Abstract
Transforming growth factor β (TGFβ) is a pleiotropic cytokine that plays critical roles to define cancer cell phenotypes, construct the tumor microenvironment, and suppress anti-tumor immune responses. As such, TGFβ is a lynchpin for integrating cancer cell intrinsic pathways and communication among host cells in the tumor and beyond that together affect responses to genotoxic, targeted, and immune therapy. Despite decades of preclinical and clinical studies, evidence of clinical benefit from targeting TGFβ in cancer remains elusive. Here, we review the mechanisms by which TGFβ acts to oppose successful cancer therapy, the reported prognostic and predictive value of TGFβ biomarkers, and the potential impact of inhibiting TGFβ in precision oncology. Paradoxically, the diverse mechanisms by which TGFβ impedes therapeutic response are a principal barrier to implementing TGFβ inhibitors because it is unclear which TGFβ mechanism is functional in which patient. Companion diagnostic tools and specific biomarkers of TGFβ targeted biology will be the key to exploiting TGFβ biology for patient benefit.
Keywords: biomarker, TGFβ signaling, cancer precision medicine, genotoxic therapy, immunotherapy, cancer prognosis
Introduction
Although tumor histology is commonly used to assign cancer stage and anatomical origin, it is widely recognized that patient outcomes and tumor response to therapy within these classifications can be dramatically different. Heterogeneous tumor cell-intrinsic genomic and molecular features interact with the complex tumor microenvironment (TME), consisting of immune cells, vasculature, fibroblasts, cytokines, and the extracellular matrix, to define the overall clinical behavior of cancer. This heterogeneity culminates in differential therapeutic responses that clearly challenge the “one size fits all” approach for many standard cancer treatments.
Transforming growth factor β (TGFβ) is often described as a pleiotropic cytokine because its myriad roles during development and homeostasis include stem cell regulation, cell fate determination, phenotypic differentiation, and proliferative potential in cells of endoderm, ectoderm, and mesoderm origin (1–3). In cancer, TGFβ has two faces: as a canonical tumor suppressor in healthy tissues, it exerts profound control of cell cycle and proliferation, but converts to a pro-tumorigenic factor during carcinogenesis during which it influences multiple aspects of tumor development (4–6).
TGFβ signaling is actively involved in tumor progression through modifying the TME, suppressing the immune response, promoting metastasis, and vascular remodeling. Indeed, both the adaptive and innate immune system are affected by TGFβ; TGFβ reduces cytotoxic activity of CD8+ T cells, promotes suppressive regulatory T cells, and broadly modulates activities of natural killer cells and macrophages. Moreover, TGFβ signaling regulates tumor cell-intrinsic phenotype switching, including epithelial-mesenchymal transition that contributes to cancer invasion and metastasis. Thus, TGFβ signaling regulates a network of interconnected signaling pathways to organize tumor components that outmaneuver anti-tumor defenses, all of which have been thoroughly reviewed recently (6). Given the broad effects of TGFβ on cancer cells, the TME and the anti-tumor immune response, drugs that inhibit TGFβ activity are postulated to improve therapeutic outcomes (5); nonetheless, a clinical strategy for TGFβ inhibition in cancer has yet to be realized (as reviewed in (7).
Clinical trials with agents that inhibit TGFβ are currently in phase I/II trials in which the desired biological effects range from greater growth control, decreased metastatic spread, better immune response, or synergism with standard of care therapy (7). Here, we briefly review the TGFβ regulation, elements of the signaling cascade, and then focus on mechanisms by which TGFβ opposes effective cancer therapy.
TGFβ regulation
In brief, mammalian TGFβ is represented by three isoforms, TGFβ 1, 2 and 3, with similar mechanisms of regulation, of which TGFβ1 is the best characterized. The TGFB1 gene encodes a single polypeptide that is cleaved and processed to form the 24kD TGFβ homodimer and the 80kD latency-associated peptide (LAP) homodimer that are non-covalently associated to form the small latent TGFβ complex. Latent TGFβ often is covalently linked to one of four latent TGFβ binding proteins, which together form the large latent complex. The secreted complexes are highly abundant in the extracellular matrix, which serves as a reservoir. Extracellular activation releases TGFβ from these latent complexes and is required for biological activity. Activation of TGFβ from the latent complex is highly controlled in normal tissues but dysregulated in cancer (3). Many factors have been identified to activate TGFβ, including proteases, integrins, pH changes, and reactive oxygen species (8–11).
TGFβ transmits signals through canonical or non-canonical pathways. TGFβ canonical signaling is initiated by binding of TGFβ ligands to the type 2 TGFβ receptor (TβRII), which causes recruitment and phosphorylation of type 1 TGFβ receptor (TβRI). Both receptors contain a cytoplasmic domain with serine/threonine protein kinase activity. The type 3 TGFβ receptor (TβRIII), betaglycan, is a non-signaling co-receptor that may increase binding of ligands to TβRII (12). Ligand binding and recruitment of TβRI activates its kinase that phosphorylates the carboxy-terminal serine residue of SMAD2 (SMAD Family Member 2) or SMAD3 (SMAD Family Member 3), which induces oligomerization of SMAD2 or SMAD3 with SMAD4 (SMAD Family Member 4), resulting in nuclear translocation for transcriptional activation or repression of target genes. SMAD4 is the only known common-mediator SMAD. However, canonical signaling undergoes modulation via feedback of multiple other mechanisms. For example, TGFβ induces SMAD7 (SMAD Family Member 7) expression, whose protein product in turn inhibits TGFβ signaling by blocking interactions between SMAD2/3 and activated TGFβ receptors, while E3-ubiqutin ligases SMURF1 and SMURF2 induce proteasomal degradation of TGFβ receptors. TGFβ also transmits signals through SMAD-independent non-canonical pathways that regulate immune surveillance and inflammation via the interferon response, cell survival and proliferation via MAPK/ERK and PI3K/Akt signaling, as well as cell migration via the RHO/ROCK pathway (3).
TGFβ signaling modulates the response to cancer therapy
Extensive studies have demonstrated that TGFβ signaling is also important in determining tumor response to chemotherapy via its actions in both tumor cells and the TME (13–17). By studying 17 matched tumor biopsies before and after neoadjuvant chemotherapy in triple-negative breast cancer, researchers found that a TGFβ-responsive gene signature and the proportion of cancer stem-like cells were enriched after therapy (18). In addition, paclitaxel, a chemotherapeutic agent that acts on microtubules, induced high autocrine TGFβ signaling and tumor-initiating capacity in breast cancer cell lines and xenografts, while TGFβ inhibitors abrogated this effect. Pronounced TGFβ signaling is also associated with reduced chemo-sensitivity in gynecologic cancers (19).
In head and neck squamous cell carcinoma (HNSC), TGFβ signaling plays a significant role in maintaining quiescence of tumor propagating cancer cells by regulating cell cycle gene transcription to control a reversible G1 cell-cycle arrest. This TGFβ signaling-related quiescence is an innate drug resistance mechanism that protects a subset of HNSC cells from chemotherapy in HNSC (13). The authors analyzed transcriptomes of 12 human papillomavirus (HPV)-negative HNSC patients with either a complete response (6 patients) or resistance (6 patients) to carboplatin or cisplatin followed by paclitaxel and found that 3316 genes were differentially expressed. They further identified TGFβ signaling as one of the most significantly activated pathways in resistant HNSC by upstream regulator analyses of the 3316 genes. HNSC cell lines also showed differential sensitivity to cisplatin (16). In colorectal cancer response to combined treatment with fluorouracil and oxaliplatin, TGFβ2-mediated signaling originating from cancer-associated fibroblasts resulted in increased stemness and intrinsic resistance to chemotherapy (20). Relapsed tumors after chemotherapy showed significantly higher expression of TβRII by immunohistochemistry compared to those without recurrence.
HER2 is a receptor tyrosine kinase that is overexpressed in approximately 20% of invasive breast cancers. The development of drug resistance to HER2 biologics such as trastuzumab remain an issue. Interestingly, continuous stimulation of HER2-positive breast cancer cells with TGFβ induces resistance to anti-HER2 therapy (21). Consistent with this, Wang et al. found that TGFβ-activated phosphatidylinositol-3 kinase/Akt signaling leads to enhanced survival and migration in HER2-positive breast cancer (22). The authors further developed an active TβRI expression signature and interrogated 22 patients with HER2-overexpressing tumors treated with neoadjuvant trastuzumab and vinorelbine. The results showed that patients achieving a complete response after treatment had tumors in which TGFβ signaling was less active based on low expression levels of the signature. Furthermore, nuclear SMAD3 expression, a marker of active canonical TGFβ signaling, was significantly associated with incomplete response in breast cancer patients receiving paclitaxel with concurrent trastuzumab, indicating that TGFβ signaling may predict response to anti-HER2 therapy (21).
The most frequently used anti-tumor treatment modalities are radiotherapy and DNA-damage inducing chemotherapy. Therapeutic control by genotoxic radio-chemotherapy is determined by the degree and type of DNA damage inflicted and the cancer cells’ intrinsic capacity to repair or tolerate that damage. Approximately two-thirds of all cancer patients receive radiotherapy, which kills tumor cells by generating DNA damage leading to DNA breaks, mutagenesis and mitotic catastrophe leading to replicative cell death (23,24). Ionizing radiation efficiently induces TGFβ activation (25), apparently activating a redox switch in the latent complex (26). Addition of TGFβ neutralizing antibody treatment to radiotherapy increases tumor control in preclinical models of breast, brain, lung cancer (27–29). Moreover, TGFβ drives fibrosis, a late tissue effect of radiotherapy, thus TGFβ inhibition could protect normal tissue and hence increase the therapeutic window (30). Radiation-induced TGFβ also blocks immunological response to radiation and synergies with immunotherapy (31). Together, these mechanisms implicate TGFβ as a barrier to the therapeutic goals of radiotherapy.
Clearly TGFβ signaling can affect the response to cancer therapy through several mechanisms, but it is its critical role in DNA damage repair (DDR) that is least appreciated. The first evidence of a role for TGFβ in genome integrity was reported by Glick et al. 25 years ago. Mouse keratinocytes with a targeted deletion of the Tgfb1 gene were highly unstable as measured by gene amplification compared to control keratinocytes (32). Subsequent studies blocking TGFβ signaling in human epithelial cells also elicited genomic instability (33). Kirshner et al. demonstrated that inhibition of TGFβ signaling attenuates kinase activity of ataxia telangiectasia (ATM), a master regulator of the DDR (34). Fanconi anemia, an inherited DNA repair disorder characterized by progressive bone marrow failure from hematopoietic stem and progenitor cell attrition can be rescued by TGFβ inhibition (35), indicating that TGFβ impacts multiple levels of DDR regulation. An adaptor protein, transforming growth factor β/mothers against decapentaplegic homolog 3 adaptor β2-Spectrin (β2SP, gene Sptbn1) is also implicated in DNA repair through β2SP-dependent activation of Fanconi anemia complementation group D2 (36).
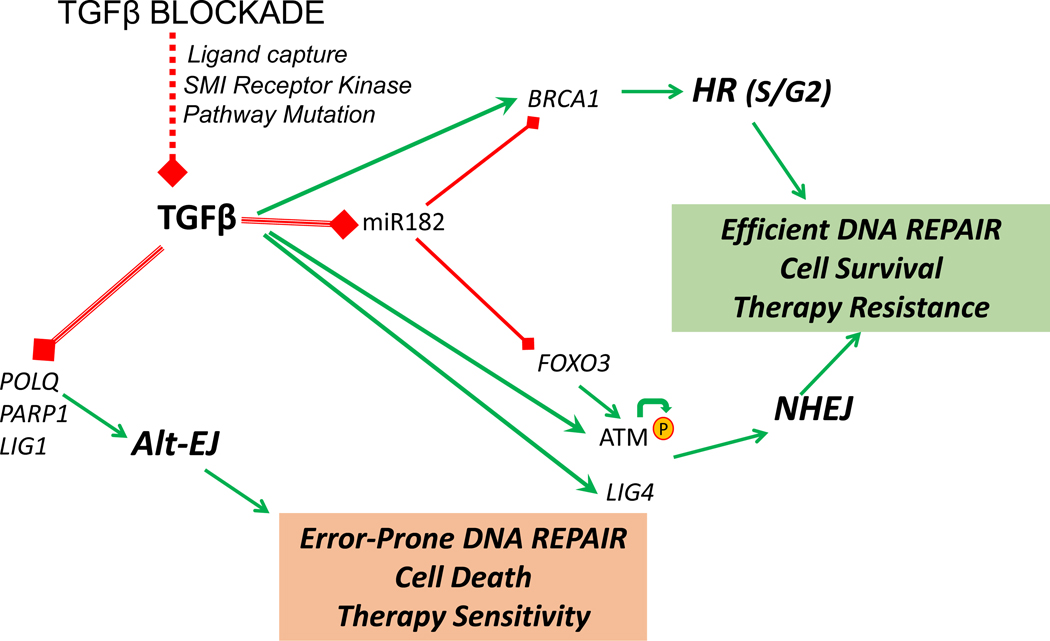
Over the last decade, TGFβ has been shown to regulate the function of ATM and BRCA1 (Breast And Ovarian Cancer Susceptibility Protein 1), and expression of ligase IV (LIG4), polymerase theta (POLQ) and poly(ADP-ribose) polymerase 1 (PARP1) (16,29,37,38)(Fig. 1). Together, these multiple targets indicate that the efficacy of TGFβ signaling should impact DNA repair via its regulation of key DDR components. Consistent with this, blocking TGFβ signaling compromises DNA repair and increases cell kill by genotoxic therapies such as radiation and platinum chemotherapy (39,40).
Figure 1. TGFβ regulates DDR pathways for effective DNA repair.
TGFβ promotes (green) HR and NHEJ in part by suppressing (red) miR-182, which causes es BRCA1 and FOXO3 degradation; FOXO3 is necessary for ATM autophosphorylation. In addition, TGFβ increases LIGIV, which together with its effects on ATM and BRCA1, promotes HR and NHEJ. TGFβ also suppresses genes POLQ, LIG1 and PARP1 that are necessary for alt-EJ. Hence the sum of TGFβ biology is to endorse efficient DNA repair and suppress error-prone repair, which together increases cell survival in response to DNA damage that is reflected in resistance to genotoxic therapy. When TGFβ signaling is compromised (red dotted line), the use of HR and NHEJ decrease and alt-EJ increases, leading to less viable cells and greater sensitivity to genotoxic therapy.
Perhaps the most compelling evidence of TGFβ’s role in DDR comes from human papilloma virus (HPV)-associated HNSC. The survival of patients with HPV-positive cancer treated with radio-chemotherapy is more than double that of HPV-negative patients (41). One of the actions of HPV viral proteins is to target TGFβ signaling components for degradation (42), which attenuates canonical TGFβ signaling (43). We found that loss of TGFβ signaling compromises the canonical DNA double strand break repair pathways homologous recombination (HR) and non-homologous end-joining (NHEJ), and that pharmaceutical TGFβ inhibition in HPV-negative cancer cells replicates the DNA repair defects exhibited by HPV-positive cancer cells and tumors to increase radioresponse (16). These data lead us to hypothesize that loss of TGFβ signaling creates specific DNA damage deficits that are exploitable within the current cancer therapy repertoire (44).
The key role that TGFβ plays in control of DDR in HNSC is generalizable in multiple other cancer types in which HPV is not involved in tumorigenesis. Indeed, multiple preclinical models show that TGFβ inhibition increases the response to ionizing radiation. In breast cancer, inhibition of TGFβ signaling by either small molecule receptor kinase inhibitors or TGFβ neutralizing antibodies, which was confirmed by suppression of SMAD2 phosphorylation, were able to radiosensitize cancer cells, as demonstrated by clonogenic cell survival assays, and increase tumor growth delay following single or fractionated radiation exposure (27). Furthermore, hormone-refractory prostate cancer cells that express more TGFβ1 are radioresistant compared to hormone-sensitive cells (45). Inhibition of TGFβ signaling also sensitized glioblastoma to radiation (28,46,47) and abolished the radioresistance of glioma-initiating cells (28).
Immunotherapy harnesses the adaptive immune system to target tumor tissue and has achieved significant success in the treatment of several tumor types. Clinically approved immune checkpoint inhibitors include antibodies against CTLA-4 or PD-1/PD-L1. However, therapeutic responses only occur in around 20–40% of patients with certain cancers (48,49). One of the biggest challenges has been to identify biomarkers that predict response to immunotherapy. TGFβ plays a critical role in the immune response and evasion mechanisms (6,50). Notably, TGFβ signaling limits T cell infiltration of tumors and anti-tumor immunity (6). TGFβ is also a fundamental regulator of the immune response following radiation. Indeed, the responsiveness of CD8+ cytotoxic T cells to endogenous tumor antigens was activated after TGFβ neutralization in combination with radiation, resulting in control of non-irradiated tumors and decreased lung metastases in mouse models through an abscopal effect (31). Thus, there is mounting evidence that TGFβ inhibition could augment the response to immunotherapy.
But as cancer and TGFβ biology is complex, it is likely that TGFβ biology is not universally tumor promoting. A comprehensive study of TGFβ inhibition in 12 immunocompetent breast cancer models reveals that TGFβ tumor-suppressive activities can be retained in advanced disease (51). Here, the study design was to measure how systemic TGFβ neutralizing antibodies affect lung metastasis post resection. TGFβ inhibition had no effect or suppressed metastasis in most (75%) tumor models but stimulated metastasis in 25%, apparently by increasing cancer stem cells. These data underscore the need for patient selection in clinical applications.
Taken together, preclinical studies in multiple cancer types have clearly demonstrated that TGFβ signaling is strongly associated the tumor composition and behaviors, resistance to chemotherapy, radiation and immunotherapy response, suggesting that biomarkers related to TGFβ signaling will be important in prognosis and to predict the therapeutic response.
Biomarkers for predicting response to therapy
Biomarkers may fall into 5 categories: diagnostic, prognostic, predictive, therapeutic, and functional. A diagnostic biomarker is usually used for cancer detection. A prognostic biomarker gives information about the potential outcome of the patient based on current capabilities in medical care. A predictive biomarker projects therapeutic response to a specific therapy. A therapeutic biomarker may be a drug target protein to determine treatment decision with the drug. A functional biomarker allows evaluating a biological function related to a specific therapy. Note that one biomarker may be useful in more than one category. The correlation of TGFβ expression or indices of signaling are correlated with poor prognosis in multiple cancer types (Table 1).
Table 1.
TGFβ signaling biomarkers associated with prognosis
| Biomarker | Detection | Specimens (n) | Cancer | Outcome | Reference |
|---|---|---|---|---|---|
|
| |||||
| TGFβ1 | IHC | 81 | Breast cancer | Invasiveness | Walker et al. 1992 (56) |
| TGFβ1 | IHC | 57 | Breast cancer | PFS | Gorsch et al. 1992 (57) |
| TGFβ1 | IHC | 34 | CRC | Metastasis | Friedman et al. 1995 (74) |
| TGFβ1 mRNA, plasma | Northern Hybridization, ELISA | 22 | CRC | Progression | Tsushima et al. 1996 (100) |
| TGFβ1,TβRI, TβRII | IHC | 73 | Prostate cancer | OS | Wikstrom et al. 1998 (77) |
| TGFβ1 serum | ELISA | 74 | Breast cancer | Invasiveness | Sheen-Chen et al. 2001 (101) |
| TGFβ1 plasma | ELISA | 302 | Prostate cancer | PFS | Shariat et al. 2004 (78) |
| TβRI, TβRII | IHC | 246 | Breast cancer | OS | Buck et al. 2004 (73) |
| TGFβ1 tumor cytosol | ELISA | 193 | Breast cancer | PFS | Desruisseau et al. 2006 (59) |
| TGFβ2 plasma | ELISA | 21 | Glioblastoma | OS | Schneider et al. 2006 (80) |
| pSMAD2 | IHC | 52 | Glioma | PFS, OS | Bruna et al. 2007 (79) |
| TβRI signature | Microarray | 295 | Breast cancer | PFS, OS | Wang et al. 2008 (22) |
| pSMAD2 | IHC | 135 | Gastric Carcinoma | Metastasis, OS | Shinto et al. 2010 (85) |
| TGFβ1 mRNA, TGFβ signature | Affymetrix | 343 | CRC | PSF | Calon et al. 2012 (75) |
| TβRI, TβRII, pSMAD2 | IHC | 574 | Breast cancer | PFS | de Kruijf et al. 2013 (102) |
| pSMAD2 | IHC | 78 | NSCLC | OS | Chen et al. 2014 (103) |
| TGFβ1 plasma | ELISA | 644 | Pancreatic Cancer | OS | Javle et al. 2014 (86) |
| fibroblast TβRII | IHC | 252 | Breast cancer | PFS | Busch et al. 2015 (95) |
| TGFβ | IHC | 407 | Liver cancer | PFS, OS | Huang et al. 2017 (81) |
| TGFβ signature | TCGA | 279 | HNSC | OS | Liu et al. 2018 (16) |
Predictive biomarkers are rapidly emerging and often approved together with specific therapies. For example, DDR deficits represent an exploitable vulnerability in cancer that are often empirically revealed by trial and error, e.g., use of cisplatin in HNSC. Recognition that the nature of specific DDR deficits can be the basis for selection of treatment led to the concept of synthetic lethality. The canonical example is synthetic lethality from PARP inhibitors in the context of homologous recombination (HR) deficits originally defined for germline BRCA mutations in breast and ovarian cancer patients. The utility of PARP inhibitors has been expanded to cancers with somatic mutations in BRCA1/2, or other HR components, defined as HR deficit phenotype, but patients with such tumors also respond well to standard of care inter-strand crosslinking platinum therapies (52). Indeed, patient selection for clinical trials for PARP inhibitors currently includes prior response to platinum agents, genomic scars (i.e., sequence alterations), and mutations in genes that are necessary for homologous recombination (53). Although some benefit is expected to occur in all patients treated with chemoradiation, the clinical outcome for each patient is variable (24). This suggests that individual tumors have different capacities for DDR that represent exploitable vulnerabilities (54).
Likewise, biomarkers that predict response to immunotherapy are of significant interest. The therapeutic efficacy of immune checkpoint inhibitors that target PD-1/PD-L1 is generally expected to be associated with the expression levels of their targets, as measured by immunohistochemistry (IHC), but the value of PD-(L)1 IHC as biomarkers has been controversial; additional biomarkers associated with response to immunotherapy include microsatellite instability, which is caused by mismatch repair defects, and mutational burden (55). Given its immunosuppressive effects, TGFβ activity in cancer is widely thought to oppose effective immunotherapy response, which underscores the potential clinical value of a robust TGFβ biomarker.
Thus, a TGFβ biomarker may be prognostic without reference to mechanism or predictive if it either reflect specific targeted activity in a cancer (e.g., immunosuppression, metastasis) or estimates vulnerability to a therapeutically targeted process like DDR.
Three decades ago, Walker and Dearing reported that TGFβ1 expression detected by IHC associates with invasion and metastasis in breast cancer (56), whereas Gorsch et al. reported that higher TGFβ1 IHC expression, but not that of TGFβ2 and TGFβ3, associates with shorter PFS (57). Multivariate analysis suggested that TGFβ1 IHC was significantly correlated with disease progression independently of established prognostic variables. Since that time, different markers of TGFβ abundance or signaling have been associated with cancer prognosis (Table 2).
Table 2.
TGFβ biomarkers associated with response to therapy
| Biomarker | Detection | Cancer | Therapy | Outcome | Reference |
|---|---|---|---|---|---|
|
| |||||
| TGFβ1 | IHC, qRT-RCR | Prostate cancer | IR | Tumor sensitivity | Wu et al. 2015 (104) |
| pSMAD2 | IF | HNSC | IR | Cell sensitivity | Liu et al. 2018 (16) |
| pSMAD2 | Western blot | Breast cancer | TβR inhibition, IR | Cell sensitivity | Bouquet et al.2011 (27) |
| serum TGFβ1 | ELISA | Cervical Carcinoma | Cisplatin-based chemoradiation | Tumor response | Yang et al. 2006 (105) |
| TGFβ signature | NanoString analysis | Breast cancer | Paclitaxel, docetaxel | Proportion of stem cells | Bhola et al. 2013 (18) |
| Nuclear SMAD3 | IHC | Breast cancer | Paclitaxel, trastuzumab | Pathological response | Chihara et al. 2017 (21) |
| TGFβ related mRNA | Gene sequencing | HNSC | Carboplatin or cisplatin followed by paclitaxel | Tumor response | Brown et al. 2017 (13) |
| TGFβ2 | IHC | CRC | Oxaliplatin-based treatment | Disease free survival | Tang et al. 2018 (20)] |
| Active TβRI signature | Microarray | Breast cancer | Trastuzumab, vinorelbine | Pathological response | Wang et al. 2008 (22) |
| TGFβ secretion | ELISA | HNSC | Cetuximab | Cell toxicity | Bedi et al 2012 (106) |
| TGFB1 | RNA sequencing | Urothelial cancer | anti-PD-L1 and standard-of-care | Tumor response | Mariathasan et al. 2018 (87) |
| TGFβ signature | RNA sequencing | Urothelial cancer | anti-PD-L1 and standard-of-care | Tumor response | Mariathasan et al. 2018 (87) |
Circulating TGFβ has been correlated with clinical outcomes in patients whose cancer is in the prostate (58), breast (59), colorectal (60,61), lung (60), liver (62), gastric (63), head and neck (64), bladder (65) and the central nervous system (66). Serum TGFβ levels associate with breast cancer stages (67). Higher serum levels of TGFβ1 correlated with more advanced lymph node status, more advanced stage, and more aggressive histologic grade. Multivariate analysis revealed that staging was an independent factor related to higher serum levels of TGFβ1.
Although blood is an attractive because it is accessible and can be measured iteratively during the course of disease or therapy, the reported measurements of circulating TGFβ protein has been inconsistent. The systemic half-life of active TGFβ1 is a matter of minutes (68). However, latent TGFβ is stored in platelets and is released upon degranulation (69); thus clotting during specimen prep can lead to spurious measurements (70,71). The relationship between short-lived active TGFβ and abundant latent TGFβ may give rise to differences due to assays or specimen preparation. Many studies employed immunoassays based on antibodies that specifically detect active TGFβ that a priori requires exogenous activation (e.g., temperatures that exceed −80°C or pH below 3), which is also is a source of additional technical discrepancies. A means to circumvent these confounders is to measure the latent complex using antibodies to LAP, which also provides discrimination of the isoforms, thereby avoiding the necessity of exogenous activation. Use of plasma rather than serum and inclusion of a control for platelet activation to assess specimen quality provides a more robust assay of circulating TGFβ levels (72).
TGFβ1 protein levels by enzyme-linked immunosorbent assay (ELISA) from 193 breast tumor samples found that TGFβ1 is an independent prognostic biomarker in which higher TGFβ1 levels correlated with shorter disease-free survival, especially for lymph node-negative patients (59). Buck et al. analyzed TβRI and TβRII expression in 246 breast cancer patients retrospectively and found that TβRII is an independent prognostic biomarker for overall survival (OS) in estrogen receptor-negative breast cancer patients. In this cohort, low expression of TβRII was associated strongly with favorable prognosis, tumor size and nodal status, and weakly with OS (73). Wang et al. used a 271 gene active TGFβ type I receptor signature to interrogate 295 breast cancers and found active TGFβ signaling correlated with poor clinical outcome (22).
TGFβ signaling in colorectal cancer (CRC) showed a similar correlation with prognosis. In a case-control study of patients with progressive colon cancer vs. patients in remission, high TGFβ1 protein expression but not that of TGFβ2 or TGFβ3, measured in the primary site correlated with disease progression to metastasis. This effect was independent of lymph node status and the degree of differentiation of the primary tumor (74). Colorectal cancers with high TGFβ1 protein levels were 18 times more likely to recur than those exhibiting low levels of TGFβ1. Calon et al. discovered that TGFβ signaling promotes colorectal cancer metastasis through activation of cancer-associated fibroblasts (75). By examining a colorectal cancer cohort of 345 cases, the authors sub-grouped tumors with high, medium, or low TGFB1 expression, and found that only patients with medium or high TGFB1 expression in the primary tumors suffered cancer recurrence during 10 years of follow-up; by comparison, all patients with low TGFB1-expressing tumors remained disease-free. The researchers in this study also established TGFβ-induced gene expression programs for 4 types of stroma cells, in which TGFβ signaling in T cells, macrophages, and fibroblasts inversely correlated with relapse-free survival, suggesting poor prognosis with high stroma TGFβ signaling. Another study also confirmed that a gene program induced by TGFβ in tumor stromal cells was associated with CRC subtypes that have a poor prognosis (76).
A retrospective study showed that overproduction of TGFβ1 in prostate cancer is associated with tumor grade, metastasis, and shorter median survival (5 vs. 10 years) compared with lower levels of TGFβ1 immunoreactivity (77). Interestingly, patients with loss of tumor TGFβ type II receptor expression in concert with TGFβ1 overproduction showed particularly short survival (2.6 vs. 10 years) (77). Shariat et al. also reported that postoperative plasma TGFβ1 levels are a significant predictor of OS and aggressive disease progression (78). In the 302 patients undergoing radical prostatectomy, preoperative plasma TGFβ1 was associated with metastases to regional lymph nodes and disease progression.
In glioma patients, high canonical TGFβ signaling is associated with aggressive clinical behavior and confers poor prognosis (79). In this study, 52 patient glioma biopsies were analyzed for nuclear pSMAD2 expression. Patients whose tumors had low levels of pSMAD2 behaved significantly better with regards to both PFS and OS, indicating that a hyperactive canonical TGFβ signaling pathway is a poor prognostic factor. Schneider et al. examined plasma TGFβ1 and TGFβ2 in 21 glioblastoma patients before and after extensive resection and found that TGFβ2 levels prior to surgery correlated with patient survival, while the difference of latent TGFβ2 levels prior to surgery minus the TGFβ2 concentration 7 days after surgery strongly correlated with patient survival (80).
Several studies also highlight the prognostic value of TGFβ activity for other cancer types. In hepatocellular carcinoma, Huang et al. demonstrated that TGFβ is a poor prognostic factor due to its inhibitory effects on CD8+ cytotoxic T cells (81). They examined TGFβ expression in 407 hepatocellular carcinoma patients and found that lower TGFβ expression was associated with better OS and recurrence-free survival, with more CD8+ T cells seen in tumors with low expression of TGFβ. In non-small cell lung cancer (NSCLC), Chen et al. examined phospho-SMAD2 expression in 78 surgical resection specimens and found that active TGFβ signaling in cancer-associated fibroblasts is associated with poor OS (82). IHC analysis of 205 NSCLC tumors also revealed that high TGFβ1 tumor expression correlated with poor prognosis (83). Consistent with this evidence, a meta-analysis of lung cancer studies confirmed that high TGFβ expression in tumors was an indicator of poor survival (84). Shinto et al. showed that 47% of 135 gastric carcinomas had high pSMAD2 expression, which correlated with peritoneal and lymph node metastasis (85). In another study, researchers evaluated plasma level of TGFβ1 in 644 patients, and found that high TGFβ1 was associated with reduced survival (86).
The status of TGFβ signaling may predict response to immune checkpoint inhibitors. Mariathasan et al. analysis of RNA sequencing data from pre-treatment tumor samples from a phase 2 clinical trial testing anti-PD-L1 effects with atezolizumab in metastatic urothelial cancer and found that genes involved in the TGFβ signaling pathway associated with lack of response (87). Two top scoring TGFβ pathway genes, TGFB1 and TGFBR2, showed increased expression in non-responders with reduced OS. Furthermore, they found TGFβ was associated with lack of response in tumors with the excluded immune phenotype. Nevertheless, concurrent TGFβ inhibition and anti-PD-L1 treatment increased T cell infiltration and provoked anti-tumor immunity with tumor regression. Another study based on a pan-cancer extracellular matrix dysregulation gene signature, revealed that a higher TGFβ-driven signature identifies a poor prognosis phenotype with cancer-associated fibroblast activation (88). The TGFβ-associated extracellular matrix program also correlated with checkpoint inhibitor failure.
TGFβ opposition to anti-tumor immunity following radiotherapy was tested in metastatic breast cancer patients by combining a humanized TGFβ neutralizing monoclonal antibody, fresolimumab, with focal fractionated radiotherapy to individual metastatic lesions (89). This phase 1 trial in which patients were randomized to 1 mg/kg vs 10 mg/kg fresolimumab met the criteria for feasibility. Notably, patients receiving the higher fresolimumab dose had a favorable systemic immune response, indicated by lower peripheral blood mononuclear cell counts and a striking boost in the CD8 central memory pool. Moreover, the median overall survival of patients who received the high dose of fresolimumab was significantly longer, i.e., double, that of patients who received the low dose. As irradiation of one or two lesions would not be expected to increase survival, this trial suggests that TGFβ inhibition synergized to elicit a systemic response.
Our group showed that TGFβ endorses effective DNA repair via canonical repair by HR and NHEJ and actively suppresses error-prone repair by alternative end-joining (alt-EJ), which is less accurate(16,90). Alt-EJ also called microhomology mediated end-joining, is a vulnerability of cancers that can be exploited (91). To interrogate whether TGFβ control of DDR affects cancer outcomes, we analyzed The Cancer Genome Atlas (TCGA) using a chronic TGFβ pathway activity gene expression signature and a curated alt-EJ signature. Consistent with TGFβ functional control of alt-EJ, the two signatures were significantly negatively correlated. More intriguingly, this was evident in 16 out of 17 solid cancer types, supporting the hypothesis that cancers that are unresponsiveness to TGFβ shift the DDR to inefficient alt-EJ, as found in HPV-positive HNSC (16). To evaluate whether this relationship affected outcomes in patients treated with genotoxic therapy, patients were stratified according to the degree of anti-correlation using a score called βAlt. Glioblastoma, ovarian carcinoma and lung squamous cell carcinoma patients whose tumors scored high for βAlt (i.e., low TGFβ and high alt-EJ) had significantly better outcomes in response to genotoxic treatment. Moreover, high βAlt correlated with better OS in a pan-cancer cohort treated with radiotherapy or chemotherapy (38).
Although most studies demonstrate that elevated TGFβ signaling correlates with poor prognosis, a few studies are conflicting (92–95). For example, Auvinen et al. reported that, based on the IHC of 273 breast cancer biopsies, the expression of TGFβ1 and TGFβ2 were both uniformly related to some favorable prognostic factors, while the expression of TGFβ2 without the expression of TGFβ1 was related to favorable disease outcome (92). Another study with 167 breast cancer patients showed that high TGFβ1 mRNA expression was inversely correlated with lymph node status and associated with longer disease-free interval, although the relapse-free survival was not increased (93). Analysis of TGFB1 and TBRII transcript and respective protein expression in 324 breast cancer sections indicated that absence of TGFβ1 and TβRII proteins was significantly associated with metastasis (94). They also showed that in multivariate analysis, TGFβ1-positive tumors were associated with increased disease-free survival, while TβRII-positive tumors were associated with increased disease-free survival and OS in human epidermal growth factor receptor-2 (HER2)-negative patients. However, TβRII expression in cancer associated fibroblasts correlated with improved recurrence-free survival in breast cancer (95). The differences in associations may be due to characteristics of the patient cohort, the tissue preservation and processing conditions, the methodology and reagents for analyzing samples.
Hence, despite several decades of investigation, the lack of a robust TGFβ prognostic biomarker is challenging to reconcile with the abundant preclinical evidence of its detriment in most cancers. It is well-appreciated by those in the field that this is in part due to the TGFβ’s manifold roles in cancer that complicate translation into a universal means to ascertain biological activity per se that can be associated with outcomes. Technical challenges with protein measurements are also a factor (96). Advances in TGFβ biomarkers in which more precise technology or specific processes or events are defined as discrete targets will eventually clarify the inconsistencies among these results.
Summary and perspectives
Although TGFβ signaling competency varies dramatically across cancer types (97), the tumor-promoting effects of TGFβ signaling in both cancer cells and stromal cells in the TME provide a broad target for therapeutic manipulation. Despites strong preclinical rationale, clinical efficacy of TGFβ inhibition in cancer has yet to be demonstrated (reviewed in (7). Nonetheless, the mounting evidence that TGFβ signaling affects immune checkpoint inhibitor efficacy is providing new traction for TGFβ inhibition in cancer. One development is bintrafusp alfa, a first-in-class bifunctional fusion protein composed of the extracellular domain of the TGFβ receptor II (a TGFβ “trap“) fused to a human antibody blocking PD-L1 (98). In addition, new strategies blocking latent TGFβ activation on certain cells are innovative approaches to increase cell and isoform specificity (99).
Given the pleiotropic actions of TGFβ in cancer, this embarrassment of riches is a principal factor in effectively implementing these new agents. Our view is that lack of clarity as to which TGFβ mechanism is functionally opposing therapy in which patient is impeding advancement of TGFβ inhibition in cancer therapy. Companion diagnostic tools or specific biomarkers of TGFβ targeted biology, whether protein measurements, transcriptomic signatures, pathway mutations, remain undervalued, although not unexplored. Evaluation of TGFβ signaling and consequences via biomarkers that report specific molecular pathways will likely provide leverage for personalized cancer medicine. Exploiting TGFβ biology for patient benefit is an achievable goal, once the landmarks for a precise route is chosen.
Acknowledgements
This preparation of this review was funded by NIH R01-CA239235, UCSF Resource Allocation program and Department of Radiation Oncology seed funding to MHBH.
Financial Support: This study was funded by a grant to MHBH from the National Institutes of Health (R01 CA239235).
Conflict of Interests: MHBH has received honoraria for speaker bureau or advisory boards from EMD Serano, Varian, Inc., and Genentech on topics related to this manuscript.
Footnotes
The other authors have declared that no conflict of interest exists.
References
- 1.Sporn MB, Roberts AB. Transforming growth factor-b: recent progress and new challenges. The Journal of Cell Biology 1992;119(5):1017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Current opinion in cell biology 2009;21(2):166–76 doi 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Massague J. TGFbeta signalling in context. Nature reviews Molecular cell biology 2012;13(10):616–30 doi 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006;6(7):506–20. [DOI] [PubMed] [Google Scholar]
- 5.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 2012;11(10):790–811 doi 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batlle E, Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019;50(4):924–40 doi 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciardiello D, Elez E, Tabernero J, Seoane J. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2020;31(10):1336–49 doi 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor b1 by plasmin. J Cell Biol 1990;110:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence DA. Identification and activation of latent transforming growth factor b. Meth Enzym 1991;198:327–36. [DOI] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH. Latency and activation in the regulation of TGF-b. Journal of mammary gland biology and neoplasia 1996;3(1):353–63. [PubMed] [Google Scholar]
- 11.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alpha vbeta1. Molecular biology of the cell 1998;9:2627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilandzic M, Stenvers KL. Betaglycan: A multifunctional accessory. Mol Cell Endocrinol 2011;339(1–2):180–9 doi 10.1016/j.mce.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Brown JA, Yonekubo Y, Hanson N, Sastre-Perona A, Basin A, Rytlewski JA, et al. TGF-beta-Induced Quiescence Mediates Chemoresistance of Tumor-Propagating Cells in Squamous Cell Carcinoma. Cell stem cell 2017;21(5):650–64.e8 doi 10.1016/j.stem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagyaraj E, Ahuja N, Kumar S, Tiwari D, Gupta S, Nanduri R, et al. TGF-beta induced chemoresistance in liver cancer is modulated by xenobiotic nuclear receptor PXR. Cell Cycle 2019;18(24):3589–602 doi 10.1080/15384101.2019.1693120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang Y, Geng L, Yi H, Huo W, Talmon G, et al. Transforming Growth Factor beta Mediates Drug Resistance by Regulating the Expression of Pyruvate Dehydrogenase Kinase 4 in Colorectal Cancer. The Journal of biological chemistry 2016;291(33):17405–16 doi 10.1074/jbc.M116.713735. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Liu Q, Ma L, Jones T, Palomero L, Pujana MA, Martinez-Ruiz H, et al. Subjugation of TGFb Signaling by Human Papilloma Virus in Head and Neck Squamous Cell Carcinoma Shifts DNA Repair from Homologous Recombination to Alternative End Joining. Clin Cancer Res 2018;24(23):6001–14 doi 10.1158/1078-0432.ccr-18-1346. [DOI] [PubMed] [Google Scholar]
- 17.Brunen D, Willems SM, Kellner U, Midgley R, Simon I, Bernards R. TGF-beta: an emerging player in drug resistance. Cell Cycle 2013;12(18):2960–8 doi 10.4161/cc.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, et al. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest 2013;123(3):1348–58 doi 10.1172/jci65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Gu X, Xia L, Zhou Y, Bouamar H, Yang J, et al. A Novel TGFbeta Trap Blocks Chemotherapeutics-Induced TGFbeta1 Signaling and Enhances Their Anticancer Activity in Gynecologic Cancers. Clin Cancer Res 2018;24(12):2780–93 doi 10.1158/1078-0432.CCR-17-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang YA, Chen YF, Bao Y, Mahara S, Yatim S, Oguz G, et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America 2018;115(26):E5990–E9 doi 10.1073/pnas.1801348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chihara Y, Shimoda M, Hori A, Ohara A, Naoi Y, Ikeda JI, et al. A small-molecule inhibitor of SMAD3 attenuates resistance to anti-HER2 drugs in HER2-positive breast cancer cells. Breast cancer research and treatment 2017;166(1):55–68 doi 10.1007/s10549-017-4382-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Molecular and cellular biology 2008;28(18):5605–20 doi 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012;9(3):193–9 doi 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016;16(4):234–49 doi 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 25.Ehrhart EJ, Carroll A, Segarini P, Tsang ML-S, Barcellos-Hoff MH. Latent transforming growth factor-b activation in situ: Quantitative and functional evidence following low dose irradiation. FASEB J 1997;11:991–1002. [DOI] [PubMed] [Google Scholar]
- 26.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiation research 2006;166(6):839–48 doi 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 27.Bouquet SF, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, et al. Transforming growth factor b1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 2011;17(21):6754–65 doi 10.1158/1078-0432.CCR-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer research 2012;72(16):4119–29 doi 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du S, Bouquet F, Lo C-H, Pellicciotta I, Bolourchi S, Parry R, et al. Attenuation of the DNA Damage Response by TGFβ Inhibitors Enhances Radiation Sensitivity of NSCLC Cells In Vitro and In Vivo Int J Radiat Oncol Biol Phys 2014;91(1):91–9 doi 10.1016/j.ijrobp.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Anscher MS. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 2010;15(4):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanpouille-Box C, Diamond J, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. Transforming growth factor (TGF) β is a master regulator of radiotherapy-induced anti-tumor immunity. Cancer research 2015;75(11):2232–42 doi 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glick AB, Weinberg WC, Wu IH, Quan W, Yuspa SH. Transforming growth factor beta 1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer research 1996;56(16):3645–50. [PubMed] [Google Scholar]
- 33.Maxwell CA, Fleisch MC, Costes SV, Erickson AC, Boissiere A, Gupta R, et al. Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer research 2008;68(20):8304–11. [DOI] [PubMed] [Google Scholar]
- 34.Kirshner J, Jobling MF, Pajares MJ, Ravani SA, Glick AB, Lavin MJ, et al. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer research 2006;66(22):10861–9 doi 10.1158/0008-5472.CAN-06-2565. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Kozono DE, O’Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, et al. TGF-beta Inhibition Rescues Hematopoietic Stem Cell Defects and Bone Marrow Failure in Fanconi Anemia. Cell stem cell 2016;18(5):668–81 doi 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Shukla V, Farci P, Andricovich J, Jogunoori W, Kwong LN, et al. Loss of the transforming growth factor-β effector β2-Spectrin promotes genomic instability. Hepatology 2017;65(2):678–93 doi 10.1002/hep.28927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim AH, Lebman DA, Dietz CM, Snyder SR, Eley KW, Chung TD. Transforming growth factor-beta is an endogenous radioresistance factor in the esophageal adenocarcinoma cell line OE-33. International journal of oncology 2003;23(6):1593–9. [PubMed] [Google Scholar]
- 38.Liu Q, Palomero L, Moore J, Guix I, Espín R, Aytés A, et al. Loss of TGFβ signaling increases alternative end-joining DNA repair that sensitizes to genotoxic therapies across cancer types. Science translational medicine 2021;13(580):eabc4465 doi 10.1126/scitranslmed.abc4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andarawewa KL, Kirshner J, Mott JD, Barcellos-Hoff MH. TGFb: Roles in DNA damage responses. In: Jakowlew S, editor. Transforming Growth Factor-Beta in Cancer Therapy, Volume II Cancer Treatment and Therapy. Volume II, Cancer Drug Discovery and Development. Totowa: Humana Press; 2007. p 321–34. [Google Scholar]
- 40.Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: Impact of TGFbeta on the DNA damage response and genomic stability. Science signaling 2014;7(341):re5 doi 10.1126/scisignal.2005474. [DOI] [PubMed] [Google Scholar]
- 41.Lohaus F, Linge A, Tinhofer I, Budach V, Gkika E, Stuschke M, et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiotherapy and Oncology 2014;113(3):317–23 doi 10.1016/j.radonc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 42.French D, Belleudi F, Mauro MV, Mazzetta F, Raffa S, Fabiano V, et al. Expression of HPV16 E5 down-modulates the TGFbeta signaling pathway. Molecular cancer 2013;12:38 doi 10.1186/1476-4598-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levovitz C, Chen D, Ivansson E, Gyllensten U, Finnigan JP, Alshawish S, et al. TGFbeta receptor 1: an immune susceptibility gene in HPV-associated cancer. Cancer research 2014;74(23):6833–44 doi 10.1158/0008-5472.can-14-0602-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Lopez K, Murnane J, Humphrey T, Barcellos-Hoff MH. Misrepair in Context: TGFbeta Regulation of DNA Repair. Frontiers in oncology 2019;9:799 doi 10.3389/fonc.2019.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CT, Hsieh CC, Yen TC, Chen WC, Chen MF. TGF-beta1 mediates the radiation response of prostate cancer. J Mol Med (Berl) 2015;93(1):73–82 doi 10.1007/s00109-014-1206-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, et al. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer research 2011;71(23):7155–67 doi 10.1158/0008-5472.can-11-1212. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Junca A, Reiners O, Borrero-Garcia LD, Beckford-Vera D, Lazar AA, Chou W, et al. Positron Emission Tomography Imaging of Functional Transforming Growth Factor β (TGFβ) Activity and Benefit of TGFβ Inhibition in Irradiated Intracranial Tumors. Int J Radiat Oncol Biol Phys 2021;109(2):527–39 doi 10.1016/j.ijrobp.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161(2):205–14 doi 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma P, Allison JP. The future of immune checkpoint therapy. Science (New York, NY) 2015;348(6230):56–61 doi 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 50.Teicher BA. Transforming Growth Factor-β and the Immune Response to Malignant Disease. Clinical Cancer Research 2007;13(21):6247–51 doi 10.1158/1078-0432.ccr-07-1654. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Yang HH, Tang B, Wu AML, Flanders KC, Moshkovich N, et al. The Outcome of TGFβ Antagonism in Metastatic Breast Cancer Models In Vivo Reflects a Complex Balance between Tumor-Suppressive and Proprogression Activities of TGFβ. Clin Cancer Res 2020;26(3):643–56 doi 10.1158/1078-0432.ccr-19-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoppe MM, Sundar R, Tan DSP, Jeyasekharan AD. Biomarkers for Homologous Recombination Deficiency in Cancer. J Natl Cancer Inst 2018. doi 10.1093/jnci/djy085. [DOI] [PubMed] [Google Scholar]
- 53.Ganguly B, Dolfi SC, Rodriguez-Rodriguez L, Ganesan S, Hirshfield KM. Role of Biomarkers in the Development of PARP Inhibitors. Biomark Cancer 2016;8(Suppl 1):15–25 doi 10.4137/BIC.S36679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connor MJ. Targeting the DNA Damage Response in Cancer. Molecular cell 2015;60(4):547–60 doi 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 55.Hodges TR, Ott M, Xiu J, Gatalica Z, Swensen J, Zhou S, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro-oncology 2017;19(8):1047–57 doi 10.1093/neuonc/nox026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker RA, Dearing SJ. Transforming growth factor beta 1 in ductal carcinoma in situ and invasive carcinomas of the breast. European journal of cancer 1992;28(2–3):641–4. [DOI] [PubMed] [Google Scholar]
- 57.Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor b1 associates with disease progression in human breast cancer. Cancer Res 1992;52:6949–52. [PubMed] [Google Scholar]
- 58.Shariat SF, Walz J, Roehrborn CG, Montorsi F, Jeldres C, Saad F, et al. Early postoperative plasma transforming growth factor-beta1 is a strong predictor of biochemical progression after radical prostatectomy. The Journal of urology 2008;179(4):1593–7 doi 10.1016/j.juro.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 59.Desruisseau S, Palmari J, Giusti C, Romain S, Martin PM, Berthois Y. Determination of TGFbeta1 protein level in human primary breast cancers and its relationship with survival. British journal of cancer 2006;94(2):239–46 doi 10.1038/sj.bjc.6602920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 2001;91(5):964–71. [PubMed] [Google Scholar]
- 61.Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, et al. Circulating Transforming Growth Factor {beta}1 as a Predictor of Liver Metastasis after Resection in Colorectal Cancer. Clin Cancer Res 2001;7(5):1258–62. [PubMed] [Google Scholar]
- 62.Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor b1 expression in irradiated liver. Radiat Res 1990;122:77–85. [PubMed] [Google Scholar]
- 63.Nakamura M, Katano M, Kuwahara A, Fujimoto K, Miyazaki K, Morisaki T, et al. Transforming growth factor beta1 (TGF-beta1) is a preoperative prognostic indicator in advanced gastric carcinoma. British journal of cancer 1998;78(10):1373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feltl D, Zavadova E, Pala M, Hozak P. Post-treatment plasma transforming growth factor beta 1 (TGF-beta1) level predicts for late morbidity in patients with advanced head and neck cancer. Neoplasma 2005;52(5):393–7. [PubMed] [Google Scholar]
- 65.Shariat SF, Kim JH, Andrews B, Kattan MW, Wheeler TM, Kim IY, et al. Preoperative plasma levels of transforming growth factor beta(1) strongly predict clinical outcome in patients with bladder carcinoma. Cancer 2001;92(12):2985–92. [DOI] [PubMed] [Google Scholar]
- 66.Mabrouk GM, Ali EM, El-Rehany MA, El-Samoly HM. TGF-beta1, TNF-alpha and cytochrome c in human astrocytic tumors: a short-term follow up and correlation with survival. Clin Biochem 2007;40(3–4):255–60 doi 10.1016/j.clinbiochem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Sheen-Chen SM, Chen HS, Eng HL, Sheen CC, Chen WJ. Serum levels of matrix metalloproteinase 2 in patients with breast cancer. Cancer letters 2001;173(1):79–82 doi S0304383501006577 [pii]. [DOI] [PubMed] [Google Scholar]
- 68.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor b1 has a longer plasma half-life in rats than active transforming growth factor b1, and a different tissue distribution. J Clin Invest 1990;86:1976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. The Journal of biological chemistry 1983;258(11):7155–60. [PubMed] [Google Scholar]
- 70.Flaumenhaft R, Abe M, Sato Y, Miyzono K, Harpel J, Heldin C-H, et al. Role of the latent TGF-b binding protein in the activation of latent TGF-b by co-cultures of endothelial and smooth muscle cells. J Cell Biol 1993;120(4):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kojima S, Rifkin DB. Mechanism of retinoid-induced activation of latent transforming growth factor-b in bovine endothelial cells. J Cell Physiol 1993;155:323–32. [DOI] [PubMed] [Google Scholar]
- 72.Pellicciotta I, Marciscano AE, Hardee ME, Francis D, Formenti S, Barcellos-Hoff MH. Development of a novel multiplexed assay for quantification of transforming growth factor-β (TGFβ). Growth Factors 2014;33(2):79–91 doi 10.3109/08977194.2014.999367. [DOI] [PubMed] [Google Scholar]
- 73.Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C. Prognostic Significance of Transforming Growth Factor {beta} Receptor II in Estrogen Receptor-Negative Breast Cancer Patients. Clin Cancer Res 2004;10(2):491–8. [DOI] [PubMed] [Google Scholar]
- 74.Friedman E, Gold LI, Klimstra D, Zeng ZS, Winawer S, Cohen A. High levels of transforming growth factor beta 1 correlate with disease progression in human colon cancer. Cancer Epidemiol Biomarkers Prev 1995;4(5):549–54. [PubMed] [Google Scholar]
- 75.Calon A, Espinet E, Palomo-Ponce S, Tauriello Daniele VF, Iglesias M, Céspedes María V, et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell 2012;22(5):571–84 doi 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nature genetics 2015;47(4):320–9 doi 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 77.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998;37(1):19–29 doi . [DOI] [PubMed] [Google Scholar]
- 78.Shariat SF, Kattan MW, Traxel E, Andrews B, Zhu K, Wheeler TM, et al. Association of Pre- and Postoperative Plasma Levels of Transforming Growth Factor {beta}1 and Interleukin 6 and Its Soluble Receptor with Prostate Cancer Progression. Clin Cancer Res 2004;10(6):1992–9 doi 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- 79.Bruna A, Darken RS, Rojo F, Ocaña A, Peñuelas S, Arias A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 2007;11(2):147–60. [DOI] [PubMed] [Google Scholar]
- 80.Schneider T, Sailer M, Ansorge S, Firsching R, Reinhold D. Increased concentrations of transforming growth factor beta1 and beta2 in the plasma of patients with glioblastoma. Journal of neuro-oncology 2006;79(1):61–5 doi 10.1007/s11060-005-9116-7. [DOI] [PubMed] [Google Scholar]
- 81.Huang CY, Wang H, Liao W, Han F, Li YQ, Chen SW, et al. Transforming Growth Factor beta is a Poor Prognostic Factor and Inhibits the Favorable Prognostic Value of CD8+ CTL in Human Hepatocellular Carcinoma. Journal of immunotherapy 2017;40(5):175–86 doi 10.1097/CJI.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Zou L, Zhang Y, Chen Y, Xing P, Yang W, et al. Transforming growth factor-beta1 and alpha-smooth muscle actin in stromal fibroblasts are associated with a poor prognosis in patients with clinical stage I-IIIA nonsmall cell lung cancer after curative resection. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014;35(7):6707–13 doi 10.1007/s13277-014-1908-y. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Zeng Y, Liu T, Du W, Zhu J, Liu Z, et al. The canonical TGF-beta/Smad signalling pathway is involved in PD-L1-induced primary resistance to EGFR-TKIs in EGFR-mutant non-small-cell lung cancer. Respir Res 2019;20(1):164 doi 10.1186/s12931-019-1137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J, Shen C, Wang X, Lai Y, Zhou K, Li P, et al. Prognostic value of TGF-beta in lung cancer: systematic review and meta-analysis. BMC cancer 2019;19(1):691 doi 10.1186/s12885-019-5917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shinto O, Yashiro M, Toyokawa T, Nishii T, Kaizaki R, Matsuzaki T, et al. Phosphorylated smad2 in advanced stage gastric carcinoma. BMC cancer 2010;10:652 doi 10.1186/1471-2407-10-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Javle M, Li Y, Tan D, Dong X, Chang P, Kar S, et al. Biomarkers of TGF-beta signaling pathway and prognosis of pancreatic cancer. PloS one 2014;9(1):e85942 doi 10.1371/journal.pone.0085942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554(7693):544–8 doi 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nature communications 2018;9(1):4692 doi 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, et al. Focal Irradiation and Systemic TGFbeta Blockade in Metastatic Breast Cancer. Clin Cancer Res 2018;24(11):2493–504 doi 10.1158/1078-0432.ccr-17-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim MR, Lee J, An YS, Jin YB, Park IC, Chung E, et al. TGFbeta1 Protects Cells from gamma-IR by Enhancing the Activity of the NHEJ Repair Pathway. Molecular cancer research : MCR 2015;13(2):319–29 doi 10.1158/1541-7786.mcr-14-0098-t. [DOI] [PubMed] [Google Scholar]
- 91.Patterson-Fortin J, D’Andrea AD. Exploiting the Microhomology-Mediated End-Joining Pathway in Cancer Therapy. Cancer research 2020;80(21):4593–600 doi 10.1158/0008-5472.can-20-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Auvinen P, Lipponen P, Johansson R, Syrjanen K. Prognostic significance of TGF-beta 1 and TGF-beta 2 expressions in female breast cancer. Anticancer research 1995;15(6B):2627–31. [PubMed] [Google Scholar]
- 93.Murray PA, Barrett-Lee P, Travers M, Luqmani Y, Powles T, Coombes RC. The prognostic significance of transforming growth factors in human breast cancer. British journal of cancer 1993;67(6):1408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paiva CE, Drigo SA, Rosa FE, Moraes Neto FA, Caldeira JR, Soares FA, et al. Absence of transforming growth factor-beta type II receptor is associated with poorer prognosis in HER2-negative breast tumours. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2010;21(4):734–40 doi 10.1093/annonc/mdp518. [DOI] [PubMed] [Google Scholar]
- 95.Busch S, Acar A, Magnusson Y, Gregersson P, Ryden L, Landberg G. TGF-beta receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene 2015;34(1):27–38 doi 10.1038/onc.2013.527. [DOI] [PubMed] [Google Scholar]
- 96.Flanders KC, Yang YA, Herrmann M, Chen J, Mendoza N, Mirza AM, et al. Quantitation of TGF-beta proteins in mouse tissues shows reciprocal changes in TGF-beta1 and TGF-beta3 in normal vs neoplastic mammary epithelium. Oncotarget 2016;7:38164–79 doi 10.18632/oncotarget.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korkut A, Zaidi S, Kanchi RS, Rao S, Gough NR, Schultz A, et al. A Pan-Cancer Analysis Reveals High-Frequency Genetic Alterations in Mediators of Signaling by the TGF-beta Superfamily. Cell systems 2018;7(4):422–37.e7 doi 10.1016/j.cels.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J, Gulley JLMD, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. Journal for immunotherapy of cancer 2020;8(1):e000433 doi 10.1136/jitc-2019-000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin CJ, Datta A, Littlefield C, Kalra A, Chapron C, Wawersik S, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Science translational medicine 2020;12(536) doi 10.1126/scitranslmed.aay8456. [DOI] [PubMed] [Google Scholar]
- 100.Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, et al. High levels of transforming growth factor b1 in patients with colorectal cancer: association with disease progression. Gastroenterology 1996;110:375–82. [DOI] [PubMed] [Google Scholar]
- 101.Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ. Serum levels of transforming growth factor beta1 in patients with breast cancer. Archives of surgery 2001;136(8):937–40 doi soa0240 [pii]. [DOI] [PubMed] [Google Scholar]
- 102.de Kruijf EM, Dekker TJ, Hawinkels LJ, Putter H, Smit VT, Kroep JR, et al. The prognostic role of TGF-beta signaling pathway in breast cancer patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2013;24(2):384–90 doi 10.1093/annonc/mds333. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Xing P, Chen Y, Zou L, Zhang Y, Li F, et al. High p-Smad2 expression in stromal fibroblasts predicts poor survival in patients with clinical stage I to IIIA non-small cell lung cancer. World J Surg Oncol 2014;12:328 doi 10.1186/1477-7819-12-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu CT, Chang YH, Lin WY, Chen WC, Chen MF. TGF Beta1 Expression Correlates with Survival and Tumor Aggressiveness of Prostate Cancer. Annals of surgical oncology 2015;22 Suppl 3:S1587–93 doi 10.1245/s10434-015-4804-9. [DOI] [PubMed] [Google Scholar]
- 105.Yang YC, Wang KL, Su TH, Liao HF, Wu MH, Chen TC, et al. Concurrent cisplatin-based chemoradiation for cervical carcinoma: tumor response, toxicity, and serum cytokine profiles. Cancer Invest 2006;24(4):390–5 doi 10.1080/07357900600705359. [DOI] [PubMed] [Google Scholar]
- 106.Bedi A, Chang X, Noonan K, Pham V, Bedi R, Fertig EJ, et al. Inhibition of TGF-β Enhances the In Vivo Antitumor Efficacy of EGF Receptor–Targeted Therapy. Molecular cancer therapeutics 2012;11(11):2429–39 doi 10.1158/1535-7163.mct-12-0101-t. [DOI] [PMC free article] [PubMed] [Google Scholar]