Abstract
Background:
We sought to determine whether dementia is associated with treatment intensity and mortality in patients hospitalized with COVID-19.
Methods:
Review of the medical records for patients > 60 years of age (n=5,394) hospitalized with COVID-19 from 132 community hospitals between March and June, 2020. We examined the relationships between dementia and treatment intensity (including intensive care unit admission (ICU) and mechanical ventilation (MV) and care processes that may influence them, including advance care planning (ACP) billing and do-not-resuscitate (DNR) orders) and in-hospital mortality adjusting for age, sex, race/ethnicity, comorbidity, month of hospitalization, and clustering within hospital. We further explored the effect of ACP conversations on the relationship between dementia and outcomes, both at the individual patient level (effect of having ACP) and at the hospital level (effect of being treated at a hospital with low: <10%, medium 10–20%, or high >20% ACP rates).
Results:
Ten percent (n=522) of the patients had documented dementia. Dementia patients were older (> 80yo: 60% vs. 27%, p< 0.0001), had a lower burden of comorbidity (3+ comorbidities: 31% vs. 38%, p=0.003), were more likely to have ACP (28% vs. 17%, p<0.0001) and a DNR order (52% vs. 22%, p<0.0001), had similar rates of ICU admission (26% vs. 28%, p=0.258), were less likely to receive MV (11% vs. 16%, p=0.001), and more likely to die (22% vs. 14%, p<0.0001). Differential treatment intensity among patients with dementia was concentrated in hospitals with low, dementia-biased ACP billing practices (risk-adjusted ICU use: 21% vs 30%, OR=0.6, p=0.016; risk-adjusted MV use: 6% vs 16%, OR=0.3, p<0.001).
Conclusions:
Dementia was associated with lower treatment intensity and higher mortality in patients hospitalized with COVID-19. Differential treatment intensity was concentrated in low-ACP billing hospitals suggesting an interplay between provider bias and “preference-sensitive” care for COVID-19.
Keywords: advance care planning, intensive care unit, do not resuscitate order, mortality, acute care hospitalization, older adults, terminal care, COVID-19, hospital medicine, medical decision making
INTRODUCTION
Older adults are at elevated risk for severe illness and death from COVID-19. Those living in nursing homes, including patients with dementia, have been most severely affected. [1] This is likely an underestimate of the mortality impacts of COVID-19 in dementia populations, for whom non-COVID-19 attributed excess mortality increased significantly during the 2020 Spring and Summer surges. [2] Many nursing home residents received end-of-life palliation in place, electing to avoid hospital transfer, [3] consistent with preferences to avoid life-sustaining treatments. [4–8] However, among patients with dementia who were hospitalized, many of whom may have been community dwelling, little is known about their treatment intensity and outcomes.
Patients who carry a diagnosis of dementia may be treated less aggressively in the acute care setting than those without a diagnosis, even if their disease is not advanced. This may be due to provider bias, patient and family treatment preferences, or both. Heightened concerns regarding bias arose in response to hospital policies that identified advanced age, frailty and dementia as criteria for de-prioritization in the event of ventilator rationing for COVID-19. [9–11] While few U.S. states actually activated crisis standards of care in 2020, [12] evidence is emerging that frontline providers were faced with making triage decisions. [13] If hospitalized COVID-19 patients with dementia received less life-sustaining treatment, this could reflect a process of shared decision making involving deliberation regarding treatment benefits and burdens informed by patient preferences documented in advance care plans, provider bias, or both.
In this study we sought to determine whether a diagnosis of dementia was associated with lower treatment intensity and greater mortality in a national sample of patients hospitalized with COVID-19 and if so, whether inpatient advance care planning (ACP) practices could explain these differences.
Materials and Methods
Setting
The data for this study comes from a national medical group that specializes in hospital medicine, critical care, and emergency medicine. At most of the 200 community hospitals where it is based, this group is the only hospital medicine provider and manages the majority of medical admissions and discharges. The medical group serves many hospitals in states that were impacted by the early COVID-19 surge, including Washington, Michigan, and Ohio as well as several in the broader metropolitan area of New York City.
Since the introduction of billing codes for advance care planning (ACP) in 2016, the medical group has been engaged in a quality improvement effort aimed at increasing the use of ACP, especially among those with advanced age, serious illness, and functional status changes that confer an increased risk of dying. The quality improvement program includes mandatory education in the use of ACP billing codes, small financial incentives for ACP documentation, priming physicians to reflect on the patients risk of dying in the next year at the time of hospital admission, and feedback to hospitalist chiefs regarding their hospitalists’ ACP frequency among older adults. Between January 2016-December 2018, rates of ACP for patients 65 and older in hospitals continuously staffed by the medical group increased from 0.3% to 9.44%, compared to 0.24% to 2.20% in all hospitals. Nevertheless, considerable variation in ACP across hospital staffed by the medical group persist.
Conceptual Framework
Preference for discussing and/or limiting life supporting treatment for patients with a diagnosis of dementia is a type of bias. This bias may be conscious or unconscious, and may include explicit or implicit valuations of the quality of life for patients with a diagnosis of dementia. It could be manifest through providers being more likely to broach ACP conversations with hospitalized COVID-19 patients with dementia or being more likely to assume that treatment limitations (e.g., a “do not resuscitate” (DNR) order) extend to other treatments (e.g., ICU admission and mechanical ventilation MV). [14] Bias may not always be pernicious; clinicians’ preference for discussing ACP with older patients and those with chronic illness is codified by clinical practice guidelines. [15] However, in some instances, it may involve unexamined projection of one’s own preferences or assumptions about patients’ preferences. [16]
While provider bias is difficult to observe in clinical practice, particularly because it may be unconscious, we seek to measure it based on observed outcomes of patients. We test our hypothesis in two ways. First we ask: Do patients with a diagnosis of dementia have more ACP billed and documented, and is this association independent of other observable factors such as age and other serious illnesses? This first approach has the appeal of simplicity, but is subject to a variety of statistical confounding, such as (a) unmeasured illness severity that influences a provider’s interest in ACP discussions (e.g., dementia, other comorbidity, or COVID-19 severity that influence prognosis and benefit/burden ratio of life-sustaining treatment) and (b) patient preferences that influences a patient’s interest in ACP discussions.
We address (a) and (b) by the use of an instrumental-variable type of approach. We take advantage of the natural variation in hospital ACP rates that do not appear to be associated with underlying differences in comorbidities across hospitals; thus differential rates of ACP are likely driven by variation in hospital-level norms of clinician behavior. We believe this to be a particularly good assumption given the efforts by the national medical group in this study to increase ACP for hospitalized older adults, with varying success across hospitals.
Documented ACP, as documented by time-based CPT billing codes, is an accurate measure of conversations about treatment preferences because it requires adherence to time and documentation requirements. In prior work, we found that two-thirds of notes associated with inpatient ACP billing codes would be classified as “goals of care” conversations (e.g., conversations about preferences for treatment during the current hospitalization, rather than preferences for treatment in the distant future). [17] [18]
Our approach improves upon prior work using variation in the use of DNR orders to capture this phenomenon. [19, 20] A DNR order – which may be the outcome of an ACP conversation – is often placed when a patient’s death is imminent in order to prevent CPR at the time of natural death. Therefore DNR may be downstream of treatment decisions whereas ACP may be upstream of treatment decisions.
Patients
This analysis is based on a database that includes review of the electronic health records (EHR) for more than 12,000 adult patients who were hospitalized for treatment of COVID-19 infection between March and June, 2020. Patients being treated for COVID-19 were identified using the medical group’s electronic billing platform, which provides clinical diagnoses supplied by treating physicians who are prompted on patient admission to identify whether or not patients are being treated for COVID-19. Random sampling of COVID-19 patients was used to restrict the number of records for review to 100 patients per hospital.
The EHR review was performed by trained abstractors at each hospital using a templated instrument specific to the EHR used in their hospital.
Variables
The data abstracted from the EMR included: patient demographics (age, sex, race/ethnicity) and comorbidity (cancer, coronary artery disease/myocardial infarction, cardiovascular disease/stroke, dementia, diabetes, HIV/AIDS, hypertension, heart failure, kidney disease, liver disease, respiratory disease, obesity, and smoking, information regarding the elicitation (presence or absence of a billed (CPT codes 99497 or 99498) ACP conversation) or documentation of treatment preferences (code status: do not resuscitate (DNR), full code, or other), use of intensive treatments including intensive care unit (ICU) admission and mechanical ventilation (MV), and patient outcome (in-hospital mortality). We collected race/ethnicity data in compliance with requirements of the funding agency, the National Institutes of Health (NIH), and use it in our regressions to account for how having a racialized identity impacts health risks and interactions with the healthcare system.
Statistical analyses
We restricted analyses for this study to those 60 and older (n=5,394) treated at hospitals (n=132) with electronic health record systems that allowed chart reviewers employed by the medical group to access the ICU portions of the health record. Standard statistical methods including t-tests for continuous variables and chi-square tests for categorical variables were used to evaluate the statistical significance of differences in demographic characteristics and comorbidity for patients by the presence of dementia and across each ACP tercile. We used mixed effects logistic regression to examine the relationships between dementia and treatment intensity and mortality for adjusting for age, sex, race, ethnicity, comorbidity, month of hospitalization, and accounting for clustering within hospital. In subsequent analyses, we additionally adjusted for ACP and stratified risk-adjusted outcomes across terciles of ACP frequency among all COVID-19 admissions. We adjusted for race and ethnicity because black and Hispanic persons presented with worse COVID-19 illness severity, have lower-quality ACP conversations due to lack of racially-concordant care, and may express greater preferences for life-supporting treatment for many reasons, including the lack of trustworthiness of the healthcare system. All statistical work was performed with STATA, version 15.1.
Ethical review and approval
The analysis was approved by the Dartmouth College Committee for the Protection of Human Subjects. The funding agency, the National Institute on Aging, expressed a specific interest in studying dementia patients with COVID-19, but had no role in data analysis or reporting.
RESULTS
There was a chart-recorded diagnosis of dementia in 522/5,394 (10%) of COVID-19 patients over age 60. Dementia patients were older (60% vs 27% >= 80 years, p<0.0001), less likely to be a racialized minority (16% vs 25% non-white race, p<0.0001), more likely to have CVA/Stroke (13% vs 8%, p<0.0001) and chronic kidney disease (15% vs 11%, p=0.004), less likely to have cancer (7% vs 11%, p=0.002), renal failure (1% vs 5%, p<0.0001), to be smokers (12% vs 19%, p<0.0001), and to be obese (5% vs 11%, p<0.0001), but had a lower overall burden of comorbidity (31% vs 38% >= 3 comorbid conditions, p=0.003) (Table 1).
Table 1.
Demographic and clinical comorbidities among hospitalized older adults with COVID-19 March-June 2020, by dementia status and by tercile of hospital ACP billing.
| Dementia Patient | Hospital ACP Tercile | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Variable | No | Yes | p-value | Low (<10%) | Medium (10–20%) | High (>20%) | p-trend |
| Patients, n | 522 | 4,872 | 1,576 | 1,750 | 2,068 | ||
| Dementia (%) | 0 | 100 | 11 | 7 | 11 | 0.928 | |
| Age >80 years (%) | 27 | 60 | <0.0001 | 29 | 29 | 31 | 0.175 |
| Male (%) | 48 | 45 | 0.139 | 45 | 50 | 49 | 0.048 |
| Non-White Race* (%) | 25 | 16 | <0.0001 | 18 | 27 | 26 | <0.0001 |
| Hispanic ethnicity* (%) | 9 | 8 | 0.455 | 10 | 9 | 7 | <0.0001 |
| Cancer (%) | 11 | 7 | 0.002 | 12 | 9 | 12 | 0.853 |
| Cirrhosis (%) | 2 | 1 | 0.413 | 2 | 1 | 2 | 0.920 |
| CAD/MI (%) | 18 | 17 | 0.684 | 18 | 16 | 19 | 0.208 |
| CVA/Stroke (%) | 8 | 13 | <0.0001 | 7 | 9 | 9 | 0.132 |
| Diabetes (%) | 33 | 29 | 0.036 | 32 | 34 | 32 | 0.708 |
| HIV/AIDS (%) | 0 | 0 | 0.777 | 0.4 | 0.5 | 0.5 | 0.505 |
| Hypertension (%) | 56 | 59 | 0.137 | 57 | 56 | 55 | 0.188 |
| Heart Failure (%) | 18 | 16 | 0.256 | 16 | 18 | 19 | 0.010 |
| Chronic Kidney Disease (%) | 11 | 15 | 0.004 | 12 | 10 | 12 | 0.749 |
| Renal Failure (%) | 5 | 1 | <0.0001 | 4 | 6 | 5 | 0.017 |
| Asthma (%) | 6 | 2 | 0.001 | 7 | 5 | 6 | 0.166 |
| Emphysema/COPD (%) | 21 | 14 | <0.0001 | 20 | 17 | 22 | 0.138 |
| Smoker (%) | 19 | 12 | <0.0001 | 18 | 13 | 22 | <0.0001 |
| Obesity (%) | 11 | 5 | <0.0001 | 10 | 10 | 12 | 0.044 |
| Total Comorbidities >=3 (%) | 38 | 31 | 0.003 | 38 | 35 | 38 | 0.712 |
We collected race/ethnicity data in compliance with requirements of the funding agency, the National Institutes of Health (NIH), and to account for different experiences with the healthcare system by racialized minority groups. In the US, non-white race and Hispanic ethnicity are associated with adverse health exposures, poorer access to health care, and discrimination in their interactions with the health system due to systemic racism. Inclusion of these terms in our models should not be interpreted as reflecting any genetic or biologic risk for COVID-19 illness severity.
Dementia patients were more likely to have ACP (28% vs. 17%, p<0.0001) and to have a DNR order (52% vs. 22%, p<0.0001), equally likely to be admitted to the ICU (26% vs. 28%, p=0.258), less likely to receive MV (11% vs. 16%, p=0.001), and more likely to die (22% vs. 14%, p<0.0001) (Table 2). After risk adjustment, dementia patients remained more likely to have ACP (26% vs. 17%, p<0.0001) and a DNR order (47% vs. 21%, p<0.0001), had similar rates of ICU admission (26% vs. 28%, p=0.487), were less likely to receive MV (12% vs 16%, p=0.044) and more likely to die (19% vs 13%, p<0.0001) (Table 2). After further adjustment for having had an ACP conversation billed during the hospitalization, dementia patients remained more likely to have a DNR order (46% vs. 21%, p<0.0001), still had similar rates of ICU admission (26% vs. 28%, p=0.539), were no longer statistically significantly less likely to receive MV (12% vs 16%, p=0.05), but remained more likely to die (18% vs 13%, p=0.002) (Table 2).
Table 2.
Crude and risk-adjusted advance care planning, DNR orders, treatment intensity and mortality rates among hospitalized older adults with COVID-19 March-June 2020, by dementia status
| Variable | No Dementia | Dementia | OR | LB 95% CI | UB 95% CI | p-value |
|---|---|---|---|---|---|---|
| Crude | ||||||
| ACP | 17% | 28% | 2.1 | 1.7 | 2.7 | <0.0001 |
| DNR | 22% | 52% | 4.3 | 3.5 | 5.2 | <0.0001 |
| ICU | 28% | 26% | 0.9 | 0.7 | 1.1 | 0.258 |
| MV | 16% | 11% | 0.6 | 0.5 | 0.8 | 0.001 |
| Mortality | 14% | 22% | 1.9 | 1.5 | 2.3 | <0.0001 |
| Risk-Adjusted * | ||||||
| ACP | 17% | 26% | 1.8 | 1.4 | 2.3 | <0.0001 |
| DNR | 21% | 47% | 3.6 | 2.9 | 4.4 | <0.0001 |
| ICU | 28% | 26% | 0.9 | 0.7 | 1.2 | 0.487 |
| MV | 16% | 12% | 0.7 | 0.5 | 1.0 | 0.044 |
| Mortality | 13% | 19% | 1.5 | 1.2 | 2.0 | <0.0001 |
| Risk + ACP Adjusted ** | ||||||
| DNR | 21% | 46% | 3.4 | 2.7 | 4.2 | <0.0001 |
| ICU | 28% | 26% | 0.9 | 0.7 | 1.2 | 0.539 |
| MV | 16% | 12% | 0.7 | 0.5 | 1.0 | 0.050 |
| Mortality | 13% | 18% | 1.5 | 1.1 | 1.9 | 0.002 |
Adjusted for age, sex, race, ethnicity, and comorbidities (Cancer, Cirrhosis, CAD/MI, CVA/Stroke, Diabetes, HIV/AIDS, Hypertension, Heart Failure, Chronic Kidney Disease, Renal Failure, Asthma, Emphysema/COPD, Smoker, Obesity), month hospitalization, and clustering by hospital.
Also adjusted for advance care planning (ACP) conversation billing and documentation during the hospitalization.
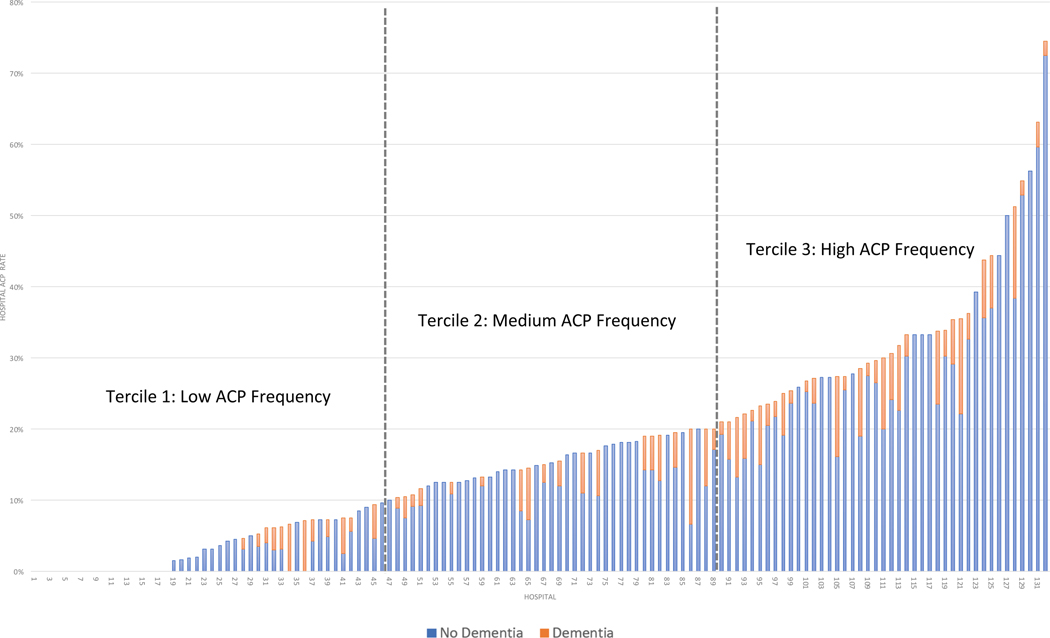
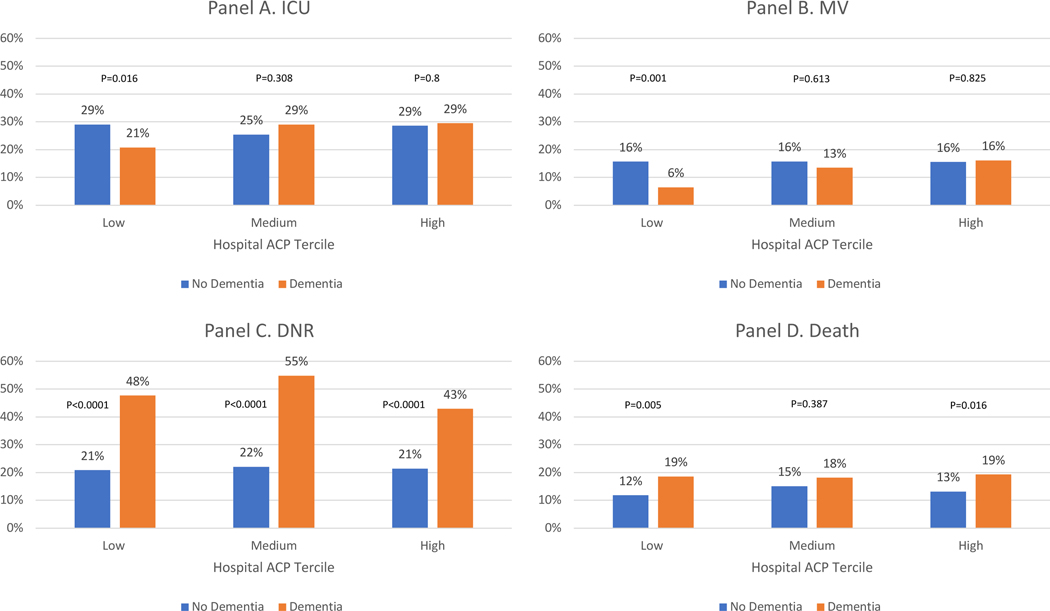
There was significant hospital-level variation in ACP among COVID-19 patients (Figure 1). The 132 hospitals included in this analysis were divided into terciles of ACP among COVID-19 patients in the time period of this study (Figure 2): low (<10%), medium (10%−20%), and high (>20%). The distribution of clinical co-morbidities were similar across the three terciles with a few exceptions (Table 1). The independent effect of dementia on the likelihood of ACP decreased across ACP terciles (low: 3% vs 8%, OR 3.2 [1.7–6.1], p<0.0001; medium: 14% vs 25%, OR 2.0 [1.3–3.2], p=0.003; high: 31% vs 41%, OR 1.6 [1.2–2.2], p=0.003), suggesting that bias in ACP for dementia patients – regardless of the source of that bias – decreases as ACP becomes more common. Other than ACP, there were no consistent directional trends observed across terciles of ACP (Figure 2; Supplementary Table S1). Dementia patients were less likely to be admitted to the ICU (21% vs 30%, OR=0.6 [0.4–0.9], p=0.016) and to receive MV (6% vs 16%, OR=0.3 [0.2–0.7], p=0.001) only in the lowest ACP tercile hospitals. Dementia patients were more likely to be DNR in all three terciles (low: 48% vs 21%, OR=3.9 [2.7–5.6] p=0.<0.0001; medium: 55% vs 22%, OR=4.7 [3.0–7.4], p<0.0001; high: 43% vs 21%, OR=3.0 [2.1–4.1], p<0.0001). Dementia patients were more likely to die in the low (19% vs. 12%, OR=1.8 [1.2–2.8], p=0.005) and high terciles (19% vs 13%, OR=1.6 [1.1–2.2], p=0.016, high).
Figure 1. Variation rates of ACP billing among hospitalized older adults with COVID-19 March-June 2020, by dementia status and hospital.
The 132 hospitals in this study are arrayed left to right from lowest ACP rates (0%) to highest (74%). Blue sections of the bars represent non-dementia patients and orange sections represent dementia patients. For analysis purposes, we divided the hospitals into terciles of ACP: low (<10%), medium (10%−20%), and high (>20%).
Figure 2, panels A-D. – Risk-adjusted treatment intensity (ICU and MV), DNR status, and mortality among hospitalized older adults with COVID-19 March-June 2020, by dementia status and tercile of hospital ACP billing frequency.
In all graphs, the y-axis represents the rate of each measure adjusted for demographic and clinical characteristics among patients without (blue) and with (orange) dementia admitted to hospitals with low (tercile 1), medium (tercile 2), or high (tercile 3) ACP documentation and billing rates. Panel A represents ICU admission rates, Panel B MV rates, Panel C DNR code status, and Panel D death rates.
DISCUSSION
In this national sample of hospitalized COVID-19 patients between March and June 2020, patients with dementia were more likely to have a documented ACP conversation with their hospitalist, more likely to have a DNR order, less likely to receive mechanical ventilation, and more likely to die. Greater ACP could explain lower MV rates but not higher death rates. While there were no differences in the frequency of DNR orders across hospitals with different rates of documented ACP conversations, low-ACP hospitals had large differences in treatment intensity (as measured by ICU and MV) for patients with dementia.
The observed relationship between a diagnosis of dementia, treatment intensity, code status, and outcomes in hospitalized COVID-19 patients are consistent with those seen in other acute care contexts. [21, 22] In an analysis of more than 100,000 acute care hospitalizations in 2017, a diagnosis of dementia was independently associated with documented ACP conversations. [17] While experts in ACP recommend conversations for all hospitalized older adults, a diagnosis of dementia is as frequently nominated as a factor prompting prioritization of the conversation early in the hospitalization as risk of clinical deterioration that would prompt ICU admission consideration. [18] The majority of patients with advanced dementia have comfort-focused treatment preferences affirmed by their proxies [6] and express their own preferences against CPR when in earlier stages of impairment. [4, 5]
The observation that COVID-19 patients with dementia were more likely to die, yet less likely to receive life-supporting treatment, is suggestive of decisions to limit treatment. [23] If it were exclusively due to unmeasured differences in COVID-19 illness severity and risk of death, we would actually expect higher rates of ICU and MV. Some have proposed using the presence of a DNR order as a proxy for decisions to limit treatment.[24] However, a DNR order (which technically specifies no cardiopulmonary resuscitation (CPR) in the event of cardiac arrest) may be the result of many different forces: provider practices (ascertainment of treatment preferences and interpretation of advance directives), underlying patient treatment preferences, and illness severity. [14, 19, 25] Indeed, a DNR order is often placed when a patient’s death is imminent in order to prevent CPR at the time of natural death. [26] Therefore, using a DNR order to “explain” variation in treatment and outcomes is problematic.
Instead, we leveraged variation in ACP conversation billing to develop hypotheses regarding the decision making proceses underlying apparent treatment limitations. We can do this because ACP billing tercile is not driven by illness severity, since patient demographic characteristics and comorbidity rates were, with minor exceptions, similar across hospital ACP terciles, as were rates of DNR orders. Rather, we posit that variation in ACP conversation billing between hospitals is attributed to variation in provider practice patterns. [17] Hospitalists at low ACP-billing sites are more likely to associate ACP with narrow code status conversations that they conduct in a few minutes, which do not meet time-based billing code requirements. [27] In contrast, hospitalists at high ACP-billing sites are more likely to associate ACP with broader conversations about treatment goals and preferences and therefore to meet time requirements. If we assume that the lower ACP billing sites have less robust admission conversations about treatment goals and preferences, those hospitalists may be more apt to make medical decisions based upon implicit rather than explicit understanding of patient treatment preferences. [16] This could explain the disparities in treatment intensity and outcomes associated with dementia at low-ACP sites despite similar rates of DNR code status; hospitalists there may be more likely to assume that DNR means no escalation for dementia patients compared to non-dementia patients. These explanations are speculations, of course. There are alternate explanations. Low ACP sites could be limiting treatment based upon predictions of lack of benefit of life-supporting treatment for some patients with dementia. Also, low ACP sites could have systematically different levels of unmeasured confounding factors due to generally lax coding and billing practices overall.
This analysis of hospitalized COVID-19 patients is not representative of treatment choices for dementia patients with COVID-19, the majority of whom were nursing home residents never transferred to the hospital. Choices to limit life-sustaining treatment for patients with dementia typically represent informed decisions and reflect patients’ current health status having already fallen below their “minimally acceptable function.” [28, 29] Our finding that high-ACP rate sites did not demonstrate disparities in treatment intensity and mortality for dementia patients suggests that comprehensive conversations about preferences and goals for medical care, while more frequent among patients with dementia, do not result in systematically different outcomes for dementia patients.
Our study has several limitations. We relied on retrospective chart review, and dementia may not be reliably documented in the EHR problem list. Furthermore, we did not collect information regarding dementia stage and the frailty-associated conditions such as immobility and dysphagia that are risk factors for poor acute care outcomes, nor did we did collect information about COVID-19 illness severity, such as vital signs or laboratory values. We did not record timing of actions (ACP conversation, DNR order, ICU, MV). We do not know what conversations actually occurred at the bedside or whether patients had prior ACP that informed inpatient treatment choices. Finally, although we posit that differential rates of ACP conversation documentation and billing reflect differences in engagement in ACP, it is possible that they only reflect differences in billing behaviors or other systematic differences in coding practices or quality of care.
CONCLUSIONS
Differences in intensity of treatment for dementia patients were observed in hospitals in which providers are less likely to document and bill for conversations about life-sustaining treatment preferences. This suggests that provider practices, including implicit biases by clinicians regarding who to prioritize for ACP conversations and how to interpret treatment limitations, may underlie these treatment differences rather than the patients’ own preferences. While advance care planning is the best process for aligning treatment with patient goals, it is especially important during the COVID-19 pandemic when resources are scarce, clinicians are overworked, and incapacitated patients lack anyone at their bedside to speak on their behalf.
Supplementary Material
Supplementary Table S1. Risk-adjusted ACP, treatment intensity (ICU and MV), DNR status, and mortality among hospitalized dementia patients with COVID-19 March-June 2020, compared to hospitalized non-dementia patients, by tercile of hospital ACP billing frequency (low, medium, high)
Key Points:
Lower risk-adjusted ICU and mechanical ventilation use among patients with dementia was concentrated in the hospitals with the lowest rates of advance care planning.
Why does this matter?
This suggests an interplay between provider bias and preference-sensitive care for COVID-19.
ACKNOWLEDGEMENTS
Source of support: National Institute on Aging. Grant #P01AG019783
SPONSOR’S ROLE
This work was funded by an administrative supplement to an existing awarded National Institute on Aging (P01AG019783). National Institute on Aging expressed a specific interest in studying dementia patients with COVID-19, but had no role in data analysis or reporting.
The work was performed The Dartmouth Institute.
Footnotes
CONFLICT OF INTEREST
JDB is an executive at Sound Physicians which provided data for this study. NJB is married to JDB.
REFERENCES
- 1.More than 100,000 U.S. coronavirus deaths are linked to nursing homes, in The New York Times. 2020. [Google Scholar]
- 2.Woolf SH, et al. , Excess Deaths From COVID-19 and Other Causes, March-July 2020. JAMA, 2020. 324(15): p. 1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englund W, Nursing homes say the ‘treat in place.’ Then came covid-19. Why did so many nursing home residents die of covid-19 on site, and not in hospitals?, in The Washington Post. 2020. [Google Scholar]
- 4.Dening KH, Jones L, and Sampson EL, Preferences for end-of-life care: a nominal group study of people with dementia and their family carers. Palliat Med, 2013. 27(5): p. 409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison Dening K, et al. , Advance Care Planning in Dementia: Do Family Carers Know the Treatment Preferences of People with Early Dementia? PLoS One, 2016. 11(7): p. e0159056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell SL, et al. , Level of Care Preferences Among Nursing Home Residents With Advanced Dementia. J Pain Symptom Manage, 2017. 54(3): p. 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulqueen K. and Coffey A, Preferences of residents with dementia for end of life care. Nurs Older People, 2017. 29(2): p. 26–30. [DOI] [PubMed] [Google Scholar]
- 8.Sellars M, et al. , Perspectives of people with dementia and carers on advance care planning and end-of-life care: A systematic review and thematic synthesis of qualitative studies. Palliat Med, 2019. 33(3): p. 274–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antommaria AHM, et al. , Ventilator Triage Policies During the COVID-19 Pandemic at U.S. Hospitals Associated With Members of the Association of Bioethics Program Directors. Ann Intern Med, 2020. 173(3): p. 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piscitello GM, et al. , Variation in Ventilator Allocation Guidelines by US State During the Coronavirus Disease 2019 Pandemic: A Systematic Review. JAMA Netw Open, 2020. 3(6): p. e2012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelen J, et al. , Patient Triage and U.S. Ventilator Allocation Policies in Anticipation of the COVID-19 Surge. Health Security [under review], 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard M. COVID-19 crisis in Los Angeles: Why activating ‘crisis standards of care’ is crucial for overwhelmed hospitals. 2021. January 6; Available from: https://theconversation.com/covid-19-crisis-in-los-angeles-why-activating-crisis-standards-of-care-is-crucial-for-overwhelmed-hospitals-152706. [Google Scholar]
- 13.Kisner J, What the chaos in hospitals is doing to doctors: Politicians refusal to admit when hospitals are overwhelmed puts a terrible burden on health-care providers, in The Atlantic. 2021. [Google Scholar]
- 14.Stevenson EK, et al. , Association between Do Not Resuscitate/Do Not Intubate Status and Resident Physician Decision-making. A National Survey. Ann Am Thorac Soc, 2017. 14(4): p. 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson W, et al. Hospital-based prognosis and goals of care discussions with seriously ill patients: A pathway to integrate a key primary palliative care process into the workflow of hospitalist physicians and their teams. 2017; Available from: https://www.hospitalmedicine.org/globalassets/clinical-topics/clinical-pdf/ctr-17-0031-serious-illness-toolkit-m1.pdf.
- 16.Haliko S, et al. , Hospital-Based Physicians’ Intubation Decisions and Associated Mental Models when Managing a Critically and Terminally Ill Older Patient. Med Decis Making, 2018. 38(3): p. 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnato AE, et al. , Use of Advance Care Planning Billing Codes for Hospitalized Older Adults at High Risk of Dying: A National Observational Study. J Hosp Med, 2019. 14(4): p. 229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan D, et al. , A New Standard for Advance Care Planning (ACP) Conversations in the Hospital: Results from a Delphi Panel. J Gen Intern Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkey AJ, et al. , Hospital Variation in Do-Not-Resuscitate Orders and End-of-Life Healthcare Use in the United States. Ann Am Thorac Soc, 2017. 14(9): p. 1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walkey AJ, et al. , Hospital Variation in Utilization of Life-Sustaining Treatments among Patients with Do Not Resuscitate Orders. Health Serv Res, 2018. 53(3): p. 1644–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardona-Morrell M, et al. , Non-beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. Int J Qual Health Care, 2016. 28(4): p. 456–69. [DOI] [PubMed] [Google Scholar]
- 22.Torke AM, et al. , Timing of do-not-resuscitate orders for hospitalized older adults who require a surrogate decision-maker. J Am Geriatr Soc, 2011. 59(7): p. 1326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson K, et al. , Limitation of Life-Sustaining Care in the Critically Ill: A Systematic Review of the Literature. J Hosp Med, 2019. 14(5): p. 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock BD, et al. , Association of Do-Not-Resuscitate Patient Case Mix With Publicly Reported Risk-Standardized Hospital Mortality and Readmission Rates. JAMA Netw Open, 2020. 3(7): p. e2010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener RS and Barnato AE, Implications of Including Hospital Do-Not-Resuscitate Rates in Risk Adjustment for Pay-for-Performance Programs. JAMA Netw Open, 2020. 3(7): p. e2010915. [DOI] [PubMed] [Google Scholar]
- 26.Blackhall LJ, Must we always use CPR? N Engl J Med, 1987. 317(20): p. 1281–5. [DOI] [PubMed] [Google Scholar]
- 27.Sacks OA, et al. , Advance Care Planning and Professional Satisfaction From “Doing the Right Thing”: Interviews With Hospitalist Chiefs. J Pain Symptom Manage, 2020. 60(4): p. e31–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernacki R, et al. , Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open, 2015. 5(10): p. e009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried TR, et al. , Understanding the treatment preferences of seriously ill patients. N Engl J Med, 2002. 346(14): p. 1061–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Risk-adjusted ACP, treatment intensity (ICU and MV), DNR status, and mortality among hospitalized dementia patients with COVID-19 March-June 2020, compared to hospitalized non-dementia patients, by tercile of hospital ACP billing frequency (low, medium, high)