Abstract
Background
GCA is a systemic vasculitis of the elderly, viewed by many as a disease with multiple and overlapping clinical phenotypes. Retrospective studies have shown differences in clinical presentation between these phenotypes. To reflect the heterogeneity of GCA and novel diagnostic methods, new classification criteria have been proposed.
Methods
This is a retrospective study of newly diagnosed patients with GCA at the outpatient rheumatology clinics at Skåne University Hospital (Malmö and Lund) between 2012 and 2018. All patients were evaluated using two sets of classification criteria, the ACR classification criteria from 1990 and a proposed revision of these criteria requiring objective findings (positive biopsy or imaging) for classification. Patients were further classified as one of four widely used clinical phenotypes.
Results
A total of 183 patients with a new diagnosis of GCA were identified. The diagnosis was confirmed by one or two experienced rheumatologists in 116 of these patients during a review of medical records. The ACR criteria were more sensitive than the revised criteria (93.1% vs 72.4%), but the revised criteria had higher specificity (94.0% vs 28.4%). The revised criteria tended to have higher sensitivity in the phenotype with constitutional symptoms compared with cranial GCA (P = 0.08).
Conclusion
The specificity of the ACR classification criteria for GCA can be improved by using revised criteria requiring objective findings of vasculitis. In addition, the wider symptoms covered by the revised criteria may improve classification of patients with a phenotype characterized by constitutional symptoms.
Keywords: giant cell arteritis, classification criteria, phenotypes
Rheumatology key messages
Classification of giant cell arteritis can be improved by revising currently used criteria.
Giant cell arteritis includes several phenotypes with different baseline characteristics.
Introduction
GCA is a systemic vasculitis of the elderly, with the highest reported incidence in populations of Northern European ancestry [1]. In recent years, interest in extracranial manifestations of GCA has increased and many consider GCA to be a disease with several different clinical phenotypes, leading to a decline in the use of the term temporal arteritis [2, 3]. Retrospective studies have shown differences in clinical presentation between these phenotypes [4–6].
The current classification criteria for GCA were published in 1990 by the ACR and include age ≥50 years, localized pain in the head, temporal artery abnormality, ESR ≥50 mm/h and abnormal artery biopsy [7]. A suggested revision of these criteria has been published, adding extracranial symptoms, imaging of large vessels, extracranial arterial examination and elevation of CRP to the original criteria [3].
The purpose of this study was to evaluate proposed classification criteria and their performance in different clinical phenotypes of GCA.
Methods
This retrospective study included consecutive patients with a new GCA diagnosis (International Classification of Diseases, Tenth Revision: codes M31.5 and M31.6) from the outpatient rheumatology clinics at Skåne University Hospital (Malmö and Lund) between 2012 and 2018. Most patients seen at these clinics are referred from primary care or other providers in the local area (population ≥50 years of age ∼200 000). Patients who had been diagnosed with GCA before 2012 were excluded, as well as patients who were referred from other clinics for a second opinion.
All included patients were evaluated according to the 1990 ACR classification criteria [7] and the proposed revision outlined above [3]. The first set requires any three of the five criteria to be fulfilled in order to be classified as GCA, while the proposed revision requires positive temporal artery biopsy (TAB) and/or imaging (Supplementary Table S1, available at Rheumatology online). The diagnosis of GCA was confirmed or rejected based on a review of the clinical records of all included patients, including ≥9 months of follow-up, by one or two experienced rheumatologists. Sensitivity and specificity for the two sets of criteria were calculated using this review as a reference.
Patients with confirmed GCA were further classified as one of four clinical phenotypes [cranial GCA, large vessel GCA, GCA with a dominance of constitutional symptoms (‘constitutional GCA’) or GCA with isolated PMR symptoms (‘isolated PMR’)] by two reviewers based purely on the main symptoms at diagnosis. Patients with distinct cranial symptoms or symptoms from large vessels (e.g. peripheral ischaemia or chest pain) were primarily defined as cranial GCA and large vessel GCA, respectively, even if there were mild constitutional symptoms present. Constitutional symptoms included fever, malaise, weight loss and fatigue. Patients with large vessel involvement on imaging but no symptoms from large vessels were not defined as large vessel GCA. The final diagnosis and phenotype classification were based on consensus.
Additional baseline data were compared between phenotypes. The study was approved by the regional research ethics committee for southern Sweden.
Definitions
Visual symptoms at diagnosis were defined as any reported visual disturbance, such as visual loss, amaurosis fugax, double vision or blurry vision, in the weeks preceding diagnosis. PMR symptoms at diagnosis were defined as bilateral stiffness and/or pain in the neck and/or shoulders and/or pelvic girdle. Only laboratory parameters recorded before initiation of glucocorticoid treatment were documented. Positive TAB was defined as a biopsy containing a section of arterial tissue with mononuclear inflammatory cells and/or giant cells. The global judgement by the pathologist was also taken into account. Positive imaging for the purpose of the revised criteria was defined as halo sign on ultrasound examination; vessel wall thickening or contrast enhancement on MRI; aneurysm/ectasia, stenosis or vessel wall thickening on CT angiography (CTA); and increased uptake on 18-fluorodeoxyglucose (18-FDG) PET. Imaging findings that were better explained by atherosclerosis or detected more than 1 year prior to GCA diagnosis were not defined as positive imaging. Dichotomous variables were considered negative if not mentioned in the medical record, unless otherwise specified.
Statistical analysis
Differences in proportions were compared using the chi-square test or Fisher’s exact test and continuous variables were compared using the non-parametric Mann–Whitney U test or the parametric Student’s t test, as appropriate. Comparisons were made between patients with confirmed GCA and no confirmed GCA and between patients with cranial GCA and constitutional GCA. Patients with large vessel GCA and isolated PMR were too few to be assessed as separate entities in the statistical analyses. P-values <0.05 were considered statistically significant. Results were not corrected for multiple comparisons and were interpreted accordingly.
The classification criteria were characterized using sensitivity and specificity and their overall diagnostic accuracy was estimated using Youden’s J statistic (sensitivity + specificity − 1). Sensitivity and specificity in this population were presented with 95% CIs, based on the Clopper–Pearson interval.
Results
In total, 225 patients were identified. After pre-specified exclusions (see above), 183 patients remained for analysis (68.5% women, median age 74.3 years). The GCA diagnosis was confirmed in 116 patients and rejected in 67 patients. Among 100 patients evaluated by two rheumatologists, agreement on verified diagnosis was 85%. Additional baseline data are presented in Supplementary Table S2, available at Rheumatology online.
Sensitivity and specificity of the classification criteria
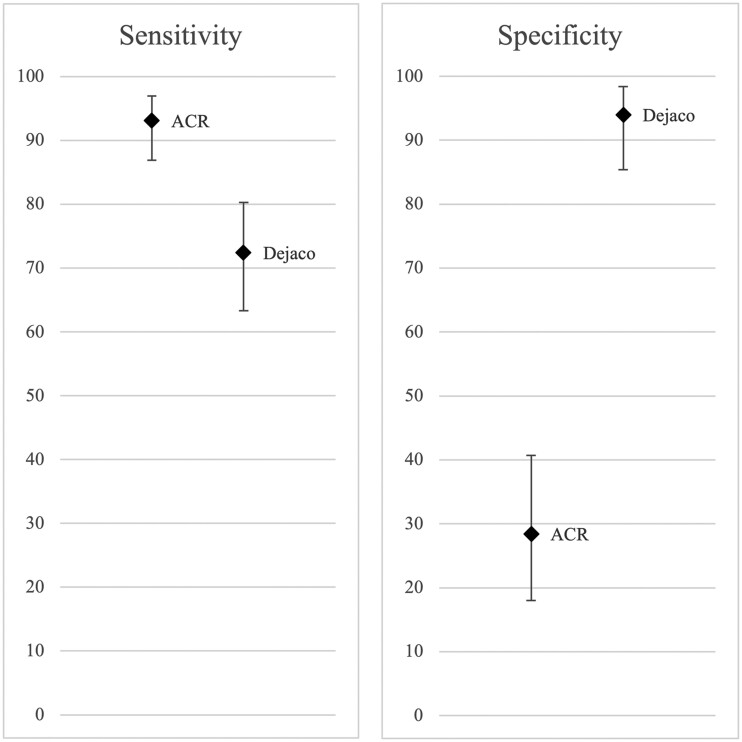
The ACR criteria had higher sensitivity (93.1% vs 72.4%), while the revised criteria had higher specificity (94.0% vs 28.4%), with non-overlapping CIs (Fig. 1). Youden’s J statistic was 0.22 for the ACR criteria and 0.66 for the revised criteria. Twenty-two patients (12%) in our cohort did not fulfil either of the criteria sets (Supplementary Table S3, available at Rheumatology online).
Fig. 1.
Sensitivity and specificity of two sets of classification criteria for GCA in %.
Error bars represent 95% CIs.
Differences between clinical phenotypes
Among the 116 patients with confirmed GCA, there were 89 patients with cranial GCA, 4 with large vessel GCA, 22 with constitutional GCA and 1 with isolated PMR. Inter-reviewer agreement for this classification was 79.4%. The sensitivity of the two sets of criteria did not differ significantly between phenotypes, but the revised criteria tended to be more sensitive among patients with constitutional GCA than with cranial GCA (86.4% vs 67.4%; P = 0.08). There was a greater difference in sensitivity between the criteria sets in cranial GCA (95.5% with ACR criteria vs 67.4% with revised criteria) than in constitutional GCA (90.9% with ACR criteria vs 86.4% with revised criteria) (Table 1).
Table 1.
Fulfilment of two sets of classification criteria in all patients with diagnosed GCA, by confirmation of GCA diagnosis and by phenotype of GCA.
| Criteria | All, n (%) | Confirmed GCA, n (%) | Rejected GCA, n (%) | P- value | Cranial, n (%) | Constitutional, n (%) | P- value |
|---|---|---|---|---|---|---|---|
| ACR 1990 [7] | 156 (85.2) | 108 (93.1) | 48 (71.6) | <0.001 | 85 (95.5) | 20 (90.9) | 1 |
| Age at disease onset ≥50 years | 183 (100) | 116 (100) | 67 (100) | NA | 89 (100) | 22 (100) | NA |
| Localized pain in the head | 145 (79.2) | 94 (81.0) | 51 (76.1) | 0.43 | 81 (91.0) | 11 (50.0) | <0.001 |
| Temporal artery abnormality | 108 (59.0) | 61 (53.0) | 47 (70.1) | 0.02 | 54 (60.7) | 7 (33.3) | 0.02 |
| Elevated ESRa | 117 (63.9) | 88 (75.9) | 29 (43.3) | <0.001 | 62 (69.7) | 21 (95.5) | 0.01 |
| Abnormal artery biopsy | 79 (43.2) | 79 (68.1) | 0 (0) | <0.001 | 59 (66.3) | 17 (77.2) | 0.32 |
| Revised (Dejaco et al. [3]) | 88 (48.1) | 84 (72.4) | 4 (6.0) | <0.001 | 60 (67.4) | 19 (86.4) | 0.08 |
| Age at disease onset ≥50 years | 183 (100) | 116 (100) | 67 (100) | NA | 89 (100) | 22 (100) | NA |
| Localized pain in the headb | 180 (98.4) | 114 (98.3) | 66 (98.5) | 1 | 89 (100) | 22 (100) | NA |
| Temporal artery abnormalityc | 111 (60.7) | 64 (55.2) | 47 (70.1) | 0.046 | 54 (60.7) | 9 (40.9) | 0.09 |
| Elevated ESRa and/or CRPd | 159 (86.9) | 110 (94.8) | 49 (73.1) | <0.001 | 83 (93.3) | 22 (100) | 0.60 |
| Abnormal artery biopsy revised e | 88 (48.1) | 84 (72.4) | 4 (6.0) | <0.001 | 60 (67.4) | 19 (86.4) | 0.08 |
ESR ≥50 mm/h. Revised criteria also included
visual symptoms, sight loss, PMR, constitutional symptoms, jaw and/or tongue claudication;
abnormality of extracranial arteries;
CRP ≥10 mg/l;
eabnormal artery biopsy or :abnormal imaging result (ultrasound, MRI and/or 18-FDG PET).
P-values are not corrected for multiple comparisons. The phenotypes large vessel GCA and isolated PMR are excluded due to too few cases. NA: not applicable.
Comparisons of individual criteria between phenotypes
Localized pain in the head and temporal artery abnormality were more common in cranial GCA (P < 0.001 and P = 0.02, respectively), while elevated ESR was more common in constitutional GCA (P = 0.01; Table 1).
Comparisons of individual criteria between confirmed and rejected GCA diagnosis
Patients with confirmed GCA had a positive TAB in 79 (68.1%) cases, while no patients without confirmed GCA were TAB positive (P < 0.001). The corresponding criterion in the revised set (positive TAB and/or imaging) was also more common in patients with confirmed GCA (72.4% vs 6.0%; P < 0.001). The patients with positive imaging in the group with rejected GCA diagnosis had possible halo sign on ultrasound (n = 1), ectasia of the ascending aorta on CTA (n = 1), aneurysm of the abdominal aorta on CTA (n = 1) and wall thickening of the aorta in combination with inferior mesenteric artery narrowing on CTA (n = 1), the latter considered to be due to atherosclerosis. Further, patients with confirmed GCA more frequently had both elevated ESR and elevated ESR and/or CRP (P < 0.001 for both), while they less frequently exhibited temporal artery abnormality and the corresponding revised criterion (P = 0.02 and P = 0.046, respectively; Table 1).
Discussion
To our knowledge, this is the first study to evaluate a proposed revision of the ACR classification criteria for GCA from 1990, previously not applied to actual patient cohorts, and to compare it with the currently used classification criteria. We also compared symptom-based clinical phenotypes of GCA in terms of classification and baseline patient characteristics.
In terms of diagnostic accuracy, the revised criteria had a higher Youden’s J statistic and higher specificity, although lower sensitivity, than the original criteria. The lower sensitivity is likely an effect of requiring a positive TAB and/or imaging, as TAB is a very specific but quite insensitive method [8] and the use of imaging for these purposes was limited at Skåne University Hospital during the study period.
No set of criteria is therefore currently more accurate than the other—the higher sensitivity of the ACR criteria would be desirable in epidemiologic studies aiming to identify all patients with the disease, for instance, while the higher specificity of the revised criteria would be better suited to patient selection for prospective studies. Furthermore, these were not meant to be diagnostic criteria, even though there is a need for such tools in clinical practice. This unmet need has led to the Diagnostic and Classification Criteria for Vasculitis (DCVAS) initiative [9, 10]. Finally, recent management guidelines emphasize the importance of objective confirmation of the disease [11, 12], which highlights the need for evaluation of new classification criteria.
Few previous studies have evaluated the distribution of clinical phenotypes of GCA based on symptoms at diagnosis [4–6]. Our results indicate that cranial GCA is the dominant phenotype in a cohort of consecutive GCA patients at a rheumatology outpatient clinic. Part of this can likely be explained by a greater awareness of the cranial phenotype of GCA among clinicians. A recent retrospective study from two French centres stratifying patients into clinical phenotypes based on symptoms reported a similar frequency of cranial GCA but more patients with large vessel GCA (9%) and fewer with constitutional GCA (9%) [5]. This difference could be a result of variations in definitions between the studies or differences in awareness of extracranial phenotypes of GCA between centres.
We did not observe a significant difference in sensitivity of the two sets of criteria between clinical phenotypes. Nonetheless, the revised criteria did show better sensitivity in the constitutional phenotype in this material. Whether the revised classification criteria are superior for patients with constitutional GCA warrants more attention in the future, especially as we move towards more widespread use of imaging in GCA patients, since this has the potential to increase the sensitivity of the revised criteria.
Limitations of this study are related to the retrospective design. There were some missing data in the medical records, which may represent information bias; namely, clinicians may have reported information that supports their preliminary diagnosis while leaving out other data. Further, clinicians may have used classification criteria for their clinical reasoning, particularly the 1990 ACR criteria, and therefore reported their findings in a way that would better fit into these criteria, which could bias the estimates. These problems are difficult to account for in a retrospective study and underline the need of prospective studies.
Another limitation is that this study only included patients from a combined secondary and tertiary centre. In Sweden, some GCA patients without complicating factors are managed in primary care. The patients in the study population may thus have more atypical symptoms than the average GCA patient, possibly leading to an overestimation of the utility of the revised classification criteria. Further, the patients in our study had a high pre-test probability of GCA, as they had received a diagnosis of GCA from a rheumatologist. This means that conclusions cannot be drawn with certainty about the larger group of patients with suspected GCA in primary care, where the pre-test probability may be lower. Consequently the new criteria also need to be evaluated in a population including patients in primary care.
A strength of this study is that it did not require a positive TAB for inclusion and consequently includes more patients with extracranial phenotypes of GCA. Another strength is the relatively large patient sample of consecutive patients from a secondary centre, thus limiting selection bias.
Conclusion
We report differences in sensitivity and specificity between two sets of classification criteria for GCA but no significant differences in sensitivity between clinical phenotypes of GCA. Our results are the first to show that the specificity of the ACR classification criteria can be improved by using a revised set of criteria requiring objective findings of vasculitis. In this population, the phenotype with constitutional symptoms constituted a minority that was more likely to fulfil criteria that include a wider range of symptoms.
Supplementary Material
Acknowledgements
The authors would like to thank Janet Kroon for assistance with the local patient register.
Disclosure statement: The authors have no conflicts of interest to declare.
Funding: This work was supported by the Swedish Research Council (grant 2015-02228), Swedish Rheumatism Association (grantR-664091) and Lund University (grant ALFSKANE-446501).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Sharma A, Mohammad A, Turesson C.. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: a systematic literature review. Semin Arthritis Rheum 2020;50:1040–8. [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 3. Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B.. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017;56:506–15. [DOI] [PubMed] [Google Scholar]
- 4. de Boysson H, Lambert M, Liozon E. et al. Giant-cell arteritis without cranial manifestations: working diagnosis of a distinct disease pattern. Medicine (Baltimore) 2016;95:e3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Boysson H, Liozon E, Ly KH. et al. The different clinical patterns of giant cell arteritis. Clin Exp Rheumatol 2019; 37 (Suppl 117):57–60. [PubMed] [Google Scholar]
- 6. Hamidou MA, Batard E, Trewick D. et al. Silent versus cranial giant cell arteritis. Initial presentation and outcome of 50 biopsy-proven cases. Eur J Intern Med 2005;16:183–6. [DOI] [PubMed] [Google Scholar]
- 7. Hunder GG, Bloch DA, Michel BA. et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–8. [DOI] [PubMed] [Google Scholar]
- 8. Rubenstein E, Maldini C, Gonzalez-Chiappe S, Chevret S, Mahr A.. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford) 2020;59:1011–20. [DOI] [PubMed] [Google Scholar]
- 9. Craven A, Robson J, Ponte C. et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol 2013;17:619–21. [DOI] [PubMed] [Google Scholar]
- 10. Watts RA. Evolving concepts in classification of systemic vasculitis: where are we and what is the way forward? Int J Rheum Dis 2019;22:1:21–7. [DOI] [PubMed] [Google Scholar]
- 11. Turesson C, Börjesson O, Larsson K, Mohammad AJ, Knight A.. Swedish Society of Rheumatology 2018 guidelines for investigation, treatment, and follow-up of giant cell arteritis. Scand J Rheumatol 2019;48:259–65. [DOI] [PubMed] [Google Scholar]
- 12. Hellmich B, Agueda A, Monti S. et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.