplifications.
An ExoSAP-IT PCR clean-up enzymatic kit from Affymetrix (Santa Clara, California, U.S.A.) was used to purify PCR products. PCR products were cycle-sequenced directly using a BrightDye Terminator Cycle Sequencing Kit (MCLAB, California, U.S.A.), purified using a BigDye Sequencing Clean Up Kit from MCLAB and run on an ABI 3130 automated capillary sequencer (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.A.).
PCR primers and the previously published 28S internal forward primer DPL600F (5’-CGG AGT GGT CAC CAC GAC CG-3’) and reverse primer DPL700R (5’-CAG CTG ATT ACA CCC AAA G-3’), together with new 28S internal forward primer DPL250F (5’-GGG TTG TTT GTG AAT GCA GCC C-3’) and internal reverse primers DPL350R (5’-GTT TAC CTC TGA GCG GTT TCA CG-3’), DPL1300R (5’-GCC TTT GGG TTT CGT AAC GCC-3’) and DPL1450R (5’-GAC GGG CCG GTG ATG CGC C-3’), designed by T.J. Achatz and V.V. Tkach, were used for sequencing reactions (Achatz et al., 2019d). Contiguous sequences were assembled using Sequencher version 4.2 software (GeneCodes Corp., Ann Arbor, Michigan, U.S.A.). Newly obtained sequences were deposited in the GenBank database (Table 1).
Sequences were initially aligned using ClustalW as implemented in MEGA7 software (Kumar et al., 2016). Initially, the phylogenetic positions of Diplostomum, Paralaria, Tylodelphys, Austrodiplostomum, Dolichorchis and one unidentified diplostomid within the Diplostomoidea Poirier, 1886 were determined using a 28S alignment with Suchocyathocotyle crocodili (Yamaguti, 1954) (Cyathocotylidae Mühling, 1896) as the outgroup based on the phylogeny published by Achatz et al. (2019d). This alignment included newly obtained sequences of species of Diplostomum (n = 3), Paralaria (n = 1), Tylodelphys (n = 2), Austrodiplostomum (n = 2), Dolichorchis (n = 2) and the unidentified diplostomid (n = 1) together with previously published sequences of Diplostomum (n = 3), Tylodelphys (n = 1) and Austrodiplostomum (n = 1), together with 15 other representatives of the Diplostomidae, 10 representatives of the Strigeidae Railliet, 1919 and two representatives of the Proterodiplostomidae Dubois, 1936.
Based on the results of the initial broader analysis, the interrelationships of Diplostomum, Paralaria, Tylodelphys and Austrodiplostomum, as currently recognized, were studied using two additional 28S alignments and two cox1 alignments with Alaria mustelae Bosma, 1931 used as the outgroup in all three analyses. One of these two 28S alignments included all newly obtained sequences of Diplostomum spp. (n = 14) and Paralaria spp. (n = 1), together with previously published sequences of Diplostomum spp. (n = 8). The other additional 28S alignment included newly obtained sequences of Tylodelphys spp. (n = 5) and Austrodiplostomum spp. (n = 2) as well as previously published sequences of Tylodelphys spp. (n = 2), Austrodiplostomum spp. (n = 3) and an unidentified diplostomid (n = 1). The first cox1 alignment included newly generated sequences of Diplostomum spp. (n = 27) and Paralaria spp. (n = 1), together with previously published sequences of species of Diplostomum (n = 53). The second cox1 alignment included newly generated sequences of Tylodelphys spp. (n = 9) and Austrodiplostomum spp. (n = 2) as well as previously published sequences of Tylodelphys spp. (n = 21), Austrodiplostomum spp. (n = 5) and an unidentified diplostomid (n = 1). Although numerous other cox1 sequences are available, we opted to include only a limited number of representatives from each of the previously published species/species-level lineages. Recent studies on Diplostomum (e.g., Georgieva et al., 2013; Blasco-Costa et al., 2014; Locke et al., 2015; Selbach et al., 2015; Kudlai et al., 2017; Hoogendoorn et al., 2020) have already explored relationships among most of these lineages. However, we ensured that all major lineages within these genera were present in our analyses.
Bayesian inference (BI) as implemented in MrBayes v3.2.6 software was used for all phylogenetic analyses (Ronquist and Huelsenbeck, 2003). The general time-reversible model with estimates of invariant sites and gamma-distributed among-site variation (GTR + G + I) model was identified as the best-fitting nucleotide substitution model for the 28S and cox1 alignments using MEGA7 (Kumar et al., 2016). BI analyses for all datasets were performed using MrBayes software as follows: Markov chain Monte Carlo (MCMC) chains were run for 3,000,000 generations with sample frequency set at 1,000. Log-likelihood scores were plotted and only the final 75% of trees were used to produce the consensus trees. The number of generations for each analysis was determined as sufficient because the standard deviation stabilized below 0.01. Pairwise comparisons for each locus were carried out using MEGA7.
To accurately and consistently identify which species-level lineage is referred to throughout the text and supplementary materials, a reference to the origin of designations of species-level lineages is provided for non-nominal species that previously were assigned a lineage identification. The following abbreviations for references for species-level lineages were used: B, Blasco-Costa et al. (2014); C, Chibwana et al. (2013); Ch, Chaudhary et al. (unpublished); Ge, Georgieva et al. (2013); Go, Gordy and Hanington (2019); H, Hoogendoorn et al. (2020); Ko, Komatsu et al. (2019); Ku, Kudlai et al. (2017); L, Locke et al. (2010a, b; 2015); M, Moszczynska et al. (2009); N, Nakao and Sasaki (2021); P, Pelegrini et al. (2019); R, Rosser et al. (2016a); Se, Sereno-Uribe et al. (2019a); Sl, Selbach et al. (2015); So, Soldánová et al. (2017).
3. Results
3.1. Molecular phylogenies
The broader 28S alignment of the Diplostomoidea was 1,118 bp long; two nucleotide positions were excluded due to indels. Similar to several recent molecular phylogenetic studies (e.g., Blasco-Costa and Locke, 2017; Hernández-Mena et al., 2017; Locke et al., 2018; Achatz et al. 2019b–d, 2020, 2021; Queiroz et al., 2020; Tkach et al., 2020), our broader 28S phylogeny (Fig. 1) demonstrated the non-monophyletic nature of the Diplostomidae and Strigeidae.
Fig. 1.
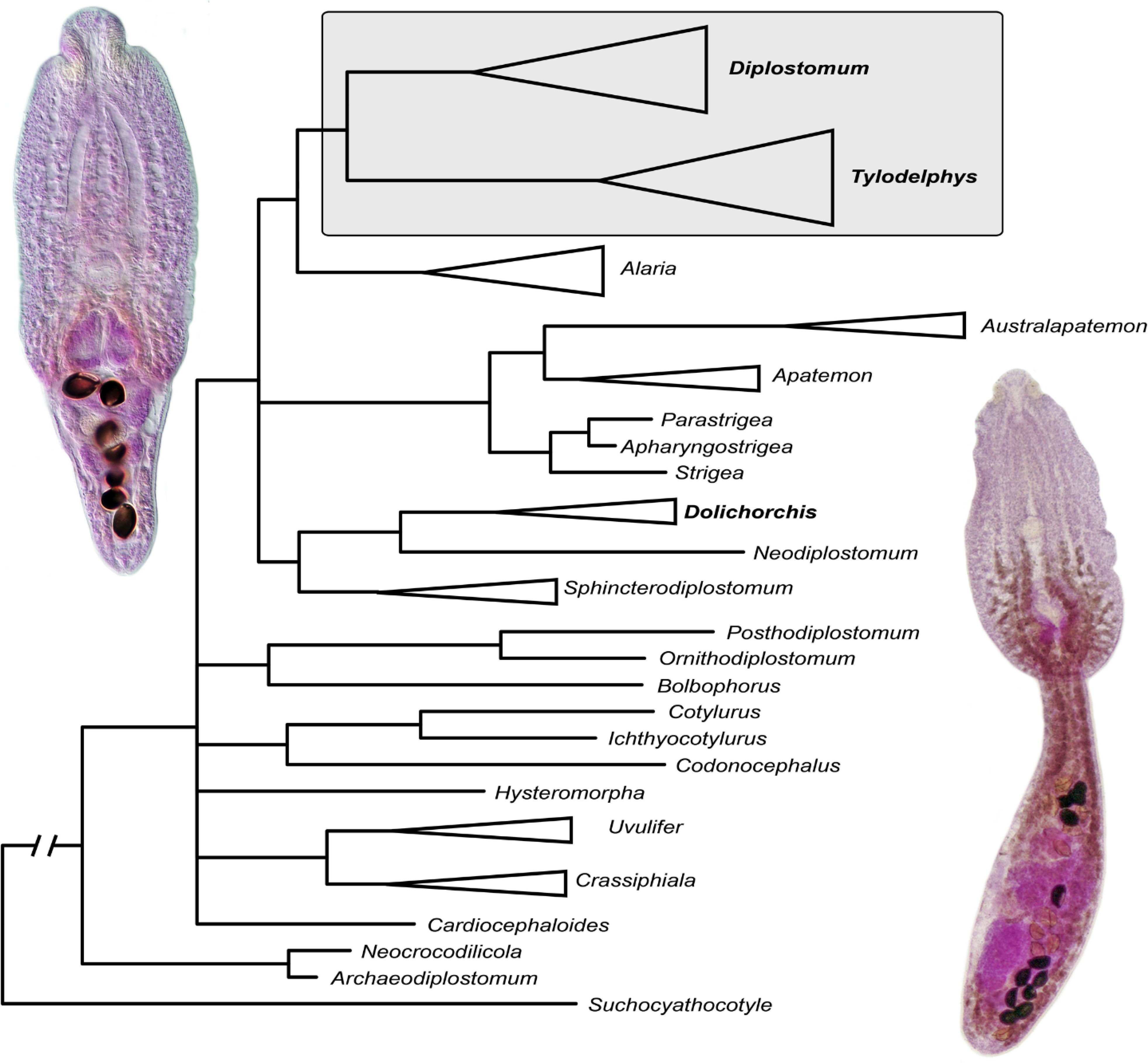
Phylogenetic interrelationships among 43 diplostomoidean taxa including 13 members of Diplostomum, Tylodelphys and Austrodiplostomum (including a former Paralaria sp.), two species-level lineages of Dolichorchis and an unknown diplostomid based on Bayesian inference analysis of partial 28S rRNA gene sequences. Members of Diplostomum + Tylodelphys are indicated by the shaded rectangle. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale bar indicates the number of substitutions per site. GenBank accession numbers are provided after the names of species. The previously accepted/published names are provided in parentheses after GenBank accession numbers.
The two sequences of the Proterodiplostomidae, Archaeodiplostomum overstreeti Tkach, Achatz & Pulis, 2020 and Neocrocodilicola georgiana (Byrd & Reiber, 1942), formed a monophyletic clade, similar to recent detailed phylogenetic analyses of the family (Tkach et al., 2020). Diplostomum, Paralaria, Tylodelphys and Austrodiplostomum formed a weakly supported clade. However, the internal topology within this clade was well-resolved. Diplostomum + Paralaria formed a 100% supported clade; similarly, Tylodelphys + Austrodiplostomum also formed a 100% supported clade. Both of these 100% supported clades had well-supported internal topologies. Tylodelphys, as currently recognized, was non-monophyletic because Tylodelphys cf. americana (Dubois, 1936), a digenean with typical Tylodelphys morphology, appeared to be more closely related to Austrodiplostomum than to other Tylodelphys spp. Members of Austrodiplostomum formed a 96% supported clade. Both Dolichorchis species-level lineages clustered together with 100% support within a 100% supported clade, which also contained Neodiplostomum Railliet, 1919. These two genera showed a very weakly supported relatedness to Sphincterodiplostomum. The unidentified diplostomid lineage (Diplostomidae gen. sp. VVT1) formed a separate branch as a part of the extensive basal polytomy of the Diplostomoidea (Fig. 1).
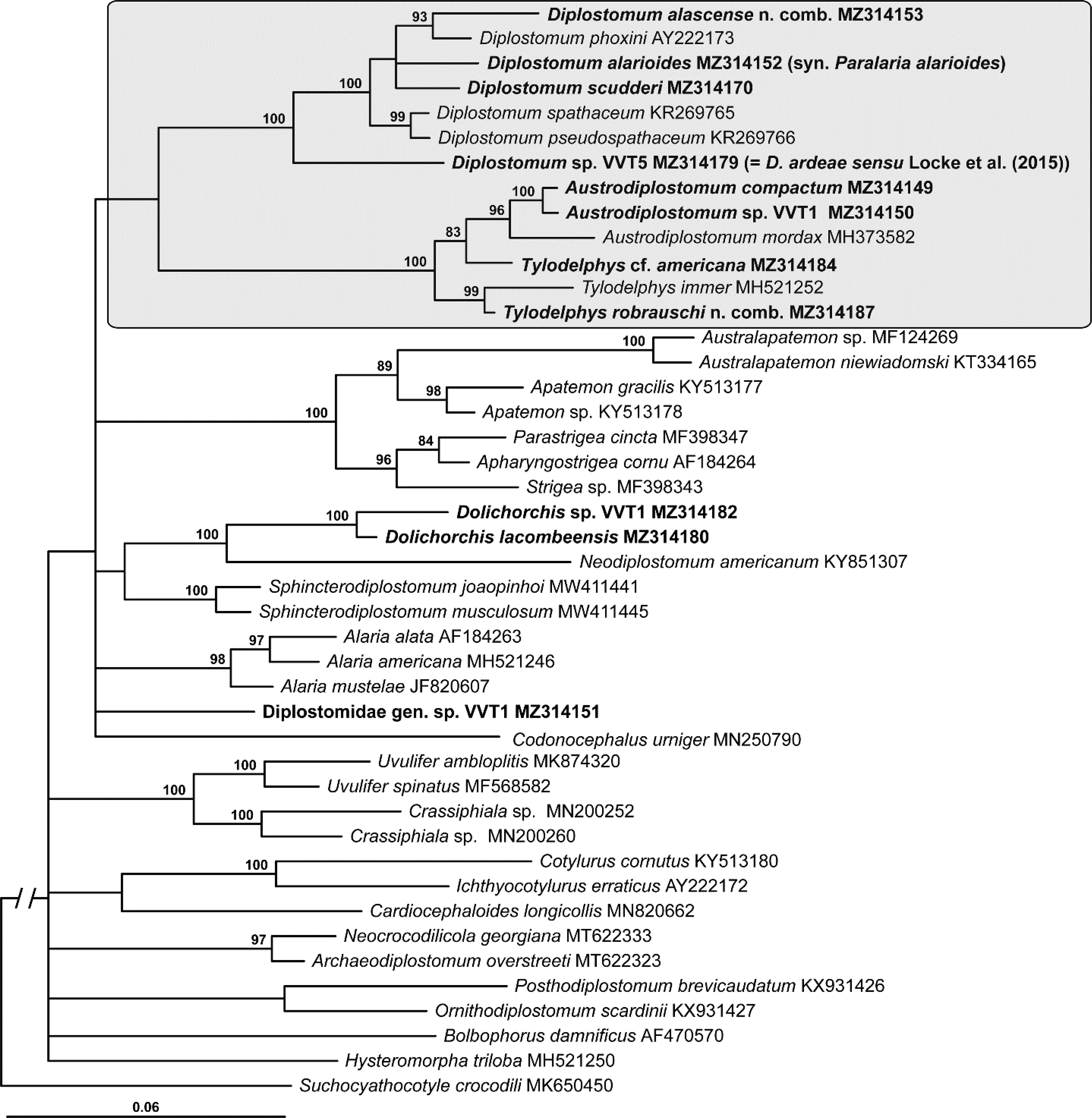
Upon trimming to the length of the shortest sequence, the second 28S alignment limited to Diplostomum and Paralaria as currently recognized, was 1,106 bp long. The internal topology within this tree was overall moderately resolved (Fig. 2). Similar to the broader 28S phylogeny (Fig. 1), Diplostomum sp. VVT5 (= D. ardeae sensu Locke et al. (2015); see section 4.3 below) appeared as a sister branch to a weakly supported clade which contained all other species of Diplostomum included in the analysis (Fig. 2); admittedly, this relationship was not well supported. A number of internal topologies were much better resolved. The two Diplostomum spp. (sp. A and B (N)) from Japan formed a 100% supported clade, which was positioned as a sister clade to the remainder of the Diplostomum taxa. The latter clade included three sub-clades: (i) Diplostomum sp. DTS1R (H); (ii) an 80% supported clade of Diplostomum sp. VVT2 + an 86% supported clade of (Diplostomum phoxini (Faust, 1918) + Diplostomum alascense Dubois, 1969 n. comb.; see section 4.3 below); and (iii) a weakly supported large clade consisting of two sub-clades (Fig. 2). The first of these sub-clades included a 100% supported cluster of (Diplostomum sp. VVT1 + Diplostomum scudderi Olivier, 1941 (94% supported) and Diplostomum marshalli Chandler, 1954 + Diplostomum sp. VVT4 (87% supported)) and a 100% supported clade of Diplostomum alarioides Dubois, 1937 + Diplostomum sp. VVT3, both from the North American river otter Lontra canadensis (Schreber). The second sub-clade (96% supported) was characterized by largely unresolved internal topology. It included a weakly supported clade of D. pseudospathaceum + Diplostomum gavium (Guberlet, 1922) and a weakly supported clade of Diplostomum indistinctum (Guberlet, 1922) + Diplostomum rauschi Shigin, 1993 + Diplostomum sp. 16 (L) + a 92% supported clade of (D. spathaceum + Diplostomum huronense (La Rue, 1927) + Diplostomum sp. 14 (L)) (Fig. 2).
Fig. 2.
Phylogenetic interrelationships among 21 taxa of Diplostomum (including a former Paralaria sp.) based on Bayesian inference analysis of partial 28S rRNA gene sequences. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale bar indicates the number of substitutions per site. GenBank accession numbers are provided after the names of species. References to origins of species numbering/naming systems are provided in parentheses after GenBank accession numbers followed by the biogeographical realms where specimens were collected, life stages of isolates and families of definitive hosts (for adult isolates and larvae molecularly matched to adult forms). Abbreviations for references to the original designations of species-level lineages: H, Hoogendoorn et al. (2020); L, Locke et al. (2010a, b; 2015); N, Nakao and Sasaki, (2021). The previously accepted/published names are provided in parentheses after GenBank accession numbers. Abbreviations for biogeographical realms: Afr, Afrotropical realm; Nea, Nearctic realm; Neo, Neotropical realm; Pal, Palaearctic realm. Abbreviations for life stage: Adu, adult; Cer, cercaria; Met, metacercaria. Abbreviations for family of definitive host: Ana, Anatidae; Ard, Ardeidae; Gav, Gaviidae; Lar, Laridae; Mus, Mustelidae; Rec, Recurvirostridae; Sco, Scolopacidae.
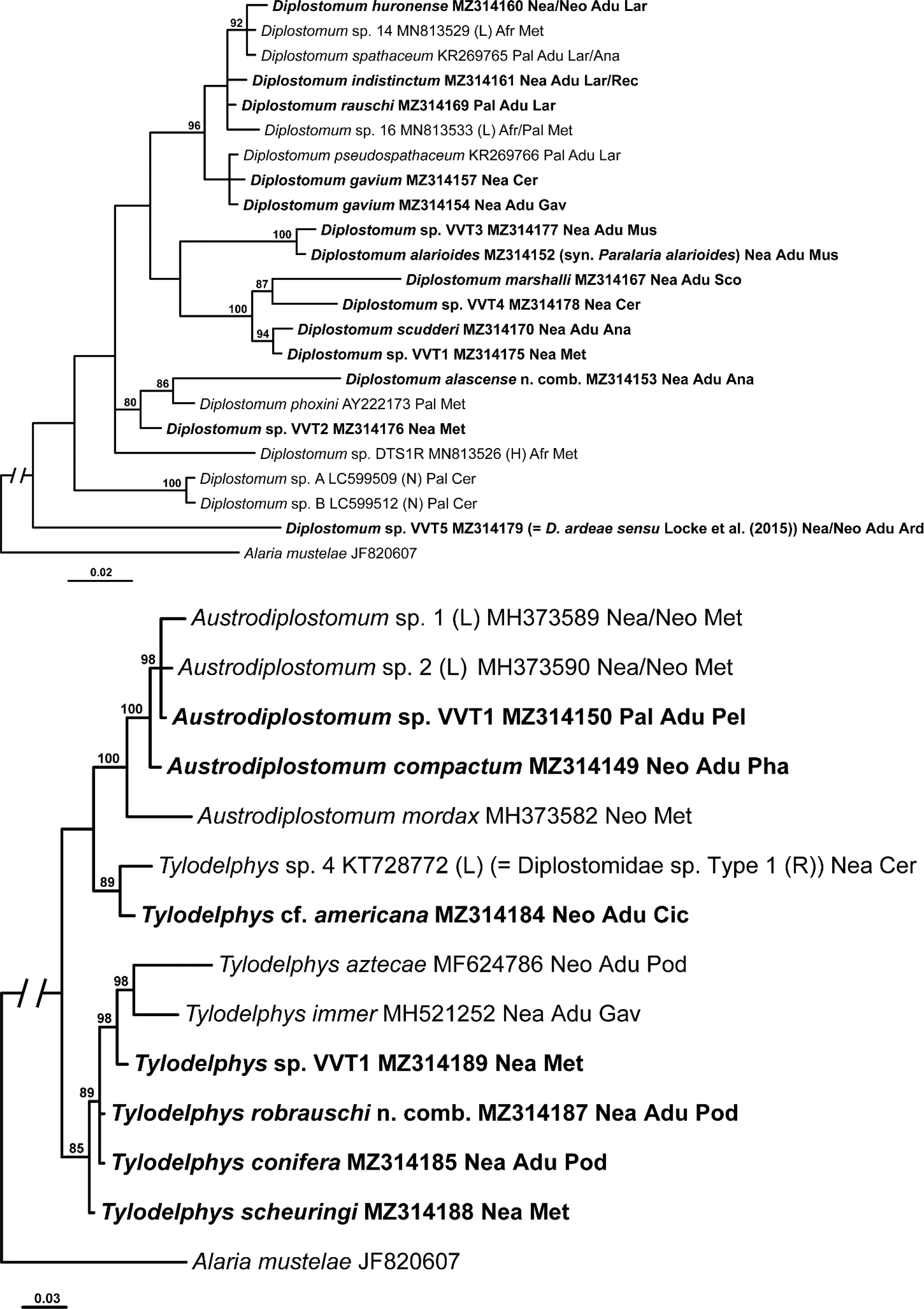
The third 28S alignment was 1,106 bp long and limited to members of Tylodelphys and Austrodiplostomum taxa, as currently recognized. The phylogenetic tree resulting from the analysis of this alignment contained two clusters (Fig. 3). Tylodelphys appeared non-monophyletic because, similar to the first 28S-based phylogeny, Tylodelphys cf. americana appeared to be more closely related to Austrodiplostomum or at least to form an independent clade in the basal polytomy. The first clade of Tylodelphys was 85% supported and contained Tylodelphys scheuringi (Hughes, 1929) and an 89% supported clade of Tylodelphys conifera (Mehlis, 1846) + Tylodelphys robrauschi Dubois, 1969 n. comb. (see section 4.5 below) + an 98% supported clade of (Tylodelphys sp. VVT1 + an 98% supported clade of (Tylodelphys immer Dubois, 1961 + Tylodelphys aztecae García-Varela, Sereno-Uribe, Pinacho-Pinacho, Hernández-Cruz & Pérez-Ponce de León, 2015)). The second clade of Tylodelphys spp. (which included T. cf. americana) formed a weakly supported cluster with Austrodiplostomum spp. Tylodelphys cf. americana and an unidentified diplostomid cercaria (Tylodelphys sp. 4 (L) (= Diplostomidae sp. 1 Type 1 (R))) formed an 89% supported clade. The Austrodiplostomum clade was strongly supported (100%). Austrodiplostomum mordax Szidat & Nani, 1951 formed a sister branch to a 100% supported clade containing the remaining Austrodiplostomum spp., including Austrodiplostomum sp. VVT1 from Pelecanus onocrotalus Linnaeus. Austrodiplostomum compactum (Lutz, 1928) formed a sister group to a 98% supported clade containing two previously published sequences of Austrodiplostomum sp. 1 and 2 (L) + Austrodiplostomum sp. VVT1 (Fig. 3).
Fig. 3.
Phylogenetic interrelationships among 13 taxa of Tylodelphys and Austrodiplostomum spp. based on Bayesian inference analysis of partial 28S rRNA gene sequences. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale bar indicates the number of substitutions per site. GenBank accession numbers are provided after the names of species. References to origins of species numbering/naming systems are provided in parentheses after GenBank accession numbers followed by the biogeographical realms where specimens were collected, life stages of isolate and families of definitive hosts (for adult isolates and larvae molecularly matched to adult forms). Abbreviations for references to the original designations of species-level lineages: L, Locke et al. (2010a, b; 2015); R, Rosser et al. (2016a). The previously accepted/published names are provided in parentheses after GenBank accession numbers. Abbreviations for biogeographical realms: Nea, Nearctic realm; Neo, Neotropical realm; Pal, Palaearctic realm. Abbreviations for life stage: Adu, adult; Cer, cercaria; Met, metacercaria. Abbreviations for families of definitive hosts: Cic, Ciconiidae; Gav, Gaviidae; Pel, Pelecanidae; Pha, Phalacrocoracidae; Pod, Podicipedidae.
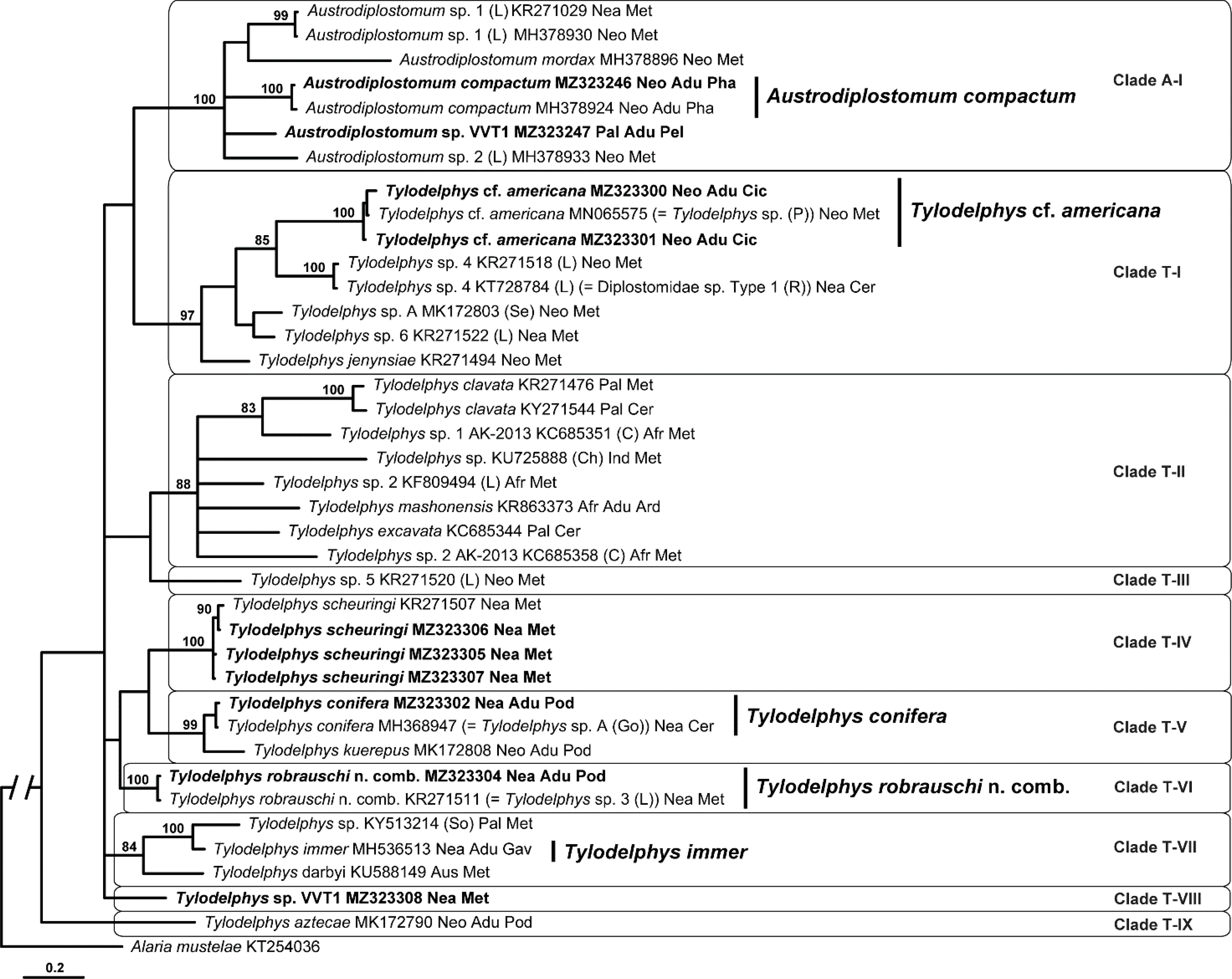
The cox1 alignments were 362 bp long. We provide the phylogenetic tree based on cox1 data from Diplostomum spp. as a supplement (Supplementary Fig. S1) due to the large size of the tree. Due to the large number of taxa and the presence of basal polytomies in both Diplostomum and Tylodelphys/Austrodiplostomum trees, we have numbered the main clades for the convenience of presenting results and following the discussion (Fig. 4; Supplementary Fig. S1).
Fig. 4.
Phylogenetic interrelationships among 27 taxa of Tylodelphys and Austrodiplostomum spp. based on Bayesian inference analysis of partial cox1 mtDNA gene sequences. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale bar indicates the number of substitutions per site. GenBank accession numbers are provided after the names of species. References to origins of species numbering/naming systems are provided in parentheses after GenBank accession numbers followed by the biogeographical realms where specimens were collected, life stages of isolate and families of definitive hosts (for adult isolates and larvae molecularly matched to adult forms). Abbreviations for references to the original designations of species-level lineages: C, Chibwana et al. (2013); Ch, Chaudhary et al. (unpublished); Go, Gordy and Hanington (2019); L, Locke et al. (2010a, b; 2015); P, Pelegrini et al. (2019); R, Rosser et al. (2016a); Se, Sereno-Uribe et al. (2019a); So, Soldánová et al. (2017). Abbreviations for biogeographical realms: Afr, Afrotropical realm; Aus, Australasian realm; Ind, Indomalayan realm; Nea, Nearctic realm; Neo, Neotropical realm; Pal, Palaearctic realm. Abbreviations for life stages: Adu, adult; Cer, cercaria; Met, metacercaria. Abbreviations for families of definitive hosts: Ard, Ardeidae; Cic, Ciconiidae; Gav, Gaviidae; Pel, Pelecanidae; Pha, Phalacrocoracidae; Pod, Podicipedidae.
The majority of Diplostomum spp. formed a 100% supported polytomous cluster with multiple well-supported internal clades, some of them well-resolved (Supplementary Fig. S1; clades D-I–D-XVI). Only a single clade (clade D-XVII) comprising D. ardeae sensu Locke et al. (2015), Diplostomum lunaschiae Locke, Drago, Núñez, Rangel e Souza & Takemoto, 2020 and Diplostomum sp. VVT5, was positioned separately from the larger polytomy. We only focus on the 10 clades containing species with newly generated DNA sequence data.
Clade D-I consisted of two strongly supported, larger sub-clades. The first major sub-clade (100% support) contained a large group of species-level lineages and two named species, D. baeri and D. phoxini. Notably, sequences of lineages belonging to the D. baeri complex appeared in three different strongly supported (100%, 92% and 100%) clusters. Within this clade, Diplostomum sp. VVT2 formed a 100% supported clade with a sequence of D. baeri sensu Galazzo et al. (2002) (MF142196; Ubels et al., 2018) (Supplementary Fig. S1). The second major sub-clade also included several species-level lineages and only a single named species, D. alascense n. comb. Within this clade, D. alascense n. comb. was clustered with a sequence of a metacercaria previously identified as Diplostomum sp. 2 (M) in a 100% supported clade.
Strongly supported (97%) clade D-II contained a polytomy with several species-level lineages, including our Diplostomum VVT1 and VVT4, as well as one named species, D. scudderi. Within this clade, Diplostomum sp. VVT4 + Diplostomum sp. 17 (L) formed a 100% supported clade.
Another weakly supported clade in the basal polytomy consisted of two strongly supported clades (D-III and D-IV). Clade D-III was split into two sub-clades (Supplementary Fig. S1) that comprised sequences of D. gavium (= Diplostomum sp. 3 (M)) and D. pseudospathaceum. The first sub-clade (99% support) contained sequences of D. gavium, while the second sub-clade (95% support) contained D. pseudospathaceum. Clade D-IV (100% support) only contained newly generated sequences of D. indistinctum + Diplostomum sp. 4 (M). Clade D-VI (100% support) only consisted of newly generated sequences of D. huronense and Diplostomum sp. 1 (M). Notably, D. indistinctum sensu Galazzo et al. (2002) and D. huronense sensu Galazzo et al. (2002) were positioned in the tree separately from our isolates of D. huronense and D. indistinctum.
Diplostomum rauschi + Diplostomum sp. Lineage 2 (B) formed a 100% supported cluster within clade D-VIII (84% support). Clade D-VIII also included a 100% supported group of Diplostomum sp. 16 (L) sequences.
The isolates of D. spathaceum formed a 100% supported cluster (clade D-IX) that appeared in the cox1 tree (Supplementary Fig. S1). In the 100% supported Clade D-X, D. alarioides was basal to the strongly supported clade of Diplostomum sp. VVT3 + Diplostomum sp. 10 (L). Clade D-XIII (100% support) consisted of D. marshalli and Diplostomum sp. A (Go).
The second major well-supported (94%) clade of Diplostomum (clade D-XVII), which was separate from the largest polytomy described above, contained D. lunaschiae + Diplostomum sp. VVT5 + D. ardeae sensu Locke et al. (2015).
This cox1 based phylogeny of Tylodelphys + Austrodiplostomum (Fig. 4) consisted of a polytomy with nine well-supported clades (clades A-I, T-I–T-VIII) and a sister clade (T-IX) which only contained T. aztecae. We opt to only discuss the six clades containing isolates with newly generated DNA sequences.
Clade A-I (100% support) contained all Austrodiplostomum taxa included in our analysis with unresolved internal topology. Within this clade, A. mordax n. comb. appeared as a sister group to a weakly supported cluster containing Austrodiplostomum sp. 1 (L).
The strongly supported (97%) clade T-I contained two nominal species, T. cf. americana and Tylodelphys jenynsiae Szidat, 1969, and a few not yet identified species-level lineages (Fig. 4). Tylodelphys jenynsiae was positioned as a sister group to a weakly supported clade consisting of a cluster of an 85% supported clade containing (a 100% supported clade of Tylodelphys sp. 4 (L) (= Diplostomidae sp. 1 Type 1 (R)) + a 100% supported clade of T. cf. americana (= Tylodelphys sp. MN065575 (P))) + an unsupported clade of (Tylodelphys sp. A (Se) + Tylodelphys sp. 6 (L)).
The clades T-IV (100% support), T-V (99% support) and T-VI (100% support) formed a weakly supported cluster. Clade T-IV contained only isolates of T. scheuringi, while clade T-V contained Tylodelphys kuerepus Sereno-Uribe, Andrade-Gómez, Ponce de León & García-Varela, 2019 + a cluster of (T. conifera + Tylodelphys sp. A (Go)). Clade T-VI only contained T. robrauschi n. comb. + Tylodelphys sp. 3 (L).
Clade T-VII (84% support) included Tylodelphys darbyi Presswell & Blasco-Costa, 2019 and a 100% supported cluster of T. immer + Tylodelphys sp. (KY513214) (So). Tylodelphys sp. VVT1 formed another independent clade (T-VIII) in the polytomy of Tylodelphys spp.
3.2. Pairwise comparisons
Due to the relatively limited amount of available 28S sequence data, it was possible to provide pairwise comparisons of all available named species or species-level lineages regardless of parasite life stage (Diplostomum: Supplementary Table S1; Tylodelphys and Austrodiplostomum: Supplementary Table S2). Due to the greater number of cox1 sequences available in GenBank, we provide pairwise comparisons only for cox1 sequences included in our phylogenetic analyses (Diplostomum: Supplementary Table S3; Tylodelphys and Austrodiplostomum: Supplementary Table S4). However, we discuss below only pairwise comparisons of Diplostomum, Tylodelphys and Austrodiplostomum cox1 sequences from morphologically identified adults or larvae matched genetically with adult specimens included in our analysis (Supplementary Table S5). Intraspecific variations of cox1 sequences were studied using two closely related representative species of Diplostomum (Supplementary Table S6) and a species of Tylodelphys (Supplementary Table S7); multiple identical sequences of species and species-level lineages were not included in analyses.
The interspecific divergence in 28S sequences of Diplostomum spp. was generally low (0–3.7%; Supplementary Table S1). Diplostomum sp. A (N) versus Diplostomum sp. B (N), D. gavium versus D. pseudospathaceum and Diplostomum sp. 14 (L) versus D. spathaceum showed the lowest levels of divergence. In contrast, D. marshalli versus Diplostomum sp. VVT5 demonstrated the greatest divergence. Similarly, the interspecific divergence in 28S sequences of Tylodelphys and Austrodiplostomum spp. was also generally low (0–1.9% and 0.1–1.3%, respectively; Supplementary Table S2). Among Tylodelphys taxa, the pair T. conifera versus T. robrauschi n. comb. were the least divergent, whereas T. aztecae versus Tylodelphys sp. 4 (L) showed the greatest difference. Among Austrodiplostomum, the pair Austrodiplostomum sp. VVT1 versus Austrodiplostomum sp. 2 (L) were the least divergent, while A. mordax versus Austrodiplostomum sp. 1 (L) and A. mordax versus Austrodiplostomum sp. 2 were the most divergent. The 28S sequences of the two Dolichorchis species-level lineages differed by 1%.
No intraspecific variation was detected among the newly obtained 28S sequences of D. huronense (n = 2), D. indistinctum (n = 6), D. spathaceum (n = 2), T. cf. americana (n = 2) and Dolichorchis lacombeensis Lunaschi & Drago, 2006 (n = 2), whereas isolates of D. gavium (n = 4) differed by up to 0.1% (1 nucleotide).
Diplostomum spp. showed interspecific divergence levels of 2.3–16.3% in the sequenced fragment of cox1 gene (Supplementary Tables S5, S6). Diplostomum pseudospathaceum versus D. gavium had the lowest level of interspecific divergence, whereas Diplostomum sp. VVT5 versus D. rauschi (KJ726449) (= Diplostomum sp. Lineage 2 (B)) were the most divergent. The interspecific divergence levels of cox1 sequences among Tylodelphys spp. (4.4–14.6%) and Austrodiplostomum spp. (9.1–13%) were similar to the interspecific divergence of cox1 sequences among Diplostomum species. Tylodelphys conifera (MH368947) (= Tylodelphys sp. A (Go)) versus T. kuerepus had the highest level of similarity among Tylodelphys species/species-level lineages, whereas T. cf. americana versus Tylodelphys mashonensis Beverley-Burton, 1963 were most divergent. Among Austrodiplostomum, A. compactum versus Austrodiplostomum sp. VVT1 were least divergent, while A. mordax versus Austrodiplostomum sp. VVT1 were the most divergent. The two Dolichorchis species-level lineages differed by 12.9–13.6% in cox1 sequences.
As expected, intraspecific variation of the representative species of Diplostomum and Tylodelphys was lower than their respective interspecific divergences. Multiple sequences of D. pseudospathaceum (n = 14) from the Palaearctic showed up to 1.8% difference in cox1 sequences (Supplementary Table S6), while D. gavium (n = 17) from the Nearctic differed only by up to 0.8%. At the same time, these two species differed by 2.3–4.1%. Isolates of T. conifera (n = 3) varied up to 0.5% (Supplementary Table S7), while isolates of Do. lacombeensis (n = 2) had 0.7% intraspecific variation.
4. Discussion
4.1. Generation of new molecular data
Although members of Diplostomum are widely distributed and often included in ecological and evolutionary studies, few sequences of adult specimens were available (e.g., Georgieva et al., 2013; Blasco-Costa et al., 2014; Pérez-del-Olmo et al., 2014; Brabec et al., 2015; Locke et al., 2015; Hoogendoorn et al., 2020). Prior to our study, sequences of 28S from morphologically identified adults were only available for three species of Diplostomum, two species of Tylodelphys and two species of Austrodiplostomum. No DNA sequence data were previously available for a member of Paralaria or Dolichorchis. Here, we provide 28S DNA sequence data from morphologically identified adults of 10 nominal species of Diplostomum, five nominal species of Tylodelphys, one nominal species of Austrodiplostomum and one nominal species of Dolichorchis. In total, we provided new ribosomal and mitochondrial DNA sequence data of 15 species/species-level lineages of Diplostomum, six species/species-level lineages of Tylodelphys, two species/species-level lineages of Austrodiplostomum, two species/species-level lineages of Dolichorchis and an unknown diplostomid lineage. To the best of our knowledge, this is the first study to generate DNA sequence data of a Diplostomum species collected in Chile and the first to report an Austrodiplostomum species in the Palaearctic.
4.2. The status of Paralaria
Kraus (1914) established the genus Paralaria for P. clathrata (type-species) and the newly described P. pseudoclathrata, both from otters. In addition, Kraus (1914) noted the presence of a genital cone which could be inverted. Dubois (1937) described D. alarioides from the giant river otter Pteronura brasiliensis (Gmelin) (syn. Lutra brasiliensis Gmelin) collected in Brazil. Later, Dubois (1944) established the genus Enhydridiplostomum for Diplostomum fosteri McIntosh, 1939 and transferred both D. fosteri and D. alarioides into Enhydridiplostomum. Members of Enhydridiplostomum also parasitize otters but lack a genital cone. Although Dubois (1963) maintained Enhydridiplostomum as a valid genus, he later changed his opinion and considered Enhydridiplostomum a synonym of the subgenus Paralaria (see Dubois, 1968). Other authors (e.g., Yamaguti, 1971; Schoop, 1989) viewed Enhydridiplostomum as a valid genus. In the most recent systematic revision of the Diplostomidae by Niewiadomska (2002), Paralaria is considered a valid genus with Enhydridiplostomum as its synonym.
Interestingly, the generic diagnosis of Paralaria by Niewiadomska (2002) reflected features characteristic of the former Enhydridiplostomum, but not other species of Paralaria. Notably, the lack of a genital cone is typical of P. fosteri and P. alarioides, whereas the type-species P. clathrata as well as P. pseudoclathrata were originally described and clearly illustrated with a genital cone. The results of our phylogenetic analyses clearly demonstrate P. alarioides along with an unidentified species-level lineage, both from otters, as members of Diplostomum. The nested position of both species from otters among species from birds in all our analyses likely reflects a secondary evolutionary host-switching event from birds into mammalian hosts (Figs. 1, 2; Supplementary Fig. S1). Based on our molecular phylogenies along with morphological evidence, we return P. alarioides to Diplostomum as D. alarioides. We expect that P. fosteri may also belong to Diplostomum; however, DNA sequence data are needed for a well-grounded conclusion and nomenclatural action. Considering the current inaccurate generic diagnosis of Paralaria provided by Niewiadomska (2002), we provide an amended diagnosis of the genus.
Paralaria Kraus 1914
Diagnosis (after Niewiadomska, 2002, amended): Body distinctly bipartite; prosoma elongate, spatulate, shorter to equal, rarely longer than claviform opisthosoma. Pseudosuckers present. Ventral sucker smaller than, or similar in size to oral sucker; pharynx large. Holdfast organ oval, elongate, with median slit; its anterior margin extends beyond middle of prosoma. Testes two, oval, trilobed posteriorly; lateral lobes may be subdivided into dorsal and ventral lobes; anterior testis median, asymmetrical, cuneate; posterior larger, may be symmetrical or massive. Ovary oval or reniform, median, in middle or at anterior margin of opisthosoma. Vitelline follicles densely distributed in prosoma, extend from level just posterior to ventral sucker to level of ovary. Copulatory bursa with dorso-subterminal opening. Genital cone present or absent. Hermaphroditic duct opens ventrally in dorsal wall of copulatory bursa. In otters. North and South America. Mesocercariae in anurans. Cercariae with four paracetabular penetration glands; flame-cell formula 2[(1 + 1 + 1) + (1 + 1 + [2])] = 14. Metacercariae of ‘diplostomulum’ type. Type-species: P. clathrata (Diesing, 1850). Other species: P. pseudoclathrata (Krause, 1914); P. fosteri (McIntosh, 1939).
Diplostomum alarioides was previously reported from the North American river otter L. canadensis in Georgia and North Carolina, U.S.A. (Sawyer, 1961; Miller and Harkema, 1968) and Ontario, Canada (Pearson, unpublished) as well as American mink Neovison vison (Schreber) from North Carolina (Miller and Harkema, 1964). Our specimens of D. alarioides from L. canadensis collected in Mississippi closely conform to the original description from specimens collected in Brazil (Dubois, 1937, 1968). Lontra canadensis and Pt. brasiliensis (type-host) do not overlap in their geographical distributions. However, both species share some overlap in geographical distributions with the Neotropical otter Lontra longicaudis (Olfers) (Polechla and Rubio, 2009; Rheingantz et al., 2014; Bouley et al., 2015). We hypothesize that D. alarioides from the Nearctic and Neotropics will prove to be separate species once DNA sequence data from the Neotropical forms are available. However, without a genetic comparison description of our specimens as a separate species is premature. Until now, only D. alarioides has been reported from the Nearctic. Clustering of the species-level lineage Diplostomum sp. VVT3 with D. alarioides, along with their 8.8% difference in cox1 sequences, indicates the presence of a second species of Diplostomum in Nearctic otters. Collection of well-fixed adult specimens are needed for description. It is worth noting that Diplostomum sp. 10 (L) is likely conspecific with Diplostomum sp. VVT3 (Supplementary Fig. S1; Supplementary Table S3). Diplostomum sp. 10 (L) was previously found in the eyes (non-lens) of the rock bass Ambloplites rupestris (Rafinesque) and the fathead minnow Pimephales promelas (Rafinesque) (Locke et al., 2015).
4.3. Remarks on other Diplostomum species
Diplostomum ardeae sensu Locke et al. (2015) from the great blue heron Ardea herodias (Linnaeus) in Canada and Diplostomum sp. VVT5 from the little blue heron Egretta caerulea (Linnaeus) collected in Mississippi have identical 28S partial sequences and only 0.6% different partial cox1 sequences. Our specimens of Diplostomum sp. VVT5 do not fit the original morphological description of D. ardeae (Dubois, 1969b; Supplementary Fig. S2). The differences include the opisthosoma:prosoma length ratio which is 0.65 in our specimens and 0.47–0.51 in D. ardeae, together with the ventral sucker:oral sucker width ratio that is approximately 1.5 in our specimens, whereas suckers are of approximately the same size in D. ardeae. Additionally, our specimens lack a strongly defined separation between prosoma and opisthosoma as opposed to the well-defined separation between prosoma and opisthosoma in D. ardeae (see Dubois, 1969b; Supplementary Fig. S2). Diplostomum sp. VVT5 sequenced here is morphologically closest to D. scudderi (syn. Diplostomum baeri eucaliae Hoffman & Hundley, 1957); however, the two species have several morphological differences (see Hoffman and Hundley, 1957 and Supplementary Fig. S2) and differ by 2.9% between partial 28S sequences and by 11% between partial cox1 sequences (Supplementary Tables S1, S3, S5). Therefore, we believe that Diplostomum sp. VVT5 and D. ardeae sensu Locke et al. (2015) represent a currently undescribed species. Detailed descriptions of these materials will be published elsewhere.
Diplostomum sp. VVT5 formed a sister branch to all other Diplostomum species (Figs. 1, 2; Supplementary Fig. S1), as previously demonstrated in other recent molecular phylogenetic studies (e.g., Locke et al., 2015; Hernández-Mena et al., 2017; Pelegrini et al., 2019; Locke et al., 2020). Some previous studies (e.g., Pelegrini et al., 2019) have suggested that this form may belong to a separate genus; however, Locke et al. (2020) considered it to be a species of Diplostomum based on its morphology. Our morphological study of adult Diplostomum sp. VVT5 does not provide any evidence supporting its placement in a separate genus. However, it is worth noting that Diplostomum sp. VVT5 and D. lunaschiae have a weakly bipartite body, similar to Tylodelphys spp., whereas many other Diplostomum spp. have a distinctly bipartite body (e.g., Shigin, 1993; Dubois, 1968, 1969a, b; Niewiadomska, 2002; Locke et al., 2020; present study).
The morphology of our specimens of D. huronense from the kelp gull Larus dominicanus Lichtenstein collected in Chile and from the ring-billed gull Larus delawarensis Ord collected in Illinois, U.S.A. closely conforms to the original description by LaRue (1927) of specimens from the European herring gull Larus argentatus Pontoppidan collected at Lake Huron (Supplementary Table S8; Supplementary Fig. S2). Similarly, the morphology of our specimens of D. indistinctum from La. delawarensis collected in North Dakota, U.S.A. closely conforms to the original description by Guberlet (1922) of specimens from La. delawarensis collected in Oklahoma, U.S.A. (Supplementary Table S8; Supplementary Fig. S2). Our cox1 sequences of D. huronense differ from the previously published sequences of D. huronense sensu Galazzo et al. (2002) by 9.9–11% (Supplementary Tables S3 and S5). Locke et al. (2010a) published cox1 sequences of adult specimens of Diplostomum sp. 1 (M); Diplostomum sp. 1 (M) is conspecific with our isolates of D. huronense based on the similarity of partial cox1 sequences (0.3–1.9%; Supplementary Tables S3 and S5).
Similarly, our cox1 sequences from adult specimens of D. indistinctum differ by 12.2–12.7% from previously published sequences of D. indistinctum sensu Galazzo et al. (2002) (Supplementary Tables S3 and S5). Diplostomum sp. 4 (M), another form sequenced from adult specimens (e.g., HM064700), turned out to be conspecific with our isolates of D. indistinctum based on the high similarity of cox1 sequences (0.6–1.4% divergence; Supplementary Tables S3 and S5).
Galazzo et al. (2002) sequenced the ITS region of D. huronense and D. indistinctum and studied morphology of the adult forms from experimentally infected La. delawarensis. Subsequently Locke et al. (2010a, b) generated cox1 data from D. indistinctum studied by Galazzo et al. (2002) and additional specimens of D. huronense and D. indistinctum identified, in part, based on comparison of ITS region sequences, which matched sequences from Galazzo et al. (2002). Galazzo et al. (2002) stated that their specimens were nearly morphologically identical to the original descriptions. Most measurements provided by Galazzo et al. (2002) seem to be consistent with D. huronense as described by La Rue (1927). Unfortunately, neither La Rue (1927) nor Galazzo provided ratios of many characters often used for species differentiations (e.g., oral sucker:ventral sucker width ratio). Based on the line drawings, D. huronense described by La Rue has an oral sucker:ventral sucker width ratio of 0.64; in contrast, D. huronense illustrated by Galazzo et al. (2002) has oral sucker:ventral sucker width ratio of 0.9 (Supplementary Table S8). Our specimens of D. huronense have an oral sucker:ventral sucker width ratio of 0.68–0.80. La Rue (1927) described the vitellarium of D. huronense as extending anteriorly to at least the level of the ventral sucker. The vitellarium in the specimen of D. huronense illustrated by Galazzo et al. (2002) does not extend beyond the level of the holdfast organ. In contrast, the vitellarium in some of our specimens of D. huronense extends anteriorly to the level of the ventral sucker (Supplementary Fig. S2). In our opinion, the sucker ratios, and the anterior extent of vitellarium provide evidence that our specimens fit the original description of D. huronense better than those reported by Galazzo et al. (2002).
Guberlet (1922) illustrated D. indistinctum with a noticeable narrowing of the anterior part of the opisthosoma immediately posterior to the prosoma (approximately half the width of the widest part of the opisthosoma). The specimen of D. indistinctum illustrated by Galazzo et al. (2002) lacked such a narrowing, whereas all our specimens of D. indistinctum have a narrowing of the anterior part of the opisthosoma (Supplementary Table S8; Supplementary Fig. S2). In addition, the oral sucker length:pharynx length ratio of D. indistinctum based on the illustrations provided by Guberlet (1922) is 0.78–1.06, whereas the oral sucker length:pharynx length ratio of D. indistinctum based on the illustration by Galazzo et al. (2002) is 1.66. The oral sucker length:pharynx length ratio of our specimens of D. indistinctum is 1.00–1.13, which is much closer to that in the original description than in the material described by Galazzo et al. (2002) (Supplementary Table S8). In our opinion, the presence of a narrowing of the opisthosoma and more similar character ratios compared with the original description support the identification of our specimens as D. indistinctum.
Sequences from specimens of Diplostomum sp. VVT2 from the yellow perch Perca flavescens Mitchill, green sunfish Lepomis cyanellus Rafinesque and pumpkinseed Lepomis gibbosus (Linnaeus) from Minnesota formed a 100% supported clade with a sequence of D. baeri sensu Galazzo et al. (2002) (MF142196) from an isolate collected from Pe. flavescens in Michigan (Ubels et al., 2018) (Table 2). The clade that included Diplostomum sp. VVT2 + D. baeri sensu Galazzo et al. (2002) from the Nearctic was separate from other clades of the D. baeri species complex containing sequences from Palaearctic only (Supplementary Fig. S1). Diplostomum baeri was originally described from the long-tailed jaeger Stercorarius longicaudus Vieillot collected at Lake Geneva (France and Switzerland) (Dubois, 1937). We find it unlikely that D. baeri sensu Galazzo et al. (2002) (and other conspecific lineages identified as D. baeri from the Nearctic; Table 2) as well as the Diplostomum sp. VVT2 belong to D. baeri. We hypothesize that D. baeri sensu Galazzo et al. (2002) from Nearctic likely represents a new species. However, sequences of adult specimens of D. baeri from the type-host and preferably close to type-locality are needed to define which lineage actually represents D. baeri. It is worth noting that specimens of Diplostomum sp. VVT2 were found encysted on the skin as well as in the eyes (Table 1). The larvae collected from the skin were encapsulated in melanized cysts.
Table 2.
Diplostomum species/species-level lineages sequenced in the present study and the corresponding previously accepted species/species-level lineage names based on BLAST search results of cox1 sequences in GenBank. References to the original designations of species-level lineages are provided.
| Taxon | Corresponding previously accepted species/species-level lineage | Reference |
|---|---|---|
|
| ||
| Diplostomum alarioides a | – | Present study |
| Diplostomum alascense | Diplostomum sp. 2 | Moszczynska et al. (2009) |
| Diplostomum gavium | Diplostomum sp. 3 | Moszczynska et al. (2009) |
| Diplostomum baeri | Ubels et al. (2018) | |
| Diplostomum marshali | Diplostomum sp. A | Gordy and Hanington (2019) |
| Diplostomum huronense | Diplostomum sp. 1 | Moszczynska et al. (2009) |
| Diplostomum indistinctum | Diplostomum sp. 4 | Moszczynska et al. (2009) |
| D. baeri | Ubels et al. (2018) | |
| Diplostomum pseudospathaceum | D. pseudospathaceum | Behrmann-Godel (2013); Georgieva et al. (2013) |
| Diplostomum rauschi | Diplostomum sp. Lineage 2 | Blasco-Costa et al. (2014) |
| Diplostomum scudderi | Diplostomum sp. 13 | Locke et al. (2015) |
| Diplostomum sp. C | Gordy and Hanington (2019) | |
| Diplostomum spathaceum | D. spathaceum | Georgieva et al. (2013) |
| D. spathaceum LIN1 | Blasco-Costa et al. (2014) | |
| Diplostomum paracaudum | Behrmann-Godel (2013) | |
| Diplostomum sp. VVT1 | – | Present study |
| Diplostomum sp. VVT2 | D. baeri sensu Galazzo et al. (2002) | Galazzo et al. (2002) |
| D. aff. baeri LIN2 | Gordy et al. (2016) | |
| D. baeri complex LIN2 | Gordy and Hanington (2019) | |
| Diplostomum sp. VVT3 | Diplostomum sp. 10 | Locke et al. (2015) |
| Diplostomum sp. VVT4 | – | Present study |
| Diplostomum sp. VVT5 | Diplostomum ardeae sensu Locke et al. (2015) | Locke et al. (2015) |
Formerly included in Paralaria.
Diplostomum mergi alascense Dubois, 1969 was originally described from red-breasted merganser Mergus serrator Linnaeus collected in Alaska (Dubois, 1969a). This taxon can be most easily distinguished from Diplostomum mergi mergi Dubois, 1932, described from M. serrator collected in Europe, based on the oral: ventral sucker ratio (suckers about the same size in D. m. alascense while in D. m. mergi the ventral sucker is larger than the oral sucker) and the anterior extent of vitellarium (vitellarium extending to about the level of the ventral sucker in D. m. alascense versus vitellarium extending anterior to the level of the ventral sucker in D. m. mergi) (Dubois, 1932, 1969a). Our specimens of D. m. alascense clearly morphologically conform to the original description and differ by at least 9.1% in sequences of cox1 from larval specimens of the D. mergi complex collected and sequenced in the Palaearctic (Supplementary Table S9). Furthermore, in our phylogenetic analysis based on cox1 gene, Nearctic D. m. alascense was positioned separately from the D. mergi complex from the Palaearctic (Supplementary Fig. S1). Considering the morphological and genetic differences, we elevate D. m. alascense to the level of species as D. alascense n. comb.
In total, we have provided species-level identifications for seven species of Diplostomum spp. based on adult morphology which were previously published as genetic lineages only (Table 2; Supplementary Table S10).
4.4. Non-monophyly of Tylodelphys
Our phylogenetic analyses positioned members of Austrodiplostomum nested within Tylodelphys (Figs. 1, 3, 4), which indicates the paraphyletic nature of Tylodelphys, similar to what has been shown previously (e.g., Locke et al., 2015; Sereno-Uribe et al., 2019b). For instance, the phylogenetic analyses conducted by Sereno-Uribe et al. (2019b), which included only a few Tylodelphys spp., demonstrated a non-monophyly of Tylodelphys due to the position of Austrodiplostomum. Austrodiplostomum spp. and Tylodelphys spp. have some morphological differences. Austrodiplostomum spp. are characterized by a heavily reduced ventral sucker or no ventral sucker at all, and the lack a genital cone. In contrast, Tylodelphys spp. typically have a small, but well-developed ventral sucker and a small genital cone (e.g., Dubois, 1938; Szidat and Nani, 1951; Niewiadomska, 2002; Dronen, 2009; Sereno-Uribe et al., 2019a, b). It is worth noting, however, that cercariae of Austrodiplostomum spp. are known to possess ventral suckers (e.g., Rosser et al., 2016a; López-Hernández et al., 2019).
Our analysis (Fig. 3) separated Tylodelphys spp. into two distinct clades. The first clade (85% support) included majority of Tylodelphys (e.g., T. conifera and T. immer), while the second clade (89% support) only contained T. cf. americana and Tylodelphys sp. 4 (M). Tylodelphys cf. americana (which has a well-developed ventral sucker and a small genital cone) is characterized by typical Tylodelphys morphology and we failed to find morphological features which would warrant its placement into a genus separate from Tylodelphys. On the other hand, adult Austrodiplostomum spp. have clear morphological differences from adult digeneans from both Tylodelphys clades. Based on the results of our phylogenetic analysis, T. cf. americana and Tylodelphys sp. 4, as well as other members of Tylodelphys clade T-I in the analysis of cox1 (Fig. 4), appear to belong to a separate, genus-level lineage. However, as mentioned above, currently available data are insufficient for a systematic action. Additional morphological and life cycle data on these taxa are necessary to erect a new genus in the future. Therefore, we provisionally maintain T. cf. americana and Tylodelphys sp. 4 (M) within Tylodelphys.
The genus Austrodiplostomum was originally established for A. mordax from the Neotropical cormorant Phalacrocorax brasilianus (Gmelin). The genus includes only two species, A. mordax and A. compactum (syn. Austrodiplostomum ostrowskiae Dronen, 2009), parasitic in cormorants of the genus Phalacrocorax Brisson (syn. Nannopterum (Gmelin)) in the Neotropics (Szidat and Nani, 1951; Sereno-Uribe et al., 2019b). However, larval stages of Austrodiplostomum spp. have been identified as far north as the southern United States (Rosser et al., 2016a).
To the best of our knowledge, no member of Austrodiplostomum has been previously reported from pelicans. However, two morphologically similar genera Bursacetabulus Dronen, Tehrany & Wardle, 1999 and Bursatintinnabulus Tehrany, Dronen & Wardle, 1999 were described based on specimens from the brown pelican Pelecanus occidentalis Linnaeus and the northern gannet Morus bassanus Linnaeus, respectively, in the Nearctic (Dronen et al., 1999; Tehrany et al., 1999). Similar to the former species of Austrodiplostomum, members of Bursacetabulus and Bursatintinnabulus lack a ventral sucker. However, members of Bursacetabulus and Bursatintinnabulus possess a sucker-like copulatory bursa. Our specimens of Austrodiplostomum sp. VVT1 from the great white pelican Pe. onocrotalus clearly lack a ventral sucker. However, the relatively poor condition of our specimens does not allow us to unequivocally establish whether the copulatory bursa of Austrodiplostomum sp. VVT1 is sucker-like. It would not be surprising if Bursacetabulus and Bursatintinnabulus are found to be synonyms of Austrodiplostomum. However, this hypothesis needs to be tested with DNA sequence data from well-fixed adult specimens of the type-species of both genera (i.e., Bursacetabulus pelecanus Dronen, Tehrany & Wardle, 1999 and Bursatintinnabulus macrobursus (Dronen, Tehrany & Wardle, 1999)).
4.5. Remarks on Tylodelphys
Tylodelphys podicipina robrauschi Dubois, 1969 was originally described as a subspecies of Tylodelphys podicipina Kozicka & Niewiadomska, 1960 based on specimens collected from the red-necked grebe Podiceps grisegena (Boddaert) in Alaska (Dubois, 1969a). The morphology of T. p. robrauschi most notably differs from T. p. podicipina in the extent of the vitellarium; the vitellarium extends to approximately the level of the ventral sucker in T. p. robrauschi (Dubois, 1969a), while in T. p. podicipina it extends anteriorly to approximately halfway between the oral and ventral suckers (Kozicka and Niewiadomska, 1960). Heneberg and Sitko (2021) proposed T. immer to be a junior synonym of T. p. podicipina based on an inaccurate comparison of ribosomal data; while the authors claimed the ITS2 sequences of T. immer and T. p. podicipina were identicial, the GenBank sequences they refer to, are not identical. Further, Heneberg and Sitko (2021) failed to compare cox1 sequences of T. immer and T. p. podicipina. Our comparison of cox1 sequences from T. p. podicipina, T. p. robrauschi and T. immer revealed at least of 8.8% difference between these species (Supplementary Table S11). Therefore, we reject the synonymizaton of T. immer with T. p. podicipina. Based on morphological differences (e.g., distribution of the vitellarium) and the level of genetic divergence (Supplemental Table S11), we elevate T. p. robrauschi to full species rank as Tylodelphys robrauschi Dubois, 1969 n. comb.
The 28S DNA sequences are also available from T. darbyi from New Zealand (Blasco-Costa et al., 2017); however, inclusion of these sequences would require trimming of our alignment to a much shorter length (777 bp) than used in our 28S analysis (1,116 bp).
To summarize, due to the availability of adult stages, we were able to provide species-level identifications for three genetic lineages of Tylodelphys that were previously sequenced only from unidentified larvae (Table 3; Supplementary Table S10).
Table 3.
Tylodelphys and Austrodiplostomum species/species-level lineages sequenced in the present study and the corresponding previously accepted species/species-level lineage names based off BLAST search results of cox1 sequences in GenBank. References to the original designations of species-level lineages are provided.
| Taxon | Corresponding previously accepted species/species-level lineage | Reference |
|---|---|---|
|
| ||
| Austrodiplostomum compactum | A. compactum | Sereno-Uribe et al. (2019b) |
| Austrodiplostomum ostrowskiae | O’Hear et al. (2014) | |
| Austrodiplostomum sp. | Farias et al. (unpublished) | |
| Austrodiplostomum sp. VVT1 | – | Present study |
| Tylodelphys cf. americana | Tylodelphys sp. | Pelegrini et al. (2019) |
| Tylodelphys conifera | Tylodelphys sp. A | Gordy and Hanington (2016) |
| Tylodelphys immer | T. immer | Locke et al. (2018) |
| Tylodelphys robrauschi n. comb. | Tylodelphys sp. 3 | Locke et al. (2015) |
| Tylodelphys scheuringi | T. scheuringi | Moszczynska et al. (2009) |
| Tylodelphys sp. VVT1 | – | Present study |
4.6. Remarks on Dolichorchis and the Diplostominae
As previously demonstrated by other authors (e.g., Blasco-Costa and Locke, 2017; Locke et al., 2018; Achatz et al., 2021), the Diplostominae was non-monophyletic in our broader analysis of 28S (Fig. 1). Despite the general morphological similarity of Tylodelphys and Dolichorchis, these two genera were not positioned together in the phylogeny (Fig. 1). It should be noted that the two genera differ in the structure of the anterior testis (asymmetrical in Dolichorchis spp. versus symmetrical in Tylodelphys spp.) and often in the distinction between prosoma and opisthosoma (body distinctly bipartite in Dolichorchis spp. versus body typically indistinctly bipartite in Tylodelphys spp.).
Members of the Diplostominae were positioned in three distinct clades in our analysis: Diplostomum + Tylodelphys; Dolichorchis + Neodiplostomum + Sphincterodiplostomum; and Hysteromorpha Lutz, 1931. Our review of morphology did not demonstrate any obvious morphological features of adult stages which would unite Dolichorchis, Neodiplostomum and Sphincterodiplostomum separately from Alaria, Diplostomum and Tylodelphys.
Only two species of Dolichorchis are known from the New World (Do. lacombeensis and Dolichorchis bonariensis Ostrowski de Núñez, 1970). Our specimens of Do. lacombeensis from Ardea cocoi (Linnaeus) closely conform to the original description of specimens from Ar. cocoi collected in Argentina by Lunaschi and Drago (2006). Our specimen of Dolichorchis sp. VVT1 from the bare-faced ibis Phimosus infuscatus (Lichtenstein) collected in Brazil was too immature for accurate species identification. However, we suspect that Dolichorchis sp. VVT1 represents a novel species-level lineage. Dolichorchis lacombeensis and Dolichorchis sp. VVT1 are clearly separate lineages based on genetic divergence comparisons; the two species differ by 1% in sequences of 28S and 12.9–13.6% in sequences of cox1. Dolichorchis bonariensis has only been reported from cormorants (order Suliformes Sharpe), whereas Dolichorchis sp. VVT1 was collected from an ibis (order Pelecaniformes Sharpe). On the other hand, our immature specimens may be the result of accidental infection. This is the first report of a species of Dolichorchis outside of Argentina in the New World (Fernandes et al., 2015).
4.7. Pairwise comparisons
Many of the DNA sequences of many Diplostomum spp., Tylodelphys spp. and Austrodiplostomum spp. currently available in GenBank originate from larval stages, which, for the most part, cannot be reliably identified to the species based on morphology. Comparisons of numerous previously published sequences have also suggested poor quality of some sequence data in the GenBank database (i.e., numerous variable sites and indels in the protein-coding gene cox1). Adequate comparisons should use only high-quality sequence data, preferably from morphologically identified adults.
The interspecific divergence levels among 28S sequences within Diplostomum, Tylodelphys, Austrodiplostomum and Dolichorchis included in this study (0–3.7%) was similar to that demonstrated within other genera of diplostomoideans (0–4.4%) (e.g., Achatz et al., 2020 and references therein; Tkach et al., 2020).
Interspecific divergence levels in partial cox1 sequences of adult Diplostomum spp. (2.3–16.3%), Tylodelphys spp. (4.4–14.6%), Austrodiplostomum spp. (9.1–13%) and Dolichorchis (12.9–13.6%) and corresponding larvae (Supplementary Table S5) found in our study were similar to those demonstrated within other diplostomoidean genera (3.4–19.8%) (e.g., Achatz et al., 2020 and references therein; Tkach et al., 2020). The interspecific differences among cox1 sequences from adult specimens of Diplostomum spp. and corresponding larvae included in our study (2.3–16.3%) were similar to or lower than those reported by Locke et al. (2010a) (9.9–15.1%) and Hoogendoorn et al. (2020) (11.8–14.7%).
However, the interspecific differences in cox1 from adult specimens in the present study was slightly higher than those provided by Georgieva et al. (2013) (4.6–14.9%) and Selbach et al. (2015) (4.3–14.7%). It is worth noting, that these studies (Locke et al., 2010a; Georgieva et al., 2013; Selbach et al., 2015; Hoogendoorn et al., 2020) were primarily based on larval specimens. The interspecific differences among cox1 sequences from adult specimens of Tylodelphys and Austrodiplostomum along with the corresponding larvae (4.4–14.6%) obtained in the present study were similar to those previously reported by Blasco-Costa et al. (2017) (8.0–16.5%) and Sereno-Uribe et al. (2019a) (5.0–15%).
Interestingly, Locke et al. (2015) reported 2.10–11.22% interspecific difference among cox1 sequences (Diplostomum and Tylodelphys spp.) between the nearest neighbors in their analysis. Our comparison of partial cox1 sequences of morphologically identified adults and corresponding larvae did not show interspecific divergence lower than 2.3% (Supplementary Tables S5, S6). However, it is important to note that almost all comparisons of cox1 sequences of species with morphologically identified adults differed by at least 4.4%. The only exception was D. gavium and D. pseudospathaceum which differ by 2.3–4.1% (Supplementary Table S6) while being very distinct morphologically. It is worth noting that comparisons among members of other diplostomoidean genera identified based on adult morphology typically yield interspecific divergence values much greater than 2.1% (e.g., Hernández-Mena et al., 2014, Achatz et al., 2019 a).
4.8. Host associations
Our phylogenetic analyses (Figs. 1, 2; Supplementary Fig. S1) provided evidence of multiple host-switching events among Diplostomum spp. The majority of adult Diplostomum isolates included in our analyses were collected from the Laridae Rafinesque (gulls). However, our analysis also included Diplostomum spp. collected from birds belonging to the Ardeidae Leach (herons), Recurvirostridae Bonaparte (avocets), Gaviidae Forster (loons), Scolopacidae Rafinesque (sandpipers), and Anatidae Leach (ducks), as well as from the Mustelidae Waldheim (otters). Notably, in the 28S trees Diplostomum sp. VVT5 (= D. ardeae sensu Locke et al., 2015) from E. caerulea formed a sister group to the weakly supported clade containing all other Diplostomum species (Figs. 1, 2). In the cox1 tree, Diplostomum sp. VVT5 formed a clade with D. lunaschiae, a parasite of the rufescent tiger heron Tigrisoma lineatum (Boddaert). Unfortunately, a 28S sequence of D. lunaschiae is not available. The phylogenetic position of Diplostomum sp. VVT5 in all analyses along with the position of D. lunaschiae in the cox1 analysis suggests that the ancestral host of Diplostomum may have been an ardeid.
The Diplostomum spp. from otters and mergansers formed three of the branches within the Diplostomum clade, representing separate secondary host-switching events (Fig. 2). However, we did not collect adults of two Diplostomum spp. (VVT1, VVT4) clustered in the clade with D. scudderi, a parasite of ducks, and D. marshalli, a parasite of sandpipers. We posit that these species also parasitize anatids and scolopacids. The diversity of Diplostomum spp. in gulls and the presence of more than one clade of species from gulls in our cox1 analysis (Supplementary Fig. S1) suggests a long history of radiation within gull hosts. At the same time, in the 28S analysis all Diplostomum isolates from gulls formed a single, strongly supported clade (Fig. 2), which suggests that the transition to gulls may have occurred only once. However, this notion might change in the future because several species and species-level lineages included in the cox1 analysis lack corresponding 28S data. In addition, nine Diplostomum species-level lineages included in the second 28S analysis (Fig. 2) have DNA sequence data available only from larval stages and their definitive hosts remain unknown. It can be anticipated that more comprehensive sequence data will reveal additional host-switching events in the evolutionary history of this large, cosmopolitan genus.
Our analyses also revealed multiple host-switching events within Tylodelphys and Austrodiplostomum (Fig. 3). Members of the genus included in our analyses were collected from the Podicipedidae Bonaparte (grebes), Gaviidae Coues (loons), Ciconiidae Gray (storks), Pelecanidae Rafinesque (pelicans) and Phalacrocoracidae Reichenbach (cormorants). In the analysis of 28S (Fig. 3), adult Tylodelphys spp. from grebes and loons formed a clade separate from Tylodelphys and Austrodiplostomum parasitic in storks and cormorants + pelicans. Within this clade, it appears that Tylodelphys species likely transitioned from grebes into loons. Tylodelphys cf. americana from the jabiru Jabiru mycteria (Lichtenstein) formed a sister group to Austrodiplostomum spp. from cormorants and pelicans. Interestingly, Austrodiplostomum sp. VVT1 from pelicans was nested among multiple Tylodelphys spp. from cormorants in both 28S and cox1 analyses (Figs. 3, 4) suggesting a transition from cormorants to pelicans.
4.9. Biogeography
Previous studies (e.g., Locke et al., 2015, 2020; Hoogendoorn et al., 2020) have demonstrated that some Diplostomum spp. are distributed across multiple biogeographic realms (i.e., Palaearctic and Afrotropics; Nearctic and Neotropics) and continents (i.e., Europe and Asia; Africa and Asia). Gibson (1996) proposed that many Diplostomum spp. may have a Holarctic distribution based on the mobility and distribution of their avian hosts; however, this has not been previously tested based on molecular data. To date, only Locke et al. (2020) has demonstrated using molecular data that a species of Diplostomum (i.e., D. ardeae sensu Locke et al. (2015)) is distributed in the Nearctic + Neotropics. In the latter study, the Nearctic samples were collected in Quebec, Canada, and those from the Neotropics were collected in Puerto Rico, near the northern edge of the Neotropics. Our Nearctic samples of D. huronense originated from the northern United States and the Neotropic specimens were collected in Chile, substantially farther south than Puerto Rico. This provides a convincing evidence that some Diplostomum spp. are broadly distributed throughout the New World.
The broad distribution of Diplostomum may be promoted, in part, by the extensive overlapping of bird migration flyways. For instance, the overlap in Atlantic Americas and East Atlantic flyways can facilitate dispersal of species between the New World and Europe (Olsen et al., 2006; Dusek et al., 2014; Ramey et al., 2015, 2016). Blasco-Costa et al. (2014) suggested that the common ancestor of Diplostomum spp. may have originated in North America and subsequently dispersed into the Palaearctic. The position of Diplostomum sp. VVT5 in our 28S and cox1 analyses (Figs. 1, 2; Supplementary Fig. S1), together with D. lunaschiae in the cox1, provide some support for this hypothesis (Figs. 1, 2). This is further supported by the presence of three other clades of Diplostomum spp. from the Nearctic in the broader clade of Diplostomum (Fig. 2). Most Diplostomum spp. from the Palaearctic formed a single, strongly supported clade in our analysis of 28S (Fig. 2). This clade also contained Diplostomum spp. from the Nearctic, Neotropics and Afrotropics. Only D. phoxini, from the Palaearctic, appeared on the tree separately from other Palaearctic forms, in a clade with Diplostomum sp. VVT2 from the Nearctic. Patterns related to biogeography of Diplostomum spp. were less pronounced in the cox1 analysis (Supplementary Fig. S1).
The majority of Tylodelphys and Austrodiplostomum spp. included in our 28S analyses originated from the New World. However, the single species from the Palaearctic (Austrodiplostomum sp. VVT1) was deeply nested within a clade of species from the Nearctic + Neotropics (Fig. 3). This provides some evidence that the ancestor of this group also likely originated in the New World. However, our understanding of the biogeographical patterns within these genera may potentially change once ribosomal data (i.e., 28S) from a greater diversity of species from other biogeographical realms become available. Similar to Diplostomum, the cox1 results did not reveal any well-defined biogeographical patterns for Tylodelphys spp. (Fig. 4).
4.10. Conclusions
We provided new ribosomal and mitochondrial DNA sequence data of 15 species/species-level lineages of Diplostomum, six species/species-level lineages of Tylodelphys, two species/species-level lineages of Austrodiplostomum, two species/species-level lineages of Dolichorchis and one species-level lineage of an unidentified diplostomid. Our study has thus significantly expanded the available sequence data from morphologically identified adult stages of Diplostomum and Tylodelphys.
Our phylogenetic analyses demonstrated some incongruences between the results based on 28S and cox1 sequence data (Figs. 2–4; Supplementary Fig. S1). For instance, Diplostomum spp. from gulls formed a monophyletic clade in the 28S tree (Fig. 2) but appear in more than one clade in the cox1 tree (Supplementary Fig. S1). Similarly, D. marshalli and D. scudderi appeared within the same 100% supported clade in the 28S tree while being separated in clades D-II and D-XIII within the major polytomy in the cox1 tree. Overall, the cox1 trees had high support for distal branches and low support for basal branches, while 28S trees were somewhat better resolved despite still containing polytomies close to the base of the trees. A similar discordance and notably lower branch support in cox1-based phylogenies have been previously reported in studies of Diplostomum spp. (e.g., Brabec et al., 2015, Hoogendoorn et al., 2020) and other diplostomoideans (e.g., Hernández-Mena et al., 2017; Achatz et al., 2019a, 2020; Hoogendoorn et al., 2019, 2020; Heneberg et al., 2020). Although cox1 sequences remain an excellent source of data for species differentiation, caution must be taken when cox1 sequences are used for phylogenetic inference at taxonomic levels above genus. Unfortunately, the avilability of 28S sequence data for diplostomoideans is lagging far behind the cox1 data, which are being generated at a much higher rate.
Our data demonstrated that P. alarioides along with an unidentified digenean, both from otters, belong to Diplostomum (Fig. 2; Supplementary Fig. S1). Importantly, molecular phylogenetic analyses have demonstrated the non-monophyly of Tylodelphys and suggested the need to establish a novel genus which contains T. cf. americana, despite the lack of clear morphological differences in adult stages (Figs. 3, 4).
Our broader 28S analysis of diplostomoideans (Fig. 1) positioned Dolichorchis spp. and the unknown diplostomid separate from Diplostomum spp. and Tylodelphys spp.; the results of the 28S analysis supported Dolichorchis as a distinct genus. Sequences of 10 species of Diplostomum and Tylodelphys identified based on adult morphology matched previously published sequences of species/species-level lineages identified based on larval morphology only or not identified to species (Tables 2, 3; Supplementary Table S10). In addition, we provided the first DNA sequence data for three species/species-level lineages of Diplostomum, one species-level lineage of Tylodelphys, one species-level lineage of Austrodiplostomum, two species/species-level lineages of Dolichorchis and one currently unknown diplostomid.
The results of our phylogenetic analyses revealed multiple host-switching events, notably from avian definitive hosts to otters along with switching between major avian groups. In addition, our results provide evidence for multiple dispersal events between biogeographical realms in the evolutionary history of the Diplostomum, Tylodelphys and Austrodiplostomum (Figs. 2, 3), together with the molecular evidence that some species are distributed throughout the Nearctic and Neotropics.
Future studies should provide additional DNA sequence data from well-fixed adult specimens to study the interrelationships within the Diplostomidae comprehensively. This approach will help clarify the taxonomy of a large number (at least 30) of yet unidentified species-level lineages of Diplostomum, Tylodelphys and Austrodiplostomum with predominantly Nearctic distribution (Moszczynska et al., 2009; Locke et al., 2010a, b, 2015; Gordy and Hanington, 2019). Furthermore, of particular interest is species identification within the two species complexes: the D. baeri species complex as defined by Blasco-Costa et al. (2014) for which the phylogenetic reconstructions have suggested North America as an ancestral area (see Blasco-Costa et al., 2014) and the D. mergi species complex as defined by Georgieva et al. (2013) and Selbach et al. (2015) which may appear to be restricted to the Palaearctic. Finally, many lineages of Tylodelphys with sequence data reported from larval stages, predominantly metacercariae, still await further taxonomic scrutiny.
Although larval Diplostomum spp. and Tylodelphys spp. are very commonly reported in ecological studies of fish and mollusk intermediate hosts, limited data from adult Diplostomum spp. and Tylodelphys spp. were available. Our newly generated DNA sequences from morphologically identified adults significantly expand the reference set of diplostomids at the species level. This knowledge is critical for future ecological studies of larval diplostomids, many of which are agents of fish diseases.
Supplementary Material
Supplementary Fig. S1. Phylogenetic interrelationships among 80 sequences from members of Diplostomum (including a former Paralaria sp.) based on Bayesian inference analysis of partial cox1 mtDNA gene sequences. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale bar indicates the number of substitutions per site. References to origins of species numbering/naming systems are provided in parentheses after GenBank accession numbers, followed by biogeographical realms where specimens were collected, life stages of isolates and families of definitive hosts (for adult isolates and larvae molecularly matched to adult forms). Black bars are positioned beside taxa for which we have provided nominal species identifications based on adult morphology. The previously accepted/published names are provided in parentheses after GenBank accession numbers.
Abbreviations for references to the original designations of species-level lineages: B, Blasco-Costa et al. (2014); C, Chibwana et al. (2013); Ge, Georgieva et al. (2013); Go, Gordy and Hanington (2019); H, Hoogendoorn et al. (2020); Ko, Komatsu et al. (2019); Ku, Kudlai et al. (2017); L, Locke et al. (2010a, b; 2015); M, Moszczynska et al. (2009); P, Pelegrini et al. (2019); Sl, Selbach et al. (2015). Abbreviations for biogeographical realms: Afr, Afrotropical realm; ANea, Nearctic realm; Neo, Neotropical realm; Pal, Palaearctic realm. Abbreviations for life stages: Adu, adult; Cer, cercaria; Met, metacercaria. Abbreviations for families of definitive hosts: Ana, Anatidae; Ard, Ardeidae; Gav, Gaviidae; Lar, Laridae; Mus, Mustelidae; Rec, Recurvirostridae; Sco, Scolopacidae.
Supplementary Fig. S2. Photographs of our specimens of Diplostomum sp. VVT5, Diplostomum huronense and Diplostomum indistinctum. (A) Diplostomum sp. VVT5, entire, ventral view. Scale bar = 250 μm. (B) Diplostomum huronense, entire, ventral view. Scale bar = 500 μm. (C) Posterior portion of the prosoma of D. huronense showing anterior extent of vitellarium, lateral view. Scale bar = 150 μm. (D) Ventral sucker of D. huronense showing anterior extent of vitellarium, ventral view. Scale bar = 100 μm. (E) Diplostomum indistinctum, entire, ventral view. Scale bar = 500 μm. Vit, vitellarium; VS, ventral sucker.
Highlights.
Novel sequence data for Diplostomum, Tylodelphys and related genera are provided
Molecular phylogenies of the group based on 28S and cox1 sequences are presented
Sequences of 10 previously unidentified larvae were matched with those from adults
Paralaria alarioides was transferred to Diplostomum
Phylogenetic analysis reveals several host switching events in the history of the group
Acknowledgments
We are grateful to our late colleague Daniel González-Acuña (Facultad de Ciencias Veterinarias, Universidad de Concepción, Chillán, Chile) for organizing logistics, obtaining permits and providing laboratory space, and Nicolás Martin (the same institution) for assistance in collecting birds in Chile. We also thank Erik Fritzell (University of North Dakota, Grand Forks, U.S.A.) for donating duck carcasses from hunting. This work was supported by the National Science Foundation, U.S.A. (VVT, grant DEB-1120734), the National Institutes of Health, U.S.A. (VVT, grant R15AI092622), University of North Dakota (TJA, Joe K. Neel Memorial Award, Esther Wadsworth Hall Wheeler Award, Student Research Stipend and Summer Doctoral Fellowship; JRM, Student Research Stipend) and the American Society of Parasitologists (TJA, Willis A. Reid, Jr. Student Research Grant). JRM was supported by the National Science Foundation (REU Site award number 1852459) and the National Institute of General Medical Sciences of the National Institutes of Health (Institutional Development Award (IDeA) grant number P20GM103442) the University of North Dakota School of Medicine & Health Sciences.
Footnotes
Note: Nucleotide sequence data reported in this paper are available in the GenBank™ under accession numbers MZ314149–MZ314189 (28S) and MZ323246–MZ323308 (cox1)
Note: Supplementary data associated with this article
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achatz TJ, Curran SS, Patitucci KF, Fecchio A, Tkach VV, 2019a. Phylogenetic affinities of Uvulifer spp. (Digenea: Diplostomidae) in the Americas with description of two new species from Peruvian Amazon. J. Parasitol. 105, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz TJ, Dmytrieva I, Kuzmin Y, Tkach VV, 2019b. Phylogenetic position of Codonocephalus Diesing, 1850 (Digenea, Diplostomoidea), an unusual diplostomid with progenetic metacercariae. J. Parasitol. 105, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz TJ, Pulis EE, Fecchio A, Schlosser IJ, Tkach VV, 2019c. Phylogenetic relationships, expanded diversity and distribution of Crassiphiala spp. (Digenea, Diplostomidae), agents of black spot disease in fish. Parasitol. Res. 118, 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz TJ, Pulis EE, Junker K, Tran BT, Snyder SD, Tkach VV, 2019d. Molecular phylogeny of the Cyathocotylidae (Digenea, Diplostomoidea) necessitates systematic changes and reveals a history of host and environment switches. Zool. Scr. 48, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz TJ, Pulis EE, González-Acuña D, Tkach VV, 2020. Phylogenetic relationships of Cardiocephaloides spp. (Digenea, Diplostomoidea) and the genetic characterization of Cardiocephaloides physalis from Magellanic penguin, Spheniscus magellanicus, in Chile. Acta Parasitol. 65, 525–534. [DOI] [PubMed] [Google Scholar]
- Achatz TJ, Bell JA, Melo FTV, Fecchio A, Tkach VV, 2021. Phylogenetic position of Sphincterodiplostomum Dubois, 1936 (Digenea: Diplostomoidea) with description of a second species from Pantanal, Brazil. J. Helminthol. 95, 1–8. [DOI] [PubMed] [Google Scholar]
- Achatz TJ, Brito ES, Fecchio A, Tkach VV, in press. Description and phylogenetic position of a new species of Herpetodiplostomum from Phyrnops geoffroanus in Brazil and a re-evaluation Cheloniodiplostomum. J. Parasitol. 107, 455–462. [DOI] [PubMed] [Google Scholar]
- Behrmann-Godel J, 2013. Parasite identification, succession and infection pathways in perch fry (Perca fluviatilis): New insights through a combined morphological and genetic approach. Parasitology 140, 509–520. [DOI] [PubMed] [Google Scholar]
- Blasco-Costa I, Faltýnková A, Georgieva S, Skírnisson K, Scholz T, Kostadinova A, 2014. Fish pathogens near the Arctic Circle: molecular, morphological and ecological evidence for unexpected diversity of Diplostomum (Digenea: Diplostomidae) in Iceland. Int. J. Parasitol. 44, 703–715. [DOI] [PubMed] [Google Scholar]
- Blasco-Costa I, Locke SA, 2017. Life history, systematics and evolution of the Diplostomoidea Poirier, 1886: progress, promises and challenges emerging from molecular studies. Adv. Parasitol. 98, 167–225. [DOI] [PubMed] [Google Scholar]
- Blasco-Costa I, Poulin R, Presswell B, 2017. Morphological description and molecular analyses of Tylodelphys sp. (Trematoda: Diplostomidae) newly recorded from the freshwater fish Gobiomorphus cotidianus (common bully) in New Zealand . J. Helminthol. 91, 332–345. [DOI] [PubMed] [Google Scholar]
- Bouley P, Isadore M, Carroll T, 2015. Return of North American river otters, Lontra canadensis, to coastal habitats of the San Francisco Bay Area, California. Northwest. Nat. 96, 1–12. [Google Scholar]
- Brabec J, Kostadinova A, Scholz T, Littewood TJ, 2015. Complete mitochondrial genomes and nuclear ribosomal RNA operons of two species of Diplostomum (Platyhelminthes: Trematoda): a molecular resource for taxonomy and molecular epidemiology of important fish pathogens. Parasit. Vectors 8, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibwana FD, Blasco-Costa I, Georgieva S, Hosea KM, Nkwengulila G, Scholz T, Kostadinova A, 2013. A first insight into the barcodes for African diplostomids (Digenea: Diplostomidae): brain parasites in Clarias gariepinus (Siluriformes: Clariidae). Infect. Genet. Evol. 17, 62–70. [DOI] [PubMed] [Google Scholar]
- Chibwana FD, Nkwengulila G, Locke SA, McLaughlin JD, Marcogliese DJ, 2015. Completion of the life cycle of Tylodelphys mashonense (Sudarikov, 1971) (Digenea: Diplostomidae) with DNA barcodes and rDNA sequences. Parasitol. Res. 114, 3675–3682. [DOI] [PubMed] [Google Scholar]
- Derycke S, Remerie T, Vierstraete A, Backeljau T, Vanfleteren J, Vincx M, Moens T, 2005. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 300, 91–103. [Google Scholar]
- Dronen NO, 2009. Austrodiplostomum ostrowskiae n. sp. (Digenea: Diplostomidae: Diplostominae) from the double-crested cormorant, Phalacrocorax auritus (Phalacrocoracidae) from the Galveston, Texas area of the Gulf of Mexico, U.S.A. Comp. Parasitol. 76, 34–39. [Google Scholar]
- Dronen NO, Tehrany MR, Wardle WJ, 1999. Diplostomes from the brown pelican, Pelecanus occidentalis (Pelecanidae), from the Galveston Texas Area, including two new species of Bursacetabulus. gen. n. J. Helminthol. Soc. Wash. 66, 21–24. [Google Scholar]
- Dubois G, 1932. Revision des Hemistomes et étude de forms nouvelles. Bull. Soc. Neuchâtel. Sci. Nat. 56, 375–412. [Google Scholar]
- Dubois G, 1937. Sur quelques Strigidés Rev. Suisse Zool. 44, 391–396. [Google Scholar]
- Dubois G, 1938. Monographie des Strigeida (Trematoda). Mem. Soc. Sci. Nat. Neuchâtel 6, 1–535. [Google Scholar]
- Dubois G, 1944. A propos de la spécificité parasitaire des Strigeida. Bull. Soc. Neuchâtel. Sci. Nat. 69, 5–103. [Google Scholar]
- Dubois G, 1968. Synopsis des Strigeidae et des Diplostomatidae (Trematoda). Mem. Soc. Sci. Nat. Neuchâtel. 10, 1–258. [Google Scholar]
- Dubois G, 1969a. Notes helminthologiques. II: Diplostomatidae Poirier et Cyathocotylidae Poche (Trematoda). Rev. Suisse Zool. 76, 3–21. [DOI] [PubMed] [Google Scholar]
- Dubois G, 1969b. Les Strigeata (Trematoda) de la collection Elizabeth M. Boyd. Bull. Soc. Neuchâtel. Sci. Nat. 92, 5–12. [Google Scholar]
- Dubois G, 1970. Les fondements de la taxonomie des Strigeata La Rue (Trematoda: Strigeida). Rev. Suisse Zool. 77, 663–685. [PubMed] [Google Scholar]
- Dubois G, 1982. Répertoire des synonymes récents de genres et d’espéces de la superfamille des Strigeoidea Railliet, 1919 (Trematoda). Bull. Soc. Neuchâtel. Sci. Nat. 105, 163–183. [Google Scholar]
- Dusek RJ, Hallgrimsson GT, Ip HS, Jónsson JE, Sreevatsan S, Nashold SW, TeSlaa JL, Enomoto S, Halpin RA, Lin X, Fedorova N, Stockwell TB, Dugan VG, Wentworth DE, Hall JS, 2014. North Atlantic migratory bird flyways provide routes for intercontinental movement of avian influenza viruses. PloS One 9, e92075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enabulele EE, Awharitoma AO, Lawton SP, Kirk RS, 2018. First molecular identification of an agent of diplostomiasis, Diplostomum pseudospathaceum (Niewiadomska 1984) in the United Kingdom and its genetic relationship with populations in Europe. Acta Parasitol. 63, 444–453. [DOI] [PubMed] [Google Scholar]
- Faltýnková A, Georgieva S, Kostadinova A, Blasco-Costa I, Scholz T, Skirnisson K, 2014. Diplostomum von Nordmann, 1832 (Digenea: Diplostomidae) in the sub-Arctic: descriptions of the larval stages of six species discovered recently in Iceland. Syst. Parasitol. 89, 195–213. [DOI] [PubMed] [Google Scholar]
- Fernandes BMM, Justo MCN, Cárdenas QC, Cohen SC, (Eds), 2015. South American trematodes parasites of birds and mammals. Oficina de Livros, Rio de Janeiro. [Google Scholar]
- Galazzo DE, Dayanandan S, Marcogliese D, McLaughlin J, 2002. Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can. J. Zool. 80, 2207–2217. [Google Scholar]
- García-Varela M, Sereno-Uribe AL, Pinacho-Pinacho CD, Domínguez-Domínguez O, Pérez-Ponce de León G, 2016. Molecular and morphological characterization of Austrodiplostomum ostrowskiae Dronen, 2009 (Digenea: Diplostomatidae), a parasite of cormorants in the Americas. J. Helminthol. 1, 1–12. [DOI] [PubMed] [Google Scholar]
- Georgieva S, Soldánová M, Pérez-del-Olmo A, Dangel DR, Sitko J, Sures B, Kostadinova A, 2013. Molecular prospecting for European Diplostomum (Digenea: Diplostomidae) reveals cryptic diversity. Int. J. Parasitol. 43, 57–72. [DOI] [PubMed] [Google Scholar]
- Gibson DI, 1996. Guide to the parasites of fishes of Canada. Part IV. Trematoda. NRC Research Press, Ottawa. [Google Scholar]
- Gordy MA, Hanington PC, 2019. A fine‐scale phylogenetic assessment of digenean trematodes in central Alberta reveals we have yet to uncover their total diversity. Ecol. Evol. 9, 3153–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordy MA, Kish L, Tarrabain M, Hanington PC, 2016. A comprehensive survey of larval digenean trematodes and their snail hosts in central Alberta, Canada. Parasitol. Res.115, 3867–3880. [DOI] [PubMed] [Google Scholar]
- Guberlet JE, 1922. Three New Species of Holostomidae J. Parasitol. 9, 6–14. [Google Scholar]
- Gupta R, 1962. Two new species of the rare genus Schwartz itrema (Vigueras, 1940) Vigueras, 1941 (Trematoda: Strigeidae). Proc. Natl. Acad. Sci. India. 32, 387–392. [Google Scholar]
- Heneberg P, Sitko J, Těšínský M, 2020. Paraphyly of Conodiplostomum Dubois, 1937. Parasitol. Int. 76, 102033. [DOI] [PubMed] [Google Scholar]
- Heneberg P, Sitko J, 2021. Cryptic speciation among Tylodelphys spp.: the major helminth pathogens of fish and amphibians. Parasitol. Res. 120, 1687–1697. [DOI] [PubMed] [Google Scholar]
- Hernández-Mena DI, García-Prieto L, García-Varela M, 2014. Morphological and molecular differentiation of Parastrigea (Trematoda: Strigeidae) from Mexico, with the description of a new species. Parasitol. Int. 63, 315–323. [DOI] [PubMed] [Google Scholar]
- Hernández-Mena DI, García-Varela M, Pérez-Ponce de León G, 2017. Filling the gaps in the classification of the Digenea Carus, 1863: systematic position of the Proterodiplostomidae Dubois, 1936 within the superfamily Diplostomoidea Poirier, 1886, inferred from nuclear and mitochondrial DNA sequences. Syst. Parasitol. 94, 833–848. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn C, Smit NJ, Kudlai O, 2019. Molecular and morphological characterization of four diplostomid metacercariae infecting Tilapia sparrmanii (Perciformes: Cichlidae) in the North West Province, South Africa. Parasitol. Res. 118, 1403–1416. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn C, Smit NJ, Kudlai O, 2020. Resolution of the identity of three species of Diplostomum (Digenea: Diplostomidae) parasitizing freshwater fishes in South Africa, combining molecular and morphological evidence. Int. J. Parasitol. Parasit. Wildl. 11, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RC, 1929. Studies on the Trematode family Strigeidae (Holostomidae) No. XIV. Two new species of Diplostomula. Occas. Pap. Mus. Zool. Univ. Michigan 202, 1–29. [Google Scholar]
- Inchausty V, Foutz M, Heckmann R, Ruas C, Ruas P, 1997. Diplostomiasis in native and introduced fishes from Yellowstone Lake, Wyoming. Great Basin Naturalist. 57, 178–183. [Google Scholar]
- Karvonen A, Kirsi S, Hudson PJ, Valtonen ET, 2004. Patterns of cercarial production from Diplostomum spathaceum: terminal investment or bet hedging? Parasitology 129, 87–92. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Savolainen M, Seppälä O, Valtonen ET, 2006. Dynamics of Diplostomum spathaceum infection in snail hosts at a fish farm. Parasitol. Res. 99, 341–345. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Itoh N, Ogawa K, 2019. Worm cataract of hatchery-reared Japanese dace Tribolodon hakonensis caused by Diplostomum sp. (Digenea: Diplostomidae). Fish Pathol. 54, 1–11. [Google Scholar]
- Kraus R, 1914. Beitrag zur Kenntnis der Hemistominen. Zetschr. Wiss. Zool. 112, 93–238. [Google Scholar]
- Kudlai O, Kostadinova A, Pulis EE, Tkach VV, 2015. A new species of Drepanocephalus Dietz, 1909 (Digenea: Echinostomatidae) from the double-crested cormorant Phalacrocorax auritus (Lesson) (Aves: Phalacrocoracidae) in North America. Syst. Parasitol. 90, 221–230. [DOI] [PubMed] [Google Scholar]
- Kudlai O, Oros M, Kostadinova A, Georgieva S, 2017. Exploring the diversity of Diplostomum (Digenea: Diplostomidae) in fishes from the River Danube using mitochondrial DNA barcodes. Parasit. Vectors 10, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K, 2016. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Bio. Evo. 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue GR, 1927. Studies on the Trematode Family Strigeidae (Holostomidae). No. V. Proalaria huronensis, sp. nov. Trans. Am. Micros. Soc. 46, 26–35. [Google Scholar]
- Landeryou T, Kett SM, Ropiquet A, Wildebore D, Lawton SP, 2020. Characterization of the complete mitochondrial genome of the Diplostomum baeri. Parasitol. Int. 79, 102166. [DOI] [PubMed] [Google Scholar]
- Locke SA, Al-Nasiri FS, Caffara M, Drago F, Kalbe M, Lapierre AR, McLaughlin JD, Nie P, Overstreet RM, Rangel e Souza GT, Takemoto RM, Marcogliese DJ, 2015. Diversity, specificity and speciation in larval Diplostomidae (Platyhelminthes: Digenea) in the eyes of freshwater fish, as revealed by DNA barcodes. Int. J. Parasitol. 45, 841–855. [DOI] [PubMed] [Google Scholar]
- Locke SA, McLaughlin JD, Marcogliese DJ, 2010b. DNA barcodes show cryptic diversity and a potential physiological basis for host specificity among Diplostomoidea (Platyhelminthes: Digenea) parasitizing freshwater fishes in the St. Lawrence River, Canada. Mol. Ecol. 19, 2813–2827. [DOI] [PubMed] [Google Scholar]
- Locke SA, Drago FB, Núñez V, Rangel e Souza GT, Takemoto RM, 2020. Phylogenetic position of Diplostomum spp. from New World herons based on complete mitogenomes, rDNA operons, and DNA barcodes, including a new species with partially elucidated life cycle. Parasitol. Res. 119, 2129–2137. [DOI] [PubMed] [Google Scholar]
- Locke SA, McLaughlin JD, Dayanandan S, Marcogliese DJ, 2010a. Diversity and specificity in Diplostomum spp. metacercariae in freshwater fishes revealed by cytochrome c oxidase 1 and internal transcribed spacer sequences. Int. J. Parasitol. 40, 333–343. [DOI] [PubMed] [Google Scholar]
- Locke SA, Van Dam AR, Caffara M, Pinto HA, López-Hernández D, Blanar CA, 2018. Validity of the Diplostomoidea and Diplostomida (Digenea, Platyhelminthes) upheld in phylogenomic analysis. Int. J. Parasitol. 48, 1043–1059. [DOI] [PubMed] [Google Scholar]
- Lockyer AE, Olson PD, Østergaard P, Rollinson D, Johnston DA, Attwood SW, Southgate VR, Horak P, Snyder SD, Le TH, Agatsuma T, McManus DP, Carmichael AC, Naem S, Littlewood DTJ, 2003. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology 126, 203–224. [DOI] [PubMed] [Google Scholar]
- López-Hernández D, Locke SA, de Assis JCA, Drago FB, de Melo AL, Rabelo EML, Pinto HA, 2019. Molecular, morphological and experimental-infection studies of cercariae of five species in the superfamily Diplostomoidea (Trematoda: Digenea) infecting Biomphalaria straminea (Mollusca: Planorbidae) in Brazil. Acta Trop. 199, 105082. [DOI] [PubMed] [Google Scholar]
- Lunaschi L, Drago FB, 2004. Descripción de una especie nueva de Tylodelphys (Digenea: Diplostomidae) parásita de Podiceps major (Aves: Podicepedidae) de Argentina. Anales Instit. Biol. Univ. Nacional Autónoma México, Ser. Zool. 75, 245–252. [Google Scholar]
- Lunaschi L, Drago FB, 2006. First report of adult specimens of Sphincterodiplostomum musculosum (Digenea, Diplostomidae). Parasitol. Int. 55, 7–10. [DOI] [PubMed] [Google Scholar]
- Lutz HL, Tkach VV, Weckstein JD, 2017. Methods for specimen-based studies of avian symbionts. In: Webster M (Ed.), The role of collections in ornithology: The extended specimen. Studies in avian biology. CRC Press, Boca Raton, pp. 157–183. [Google Scholar]
- McCloughlin T, 2016. A sight for sore eyes: Diplostomum and Tylodelphys in the eyes of fish. Iranian J. Parasitol. 11, 429–430. [PMC free article] [PubMed] [Google Scholar]
- Miller GC, Harkema R, 1964. Studies on helminths of North Carolina vertebrates. V. Parasites of the mink, Mustela vison Schreber. J. Parasitol. 50, 717–720. [PubMed] [Google Scholar]
- Miller GC, Harkema R, 1968. Helminths of some wild mammals in the South-Eastern United States. Proc. Helminthol. Soc. Wash. 35, 118–125. [Google Scholar]
- Moszczynska A, Locke SA, McLaughlin JD, Marcogliese DJ, Crease TJ, 2009. Development of primers for the mitochondrial cytochrome c oxidase 1 gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol. Ecol. Resources 9, 75–82. [DOI] [PubMed] [Google Scholar]
- Nakao M, Sasaki M, 2021. Trematode diversity in freshwater snails from a stopover point for migratory waterfowls in Hokkaido, Japan: An assessment by molecular phylogenetic and population genetic analyses. Parasitol. Int. 83, 102329. [DOI] [PubMed] [Google Scholar]
- Niewiadomska K, 2002. Family Diplostomidae Poirier, 1886. In: Gibson DI, Jones A, Bray RA (Eds.), Keys to the Trematoda. Vol. I. CAB International, Wallingford, UK. pp. 167–196. [Google Scholar]
- Niewiadomska K, 2010. Freshwater fauna of Poland. 34A. Trematodes (Trematoda). General part; Systematic part - Aspidogastrea, Digenea: Strigeida. Polish Hydrobiological Society, University of Łódź, Łódź. (In Polish) [Google Scholar]
- Nigrelli RF, Maraventano LW, 1944. Pericarditis in Xenopus laevis caused by Diplostomulum xenopi sp. nov. a larval strigeid. J. Parasitol. 30, 184–190. [Google Scholar]
- O’Hear M, Pote L, Yost M, Doffitt C, King T, Panuska C, 2014. Morphologic and molecular identifications of digenetic trematodes in double-crested cormorants (Phalacrocorax auritus) from the Mississippi Delta, USA. J. Wildl. Dis. 50, 42–49. [DOI] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus ADME, Fauchier RAM, 2006. Global patterns of influenza A virus in wild birds. Science. 312, 384–388. [DOI] [PubMed] [Google Scholar]
- Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ, 2003. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 33, 733–755. [DOI] [PubMed] [Google Scholar]
- Pelegrini LS, Gião T, Vieira DMD, Müller MI, José da Silva R, Pérez-Ponce de León G, Kozlowiski de Azevedo R, Abdallah VD, 2019. Molecular and morphological characterization of the metacercariae of two species of diplostomid trematodes (Platyhelminthes, Digenea) in freshwater fishes of the Batalha River Brazil. Parasitol. Res. 118, 2169–2182. [DOI] [PubMed] [Google Scholar]
- Pérez-del-Olmo A, Georgieva S, Pula HJ, Kostadinova A, 2014. Molecular and morphological evidence for three species of Diplostomum (Digenea: Diplostomidae), parasites of fishes and fish-eating birds in Spain. Parasit. Vectors 7, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polechla PJ Jr., Carrillo-Rubio E, 2009. Historic and current distributions of river otters (Lontra canadensis) and (Lontra longicaudis) in the Rio Grande or Rio Bravo Del Norte Drainage of Colorado and New Mexico, USA and of Chihuahua, Mexico and adjacent areas. IUCN Otter Spec. Group. 26, 82–96. [Google Scholar]
- Queiroz MS, López-Hernández D, Locke SA, Pinto HA, Anjos LA, 2020. Metacercariae of Heterodiplostomum lanceolatum (Trematoda: Proterodiplostomidae) found in Leptodactylus podicipinus (Anura: Leptodactylidae) from Brazil: a morphological, molecular and ecological study. J. Helminthol. 94, E66. [DOI] [PubMed] [Google Scholar]
- Ramey AM, Reed JA, Walther P, Link P, Schmutz JA, Douglas DC, Stallknecht DE, Soos C, 2016. Evidence for the exchange of blood parasites between North America and the Neotropics in blue-winged teal (Anas discors). Parasitol. Res. 115, 3923–3939. [DOI] [PubMed] [Google Scholar]
- Ramey AM, Schmutz JA, Reed JA, Fujita G, Scotton BD, Casler B, Fleskes JP, Konishi K, Uchida K, Yabsley MJ, 2015. Evidence for intercontinental parasite exchange through molecular detection and characterization of haematozoa in northern pintails (Anas acuta) sampled throughout the North Pacific Basin. Int. J. Parasitol: Parasit. Wild. 4, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheingantz ML, Saraiva de Menezes JF, Benoit de Thoisy, 2014. Defining Neotropical otter Lontra longicaudis distribution, conservation priorities, and ecological frontiers. Trop. Conserv. Sci. 7, 214–229. [Google Scholar]
- Ronquist F, Huelsenbeck JP, 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19, 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rosser TG, Alberson NR, Khoo LH, Woodyard ET, Pote LM, Griffin MJ, 2016a. Characterization of the life cycle of a fish eye fluke, Austrodiplostomum ostrowskiae (Digenea: Diplostomidae), with notes on two other diplostomids infecting Biomphalaria havanensis (Mollusca: Planorbidae) from catfish aquaculture ponds in Mississippi, USA. J. Parasitol. 102, 260–274. [DOI] [PubMed] [Google Scholar]
- Rosser TG, Baumgartner WA, Alberson NR, Woodyard ET, Reichley SR, Wise DJ, Pote LM, Griffin MJ, 2016b. Austrodiplostomum sp., Bolbophorus sp. (Digenea: Diplostomidae), and Clinostomum marginatum (Digenea: Clinostomidae) metacercariae in inland silverside Menidia beryllina from catfish aquaculture ponds, with notes on the infectivity of Austrodiplostomum sp. cercariae in channel catfish Ictalurus punctatus. Parasitol. Res. 115, 4365–4378. [DOI] [PubMed] [Google Scholar]
- Rudko SP, Reimink RL, Froelich K, Gordy MA, Blankespoor CL, Hanington PC, 2018. Use of qPCR-based cercariometry to assess swimmer’s itch in recreational lakes. EcoHealth 15, 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer TK, 1961. The American otter, Lutra canadensis vaga, as a host for two species of trematodes previously unreported from North America. Proc. Helminthol. Soc. Wash. 28, 175–176. [Google Scholar]
- Schoop WL, 1989. Systematic analysis of the Diplostomidae and Strigeidae (Trematoda). J. Parasitol. 75, 21–32. [PubMed] [Google Scholar]
- Selbach C, Soldánová M, Georgieva S, Kostadinova A, Sures B, 2015. Integrative taxonomic approach to the cryptic diversity of Diplostomum spp. in lymnaeid snails from Europe with a focus on the ‘Diplostomum mergi’ species complex. Parasit. Vectors 8, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä O, Karvonen A, Valtonen TE, 2008. Shoaling behavior of fish under parasitism and predation risk. Animal Behav. 75, 145–15. [Google Scholar]
- Sereno-Uribe AL, Anrade-Gómez L, Ponce de Leon GP, García-Varela M, 2019a. Exploring the genetic diversity of Tylodelphys (Diesing, 1850) metacercariae in the cranial and body cavities of Mexican freshwater fishes using nuclear and mitochondrial DNA sequences, with the description of a new species. Parasitol. Res. 118, 203–217. [DOI] [PubMed] [Google Scholar]
- Sereno-Uribe AL, Andrade-Gómez L, Ostrowski de Núñez M, Pérez-Ponce de León GP, García-Varela M, 2019b. Assessing the taxonomic validity of Austrodiplostomum spp. (Digenea: Diplostomidae) through nuclear and mitochondrial data. J. Parasitol. 105, 102–112 [PubMed] [Google Scholar]
- Shigin AA, 1986. Trematode fauna of the USSR. Genus Diplostomum. Metacercariae. Nauka, Moscow (In Russian). [Google Scholar]
- Shigin AA, 1993. Trematodes of the fauna of Russia and neighbouring regions.Genus Diplostomum. Adults. Nauka, Moscow (In Russian). [Google Scholar]
- Soldánová M, Georgieva S, Roháčová J, Knudsen R, Kuhn JA, Henriksen EH, Siwertsson A, Shaw JC, Kuris AM, Amundsen P-A, Scholz T, Lafferty KD, Kostadinova A, 2017. Molecular analyses reveal high species diversity of trematodes in a sub-Arctic lake. Int. J. Parasitol. 47, 327–345. [DOI] [PubMed] [Google Scholar]
- Szidat L, Nani A, 1951. Diplostomiasis cerebralis del pejerrey. Rev. Mus. Argentino Cien. Nat. Bernardino Rivadavia 1, 323–384. [Google Scholar]
- Tehrany MR, Dronen NO, Wardle WJ, 1999. Revision of Bursacetabulus (Diplostomidae: Diplostominae) with the proposal of Bursatintinnabulus n. gen., and description of Bursatintinnabus bassanus n. sp. and Bursacetabulus morus n. sp. from northern gannet, Morus bassanus (AVES), from the Texas Gulf Coast. J. Parasitol. 85, 531–533. [PubMed] [Google Scholar]
- Tkach VV, Achatz TJ, Pulis EE, Junker K, Snder SD, Bell JA, Halajan A, Melo FTV., 2020. Phylogeny and systematics of the Proterodiplostomidae Dubois, 1936 (Digenea: Diplostomoidea) reflect the complex evolutionary history of the ancient digenean group. Syst. Parasitol. 97, 409–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM, Swiderski Z, 2003. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 56, 1–15. [DOI] [PubMed] [Google Scholar]
- Tkach VV, Pawlowski J, 1999. A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitol. 44, 147–148. [Google Scholar]
- Ubels JL, DeJong RJ, Hoolsema B, Wurzberger A, Nguyen T-T, Blankespoor HD, Blankespoor CL, 2018. Impairment of retinal function in yellow perch (Perca flavescens) by Diplostomum baeri metacercariae. Int. J. Parasitol. Parasit. Wildl. 7, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas Muñoz JC, Feld CK, Hilt S, Manfrin A, Nachev M, Köster D, Jochmann MA, Schmidt TC, Sures B, Ziková A, Knopf K, 2021. Eye fluke infection changes diet composition in juvenile European perch (Perca fluviatilis). Sci. Rep. 11, 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguti S, 1971. Synopsis of the Digenetic Trematodes of Vertebrates. Vols. 1 and II. Keigaku Publishing, Tokyo. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Phylogenetic interrelationships among 80 sequences from members of Diplostomum (including a former Paralaria sp.) based on Bayesian inference analysis of partial cox1 mtDNA gene sequences. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale bar indicates the number of substitutions per site. References to origins of species numbering/naming systems are provided in parentheses after GenBank accession numbers, followed by biogeographical realms where specimens were collected, life stages of isolates and families of definitive hosts (for adult isolates and larvae molecularly matched to adult forms). Black bars are positioned beside taxa for which we have provided nominal species identifications based on adult morphology. The previously accepted/published names are provided in parentheses after GenBank accession numbers.
Abbreviations for references to the original designations of species-level lineages: B, Blasco-Costa et al. (2014); C, Chibwana et al. (2013); Ge, Georgieva et al. (2013); Go, Gordy and Hanington (2019); H, Hoogendoorn et al. (2020); Ko, Komatsu et al. (2019); Ku, Kudlai et al. (2017); L, Locke et al. (2010a, b; 2015); M, Moszczynska et al. (2009); P, Pelegrini et al. (2019); Sl, Selbach et al. (2015). Abbreviations for biogeographical realms: Afr, Afrotropical realm; ANea, Nearctic realm; Neo, Neotropical realm; Pal, Palaearctic realm. Abbreviations for life stages: Adu, adult; Cer, cercaria; Met, metacercaria. Abbreviations for families of definitive hosts: Ana, Anatidae; Ard, Ardeidae; Gav, Gaviidae; Lar, Laridae; Mus, Mustelidae; Rec, Recurvirostridae; Sco, Scolopacidae.
Supplementary Fig. S2. Photographs of our specimens of Diplostomum sp. VVT5, Diplostomum huronense and Diplostomum indistinctum. (A) Diplostomum sp. VVT5, entire, ventral view. Scale bar = 250 μm. (B) Diplostomum huronense, entire, ventral view. Scale bar = 500 μm. (C) Posterior portion of the prosoma of D. huronense showing anterior extent of vitellarium, lateral view. Scale bar = 150 μm. (D) Ventral sucker of D. huronense showing anterior extent of vitellarium, ventral view. Scale bar = 100 μm. (E) Diplostomum indistinctum, entire, ventral view. Scale bar = 500 μm. Vit, vitellarium; VS, ventral sucker.


