Abstract
Objectives
Cognitive dysfunction is common in patients with aPL (including primary APS or APS associated with SLE). Neuroimaging biomarkers may contribute to our understanding of mechanisms of cognitive dysfunction in these cohorts. This review aimed to investigate: (i) the prevalence of cognitive dysfunction in studies including neuroimaging biomarkers; and (ii) associations between cognition and neuroimaging biomarkers in patients with APS/aPL.
Methods
We conducted a systematic search of electronic databases PubMed, Science Direct, Scopus and PsycINFO, and included studies with descriptions of neuroimaging findings, cognitive dysfunction or both, in patients with aPL positivity (LA, IgG and IgM aCL and anti-β2 glycoprotein-I antibodies).
Results
Of 120 search results we included 20 eligible studies (6 APS, 4 SLE with APS/aPL and 10 NPSLE). We identified a medium risk of bias in 6/11 (54%) of cohort studies and 44% of case–control studies, as well as marked heterogeneity in cognitive assessment batteries, APS and aPL definitions, and neuroimaging modalities and protocols. The prevalence of cognitive dysfunction ranged between 11 and 60.5%. Structural MRI was the most common imaging modality, reporting cognitive dysfunction to be associated with white matter hyperintensities, ischaemic lesions and cortical atrophy (four with cerebral atrophy, two with white matter hyperintensities and two with cerebral infarcts).
Conclusion
Our findings confirm that cognitive impairment is commonly found in patients with aPL (including APS, SLE and NPSLE). The risk of bias, and heterogeneity in the cognitive and neuroimaging biomarkers reported does not allow for definitive conclusions.
Keywords: antiphospholipid syndrome, antiphospholipid antibodies, cognitive dysfunction, neuroimaging biomarkers, assessment
Rheumatology key messages
Limited reporting of cognitive dysfunction in APS compared with SLE and NPSLE with aPL positivity.
Studies including neuroimaging biomarkers in APS/aPL-positive patients with cognitive dysfunction were scarce and heterogeneous.
Multicentre studies with standardized image acquisition and international APS clinical and laboratory criteria are required.
Introduction
APS is an autoimmune antibody-mediated disease, characterized by recurrent vascular thrombosis (venous, arterial and microvascular), pregnancy morbidity and thrombocytopenia [1–3]. A characteristic indicator of APS is the presence of aPL, including LA, as well as IgG and IgM aCL, and anti-β2 glycoprotein-I antibodies (anti-β2GPI) [2, 4, 5], and diagnosis is made in accordance with the International updated Sapporo (Sydney) classification criteria [6]. APS can occur in isolation, where the disease is classified as occurring alone [primary APS (PAPS)], or in the context of other autoimmune conditions [secondary APS (SAPS)], most notably SLE [7].
Cognitive dysfunction is a common neurological manifestation of APS, particularly in SAPS associated with SLE. Evidence regarding the prevalence of cognitive dysfunction and PAPS is limited [8]. One review reported frequency of cognitive dysfunction to range between 15–80% in cohorts of aPL carriers, PAPS and SLE [9]. The association of cognitive dysfunction with APS has mainly been discussed in the context of NPSLE [10], which according to the ACR consists of 19 neurologic syndromes of the central, peripheral and autonomic nervous systems including cognitive dysfunction or psychiatric syndromes, where other causes have been excluded [11]. Using the ACR consensus criteria, the prevalence of cognitive dysfunction for SLE was reported as 43, 30 and 6% for mild, moderate and severe disease, respectively [12]. Cognitive dysfunction is also common in SLE where there are no neuropsychiatric symptoms [13].
Although neuroimaging biomarkers are a potentially powerful way to understand mechanisms of cognitive impairment, evidence summarizing neuroimaging characteristics of APS is also scare [2, 14]. One review article described the relationship between cognitive dysfunction and magnetic resonance abnormalities (MRI) specific to patients with SLE [8]. More recently, there has been increasing interest in examining the associations between SLE and aPL with dementia [15, 16].
Given the limited evidence regarding the prevalence and mechanisms of cognitive dysfunction in patients with a diagnosis of APS or aPL positivity, there remains scope to examine available studies reporting detailed cognitive assessment and neuroimaging biomarkers. The objectives of this systematic review were to determine: (i) the prevalence of cognitive dysfunction in studies including neuroimaging biomarkers; and (ii) associations between cognition and neuroimaging biomarkers in patients with APS/aPL.
Methods
Literature search and selection strategy
We electronically searched PubMed, Science Direct, Scopus and PsycINFO up to January 2021 using key terms ‘antiphospholipid syndrome’, ‘neuroimaging’, ‘cognitive impairment’ and ‘neuropsychiatric systemic lupus erythematosus [NPSLE]’, combined using Boolean operators (supplementary Table S1, available at Rheumatology online). In addition to the database searches, reference lists of selected articles were checked for their included relevant research papers.
Publication selection criteria
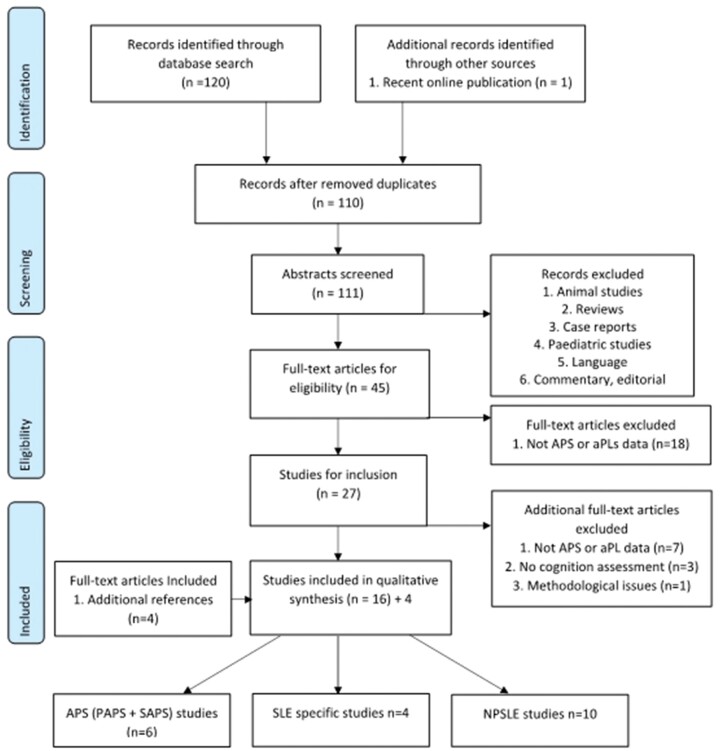
Publication inclusion criteria were: adult cohorts ≥18 years of age; studies including patients defined as diagnosed with APS (PAPS and SAPS); cohorts with aPL (various combinations of LA, aCL, anti-β2GPI) positivity; and studies reporting both cognitive assessment and neuroimaging biomarkers. Exclusion criteria were: animal studies; paediatric cohort studies; review articles and reports; case reports and case studies (fewer than five subjects); editorials; letters; and commentaries. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [17] for the search strategy, study selection and inclusion, as well as data extraction and analysis (see Fig. 1) (supplementary Table S2, available at Rheumatology online).
Fig. 1.
Workflow diagram of publication selection process using PRISMA guidelines
n, number of articles after each screening stage; PAPS: primary SLE; SAPS: secondary SLE; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Quality assessment
We appraised the quality of included studies using the Newcastle-Ottawa Quality Assessment Scale (NOS) for case–control and longitudinal cohort studies [18] and adapted version for cross-sectional cohort studies [19]. The NOS allocates a maximum score of 9 points indicating very high quality and a low risk of bias, whereas a minimum score of 1, 2 or 3 indicates low quality and a high risk of bias. The scoring system allocates up to 4 points for selection of subjects, 2 points for comparability and 3 points for exposure (in case–control cohort studies) and outcome (in cohort studies). Studies scoring above the median value were considered high quality (low risk of bias) and those below the median as low quality (high risk of bias).
Data extraction
For each study we extracted data on: first author and year (study ID); study design; number of patients and controls (if included); mean age in years; percentage female; types and isotypes of aPL and cut-off values; cognitive dysfunction prevalence, cognitive domains assessed; neuroimaging modality and neuroimaging biomarkers assessed; cognitive domains affected; and associations between neuroimaging biomarkers, cognitive dysfunction and aPL positivity.
Results
Search results and publication selection
We identified 120 articles through the electronic search. A detailed search strategy is presented in Fig. 1. Two independent raters (C.D. and D.J.W.) evaluated the studies at the eligibility and inclusion phases of the review where there was full agreement for publication selection.
Quality assessment results for selected studies
Quality assessments of the included studies were undertaken by C.D. using the NOS criteria for cohort and case–control studies are shown in Tables 1 and 2. The median score of NOS was 6 for cohort studies and 7 for case–control studies. Among the 11 cohort studies, 7 were considered of medium to higher methodological quality, scoring ≥6, and for the 9 case–control studies, 5 were considered of medium to higher methodological quality, scoring ≥7. Overall, there were 8 included studies considered of lower methodological quality, and therefore a higher risk of bias in 6/11 (54%) of cohort studies and in 4/9 (44%) of case–control studies.
Table 1.
Risk of bias assessment of included studies according to the modified Newcastle-Ottawa Scale—Version for cohort studies (n = 11)
| Quality assessment | APS studies |
SLE studies | NPSLE studies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arvanitakis et al. (2019) [20] | Homayoon et al. (2014) [21] | Zamproni et al. (2013) [22] | Erkan et al. (2010) [23] | Chapman et al. (2002) [24] | Whitelaw et al. (1999) [25] | Sarbu et al. (2015) [26] | Steup-Beekman et al. (2013) [27] | Abda et al. (2013) [28] | Zirkzee et al. (2012) [29] | Cantú-Brito et al. (2010) [30] | ||
| Selection | ||||||||||||
| 1. Is the case definition adequate | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | |
| 2. Representativeness of cases | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ● | ○ | ○ | |
| 3. Ascertainment of exposure | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| 4. Outcome of interest was not present at start of study | ● | ● | ○ | ● | ● | ● | ● | ● | ○ | ○ | ● | |
| Comparability | ||||||||||||
| 5. Study controls for most important factor | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | |
| 6. Study controls for second important factor | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | |
| Outcome | ||||||||||||
| 7. Assessment of outcome | ● | ●● | ●● | ●● | ●● | ●● | ●● | ●● | ●● | ●● | ● | |
| 8. Statistical test (CS only) | ● | ● | ○ | ○ | ● | ● | ○ | ● | ● | ● | ||
| 9. Adequate follow up period for outcome of interest (LS only) | ● | |||||||||||
| 10. Adequacy of follow up of cohorts (LS only) | ● | ● | ||||||||||
| Total score | 9/9 | 9/9 | 6/9 | 7/9 | 4/9 | 5/9 | 9/9 | 5/9 | 6/9 | 5/9 | 6/9 | |
CS: cross-sectional studies; LS: longitudinal studies.
Table 2.
Risk of bias assessment of included studies according to the modified Newcastle-Ottawa Scale—Version for case-control studies (n = 9)
| Quality assessment | APS studies | SLE studies |
NPSLE studies |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tektonidou et al. (2006) [31] | Kozora et al. (2014, 2016) [32],[33] | Appenzeller et al. (2007) [34] | Tomietto et al. (2007) [35] | Shulman et al. (2017) [36] | Emmer et al. (2008) [37] | Cho et al. (2007) [38] | Roldan et al. (2006) [39] | Appenzeller et al. (2005) [40] | |
| Selection | |||||||||
| 1. Is the case definition adequate | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| 2. Representativeness of the cases | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| 3. Selection of controls | ● | ● | ● | ● | ● | ○ | ○ | ○ | ● |
| 4. Definition of controls | ○ | ○ | ● | ● | ● | ○ | ● | ○ | ● |
| Comparability | |||||||||
| 5. Study controls for most important factor | ● | ● | ● | ● | ○ | ● | ○ | ● | ● |
| 6. Study controls for second important factor | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● |
| Exposure | |||||||||
| 7. Measurement method of variables of interest described | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| 8. Methods of measurements same for cases and controls | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| 9. Non-response rate | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● |
| Total score | 7/9 | 7/9 | 8/9 | 8/9 | 6/9 | 6/9 | 5/9 | 5/9 | 9/9 |
Characteristics of studies included in review
Of the 20 studies included, the disease groups were n = 6 APS (mixed PAPS and SAPS), n = 4 SLE specific and n = 10 NPSLE (see Tables 3 and 4). More than half of the included studies were cohort studies and n = 9 were case–control (n = 2 APS/aPL positive, n = 3 SLE, n = 4 NPSLE) [20–29]. Three studies were longitudinal in design [30, 31, 23] and at least seven studies were reported as retrospective where patient cohorts and data were extracted from case notes and patient-held registries [32, 33, 34, 35, 36, 27, 28]. Cohort sizes within studies were generally small with the exception of the two most recent included studies [30, 37], with mean age ranging from 31 to 81 years, and >75% were female.
Table 3.
Characteristics of studies describing APS (n = 6) and SLE (n = 4) specific studies
| Author and year | Study design | Sample (n) | Mean age (years) | % Female |
|
aPL types (isotypes; cut-offs) | % Cognitive dysfunction | Cognitive domains | Imaging modality | Imaging biomarkers | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APS [mixed—PAPS, SAPS and aPL carriers (+)] studies (n = 6) | ||||||||||||
| Arvanitakis et al. (2019) [20] | Longitudinal cohort | 956 | 81.1 | 72 |
|
aCL anti-β2GPI (IgG/M) | NR | Global, perceptual speed, working memory, episodic memory, semantic memory, visuospatial ability | MRI | WMH total volume, infarcts with volume of ≥3mm | ||
| Homayoon et al. (2014) [21] | Cross-sectional, prospective cohort | 1895 | 64.6 | 58 |
|
aCL (IgG >21 U/ml, IgM >12 U/ml) | NR | Global | MRI | WMH, silent cortical infarcts, lacunes, hippocampus volume (CA1–CA4) | ||
| Zamproni et al. (2013) [22] | Cross-sectional, observation cohort | 27 | 42 (non-RLS), 35 (RLS) | 70 |
|
aCL (IgG/M >40 GPL); LA (INR >1, or 3 on AC Rx) | 30 | Global, learning memory, visuospatial, nonverbal memory and fluency, executive function, attention, frontal function | TCD | Presence of RLS | ||
| Erkan et al. (2010) [23] | Cross-sectional, retrospective cohort | 143 | NR | 88 |
|
LA; aCL, anti-β2GPI (≥40 U IgG/M/A) | 15 | NR | MRI | WM changes | ||
| Tektonidou et al. (2006) [31] | Cross-sectional, case-control | 60 (cases), 60 (controls) | 41.1 (cases), 40.6 (controls) | 77 |
|
LA; aCL (IgG/M), anti-β2GPI | 42 | Global, attention, immediate word span, learning, retrieval efficiency, visuospatial, psychomotor speed, verbal fluency, abstract reasoning, conceptual flexibility | MRI | WML, infarcts, cortical atrophy, haemorrhages | ||
| Chapman et al. (2002) [24] | Cross-sectional, retrospective cohort | 23 | 57.5 | 56 |
|
aCL (10–20 (elevated), > 20 (high) GPL) | 39 | Global, dementia criteria | CT, EEG | Generalized pathology, focal pathology | ||
| SLE-specific studies (n = 4) | ||||||||||||
| Kozora et al. (2014, 2016)1 [32, 33] | Cross-sectional, case–control | 20 (SLE), 20 (aPL+), 10 (control) | 36.5 (SLE), 37.6 (aPL+), 40.8 (control) | All |
|
LA; aCL, anti-β2GPI (IgG/M) | 40 | Global, learning, memory, attention, working memory, executive function, verbal fluency, visuo-constructive, motor functioning | MRI, fMRI | WMH, cerebral atrophy | ||
| Appenzeller et al. (2007) [34] | Longitudinal case-control | 75 (cases), 44 (controls) | 32.3 (cases), 33.8 (controls) | 93 |
|
NR | NR | Global, simple/complex attention, memory, visuospatial processing, language, reasoning/problem solving, psychomotor speed, executive function | MRI | Cerebral atrophy | ||
| Tomietto et al. (2007) [35] | Cross-sectional, prospective case–control | 52 (SLE), 20 (RA) | 36.3 (SLE), 41 (RA) | 90 |
|
LA (aPTT); aCL (>15 IgG IU/ml) anti-β2GPI (>20 IgG IU/ml) | 60 | Global, simple/complex attention, memory, visuospatial processing, language, reasoning/problem solving, psychomotor speed, executive function | MRI | Cortical atrophy, focal lesions | ||
| Whitelaw et al. (1999) [25] | Cross-sectional, prospective cohort | 69 | 34.0 | 97 |
|
aPL (IgG) | NR | Intelligence, logical memory, visual reproduction, learning, executive function, auditory verbal learning | MRI | Diffuse and focal ischaemic change, WM lesions, UBOs | ||
Same cohort in both publications. AC: anticoagulants; anti-β2GPI: anti-β2 glycoprotein-I antibodies; CA: cornu ammonis; EEG: electroencephalogram; fMRI: functional MRI; INR: international normalized ratio; NR: not reported; PAPS: primary APS; RLS: right to left shunt; Rx: treatment; SAPS: secondary APS; TCD: transcranial Doppler; UBOs: unidentified bright objects; WM: white matter; WMH: white matter hyperintensities; WML: white matter lesions.
Table 4.
Characteristics of studies describing NPSLE (n = 10) specific cohort studies
| Author and year | Study design | (n) sample | Mean age (years) | % Female |
|
aPL types (isotypes; cut-offs) | % Cognitive dysfunction | Cognitive domains | Imaging modality | Imaging biomarkers |
|---|---|---|---|---|---|---|---|---|---|---|
| NPSLE studies (n = 10) | ||||||||||
| Shulman et al. (2017) [36] | Cross-sectional, case–control | 21 (cases), 11 (controls) | 40.14 (cases), 39.6 (controls) | NR |
|
LA; aCL, anti-β2GPI (IgG/M) | 47.6 | Global, memory, information processing speed, executive function, visual spatial, verbal function, motor skills, problem solving, attention | MRI, OCT | Infarcts, UBOs, retinal nerve fiber layer thickness (biomarker for white matter damage) |
| Sarbu et al. (2015) [26] | Cross-sectional, retrospective cohort | 108 | 40.6 | 92 |
|
LA; aCL (IgG/M) | 11 | Global, simple/complex attention, memory, visuospatial processing, language, reasoning/problem solving, psychomotor speed, executive function | MRI | Inflammatory lesions, LVD, SVD |
| Steup-Beekman et al. (2013) [37] | Cross-sectional, retrospective cohort | 155 | 29.7 (median) | 90 |
|
LA; aCL (IgG/M) | 25.6 | Global, simple/complex attention, memory, visuospatial processing, language, reasoning/problem solving, psychomotor speed, executive function | MRI | WMH, infarcts, atrophy |
| Abda et al. (2013) [28] | Cross-sectional, prospective cohort | 34 | 33.2 | 94 |
|
aPL | 42.86 | Global, attention, memory, problem solving, visuospatial processing, psychomotor speed | MRI, DWI, MRA | Ischaemic brain lesions and demyelination, infarctions, diffuse brain atrophy |
| Zirkzee et al. (2012) [29] | Cross-sectional, retrospective cohort | 71 (SLE) | 42 | 90 |
|
LA; aCL | 60.5 | Global intelligence, memory, executive function, psychomotor speed | MRI | Infarction, inflammation |
| Cantú-Brito et al. (2010) [30] | Longitudinal, prospective cohort | 109 | 34 | 95 |
|
aCL (IgG) | 38.5 | Memory, language, calculation, construction, reasoning | TCD | Microembolic signals—vascular damage |
| Emmer et al. (2008) [37] | Cross-sectional, prospective case–control | 52 | 38.5 (cases), 44.7 (controls) | 90 |
|
aCL (IgG/M) | 13.5 | NR | MTI, MRS | Histogram peak height, NAA:Cr ratio |
| Cho et al. (2007) [38] | Cross-sectional, retrospective case–control | 25 (NPSLE), 18 (NBD) | 31 (NPSLE), 38 (NBD) | 67 |
|
aCL, anti-β2GPI | 25.5 | NR | MRI | WMH, infarcts, parenchymal haemorrhage, atrophy, abnormal intracranial and meningeal enhancement |
| Roldan et al. (2006) [39] | Cross-sectional, retrospective case–control | 28 (SLE), 28 (controls) | 40 (SLE), 37 (controls) | 82 |
|
LA; aCL; aPL (IgG/M/A) | 57 | NR | MRI | Infarcts, periventricular and WMH, cortical atrophy, ventricular dilation |
| Appenzeller et al. (2005) [40] | Cross-sectional, prospective case–control | 115 (SLE), 44 (controlss) | 33.5 (cases), 33.8 (controls) | 95 |
|
LA; aCL (IgG/M) | 30 | Global, simple/complex attention, memory, visuospatial processing, language, reasoning/problem solving, psychomotor speed, executive function | MRI | Cerebral atrophy, infarcts |
Anti-β2GPI: anti-β2 glycoprotein-I antibody; Cr: creatinine; DWI: diffusion-weighted imaging; LVD: large vessel disease; MRA: magnetic resonance angiography; MRS: magnetic resonance spectroscopy; MTI: magnetization transfer imaging; NAA: N-acetylaspartate; NBD: neuroBehçet’s disease; NR: not reported; OCT: optical coherence tomography; SVD: small vessel disease; TCD: transcranial Doppler; UBOs: unidentified bright objects; WMH: white matter hyperintensities.
Prevalence and assessment of cognitive dysfunction and/or dementia
The prevalence of cognitive dysfunction for all included studies across all patient groups ranged from 11% [34] to 60.5% [36], although some studies did not report this [30, 37, 39, 23] (see Tables 4 and 5). The prevalence of cognitive dysfunction in APS [mixed—PAPS, SAPS and aPL carriers (+); six studies including 3104 patients] ranged from 15 to 42%. The prevalence of cognitive dysfunction in SLE (4 studies, 236 patients) ranged from 40 to 60%, and in NPSLE (10 studies, 718 patients) from 11 to 47.6%.
Table 5.
Associations between neuroimaging biomarkers, cognitive dysfunction and APS or persistent aPL+
| Author and year | Sample (n) | Cognitive domain(s) affected | Statistical analysis | Cognitive dysfunction (exposure) and imaging biomarkers (outcome) | Imaging biomarkers (exposure) and aPL+ (outcome) | Cognitive dysfunction (exposure) and aPL+ (outcome) |
|---|---|---|---|---|---|---|
| Structural MRI (n = 16) | ||||||
| Arvanitakis et al. (2019) [20] | 956 | No specific domains reported | Linear regression, logistic regression | Association not assessed | Presence of brain infarcts and aPL+ (OR = 1.007, P = 0.97) | Global cognitive function and aPL+ (beta = −0.062, P = 0.203) |
| Homayoon et al. (2014) [21] | 1895 | No specific domains reported | Linear regression | Association not assessed | Hippocampal volume and aCL (IgG) (beta = −0.071, CI 0.013, 0.007, P = 0.003) | Global cognition and; aCL status (beta = −0.361, CI 0.666, 0.058, P = 0.020); aCL (IgG) (beta = −0.591, CI 1.058, 0.124, P = 0.01) |
| Erkan et al. (2010) [23] | 143 | No specific domains reported | χ 2 statistic (Fisher’s exact test) | Association not assessed | WM changes and high titer aCL (RR 2.03, CI 1.04, 3.94, P = 0.02) | Cognitive dysfunction and high titer aCL (P = 0.12) |
| Tektonidou et al. (2006) [31] | 60 (cases), 60 (controls) | Complex attention and verbal fluency | Logistic regression | Cognitive deficits and; WMLs (OR 4.18, CI 1.33, 13.11, P = 0.01); infarcts (OR 1.22, CI 0.35, 4.20, P = 0.76) | Association not assessed | Cognitive deficits and; aCL (IgG) (OR 1.92, CI 0.34, 10.78, P = 0.46); aCL (IgM) (OR 0.63, CI 0.22, 1.78, P = 0.38); LA (OR 2.38, CI 0.76, 7.40, P = 0.14); anti-β2GPI (OR 2.11, CI 0.74, 6.05, P = 0.16) |
| Kozora et al. (2014) [32] | 20 (SLE), 20 (aPL+) | Highest frequency of impairment in visual learning and memory, visuomotor speed and flexibility, verbal fluency, visuoconstruction and rapid auditory information processing | Spearman’s correlation | Cognitive impairment and abnormal/incidental MRI findings (P = 0.75) | Association not assessed | Cognitive impairment and aPL+ (P > 0.232) |
| Appenzeller et al. (2007) [34] | 75 (cases), 44 (controls) | General memory | t-statistic [SPM(t)] | Severe cognitive dysfunction and reduced WM and GM (statistical result not reported) | Reduced WM and GM and aPL+ (statistical result not reported) | Association not assessed |
| Appenzeller et al. (2005) [40] | 115 (cases), 44 (controls) | No specific domains significant | Linear regression | Cognitive dysfunction and reduced corpus callosum and cerebral volumes (P = 0.001) | Cerebral and corpus callosum volumes and aPL+ (P = 0.1) | Association not assessed |
| Tomietto et al. (2007) [35] | 52 (SLE), 20 (RA) | Memory, complex attention and executive function | Logistic regression | Severity of cognitive deficits and MRI severity (cerebral atrophy and ischaemic lesions) (OR 33.5, CI 3.23–348.3, P < 0.01) | MRI severity (cerebral atrophy and ischaemic lesions) (OR 7.9, CI 1.5, 4.1, P = 0.01); macro-ischaemic lesions (OR 8.8 CI 1, 76, P = 0.03); and aPL+ | Severity of cognitive deficits (OR 4.9, CI 1.2, 20.3, P = 0.03); executive function (OR 9.4, CI 1.1, 80, P = 0.02); complex attention (OR 6.22, CI 1.5, 25.6, P = 0.009); and aPL+ |
| Whitelaw et al. (1999) [25] | 69 | Intelligence, visual reproduction, learning, executive function, auditory verbal learning | Pearson’s correlation, | Association not assessed | VBRs and aPL+ (r = −1.01, P = 0.0004) | Intelligence (r = 0.72, P = 0.0007); visual reproduction (r = −0.63, P = 0.003); learning (easy) (r = −0.71, P = 0.0009); executive function (r = −0.32, P = 0.05); auditory verbal learning (r =−0.69, P = 0.001); and aPL+ |
| Sarbu et al. (2015) [26] | 108 | No specific domains reported | χ 2 statistic (Fisher’s exact test) | Cognitive dysfunction and WMH (P = 0.045) | WMH (P = 0.018); microbleeds (P = 0.002); cortical atrophy (P = 0.008); and LA | Association not assessed |
| Steup-Beekman et al. (2013) [37] | 155 | No specific domains reported | Descriptive statistics | Association not assessed | Association not assessed | Association not assessed |
| Abda et al. (2013) [28] | 34 | Attention, memory, problem solving, visual-spatial processing, psychomotor speed | χ 2 statistic (Fisher’s exact test) | No statistical differences cognitive deficits and MRI abnormalities | Association not assessed | Association not assessed |
| Zirkzee et al. (2012) [29] | 71 | No specific domains reported | χ 2 statistic | Association not assessed | Association not assessed | Association not assessed |
| Emmer et al. (2008) [37] | 52 | No specific domains reported | Linear regression | Cognitive dysfunction and; lower MTR histogram peak for brain parenchyma (beta = −0.435, R = 0.664, P < 0.001); WM (beta = −0.445, R = 0.647, P < 0.001); GM (beta = −0.306, R = 0.663, P < 0.01) | aCL on MTR histogram parameters (ns) | Association not assessed |
| Cho et al. (2007) [38] | 25 (NPSLE), 18 (NBD) | No specific domains reported | χ 2 statistic | Association not assessed | Association not assessed | n = 3 patients with cognitive dysfunction were aPL+ (association not assessed) |
| Roldan et al. (2006) [39] | 28 (SLE), 28 (controls) | No specific domains reported | Fisher’s exact test | Association not assessed | Old cerebral infarcts and aPL+ and aCL (P < 0.001) | Association not assessed |
| fMRI (n = 1) | ||||||
| Kozora et al. (2016) [22] | 40 (cases), 10 (controls) | Executive function, working memory | Wilcoxon rank-sum test | Higher activation in bilateral frontal, temporal and parietal cortices during working memory and executive function tasks (P < 0.001) | Higher activation in bilateral frontal, temporal and parietal cortices and aPL+ (P < 0.001) | Higher activation in bilateral frontal, temporal and parietal cortices during working memory and executive function tasks for aPL+ (P < 0.001) |
| TCD (n = 2) | ||||||
| Zamproni et al. (2013) [22] | 27 | Global cognition and executive function | Mann–Whitney U test | Worse global cognition and executive function with sRLS (P < 0.05) | Association not assessed | Association not assessed |
| Cantú-Brito et al. (2010) [30] | 109 | Memory, attention, visuospatial construction | χ 2 statistic, logistic regression | Cognitive dysfunction and MES (P = 0.036), cognitive dysfunction and MES (beta = 0.61, P = 0.19) | MES and aCL (IgG) (ns) | Association not assessed |
| EEG and CT (n = 1) | ||||||
| Chapman et al. (2002) [24] | 23 | No specific domains reported | Fisher’s exact test | Association not assessed | Association not assessed | Association not assessed |
| OCT (n = 1) | ||||||
| Shulman et al. (2017) [36] | 21 (cases), 11 (controls) | No specific domains significant | Pearson correlation | RNFL thickness and global cognition (r = −0.17, P = 0.45); memory (r = 0.08, P = 0.70); executive function (r = −0.25, P = 0.26); attention (r = 0.14, P = 0.53); information processing speed (r = −0.18, P = 0.46); visual spatial (r = −0.26, P = 0.26); verbal function (r = 0.19, P = 0.42); motor skills (r = −0.28, P = 0.21) | Association not assessed | Association not assessed |
Anti-β2GPI: anti-β2 glycoprotein-I antibody; beta: coefficient for a multiple linear regression; EEG: electroencephalogram; fMRI: functional MRI; GM: grey matter; MES: micro-embolic signals; MTR: magnetization transfer ratio; NBD: neuroBehçet’s disease; ns: not statistically significant; OCT: optical coherence tomography; OR: odds ratio; P: statistical significance probability; R: correlation between predicted and observed values; RNFL: retinal nerve fiber layer; RR: relative risk ratio; r: Pearson’s correlation; SPM: statistical parametric mapping; sRLS: significant right to left shunt; TCD: transcranial Doppler; VBR: ventriculo brain ratios; WM: white matter; WMH: white matter hyperintensities; WML: white matter lesions. Bold text indicates results statistically significant.
Two studies assessed cognition using a global measure such as the Mini-Mental State Examination [37] or the Short Mental Test [33], whereas other studies included global cognition and other detailed neuropsychological batteries [30, 38, 40, 36, 20–23, 25, 29]. Some studies [34, 35, 21–24, 29] reported adherence to the neuropsychological battery for SLE suggested by the ACR and included the cognitive domains global cognition, simple/complex attention, memory, visuospatial processing, language, reasoning/problem solving, psychomotor speed, executive function [11]. There was heterogeneous use of neuropsychological batteries and in turn cognitive domains assessed across studies, except for where there was consistent use of the recommended ACR neuropsychological battery [34, 35, 21, 22, 24, 29]. A limited number of studies report specific cognitive domains affected and for those that did, memory and/or executive function were the most common domains to be identified [38, 39, 40, 22–24], followed by attention [40, 20, 24] (see Table 5). One study [33] examined the association of APS with dementia and included the Diagnostic and Statistical Manual of Mental Disorders, 4th edition [41] criteria for dementia to select the dementia cohort (56%).
APS criteria and aPL assessment
Eight studies included cohorts with APS [38, 32, 35, 31, 20, 25, 26], with three studies inclusive of patients with PAPS [38, 32, 20]; of the five NPSLE studies, ≤25% of these studies’ cohorts were defined as APS. Only three studies [32, 33, 20] were inclusive of cohorts that were aPL carriers and the frequency of aPL carriers ranged between 6 and 73% in the remaining studies (see Tables 3 and 4). Seven studies adhered to the Sapporo Criteria for inclusion of patients with APS or to indicate presence of aPL positivity at least twice, measured 12 weeks apart. Some studies [35, 31, 20, 27] reported using the original preliminary classification criteria for definite APS [42], whereas others, including some recent studies [38, 32, 21, 22], used the updated Sydney classification criteria [6]. The remaining other 13 studies included patients with aPL positivity and only one of these studies [25] reported that the presence of aPL was recorded at least twice over 12 weeks apart, whereas all other studies [30, 37, 33,34,39, 40, 36, 23, 24, 26, 27, 29] recorded the presence of aPL following a single sample and did not specify that aPL was retested to confirm persistence. A small number of studies included all three criteria aPL (LA; IgG and IgM aCL; and anti-β2GPI) [32, 20–22, 24, 25], with the combination aCL and LA as the most common included antibodies [38, 34, 35, 36, 28, 29] or aCL as the only included aPL [37, 33, 31, 26, 27]. Only five studies indicated their cut-off values for aPL [32,33,37,38, 24], with two of these studies using the Sapporo/Sydney laboratory criteria [38, 32]. One study made reference to single, double and triple aPL-positivity and reported these as 3 (15%), 6 (30%) and 11 (55%), respectively [21, 22]. Where aPL methods were specified, the analysis reported referred to the DRVVT and/or aPTT and Kaolin clotting time for LA, and the use of ELISA for aCL and anti-β2GPI.
Associations between imaging biomarkers and cognitive dysfunction
For studies inclusive of MRI biomarkers, these reported associations between white matter hyperintensities (WMH) or white matter lesions, ischaemic lesions, cerebral atrophy and cognitive dysfunction [34, 20]. Three studies [23, 24, 29] reported statistically significant associations between cortical atrophy and cognitive dysfunction. Studies including other imaging modalities also reported associations with cognitive dysfunction [38, 31, 26]. Four studies [33, 40, 21, 25] found no association between imaging biomarkers and cognitive dysfunction. Some studies did not examine the association between imaging biomarkers and cognitive function [30, 37, 32, 39, 35, 36, 27, 28] (see Table 5).
Associations between imaging biomarkers and aPL positivity
Two studies [32, 34] found associations between white matter changes and aPL positivity. [24, 28]. Four studies [37, 39, 34, 23] reported associations between cerebral atrophy and aPL positivity [37, 39, 34, 24] while other studies [30, 33, 31, 21, 26, 29] found no association between imaging biomarkers and aPL positivity. Some studies did not examine associations between imaging biomarkers and aPL positivity [38, 33, 35,36,40, 20, 25, 27] (see Table 5).
Associations between cognitive dysfunction and aPL positivity
For associations between cognitive dysfunction and aPL positivity, one study reported statistically significant associations for global cognition with positive aCL [participants were classified as aCL positive if the aCL titre (any isotype) was positive in the blood sample] [37]. Other studies found severity of cognitive deficits; executive dysfunction, complex attention, intelligence, visual reproduction, learning (easy) and auditory verbal learning to be associated with aPL positivity (aPL positivity was defined as levels of aCL >15 IgG phospholipid units/ml and levels of anti-β2GPI I IgG >20 IU/ml) [24], or aPL positivity not defined) [39]. One study reported that in aPL-positive patients (defined as a positive LA test; aCL IgG/IgM >40 units; and/or anti-β2GPI IgG/IgM >40 units; on two or more occasions), 45.5% with abnormal MRI findings were cognitively impaired [21], while another study reported 39% of APS patients had cognitive dysfunction and a trend towards higher levels of aPL [aCL 10–20 (elevated), >20 (high) GPL units] in demented APS patients but did not report it as statistically significant [33]. Six studies [30, 32, 33, 20, 21, 27] found no association between cognitive dysfunction and aPL positivity and over half of the included studies [38, 31,34–36,40, 23, 25, 26, 28, 29] did not examine this association (see Table 5).
Discussion
In this review, we summarized the literature regarding neuroimaging biomarkers used to identify neuropathology and cognitive dysfunction in APS/aPL-positive patients. Few studies have been inclusive of cognitive function and neuroimaging biomarker data in primary APS patients, and most studies available include SLE and NPSLE cohorts with aPL. There was vast heterogeneity between the 20 observational (case–control and cohort) included studies on various levels, from use of different cognitive assessment batteries, APS and aPL definitions and criteria, to wide variation in neuroimaging modalities. The quality assessment results for half of included studies was of a lower methodological quality, resulting in a higher risk of bias. There were more studies that included NPSLE cohorts in comparison with studies exclusive for PAPS and SAPS, which were all SLE-specific cohorts.
Prevalence and assessment of cognitive dysfunction in APS and aPL-positive patients
The prevalence range of cognitive dysfunction reported for APS and aPL-positive patients was diverse, with half of the studies documenting the rate to be 30% or higher in all APS, SLE and NPSLE cohorts. Similar figures have been previously reported for APS and aPL carriers [9, 43], and even higher rates of cognitive dysfunction for SLE and NPSLE patient cohorts [12]. Although there has been previous reporting of cognitive dysfunction in these patient groups [8, 13], only a limited number of studies, mainly with small sample sizes, have assessed cognitive function using standardized batteries, e.g. the ACR neuropsychological battery [11]. We included only one study that reported prevalence of dementia associated with APS to be 56% [33], which was also reported to be high in previous reviews [16, 44, 45]. It was not evident from the studies reviewed whether factors such as age, gender, education levels and possible cardiovascular risk factors are associated with cognitive dysfunction in APS and aPL carriers, as these variables were rarely controlled for where multivariate analysis was conducted.
Consistent patterns of cognitive dysfunction among the included studies were for specific domains memory, executive function and attention, where reported. This pattern of cognitive domains affected has been previously reported for APS and aPL carriers [9], and executive function for SLE, whereas verbal reasoning and visuo-spatial organization was found to be associated with NPSLE diagnosis [13]. The evidence indicates that patients with APS and/or aPL (including associated autoimmune conditions, i.e. SLE or NPSLE) have some degree of cognitive dysfunction. The clinical presentation in terms of cognitive domains affected is similar to patterns associated with vascular cognitive impairment, including large vessel disease [46], subcortical small vessel disease and dementia [47, 48]. More importantly, none of the studies included in this review or those previously reported has assessed or detected the onset of a diagnosis of mild cognitive impairment. Insidious cognitive decline may be of great benefit to assess clinically for planning treatment interventions and where detected, offer further insight into the neuropathological basis of cognitive dysfunction in APS and aPL carriers.
APS criteria and aPL assessment
This review highlights the dearth of studies available focusing on primary APS and aPL carriers that examine cognitive dysfunction and include neuroimaging biomarker data. We found there was also a limited number of studies that assessed the presence of all three criteria aPL, adhered to the Sapporo Criteria, specified that aPL were persistent, or made reference to single, double and triple aPL-positivity [5] In order to determine the pattern of cognitive dysfunction, it is important to establish more homogeneous APS and aPL cohorts before extracting meaningful conclusions regarding associated cognitive status. There were also wide variations in technical differences in antibodies quantification, adding further to the heterogeneity issue in the cohorts included. Stricter adherence to the Sydney (update Sapporo) criteria, particularly the laboratory criteria, when selecting cohorts for inclusion in APS and aPL studies [4, 5] would improve generalizability when drawing conclusions from these patients’ groups.
Associations between neuroimaging biomarkers and cognitive dysfunction
As expected, cognitive dysfunction was found to be associated with white matter lesions or WMH, ischaemic lesions and cortical atrophy from studies inclusive of structural MRI. The high burden of WMH in APS patients has been referred to as resembling multi-infarct dementia as a result of vascular damage [49]. In other disease pathologies cognitive decline strongly correlates with cortical atrophy [50], which is also the finding for APS patients in this review indicating degenerative brain changes. The cognitive dysfunction may be explained by the small vessel ischaemic events and also by the underlying pathophysiology as a result of brain volume loss. Most of the studies did not examine or report if there were particular associations between specific cognitive domains affected and neuroimaging biomarkers’ findings. The only reported magnetization transfer imaging study revealed lower magnetization transfer ratio peak height of brain parenchyma, white matter and grey matter for NPSLE patients compared with healthy controls suggestive of axonal dysfunction and demyelination [26]. The transcranial Doppler studies were also supportive of the association between cognitive dysfunction and vascular damage, with patients that had significant right to left shunt or presence of microembolic signals having worse cognitive function. Although the evidence is targeted at understanding explanations for cognitive dysfunction in APS, the actual rate of cognitive change progression has not been studied despite the potential of neuroimaging biomarkers to detect pathological brain changes from a mild cognitive impairment diagnosis onwards.
Associations between neuroimaging biomarkers and aPL positivity
Significant associations were reported for WMH, cerebral infarcts and cortical atrophy with aPL positivity. WMH, microbleeds and cortical atrophy were associated with LA, and old cerebral infarcts and hippocampal volume loss with aCL. These findings are consistent with neuroimaging studies of patients with APS, in that Zhu et al. [2] found the main characteristics of neurological APS in the brain were ischaemic changes as in multifocal cerebral infarctions, white matter demyelination and cerebral atrophy. Kaichi et al. [14] also found similar MRI abnormalities, including large territorial infarctions, lacunar infarctions in the deep white matter, localized cortical infarctions in the middle cerebral artery territory, bilateral border zone infarctions, anterior basal ganglia lesions and stenotic arterial lesions, all of which were more common in SLE patients with APS. In an earlier review, Sanna et al. [51] also outlined similar brain involvement in aPL-positive patients. However, another recent study reported finding no difference in structural and functional brain connectivity in SLE patients vs controls according to neuropsychiatric involvement or aPL status [52]. Although we reported associations between neuroimaging biomarkers and aPL positivity, it is worth noting that the same number of studies found no association.
Associations between cognitive dysfunction and aPL positivity
Over half of the studies did not examine associations between cognitive dysfunction and aPL, despite inclusion of both variables in each of the studies in addition to neuroimaging biomarkers. Deficits in global cognition were found to be associated with aCL positivity and in terms of deficits in specific cognitive domains, executive dysfunction, complex attention, intelligence, visual reproduction and learning were associated with aPL positivity. The single study that used functional MRI reported higher brain activation in bilateral frontal, temporal and parietal regions during working memory and executive function tasks; the authors explained cortical over-activation as a compensatory mechanism for early white matter neuropathology [22]. There are no other reviews to our knowledge that compare specific cognitive domains affected with aPL positivity. The associations found between neuroimaging biomarkers and cognitive dysfunction are possibly best explained by neuronal impairments through vascular disease, e.g. thrombotic, immune or neuronal effects. There is increasing interest in understanding the pathophysiological process for cognitive dysfunction and APS, and more recent reviews have explored the association between APS and dementia, e.g. aPL and dementia [16] and the evidence between SLE and dementia [15]. Cognitive dysfunction and APS has been mainly explained by hypercoagulability, as aPL are likely to attack vascular endothelial cells, activating the inflammatory response and coagulation cascade, which results in occlusive thrombosis leading to progressive compromise of neural activity and a resulting decline in cognitive function and ultimately vascular dementia [15]. Despite the fact that cognitive dysfunction cannot be explained exclusively by thrombotic events or hypercoagulability, stroke and transient ischaemic attack are the only included neurological manifestations in the 2006 APS criteria [53].
Limitations and other confounders for consideration
Seven of the included studies were retrospective with cohorts selected from referrals (potentially leading to selection bias) or patient registries. Moreover, the duration of disease varied widely across studies and was not controlled for in multivariate analysis. Given the association between cognition and mood, greater inclusion and investigation of depression scales are also warranted in future. Regional or ethnic differences were also not identified in the cohorts included, which adds further to the sampling heterogeneity within APS studies [54]. Other antibodies, either non-criteria aPL or other antibodies, may play a role in the pathogenesis of neural damage and associated brain pathology, and thus also account for cognitive dysfunction in patients with APS, e.g. noncriteria aPL such as anti-phosphatidylserine/prothrombin antibodies, lymphocytotoxic antibodies [55], antiglutamate receptor antibodies [56], brain-derived neurotrophic factor [57], anti-ribosomal P [58] and MMP-9 [59]. Other confounders that may interfere with results reported is the use of medications such as thrombolytic and CS therapies. Correlations between cognition and neuroimaging were inconsistent; indeed, six of the studies included found no correlation. We acknowledge the small sample sizes, which limit the precision of studies reporting correlations between cognitive and brain imaging findings; moreover, heterogeneity of cognitive measures and neuroimaging ratings do not allow definitive conclusions on these complex relationships. In conclusion, multicentre studies in representative populations with standardized image acquisition and protocols, including clearer definitions of the clinical populations using international clinical and laboratory criteria for APS, are required.
Nevertheless, our findings confirm that cognitive impairment is commonly found in patients with aPL (including those with APS, SLE and NPSLE). The correlations of cognition with neuroimaging biomarkers suggest that neuroimaging studies should be incorporated in research and clinical practice to understand mechanisms of cognitive impairment in patients with aPL. Ultimately, determining and investigating the strength of the association between neuroimaging biomarkers and cognitive impairment in APS/aPL-positive patients could in future guide clinicians in symptomatic or disease-modifying treatment strategies.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: C.D. declares no conflicts of interest. H.C. reports institutional research support and support to attend scientific meetings from Bayer Healthcare, with honoraria for lectures from Bayer Healthcare and consultancy fees from Union Chimique Belge Biopharma paid to University College London Hospitals Charity, outside the submitted work. D.J.W. has received honoraria from Bayer, Alnylam and Portola, outside the submitted work.
Data availability statement
The authors confirm that the data supporting the findings of this review are available within the article.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Rodrigues CE, Carvalho JF, Shoenfeld Y.. Neurological manifestations of antiphospholipid syndrome. Eur J Clin Invest 2010;40:350–9. [DOI] [PubMed] [Google Scholar]
- 2. Zhu DS, Fu J, Zhang Y. et al. Neurological antiphospholipid syndrome: clinical, neuroimaging, and pathological characteristics. J Neurol Sci 2014;346:138–44. [DOI] [PubMed] [Google Scholar]
- 3. Gaspar P, Cohen H, Isenberg DA.. The assessment of patients with the antiphospholipid antibody syndrome: where are we now? Rheumatology (Oxford) 2020;59:1489–94. [DOI] [PubMed] [Google Scholar]
- 4. Devreese KMJ, de Groot PG, de Laat B. et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2020;18:2828–39. [DOI] [PubMed] [Google Scholar]
- 5. Devreese KMJ, Ortel TL, Pengo V, de Laat B; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost 2018;16:809–13. [DOI] [PubMed] [Google Scholar]
- 6. Miyakis S, Lockshin MD, Atsumi T. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 7. Cervera R, Serrano R, Pons-Estel GJ. et al. ; Euro-Phospholipid Project Group (European Forum on Antiphospholipid Antibodies). Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2015;74:1011–8. [DOI] [PubMed] [Google Scholar]
- 8. Erkan D, Kozora E, Lockshin MD.. Cognitive dysfunction and white matter abnormalities in antiphospholipid syndrome. Pathophysiology 2011;18:93–102. [DOI] [PubMed] [Google Scholar]
- 9. Yelnik CM, Kozora E, Appenzeller S.. Cognitive disorders and antiphospholipid antibodies. Autoimmun Rev 2016;15:1193–8. [DOI] [PubMed] [Google Scholar]
- 10. Popescu A, Kao AH.. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol 2011;9:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Rheumatology. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 12. Brey RL, Holliday SL, Saklad AR. et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology 2002;58:1214–20. [DOI] [PubMed] [Google Scholar]
- 13. Leslie B, Crowe SF.. Cognitive functioning in systemic lupus erythematosus: a meta-analysis. Lupus 2018;27:920–9. [DOI] [PubMed] [Google Scholar]
- 14. Kaichi Y, Kakeda S, Moriya J. et al. Brain MR findings in patients with systemic lupus erythematosus with and without antiphospholipid antibody syndrome. Am J Neuroradiol 2014;35:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Z, Rocha NP, Salem H, Diniz BS, Teixeira AL.. The association between systemic lupus erythematosus and dementia A meta-analysis. Dement Neuropsychol 2018;12:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Islam MA, Alam F, Kamal MA. et al. Presence of anticardiolipin antibodies in patients with dementia: a systematic review and meta-analysis. Front Aging Neurosci 2017;9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells GA, Shea B, O’Connell D. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19. Modesti PA, Reboldi G, Cappuccio FP. et al. ; ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS One 2016;11:e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arvanitakis Z, Capuano AW, Brey R. et al. Antiphospholipid antibodies: cognitive and motor decline, neuroimaging and neuropathology. Neuroepidemiology 2019;53:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Homayoon N, Schwingenschuh P, Hofer E, Katschnig-Winter P, Schmidt R.. Anticardiolipin antibodies are associated with cognitive dysfunction in stroke-free individuals. Eur J Neurol 2014;21:427–32.e21-2. [DOI] [PubMed] [Google Scholar]
- 22. Zamproni LN, Rubert MC, Zétola VF, Mader-Joaquim MJ, Lange MC.. Cognitive impairment and antiphospholipid syndrome: is paradoxical embolism the rule? Neurol Res 2013;35:890–4. [DOI] [PubMed] [Google Scholar]
- 23. Erkan D, Barbhaiya M, George D, Sammaritano L, Lockshin M.. Moderate versus high-titer persistently anticardiolipin antibody positive patients: are they clinically different and does high-titer anti-beta 2-glycoprotein-I antibody positivity offer additional predictive information? Lupus 2010;19:613–9. [DOI] [PubMed] [Google Scholar]
- 24. Chapman J, Abu-Katash M, Inzelberg R. et al. Prevalence and clinical features of dementia associated with the antiphospholipid syndrome and circulating anticoagulants. J Neurol Sci 2002;203–204:81–4. [DOI] [PubMed] [Google Scholar]
- 25. Whitelaw DA, Spangenberg JJ, Rickman R, Hugo FH, Roberts M.. The association between the antiphospholipid antibody syndrome and neuropsychological impairment in SLE. Lupus 1999;8:444–8. [DOI] [PubMed] [Google Scholar]
- 26. Sarbu N, Alobeidi F, Toledano P. et al. Brain abnormalities in newly diagnosed neuropsychiatric lupus: systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort. Autoimmun Rev 2015;14:153–9. [DOI] [PubMed] [Google Scholar]
- 27. Steup-Beekman GM, Zirkzee EJ, Cohen D. et al. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: epidemiology and radiology pointing to an immune-mediated cause. Ann Rheum Dis 2013;72: ii76–9. [DOI] [PubMed] [Google Scholar]
- 28. Abda EA, Selim ZI, Radwan ME. et al. Markers of acute neuropsychiatric systemic lupus erythematosus: a multidisciplinary evaluation. Rheumatol Int 2013;33:1243–53. [DOI] [PubMed] [Google Scholar]
- 29. Zirkzee EJ, Steup-Beekman GM, van der Mast RC. et al. Prospective study of clinical phenotypes in neuropsychiatric systemic lupus erythematosus; multidisciplinary approach to diagnosis and therapy. J Rheumatol 2012;39:2118–26. [DOI] [PubMed] [Google Scholar]
- 30. Cantú-Brito C, Baizabal-Carvallo JF, Alonso-Juárez M, García-Ramos G.. The clinical significance of microembolic signals in patients with systemic lupus erythematosus. Neurol Res 2010;32:134–8. [DOI] [PubMed] [Google Scholar]
- 31. Tektonidou MG, Varsou N, Kotoulas G, Antoniou A, Moutsopoulos HM.. Cognitive deficits in patients with antiphospholipid syndrome: association with clinical, laboratory, and brain magnetic resonance imaging findings. Arch Intern Med 2006;166:2278–84. [DOI] [PubMed] [Google Scholar]
- 32. Kozora E, Erkan D, Zhang L. et al. Cognitive dysfunction in antiphospholipid antibody (aPL)-negative systemic lupus erythematosus (SLE) versus aPL-positive non-SLE patients. Clin Exp Rheumatol 2014;32:34–40. [PubMed] [Google Scholar]
- 33. Kozora E, Uluğ AM, Erkan D. et al. Functional magnetic resonance imaging of working memory and executive dysfunction in systemic lupus erythematosus and antiphospholipid antibody-positive patients. Arthritis Care Res (Hoboken) 2016;68:1655–63. [DOI] [PubMed] [Google Scholar]
- 34. Appenzeller S, Bonilha L, Rio PA. et al. Longitudinal analysis of gray and white matter loss in patients with systemic lupus erythematosus. Neuroimage 2007;34:694–701. [DOI] [PubMed] [Google Scholar]
- 35. Tomietto P, Annese V, D’Agostini S. et al. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum 2007;57:1461–72. [DOI] [PubMed] [Google Scholar]
- 36. Shulman S, Shorer R, Wollman J, Dotan G, Paran D.. Retinal nerve fiber layer thickness and neuropsychiatric manifestations in systemic lupus erythematosus. Lupus 2017;26:1420–5. [DOI] [PubMed] [Google Scholar]
- 37. Emmer BJ, Steup-Beekman GM, Steens SC. et al. Correlation of magnetization transfer ratio histogram parameters with neuropsychiatric systemic lupus erythematosus criteria and proton magnetic resonance spectroscopy: association of magnetization transfer ratio peak height with neuronal and cognitive dysfunction. Arthritis Rheum 2008;58:1451–7. [DOI] [PubMed] [Google Scholar]
- 38. Cho B-S, Kim H-S, Oh S-J. et al. Comparison of the clinical manifestations, brain MRI and prognosis between neuroBeçhet’s disease and neuropsychiatric lupus. Korean J Intern Med 2007;22:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roldan CA, Gelgand EA, Qualls CR, Sibbitt WL Jr.. Valvular heart disease is associated with nonfocal neuropsychiatric systemic lupus erythematosus. J Clin Rheumatol 2006;12:3–10. [DOI] [PubMed] [Google Scholar]
- 40. Appenzeller S, Rondina JM, Li LM, Costallat LT, Cendes F.. Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis Rheum 2005;52:2783–9. [DOI] [PubMed] [Google Scholar]
- 41.Diagnostic and statistical manual of mental disorders: DSM-IV: 4th edn. Washington, DC: American Psychiatric Association 1994.
- 42. Wilson WA, Gharavi AE, Koike T. et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 1999;42:1309–11. [DOI] [PubMed] [Google Scholar]
- 43. Yelnik CM, Kozora E, Appenzeller S.. Non-stroke central neurologic manifestations in antiphospholipid syndrome. Curr Rheumatol Rep 2016;18:11. [DOI] [PubMed] [Google Scholar]
- 44. Gómez-Puerta JA, Cervera R, Calvo LM. et al. Dementia associated with the antiphospholipid syndrome: clinical and radiological characteristics of 30 patients. Rheumatology (Oxford) 2005;44:95–9. [DOI] [PubMed] [Google Scholar]
- 45. Mosek A, Yust I, Treves TA. et al. Dementia and antiphospholipid antibodies. Dement Geriatr Cogn Disord 2000;11:36–8. [DOI] [PubMed] [Google Scholar]
- 46. Hayes S, Donnellan C, Stokes E.. Executive dysfunction and balance function post-stroke: a cross-sectional study. Physiotherapy 2016;102:64–70. [DOI] [PubMed] [Google Scholar]
- 47. Wallin A, Román GC, Esiri M. et al. Update on vascular cognitive impairment associated with subcortical small-vessel disease. J Alzheimers Dis 2018;62:1417–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Donnellan C, Al Banna M, Redha N. et al. Predictors of vascular cognitive impairment poststroke in a Middle Eastern (Bahrain) cohort: a proposed case-control comparison. JMIR Res Protoc 2016;5:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fleetwood T, Cantello R, Comi C.. Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy. Front Neurol 2018;9:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mouton PR, Martin LJ, Calhoun ME, Dal Forno G, Price DL.. Cognitive decline strongly correlates with cortical atrophy in Alzheimer’s dementia. Neurobiol Aging 1998;19:371–7. [DOI] [PubMed] [Google Scholar]
- 51. Sanna G, Bertolaccini ML, Hughes GR.. Hughes syndrome, the antiphospholipid syndrome: a new chapter in neurology. Ann NY Acad Sci 2005;1051:465–86. [DOI] [PubMed] [Google Scholar]
- 52. Preziosa P, Rocca MA, Ramirez GA. et al. Structural and functional brain connectomes in patients with systemic lupus erythematosus. Eur J Neurol 2020;27:113. [DOI] [PubMed] [Google Scholar]
- 53. D’Angelo C, Franch O, Fernández-Paredes L. et al. Antiphospholipid antibodies overlapping in isolated neurological syndrome and multiple sclerosis: neurobiological insights and diagnostic challenges. Front Cell Neurosci 2019;13: 107. doi: 10.3389/fncel.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uthman I, Khamashta M.. Ethnic and geographical variation in antiphospholipid (Hughes) syndrome. Ann Rheum Dis 2005;64:1671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Denburg SD, Behmann SA, Carbotte RM, Denburg JA.. Lymphocyte antigens in neuropsychiatric systemic lupus erythematosus. Relationship of lymphocyte antibody specificities to clinical disease. Arthritis Rheum 1994;37:369–75. [DOI] [PubMed] [Google Scholar]
- 56. Gerosa M, Poletti B, Pregnolato F. et al. Antiglutamate receptor antibodies and cognitive impairment in primary antiphospholipid syndrome and systemic lupus erythematosus. Front Immunol 2016;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diniz BS, Teixeira AL.. Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. Neuromolecular Med 2011;13:217–22. [DOI] [PubMed] [Google Scholar]
- 58. Agmon-Levin N, Shoenfeld Y.. The spectrum between antiphospholipid syndrome and systemic lupus erythematosus. Clin Rheumatol 2014;33:293–5. [DOI] [PubMed] [Google Scholar]
- 59. Ainiala H, Hietaharju A, Dastidar P. et al. Increased serum matrix metalloproteinase 9 levels in systemic lupus erythematosus patients with neuropsychiatric manifestations and brain magnetic resonance imaging abnormalities. Arthritis Rheum 2004;50: 858–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this review are available within the article.